Abstract
Many reports indicate medicinal value of garlic (Allium sativum), a popular herbal medicine used worldwide, and its therapeutic effect against several diseases. Earlier studies in our laboratory have shown a potential therapeutic role of garlic–artemisinin combination in mice infected with Plasmodium berghei. A single dose of α, β-arteether with three oral doses of garlic provides almost 95% protection. The present study aims to understand the mode of action of this combination. We have documented the level of nitric oxide (NO), a key molecule of protection and have seen in the reversal of organ morphology caused by malaria infection. The combination effects on the (a) survival rate and degree of parasitemia and (b) NO levels in blood, liver, spleen and thymus of malaria-infected mice were investigated. During the study, liver, spleen and thymus cell suspensions were assessed for immunobiochemical alterations of NO levels. The increase in NO level after infection appears to be unable to protect, whereas striking increase in spleen and thymus leads to protection against infection, and is further confirmed by detection of increased inducible nitric oxide synthase mRNA expression levels in different organs by RT-PCR. In addition, the role of T cell subsets during combination treatment was also studied. All these results indicate a potential mechanism of protection through NO pathway in combination-treated animals after malaria infection and may lead to an immunotherapy trial of malaria disease.
Keywords: Malaria, Plasmodium berghei, Garlic, Arteether, Nitric oxide, INOS
Introduction
Malaria, a parasitic disease, is very common in tropical and subtropical regions of the world (Evans 2009). Plasmodium species, causative agent of malaria, exerts a greater influence on the world population with millions of people being at risk every year, especially children below 5 years and the pregnant women. Owing to the drug resistance, research groups around the world are focused on identifying new antimalarials to be used in combination with a potent antimalarial drug, artemisinin (Adjuik et al. 2004; Nandakumar et al. 2006). Our laboratory has entered this group by showing garlic (Allium sativum) in combination with artemisinin has significant antimalarial effect (Palakkod Govindan et al. 2016). In continuation, the current study focuses on identifying mechanism of protection by this combination against malaria infection in the experimental rodent model of malaria. Garlic (Allium sativum), for years, has been widely used as a medicine to reduce various risk factors associated with several diseases. It contains bioactive components that have proven beneficial in treatment of cancer, infection, inflammation, etc. (Amagase 2006). It is also known to exhibit anticoagulant, antioxidant, antibiotic, hypocholesterolemic, hypoglycemic and hypotensive activities (Mikaili et al. 2013). There is a clear necessity for standardization of garlic preparations, especially for use in biological studies. More recent biological studies have attempted to rectify this problem. Hence, we have used the ayurvedic medicine garlic pearl oil, locally available in India which is already in use for management of cholesterol, hypertension, digestion and to improve immunity. Garlic pearls are made from extract of concentrated source of a special variety of garlic. It is well known that malaria infection leads to modulation of host immune responses (Coban et al. 2007) and one such mechanism explored in this study is nitrogen and oxygen double-bonded molecule, nitric oxide (NO). This biologically less half-life molecule is a primary product of nitric oxide synthase (NOS) enzyme which exists in three isoforms: two are constitutively expressed and one is inducible (iNOS) (Griffith and Stuehr 1995). In this study, the main focus is on the role of NO in conferring protection during garlic and arteether combination treatment in malaria-infected mice.
Several immunological and non-immunological interactions are known to mediate mechanisms of host defense during infections (Deans and Cohen 1983), and NO is one such versatile molecule which is shown to have a lead role in pathology or protection during malaria infection. NO has been well known to be an effecter molecule involved in several biological processes (Amini et al. 2009; Iadecola et al. 1994) especially in host defense against numerous pathogens (Convit et al. 2004; Machado-Pinto et al. 2002; Toledo et al. 2001). Plasmodium berghei is one such pathogen of rodent malaria model which is reported to be susceptible to NO mediators (Ali et al. 2010). We have used this model for demonstrating the mechanism for protection after the drug treatment by manipulating NO levels in the host. Previous reports showed manipulation of NO levels in mosquito’s gut, which will provide to control malaria transmission (Luckhart et al. 1998). Nitric oxide (NO), derived from molecular oxygen and the guanidino nitrogen of l-arginine, in a reaction catalyzed by NO-synthase (NOS) displays manifold activities (Badaro et al. 2001; Murray et al. 2003). NO has been well known to be an effector molecule of immunological reactions in host defense against numerous pathogens (Convit et al. 2004; Machado-Pinto et al. 2002; Toledo et al. 2001). NO has been implicated in different aspects of malaria parasite infection. NO-mediated non-specific host defense contributes to the protective immunity to plasmodium exoerythrocytic forms and blood-stage malaria parasites during early phase of infection (Acosta and Cazorla 2004; Borja-Cabrera et al. 2004; Gamboa-León et al. 2006; Santos et al. 2003). However, the role of NO in the protection against malaria remained to be debated. In the current study, the immunomodulation of host during malaria infection and protection after garlic and arteether combination treatment was investigated via NO pathway and its possible relationship with NO production and protection.
Materials and methods
Drugs and chemicals
Commercially available antimalarial drug α, β-arteether (E MAL™, Themis Medicare Ltd., Uttarakhand, India) and ayurvedic medicine garlic pearl oil (Sun Pharmaceutical Ind. Ltd., Mumbai, India) were procured locally. Roswell Park Memorial Institute (RPMI) medium 1640 and heat-inactivated fetal bovine serum (FBS) are procured from Gibco by Life Technologies (NY, USA) and 5000 U/mL penicillin, 5 mg/mL streptomycin from HiMedia Laboratories (Mumbai, India), Griess reagent (5% phosphoric acid, 1% sulfanilamide and 0.1% N-(1-naphthyl)-ethylenediamine dihydrochloride (NED) from Sigma, anti-mouse CD4+-FITC, anti-mouse CD8+-PE and anti-mouse CD3+-PE were procured from eBioscience (TM, USA), RBC lysis buffer from BioLegend Inc. (CA, USA), Revert Aid first strand cDNA synthesis kit and PCR master mix (2X) from Thermo Scientific (MA, USA) and iNOS and GAPDH Primers from Sigma (MI, USA).
Test animals
Swiss albino male mice of 4–5 week old (25 g) were used in all the experiments. They were obtained from the Central Animal Facility, Indian Institute of Science, Bangalore. All precautions were undertaken to minimize suffering. Animal experiments were carried out as per the guidelines of the “Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA)” Government of India (Registration No: 48/1999/CPCSEA) and as approved by the Institutional Animal Ethics Committee (IAEC) (CAF/Ethics/282/2012 and 585/2018).
