Abstract
Cerebral toxoplasmosis is one of the neurological infections with high morbidity and mortality in patients with AIDS, so the accurate method for diagnosis of cerebral toxoplasmosis seems necessary. In this study, nested PCR assay using B1 gene was evaluated in diagnosis of toxoplasmosis in serum and peripheral blood mononuclear cell (PBMC) among HIV/AIDS patients. One hundred eight blood samples from HIV/AIDS patients, including four patients with cerebral toxoplasmosis and 104 HIV/AIDS patients without cerebral toxoplasmosis were evaluated for the Toxoplasma gondii antibodies using Enzyme Linked immunosorbent Assay. DNA of serum and PBMC of these patients were extracted and nested-PCR was carried out. Of 108 participants, 95 cases (88%) were positive for Toxoplasma IgG antibodies and one patient was found positive for Toxoplasma IgM antibody. In general, four patients, including three patients with cerebral toxoplasmosis, who were positive for Toxoplasma IgG antibodies and one patient without cerebral toxoplasmosis who was positive for Toxoplasma IgM antibody were found to be PCR positive. DNA of T. gondii was detected in both serum and PBMC in two cerebral toxoplasmosis patients; however DNA was detected in only PBMC in other cerebral toxoplasmosis patient. All cases with cerebral toxoplasmosis were also diagnosed by clinical and radiological manifestations. The results of this study showed that the numbers of positive samples by PCR in PBMC were higher than serum specimens for diagnosis of toxoplasmosis. If molecular method and immunological assay are complemented with magnetic resonance imaging, the results can be useful for diagnosis of cerebral toxoplasmosis.
Keywords: Toxoplasmosis, HIV, B1, PCR, Parasite, Opportunistic infection
Introduction
Opportunistic protozoan parasitic infections are among the most serious infections in patients with AIDS, organ transplant recipients and also cancer patients (Esteghamati et al. 2019; Khanaliha et al. 2015; Masoumi-Asl et al. 2019). Toxoplasma gondii (T. gondii) is an opportunistic apicomplexan protozoa that infects human and mammals worldwide. Almost one-third of the world’s population are infected by T. gondii in Europe, South America, Africa and Asia (Nissapatorn 2008).
Toxoplasma gondii can cause congenital toxoplasmosis during pregnancy in fetus and is dangerous for pregnant women with acquired toxoplasmosis (Dubey 2008). In addition, the relationship between depression and T. gondii infection and severity of depressive clinical symptoms in pregnant women has been reported (Shiadeh et al. 2016). T. gondii is a threatening disease in immunocompromised patients, like patients with acquired immune deficiency syndrome (AIDS) (Dubey 2008; Lee and Lee 2017; Luft et al. 1984), organ transplant recipients (Fricker-Hidalgo et al. 2009) and those with malignancy (Sayyahfar et al. 2015).
The reactivation of latent infection occurs in HIV/AIDS patients, causing life-threatening disease, like encephalitis (Hellerbrand et al. 1996). Cerebral toxoplasmosis is one of the most prevalent neurological opportunistic infections in AIDS patients in the last stages of disease (Luft and Chua 2000) and it is associated with the prevalence of T. gondii antibodies in a population (Holliman 1988).
Diagnosis is based on clinical manifestation, histological examination and serological tests using Enzyme Linked Immunosorbent Assay (ELISA) with various antigens (Khanaliha et al. 2012, 2014); however molecular methods are effective to detect the parasite in biological samples, such as Blood (Lamoril et al. 1996), Aqueous Humor (Bourdin et al. 2014), cerebrospinal fluids (Novati et al. 1994) using oligonucleotide primers. Molecular methods were used in several studies for diagnosis of toxoplasmosis in acute phase when serological tests are not sufficient. Nested-polymerase chain reaction (PCR) is a helpful technique for diagnosis of toxoplasmosis (Wahab et al. 2010). A serological assay is considered as the most challenging procedure, because specific antibodies may not be present in the early stages of infection, especially in immune deficient or pregnant patients (Sensini 2006).
The result of some previous studies have reported the usefulness of PCR with the B1 target gene (Burg et al. 1989; Jones et al. 2000). The improvement of a sensitive PCR protocol to identify T. gondii B1 gene can be effective in diagnosis of toxoplasmosis. Overall, the results of serology tests, molecular methods and imaging technologies, like computed tomography and magnetic resonance imaging (MRI) can be useful to diagnose toxoplasmosis (Rostami et al. 2018).
