Abstract
Background
Granular cell tumors (GCT) in the gastrointestinal tract are rare. Herein, we describe a case of a gastric GCT diagnosed preoperatively by endoscopic ultrasound-guided fine needle aspiration biopsy (EUS-FNAB) and successfully resected by single-incision laparoscopic surgery (SILS).
Case presentation
A 46-year-old Japanese woman had a tumor located in the angle of the stomach that was approximately 1.5 cm in diameter. Abdominal computed tomography (CT) revealed a submucosal tumor (SMT), which was finally diagnosed as a gastric GCT using EUS-FNAB. The tumor was not identified by CT 1 year and 4 months before diagnosis; therefore, because there was a possibility that the tumor was malignant, we performed surgical wedge resection using SILS. The patient had an uneventful recovery postoperatively and was discharged without complications 3 days after surgery. The tumor was pathologically diagnosed as a benign GCT that remained within the muscular layer. No recurrence or complications have occurred in the first 16 months since the surgery.
Conclusion
Because gastric GCTs are generally benign and are rarely associated with lymph node metastasis, SILS seems to be a safe and feasible surgical approach for treating GCTs.
Keywords: Granular cell tumor, Stomach, SILS, EUS-FNAB
Background
Granular cell tumors (GCTs), which were first reported by Weber in 1854 [1], are infrequent mesenchymal soft-tissue tumors derived from Schwann cells and are generally benign [2–4]. They can be found at many sites, including the skin, tongue, subcutaneous tissues of the chest, upper extremities, and female genital area [5, 6]. However, GCTs in the gastrointestinal tract are uncommon and comprise approximately 4–6% of all GCTs [6]. The most common location for GCTs in the gastrointestinal tract is the esophagus, while the second most common site is the colon [7]; gastric localization is very uncommon for GCTs.
Herein, we describe a case of GCT that was successfully diagnosed by endoscopic ultrasound-guided fine needle aspiration biopsy (EUS-FNAB) preoperatively and resected by single-incision laparoscopic surgery (SILS), which is a minimally invasive surgical technique.
Case presentation
A 46-year-old Japanese woman underwent computed tomography (CT) or ultrasonography (US) examinations every year since the detection of a giant hepatic hemangioma (Fig. 1a, b). A follow-up CT scan incidentally detected a tumor that was approximately 1.5 cm in diameter at the angle of the stomach in the anterior gastric wall (Fig. 1a, b), which had not appeared in the CT image 1 year and 4 months previously (Fig. 1c, d). The subsequent examinations by upper gastrointestinal endoscopy (UGE) and abdominal US revealed that it was a submucosal tumor (SMT) with homogeneous low echoes (Fig. 1e, f). Physical examination, biochemical data, tumor markers (including carcinoembryonic antigen (CEA), carbohydrate antigen 19-9 (CA19-9), cancer antigen 125 (CA125), and squamous cell carcinoma (SCC)), and chest and abdominal X-ray films were all within normal limits. Endoscopic US (EUS) (20 MHz) was also performed to evaluate clinical characteristics of this tumor; it revealed that the tumor had developed from the fourth layer of the gastric wall, which corresponds to the muscular layer, and had homogeneous low echoes (Fig. 1g). A small amount of tissue sample was taken using the EUS-FNAB technique (Fig. 1h) and was provided for pathological examinations. As a result, the pathological findings showed several spindle-shaped cells with small circular nuclei and a granular eosinophilic cytoplasm. Furthermore, immunohistochemical staining revealed that S-100 was positive (Fig. 2a, b); c-kit, CD34, and desmin were negative. Based on these results, the tumor was diagnosed as a GCT.
Fig. 1.