In vivo infection and drug treatment protocols
Our laboratory has shown the in vivo antimalarial activity and protection after garlic pearl oil and arteether combination treatment, and we have followed the same protocol as previously described elsewhere (Palakkod Govindan et al. 2016). In brief, experimental infections were initiated with 60–70% parasitized erythrocytes obtained from a passaged donor animal and injected intraperitoneally (i.p.) on start of experiment (Day 0) after appropriate dilution. All the control mice died between Day 5 and Day 6 post-infection. 72 h later (Day 3), when the parasitemia was about 2–4%, infected mice were treated with either single dose of arteether (500 μg) intramuscularly (i.m.) and/or three oral doses of garlic pearl oil (100 µL/mouse on Day 3, 4 and 5) to non-anesthetized mice. Blood was drawn from tail vein to check the parasitemia progression or inhibition at regular time intervals, and mouse mortality was noted daily by staining blood smears with Giemsa. Blood was drawn from tail vein of infected animals every alternate day and further 5 days post-treatment. In vivo antimalarial activity was examined in groups of ten male mice in three independent experiments for the study duration (30 days). Tables 1, 2 and 3 show the clear picture of the workflow of the study.
Table 1.
Experimental mice in different groups
| Group | Treatment | Dosage | Route of administration |
|---|---|---|---|
| Group 1 | Uninfected | Normal animals | |
| Group 2 | P. berghei infected | 100 µL of 106/mL | i.p.a |
| Group 3 | Infected and garlic treated | 100 µL (3 doses at 24 h interval) | Oral |
| Group 4 | Infected and arteether treated | 500 µg (single dose) | i.m.b |
| Group 5 | Infected and garlic + arteether treated | 500 µg (single dose) + 100 µL (3 doses at 24 h interval) | i.m.b + oral |
aIntraperitoneal
bIntramuscular
Table 2.
Procedure overview of the study
| Day 0 | P. berghei infection | Group 2, 3, 4 and 5 |
| Day 3 | 1st treatment |
Group 3: Ga-100 µL Group 4: AEb-500 µg Group 5: AEb-500 µg + Ga-100 µL |
| Day 4 | 2nd treatment |
Group 3: Ga-100 µL Group 5: Ga-100 µL |
| Day 5 | 3rd treatment |
Group 3: Ga-100 µL Group 5: Ga-100 µL |
aGarlic
bArteether
Table 3.
Short-term culture supernatant collection time points
| In vitro culturing of blood, spleen and thymus cells from treated mice | |
|---|---|
| Day 4 | From Day 3 treated mice |
| Day 5 | From Day 4 treated mice |
| Day 6 | From Day 5 treated mice |
| Collection of culture supernatant with day and different time points | |||
|---|---|---|---|
| 24 h | 48 h | 72 h | |
| Day 5 | From Day 4 culture | – | – |
| Day 6 | From Day 5 culture | From Day 4 culture | – |
| Day 7 | From Day 6 culture | From Day 5 culture | From Day 4 culture |
| Day 8 | – | From Day 6 culture | From Day 5 culture |
| Day 9 | – | – | From Day 6 culture |
Histopathological investigation
Experimental mice were anesthetized by inhalation of diethyl ether and killed for organ collection, which includes the brain, liver and spleen. The organs excised were immediately fixed with 4% paraformaldehyde, embedded in paraffin, cut into 5 µm sections and finally stained with hematoxylin and eosin (H&E). The morphological alterations in the tissues of P. berghei-infected and treated mice were examined under light microscopy at 10× magnification. The results of histopathological examination were confirmed by observation of not less than 10 fields per section.
Short-term culturing of cells
Peripheral blood cell culture
Blood was collected from mouse by cardiac puncture in a heparinized tube and centrifuged 350 × g for 5 min; RBC pellet was resuspended in RPMI 1640 medium for short-term culture. 1 × 106 cells/mL of medium (pH = 7.4) was propagated in vitro at 37 °C in an incubator with 5% CO2 for 24, 48 and 72 h (in triplicates). After the terminal time as mentioned in Table 3, 500 µL of cell culture medium was aliquoted, centrifuged, and supernatant was used for NO analysis.
Primary cell culturing of splenocytes and thymocytes
Mouse spleen was excised aseptically and disrupted in the sterile culture dish with 10 mL of RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/mL of penicillin and 100 µg/mL of streptomycin. Cells were filtered through a sterile fine mesh and later centrifuged at 350 × g for 10 min at room temperature. RBCs were lysed with cold RBC lysis buffer, and cells were washed twice with fresh medium. Cell viability was determined by Trypan Blue exclusion and was 95%. Splenocytes were adjusted to a final concentration of 1 × 107 cells/mL in RPMI, and 1.5 mL aliquots of the suspended cells per well were incubated in 12-well flat-bottom tissue culture plates (Tarsons Products Pvt. Ltd., Kolkata, India) in triplicates for 24, 48 and 72 h. At each terminal time point, after thorough suspension of cells, 500 µL was collected from each well and was centrifuged at 350 × g for 10 min at room temperature. Supernatant aliquots were stored at − 80 °C until further analysis. Cell pellet was used for surface phenotype analysis by FACS.
Thymocytes were released from aseptically removed thymus by disrupting in RPMI medium using a pair of blunt ended forceps and passed through fine wire mesh in order to obtain a single-cell suspension and centrifuged 350 × g for 10 min. Thymocytes were suspended with RPMI to get the final concentration of 1 × 106 cells/mL and followed the same protocol like splenocytes culture (as above). Pooled blood, splenocytes and thymocytes from two mice were subjected to analysis.
Estimation of nitric oxide by Griess reagent
As a measure of NO production, concentrations of nitrite (NO2−) in cell supernatants were determined by the Griess reaction. The standard Griess reaction protocol was adapted to assay nitrite. 100 μL of culture supernatants was transferred to a 96-well flat-bottomed microplate (Nunc) in triplicate, added 100 μL of Griess reagent, incubated at room temperature for 10 min in dark, and absorbance was read at 550 nm using a spectrophotometer (SpectraMax 190 Microplate Reader, Molecular Devices). Values for the concentration of nitrite assayed were calculated from standard calibration plots for NaNO2 and NaNO3 following nitrate reductase action. For each assay run in this study, the efficiency of nitrate reductase activity and the recovery of nitrite from biological samples were determined using internal standards. Values presented for nitrite concentrations, as a measure of NO, have been corrected for these losses.
