Abstract
The fundamental goal of prosthesis is to achieve optimal levels of performance and enhance the quality of life of amputees. Socket type prostheses have been widely employed despite their known drawbacks. More recently, the advent of osseointegrated prostheses have demonstrated potential to be a better alternative to socket prosthesis eliminating most of the drawbacks of the latter. However, both socket and osseointegrated limb prostheses are prone to superficial infections during use. Infection prone skin lesions from frictional rubbing of the socket against the soft tissue are a known problem of socket type prosthesis. Osseointegration, on the other hand, results in an open wound at the implant-stump interface. The integration of infection sensors in prostheses to detect and prevent infections is proposed to enhance quality of life of amputees. Pathogenic volatiles having been identified to be a potent stimulus, this paper reviews the current techniques in the field of infection sensing, specifically focusing on identifying portable and flexible sensors with potential to be integrated into prosthesis designs. Various sensor architectures including but not limited to sensors fabricated from conducting polymers, carbon polymer composites, metal oxide semiconductors, metal organic frameworks, hydrogels and synthetic oligomers are reviewed. The challenges and their potential integration pathways that can enhance the possibilities of integrating these sensors into prosthesis designs are analysed.
Keywords: Prosthesis, Amputee, Infection, Sensing, Materials
Introduction
Prosthetic devices date back thousands of years and they have been in use with varying levels of success since their inception [1, 2]. The advancements in the field of robotics and healthcare have revolutionized the field of prosthesis in recent years and are continuing to improve the quality of life for amputees [3–6]. The constantly increasing research interests on these prosthetic devices can be attributed to the need for further improvement of the functionality and safety of these devices.
The functionality of these devices could be measured through a measure of their everyday performance levels. The close replicability of the full range of motion (RoM) and amputee specific design with appropriate parameters such as physical dimensions and mass can be construed as a measure of the biomechanical performance of the prosthetic device. Apart from mechanical functionality of prosthetic device, the second main measure of the goodness of a prosthetic is the comfort level they can provide to the amputee. This encompasses the goodness of fit and the extent of seamless integration to the residual limb of the amputee with minimal tissue damage.
The functionality improvement in prosthetics has been constantly evolving in parallel with innovative technologies. From sixteenth-century, the increasing use of mechanisms and gears have resulted in replacement of single link prosthesis [7] to a multilink prosthesis [8] and in more recent times, with the advent of information age and microelectromechanical systems (MEMS), the ability to integrate complicated controllers and sensors to the prosthetics [5, 6] has resulted in improved functionality. Furthermore, in the late twentieth-century, the advancements in 3D printing technology have enabled fabrication of numerous prosthesis designs that were deemed complex for traditional fabrication methods [9–13].
Limb prosthesis
Socket prosthesis has been the dominant form of prosthesis since the early days of prosthetics [1, 8] and is still to date the widely prescribed form of prosthesis. With the increase in computational capabilities, socket prosthesis design has been optimized through finite element models [14, 15]. Improvements in wireless communications technology and the onset of Internet of Things have further enabled prosthetic manufacturers and researchers to embed various sensors capable of measuring prosthetic loads [16–18], the RoM/gait of the patients [19–21] and in some cases even provide sensory feedback to the patients [22]. Despite the various improvements to the socket prosthesis designs over time, studies of periodic surveys of amputees who have been prescribed socket type prosthesis [23, 24] clearly indicate that despite their long established history as a prosthetic device, they are extremely uncomfortable.
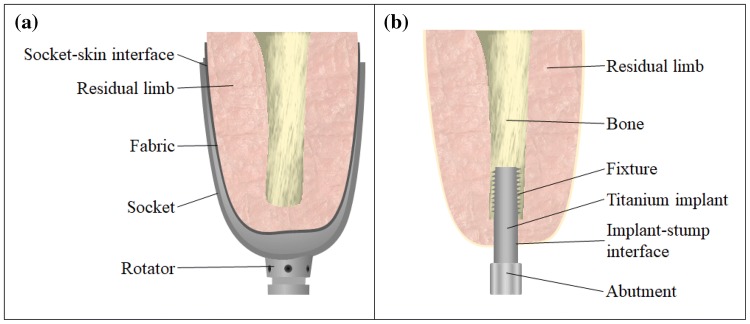
Socket based prosthesis essentially wrap around the residual limb of an amputee as schematically shown in Fig. 1a. This constant contact between the socket and the soft tissue combined with the movement-induced shearing between the socket surface and the residual limb, the load-induced changes in frictional forces on the soft tissue and slippage of socket have been commonly reported as causality for discomfort. These factors not only induce discomfort to the amputees, but have also been reported to be a contributing factor for various ailments such as but not limited to edema, skin irritation, cysts and blisters as reviewed by Lyon et al., Levy et al., and Meulenbelt et al. [25–27]. These complications when left unchecked can result in infection of the socket-tissue interface areas making use of socket prosthesis impossible until the infection is treated and the skin complication is healed.
Fig. 1.
Representative cut-section showing a socket type prosthesis and b osseointegrated implants
The introduction of osseointegration process in the early 1950s and a pioneering surgical procedure of osseointegrated dental implants by Brånemark demonstrating osseointegration as a technique for rehabilitations, have led way to a far better and more functional alternative to traditional prosthetics [28]. While over 800,000 patients have undergone dental osseointegration, since 1965, with long-term success rates [29] and over 2000 published medical articles, strong basis for this surgical procedure has been laid out. Following this, more recently this surgical procedure administered for upper and lower limb amputees. A schematic of a typical osseointegrated implant is shown in Fig. 1b. With the implant being in contact directly with the bone tissue, the microcirculation of the bone around the load carrying implant provides a much stronger musculoskeletal level integration. As shown in Fig. 1b, the titanium implant acts as a direct extension of the residual bone and would thus enable far greater mechanical loading with minimal discomfort for the patient. This transcutaneous implant architecture has demonstrated far greater comfort levels and patient satisfaction (95%) over traditional socket type prostheses [30, 31]. In a study carried out by Meent et al. [32] on the walking ability of amputees with prosthetics, the patients with osseointegrated prosthesis were capable of walking 44% faster and 27% longer than patients with socketed prosthetics. Despite being a newer medical procedure, the average osseointegrated prosthesis medical cost, according to Haggstrom et al. [33] is approximately €500/year lesser than socket prostheses.
Osseointegrated prosthesis having been demonstrated to be far superior to that of socketed prostheses, is not free of drawbacks. The transcutaneous architecture of the prosthesis demands a surgical procedure for the insertion of the titanium implant into the residual bone. This results in an open wound at the implant-stump interface. While successful healing of this wound can result in a complete and strong osseointegration, any incomplete healing of this wound might result in repeat surgery and in some cases even complete removal of the titanium structure. Clinical reviews conducted following osseointegration process clearly indicate the main reason behind unsuccessful osseointegration as tabulated in Table 1.
Table 1.
Complications from follow up studies on patients with osseointegrated prosthesis
The main complication associated with osseointegrated implants was clearly infections (both superficial and deep infections). Deep implant infections are typically a result of nosocomial (hospital environment related) pathogens that can attach on to the implant or the open wound during surgical procedure. Superficial infections however can be picked up by the patient both while in the hospital and from external sources following the surgical procedure. The superficial infections when left unchecked can spread and manifest as deep implant infections. Branemark et al. [35] in his recent study reported that among 11 patients with deep implant infections, the infection-impaired bone-implant bonding resulted in an early loosening of the implant. While non-infectious complications due to improper loading and insufficient rehabilitation associated with this relatively newer surgical procedure are fairly low (< 10%), the primary cause of repeat surgeries and implant removal could be associated to infectious complications.
Infectious complications
Infection related complications being a commonplace in both the traditional socket prostheses and the more recent osseointegrated prosthesis, it becomes critical to address this issue to ensure these prostheses are more robust, reliable and safe. The pathogens (mostly bacteria and in some cases viruses) are present even in cleaner and hygienic hospitals in developed countries [37]. It was identified that most nosocomial bacteria and viruses can survive on inactive surfaces for months together and can be a source of transmission unless the surface is regularly disinfected [38]. A complete and thorough disinfection is not possible and furthermore, the threat of antimicrobial resistant bacteria [39] further complicates this issue. Biofilm formation on implants have also been identified to be the most devastating complication and the review by Gbejuade et al., clearly outlines the formation of biofilms and their effect on a successful joint arthroplasty [40]. Among 39 patients studied, it was identified that over 40% of the patients showed signs of Staphylococcus aureus (S. aureus) infections with 25% of the patients showing signs of Coagulase-Negative Staphylococci infections. The remaining patients exhibited signs of various Streptococci strains, Enterococcus faecalis, Escherichia coli (E. coli) and Pseudomonas aeruginosa infections at the implant-stump interface with several patients were identified to be infected with more than one bacterial species [40]. Superficial infections, if identified in early stages can be easily cured, but deep implant infections if present poses a more serious threat.
The implant during the surgical procedure is not completely isolated from any air-borne or contact-transferred pathogenic contaminants. Their instantaneous adhesion onto the titanium surface has been identified as the most critical step for introducing pathogens into the soft tissue of the amputee [41]. With the release of such findings, novel self-sterilizing coatings such as those created by Ohko et al. [42] and antibacterial coatings [43], especially those that specifically prevent S. aureus adhesion to implants [44] have been developed and implemented. Furthermore, to deter deep implant infections, antibiotic pellets are also placed before the wound is sutured during the surgical procedure. After released from surgical care, any presence of infection and lack of detection of early stage infections can lead to propagation of infection into the implant-stump interface. Patient carelessness and oversight on medication could be attributed as a major source of superficial infections and further complications resulting from these infections.
