Abstract
Echinococcosis/hydatidosis is one of the most important parasitic zoonotic diseases in the world. Cystic echinococcosis increases public health and socio-economic concern due to considerable morbidity rates that give rise to elevated economic losses both in the public health part and in the farm animal field. The enzyme linked immunosorbent assay (ELISA) is consider the more accurate tool for diagnosis of hydatidosis in camels. In the present study, affinity purified Echinococcus granulosus (E. granulosus) antigens (APA) were purified from crude hydatide E. granulosus germinal layer proteins for detection of E. granulosus antibodies in infected camels, using affinity matrix (camel IgGs coupled to CNBr-activated Sepharose). The electrophoretic profile of the APA showed that it was separated into two bands; one major band of 130 kDa and one minor band at 55 kDa. These antigens were used successfully as specific coating antigenic proteins in detection of echinococcosis in camel. In a trial to prepare an anti-camel IgGs peroxidase conjugate; peroxidase enzyme was purified from turnip roots (TPOD) using ammonium sulfate precipitation and affinity chromatography on phenyl Sepharose CL-4B. The purified TPOD showed a major band at 35 kDa. Rabbit anti-camel IgG antibodies (AC IgGs) were prepared then purified using affinity chromatography on Protein G-Sepharose. The TPOD, and commercial HRP for comparison, enzymes were conjugated to AC IgGs using 1%, 5% and 10% glutaraldehyde. The results revealed that the HRP was much better than TPOD in conjugation with AC-IgG antibodies and the 10% glutaraldehyde concentration was the most efficient concentration with ELISA titer 1:50.
Keywords: Hydatidosis, Conjugates, ELISA, Camel, HRP, Peroxidase
Introduction
The camel has an important position among animals in which human has neglected and has certainly undiscovered. From a worldwide point of view, little concern with the financial value of camel production in comparison with that of other domestic animals. Nevertheless, camel rearing could be taken into consideration effectively.
Cystic echinococcosis (CE) is one of most common parasitic zoonotic diseases that is caused by E. granulosus aggregate larval phase (Craig et al. 2003). CE can be diagnosed using different imaging methods such as ultrasound (US), computed tomography (CT) and magnetic resonance imaging (MRI). Even so, U.S.-based testing reliability depends heavily on the ultra-sonograph’s capacity (Yu et al. 2008). Therefore, it may be hard to differentiate certain cyst stages from normal non-parasitic cysts (Brunetti et al. 2011). Appropriate sero-diagnostic aids are available to help in diagnosing of a wide range of infectious diseases that affect camels (Al-Ruwaili et al. 2012). Nevertheless, the quality of U.S. research depends heavily on the capability of the ultra-sonograph (Yu et al. 2008). Hence, separating these cyst phases from normal non-parasitic cysts may be difficult (Brunetti et al. 2011). Suitable sero-diagnostic aids are available to assist in the treatment of a wide range of infectious diseases affecting camels (Al-Ruwaili et al. 2012).
Enzymes antibodies conjugation include the construction of a steady and covalent bond between both of them. The most commonly used enzyme in the conjugation process is horseradish peroxides (HRP). Although other sources rich in POD are locally available in Egypt, turnip roots were chosen for this study because of its availability at local markets in low price almost throughout the year, and the juicy nature of turnip roots compared with the fibrous nature of radish roots (Mazza et al. 1968; Hamed et al. 2009). In general, POD is cheap and can be attached to antigen-specific monoclonal or polyclonal antibodies by a variety of methods. Besides, many chromogenic substrates are also available for its detection (Jeanson et al. 1988; Ramesh et al. 2014). In enzymatic and immunodiagnostic kits, POD has been generally used clinically as a part of them. POD antibody conjugates are used in about 90% of the immunoassay kits (Rashimaw 1982; Hamed et al. 2009). The present study aims firstly to compare the crude and affinity-purified antigens from the germinal layer of hydatid cyst isolated from camel, secondly comparison between horse raddish and turnip roots peroxidase enzymes for the preparation of hydatidosis immuno- diagnostic kit for camels.
Materials and methods
Rabbit anti-camel IgG antibodies (AC IgGs) preparation
Camel immunoglobulin Gs (C IgGs) were purified using the method of Khamehchian et al. (2014) with slight modification. The positive hydatid camel serum was kindly donated by Kandil et al. (2018) and was precipitated using 55% ammonium sulfate; ion-exchange chromatography on DEAE-Sepharose CL-6B was used to purify the precipitate. The unbound proteins were washed with 50 mM Tris–HCl, pH 7.8, while the other bound proteins were washed out using 1 M NaCl in 50 mMTris-HCl, pH 7.8. Fractions of five ml were collected at constant flow rate of 48 ml/h.
The Anti-camel IgG antibodies (AC IgGs) were prepared by intra-muscular injection of 20 µg of camel IgGs dissolved in 0.5 ml 0.9% saline and an equal volume of Freund’s complete adjuvant (FCA) (Sigma Chemical Co., St. Louis, MO, USA) into three male rabbits on day 0. The control group was immunized only with saline mixed with the Freund’s complete adjuvant. The rabbits were boosted with 20 μg of camel IgGs mixed with Freund’s incomplete adjuvant (FIA) (Sigma Chemical Co., St. Louis, MO, USA) on days 14 and 28 using the same method. Two weeks after boosting, blood samples were collected from the rabbit marginal ear vein, the serum was separated, pooled and stored at − 20 °C (Hudson and Hay 1989).
The rabbit anti-camel IgGs (AC IgGs) were purified using the Protien G-Sepahrose affinity chromatography. Briefly, 5 ml of rabbit anti-sera were applied to a protein G-Sepharose column (5 × 1.5 cm) (Sigma Chemical Co.), the adsorbed fractions were washed out using 100 mM glycine–HCl, pH 2.9 in tubes containing 1 M PBS pH 9 at a flow rate of 36 ml/h.(Hudson and Hay 1989).