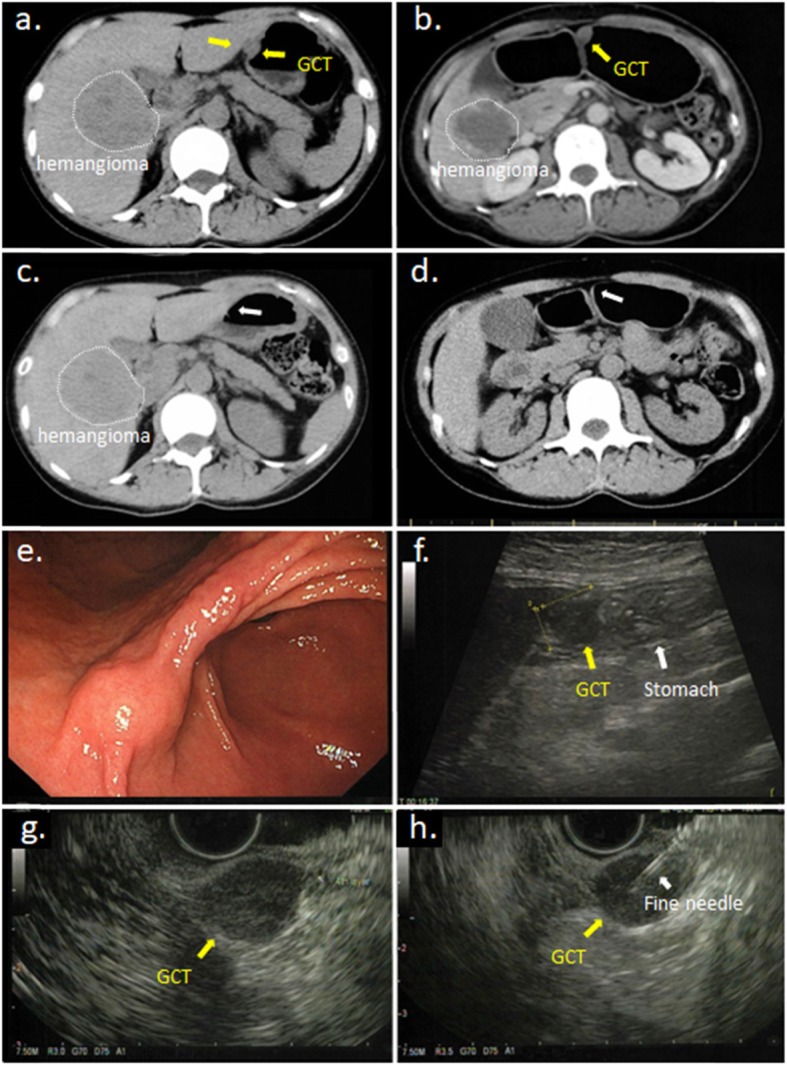
a Abdominal computed tomography (CT) revealed a solid extramural tumor situated at the lesser curvature of the lower body of the stomach (yellow arrow). The portion surrounded by white dotted line is hepatic hemangioma. b CT using foaming agent showed it more clearly. c The CT scan performed one year ago and d 4 years ago showed the tumor did not exist (white arrow). e Gastroscopy revealed submucosal tumor at the anterior side of the angle of the stomach. f Abdominal ultrasound sonography showed a low echoic mass measuring 15 mm in diameter existed in contact with the stomach. g Endoscopic ultrasound sonography (EUS) showed that the tumor of about 15 mm in diameter occurred from the fourth layer of the gastric wall and h the needle of EUS-guided fine needle aspiration biopsy (EUS-FNA) surely punctured to the tumor
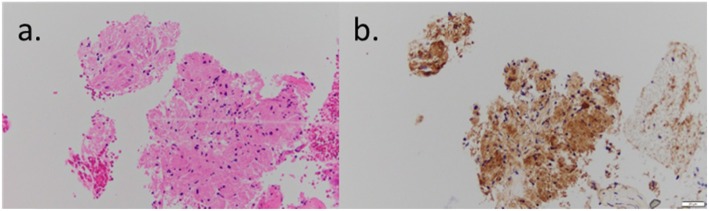
Fig. 2.
The specimens by endoscopic ultrasound sonography-guided fine needle aspiration biopsy (EUS-FNAB) using a hematoxylin-eosin staining (× 400) and b immunohistochemical staining for S-100 protein (× 400)
Conservative management was considered as a therapeutic option due to the benign nature of most GCTs; in addition, the tumor size was smaller than 2 cm and no swollen lymph nodes were observed. However, it was clear that this tumor was new and had grown quickly between CT examinations; thus, there was a possibility that this tumor was malignant. Consequently, we decided to resect the tumor using local resection without lymph node dissection. Considering the cosmetic appearance of the wound in the young female patient, we decided to use the SILS method to perform a minimally invasive surgery.