From sensors that can accurately monitor the pressure levels exerted on the prosthesis to sensors that can monitor the structural health, the prosthesis industry would greatly benefit from a novel sensor that can accurately identify the early stage infections irrespective of the species of pathogen present. Detection of pathogen is however not a simplistic procedure. Traditional approach for infection diagnosis is through periodic physical swab sample collection. The patient is required to visit the hospital to provide a cotton swab sample from the open wound. This swab sample is then cultured on a nutrient medium which might take anywhere between 24 and 48 h depending upon the potency of pathogens present. As the sample collection and culture preparation locations are usually not at the same place, the contamination of swab sample during the transit or handling process can result in false positives. This process has already been deemed too slow and ineffective in most cases when the culture’s nutrient medium may not support multiplication of certain bacterial species. Hence, alternative approaches have also been established such as use of enzyme immunoassays [45], polymerase chain reactions [46] and ligase chain reaction assays [47]. While these approaches have been demonstrated to be more effective in diagnosing bacterial presence, the number of steps involved in identifying the strain such as selection of primer sequences for effective amplification of the sampled DNA and in assay preparation increases the complexity of the process. Any wrong selection of the primer sequence or the contamination introduction can again result in false positives. Even when carefully controlled, these approaches still make use of chemical assays. The use of such assays makes it extremely difficult for integration into prosthesis designs. Hence, an alternative approach to detecting bacterial presence is needed.
Infection sensing through olfaction
As ways of determining wound infections, albeit only the late stages of infections, the Greek and the Chinese, as early as 2000 BCE, have used olfaction or ‘smell’. An infected wound smells vastly different (in most cases pungent smelling) than that of a healthy wound which was used as an indicator of infection presence [48]. Pathogens, being a basic lifeform, metabolize nutrients for survival. The nutrient metabolism results in the release of metabolites from pathogens. These metabolites are in essence volatile organic compounds (VOCs) that emanate from the wound surface. This ancient method of infection diagnosis has been long discarded, as this approach is only suitable for later stages of infections only when significant number of bacterial colony forming units (CFUs) were present. Recently the advancement in gas chromatography and mass spectrometry (GC/MS) techniques have been demonstrated to be capable of sampling broad range of volatiles released from bacterial colonies with high sensitivity and accuracy.
Increasing interest in exploiting the volatile analysis technique to detect pathogen presence is evident from increasing number of research articles published in the field of breath research focussing on capturing and analysing breath volatiles [49–51]. Zhu et al. [51] have demonstrated the ability of volatile analysis technique to distinguish Pseudomonas aeruginosa infection from S. aureus infection in mice models from sampling their exhaled breath. While these research articles demonstrate the viability of using volatiles to identify pathogen presence, the use of solid phase micro extraction for sampling volatiles restricts the range and detection limits. Furthermore, the forced induction of volatiles into the sampling instrument for analysis make these approaches unsuitable for sampling volatiles directly from open-wounds. In these lines, our recent work makes use of a more recently developed sampling technique called stir-bar sorptive extraction (SBSE) [52]. This technique allows sampling of volatiles released from a static substrate with minimal need for forced induction. The large sorptive volume of the sampling media allowed identification of the chemical composition of the volatiles released from E. coli. Over 145 different volatile compounds with trace elements down to picomolar concentrations were identified [52]. Further work on four different bacterial species paved way for the development of a database of bacterial volatiles, demonstrating the ability to use the unique volatile biomarkers as an indicator for the presence of particular species of bacteria. With volatile biomarkers having been identified and established in being capable of providing information on not only the species of the pathogen but differentiate between strains of a particular pathogenic species [50], the potential for using these volatile biomarkers as a sensing stimulus is promising.
Volatile biomarker sensing
Volatile biomarkers, while being a potent stimulus for signalling infection presence and identifying infection type (both pathogenic species and strain), the research so far on sampling and analysing these volatiles has been reliant upon highly expensive and bulky GC/MS instruments that would take up the size of a small sized car. Though researches in developing lab-on-chip MS [53] and MEMS GC columns [54] have been underway, they have been limited to liquid phase separation with a noticeable loss of resolution due to miniaturization. The results from a MS instrument as detailed in [52] still demand further data analysis, to identify individual fragments of the component ions and compound identification from the spectral data by matching them against an existing database with specific software. To extract the resolution and accuracy of such a bulky setup and automating the compound identification process towards integrating them into a prosthetic device is far from achievable at present. Hence, alternative means of identifying these volatiles is necessary. The following sections analyse the suitability of potential volatiles/gas sensing functional materials that demonstrate potential for miniaturization and integration into future prosthetic designs. While a gamut of gas sensing techniques are available, owing to the target application’s demands, this review only focusses on fundamental sensing techniques, materials and processes used that would allow potential integration into future prosthetic designs. In these lines, only sensing techniques that can be realized in the form of flexible/elastic architecture with intention of embedding the potential sensing architecture into a fabric/elastic bandage that wraps around the osseointegrated prosthesis-residual limb interface for detecting infection are reviewed.
Electronic noses
The term electronic nose or simply E-nose, coined in the 1980s, represents an instrument that contains a heterogeneous array of electrochemical sensors with partial specificity and a pattern recognition system [55]. Electrochemical gas sensors produce a change in their electrical activity (change in conductivity) upon exposure to certain gases. These gas molecules bind to the functional chemical compound (sensing material) which may be purely sorptive (surface adsorption) or a combination of adsorption with physical absorption or through chemisorption (chemical reaction induced binding). The selection of the functional material will depend on the mode of binding preferred.
Binding of target gas molecules through chemisorption is only used in applications where high selectivity is preferred which is a necessity in infection sensors. This selectivity would ensure change in electrochemical response of the functional material only in the presence of the target volatile biomarker. For instance, the presence of indole (C8H7N) in the wound volatiles is a positive indicator for the presence of E. coli infection. Any chemical capable of a strong reaction to indole would thus be an ideal functional material/sensing element in an E-nose for E.coli infection detection. The most sensitive reagent used in laboratory tests that reacts with dissolved indole is p-dimethylaminocinnamaldehyde [56]. However, the use of this reagent as a freestanding electrochemical sensor capable of reacting with indole in gaseous phase is not possible. Moreover, chemical reaction of such reagents with indole molecule is an irreversible reaction. Hence, any E-nose based on such chemisorption-based mechanism cannot be implemented as a reusable sensing device to detect pathogens in future prosthetic devices. Physisorption and weak chemisorption based E-noses, which typically use metal oxide semiconductors (MOx), conducting polymers (CPs) and carbon based composites are seeing a strong growth. Their sensing mechanism and their potential for use in infection sensing is analysed in the upcoming sections.
Metal oxide semiconductor (MOx)
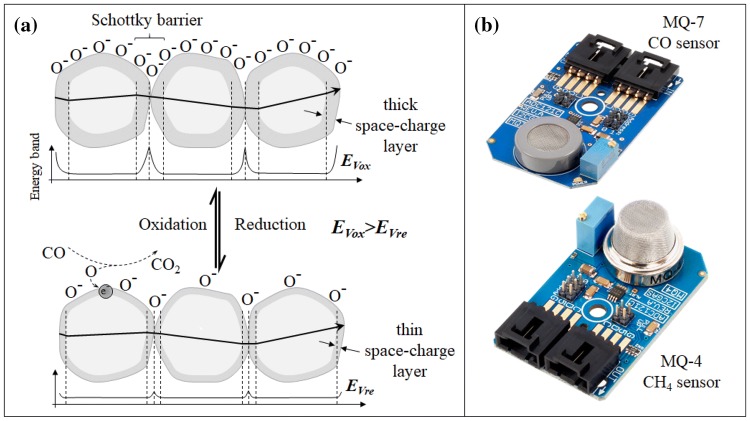
This type of functional material is the most widely used sensing element in E-nose applications. It demonstrates high levels of sensitivity to various organic vapours with a fast response time. Having a simpler chemical composition as opposed to other functional materials used in E-nose applications, MOx sensors have a stable lifetime and are readily available commercially with little to no further chemical processing needed. The operating principles of metal oxide semiconductors are schematically represented as in Fig. 2a and commercially available MOx based gas sensors are shown in Fig. 2b. The metal oxides have a large band gap due to a wide space-charge region with oxygen ions surrounding the metallic core in case of an n-type MOx. The ionosorbed oxygen (O2) restricts electron flow among the metal oxides. However, at temperatures greater than 150 °C (in some cases even higher temperatures up to 300 °C is required [57]), it becomes ionosorbed as O−. This ionosorbed oxygen can easily combine with a reducing gas (n-type MOx—typical examples include tin oxide, zinc oxide or iron oxides) which would allow a release of free electron into the space-charge region as shown in Fig. 2a. Thus, in the presence of a reducing gas and at an operating temperature above 150 °C, the excess electrons released into the space-charge region deplete this potential barrier allowing increased conductivity. The similar process is reversed in case of p-type semiconductors usually comprising oxides of nickel or cobalt where the hole transfer occurs in presence of oxidizing gases. This increased conductivity is measured as a decrease in resistance by the accompanying electronics to read out rapid and sensitive detection of the analyte gases.
Fig. 2.
a Schematic representation of conduction mechanism during oxidation and reduction. b Commercially available MOx based gas sensors
The reversible mechanism at lower temperatures allows rapid desorption of the reducing/oxidizing gases from the surface of the metal oxide semiconductors. While surface adsorption of the gases has been identified to be highly sensitive, research into further improving their sensitivity through converting bulk MOx into nanostructured particles has also been underway. Table 2 summarizes various highly sensitive MOx based gas sensors with improved selectivity, through use of catalysts or hybrid mixtures that have been published. However, owing to the extremely large number of published research on MOx sensors, this list is not exhaustive.