Purification of turnip peroxidase
Turnip (TPOD) homogenate was concentrated by ammonium sulfate precipitation to obtain turnip peroxidase enzyme which was purified using Phenyl Sepharose column.Briefly, the enzyme samplewas dissolved in 0.05 M sodium phosphate buffer containing 1 M ammonium sulfate, pH 6.8 (equilibration buffer, EB), and applied onto degassed preswollen Phenyl Sepharose CL-4B column (20 × 1.6 cm i.d.), previously equilibrated with the same EB. The proteins bound to the hydrophobic resin were eluted using a stepwise decrease in ammonium sulfate concentration (1 M, 0.8 M of ammonium sulphate and phosphate buffer, pH 6.8) (Hamed et al. 2009). Fractions of 2 ml were collected at a flow rate of 30 ml/h. Absorbance of each fraction was determined at 280 nm for protein detection using a Shimadzu spectrophotometer UV-100-2. The POD activity was assayed in each tube. Fractions containing enzyme activity were pooled and stored at − 20 °C until use.
Peroxidase assay
POD activity was determined according to the method of Childs and Bardsley (1975), using ABTS as a reducing substrate, in a reaction mixture (1 ml) containing 6 mM H2O2, 0.36 mM ABTS, 100 mM sodium acetate buffer (pH 6) and POD concentration which gave linear reaction over a period of 3 min. The change in absorbance at 414 nm was recorded at one min intervals. One POD unit activity was defined as the quantity of enzyme that oxidized 1 mmol ABTS/min at 25 °C under the same conditions of the assay.
Conjugation of AC IgGs with peroxidase enzymes
The rabbit AC IgGs were conjugated to turnip peroxidase (TPOD) (300 units and 10 mg) according to the one-step method of Boorsma and Kalsbeek (1975). Briefly, glutaraldehyde (0.05 ml) was added under gentle stirring to one ml of 0.1 M phosphate buffer, pH 6.8, containing 3.3 mg protein of AC IgGs and TPOD enzyme. After incubation for 2 h at room temperature, the reaction mixture was dialyzed overnight against 0.1 M PBS, pH 7.2. Next, 1% BSA was added as stabilizer. Also, commercial HRP (Sigma) was used for conjugation with the prepared AC-IgG antibodies using the same ratios and concentrations (1%, 5% and 10%) of glutaraldehyde.
Germinal layer antigens preparation
Hydatid cysts were collected from the lungs and livers of slaughtered camels at EL-Bassatin abattoir, Cairo, Egypt. Germinal layers were obtained from dissected collected cysts. After washing with phosphate buffer saline (PBS), pH 7.2, the germinal layers were grinded and centrifuged for 14,000 rpm/30 min. at 4 °C. The supernatant was obtained and preserved in Aliquots at − 20 °C as crude germinal layer antigen (CGLA) (Kandil et al. 2018).
The affinity purified germinal layer antigen was prepared using affinity chromatography column. Briefly, the A column (3.5 × 1.6 cm) was packed with immobilized camel IgGs on CNBr-activated Sepharose 4B. 33 mg of CGLA was applied to the column. The non adsorbed proteins were washed out using 20 mM phosphate buffer, pH 7.4, while the adsorbed proteins were eluted at a constant flow rate using 0.1 M glycine–HCl buffer pH 2.9. Fractions of two ml were collected in tubes containing 100 µl 1 M Tis-HCl pH 9. The fractions of the bound proteins were collected, pooled, concentrated and designated as affinity purified germinal layer antigens (APGLA) (El Hakim et al. 2007).
Sodium dodecylesulphate gel electrophoresis profile
Electrophoretic analysis was examined following the method of Laemmli (1970) using the Mini-Protean II Dual-Slab Cell (BioRad, USA) to characterize the separated purified antigens and/or the separated IgGs under reducing conditions. Pre-stained molecular weights protein markers (Genedirex BLUltra, USA) were used.
Enzyme linked immunosorbent assay (ELISA)
The affinity purified germinal layer antigen was evaluated using ELISA described by Kandil et al. (2016). Briefly, ELISA plates were coated overnight with different concentrations (0.25 µg to 8 µg/well) of CGLA or APGLA dissolved in 50 mM sodium carbonate, pH 9.6 (coating buffer). After blocking, each well was incubated with 100 µl of 1:200 +ve camel serum obtained from Kandil et al. (2018). Commercial protein A peroxidase conjugate (1:1000) was added to each well and incubated for 60 min at 37 °C. Finally, 100 µl of 0.33 mg OPD/ml in citrate buffer, pH 4.5 containing 0.04% hydrogen peroxide (substrate buffer) were added to each well. After 20 min, the reaction was stopped and the absorbance values were determined at 450 nm with ELISA reader.
For evaluation of prepared rabbit AC IgG-TPOD and AC IgG-HRP conjugates, the ELISA plates were coated with 2 µg/well of crude germinal layer of camel hydatid cyst dissolved in washing buffer, each well was incubated with 100 µl of 1:200 +ve camel serum dissolved in washing buffer at 37 °C for 60 min and serial dilutions from the prepared conjugates were applied to the wells and incubated at 37 °C for 60 min. and the reaction was performed as above. The mean result values were obtained after recording the result values in duplicate. A standard curve was plotted between log antigen concentrations, or conjugates dilutions, and log optical density (OD). The ELISA titers were defined as the dilutions that gives 0.5 OD at 450 nm.
Protein determination
Total protein content of CGLA and APGLA and antibodies concentration were estimated using the method of Lowry et al. (1951).
Results
Rabbit anti-camel IgG antibodies (AC IgGs) preparation
The normal camel serum and positive hydatid cyst camel anti-sera were precipitated using 55% ammonium sulfate, the resultant precipitates were then purified using ion-exchange chromatography on DEAE-Sepharose CL-6B (Fig. 1). Two major protein peaks were obtained and the camel IgGs were detected in the unbound fractions.
Fig. 1.