The aim of this study was evaluation of a PCR assay using B1 gene for diagnosis of toxoplasmosis in serum and PBMC among HIV/AIDS patients.
Materials and methods
One hundred eight blood samples from HIV/AIDS patients including four patients with cerebral toxoplasmosis and 104 HIV/AIDS patients without cerebral toxoplasmosis who were referred to hospitals associated with Iran University of Medical Sciences were collected from August 2017 to September 2018 and evaluated for the of T. gondii antibodies using Enzyme Linked immunosorbent Assay. One hundred and eight DNA samples of these patients were extracted and carried out by nested-PCR.
About 10 mL of peripheral blood with EDTA was collected for DNA extraction and PBMC preparation and 5 mL was used for immunological tests. The PBMC isolation was performed from 5 mL of blood with EDTA by density gradient centrifugation using Fycoll (Amersham Biosciences Europe Gmbh, Milan, Italy), according to the manufacturer protocol (Donyavi et al. 2019).
Blood samples were collected before special treatment for three patients with cerebral toxoplasmosis and in one of them Blood sample was collected on the first week of anti-toxoplasma therapy.
DNA extraction and PCR
The genomic DNA was extracted from the patient serum and PBMC obtained by using the QIAamp DNA minikit (Qiagen, Hilden, Germany); the primers were designed within B1 gene with amplification of 194-bp fragment.
B1-nested PCR
5′-TCAAGCAGCGTATTGTCGAG 663–682
5′-CCGCAGCGACTTCTATCTCT 949–930
5′-GGAACTGCATCCGTTCATGAG 694–714
5′-TCTTTAAAGCGTTCGTGGTC 887–868
PCR was performed in a 25 µL mixture containing the template (3 µL of DNA), 2.5 U Taq DNA polymerase, and 2.5 µL of 10 × PCR buffer, 20 pmol of each primer, 100 µmol dNTPs and 0.15 mmol MgCl2. Amplification was performed as follows with denaturation at 94 °C for 30 s followed by annealing at 54 °C for 45 s and finally an extension step at 72 °C for 30 s cycled 35 times and expected band was 287 bp. The second step was performed similar with first step except annealing temperature that was carried out with 45 °C, initial denaturation at 94 °C for 5 min and end temperature at 72 °C for 5 min was performed and the expected band was 194 bp. The PCR positive and negative control samples were tachyzoite of T. gondii RH strain and distilled water. The PCR products were electrophoresed in a 1.5% agarose gel (Fallahi et al. 2014) .
Sequencing of PCR products
A second round of PCR was performed for more confirmation of the T. gondii PCR result and the product was purified using the High Pure PCR Product Purification Kit (Roche Diagnostic, Mannheim, Germany) according the Kit procedure and were used for direct sequencing using the dye termination method and an ABI 3730xl sequencer and finally DNA sequence was analyzed with genius software version 10.1 and the results was compared and blasted with deposited sequences in PubMed.
Enzyme linked immunosorbent assay (ELISA)
Specific IgG and IgM against T. gondii was detected using Enzyme Linked Immunosorbent Assay (Uroimmun, Germany) for 108 patients with HIV/AIDS according manufacture instruction. The tests determined the presence or absence of anti T. gondii immunoglobulin (IgG) and (IgM) antibodies.
Statistical analysis
Analysis was done using SPSS version 18 (Chicago, IL, USA) and Chi-square, Predictive values test were used to analyze statistical relationship.
Results
In this study, 108 HIV/AIDS patients were evaluated for toxoplasmosis infection. The age of patients was between 5 and 60 years with the mean age of 39.7 ± 10.5 years. Of the 108 participants, 66 cases were male (61.1%) and 42 cases were female (38.9%).
Enzyme linked immunosorbent assay
All 108 HIV/AIDS patients were evaluated for Toxoplasma specific IgG and IgM antibodies and 95 subjects (88%) were positive for IgG, including four patients with cerebral toxoplasmosis and 91 subjects (87.5%) of 104 AIDS patients without cerebral toxoplasmosis had Toxoplasma-specific IgG antibodies. In the present study, Toxoplasma-specific antibodies (IgG) were detected in the cerebral patients that for two cases, IgG titers were high, but IgM antibodies were negative in the cerebral toxoplasmosis patients and one patient without cerebral toxoplasmosis was IgM positive.