Table 2.
Metal oxide semiconductor based gas sensors and their operating parameters
| Sensing Gas | MOx type | Sensing temperature | Detection Limits | Response Time | References |
|---|---|---|---|---|---|
| Acetone | CdO | 300 | < 20 ppm | 3 s | [58] |
| Fe2O3 + Pt/Pd/RuO2 | 300 | 0.1–20 ppm | 3 s | [58] | |
| Butane | ZnO (Al, In, Ga) | 200–350 | 2–1000 ppm | 2 min | [59] |
| Ga2O3 + SnO2 | 500–950 | 100 ppm | 1 min | [61] | |
| Methane | Ga2O3 + SnO2 | 500–950 | 10,000 ppm | 1 min | [61] |
| Chlorine | In2O3 + Fe2O3 | 250–500 | 0.1–5 ppm | 2 min | [62] |
| Carbon monoxide | CuO/ZnO | 200–400 | 4000 ppm | 1.5 min | [63] |
| Bi2O3 + SnO2 | 200–350 | < 500 ppm | 80–90 s | [64] | |
| SnOx | 200–500 | 1–100 ppm | 1 min | [65] | |
| SnO2 | 131–313 | 1–20 ppm | 1 h | [66] | |
| SnO2 | 25–400 | 200–3000 ppm | 1 min | [67] | |
| SnO2 | 200–420 | 50–420 ppm | 5 ms | [68] | |
| Ethanol | CuO | 350 | 1–1000 ppm | – | [70] |
| Ga2O3 +Rh, Ru, Ir | 540–800 | 25–50 ppm | 1 min | [71] | |
| ZnO +SnO2 | 20 | 0.1–5 ppm | – | [72] | |
| Fe2O3 + SnO2 | 170–340 | 10–1000 ppm | – | [73] | |
| TiO2 | 200–400 | 400–2000 ppm | 3 min | [74] | |
| TiO2 + Pt/Nb | 300–500 | 500–1250 ppm | 5 min | [75] | |
| Hydrogen Sulphide | CeO2 + SnO2 | 10–125 | 5–25 ppm | 30 s | [76] |
| CuO + SnO2 | 100 | 5–100 ppm | 60–140 s | [77] | |
| SnO2 | 300–350 | 0–9 ppm | – | [78] | |
| ZnO + Sb2O5 | 200–400 | 0.01–40 ppm | 15 min | [79] | |
| Humidity (Water vapour) | Ta2O5 | 400–450 | 45–100% RH | 40 ms | [80] |
| LPG | ZnGa2O4 | 200–400 | 500 ppm | 1 min | [81] |
| Methanol | TiO2 | 200–400 | 100–500 ppm | 3 min | [74] |
| Ammonia | Cr2O3 + TiO2 | 200–500 | 10,000 ppm | 2–5 min | [82] |
| CoOx | 27 | 1–200 ppm | 2–4 min | [83] | |
| MoO3 | 450 | 3–400 ppm | 1 min | [84] | |
| Nitrous oxides | SnO2 | 131–313 | 0.01–0.25 ppm | 1 h | [66] |
| SnO2 | 300–350 | 0–9 ppm | – | [78] | |
| SnO2 | 200–420 | 1–2 ppm | 5 ms | [68] | |
| SnO2 | – | 100 ppb | – | [85] | |
| SnO2 | 100–350 | 5–800 ppb | 30 min | [86] | |
| CoO + In203 | 125 | 100 ppm | 4 min | [87] | |
| Ozone | In2O3 + Fe2O3 | 300–550 | 10–300 ppb | 2 min | [88] |
| Petrol | SnO2 | 20–320 | 1500 ppm | – | [89] |
| Propane | In2O3 | 350 | 1000 ppm | 1.5 min | [60] |
| ZnO | 300 | 0–8000 ppm | – | [90] | |
| Propanol | TiO2 | 200–400 | 400–200 ppm | 3 min | [74] |
| Trimethyl amine | SnO2 | 290 | 10–300 ppm | 12 s | [91] |
| In2O3 | 300–640 | – | – | [69] | |
| Xylene | SnO2 | – | 10–100 ppm | 10 s | [92] |
From Table 2, it can be observed that the most commonly sensed gases are carbon monoxide (CO) [63–68] and nitrous oxides (NO, NO2, NOx) [66, 68, 78, 85, 86] which are fairly small molecule gases. MOx based sensors for slightly more complex molecules such as Xylene [92], trimethylamine [69, 91] are not that common. While sensitivities down to 5 ppb for some gases have been realized with MOx sensors, the selectivity of this sensing architecture is poor. As observed from the table, the similar fabricated tin oxide (SnO2) based sensors show a wide range of response to gases such as butane [61], carbon monoxide [64–68], ethanol [72, 73], ammonia, nitrous oxides at similar operating temperatures but with relatively varying response times. Being capable of synthesizing into nanostructured crystalline materials for integration in MEMS devices for improved sensitivity and lower operating temperatures with very low power consumption, as demonstrated by Bhattacharyya et al. [93], these devices show a huge potential for integration into many devices.
Conducting polymers
Conducting polymers are relatively newer functional materials compared to MOx that have been employed as gas sensors since early 80s [94]. As opposed to MOx based gas sensors that operate at high temperatures, CPs have demonstrated better response to target analytes at room temperatures with improved sensitivities and shorter response times. With good mechanical properties and artificial synthesis process to control the electrical properties, CPs have in some cases demonstrated better and more tuneable response than those which can be obtained from traditional MOx based sensors. These conducting polymers are long chain polymers with alternating single (σ) and double (σ–π) bonds. The alternating structure with loosely bonded π electrons that can shift between the bonds as shown in Fig. 3, results in the formation of delocalized π electrons clouds which make them intrinsically conducting.
Fig. 3.
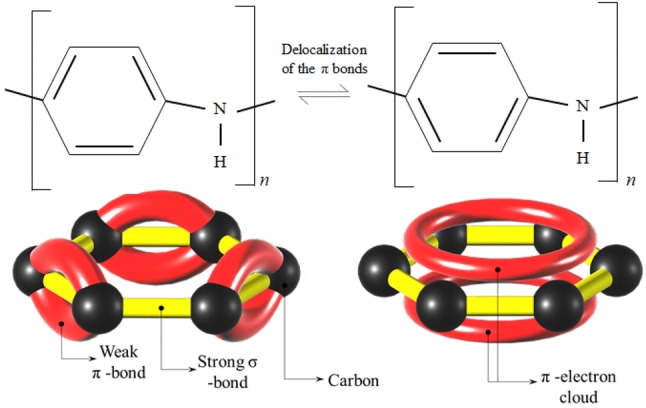
Above: PAni’s structure’s alternating double and single bonds showing delocalization induced movement of the π-bond electrons. Below: 3D representation of a π-electron cloud as a result of delocalization
Through doping and functionalization processes, the behaviour of the conducting polymer can be chemically controlled better than MOx. Synthesis techniques of conducting polymers have been vastly improved from early electrochemical deposition processes, such as those demonstrated by Lu et al. [95], to highly controlled three-dimensional patterning down to microstructure levels as demonstrated by us previously [96, 97], which allow fabrication of more complex sensor architectures using these functional materials. CPs such as polyaniline (PAni), polythiophenes (PTh) and polypyrrole (PPy) are the most widely researched CPs owing to their excellent electromechanical properties. Upon exposure to similarly reducing or oxidizing gases, similar to MOx based sensors, the change in the electrical response of these functional materials have been successfully read out to sense and quantify the volatile concentration. Table 3 summarizes some of the developed CP gas sensors and their characteristic properties for sensing gases.
Table 3.
Conducting polymer based gas sensors and their operating parameters
| Gas Sensed | Functional CP | Detection limits | Response time | Recovery time | References |
|---|---|---|---|---|---|
| Acetone | PTh | 200–300 ppm | 3–5 min | 3–5 min | [98] |
| Ammonia | PAni | 50 ppb | 1–5 min | 3 h | [99] |
| PPy | 38–290 ppm | 20 s | 15 min | [100] | |
| Carbon monoxide | PAni | 10 ppm | 5 s | 7 s | [101] |
| PAni | 60 ppm | 24 s | 36 s | [102] | |
| Carbon dioxide | PPy | 100–700 ppm | 210–270 s | 25–30 min | [103] |
| Hydrochloric acid | PAni | 200 ppb | 6 s | 10 s | [104] |
| Hydrogen Sulphide | PAni | 10 ppm | 1.1 min | [105] | |
| Methanol | PAni | 1 ppm | 2–20 s | 2–15 s | [106] |
| Nitrous oxides | PPy | 20 ppm | < 5 s | 4–5 min | [107] |
| PTh | 4 ppm | < 1 min | 10 min | [108] | |
| PAni | 500 ppb | 30 s | 65 s | [102] | |
| Toluene | PTh | 20 ppm | 3–5 min | 3–5 min | [98] |
| Water vapour | PAni | 25–400 ppm | 3–10 s | 3–10 s | [109] |
Although CP gas sensors have quicker response-times sensors with noticeably better sensitivities, the recovery following removal of the gases takes a much longer time compared to MOx sensors, which have similar adsorption and desorption rates. All laboratory results of the recovery following exposure to gases are measured by flushing the sensing element with an inert gas such as argon or helium [98–109]. This is to ensure the complete removal of residual sensed gas not only from the chamber but from the CP sensing element as well. Repeatable exposure to the same gases has also been observed to induce permanent irreversible reaction of the gas to the CP [110].