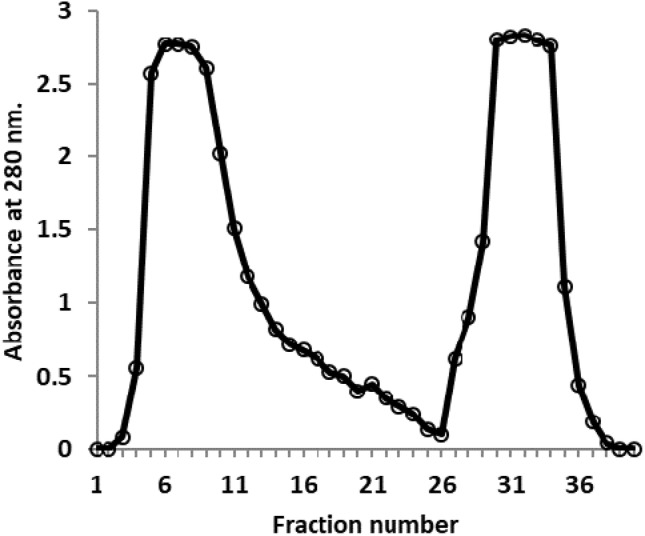
Ion exchange chromatography of precipitated camel serum on DEAE Sepharose (1.6 × 15 cm)
The rabbit anti-camel IgGs were purified using Protein G-Sepharose column. The chromatographic profile of the rabbit anti camel IgGs on Protein G-Sepharose showed the separation of the rabbit anti-serum into one unbound and one bound protein beaks. The buffer 0.1 M Tris–HCl, pH 7.4 was used to wash out the unbound proteins, while the bound ones were eluted using 0.1 M glycine HCl, pH 2.9 at a flow rate of 60 ml/h (Fig. 2).
Fig. 2.
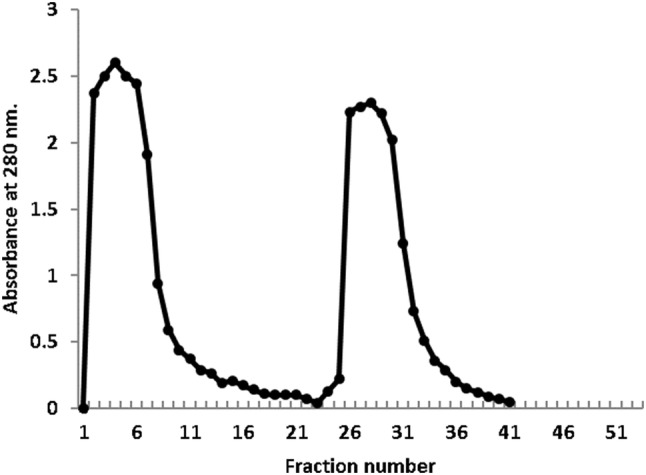
Affinity chromatography of rabbit anti-camel IgGs on Protein G-Sepharose column (1.6 × 4 cm)
Conjugation of AC IgGs with peroxidase enzymes
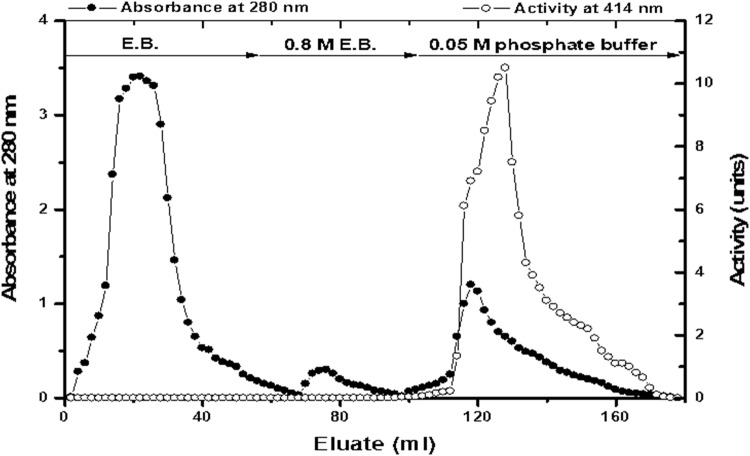
TPOD was prepared from 1.5 kg of turnip roots. The initial specific activity was 3.58 units/mg of protein. The ammonium sulfate precipitation of TPOD increased the specific activity to 6.29 units/mg of protein with 1.75-fold purification and 12.7% recovery. The ammonium sulfate precipitate of TPOD was applied on Phenyl Sepharose CL-4B. Most of the TPOD activity was bound to the matrix and eluted by buffer lacking ammonium sulfate. The typical elution profile is shown in Fig. 3. Most of the TPOD activity was recovered in the third peak with 299 units and 42 mg of protein with specific activity of 7.12 units/mg of protein and fold purification of 2.0 from the crude extract (Table 1). The fractions were collected in 2 ml volume at a flow rate of 30 ml/h. Absorbance of each fraction was measured at 280 nm and TPOD activity was detected using ABTS.
Fig. 3.
Typical elution profile for the hydrophobic interaction chromatography of TPOD (ammonium sulfate precipitate) on Phenyl Sepharose CL-4B column (20 × 1.6 cm)
Table 1.
Summary of purification of turnip (B. rapa) POD
| Sample | Activity (units)a | Protein (mg) | Specific activityb | % Recovery | Fold of purification |
|---|---|---|---|---|---|
| Crude homogenate | 5950 | 1660 | 3.58 | 100 | 1 |
| Ammonium sulfate precipitate | 755 | 120 | 6.29 | 12.7 | 1.75 |
| Phenyl Sepharose | 299 | 42 | 7.12 | 5 | 2 |
aOne unit of POD activity is the amount of the enzyme that oxidized 1 mmol ABTS/min at 25 °C under assay conditions
b(units/mg protein)
Tables 2 and 3 show characteristics of standard HPR and TPOD peroxidases before and after conjugation process. At the beginning, standard HRP and TPOD peroxidases (2.5 and 10 mg protein) with total activity (1000 and 190 U) and specific activity (400 and 19 U/mg protein), respectively, were conjugated to 0.75 mg of AC-IgGs in a ratio of 3:1 (w/w). After conjugation, the HRP-IgG conjugate (about 3.2, 3.2, 3 mg proteins/ml) had total activity and specific activity of 750, 850, 800 U and 234, 265, 266 U/mg protein using 1%, 5% and 10% glutraldehyde, respectively, compared with the TPOD-IgG conjugate (13, 13, 17.6 mg proteins/ml) had total activity and specific activity of180, 175, 150 U and 14, 13.5, 8.5 U/mg protein, respectively, for the same concentrations of glutaraldehyde. Moreover the higher percentage recovery was showed with TPOD-conjugate rather than the HRP-conjugate (Tables 2, 3).