PCR
One hundred and eight HIV/AIDS patients were evaluated for detection of B1 gene using PCR. In general, four patients, including three patients with cerebral toxoplasmosis who were positive for Toxoplasma IgG antibodies and one patient without cerebral toxoplasmosis were found to be PCR positive and one patient without cerebral toxoplasmosis was positive for Toxoplasma IgM antibody. DNA of T. gondii was detected in both serum and PBMC in two patients with cerebral toxoplasmosis and one patient without cerebral toxoplasmosis. Furthermore, DNA was detected only in PBMC in another patient with cerebral toxoplasmosis.
PCR product of B1 gene in PBMC of HIV/AIDS patients is shown in Fig. 1. A band of 194 bp indicating B1 gene was detected in agarose gel electrophoresis.
Fig. 1.
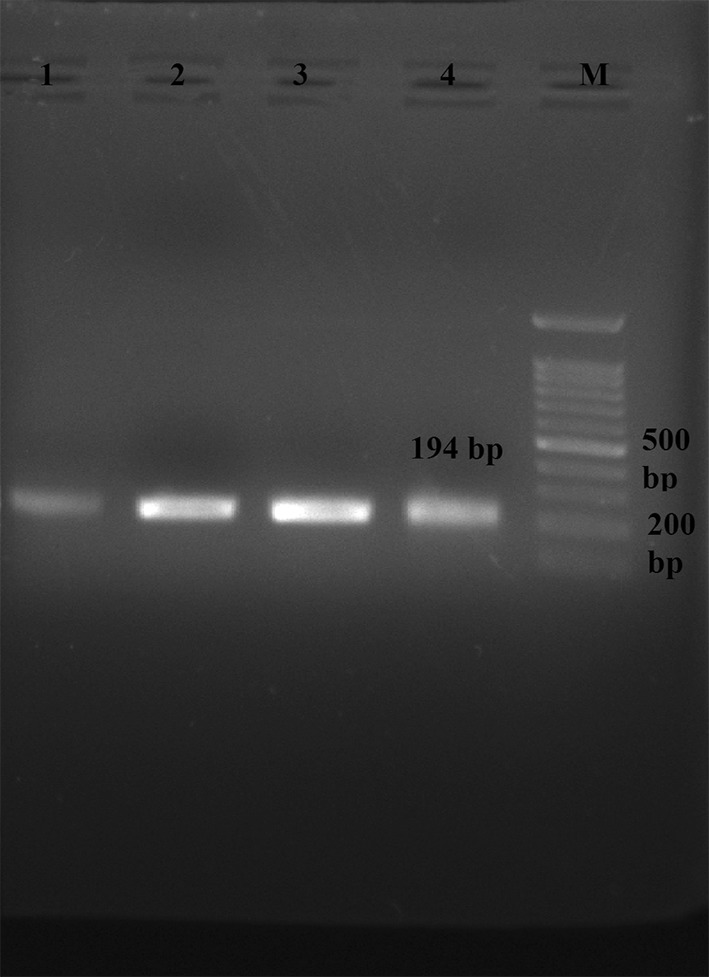
Agarose gel electrophoresis of 194 bp B1 PCR product of Toxoplasma gondii in PBMC samples of HIV/AIDS patients, M-100 bp DNA ladder marker
Result of clinical laboratory tests
All four patients with cerebral toxoplasmosis were admitted to the infectious diseases ward, Rasoul Akram hospital, Iran University of medical sciences. Cerebral toxoplasmosis was diagnosed based on the criteria of the Centers for Disease Prevention and Control (CDC) with clinical symptoms, including focal neurological abnormality, decreased level of consciousness, ataxia and hemiplegia. Multiple ring-enhancing lesions were observed in Magnetic resonance imaging (MRI) results in these four patients and consequently, the result of brain biopsy was positive for toxoplasmosis in three of these patients. The images showed multiple lesions in the involved cerebral hemispheres, the left and right and occipital regions of hemispheres in four patients. The results of clinical laboratory tests showed positive Toxoplasma specific IgG antibody and negative IgM results for patients with cerebral toxoplasmosis and also CD4+ T lymphocyte cell count of less than 100 cells. CD4 level in HIV-positive patients with toxoplasmosis was 51.5 ± 34.5 CD4 cells/µL of blood ranged between 17 and 98, which was significantly lower than HIV-positive patients without clinical manifestation of toxoplasmosis (415.1 ± 211.3 CD4 cells/µL of total blood ranged between 89 and 900) (P = 0.001).