Carbon nanomaterial and carbon composites
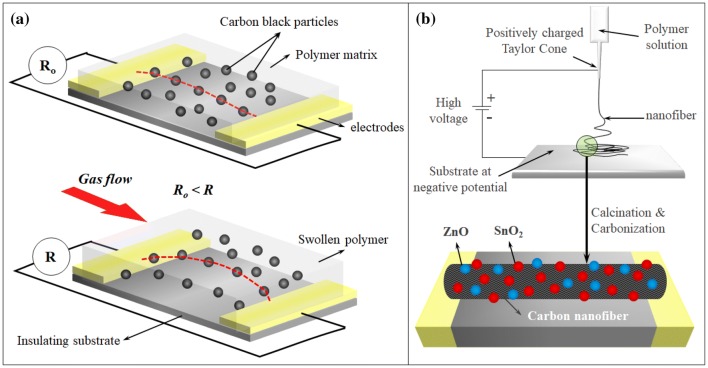
Among carbon based nanomaterials, the most widely researched materials for their gas sensing properties are carbon black (CB), carbon nanofibers (CNF), carbon nanotubes (CNT) both single-walled (SWCNT) and multi-walled (MWCNT) and graphene (Gp). These nanomaterials are typically used in a composite matrix, except for CNTs, which are sometimes used in form of composites, electrospun fibres [111] or as freestanding forests, either in their pristine form or as functionalized/decorated with other nanoparticles [112]. CB and CNF in the form of CB/polymer and CNF/polymer composites have also been demonstrated for gas sensing applications. A schematic representation of the sensing mechanism CB/polymer composites is shown in Fig. 4a. A predetermined amount of CB dispersed into an insulating polymer matrix is laid out as thin films. The CB’s mass fraction would characterize the electrical properties of the film, which is defined by percolation theory [113]. The target volatile determines the chemical composition of the insulating polymer matrix as the polymer matrix needs to swell in presence of the target volatiles. Once the CB/polymer composite sensor is synthesized, in presence of the target volatile the polymer matrix swells. This increases the antiparticle distances between the conductive CB particles thus increasing their tunnelling resistance as shown in Fig. 4a.
Fig. 4.
a CB/polymer composite gas sensor swelling under gas flow increasing sensor resistance. b MOx coated electrospun CNT for gas sensing
Using an array of swelling polymers composed of vinylated phenols, styrene–co-alcohols, acrylates and bisphenols, Lonergan et al. [114] demonstrated that the CB/polymer composites were capable of responding to a wide range of volatile organic compounds among which the characteristic response of individual CB/polymers to toluene, methanol, 2-propanol, hexane, ethyl acetate, ethanol, chloroform, benzene and acetone were analysed. As the insulating polymers in the composites swell differently to different volatiles, the consolidated responses were identified through principal component analysis [114]. Once the CB/polymer sensing array elements were characterized, to identify the type of volatile present, a simple cross reference to the previously established principal component space is carried out. However, the macromolecular movement of the CB within the matrix results in rearrangement of this conductive filler within the polymer matrix thus inducing a steady signal drift upon consecutive swelling and shrinking process.
With relatively low sensitivity of CB/polymer composites (in few parts per thousand ranges), research into CNF/polymers based sensors synthesized in a similar fashion were carried out [115, 116]. While pristine CNF/polymer gas sensors show stable performance due to increased resistance to macroscopic movement of the high-aspect ratio fibres [115], works by Zhang et al. [117] and Lee et al. [118] demonstrate use of metal and metal oxide decorated CNFs fabricated through electrospinning technique to further improve the sensitivity of their gas sensors. A schematic representation of this sensing architecture is shown in Fig. 4b. ZnO and SnO2 decorated electrospun CNFs were directly deposited as thin films without the need for an insulating matrix. This allowed direct contact of the target volatiles to these functional materials. Dimethyl methyl phosphonate were detected down to 0.1 ppb using this sensor [118].
Numerous SWCNT and MWCNT based gas sensors have also been developed alongside CB and CNF based sensors. Earliest research using SWCNTs as gas sensors, that are capable of responding to nitrous oxide and ammonia vapours, was carried out by Tan et al. [119]. By fabricating a transistor like architecture using the vapour sensitive SWCNT as the transistor base layer, Kong et al. [120] demonstrated increase in conductance by nearly a factor of 10 upon exposure to 200 ppm NO2 gas. Upon exposure to 1% NH3 in Argon, the conductance of sensor nearly dropped to zero. The turn on time upon exposure to NO2 was less than 5 s while the turn off time upon exposure to NH3 was about 3 min. While Palladium (Pd) functionalized CNT gas sensors showed high sensitivities to hydrogen [121], CNT’s decorated with metals such as but not limited to Zn, Cr, Pt, Rh demonstrated improved sensitivities to CO, NO2 methane and hydrogen sulphide and benzene. Rh-CNT gas sensors were fabricated by Leghrib et al. [122] for detecting benzene vapours. The Rh-CNT gas sensors were exposed to benzene as low as 50 ppb with a sensor response time of less than a minute. To remove the benzene from the Rh-CNT, the setup was heated to 150 °C and this resulted in a nearly instantaneous drop in resistance. For successive exposure to benzene vapours, the Rh-CNT sensor was required to be brought to room temperature, which took over 10 min [122].
Gp based gas sensors are relatively new with the first reported use being as late as 2007 compared to other carbon nanoparticles [123]. Similar to performance levels of CNT gas sensors, Gp gas sensors also required reheating following sensing of target gases. With doping and nanoparticle decoration to enhance carbon nanoparticle properties having already been demonstrated in the field of CNF and CNT based sensing applications, boron or sulphur doped Gp were identified in silico to be capable of detecting nitric oxides [124]. With further research into reduced graphene oxide (rGpO), Pd nanoparticle decorated rGpO sensors were fabricated by Li et al. [125] using chemical vapour deposited Gp sheets as the contact material for the Pd-rGpO sheets. The demonstrated sensor showed high levels of sensitivity (2–420 ppb) to NO gas with 50 min response and 45 min of recovery time.
Metal organic frameworks and covalent organic frameworks
Metal organic frameworks (MOFs) and covalent organic frameworks (COFs) are a new class of functional materials that have diverse assembly of building units similar to zeolites [126]. Research into MOFs has exploded since their inception owing to their high-density storage capabilities of gases like H2 and CH4. MOFs are in essence a highly structured (2D and 3D) and nanoporous network of polyatomic metallic clusters linked together by polytopic linkers [127–129]. Numerous reviews published on this class of functional material [130, 131] exploring their possible applications as gas storage media in transportation and chemical processing plants and a large database on various MOFs indicate their extremely selective and robust gas storage capabilities.
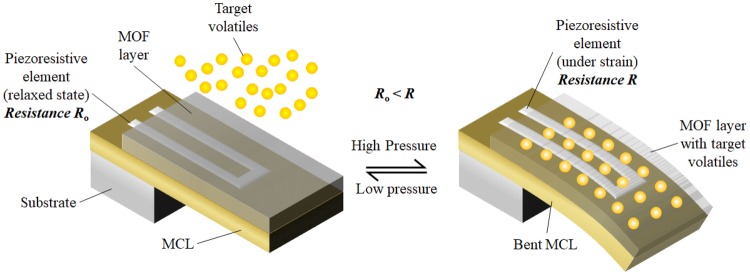
The controlled nanoporous structure makes MOFs extremely selective to a particular chemical, a feature that has been exploited for use as chemical sensors. When chromophores (a colour producing atom/group) are used as linkers for the metallic clusters during the synthesis of MOF, upon exposure to a sorptive fluid, a solvatochormic (in case of liquids)/vapochromic (in case of gases) shift occurs which can be used as an indicator for the presence of the sorptive fluid. Lu et al. have synthesized a Cu-MOF containing 3, 6-di (pyridine-4-yl)-1, 2, 4, 5-tetrazine as the polytopic linker which can produce a solvatochormic shift in the visible spectrum when exposed to different organic compounds [132]. Upon exposure to acetonitrile, the synthesized MOF appears as bright red while exposure to trichloromethane results in absorption of all wavelengths of the visible spectrum except dark green. Among various sensing approaches using MOF under the presence of a sorptive fluid as reviewed by Kreno et al., [133], the most idealistic form of implementation of these MOF sensors in sensing pathogenic biomarkers can be through use of micro-cantilever structures (MCLs) as demonstrated by Lee et al. [134]. These MCLs comprises an extremely thin coating of a suitable MOF (MOF selection depends on the target volatile to be sensed) on a MCL with highly sensitive piezoresistive elements patterned using photolithography techniques. During sorption of target gases, the changes in unit cell dimensions result in a large tensile and compressive stress on the cantilever and this change in stress is measured by the piezoresistive element as schematically represented in Fig. 5.
Fig. 5.
Piezoresistive MCL with MOF coating showing adsorption induced response change for gas sensing
Similar to MOFs, COFs are made up of porous crystalline polymers that are linked together via covalent bonds. The ability to synthesize these COFs akin to MOFs with relatively higher mechanical and thermal stability due to strong covalent bonds makes this material more attractive than MOFs [135]. With all the advantages of MOFs, COFs have also been increasingly synthesized since first reported in 2005. A detailed review of various COFs with their chemical structures and properties has been carried out by Ding and Wang [136] and Feng et al. [137].
It is only as recent as the early 2010s that the potential of these COFs as a sensing element has been reported. The first potential use of COFs as a sensing element for 2, 4, 6-trinitrophenol (TNP) was proposed and identified by Das et al. [138]. Using TfpBDH covalent organic nanosheets, the group was able to visualize a complete shift in the spectral emission of the pristine nanosheet turning it from a bright blue sheet to a dark bluish grey sheet upon exposure to TNP [138]. This process was identified to be reversible upon exposure to triethylamine. The colour shift occuring within 36 ns when exposed to TNP indicated extremely fast response times. With the group focusing primarily on COF synthesis, only a part of their research was on using the synthesized COF as a TNP sensor.