Surgical procedure by SILS
We placed a 3-cm skin incision on the transumbilical plane and equipped the incision site with the Lap Protector mini™ (Hakko Co. Ltd., Tokyo, Japan) and EZ access (Hakko Co. Ltd., Tokyo, Japan). We used an 11-mm trocar and two 5-mm trocars and inserted them into the abdominal cavity through the EZ access. No other assist ports were placed. When the flexible scope for laparoscopic surgery was inserted into the abdominal cavity, the tumor could be seen at the angle of the anterior wall of the stomach as a small white colored nodule slightly protruding from the gastric wall. It was covered with a thin serosal capsule and had a smooth surface (Fig. 3a). The lesser omentum near the tumor was dissected using a bipolar-tissue sealing system (ENSEAL®G2 with Articulating head, Ethicon Endo-Surgery Inc., Cincinnati, OH) in order to perform complete resection with an adequate surgical margin. The cutting line was confirmed with forceps before resection of the tumor (Fig. 3b). After these procedures, the tumor was resected with an auto-suturing device (Signia™ Stapling System, Covidien Japan, Inc., Tokyo, Japan) (Fig. 3c). Finally, after verifying that hemostasis was achieved and that there was no suture leakage of the resected stump, the wound was closed in two layers, using a standard protocol.
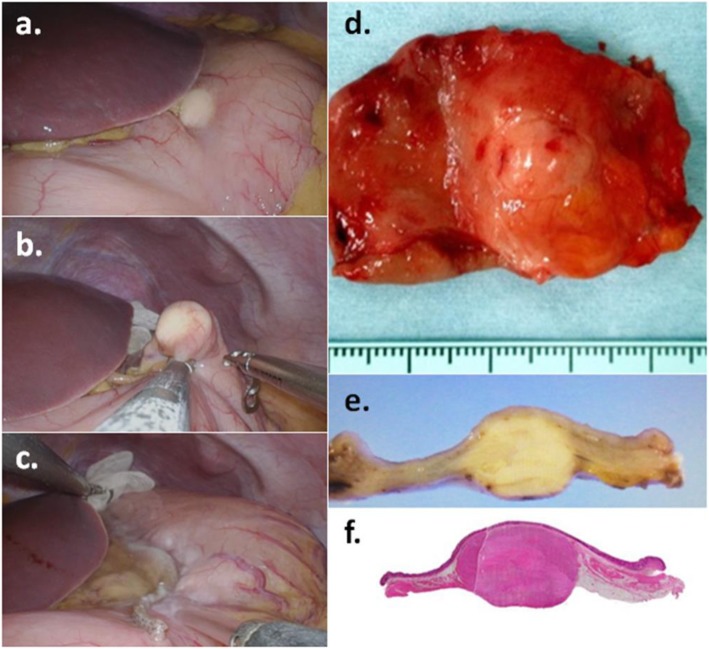
Fig. 3.
The tumor could be seen at the angle of the stomach (a) and was resected with auto suturing device; Signia™ Stapling System (b, c). The tumor was an elastic soft mass with a thin fibrous capsule and measured 15 × 15 mm in diameter (d, e, f)
The resected tumor was an elastic soft mass with a thin fibrous capsule, was 15 × 15 mm in size (cross-section), and was located in the proper muscular layer of the stomach wall (Fig. 3d–f). Hematoxylin and eosin staining revealed circular-shaped or spindle-shaped cells with small circular nuclei and a granular eosinophilic cytoplasm, which was proliferated densely within the tumor (Fig. 4a); the tumor had only a partial capsule (Fig. 4b). Immunohistochemical staining showed that the S-100 protein was detected in nearly all cells of the tumor (Fig. 4c), while c-kit and CD34 were negative (Fig. 4d, e). In addition, Ki-67 expression was observed in less than 1% of cells (Fig. 4f). Based on the above results, the resected tumor was diagnosed as a benign GCT. The patient had an uneventful recovery postoperatively and was discharged without complications three days after surgery. No recurrence or complications have occurred in the last 16 months since the surgery.