RNA isolation and semiquantitative RT-PCR
Total RNA was isolated from blood, liver, spleen, thymus and brain tissues of P. berghei-infected and treated animals using TRI reagent (Sigma) following manufacturer’s instructions, and semiquantitative RT-PCR was used to determine iNOS mRNA expression. cDNA was synthesized using an RevertAid first strand cDNA synthesis kit (Thermo Scientific) following the manufacturer’s instructions. The primer sequences for iNOS and GAPDH are: 5′-ATGGCTTGCCCCTGGAAGTTTCTC-3′ (forward), 5′-CAGCTTCCAGCCTGGCCAGATG-3′ (reverse) (iNOS) and 5′-ATGGTGAAGGTCGGTGTGAACGGA-3′ (forward), 5′-TTACTCCTTGGAGGCCATGTAGG-3′ (reverse) (GAPDH). PCR program conditions were 1.5 min at 94 °C, 1.5 min at 55 °C, 2 min at 72 °C for 40 cycles for iNOS and 30 s at 94 °C, 30 s at 58 °C, 45 s at 72 °C for 35 cycles for GAPDH. The predicted PCR products were 486 bp and 496 bp for iNOS and GAPDH, respectively. 10-µL PCR product from a 50-µL reaction mixture was analyzed by electrophoresis on 1.5% agarose gels. The product was visualized on ChemiDoc XRS (Bio-rad).
Flow cytometric analysis
A single-cell suspension of splenocytes (5 × 106 cells) and thymocytes (0.5 × 106 cells) in 500 μL of medium was stained for cell surface expression of the T cell co-receptors, CD4+, CD8+ and CD3+ using the fluorochrome-conjugated antibodies, anti-mouse CD4+-FITC, anti-mouse CD8+-PE and anti-mouse CD3+-PE. Staining was done at 4 °C for 45 min in dark, and then, cells were washed twice with PBS, fixed in 0.5% paraformaldehyde and subjected to FACS analysis using BD FACS Verse TM flow cytometer. Approximately 20,000 events in the singlet (FSC-A vs. FSC-H) and live gate (FSC-A vs. SSC-A) were acquired. The data were analyzed as dot plots, where the cell subsets in the splenocytes and thymocytes were quantified based on percent cell population.
Statistical analysis
The data were plotted using Graph Pad Prism 5 software. The data are represented as mean ± standard error of mean (SEM), consisting of two or more independent experiments. Experiments with multiple groups were analyzed using one-way analysis of variance (ANOVA), two-way ANOVA to compare the means of different unpaired or paired values, respectively, followed by Tukey’s multiple comparison test. Values of p < 0.05 were considered as statistically significant.
Results
Effect of garlic and artemisinin combination on parasitemia and mortality
Giemsa-stained blood smear observation confirms the P. berghei infection as compared with uninfected control (Fig. 1). As we have reported earlier (Palakkod Govindan et al. 2016), between day 5 and day 6 post-infection, the infected animals will show clinical signs of malaria like pale, inactive, biologically weak and death due to anemia with 80–90% mortality rate, whereas infected mice treated with garlic survived between day 8 and day 10 and arteether-treated animals survived anywhere between day 18 and day 21. At the same time, garlic and arteether combination-treated animals were healthy and no clinical symptoms with 0% parasitemia (Fig. 2a) and the survival rate was 100% (Fig. 2b) for the study duration (30 days).
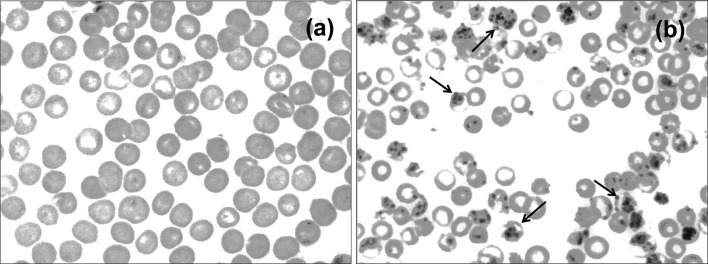
Fig. 1.
Confirmation of P. berghei infection in mice: Giemsa-stained thin blood smears of uninfected (a) and P. berghei-infected mice (b) (black arrow indicates malaria parasites)
Fig. 2.
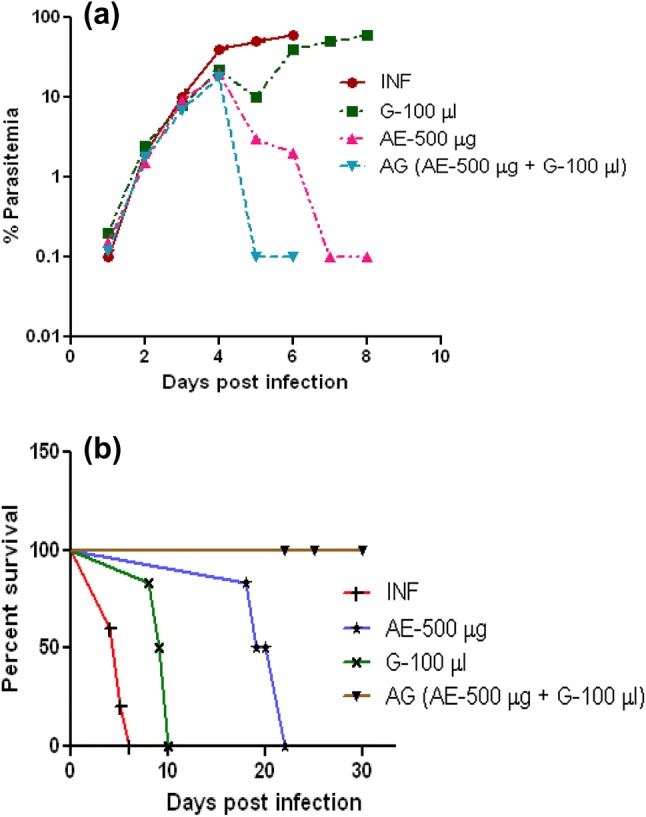
Effect of garlic–arteether combination on parasitemia and mortality: Percent parasitemia (a) and survival patterns (b) of P. berghei-infected mice after arteether (500 μg, single dose), garlic oil (100 μL, three doses) and arteether and garlic oil combination drug treatment. Data represent a mean of ten mice per group in each experiment and result of three independent experiments. INF—P. berghei-infected, G—garlic oil, AE—arteether, AG—arteether + garlic oil
Histopathological findings
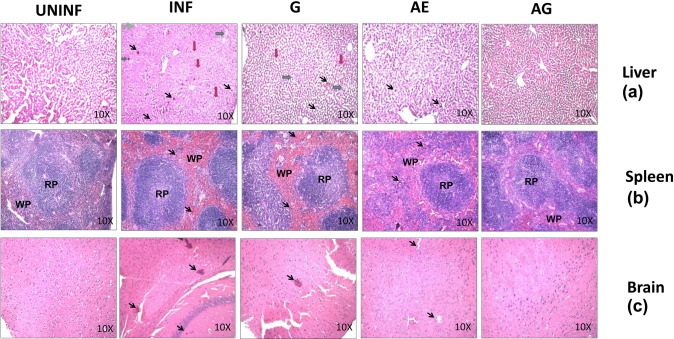
Sequestration of pRBCs in the microvasculature of the tissues was observed. In liver, along with pRBC sequestration, centrilobular region degeneration, necrosis with swollen hepatocytes and abundant kupffer cells containing numerous malarial pigment grains were observed. These reflect the pathological changes due to malaria infection in the liver sections. When these changes were compared with monotherapy-treated sections, slight improvement was observed, whereas in combination drug-treated sections, significant improvement in morphology and liver architecture was observed and was comparable with that of uninfected sections with normal hepatocytes, kupffer cells without any malarial pigment (Fig. 3a).