Associated demographic, laboratory and epidemiological parameters in 108 HIV/AIDS patients are summarized in Table 1.
Table 1.
Demographic, laboratory, and epidemiological parameters of individuals with human immunodeficiency virus infection
| HIV/AIDS patients | Toxoplasma DNA positive | Toxoplasma DNA negative | Total | P value | |
|---|---|---|---|---|---|
| No. of patients | |||||
| Male | 4 (100%) | 62 (59.6%) | 66 (61.1%) | 0.134 | Fisher’s exact test |
| Female | 0 (0.0%) | 42 (40.4%) | 42 (38.9%) | ||
| Age (year) ± SD | 49.5 ± 3.1 (46–53) | 39.4 ± 10.5 (5–60) | 39.7 ± 10.5 (5–60) | 0.059 | T test |
| Laboratory parameters | |||||
| Viral load IU/mL (median) | 50610.0 (39564–58325) | 4593.0 (75–1011793) | 6118.5 (75–1011793) | 0.163 | Mann–Whitney U |
| CD4 count | 51.5 ± 34.5 (17–98) | 415.1 ± 211.3 (89–900) | 401.4 ± 218.7 (17–900) | 0.001a | T test |
| ALT1 (IU/L) | 45.7 ± 8.4 (38–54) | 48.5 ± 18.6 (21–91) | 48.4 ± 18.3 (21–91) | 0.948 | Mann–Whitney U |
| AST2 (IU/L) | 41 ± 8.2 (33–49) | 43.1 ± 17.3 (19–83) | 43.0 ± 17.0 (19–83) | 0.896 | Mann–Whitney U |
| Toxoplasma IgG antibody | 4 (100%) | 91 (87.5%) | 95 (88%) | 0.594 | Fisher’s exact test |
| Toxoplasma IgM antibody | 1 (25%) | 0 (0%) | 1 (0.92%) | 0.963 | |
| Epidemiological parameters | |||||
| IVDU | 2 (50.0%) | 50 (48.1%) | 52 (48.1%) | 0.662 | Fisher’s exact test |
| IVDU partner | 0 (0%) | 32 (30.8%) | 32 (29.6%) | 0.239 | Fisher’s exact test |
| History of imprison | 1 (25.0%) | 46 (44.2%) | 47 (43.5%) | 0.413 | Fisher’s exact test |
| Tattooing | 0 (0%) | 18 (17.3%) | 18 (16.7%) | 0.477 | Fisher’s exact test |
| Needle stick | 0 (0%) | 16 (15.4%) | 16 (14.8%) | 0.521 | Fisher’s exact test |
| Unprotected Sex | 0 (0%) | 40 (38.5%) | 40 (37.0%) | 0.152 | Fisher’s exact test |
| Mother to child | 0 (0%) | 2 (1.9%) | 2 (1.9%) | 0.927 | Fisher’s exact test |
| Blood transfusion | 0 (0%) | 2 (1.9%) | 2 (1.9%) | 0.927 | Fisher’s exact test |
1Alanine aminotransferase (ALT); 2 Aspartate aminotransferase (AST); a Statistically Significant
Results of sequencing
The new sequences were deposited in GenBank with the accession numbers of MK031698, MK031699, MK031700 and MK031701. The results of new sequences were compared and blasted with deposited sequences in PubMed. These obtained sequences had 90–100% similarities with KX270388, KX270387, KX270386 and KX270385.
The patients were treated with Trimethoprim/Sulfamethoxazole (TMP-SMX) for 6 weeks and relative remissions were observed after 2 weeks period.
Discussion
Cerebral toxoplasmosis is the most common brain focal lesions in AIDS patient (Vidal et al. 2004). Brain biopsy and PCR for cerebrospinal fluid (CSF) sample have been used previously in diagnosis of cerebral toxoplasmosis, but the accurate, less invasive and rapid method is necessary to diagnose cerebral toxoplasmosis (Vidal et al. 2004).
These days, due to highly active antiretroviral combination therapy (HAART), the frequency of cerebral toxoplasmosis has decreased, however focal brain disorders are still observed in regions using HAART among AIDS patients in developing countries (Ammassari et al. 2000; Sacktor 2002).
The results of some previous studies have demonstrated the usefulness of the PCR assay using B1 gene as one of the most conserved gene sequences among different strains for diagnosis of T. gondii (Bretagne et al. 2000; Burg et al. 1989; Fallahi et al. 2014).