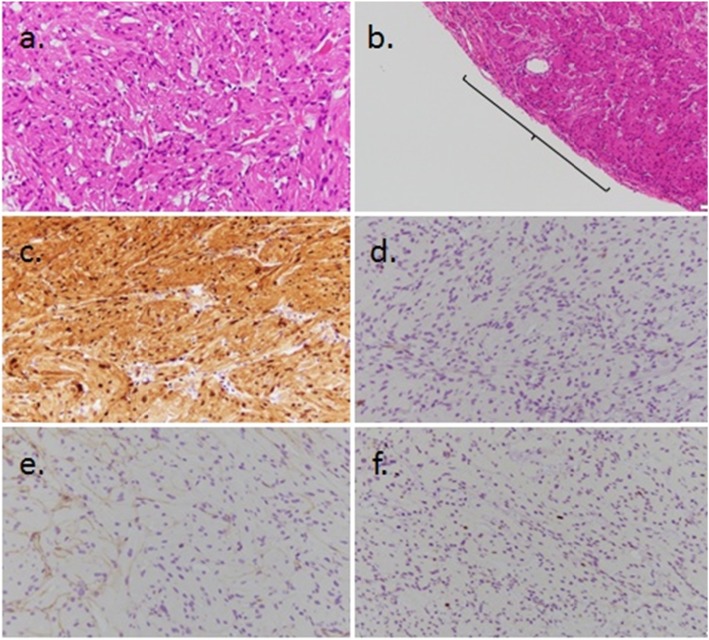
Fig. 4 .
a The specimens of surgically resected tumor using hematoxylin-eosin staining (× 400). b The capsule of the tumor was partially lacking using hematoxylin-eosin staining (× 100). c The specimens of surgically resected tumor using immunohistochemical staining for S-100 protein (× 400). d The specimens using immunohistochemical staining for c-kit (× 400). e The specimens using immunohistochemical staining for CD34 (× 400). f The specimens using immunohistochemical staining for Ki-67 (× 400)
Discussion
We have reported a case of a GCT that was diagnosed preoperatively using EUS-FNAB and treated with local excision using the SILS technique.
In recent years, advancements in endoscopy-related equipment have led to frequent detection of small SMTs. However, in cases of small SMTs, it is difficult to determine the most effective treatment strategy, even when considering the treatment policies and clinical guidelines for gastric SMTs and GCTs [8]; that is, many issues still remain. First, it may be challenging to diagnose SMT qualitatively and accurately. According to the guidelines, we added EUS and EUS-FNAB due to the presence of an SMT less than 2 cm in diameter and confirmation of tumor enlargement, in comparison to previous images. Fortunately, we were able to diagnose a GCT based on the pathological examination of the EUS-FNAB sample; however, in some cases, it can be challenging to obtain sufficient tissue to make an accurate pathological diagnosis. In Japan, there are only 9 known cases that have used EUS-FNAB to diagnose GCT. Smaller tumors are more difficult to diagnose accurately. Furthermore, because GCTs of the stomach are rare, there are no distinctive EUS images that are used to characterize GCTs. Search for the term gastric granular cell tumor in PubMed and Ichushi-Web (Japan Medical Abstracts Society; JAMAS) from inception to 2019 yielded 39 cases, including ours, in Japan (Table 1) [9–12] and 32 cases in other countries [13, 14].
Table 1.
Case reports of GCTs of the stomach in Japan
| Case | Author | Year | Treatment | Size (mm) | Pathological depth | Malignancy | Concomitant lesion |
|---|---|---|---|---|---|---|---|
| 1 | Tuneyosi | 1978 | * | 7 | * | ||
| 2 | Tuneyosi | 1978 | * | 7 | * | ||
| 3 | Tuneyosi | 1978 | * | 15 | * | ||
| 4 | Takagi | 2004 | * | 2 | * | ||
| 5 | Takagi | 2004 | Endoscopic resection | 6 | * | ||
| 6 | Tanimura | 1980 | Endoscopic resection | 10 | ≦sm | ||
| 7 | Asagi | 1981 | Endoscopic resection | 12 | ≦sm | ||
| 8 | Eda | 1988 | Endoscopic resection | 17 | ≦sm | ||
| 9 | Toyohara | 1991 | Endoscopic resection | 13 | ≦sm | ||
| 10 | Yasuda | 1995 | Endoscopic resection | 8 | ≦sm | ||
| 11 | Matsumoto | 1996 | Endoscopic resection | 6 | ≦sm | ||
| 12 | Wakasugi | 1996 | Endoscopic resection | 6 | ≦sm | ||
| 13 | Nakazawa | 2000 | Endoscopic resection | 10 | ≦sm | ||
| 14 | Katujima | 2003 | Endoscopic resection | 12 | ≦sm | ||
| 15 | Kato | 2012 | Endoscopic resection | 20 | ≦sm | ||
| 16 | Ozawa | 2010 | Endoscopic resection | 9 | mp | ||
| 17 | Tomura | 1969 | Gastrectomy | 10 | ≦sm | Neurinoma | |
| 18 | Saito | 1975 | Gastrectomy | 3 | ≦sm | Gastric cancer and leiomyoma | |
| 19 | Arima | 1978 | Gastrectomy | 10 | ≦sm | Gastric ulcer | |
| 20 | Hamada | 1984 | Gastrectomy | 13 | ≦sm | ||
| 21 | Ozaki | 1988 | Gastrectomy | 8 | ≦sm | ||
| 22 | Tuchida | 1989 | Gastrectomy | 20 | ≦sm | Gastric ulcer | |
| 23 | Xuan | 1992 | Gastrectomy | 12 | ≦sm | Gastric cancer | |
| 24 | Hirai | 1995 | Gastrectomy | 20 | ≦sm | ||
| 25 | Fujii | 2001 | Gastrectomy | 10 | ≦sm | Gastric cancer | |