A more detailed research on using COFs as a sensor was carried out by Li et al. [139] for detecting copper ions (Cu2+). Solvothermally synthesized COF-JLU3 was used as the sensing COF with fluorescence shift based visual detection upon exposure to Cu2+ ions. The luminescence spectra of COF-JLU3 suspension in tetrahydrofuran changes shows the peak intensity at 601 nm drop from 100 a.u (normalized intensity) down to 10 a.u in the presence of Cu2+ ions. The selectivity of COF-LJU3 to Cu2+ ions were verified by testing and measuring their intensity shift with other metallic ions such as lead, silver, potassium, sodium ions. While most of the tested ions showed only less than 20% drop in the normalized luminous intensity, the COF-JLU3 also demonstrated up to 60% drop in presence of nickel, iron and cobalt ions.
Ding et al. [140] used COF-LZU8 to develop fluorescent COFs specifically for sensing and removal of mercury ions (Hg2+). The COF-LZU8 was functionalized with thio-ether group for improving the selective detection and facile removal of Hg2+. The thio ether group was used as the Hg2+ receptor and the porous structure of the framework for real-time detection. With high levels of reported sensitivity (spectral shift occurs at concentrations as small as 3.3 µM with 90% drop at 33.3 µM) and excellent selectivity (less than 15% spectral intensity drop to all other metallic ions tested [140]) the fluorescent COFs enabled easy visibility of the COFs real-time response. This research highlights the construction of functionalized COFs that can further open up these functional materials to a wider range of applications.
Hydrogels and hydrogel composites
Hydrogels are highly hydrophilic polymeric structures that upon exposure to water can swell by absorbing the water until an equilibrium state is attained. Hydrogels are themselves not a singular polymeric compound but a generalized term that encompasses all hydrophilic swell-able polymeric networks [141]. Their chemical characteristics have made them an ideal candidate for use as sensing materials and we focus on research that has been carried out using these materials as chemical sensors.
Using a MCL type architecture, pH sensitive poly (vinyl alcohol)/poly (acrylic acid) (PVA/PAA) hydrogels have been used to sense chemicals with pH levels of 7 (neutral) and 1 (strong acid) [142]. A 50 µm thick PVA/PAA hydrogel layer was deposited on a MCL with a piezoresistive bridge network fabricated using photolithography. Upon exposure to a fluid with a low pH, the hydrogel shrinks rapidly inducing a shift of the mass of the hydrogel film on the MCL. This resulting drop in output voltage is picked up by the electronics as a presence of strong acid. The extent of shrinkage depends on the strength of the acid that has been calibrated against the output voltage of the sensor demonstrating a hydrogel based pH sensor with a sensitivity of 15 mV/pH. Trinh et al. [143] further analysed the behaviour of these hydrogels under varying pH conditions in silico. Similar research with bilayer cantilevers with hydrogels coated on either side was also demonstrated to detect pH changes [142].
Gas sensing with hydrogels has also been demonstrated by modifying the hydrogel with gas sensitive fluorescent dyes [144, 145]. Using 4-amino, 5-methylamino, 2′, 7′-difluorofluorescein within a polyethylene glycol hydrogel, Zguris J et al. [145] were able to demonstrate fluorescence shift upon exposure to NO. The use of fluorescent dye did not affect the molecular structure of the hydrogel. It allowed the shift of colour within the hydrogel even at exposure limits as small as 0.5 µM. Exploiting this bio-inert character of hydrogels, the use of sodium bicarbonate in a pH-sensitive hydrogel sensor allowed sensing of CO2 gas directly without dissolution in fluids as demonstrated by Herber et al. [146].
Efforts to bring together multiple sensing elements have been demonstrated by Bai et al. [147] where graphene oxides (GpO)/CP composites based hydrogels were developed. These hybrid hydrogels were prepared by in situ chemical polymerization in aqueous dispersions of GpO sheets. Three different conducting polymers namely PAni, PPy and poly (3, 4-ethylenedioxythiophene) (PEDOT) grafted with GpO based hydrogels were synthesized with GpO/PPy based hydrogels demonstrating high sensitivity to ammonia gas at concentrations down to 800 ppm. The sensor was fabricated by using the hydrogel film in-between two conductive electrodes as a chemoresistor and demonstrated response times less than 10s. While the recovery rate after removal of ammonia is not detailed, the high electrical conductivity due the presence of GpO and CPs within the hydrogel, and the bio-inert nature of hydrogel demonstrates huge potential for use of these hybrid composite hydrogels as an electroactive material in biomedical applications.
More recently, Wu et al. [148] have developed a high performance CO2 and NO2 sensor with three-dimensional functionalized rGpO based hydrogels using molecular self-assembly in presence of hydroquinone. The group compared functionalized and non-functionalized rGpO based hydrogels and determined that functionalized rGpO exhibits comparatively faster recovery times (10 min) and lower detection limits (NO2 at 200 ppb and CO2 at 20 ppm). The works by Bai et al. [147] and Wu et al. [148], demonstrate that hybrid sensor designs using multiple functional materials can alleviate the drawbacks of either functional material allowing better sensing architectures to be realized.
Synthetic oligomers
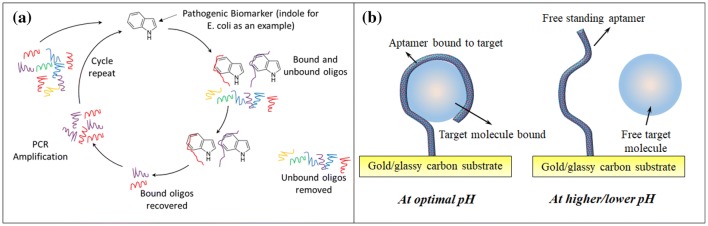
Single stranded deoxyribose/ribonucleic acids (ssDNA/ssRNA), or more commonly, aptamers, are specifically isolated strands from a large chemically synthesized oligonucleotide library on the basis of their affinity to a specific target molecule [149, 150]. When an aptamer is required for a specific target molecule, a process called systemic evolution of ligands by exponential enrichment or SELEX is used, where the target molecule in a suitable solvent is introduced into a large library (in the order of 12–15) of oligonucleotides. The single strands, which have the highest affinity to the target molecule, bind to the introduced target molecule. The unbound ssDNA are washed away and the bound ssDNA are enriched through polymerase chain reaction (PCR) amplification and purification as shown in Fig. 6a. The process is repeated until only ssDNA with the highest affinity to the target molecule remains. As the SELEX process can be tailored to suit a wide range of molecules and with techniques such as flu-mag-SELEX [151] and other non-SELEX based aptamer selection techniques [152], the target molecule range has been widely extended. These biomaterials are capable of completely folding around their target molecule at suitable pH conditions and can reverse the folding process to release the target molecule at certain pH as shown in Fig. 6b or by reversing the electric potential.
Fig. 6.
a Aptamer selection through SELEX process b reversible action of aptamer binding to target molecule through pH control
The synthesis of these aptamers tailored to a specific target molecule introduces extremely high selectivity and their intrinsically high sensitivities (with molecular level folding) make these synthetic materials as a highly potent chemical sensor. Their high levels of biocompatibility have already been utilized in synthesis of automated drug delivery devices in vivo [153–155]. For instance, Huang et al. [155], have demonstrated conjugation of anti-tumour drugs to target the antibodies by attaching the drugs to an aptamer designed for targeting tumour cells. Sgc8 aptamer [156], was truncated to form Sgc8c [155] to specifically target the protein, tyrosine kinase 7. This protein is specifically expressed in lymphoblastoids having the T cell acute lymphoblastic leukaemia. Fluorescein conjugated antitumor drug, doxorubicin (DOX), was conjugated to the Sgc8c aptamer and exposed to the T-cells in assays and the activity of the aptamer was monitored. It was observed that the aptamer prevents release of DOX at non-specific sites but unconjugated the drug only upon binding to the target T-cell, thus releasing the drug at T-cell specific sites. Apart from targeted drug delivery applications, aptamers are also starting to see increasing use as an electrochemical sensor for sensing complex proteins to small molecules and ions. Wang et al. [157] have demonstrated the use of aptamers for detecting the enzyme, thrombin at concentrations as low as 0.1 nM. Folate receptors were detected at 0.77 ng/mL by He et al. [158]. Metallic ions such as potassium ions, silver ions and mercury ions have been detected using aptamers down to 100 µM, 5 nM and 0.5 nM respectively [159–161].
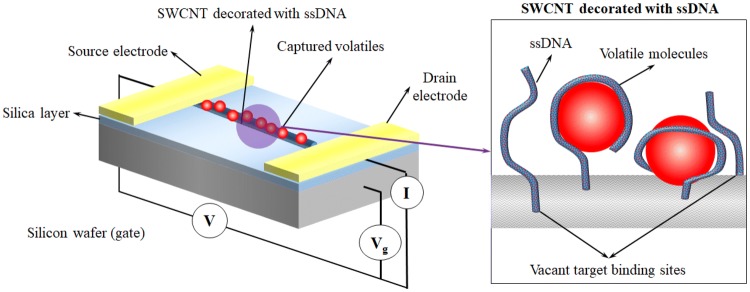
While aptamers have exhibited excellent stability and selectivity in liquid phase medium, use of ssDNA to detect gas phase medium was demonstrated by Staii and Johnson [162]. ssDNAs can effectively bind to the target molecule in the same medium selected from the oligonucleotide library. However, by decorating these ssDNA on SWCNT, the researchers managed to demonstrate acceptable levels of response from ssDNA decorated SWCNT to various gases (the schematic of the senor setup is shown in Fig. 7). By passing propionic acid vapours at 150 ppm, over bare and ssDNA decorated SWCNT based sensors the researchers observed over 8% variation in the current transfer ratio [162]. A stronger response was however observed upon the sensor being exposed to trimethylamine when a difference of 30% in current transfer ratio was observed between the bare and ssDNA decorated SWCNT indicated a better selectivity towards trimethylamine. While the reported response indicates a severe drop in selectivity and sensitivity of ssDNAs while operating in air, the research promises potential for future improvement in in-air volatile sensing capabilities of ssDNAs.