Several studies have been indicated that multi-copy genes for detection of T. gondii DNA are much more suitable than single-copy genes. It has been reported that the 35-fold repeated B1 target is more sensitive than the ribosomal genes (Burg et al. 1989; Jones et al. 2000). Sensitivities of PCR in peripheral blood samples in HIV/AIDS patients with some DNA targets have been reported between 16 and 86% (Colombo et al. 2005; Dupouy-Camet et al. 1993; Gianotti et al. 1997; Joseph et al. 2002; Pelloux et al. 1997).
In some previous studies, T. gondii DNA was detected in PBMC and serum samples from patients with toxoplasmic retinochoroiditis (Contini et al. 2005; Silveira et al. 2011). Although their results showed that the number of positive samples by PCR in PBMC was higher than serum specimens, the detection of T. gondii DNA in both PBMC and serum was almost similar (Contini et al. 2005).
In the present study, T. gondii DNA was detected by PCR using B1 gene in four blood samples of HIV/AIDS patients, including three patients with cerebral toxoplasmosis that in one of them, the result of PCR was positive only in PBMC sample and one toxoplasmosis patient with other neurological disorder showed a positive PCR and Toxoplasma IgM antibody results. Furthermore, PCR result for one cerebral toxoplasmosis patient was negative who was under anti-toxoplasma therapy.
In this study, Toxoplasma-specific IgG antibodies were detected in cerebral toxoplasmosis patients and in two of them, IgG titers were high, but IgM antibodies were negative in the cerebral toxoplasmosis patients, which supports the idea of reactivation of the latent infection that occurs in the secondary immune response.
Although the results of some previous studies proposed that different levels of Toxoplasma-specific IgG antibodies can not be used to determine a reactivation or presence of cerebral toxoplasmosis (Luft and Chua 2000; Sadler et al. 1998), other studies have suggested that the high titers of IgG may be representative of cerebral toxoplasmosis (Derouin et al. 1996; Hellerbrand et al. 1996).
PCR using B1 gene was performed in blood samples of 64 patients with cerebral toxoplasmosis and 51 cases (79.7%) were PCR positive and 58 cases (91%) had detectable IgG Toxoplasma-specific antibodies and also patients with cerebral toxoplasmosis showed higher titers of anti-T. gondii IgG than patients without cerebral toxoplasmosis, which was statistically significant. The findings of the study indicated the reactivation of the latent infection occurred in the secondary immune response in HIV patients (Colombo et al. 2005).
In a study, 18.8% (4/22) of HIV positive patients with cerebral toxoplasmosis and 7.4% (2/27) of patients with other neurological disorder had positive results using B1 gene by a Real-Time PCR Assay. These two patients with neurological disorder had active systemic toxoplasmosis and laboratory data showed IgG anti-Toxoplasma antibodies positive and IgM negative and also IgA positive in one case and in the other case, both specific anti-Toxoplasma IgM and IgA were positive (Cardona et al. 2011).
Ninety-four blood samples collected from HIV positive patients from the West of Iran were evaluated for toxoplasmosis infection by PCR using B1 gene. The results showed that 19.1% (18/94) of them were positive for Toxoplasma IgG antibodies and only one sample was positive using PCR and this sample showed the highest titer in IgG-ELISA (Rostami et al. 2014).
In a study, PCR using SAG2 gene as primer was useful in detection of T. gondii in 22/38 (57.9%) samples, including 5/12 (35.7%) samples from serum samples and 17/26 (65.8%) samples from the whole blood samples. All PCR positive samples were IgG-positive, except for two samples (Gashout et al. 2016).
Result of some studies have proposed that the sensitivity of toxoplasmosis diagnosis is decreased, when the samples are collected after the first week of anti-Toxoplasma therapy (Cingolani et al. 1996), so this subject should be considered before sample collection.
Although the results of present study showed that the numbers of positive samples by PCR in PBMC were higher than serum specimens in diagnosis of cerebral toxoplasmosis, further studies on more toxoplasmosis patients are needed for more investigations.
Conclusion
PBMCs are one of the important sources for evaluation of T. gondii infection. Although PCR is an appropriate tool in diagnosis of T. gondii, the results of the study suggest that molecular method and immunological assay, especially high titer of Toxoplasma IgG antibodies using MRI result can be useful to diagnose cerebral toxoplasmosis. This strategy may prevent more invasive approaches, such as brain biopsy.