Fig. 3.
H&E-stained sections of liver, spleen and brain tissues from P. berghei-infected animals before and after treatment. UNINF, Uninfected; INF, P. berghei infected; G, garlic treated; AE, arteether treated; AG, arteether + garlic treated; RP, red pulp; WP, white pulp (black arrow indicates malaria pigment, red arrow indicates swollen hepatocytes, blue arrow indicates kupffer cells containing malaria pigment, and green arrow indicates centrilobular region degeneration)
In case of infected spleen sections, difference in germinal center with enlarged red and white pulps containing hemozoin pigments was observed. The overall enlargement of these pulps was associated with the loss of germinal center typical structure of malaria-infected mice. Not many differences were observed with mono-drug-treated and infected sections, but improved morphological changes with no severe pathological changes were identified in combination drug-treated spleen sections (Fig. 3b).
In case of brain sections, pigmentation and sequestration of infected RBCs especially hemozoin containing red blood cells in the blood vessels were observed as brownish small spots and also in mono-drug-treated samples. There was no significant difference observed in mono-drug-treated and malaria-infected sections. However, no such changes were observed in the combination drug-treated animal sections (Fig. 3c).
Effect of combination drug treatment on nitric oxide levels in in vitro short-term cultures of P. berghei-infected animals
Nitric oxide levels in P. berghei-infected animals
NO levels in blood, spleen and thymus were estimated in P. berghei-infected animals at different time points during the course of infection. Assays showed a moderate increase in NO levels in blood and spleen during the infection when compared with the uninfected control, whereas a significant increase in NO levels was observed in thymus on Day 5 of infection (Fig. 4).
Fig. 4.
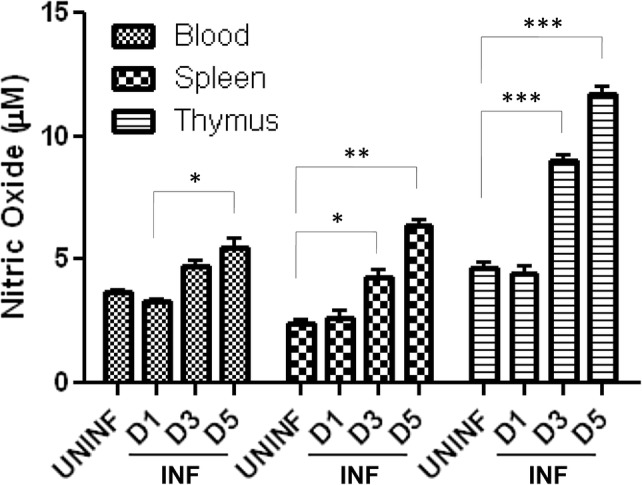
Effect of P. berghei infection on nitric oxide production in mouse tissue organs: values of NO (in µM)—derived from standard curve are presented as mean ± standard error of mean (UNINF—uninfected, INF—P. berghei infected) (*p < 0.05; **p < 0.01; ***p < 0.001) (two-way ANOVA) (D1, Day 1; D3, Day 3; D5, Day 5)
Effect of garlic and arteether combination treatment on nitric oxide levels in P. berghei-infected animals
Blood, spleen and thymus were collected 24 h after 1st, 2nd and 3rd doses of garlic and arteether combination treatment independently in vivo system. Single-cell suspension of corresponding organs was made and cultured in vitro for 72 h. After which, the cell culture supernatant was collected and NO levels were estimated. After 1st dose of treatment, there was no difference in NO levels observed when compared with infected control in blood, spleen and thymus. After 2nd dose of treatment, no significant change was observed in NO levels in blood; however, an increase was observed in all the samples of spleen and thymus, especially a maximum increase in NO levels was observed in the thymus of combination-treated animals (Fig. 5a). A similar pattern was observed after 3rd dose of treatment with very significant levels of NO (Fig. 5b). Here, we have compared NO levels of different organ tissues from treated animals.
Fig. 5.