Table 2.
Conjugation of standard HRP to AC IgG using different concentrations of glutaraldehyde
| Sample numbera | Protein (mg) | Activity (units) | Specific activity (units/mg protein) | Recovery (%) | ELISA titer |
|---|---|---|---|---|---|
| 1 | 2.5 | 1000 | 400 | 100 | – |
| 2 | 3.2 | 750 | 234 | 75 | 1: 10 |
| 3 | 3.2 | 850 | 265 | 85 | 1: 15 |
| 4 | 3 | 800 | 266 | 80 | 1: 50 |
aSample number: 1, Standard HRP; 2, HRP-AC IgG conjugate (using 1% glutaraldehyde), 3, HRP-AC IgG conjugate (using 5% glutaraldehyde) and 4, HRP-AC IgG conjugate (using 10% glutaraldehyde)
Table 3.
Conjugation of Phenyl Sepharose purified TPOD to AC IgG using different concentrations of glutaraldehyde
| Sample numbera | Protein (mg) | Activity (units) | Specific activity (units/mg protein) | Recovery (%) | ELISA titer |
|---|---|---|---|---|---|
| 1 | 10 | 190 | 19 | 100 | – |
| 2 | 13 | 180 | 14 | 95 | 1: 1 |
| 4 | 13 | 175 | 13.5 | 92 | 1: 2 |
| 5 | 17.6 | 150 | 8.5 | 79 | 1: 10 |
aSample number: 1, Phenyl Sepharose purified TPOD; 2, TPOD-AC IgG conjugate (using 1% glutaraldehyde); 3, TPOD-AC IgG conjugate (using 5% glutaraldehyde); 4, TPOD-AC IgG conjugate (using 10% glutaraldehyde)
Affinity purified germinal layer antigens (APGLA)
The affinity chromatographic profile of hydatid cyst crude germinal layer antigen proteins on CNBr-Sepharose coupled to camel IgGs showed the separation of crude antigens into two fractions; non-adsorbed proteins and adsorbed immunogens (Fig. 4). The buffer 0.1 M Tris–HCl, pH 7.4 was used to wash out the non adsorbed proteins while 0.1 M glycine HCl buffer, pH 2.9 was used to elute the adsorbed immunogens at a flow rate of 48 ml/h.
Fig. 4.
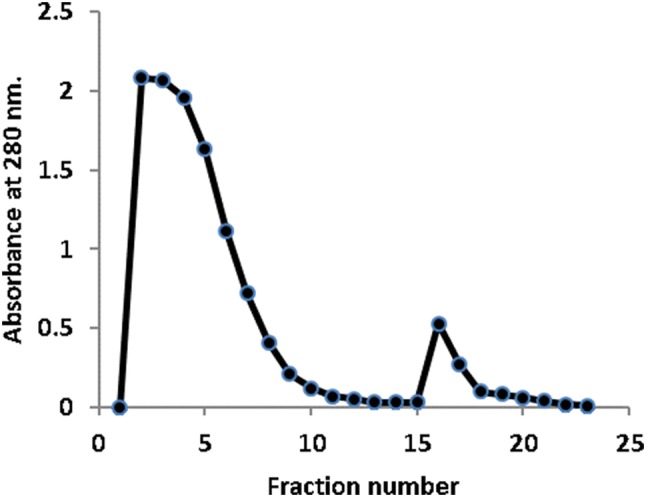
Affinity chromatography of hydatid cyst crude germinal layer proteins on CNBr-activated Sepharose coupled to camel IgGs (1.6 × 5 cm)
Sodium dodecyle sulphate gel electrophoresis profile
The separation of hydatid cyst crude germinal layer antigen on 12% SDS-PAGE showed multiple protein bands at molecular weight range of 35 to 150 kDa, while the electrophoretic profile of the affinity purified immunogens showed that the purified immunogens were separated into two bands; one major protein band of 130 kDa and the second minor protein band at 55 kDa (Fig. 5).
Fig. 5.
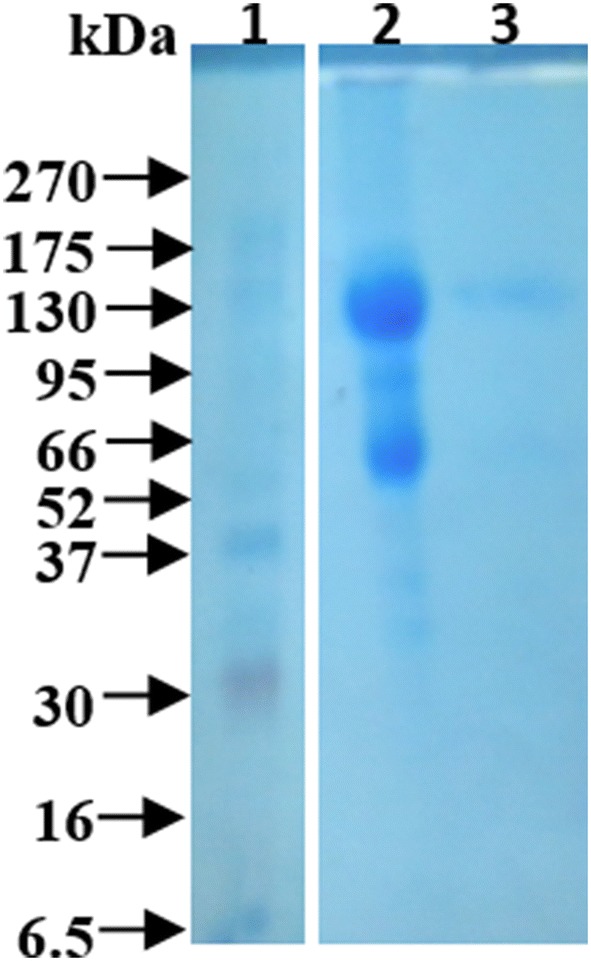
12% SDS-PAGE of camel hydatid cyst germinal layer antigens. Lane 1: pre-stained molecular weights protein markers, GenedirexBLUltra, USA); lane 2: hydatid cyst crude germinal layer antigen and lane 3: affinity purified germinal layer antigen
The electrophoretic analysis of camel IgGs
The protein peaks of the precipitated camel serum eluted from the ion exchange chromatography were run on 15% SDS-PAGE. The first peak showed purified camel IgG with 2 molecular weights under reducing conditions (heavy chain IgG with about 55 kDa and light chain with about 30 kDa), the separation of this peak under non-reducing conditions gives a major band of about 150 kDa, while the other peaks revealed non-IgG proteins (Fig. 6).