Acknowledgements
The authors would like to thank from staffs of Infectious Diseases ward, Rasoul-e-Akram Hospital, Iran University of Medical Sciences for their helpful cooperation.
Author’s contributions
KK and FBS proposed the study. KK, AE and SK designed the study. TD, SG and QA help in technical works and Borna Salami helps in sample collection. KK and FBS and SS revised the manuscript. All the authors approved the final manuscript
Sources of funding
This study was funded by Research Center of Pediatric Infectious Diseases, Iran University of Medical Sciences in Tehran, Iran with Grant Number (95-02-131-28979)
Compliance with ethical standards
Ethic approval
Ethical approval for the study and informed consent forms were approved by ethics committee of Iran University of Medical Sciences (Code Number: IR.IUMS.REC 1395.95-02-131-28979). Ethical approval for this study was obtained from IUMS’s Ethics Committee, and written informed consent was taken from all patients or Legally Authorized Representative. All patients or relatives informed the study procedures and agreed to participate in this study.
Conflict of interest
The authors declare that they have no conflicts of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ammassari A, et al. AIDS-related focal brain lesions in the era of highly active antiretroviral therapy. Neurology. 2000;55:1194–1200. doi: 10.1212/wnl.55.8.1194. [DOI] [PubMed] [Google Scholar]
- Bourdin A, et al. PCR detection of Toxoplasma gondii DNA in blood and ocular samples for the diagnosis of ocular toxoplasmosis. J Clin Microbiol. 2014;52(11):3987–3991. doi: 10.1128/JCM.01793-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretagne S, Costa JM, Foulet F, Jabot-Lestang L, Baud-Camus F, Cordonnier C. Prospective study of toxoplasma reactivation by polymerase chain reaction in allogeneic stem-cell transplant recipients. Transpl Infect Dis. 2000;2:127–132. doi: 10.1034/j.1399-3062.2000.020305.x. [DOI] [PubMed] [Google Scholar]
- Burg JL, Grover CM, Pouletty P, Boothroyd J. Direct and sensitive detection of a pathogenic protozoan, Toxoplasma gondii, by polymerase chain reaction. J Clin Microbiol. 1989;27:1787–1792. doi: 10.1128/jcm.27.8.1787-1792.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona N, Basto N, Parra B, Zea AF, Pardo CA, Bonelo A, Gómez-Marin JE. Detection of Toxoplasma DNA in the peripheral blood of HIV-positive patients with neuro-opportunistic infections by a real-time PCR assay. J Neuroinfect Dis. 2011;2:1–6. [Google Scholar]
- Cingolani A, De Luca A, Ammassari A, Murri R, Linzalone A, Grillo R, Antinori A. PCR detection of Toxoplasma gondii DNA in CSF for the differential diagnosis of AIDS-related focal brain lesions. J Med Microbiol. 1996;45:472–476. doi: 10.1099/00222615-45-6-472. [DOI] [PubMed] [Google Scholar]
- Colombo FA, et al. Diagnosis of cerebral toxoplasmosis in AIDS patients in Brazil: importance of molecular and immunological methods using peripheral blood samples. J Clin Microbiol. 2005;43:5044–5047. doi: 10.1128/JCM.43.10.5044-5047.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contini C, Seraceni S, Cultrera R, Incorvaia C, Sebastiani A, Picot S. Evaluation of a Real-time PCR-based assay using the lightcycler system for detection of Toxoplasma gondii bradyzoite genes in blood specimens from patients with toxoplasmic retinochoroiditis. Int J Parasitol. 2005;35:275–283. doi: 10.1016/j.ijpara.2004.11.016. [DOI] [PubMed] [Google Scholar]
- Derouin F, et al. Predictive value of Toxoplasma gondii antibody titres on the occurrence of toxoplasmic encephalitis in HIV-infected patients. ANRS 005/ACTG 154 Trial Group. AIDS. 1996;10:1521–1527. doi: 10.1097/00002030-199611000-00010. [DOI] [PubMed] [Google Scholar]