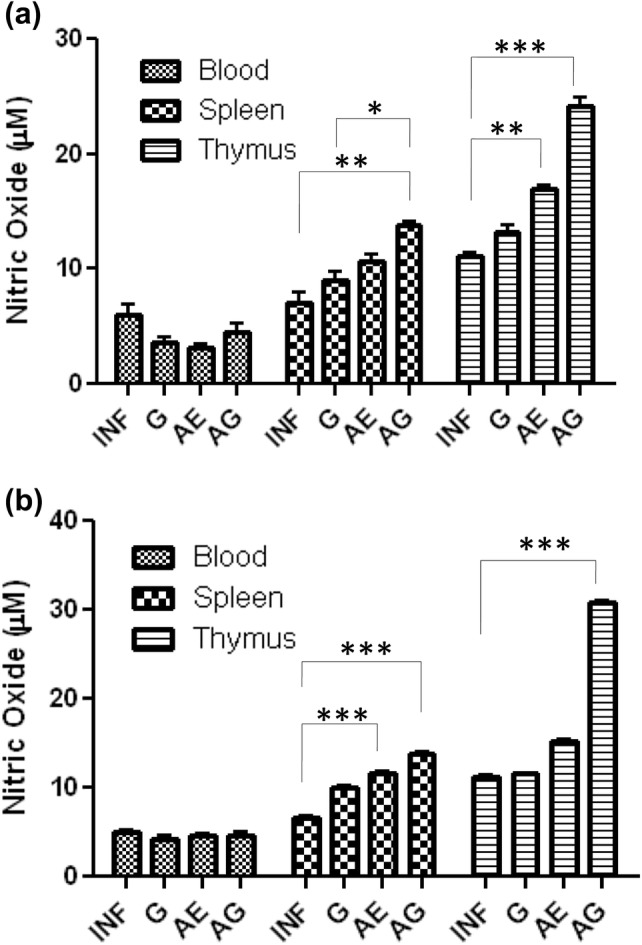
Drug-treated responses on nitric oxide production in P. berghei-infected mice: values are presented as mean ± standard error of mean of P. berghei infected (INF), garlic oil (G), arteether (AE) and arteether + garlic oil (AG) combination-treated animals (*p < 0.05; **p < 0.01; ***p < 0.001) (two-way ANOVA)
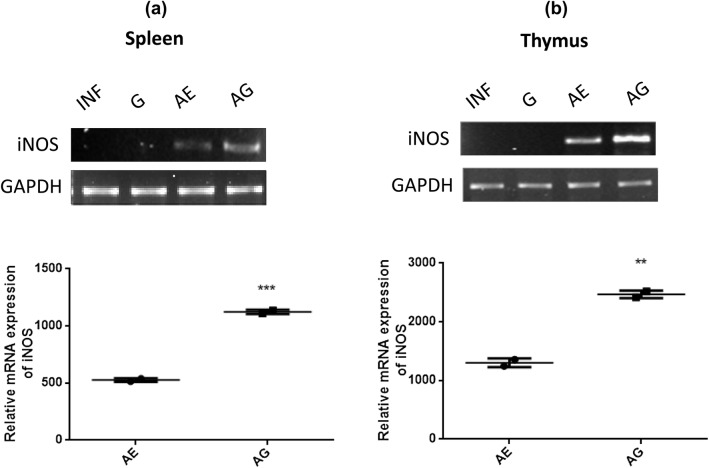
Effect of garlic and arteether combination treatment on iNOS mRNA expression
The iNOS mRNA expression was analyzed after 1st, 2nd and 3rd treatment from blood, spleen, liver, thymus and brain tissues. No expression was observed after 1st and 2nd treatment in any samples. In combination-treated animals, no change was observed in the expression of iNOS in blood, liver and brain tissues before and after treatment (data not shown), whereas a marked increase in iNOS mRNA expression was observed in spleen and thymus tissues only after the 3rd treatment. Increased expression of iNOS was observed in both spleen and thymus of AG-treated animals when compared with that of AE-treated animals (Fig. 6a, b). This correlates with the NO levels detected in respective tissues of AG-treated animals. In garlic and arteether monotherapy, no change or slight increase in iNOS mRNA expression was observed across all the tissues, respectively. Here, we have compared the increase of iNOS mRNA expression with AE-treated samples because we did not notice any iNOS mRNA expression in the infected samples. GAPDH was used as an internal control.
Fig. 6.
Relative mRNA expression levels of iNOS in spleen (a) and thymus (b) of P. berghei-infected and treated animals. INF, P. berghei infected; G, garlic treated; AE, arteether treated; AG, arteether + garlic treated (*p < 0.05; **p < 0.01; ***p < 0.001) (unpaired t test)
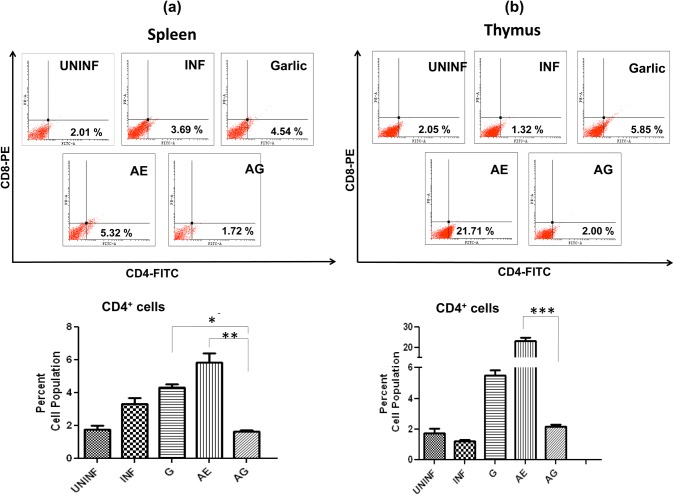
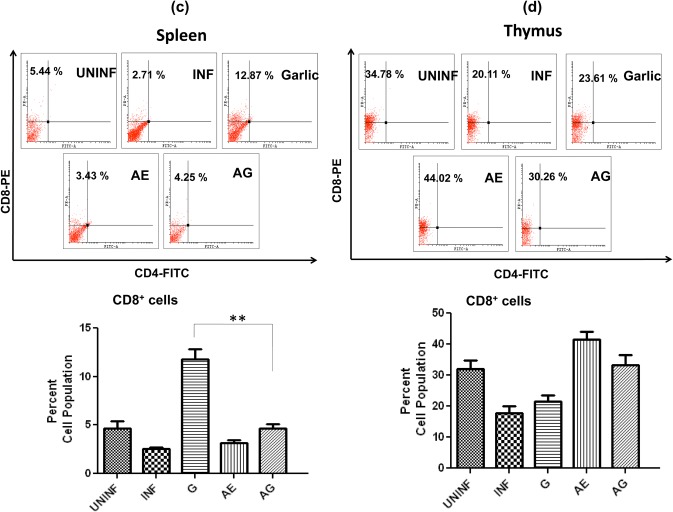
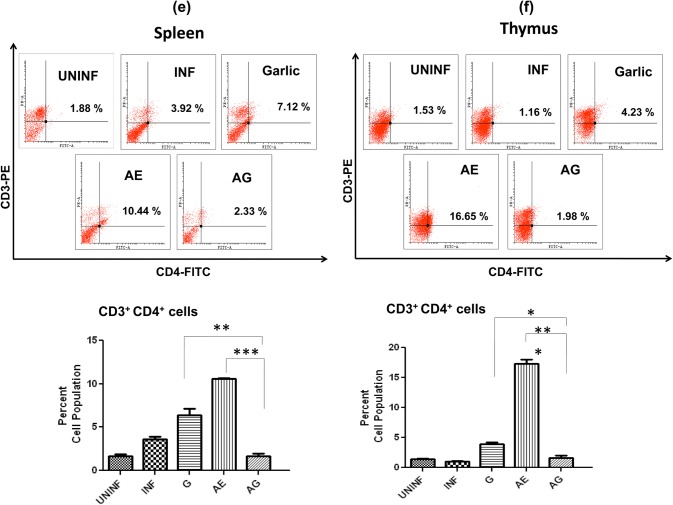
Effect of combination drug treatment on T-lymphocyte subsets levels in spleen and thymus in P. berghei-infected animals
In spleen, a slight increase in CD4+ population in garlic treated and significant increase in AE monotherapy were observed (Fig. 7a). In thymus, we observed an increase in garlic and arteether treated; however, in arteether a very significant level of CD4+ cells was observed (19-fold increase) (Fig. 7b). The CD4+ populations dropped to uninfected levels in combination treated in both spleen and thymus. With respect to CD8+ cell population in spleen, a significant increase was observed only in garlic-treated animals (Fig. 7c), whereas in thymus, an increase in both arteether-treated and combination-treated animals observed (Fig. 7d). In addition, the single-cell suspensions of spleen and thymus were stained for CD3+ + CD4+ cells. The cell population of these double-positive cells exhibited a pattern similar to that of CD4+ stained cells in both spleen (Fig. 7e) and thymus (Fig. 7f). This increase of cytotoxic T cells during monotherapy in different organs and decrease in combination-treated might be due to T cell apoptosis and absence of malarial parasites.