Fig. 6.
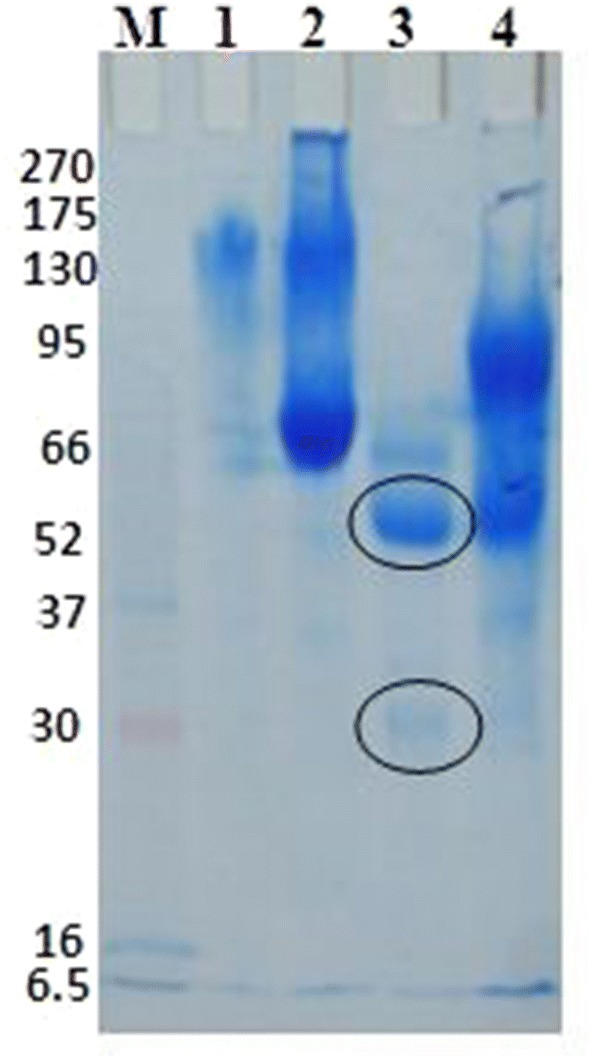
15% SDS-PAGE of the purified camel IgGs: Lane M:Pre-stained molecular weights protein markers, GenedirexBLUltra, USA; lane 1: unbound proteins and Lane 2: bound proteins after ion exchange chromatography, under non reducing conditions; lane 3: unbound proteins and Lane 4: bound proteins after ion exchange chromatography, under reducing conditions
The electrophoretic profile of the bound fractions of rabbit anti-camel IgGs under reducing conditions showed that the IgGs were separated into two main protein bands at 55 and 25 kDa (Fig. 7).
Fig. 7.
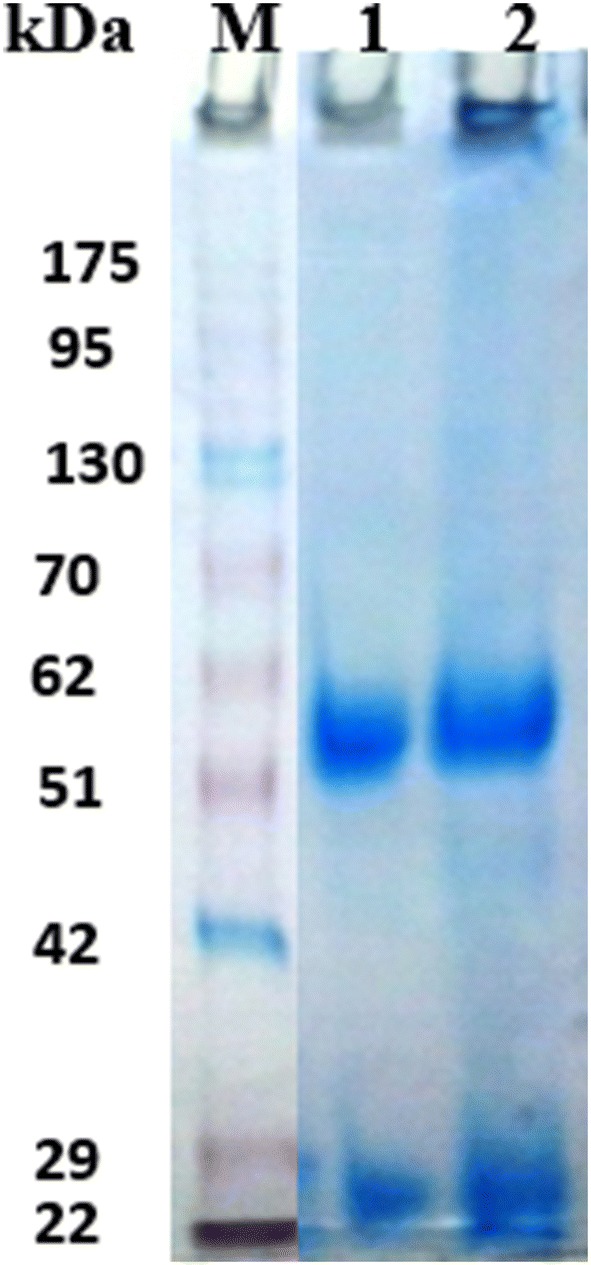
15% SDS-PAGE of rabbit anti-camel IgGs: Lane M: Pre-stained molecular weights protein markers, Vivantas and lane 1 bound proteins fraction
Enzyme linked immunosorbent assay (ELISA)
ELISA assay was designed for comparison between CGLA and APGLA as coating antigenic proteins in detection of hydatidosis in camels. The results revealed that optimum antigen concentrations of the CGLA and APGLA were found to be 2 and 3 µg/well, respectively while the ELISA titers of positive camel sera, using the two antigens, were 2.3 and 2, respectively (Table 4).