| 26 | Eguchi | 2002 | Gastrectomy | * | ≦sm | Gastric cancer and malignant lymphoma | |
| 27 | Nishimori | 1991 | Gastrectomy | 18 | mp | Duodenal ulcer | |
| 28 | Matsumoto | 1996 | Gastrectomy | 70 | mp | (+) | |
| 29 | Maekawa | 2003 | Gastrectomy | 15 | mp | Esophageal GCT | |
| 30 | Kobayasi | 1971 | Nucleation | 10 | ≦sm | Gastric ulcer | |
| 31 | Nagaoka | 1997 | Nucleation | 20 | ≦sm | (+) | |
| 32 | Ikeda | 1977 | Wedge resection | 20 | ≦sm | ||
| 33 | Yamaguti | 1989 | Wedge resection | 15 | ≦sm | Gastric cancers | |
| 34 | Sano | 2008 | Wedge resection | 11 | ≦sm | ||
| 35 | Nomura | 1973 | Wedge resection | 10 | mp | Gastric ulcer | |
| 36 | Sano | 2008 | Wedge resection | 12 | mp | ||
| 37 | Niwatari | 2009 | Wedge resection | 45 | mp | ||
| 38 | Iwanaga | 2019 | Wedge resection (LECS) | 10 | ≦sm | ||
| 39 | Our case | 2019 | Wedge resection | 15 | mp |
The second issue is that a routine treatment strategy has not been established for GCTs. It is important and valuable to get an accurate diagnosis, but it is not required when deciding the appropriate treatment plan. Although GCTs are benign in most cases, the possibility of malignancy of the SMT should not be denied preoperatively. Accordingly, GCTs should be resected and, ideally, detected early and excised using minimally invasive procedures.
The third issue involves the resection of GCTs; there is controversy regarding the resection method that should be used for GCTs. According to previous Japanese reports, endoscopic resection was performed in 12 of 34 resected gastric GCT cases, excluding our case, and no local recurrence was observed. Twenty-five of these 34 GCTs (75.6%) originated pathologically from the mucosal or submucosal layer of the stomach wall, and all tumors were ≤ 2 cm in diameter (Table 1) [9–12]; based on these findings, endoscopic resection might be a feasible treatment option. However, tumor invasion into the proper muscular layer was observed in 7 of 34 resected cases, excluding one with an unknown tumor depth; 5 of these tumors which were < 20 mm in size. Considering these data and that endoscopic resection was mainly performed for diagnostic purposes, endoscopic resection should be performed for GCTs with caution.
On the other hand, as shown in Table 1, 23 GCTs were resected surgically in Japan. Fourteen of these tumors were resected surgically due to their size or the presence of comorbidities. Gastrectomy was performed in 13 cases, wedge resection was performed in 8 cases, and enucleation was performed in 2 cases (Table 1) [9–12]. Although the short-term and long term outcomes were inconsistent in the previous reports, the cases did not show recurrence, regardless of the type of surgery performed; thus, we considered that if the tumor is completely removed, the minimum surgical margin seems to be adequate.
The fourth issue concerns the need for lymphadenectomy. There were some reports of malignant GCTs of the esophagus and the breast with regional lymph node metastasis; thus, regional lymph node dissection and tumor resection are recommended in cases of malignant GCTs [15, 16]. However, regarding GCTs in other regions, including the stomach, no studies have reported GCTs with lymph node metastasis. This is most likely because gastric GCTs are rarely malignant. There are only two cases of malignant gastric GCTs reported in Japan [14, 17], and lymph node metastasis was not observed in either of these cases. Due to the limited reported cases of malignant gastric GCTs, the clinical feasibility of lymph node dissection is not definitive.