Fig. 7.
Effect of combination drug treatment on T-lymphocyte subsets: flow cytometric analysis and comparison of percent cell populations of CD4+ (a, b), CD8+ (c, d) and CD3+ + CD4+ (e, f) cells in uninfected (UNINF), P. berghei infected (INF), garlic oil (G), arteether (AE) and arteether + garlic oil (AG) combination-treated animals in spleen and thymus. Values are presented as mean ± standard error of mean (*p < 0.05; **p < 0.01; ***p < 0.001) (one-way ANOVA)
Discussion
We have investigated the mechanism of garlic–arteether combination treated via histopathological improvement, NO pathway and also through the flow cytometric analysis of T cells occurring in mice during infection within the rodent malaria parasite. The presence of NO or its metabolites in circulating body fluids gives no indication of its production site, and it might be detected in circulating blood or in different host organs during malaria infection (Nahrevanian and Dascombe 2001). The correlation between this ubiquitous molecule (NO) and multiple tissues affected by malaria is under debate with respect to overall value of NO in the host response to malaria (Rockett et al. 1994; White 1998). NO has been shown to inhibit the growth and function of several infectious agents (Woods et al. 1994) by inactivating several metabolic enzymes in mammalian cell targets; other potential enzymatic targets may exist in parasites (Meshnick and Eaton 1981). Studies so far point to the dual role played by NO during pathology (Stevenson et al. 1995) or protection (Aikawa 1988) in P. berghei-infected mice. This uncertainty may be attributed to several factors such as strain of malaria, degree of parasitemia, tissue samples and assay methods. It is well documented that iNOS mRNA expression is an important part of host’s immune response to several known infections (Kröncke et al. 1998). The down-regulation or up-regulation of iNOS gene expression may be an effective therapeutic strategy for prevention or protection from infection. iNOS gene expression is an important proximal mechanism for the up-regulation of NO production. In this regard, the present finding of an association of increased iNOS gene expression leading to increase in NO production in case of combination-treated mice supports the possible protective role of NO in the host, along with the histopathological organ modifications. This is in addition to the other immune responses that might be responsible for protection. In case of brain samples, we did not observe any change either in NO level or in iNOS expression at any point of our treatment (data not shown). Several previous studies demonstrated the histopathological damages of the organs like liver (Meis et al. 1983). Spleen (Coquelin et al. 1999) and brain (Franke-Fayard et al. 2005) due to malaria infection. These abnormalities contribute to architecture loss and affect host immune response (Cadman et al. 2008). Our results of histopathological examination showed the combination organ morphology was as good as the uninfected control animals, and hence proven, the protection was due to combination treatment.
Number of studies has shown that CD4+ and CD8+ T cells are the main participants in the development of both malaria pathogenesis and protective immunity (Engwerda et al. 2005; Podoba and Stevenson 1991). For blood-borne infectious diseases, CD4+ T cells, from main lymphoid organs such as spleen and thymus, have been implicated in the immune responses to malaria parasite (Schofield and Grau 2005). There are reports where CD4+ and CD8+ T cells contribute to the NO responses in lymphoid organs during P. berghei infection in mice (Rockett et al. 1994). We took advantage of these facts and tried to identify whether CD4+ and CD8+ cells have a role during combination therapy. We observed variations in CD4+ and CD8+ cell populations in both garlic and arteether monotherapy in spleen and thymus, whereas the cell populations were significantly reduced in combination treated when there was a peaked level due to monotherapy. This suggests that garlic had a significant cytotoxic T cell effect during monotherapy, especially in spleen, whereas same increase was noticed in thymus with arteether monotherapy. With the combination treatment, the CD4+ and CD8+ T cell populations declined probably due to T cell apoptosis as shown elsewhere (Nussler et al. 1993; Seguin et al. 1994) and this might be due to complete elimination of parasites as reported (Liehl et al. 2015). The experiment was planned with double-positive markers to identify CD3+ and CD4+ cells in spleen and thymus to know the ratio of CD4+ T-helper population versus CD3+. CD3 is the most common T cell marker which is only expressed by T cells. By gating for CD3+ cells, it is guaranteed that no CD3+ but CD4+ cells are counted as CD4+ T cells. The result of double-positive labeling matches with the cell counts with CD4+ labeling. In double-positive ratio with CD3++CD4+ cell population, the same results with CD4+ were observed in both spleen and thymus. The pivotal role of CD4+ and CD8+ T cells in the development of protective immunity makes necessary profound comprehension of the mechanisms involved in their activation and regulation during garlic and arteether combination therapy. Thus, we conclude that the cell surface markers such as CD4+ and CD8+ T cells have a central role during the onset of malaria infection and in combination drug treatments where they are acting in parallel with the protection mechanism. Taken together, these results strongly support the potential of garlic–arteether combination for the immunotherapy of malaria infection and also complemented by the direct inhibitory effect of ajoene, a garlic component as seen in other parasitic diseases (Ledezma et al. 2002).