Fig. 7.
Device architecture of ssDNA decorated SWCNT gas sensors
As a means of infection sensing, aptamers have also been demonstrated to be capable of sensing E. coli and S. aureus. The aptamers are selected from the oligonucleotide libraries by using E. coli and S. aureus as the binding targets. So et al. [163] have demonstrated the use of aptamer functionalized SWCNTs (apt-SWCNT) to screen for presence of E. coli for eventual application in testing food samples. Using arrays of apt-SWCNT as field effect transistors that show a conductance decrease of more than 50% upon binding with E. coli, So et al. have been able to demonstrate rapid detection (< 20 min) of E. coli. By using Salmonella as a control to observe the apt-SWCNT sensor’s response in non E. coli solutions, the selectivity of the sensor was demonstrated. A dense array of apt-SWNCT to maximize the surface area was also proposed towards development of a more rapid screening tool.
S. aureus Aptamer/Gp based piezoelectric sensor was developed by Lian et al. [164] for rapid and specific detection of S. aureus. The aptamer/Gp was electrochemically deposited onto interdigital gold electrodes that were connected to a piezoelectric quartz crystal. Upon binding of the aptamers with S. aureus, the shift in electrical parameters resulted in change of resonance frequency of the quartz crystal that was used as a response for S. aureus presence. With a short response time of 60 min, the sensors demonstrated high levels of sensitivity (detection limits as small as 41 CFU/mL). These aptamer sensors [163, 164] though capable of detecting E. coli and S. aureus with high levels of selectivity and sensitivity demand the pathogens to be in a suitable buffer.
Insect odorant receptors
With attractive properties of using biomaterials such as aptamers for sensing, a more recent and novel approach involves utilizing biomaterial from living beings, in particular insects, as electrochemical sensor to explore the possibility of biomimicking the process of ‘smell’. Insects among many organisms possess a heightened sense of smell [165]. The olfactory sensory neurons of the insect antennae have membrane proteins acting as odorant receptors [166]. Montage et al., have analysed these odorant receptors expressed in cell lines and observed their sensitivity to various compounds [167]. These odorant receptors are a family of G protein-couple receptors, which unlike aptamers that are capable of binding to ligands and small molecules, display extreme affinity to a range of odour molecules. Recent research on odorant receptor grafted liposomes bound covalently to gold electrodes has demonstrated to be capable of detecting 4-ethlyguaiacol (4-EG), an odorant molecule released from wine and beer [168]. Using odorant receptors from the common fruit fly, Drosophila melanogaster, detection limits as low as 1aM were realized (10−18 mol/l) [168]. While the odorant receptors showed positive response to 4-EG no response was observed when exposed to E2-hexenal which is also an odour compound with a leaf-like odour.
Conclusion and outlook
The unique electrochemical properties and the electronic structure of novel functional materials are being constantly improved upon and newer gas sensing capabilities are being explored. Existing literature indicates that the implementation of these novel functional materials for sensing infections has not been thoroughly explored with an increasing need for further intrinsic improvements to eliminate their various drawbacks. The unique nature of the reviewed functional materials allows their synthesis into flexible/elastic matrices to act as a secondary skin that wraps around the wound-prosthesis interface. Some sensing architectures, such as carbon nano-particle/swelling polymer based sensors and piezoresistive cantilever MOFs sensors, though operate on the principle of the functional material expanding and contracting can still be used as a flexible secondary skin through prior electronic calibration. Furthermore, the sensing techniques and sensor architectures reviewed here allow realization of an integrated sensor with low power requirements (in the order of few microwatts to few milliwatts) to enable these sensors to draw power from portable energy sources (batteries or motion powered energy harvesters) that can be integrated into osseointegrated prosthesis. A brief summary of various sensing architectures reviewed and their salient features are detailed in Table 4.
Table 4.
Comparative overview of sensing architectures for potential use as pathogenic biomarker sensors
| Functional material | Selectivity | Sensitivity | Response times | Operating voltages | Biocompatibility | Degradability |
|---|---|---|---|---|---|---|
| MOx | Poor | 5 ppb–10 ppt | 5 ms–30 min | − 20 to 20 V | Low | Low |
| CP | Poor | 50 ppb–700 ppm | < 5 s (response) 30 min (recovery) | 10 V | High | Medium |
| Carbon nanoparticle composites | Poor | 0.1 ppb–420 ppb | 50 min | Resistive (5 V bias | Low (CNT-carcinogenic) | Medium |
| MOFs | Good | 5 ppb–40 ppm | 20 s–5 min | Resistive (5 V bias) | Good | Medium |
| COFs | Good | 2.8 pg/mL | 40 ns | 0.6 V | Good | Medium |
| Hydrogel/hydrogel composites | High | 1–10 ppm | Continual rise > 10 min | ~ 125 mV | High | High |
| Synthetic oligomers | Extremely High | 1 fM | Exposure 30–60 min | N/A (EIS at 1.02 V) | High | High |
| Insect odorant receptors | High | 1 aM (attomolar) | Exposure 15 min | N/A (EIS at 1.02 V) | High | High |
Implementing MOx in infection sensing applications would demand sensing a very narrow range of pathogenic volatiles, more particularly in close proximity to soft tissues. However, this is hindered by their poor selectivity and high operating temperatures. As pathogenic volatiles are an extremely complex mixture of various commonly occurring gases such as CO2, NOX, amongst other pathogen specific biomarkers [49–52], the MOx sensors would be exposed to more than one type of oxidizing/reducing gases at the same time and the electron transfer strength of these gases can greatly influence the gas to which MOx sensor responds to. In such cases where competing gases are to be selectively detected, a largely varied array with each sensing element having unique operating temperatures for tuned selectivity becomes necessary to be able to sense a specific pathogenic biomarker making this functional material an unrealistic choice for precision sensing of infection with zero false positives.
As for CPs, the requirement of flushing the sensing element with an inert gas after interaction with the target gas, to ensure a faster and near complete recovery, requires additional inert gas storage media to be introduced into portable sensors for use in remote infection sensing applications. The long-term instability and irreversibility coupled with selectivity issues akin to MOx based sensors further renders this functional material unsuitable for applications demanding a high level of selective volatile sensing but the fast response time makes certain aspects of this functional material worth further research.
Carbon nanoparticle based gas sensors demonstrate comparable sensitivities and response times to that of CP based sensors and at the same time provide long-term stability. However, the comparatively slower relaxation times coupled with carcinogenic nature of freestanding nanoparticles [169] makes these functional materials less attractive for use in biomedical applications such as infection sensing where they would be in close proximity to humans. However, research into the biocompatible elastomer encapsulated sensors fabricated from these carbon nanoparticles demonstrate a good fit for biomedical applications [20] making them an ideal choice for conductive electrodes that can conform to the prosthetic’s movements. These three most commonly used functional materials in E-nose applications demand having an array architecture as they suffer from low selectivity. Furthermore, only research on simpler volatile compounds has been demonstrated, demanding further improvement in this field for detecting complex organic volatiles.
MOFs and COFs show high sensitivities and selectivity to the target molecules with ease of chemical synthesis in laboratory environment and their high levels of tenability make them an ideal candidate for potential use in infectious biomarker sensing applications. With higher thermal stability than MOFs, the more recently introduced COFs are especially a highly promising source for future research and application as a gas sensing media. Although sensors have been demonstrated using the COFs for sensing ions liquid phase, the atmospheric pressure conditions lower the amount of target molecules adsorbed into the framework demanding the need for higher atmospheric pressures.
While hydrogels on their own have limited potential for gas sensing and lower stability in atmospheric conditions with evaporation induced sensor degradation, pioneering works by [147] and [148] demonstrate the capabilities of combining multiple functional materials to enhance their characteristics.
The high selectivity and sensitivity of aptamer show a huge promise for detecting specific volatiles such as pathogenic biomarkers. The advancements in SELEX processes to identify aptamers for a specific molecule would allow rapid synthesis of aptamers specific to individual pathogenic biomarkers. By using an array of individual pathogenic biomarker binding aptamers, an infection sensor capable of sensing pathogens at early stages of infection would be possible, in addition to aiding in diagnosis of the pathogen itself. The limitations of realization of such sensors, at present, are primarily due to inability to maintain aptamer sensors in their synthesis buffer (liquid) media while being exposed to the target pathogenic biomarkers as a solid-state sensing media.
With the introduction of aptamer based biomaterial sensors and the more recent use of olfactory receptors from insects, the potential for new and more effective sensors capable of detecting infections is on the rise. The ideal pathogenic biomarker sensors should have the selectivity of aptamers, with sensitivity of odorant receptors and a long-term stable configuration in a solid-state architecture like those of MOx, CP and carbon nanoparticle based sensors. Furthermore, the organic nature of these DNA based functional materials (aptamers and olfactory receptors) introduces innate biocompatibility when these materials are integrated into flexible wearable sleeves that can encapsulate the wound-prosthesis interface. While olfactory receptors are extremely sensitive to target molecule, unlike aptamers, they are not custom synthesizable to target a specific molecule making the selectivity and tuneability of aptamers worth future research for infection sensing applications. It can be anticipated that further research into these functional materials for potential use in infection sensing applications can reveal highly capable sensing architectures and pave a pathway for future volatile sensors.