The final issue regards the determination of the proper surgical approach; open surgery, conventional laparoscopic surgery, and SILS are the most commonly used surgical techniques for GCTs. Recently, some reports have resected gastric SMTs, including gastrointestinal stromal tumors, using the SILS technique [18–20]; we also used the SILS technique, which was established in 2006. As described above, local resection is acceptable for benign or low-grade malignant gastric SMTs without lymph node metastasis. In our case, the tumor was small (< 2 cm in diameter) and was located in the anterior wall of the angle of the stomach, which is easily accessible. Therefore, we decided that SILS could be used safely and reliably to resect the tumor. The SILS approach has great advantages, in terms of alleviation of surgical stress and better cosmetic outcomes; thus, it should be taken into consideration as a feasible surgical approach in cases of small tumors that are non-malignant (or have a very low risk of becoming malignant) or when lymph node dissection is not necessary. In the recent years, laparoscopy and endoscopy cooperative surgery (LECS) [21] was a good indication for this kind of SMT and could be one of the options in this case; however, in this study, we adopted the SILS method that has been used and become familiar.
Conclusion
In conclusion, our case study suggests that the SILS technique is a safe and feasible surgical procedure for gastric GCTs. However, because the necessity of lymph node dissection is still controversial, the malignant potential of gastric GCTs remains unclear, and the reliable implementation of SILS requires surgeons to have adequate skills and experience for conventional laparoscopic surgery, indications for surgery should be carefully tailored for each case. Because gastric GCTs are rare, it is also important to accumulate cases of GCTs and continually examine optimal treatment methods, in the future.
Acknowledgements
Not applicable.
Rights and permissions
Open Access. This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Abbreviations
- CT
Computed tomography (CT)
- EUS-FNAB
Endoscopic ultrasound-guided fine needle aspiration biopsy
- GCTs
Granular cell tumors
- SILS
Single-incision laparoscopic surgery
- SMT
Submucosal tumor
- US
Ultrasonography (US)
Authors’ contributions
AY, YK, and TY performed the surgery and perioperative management on the patient, and AY, YK, and TY drafted the manuscript. All authors read and approved the final manuscript.
Funding
There is no funding for this work.
Availability of data and materials
All datasets supporting the conclusions of this article are included within the article.
Ethics approval and consent to participate
This article does not contain any studies with human or animal subjects performed by any of the authors.
Consent for publication
Written informed consent was obtained from the patient for publication of this report and the use of any accompanying images.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weber CO. Anatomische Untersuchung einer hypertrophischen Zunge nebst Bemerkungen über die Neubildung quergestreifter Muskelfasern. Virchows Arch A Pathol Anat. 1854;7:115–125. doi: 10.1007/BF01936232. [DOI] [Google Scholar]
- 2.Cavaliere A, Sidoni A, Ferri I, Falini B. Granular cell tumor: an immunohistochemical study. Tumori. 1994;80:224–228. doi: 10.1177/030089169408000312. [DOI] [PubMed] [Google Scholar]
- 3.Stefansson K, Wollmann RL. S-100 protein in granular cell tumors (granular cell myoblastomas) Cancer. 1982;49:1834–1838. doi: 10.1002/1097-0142(19820501)49:9<1834::AID-CNCR2820490916>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 4.Fisher ER, Wechsler H. Granular cell myoblastoma–a misnomer. Electron microscopic and histochemical evidence concerning its Schwann cell derivation and nature (granular cell schwannoma) Cancer. 1962;15:936–954. doi: 10.1002/1097-0142(196209/10)15:5<936::AID-CNCR2820150509>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 5.Khansur T, Balducci L, Tavassoli M. Granular cell tumor. Clinical spectrum of the benign and malignant entity. Cancer. 1987;60:220–222. doi: 10.1002/1097-0142(19870715)60:2<220::AID-CNCR2820600217>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 6.Lack EE, Worsham GF, Callihan MD, et al. Granular cell tumor: a clinicopathologic study of 110 patients. J Surg Oncol. 1980;13:301–316. doi: 10.1002/jso.2930130405. [DOI] [PubMed] [Google Scholar]