Table 4.
ELISA titers for CGLA, APGLA Ag concentrations and camel serum
| CGLA | APGLA | ELISA titer using | ||
|---|---|---|---|---|
| CGLA (2 µg/well)a | APGLA (3 µg/well)a | |||
| Optimum Ag concentration (µg/well) | 2 | 3 | – | – |
| Positive camel serum | – | – | 2.3 | 2 |
| Normal camel serum | 0 | 0 | 0 | 0 |
aTiter is the (−Log dilution of antisera)
The optimum titers of prepared conjugates were assayed using ELISA. The results revealed that TPOD conjugate showed ELISA titers of 1:1, 1:2 and 1:10 while HRP conjugate showed ELISA titers of 1:10, 1:15 and 1:50 at 1%, 5% and 10% glutaraldehyde concentrations, respectively (Fig. 8 and Tables 2, 3).
Fig. 8.
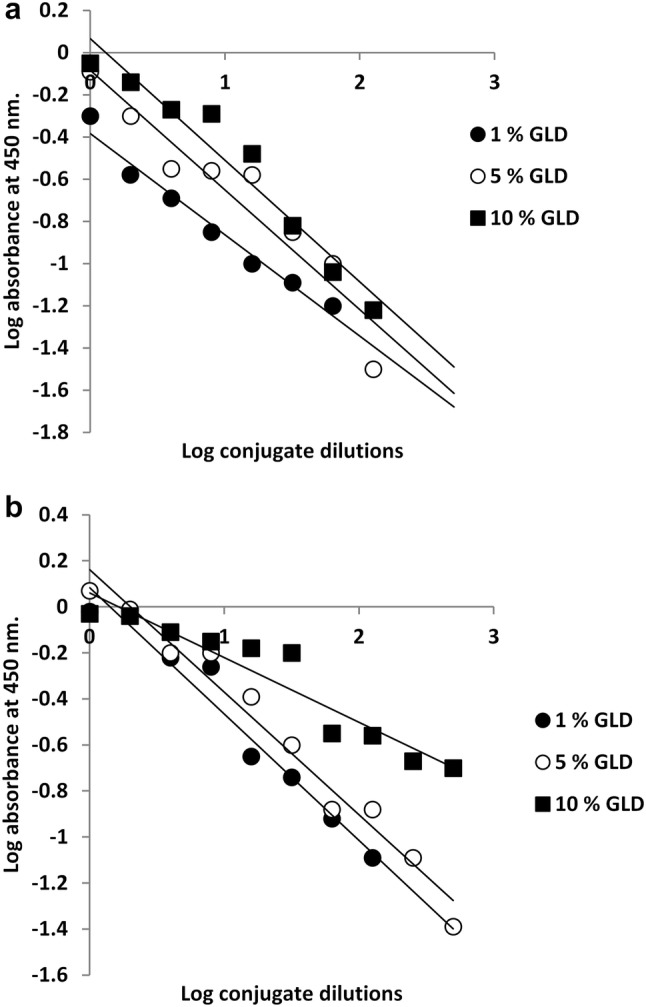
Enzyme-linked immunosorbent assay (ELISA) titration curve of the prepared conjugates; a: turnip peroxidase (TPOD) and b: horseradish peroxidase (HRP) conjugates
Discussion
In several countries, echinococcosis/hydatidosis is one of the world’s leading parasitic zoonotic diseases caused by E. granulosus. CE is a growing public health and socio-economic problem due to the high morbidity levels causing elevated economic losses in both the public health and the livestock sectors. When treatment is not given, the infection can have lethal effects in humans, so early diagnosis is necessary to facilitate care and minimize morbidity and mortality. Furthermore, parasite identification in the definitive host plays a central role in epidemiological studies and hydatidosis control surveillance programs. The classification of native and partially purified antigens currently available for immunodiagnostic purposes is given special attention. There is no diagnosis of camel diseases that require specific marked immunoglobulins (Igs) to be developed using the ELISA technique.
Identifying highly immunogenic antigens and designing active vaccines and adjuvant constructs against CE is of great interest. To this end, different methods can be used, including immune-based genomics and proteomics, immune information technology, process vaccinology, and mathematical/computational modeling (Pourseifa et al. 2018). The immunoaffinity chromatographic purification method is considered as one of the most powerful purification tools for the purification of immunogens from different pathogens (El Hakim et al. 2007). In the present study, APGLA purified from crude hydatid germinal layer proteins from camel hydatid cysts for detection of anti-hydatid cyst antibodies in infected camels, using affinity matrix (camel IgGs coupled to CNBr-activated Sepharose).The electrophoretic profile of the APGLA showed that it was separated into two bands; one major band of 130 kDa one minor band at 55 kDa. These antigens were used successfully as specific coating antigenic proteins in detection of hydatidosis in camel and they were found to be almost the same as the crude germinal layer antigens. These results suggest that the APGLA proteins can be used in the diagnosis of hydatidosis infestation in camel and could be useful in the vaccination of camel against echinococcosis infestation. Pourseifa et al. (2018) addressed important vaccine antigens with respect to their biochemical and immunological properties and vaccine building potential for E. granulosus. They also provided some insights into the latest vaccine generation and development strategies that are built through computer modeling approaches (Pourseifa et al. 2018).
HRP is used for different analytical and industrial uses among peroxidases. Certain peroxidases especially HRP had better substrate specificities, a broad pH range, thermal stability and economic viability (Pandey et al. 2017). Sweet potato peroxidase has high specific activity and uniquely usable electrochemical characters; so, it is used as a biosensor (Lindgren et al. 2000; Abdel-Aty et al. 2018). Tobacco peroxidase has been immobilized into graphite electrodes and used for aromatic compound detection (Castillo et al. 2006). Spring cabbage peroxidase has a strong preference for different substrates, high thermal and pH stability, and low extraction costs, and is therefore used as a bioelectro-catalysis (Belcarz et al. 2008; El-Gayar et al. 2012). For uric acid detection kits, turnip root peroxidases were used (Agostini et al. 2002). It is highly desirable to search for modern sources of specific peroxidase properties such as high temperature, pH range, stability, and high substrate specificity. The coupling of POD and antibodies should produce a conjugate that resembles the native proteins. Here we used the glutaraldehyde method in which glutaraldehyde links carrier molecules (POD) to the N-terminus of the antibody peptides (Saraiva et al. 2007).