Having identified the salient features of various functional materials and their drawbacks, the aim of this review is to promote research and development of more novel sensing mechanisms. Combining the promising features of the current state-of-art functional materials would allow realization of a superior class of functional materials for use in infection sensing in biomedical devices and across a wider range of applications.
Acknowledgements
The review of existing literature for this article was carried out with funding from US Office of Naval Research (Grant Number N62909-17-1-2014).
Compliance with ethical standards
Conflict of interest
The authors declare no conflicts of interest.
Ethics approval
The article does not contain any studies with human or animal participants that were carried out by the author.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Seymour R. Prosthetics and orthotics: lower limb and spinal. Philadelphia: Lippincott Williams & Wilkins; 2002. [Google Scholar]
- 2.Magee R. Amputation through the ages: the oldest major surgical operation. Aust N Z J Surg. 1998;68(9):675–678. doi: 10.1111/j.1445-2197.1998.tb04843.x. [DOI] [PubMed] [Google Scholar]
- 3.Fite K, Mitchell J, Sup F, Goldfarb M, editors. Design and control of an electrically powered knee prosthesis. In: 2007 IEEE 10th international conference on rehabilitation robotics. IEEE; 2007.
- 4.Mavroidis C, Pfeiffer C, DeLaurentis KJ, Mosley MJ. Prosthetic, orthotic, and other rehabilitative robotic assistive devices actuated by smart materials. Google Patents; 2002.
- 5.Carlson JD, Matthis W, Toscano JR, editors. Smart prosthetics based on magnetorheological fluids. Smart structures and materials 2001: industrial and commercial applications of smart structures technologies. International Society for Optics and Photonics; 2001.
- 6.Leong J, Parzer P, Perteneder F, Babic T, Rendl C, Vogl A, et al., editors. proCover: sensory augmentation of prosthetic limbs using smart textile covers. In: Proceedings of the 29th annual symposium on user interface software and technology. ACM;2016.
- 7.Finch J. The ancient origins of prosthetic medicine. Lancet. 2011;377(9765):548–549. doi: 10.1016/S0140-6736(11)60190-6. [DOI] [PubMed] [Google Scholar]
- 8.Thurston AJ. Paré and prosthetics: the early history of artificial limbs. ANZ J Surg. 2007;77(12):1114–1119. doi: 10.1111/j.1445-2197.2007.04330.x. [DOI] [PubMed] [Google Scholar]
- 9.Herbert N, Simpson D, Spence WD, Ion W. A preliminary investigation into the development of 3-D printing of prosthetic sockets. J Rehabil Res Dev. 2005;42(2):141. doi: 10.1682/JRRD.2004.08.0134. [DOI] [PubMed] [Google Scholar]
- 10.Simone F, York A, Seelecke S, editors. Design and fabrication of a three-finger prosthetic hand using SMA muscle wires. In: Bioinspiration, biomimetics, and bioreplication. International Society for Optics and Photonics; 2015.
- 11.Bahari MS, Jaffar A, Low CY, Jaafar R, Roese K, Yussof H. Design and development of a multifingered prosthetic hand. Int J Soc Robot. 2012;4(1):59–66. doi: 10.1007/s12369-011-0133-8. [DOI] [Google Scholar]
- 12.Campbell T, Williams C, Ivanova O, Garrett B. Could 3D printing change the world. Technologies, Potential, and Implications of Additive Manufacturing, Atlantic Council, Washington, DC. 2011:3.
- 13.Dodziuk H. Applications of 3D printing in healthcare. Pol J Cardio-thoracic Surg. 2016;13(3):283. doi: 10.5114/kitp.2016.62625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Childress D, Steege J. Computer-aided analysis of below-knee socket pressure. J Rehabil Res Dev. 1987;25(1):22–24. [Google Scholar]
- 15.Silver-Thorn B, Childress DS. Parametric analysis using the finite element method to investigate prosthetic interface stresses for persons with trans-tibial amputation. J Rehabil Res Dev. 1996;33(3):227–238. [PubMed] [Google Scholar]
- 16.Sonck WA, Cockrell JL, Koepke GH. Effect of liner materials on interface pressures in below-knee prostheses. Arch Phys Med Rehabil. 1970;51(11):666. [PubMed] [Google Scholar]
- 17.Appoldt FA, Bennett L. A preliminary report on dynamic socket pressures. Bull Prosthet Res. 1967;10(8):20–55. [Google Scholar]
- 18.Convery P, Buis A. Socket/stump interface dynamic pressure distributions recorded during the prosthetic stance phase of gait of a trans-tibial amputee wearing a hydrocast socket. Prosthet Orthot Int. 1999;23(2):107–112. doi: 10.3109/03093649909071621. [DOI] [PubMed] [Google Scholar]
- 19.Biddiss E, Chau T. Electroactive polymeric sensors in hand prostheses: bending response of an ionic polymer metal composite. Med Eng Phys. 2006;28(6):568–578. doi: 10.1016/j.medengphy.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 20.Devaraj H, Giffney T, Petit A, Assadian M, Aw K. The development of highly flexible stretch sensors for a robotic hand. Robotics. 2018;7(3):54. doi: 10.3390/robotics7030054. [DOI] [Google Scholar]
- 21.Young AJ, Simon AM, Fey NP, Hargrove LJ. Intent recognition in a powered lower limb prosthesis using time history information. Ann Biomed Eng. 2014;42(3):631–641. doi: 10.1007/s10439-013-0909-0. [DOI] [PubMed] [Google Scholar]
- 22.Leong J, Parzer P, Perteneder F, Babic T, Rendl C, Vogl A, et al., editors. proCover: sensory augmentation of prosthetic limbs using smart textile covers. In: Proceedings of the 29th annual symposium on user interface software and technology. ACM;2016.
- 23.McColl I. Review of artificial limb and appliance centre services: the report of an independent working party under the chairmanship of Professor Ian McColl. DHSS; 1986.
- 24.Nielsen CC. A survey of amputees: functional level and life satisfaction, information needs, and the prosthetist’s role. J Prosthet Orthot. 1991;3(3):125–129. doi: 10.1097/00008526-199106000-00009. [DOI] [Google Scholar]
- 25.Lyon CC, Kulkarni J, Zimersonc E, Van Ross E, Beck MH. Skin disorders in amputees. J Am Acad Dermatol. 2000;42(3):501–507. doi: 10.1016/S0190-9622(00)90227-5. [DOI] [PubMed] [Google Scholar]
- 26.Levy SW. Skin problems of the leg amputee. Prosthet Orthot Int. 1980;4(1):37–44. doi: 10.3109/03093648009103113. [DOI] [PubMed] [Google Scholar]
- 27.Meulenbelt HE, Dijkstra PU, Jonkman MF, Geertzen JH. Skin problems in lower limb amputees: a systematic review. Disabil Rehabil. 2006;28(10):603–608. doi: 10.1080/09638280500277032. [DOI] [PubMed] [Google Scholar]
- 28.Branemark R, Branemark P, Rydevik B, Myers RR. Osseointegration in skeletal reconstruction and rehabilitation: a review. J Rehabil Res Dev. 2001;38(2):175–182. [PubMed] [Google Scholar]
- 29.Adell R, Eriksson B, Lekholm U, Brånemark P-I, Jemt T. A long-term follow-up study of osseointegrated implants in the treatment of totally edentulous jaws. Int J Oral Maxillofacial Implants. 1990; 5(4). [PubMed]
- 30.Aschoff H-H, Clausen A, Tsoumpris K, Hoffmeister T. Implantation der Endo-Exo-Femurprothese zur verbesserung der mobilität amputierter patienten. Operative Orthopädie und Traumatologie. 2011;23(5):462–472. doi: 10.1007/s00064-011-0054-6. [DOI] [PubMed] [Google Scholar]
- 31.Aschoff HH, Kennon RE, Keggi JM, Rubin LE. Transcutaneous, distal femoral, intramedullary attachment for above-the-knee prostheses: an endo-exo device. JBJS. 2010;92(2):180–186. doi: 10.2106/JBJS.J.00806. [DOI] [PubMed] [Google Scholar]
- 32.Van de Meent H, Hopman MT, Frölke JP. Walking ability and quality of life in subjects with transfemoral amputation: a comparison of osseointegration with socket prostheses. Arch Phys Med Rehabil. 2013;94(11):2174–2178. doi: 10.1016/j.apmr.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 33.Haggstrom EE, Hansson E, Hagberg K. Comparison of prosthetic costs and service between osseointegrated and conventional suspended transfemoral prostheses. Prosthet Orthot Int. 2013;37(2):152–160. doi: 10.1177/0309364612454160. [DOI] [PubMed] [Google Scholar]
- 34.Brånemark R, Berlin Ö, Hagberg K, Bergh P, Gunterberg B, Rydevik B. A novel osseointegrated percutaneous prosthetic system for the treatment of patients with transfemoral amputation: a prospective study of 51 patients. Bone Joint J. 2014;96(1):106–113. doi: 10.1302/0301-620X.96B1.31905. [DOI] [PubMed] [Google Scholar]