- 7.Nakachi A, Miyazato H, Oshiro T, Shimoji H, Shiraishi M, Muto Y. Granular cell tumor of the rectum: a case report and review of the literature. J Gastroenterol. 2000;35(8):631–634. doi: 10.1007/s005350070064. [DOI] [PubMed] [Google Scholar]
- 8.Nishida T, Hirota S, Yanagisawa A, et al. Clinical practice guidelines for gastrointestinal stromal tumor (GIST) in Japan. Int J Clin Oncol. 2008;13(5):416–430. doi: 10.1007/s10147-008-0798-7. [DOI] [PubMed] [Google Scholar]
- 9.Ozawa T, Wachi E. A case of granular cell tumor of the stomach extending into the muscularis propria that presented with unusual findings on EUS image. Gastroenterol Endosc. 2011;53(19):3236–3294. [Google Scholar]
- 10.Kato M, Nishida T, Morii E, et al. A case of granular cell tumor of the stomach with gradual enlargement during a 30 month-follow-up. Gastroenterol Endosc. 2012;54:1819–1826. [Google Scholar]
- 11.Hirai K, Satoh N, Tamegai Y, et al. A case of granular cell tumor in the stomach. The Japanese Journal of Gastroenterological Surgery. 1995;28(5):1076–1080. doi: 10.5833/jjgs.28.1076. [DOI] [Google Scholar]
- 12.Iwanaga A, Ogada T, Kobayashi T, et al. Gastric granular cell tumor successfully treated with laparoscopic endoscopic cooperative surgery: a case report. Kurume Med Assoc. 2019;82(4-5):210–217. [Google Scholar]
- 13.Blandamura S, Altavilla G, Castoro C, et al. Cornbined granular cell tumor of the oesophagus and stomach: a case report and review of the literature. ltal J Gastroenterol. 1993;25:259–264. [PubMed] [Google Scholar]
- 14.Nagaoka S, Bandoh T, Toyoshima H, et al. A case of malignant granular cell tumor of the stomach. Gastroenterol Endosc. 1997;39:1799–1804. [Google Scholar]
- 15.Takebayashi Katsushi, Kinoshita Yoshihiro, Ozawa Tsuyoshi, Nakagawa Masatoshi, Tanaka Tsuyoshi, Ogawa Masako, Ehara Kazuhisa, Ueno Masaki, Udagawa Harushi. A Case of Malignant Granular Cell Tumor of the Esophagus with Multiple Liver Metastasis. The Japanese Journal of Gastroenterological Surgery. 2012;45(1):23–29. doi: 10.5833/jjgs.45.23. [DOI] [Google Scholar]
- 16.Akahane K, Kato K, Ogiso S, et al. Malignant granular cell tumor of the breast: case report and literature review. Breast Cancer. 2015;22(3):317–323. doi: 10.1007/s12282-012-0362-1. [DOI] [PubMed] [Google Scholar]
- 17.Matumoto H, Kojima Y, Inoue T, et al. A malignant granular cell tumor of the stomach: report of a case. Surg Today. 1996;26:119–122. doi: 10.1007/BF00311775. [DOI] [PubMed] [Google Scholar]
- 18.Hirano Yasumitsu, Watanabe Toru, Uchida Tsuneyuki, Yoshida Shuhei, Kato Hideaki, Hosokawa Osamu. Laparoendoscopic Single Site Partial Resection of the Stomach for Gastrointestinal Stromal Tumor. Surgical Laparoscopy, Endoscopy & Percutaneous Techniques. 2010;20(4):262–264. doi: 10.1097/SLE.0b013e3181e36a5b. [DOI] [PubMed] [Google Scholar]
- 19.Sasaki A, Koeda K, Nakajima J, et al. Single incision laparoscopic gastric resection for SMTs: report of three cases. Surg Today. 2011;41:133–136. doi: 10.1007/s00595-009-4204-5. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi T, Takeuchi H, Kawakubo H, et al. Single incision laparoscopic surgery for partial gastrectomy in patients with a gastric SMT. Am Surg. 2012;78:447–450. [PubMed] [Google Scholar]
- 21.Hiki N, Yamamoto Y, Fukunaga T, Yamaguchi T, Nunobe S, Tokunaga M, Miki A, Ohyama S, Seto Y. Laparoscopy and endoscopy cooperative surgery for gastrointestinal stromal tumor dissection. Surg Endosc. 2008;22(7):1729–1735. doi: 10.1007/s00464-007-9696-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All datasets supporting the conclusions of this article are included within the article.