The ways of coupling depend either on the transformation of terminal sugars in N-glycan molecules to aldehyde functions in horseradish peroxidase (HRP) and binding to amino groups in the other molecule, or on the utilization of the molecule enzyme amino groups and sufficient succinimide ester reagents. Absence of free amino groups has seriously restricted the selection of conjugation ways. It is stated that there are six amino groups per molecule of the widely used HRP preparations (O’Brien et al. 2003). Economically, the conjugated product of glutaraldehyde method was preferable, as it requires one purification step, has higher recovery, and is immunologically comparable, beside its higher stability (Hamed et al. 2009). Tables 2 and 3 describe the features of both standard HRP and TPOD peroxidases before and after the coupling process showed that most enzymatic peroxidase activities were maintained after the coupling cycle and the TPOD-conjugate displayed a higher percentage recovery over the initial activity than the HRP-conjugate. This method suggests that the glutaraldehyde method did not change the enzyme’s functionality. Similarly, TPOD showed a 58.5 percent recovery in combination with glutaraldehyde antibodies (Hamed et al. 2009). Upon conjugation, Brassica oleracea gongylodes peroxidase only recovered 54% of intial activity (Shetty et al. 2017).
In this work, ELISA experiment was designed to determine the coupling degree between peroxidase and antibody and to measure the antibody concentration in the prepared conjugate. The results revealed that the TPOD conjugates showed ELISA titers of 1:1, 1:2 and 1:10 while the HRP conjugates showed ELISA titers of 1:10, 1:15 and 1:50 at 1%, 5% and 10% glutaraldehyde concentrations, respectively suggesting that the HRP-conjugate has higher coupling affinity than the TPOD-conjugate. Variations in ELISA titers between conjugates are due to variations in conjugation methods, conjugation percent between the antibody and the enzyme, coupling agent concentration and optimization during the coupling process. (Shetty et al. 2017; Abuknesha et al. 2005). The results also indicated that the ELISA titers increased with the increasing of glutaraldehyde concentrations, this may be attributed to the increasing of the immobilized amount of peroxidase enzymes to AC IgG antibodies as a result of the activation of more amino groups with the increasing of the concentration of glutaraldehyde solution (Chen et al. 2013).
Conclusion
In this study, the APGLA based ELISA was established using APGLA purified from hydatid cyst germinal layer as a source. The test has been shown to be an effective serological diagnostic tool to investigate the prevalence of hydatidosis in camel and in the future could be an alternative serological approach to crude antigen ELISA. In order to exclude cross-reactivities to other associated pathogens, further studies are needed. The TPOD and HRP enzymes were conjugated to AC IgGs using 1%, 5% and 10% glutaraldehyde. The results revealed that the HRP was much better than TPOD in conjugation with AC-IgG antibodies and 10% glutaraldehyde was the best concentration for conjugation step with ELISA titer 1: 50. Further studies are needed to increase the sensitivity of the prepared conjugates.
Acknowledgements
This work was financially supported by National Research Centre fund in Egypt (Grant No: 11090303). This work (Rabbit anti-camel IgG peroxidase conjugate) is registered as a patent at Academy of Scientific Research and Technology-Patent Office (Registration No. 80/2018)
Authors’ contribution
KOM and E-HAE designed the plan of work. All authors carried out sample collection, sample processing, conducted the experiment and the laboratory work of the samples. KOM, E-HAE and GAM analyzed and discussed the resultant data. KOM, E-HAE, GAM and HSHM prepared, revised and reviewed the manuscript for publication. All authors read and approved the final manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional guides on the care and use.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Abdel-Aty AM, Hamed MB, Gad AM, El-Hakim AE, Mohamed SA. Ficus sycomorus latex: an efficient alternative Egyptian source for horse radish peroxidase in labeling with antibodies for immunodiagnostic kits. Vet World. 2018;11:1364–1370. doi: 10.14202/vetworld.2018.1364-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abuknesha RA, Luk C, Griffith HHM, Maragkou A, Iakovaki D. Efficient labeling of antibodies with horseradish peroxidase using cyanuric chloride. J Immunol Methods. 2005;306:211–217. doi: 10.1016/j.jim.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Agostini E, Hernandez-Ruiz J, Arnao MB, Milrad SR, Tigier HA, Acosta M. A peroxidase isoenzyme secreted by turnip (Brassica napus) hairy-root cultures: inactivation by hydrogen peroxide and application in diagnostic kits. Biotechnol Appl Biochem. 2002;35:1–7. doi: 10.1042/BA20010049. [DOI] [PubMed] [Google Scholar]
- Al-Ruwaili MA, Khalil OM, Selim SA. Viral and bacterial infections associated with camel (Camelus dromedarius) calf diarrhea in North Province, Saudi Arabia. Saudi J Biol Sci. 2012;19:35–41. doi: 10.1016/j.sjbs.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcarz A, Ginalska G, Kowalewska B, Kulesza P. Spring cabbage peroxidases-potential tool in biocatalysis and bioelectrocatalysis. Photochem. 2008;69:627–636. doi: 10.1016/j.phytochem.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Boorsma DM, Kalsbeek GL. A comparative study of horse radish peroxidase conjugates prepared with a one-step and a two-step method. J Histochem Cytochem. 1975;23:200–207. doi: 10.1177/23.3.47869. [DOI] [PubMed] [Google Scholar]