Even though it is a matter of debate whether NO is involved in pathogenicity or in protection, our study shows that NO production after garlic–arteether combination treatment is most important effecter to eliminate the parasite and is involved in several immunological interactions mediating the host defense at different production sites. Studies showing that any disruption of iNOS gene in animals failed to produce NO and in turn, failed to control parasitic disease in vivo system are perhaps an ultimate demonstration of the relevant of this effecter molecule (Wei et al. 1995). In addition, our in vivo and in vitro data show that combination of drug treatment shows immunotherapeutic effect and is mediated by several parameters like T cells (CD4+ and CD8+), different tissues activation to produce NO for parasite elimination which leads to protection. From the prospective of high output on NO production via iNOS pathway leads to inactivation of enzymes (as mentioned earlier), hence the inhibition of cell proliferation (Kwon et al. 1991).
Finally, we conclude that all these changes, subsequently, may lead to secondary effects involving NO in protection with garlic–arteether combination treatment, which may contribute to reduce organ damage and also suggests that these may have a pharmacologically and clinically important role in protection from malaria and the mechanism is via NO production, which indicates a potential novel therapy in the treatment of severe malaria. To understand fully the protection after combination treatment, studies should be carried out in host/parasite interaction taking during infection and drug treatment which may be directly relevant for aspects of protection after combination drug treatment.
Acknowledgements
Authors would like to thank the Undergraduate Programme, Indian Institute of Science, Bangalore. The study was supported by Indian Council of Medical Research (ICMR), New Delhi (Ref No. 5/10/FR/19/2015-RBMH). Authors would like to thank Dr. Vijayakumar Govindaraj for critical review of the manuscript.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Acosta M, Cazorla D. Centipede (Scolopendra sp.) envenomation in a rural village of semi-arid region from Falcon State, Venezuela. Rev Invest Clin. 2004;56:712–717. [PubMed] [Google Scholar]
- Adjuik M, Babiker A, Garner P, Olliaro P, Taylor W, White N, Group IAS Artesunate combinations for treatment of malaria: meta-analysis. Lancet. 2004;363:9–17. doi: 10.1016/s0140-6736(03)15162-8. [DOI] [PubMed] [Google Scholar]
- Aikawa M. Human cerebral malaria. Am J Trop Med Hyg. 1988;39:3–10. doi: 10.4269/ajtmh.1988.39.3. [DOI] [PubMed] [Google Scholar]
- Ali M, Al-Olayan EM, Lewis S, Matthews H, Hurd H. Naturally occurring triggers that induce apoptosis-like programmed cell death in Plasmodium berghei ookinetes. PLoS ONE. 2010;5:e12634. doi: 10.1371/journal.pone.0012634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amagase H. Clarifying the real bioactive constituents of garlic. J Nutr. 2006;136:716S–725S. doi: 10.1093/jn/136.3.716S. [DOI] [PubMed] [Google Scholar]
- Amini M, Nahrevanian H, Khatami S, Farahmand M, Mirkhani F, Javadian S. Biochemical association between essential trace elements and susceptibility to Leishmania major in BALB/c and C57BL/6 mice. Braz J Infect Dis. 2009;13:83–85. doi: 10.1590/S1413-86702009000200002. [DOI] [PubMed] [Google Scholar]
- Badaro R, Lobo I, Nakatani M, Muiños A, Netto EM, Coler RN, Reed SG. Successful use of a defined antigen/GM-CSF adjuvant vaccine to treat mucosal Leishmaniasis refractory to antimony: a case report. Braz J Infect Dis. 2001;5:223–232. doi: 10.1590/s1413-86702001000400008. [DOI] [PubMed] [Google Scholar]
- Borja-Cabrera GP, et al. Effective immunotherapy against canine visceral leishmaniasis with the FML-vaccine. Vaccine. 2004;22:2234–2243. doi: 10.1016/j.vaccine.2003.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadman ET, et al. Alterations of splenic architecture in malaria are induced independently of toll-like receptors 2, 4, and 9 or MyD88 and may affect antibody affinity. Infect Immun. 2008;76:3924–3931. doi: 10.1128/iai.00372-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coban C, Ishii KJ, Horii T, Akira S. Manipulation of host innate immune responses by the malaria parasite. Trends Microbiol. 2007;15:271–278. doi: 10.1016/j.tim.2007.04.003. [DOI] [PubMed] [Google Scholar]
- Convit J, Ulrich M, Polegre MA, Avila A, Rodríguez N, Mazzedo MI, Blanco B. Therapy of Venezuelan patients with severe mucocutaneous or early lesions of diffuse cutaneous leishmaniasis with a vaccine containing pasteurized Leishmania promastigotes and bacillus Calmette-Guerin: preliminary report. Mem Inst Oswaldo Cruz. 2004;99:57–62. doi: 10.1590/S0074-02762004000100010. [DOI] [PubMed] [Google Scholar]
- Coquelin F, Boulard Y, Mora-Silvera E, Richard F, Chabaud AG, Landau I. Final stage of maturation of the erythrocytic schizonts of rodent Plasmodium in the lungs. C R Acad Sci III. 1999;322:55–62. doi: 10.1016/S0764-4469(99)80017-1. [DOI] [PubMed] [Google Scholar]
- Deans JA, Cohen S. Immunology of malaria. Annu Rev Microbiol. 1983;37:25–49. doi: 10.1146/annurev.mi.37.100183.000325. [DOI] [PubMed] [Google Scholar]
- Engwerda C, Belnoue E, Grüner AC, Rénia L. Experimental models of cerebral malaria. Curr Top Microbiol Immunol. 2005;297:103–143. [PubMed] [Google Scholar]
- Evans W. Trease and Evans pharmacognosy. 16. Philadelphia, PA: Saunders Ltd; 2009. [Google Scholar]
- Franke-Fayard B, et al. Murine malaria parasite sequestration: CD36 is the major receptor, but cerebral pathology is unlinked to sequestration. Proc Natl Acad Sci U S A. 2005;102:11468–11473. doi: 10.1073/pnas.0503386102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamboa-León R, et al. Immunotherapy against visceral leishmaniasis with the nucleoside hydrolase-DNA vaccine of Leishmania donovani. Vaccine. 2006;24:4863–4873. doi: 10.1016/j.vaccine.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Griffith OW, Stuehr DJ. Nitric oxide synthases: properties and catalytic mechanism. Annu Rev Physiol. 1995;57:707–736. doi: 10.1146/annurev.ph.57.030195.003423. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Pelligrino DA, Moskowitz MA, Lassen NA. Nitric oxide synthase inhibition and cerebrovascular regulation. J Cereb Blood Flow Metab. 1994;14:175–192. doi: 10.1038/jcbfm.1994.25. [DOI] [PubMed] [Google Scholar]