- Donyavi T, et al. High prevalence of occult hepatitis C virus infection in injection drug users with HIV infection. Arch Virol. 2019;164:2493–2504. doi: 10.1007/s00705-019-04353-3. [DOI] [PubMed] [Google Scholar]
- Dubey JP. The history of Toxoplasma gondii—the first 100 years. J Eukaryot Microbiol. 2008;55:467–475. doi: 10.1111/j.1550-7408.2008.00345.x. [DOI] [PubMed] [Google Scholar]
- Dupouy-Camet J, et al. Detection of Toxoplasma gondii in venous blood from AIDS patients by polymerase chain reaction. J Clin Microbiol. 1993;31:1866–1869. doi: 10.1128/jcm.31.7.1866-1869.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteghamati A, Khanaliha K, Bokharaei-Salim F, Sayyahfar S, Ghaderipour M. Prevalence of Intestinal Parasitic Infection in Cancer, Organ Transplant and Primary Immunodeficiency Patients in Tehran, Iran. Asian Pac J Cancer Prev. 2019;20:495–501. doi: 10.31557/APJCP.2019.20.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallahi S, et al. Comparison of the RE and B1 gene for detection of Toxoplasma gondii infection in children with cancer. Parasitol Int. 2014;63:37–41. doi: 10.1016/j.parint.2013.08.005. [DOI] [PubMed] [Google Scholar]
- Fricker-Hidalgo H, et al. Diagnosis of toxoplasmosis after allogeneic stem cell transplantation: results of DNA detection and serological techniques. Clin Infect Dis. 2009;48:e9–e15. doi: 10.1086/595709. [DOI] [PubMed] [Google Scholar]
- Gashout A, et al. Molecular diagnosis of Toxoplasma gondii infection in Libya. BMC Infect Dis. 2016;16:157. doi: 10.1186/s12879-016-1491-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianotti N, Cinque P, Castagna A, Novati R, Moro M, Lazzarin A. Diagnosis of toxoplasmic encephalitis in HIV-infected patients. AIDS. 1997;11:1529. [PubMed] [Google Scholar]
- Hellerbrand C, Goebel F, Disko R. High predictive value of Toxoplasma gondii IgG antibody levels in HIV-infected patients for diagnosis of cerebral toxoplasmosis. Eur J Clin Microbiol Infect Dis. 1996;15:869–872. doi: 10.1007/BF01691219. [DOI] [PubMed] [Google Scholar]
- Holliman RE. Toxoplasmosis and the acquired immune deficiency syndrome. J Infect. 1988;16:121–128. doi: 10.1016/s0163-4453(88)93847-9. [DOI] [PubMed] [Google Scholar]
- Jones CD, Okhravi N, Adamson P, Tasker S, Lightman S. Comparison of PCR detection methods for B1, P30, and 18S rDNA genes of T. gondii in aqueous humor. Invest Ophthalmol Vis Sci. 2000;41:634–644. [PubMed] [Google Scholar]
- Joseph P, et al. Optimization and evaluation of a PCR assay for detecting toxoplasmic encephalitis in patients with AIDS. J Clin Microbiol. 2002;40:4499–4503. doi: 10.1128/JCM.40.12.4499-4503.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanaliha K, Motazedian M, Sarkari B, Bandehpour M, Sharifnia Z, Kazemi B. Expression and purification of P43 Toxoplasma gondii surface antigen. Iran J Parasitol. 2012;7:48. [PMC free article] [PubMed] [Google Scholar]
- Khanaliha K, Motazedian MH, Kazemi B, Shahriari B, Bandehpour M, Sharifniya Z. Evaluation of recombinant SAG1, SAG2, and SAG3 antigens for serodiagnosis of toxoplasmosis. Korean J Parasitol. 2014;52:137. doi: 10.3347/kjp.2014.52.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanaliha K, Mohebali M, Davoudi S, Hosseini OR, Tarighi F, Rezaeian T, Rezaeian M. Detection of emergence Cyclospora cayetanensis in A HIV+/AIDS patient with diarrhea from Tehran: a case report. Iran J Public Health. 2015;44:865. [PMC free article] [PubMed] [Google Scholar]
- Lamoril J, Molina J, De Gouvello A, Garin Y, Deybach J, Modai J, Derouin F. Detection by PCR of Toxoplasma gondii in blood in the diagnosis of cerebral toxoplasmosis in patients with AIDS. J Clin Pathol. 1996;49:89–92. doi: 10.1136/jcp.49.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S-B, Lee T-G. Toxoplasmic encephalitis in patient with acquired immunodeficiency syndrome. Brain Tumor Res Treat. 2017;5:34–36. doi: 10.14791/btrt.2017.5.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft BJ, Chua A. Central nervous system toxoplasmosis in HIV pathogenesis, diagnosis, and therapy. Curr Infect Dis Rep. 2000;2:358–362. doi: 10.1007/s11908-000-0016-x. [DOI] [PubMed] [Google Scholar]