- Brunetti E, Garcia HH, Junghanss T. Cystic echinococcosis: chronic, complex, and still neglected. Plos Negl Trop Dis. 2011;5:E1146. doi: 10.1371/journal.pntd.0001146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo J, Ferapontova E, Hushpulian D, Tasca F, Tishkov V, Chubar T, Gazaryan I, Gorton L. Direct electrochemistry and bioelectrocatalysis of H2O2 reduction of recombinant tobacco peroxidase on graphite: effect of peroxidase single-point mutation on Ca2+-modulated catalytic activity. J Electroanal Chem. 2006;588:112–121. doi: 10.1016/j.jelechem.2005.12.010. [DOI] [Google Scholar]
- Chen H, Zhang Q, Dang Y, Shu G. The effect of glutaraldehyde cross-linking on the enzyme activity of immobilized β-galactosidase on Chitosan bead. J Food Sci Technol. 2013;5:932–935. [Google Scholar]
- Childs RE, Bardsley WG. Steady-state kinetics of peroxidase with 2,2′-azino-di-(3-ethyl-benzthiazoline6-sulphonic acid) as chromogen. J Biochem. 1975;145:93–103. doi: 10.1042/bj1450093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig PS, Rogan MT, Campos-Ponce M. Echinococcosis: disease, detection and transmission. Parasitology. 2003;127:S5–S20. doi: 10.1017/S0031182003004384. [DOI] [PubMed] [Google Scholar]
- El Hakim AE, Shahein YE, Abouelella AM, Selim ME. Purification and characterization of two larval glycoproteins from the cattle tick, Boophilus annulatus. J Vet Sci. 2007;8:175–180. doi: 10.4142/jvs.2007.8.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Gayar KE, Ibrahim MA, Mohamed SH, Zakaria Z, Abdalhameed AS. Application of extracted peroxidase enzyme from turnip roots (Brassica napus) in clinical diagnostic kit. Int Cur Res Rev. 2012;4:17–22. [Google Scholar]
- Hamed RR, Mohamed TM, El Hakim AE, Gad AM. Biochemical studies on the conjugation of antibodies with turnip peroxidase using two different methods. J Biol Res. 2009;12:173–186. [Google Scholar]
- Hudson L, Hay FC. Practical immunol. 3. Philadelphia: Blackwell Scientific; 1989. [Google Scholar]
- Jeanson A, Cloes JM, Bouchet M, Rentier B. Comparison of conjugation procedures for the preparation of monoclonal antibody-enzyme conjugates. J Immunol Methods. 1988;111:261–270. doi: 10.1016/0022-1759(88)90135-4. [DOI] [PubMed] [Google Scholar]
- Kandil OM, Hendawy SHM, El Namaky AH, Gabrashanska MP, Nanev VN. Evaluation of different Haemonchus contortus antigens for diagnosis of sheep haemonchosis by ELISA and their cross reactivity with other helminthes. J Parasit Dis. 2016;41(3):678–683. doi: 10.1007/s12639-016-0865-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandil OM, Hassan NMF, Sedky D, Ata EB. Studies on the specific immuunodiagnosis of cystic Echinococcosis in camels using enzyme linked immunosorbent assay. BJVM. 2018;22(3):305–313. doi: 10.15547/bjvm.2136. [DOI] [Google Scholar]
- Khamehchian S, Zolfagharian H, Mohammadpour N, Majid D, Madani R. Study on camel IgG purification. Hum Vaccin Immunother. 2014;10:1633–1638. doi: 10.4161/hv.28531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindgren A, Ruzgas T, Gorton L, Csoregi E, Bautista AG, Sakharov IY, Gazaryan IG. Biosensors based on novel peroxidases with improved properties in direct and mediated electron transfer. Biosens Bioelectron. 2000;15:491–497. doi: 10.1016/S0956-5663(00)00110-X. [DOI] [PubMed] [Google Scholar]
- Lowry DH, Rosenbrough NJ, Far AL, Randal RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mazza G, Charles C, Bouchet M, Ricard J, Raynaud J. Isolation, purification and physico-chemical properties of turnip peroxidases. Biochim Biophys Acta. 1968;167:89–98. doi: 10.1016/0005-2744(68)90279-9. [DOI] [PubMed] [Google Scholar]
- O’Brien AM, Smith AT, Ó’Fágáin C. Effects of phthalic anhydride modification on horseradish peroxidase stability and activity. Biotechnol Bioeng. 2003;81:233–240. doi: 10.1002/bit.10462. [DOI] [PubMed] [Google Scholar]
- Pandey VP, Awasthi M, Singh S, Tiwari S, Dwivedi UN. A comprehensive review on function and application of plant peroxidases. Biochem Anal Biochem. 2017;6:1–16. doi: 10.4172/2161-1009.1000308. [DOI] [Google Scholar]
- Pourseifa MM, Moghaddamc G, NazliSaeedi N, Barzegaria A, Dehghania J, Omidi Y. Current status and future prospective of vaccine development against Echinococcus granulosus. Biologicals. 2018;51:1–11. doi: 10.1016/j.biologicals.2017.10.003. [DOI] [PubMed] [Google Scholar]
- Ramesh KK, Xiavour S, Latha S, Kumar V. Sukumaran (2014) Anti-human IgG-horseradish peroxidase conjugate preparation and its use in ELISA and western blotting experiments. J Chromatogr Separat Tech. 2014;5:211–230. [Google Scholar]
- Rashimaw JAM. Nucleic acids and proteins in plants I. Structure, biochemistry and physiology of proteins. In: Boulter D, Parthier B, editors. Encyclopedia of plant physiology. New York: Springer; 1982. pp. 237–239. [Google Scholar]
- Saraiva JA, Nunes CS, Coimbra MA. Purification and characterization of olive (Oleaeuropaea L.) peroxidase—evidence for the occurrence of a pectin binding peroxidase. Food Chem. 2007;101:1571–1579. doi: 10.1016/j.foodchem.2006.04.012. [DOI] [Google Scholar]
- Shetty P, D’Souza A, Geethu CP. Conjugation of peroxidase from Brassica oleraceagongylodes for use as a label-prospect of a novel enzyme tag for immunoassay systems. Int J Appl Sci Biotechnol. 2017;5:59–65. doi: 10.3126/ijasbt.v5i1.17009. [DOI] [Google Scholar]
- Yu SH, Wang H, Wu XH, Ma X, Liu PY, Liu YF, Zhao YM, Morishima Y, Kawanaka M. Cystic and alveolar echinococcosis: an epidemiological survey in a tibetan population in southeast Qinghai, China. Jpn J Infect Dis. 2008;61:242. [PubMed] [Google Scholar]