- Kröncke KD, Fehsel K, Kolb-Bachofen V. Inducible nitric oxide synthase in human diseases. Clin Exp Immunol. 1998;113:147–156. doi: 10.1046/j.1365-2249.1998.00648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon NS, Stuehr DJ, Nathan CF. Inhibition of tumor cell ribonucleotide reductase by macrophage-derived nitric oxide. J Exp Med. 1991;174:761–767. doi: 10.1084/jem.174.4.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledezma E, Jorquera A, Bendezú H, Vivas J, Pérez G. Antiproliferative and leishmanicidal effect of ajoene on various Leishmania species: ultrastructural study. Parasitol Res. 2002;88:748–753. doi: 10.1007/s00436-002-0649-9. [DOI] [PubMed] [Google Scholar]
- Liehl P, et al. Innate immunity induced by Plasmodium liver infection inhibits malaria reinfections. Infect Immun. 2015;83:1172–1180. doi: 10.1128/iai.02796-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luckhart S, Vodovotz Y, Cui L, Rosenberg R. The mosquito Anopheles stephensi limits malaria parasite development with inducible synthesis of nitric oxide. Proc Natl Acad Sci U S A. 1998;95:5700–5705. doi: 10.1073/pnas.95.10.5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Pinto J, Pinto J, da Costa CA, Genaro O, Marques MJ, Modabber F, Mayrink W. Immunochemotherapy for cutaneous leishmaniasis: a controlled trial using killed Leishmania (Leishmania) amazonensis vaccine plus antimonial. Int J Dermatol. 2002;41:73–78. doi: 10.1046/j.1365-4362.2002.01336.x. [DOI] [PubMed] [Google Scholar]
- Meis JF, Verhave JP, Jap PH, Sinden RE, Meuwissen JH. Malaria parasites–discovery of the early liver form. Nature. 1983;302:424–426. doi: 10.1038/302424a0. [DOI] [PubMed] [Google Scholar]
- Meshnick SR, Eaton JW. Leishmanial superoxide dismutase: a possible target for chemotherapy. Biochem Biophys Res Commun. 1981;102:970–976. doi: 10.1016/0006-291x(81)91633-8. [DOI] [PubMed] [Google Scholar]
- Mikaili P, Maadirad S, Moloudizargari M, Aghajanshakeri S, Sarahroodi S. Therapeutic uses and pharmacological properties of garlic, shallot, and their biologically active compounds. Iran J Basic Med Sci. 2013;16:1031–1048. [PMC free article] [PubMed] [Google Scholar]
- Murray HW, Brooks EB, DeVecchio JL, Heinzel FP. Immunoenhancement combined with amphotericin B as treatment for experimental visceral leishmaniasis. Antimicrob Agents Chemother. 2003;47:2513–2517. doi: 10.1128/aac.47.8.2513-2517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahrevanian H, Dascombe MJ. Nitric oxide and reactive nitrogen intermediates during lethal and nonlethal strains of murine malaria. Parasite Immunol. 2001;23:491–501. doi: 10.1046/j.1365-3024.2001.00406.x. [DOI] [PubMed] [Google Scholar]
- Nandakumar DN, Nagaraj VA, Vathsala PG, Rangarajan P, Padmanaban G. Curcumin-artemisinin combination therapy for malaria. Antimicrob Agents Chemother. 2006;50:1859–1860. doi: 10.1128/aac.50.5.1859-1860.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussler AK, Rénia L, Pasquetto V, Miltgen F, Matile H, Mazier D. In vivo induction of the nitric oxide pathway in hepatocytes after injection with irradiated malaria sporozoites, malaria blood parasites or adjuvants. Eur J Immunol. 1993;23:882–887. doi: 10.1002/eji.1830230417. [DOI] [PubMed] [Google Scholar]
- Palakkod Govindan V, Panduranga AN, Krishna Murthy P. Assessment of antimalarial activity of arteether and garlic oil combination therapy. Biochem Biophys Rep. 2016;5:359–364. doi: 10.1016/j.bbrep.2016.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podoba JE, Stevenson MM. CD4+ and CD8+ T lymphocytes both contribute to acquired immunity to blood-stage Plasmodium chabaudi AS. Infect Immun. 1991;59:51–58. doi: 10.1128/IAI.59.1.51-58.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockett KA, Awburn MM, Rockett EJ, Cowden WB, Clark IA. Possible role of nitric oxide in malarial immunosuppression. Parasite Immunol. 1994;16:243–249. doi: 10.1111/j.1365-3024.1994.tb00346.x. [DOI] [PubMed] [Google Scholar]
- Santos WR, Aguiar IA, Paraguai de Souza E, de Lima VMF, Palatnik M, Palatnik-de-Sousa CB. Immunotherapy against murine experimental visceral leishmaniasis with the FML-vaccine. Vaccine. 2003;21:4668–4676. doi: 10.1016/s0264-410x(03)00527-9. [DOI] [PubMed] [Google Scholar]
- Schofield L, Grau GE. Immunological processes in malaria pathogenesis. Nat Rev Immunol. 2005;5:722–735. doi: 10.1038/nri1686. [DOI] [PubMed] [Google Scholar]
- Seguin MC, et al. Induction of nitric oxide synthase protects against malaria in mice exposed to irradiated Plasmodium berghei infected mosquitoes: involvement of interferon gamma and CD8+ T cells. J Exp Med. 1994;180:353–358. doi: 10.1084/jem.180.1.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson MM, Tam MF, Wolf SF, Sher A. IL-12-induced protection against blood-stage Plasmodium chabaudi AS requires IFN-gamma and TNF-alpha and occurs via a nitric oxide-dependent mechanism. J Immunol. 1995;155:2545–2556. [PubMed] [Google Scholar]
- Toledo VP, et al. Immunochemotherapy in American cutaneous leishmaniasis: immunological aspects before and after treatment. Mem Inst Oswaldo Cruz. 2001;96:89–98. doi: 10.1590/S0074-02762001000100010. [DOI] [PubMed] [Google Scholar]
- Wei XQ, et al. Altered immune responses in mice lacking inducible nitric oxide synthase. Nature. 1995;375:408–411. doi: 10.1038/375408a0. [DOI] [PubMed] [Google Scholar]
- White NJ. Malaria parasite biology, pathogenesis and protection. Washington DC: American Society Microbiology Press; 1998. [Google Scholar]
- Woods ML, Mayer J, Evans TG, Hibbs JB. Antiparasitic effects of nitric oxide in an in vitro murine model of Chlamydia trachomatis infection and an in vivo murine model of Leishmania major infection. Immunol Ser. 1994;60:179–195. [PubMed] [Google Scholar]