- Luft BJ, Brooks RG, Conley FK, McCabe RE, Remington JS. Toxoplasmic encephalitis in patients with acquired immune deficiency syndrome. JAMA. 1984;252:913–917. [PubMed] [Google Scholar]
- Masoumi-Asl H, Khanaliha K, Bokharaei-Salim F, Esteghamati A, Kalantari S, Hosseinyrad M. Enteric Opportunistic Infection and the Impact of Antiretroviral Therapy among HIV/AIDS Patients from Tehran, Iran. Iran J Public Health. 2019;48:730. [PMC free article] [PubMed] [Google Scholar]
- Nissapatorn V. Lessons learned about opportunistic infections in southeast Asia. Southeast Asian J Trop Med Public Health. 2008;39:625. [PubMed] [Google Scholar]
- Novati R, et al. Polymerase chain reaction for Toxoplasma gondii DNA in the cerebrospinal fluid of AIDS patients with focal brain lesions. AIDS. 1994;8:1691–1694. doi: 10.1097/00002030-199412000-00008. [DOI] [PubMed] [Google Scholar]
- Pelloux H, Dupouy-Camet J, Derouin F, Aboulker J, Raffi F, Group B-TS. A multicentre prospective study for the polymerase chain reaction detection of Toxoplasma gondii DNA in blood samples from 186 AIDS patients with suspected toxoplasmic encephalitis. AIDS. 1997;11:1888–1890. doi: 10.1097/00002030-199715000-00018. [DOI] [PubMed] [Google Scholar]
- Rostami A, Keshavarz H, Shojaee S, Mohebali M, Meamar AR. Frequency of Toxoplasma gondii in HIV positive patients from West of Iran by ELISA and PCR. Iran J Parasitol. 2014;9:474. [PMC free article] [PubMed] [Google Scholar]
- Rostami A, Karanis P, Fallahi S. Advances in serological, imaging techniques and molecular diagnosis of Toxoplasma gondii infection. Infection. 2018;46:303–315. doi: 10.1007/s15010-017-1111-3. [DOI] [PubMed] [Google Scholar]
- Sacktor N. The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. J Neurovirol. 2002;8(Suppl 2):115–121. doi: 10.1080/13550280290101094. [DOI] [PubMed] [Google Scholar]
- Sadler M, Brink N, Gazzard B. Management of intracerebral lesions in patients with HIV: a retrospective study with discussion of diagnostic problems. QJM. 1998;91:205–217. doi: 10.1093/oxfordjournals.qjmed.a030150. [DOI] [PubMed] [Google Scholar]
- Sayyahfar S, Karimi A, Gharib A, Fahimzad A. Association of systemic anaplastic large cell lymphoma and active toxoplasmosis in a child. Iran J Cancer Prev. 2015;8(4):e3438. doi: 10.17795/ijcp-3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensini A. Toxoplasma gondii infection in pregnancy: opportunities and pitfalls of serological diagnosis. Clin Microbiol Infect. 2006;12:504–512. doi: 10.1111/j.1469-0691.2006.01444.x. [DOI] [PubMed] [Google Scholar]
- Shiadeh MN, et al. The correlation between Toxoplasma gondii infection and prenatal depression in pregnant women. Eur J Clin Microbiol Infect Dis. 2016;35:1829–1835. doi: 10.1007/s10096-016-2734-5. [DOI] [PubMed] [Google Scholar]
- Silveira C, et al. Toxoplasma gondii in the peripheral blood of patients with acute and chronic toxoplasmosis. Br J Ophthalmol. 2011;95:396–400. doi: 10.1136/bjo.2008.148205. [DOI] [PubMed] [Google Scholar]
- Vidal JE, Colombo FA, de Oliveira ACP, Focaccia R, Pereira-Chioccola VL. PCR assay using cerebrospinal fluid for diagnosis of cerebral toxoplasmosis in Brazilian AIDS patients. J Clin Microbiol. 2004;42:4765–4768. doi: 10.1128/JCM.42.10.4765-4768.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahab T, Edvinsson B, Palm D, Lindh J. Comparison of the AF146527 and B1 repeated elements, two real-time PCR targets used for detection of Toxoplasma gondii. J Clin Microbiol. 2010;48:591–592. doi: 10.1128/JCM.01113-09. [DOI] [PMC free article] [PubMed] [Google Scholar]