- 35.Brånemark RP, Hagberg K, Kulbacka-Ortiz K, Berlin Ö, Rydevik B. Osseointegrated percutaneous prosthetic system for the treatment of patients with transfemoral amputation: a prospective five-year follow-up of patient-reported outcomes and complications. J Am Acad Orthop Surg. 2019;27(16):e743–e751. doi: 10.5435/JAAOS-D-17-00621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aschoff H, Juhnke D. Evaluation of 10 years experience with endo-exo femur prostheses-background, data and results. Zeitschrift fur Orthopadie und Unfallchirurgie. 2012;150(6):607–614. doi: 10.1055/s-0032-1327932. [DOI] [PubMed] [Google Scholar]
- 37.Boyce JM. It is time for action: improving hand hygiene in hospitals. Ann Intern Med. 1999;130(2):153–155. doi: 10.7326/0003-4819-130-2-199901190-00011. [DOI] [PubMed] [Google Scholar]
- 38.Kramer A, Schwebke I, Kampf G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis. 2006;6(1):130. doi: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Holmes AH, Moore LS, Sundsfjord A, Steinbakk M, Regmi S, Karkey A, et al. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet. 2016;387(10014):176–187. doi: 10.1016/S0140-6736(15)00473-0. [DOI] [PubMed] [Google Scholar]
- 40.Gbejuade HO, Lovering AM, Webb JC. The role of microbial biofilms in prosthetic joint infections: a review. Acta Orthop. 2015;86(2):147–158. doi: 10.3109/17453674.2014.966290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.An YH, Friedman RJ. Concise review of mechanisms of bacterial adhesion to biomaterial surfaces. J Biomed Mater Res. 1998;43(3):338–348. doi: 10.1002/(SICI)1097-4636(199823)43:3<338::AID-JBM16>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 42.Ohko Y, Utsumi Y, Niwa C, Tatsuma T, Kobayakawa K, Satoh Y, et al. Self-sterilizing and self-cleaning of silicone catheters coated with TiO2 photocatalyst thin films: a preclinical work. J Biomed Mater Res. 2001;58(1):97–101. doi: 10.1002/1097-4636(2001)58:1<97::AID-JBM140>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 43.Shirtliff ME, Calhoun JH, Mader JT. Experimental osteomyelitis treatment with antibiotic-impregnated hydroxyapatite. Clin Orthop Relat Res. 2002;401:239–247. doi: 10.1097/00003086-200208000-00027. [DOI] [PubMed] [Google Scholar]
- 44.Arciola CR, Bustanji Y, Conti M, Campoccia D, Baldassarri L, Samori B, et al. Staphylococcus epidermidis–fibronectin binding and its inhibition by heparin. Biomaterials. 2003;24(18):3013–3019. doi: 10.1016/S0142-9612(03)00133-9. [DOI] [PubMed] [Google Scholar]
- 45.Voller A, Bidwell D, Bartlett A. Enzyme immunoassays in diagnostic medicine: theory and practice. Bull World Health Organ. 1976;53(1):55. [PMC free article] [PubMed] [Google Scholar]
- 46.Gomez E, Cazanave C, Cunningham SA, Greenwood-Quaintance KE, Steckelberg JM, Uhl JR, et al. Prosthetic joint infection diagnosis using broad-range PCR of biofilms dislodged from knee and hip arthroplasty surfaces using sonication. J Clin Microbiol. 2012;50(11):3501–3508. doi: 10.1128/JCM.00834-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee HH, Burczak J, Muldoon S, Leckie G, Chernesky M, Schachter J, et al. Diagnosis of Chlamydia trachomatis genitourinary infection in women by ligase chain reaction assay of urine. Lancet. 1995;345(8944):213–216. doi: 10.1016/S0140-6736(95)90221-X. [DOI] [PubMed] [Google Scholar]
- 48.Majno G. The ancient riddle of σñψις (sepsis) J Infect Dis. 1991;163(5):937–945. doi: 10.1093/infdis/163.5.937. [DOI] [PubMed] [Google Scholar]
- 49.Bean HD, Dimandja JMD, Hill JE. Bacterial volatile discovery using solid phase microextraction and comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry. J Chromatogr B. 2012;901:41–46. doi: 10.1016/j.jchromb.2012.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao W, Duan Y. Breath analysis: potential for clinical diagnosis and exposure assessment. Clin Chem. 2006;52(5):800–811. doi: 10.1373/clinchem.2005.063545. [DOI] [PubMed] [Google Scholar]
- 51.Zhu J, Jiménez-Díaz J, Bean HD, Daphtary NA, Aliyeva MI, Lundblad LK, et al. Robust detection of P. aeruginosa and S. aureus acute lung infections by secondary electrospray ionization-mass spectrometry (SESI-MS) breathprinting: from initial infection to clearance. J Breath Res. 2013;7(3):037106. doi: 10.1088/1752-7155/7/3/037106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Devaraj H, Pook C, Swift S, Aw KC, McDaid AJ. Profiling of headspace volatiles from Escherichia coli cultures using silicone-based sorptive media and thermal desorption GC–MS. J Sep Sci. 2018;41(22):4133–4141. doi: 10.1002/jssc.201800684. [DOI] [PubMed] [Google Scholar]
- 53.Malcolm A, Wright S, Syms RR, Moseley RW, O’Prey S, Dash N, et al. A miniature mass spectrometer for liquid chromatography applications. Rapid Commun Mass Spectrom. 2011;25(21):3281–3288. doi: 10.1002/rcm.5230. [DOI] [PubMed] [Google Scholar]
- 54.Radadia A, Salehi-Khojin A, Masel R, Shannon M. The fabrication of all-silicon micro gas chromatography columns using gold diffusion eutectic bonding. J Micromech Microeng. 2009;20(1):015002. doi: 10.1088/0960-1317/20/1/015002. [DOI] [Google Scholar]
- 55.Persaud K, Dodd G. Analysis of discrimination mechanisms in the mammalian olfactory system using a model nose. Nature. 1982;299(5881):352. doi: 10.1038/299352a0. [DOI] [PubMed] [Google Scholar]
- 56.Lombard GL, Dowell V. Comparison of three reagents for detecting indole production by anaerobic bacteria in microtest systems. J Clin Microbiol. 1983;18(3):609–613. doi: 10.1128/JCM.18.3.609-613.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Barsan N, Schweizer-Berberich M, Göpel W. Fundamental and practical aspects in the design of nanoscaled SnO2 gas sensors: a status report. Fresenius’ J Anal Chem. 1999;365(4):287–304. doi: 10.1007/s002160051490. [DOI] [Google Scholar]
- 58.Ryabtsev S, Shaposhnick A, Lukin A, Domashevskaya E. Application of semiconductor gas sensors for medical diagnostics. Sens Actuat B Chem. 1999;59(1):26–29. doi: 10.1016/S0925-4005(99)00162-8. [DOI] [Google Scholar]
- 59.Nanto H, Minami T, Takata S. Zinc-oxide thin-film ammonia gas sensors with high sensitivity and excellent selectivity. J Appl Phys. 1986;60(2):482–484. doi: 10.1063/1.337435. [DOI] [Google Scholar]
- 60.Chung W-Y, Sakai G, Shimanoe K, Miura N, Lee D-D, Yamazoe N. Preparation of indium oxide thin film by spin-coating method and its gas-sensing properties. Sens Actuat B Chem. 1998;46(2):139–145. doi: 10.1016/S0925-4005(98)00100-2. [DOI] [Google Scholar]
- 61.Frank J, Fleischer M, Meixner H. Gas-sensitive electrical properties of pure and doped semiconducting Ga2O3 thick films. Sens Actuat B Chem. 1998;48(1–3):318–321. doi: 10.1016/S0925-4005(98)00064-1. [DOI] [Google Scholar]
- 62.Tamaki J, Naruo C, Yamamoto Y, Matsuoka M. Sensing properties to dilute chlorine gas of indium oxide based thin film sensors prepared by electron beam evaporation. Sens Actuat B Chem. 2002;83(1–3):190–194. doi: 10.1016/S0925-4005(01)01039-5. [DOI] [Google Scholar]
- 63.Jung S-J, Yanagida H. The characterization of a CuO/ZnO heterocontact-type gas sensor having selectivity for CO gas. Sens Actuat B Chem. 1996;37(1–2):55–60. doi: 10.1016/S0925-4005(96)01986-7. [DOI] [Google Scholar]
- 64.Devi GS, Manorama S, Rao V. SnO2/Bi2O3: a suitable system for selective carbon monoxide detection. J Electrochem Soc. 1998;145(3):1039–1044. doi: 10.1149/1.1838385. [DOI] [Google Scholar]
- 65.Windischmann H, Mark P. A model for the operation of a thin-film SnOx conductance-modulation carbon monoxide sensor. J Electrochem Soc. 1979;126(4):627–633. doi: 10.1149/1.2129098. [DOI] [Google Scholar]
- 66.Martin MA, Santos J, Vasquez H, Agapito J. Study of the interferences of NO2 and CO in solid state commercial sensors. Sens Actuat B Chem. 1999;58(1):469–473. doi: 10.1016/S0925-4005(99)00128-8. [DOI] [Google Scholar]
- 67.Tang Z, Fung SK, Wong DT, Chan PC, Sin JK, Cheung PW. An integrated gas sensor based on tin oxide thin-film and improved micro-hotplate. Sens Actuat B Chem. 1998;46(3):174–179. doi: 10.1016/S0925-4005(98)00118-X. [DOI] [Google Scholar]
- 68.Heilig A, Barsan N, Weimar U, Schweizer-Berberich M, Gardner J, Göpel W. Gas identification by modulating temperatures of SnO2-based thick film sensors. Sens Actuat B Chem. 1997;43(1–3):45–51. doi: 10.1016/S0925-4005(97)00096-8. [DOI] [Google Scholar]
- 69.Egashira M, Shimizu Y, Takao Y. Trimethylamine sensor based on semiconductive metal oxides for detection of fish freshness. Sens Actuat B Chem. 1990;1(1–6):108–112. doi: 10.1016/0925-4005(90)80182-Y. [DOI] [Google Scholar]
- 70.Frietsch M, Zudock F, Goschnick J, Bruns M. CuO catalytic membrane as selectivity trimmer for metal oxide gas sensors. Sens Actuat B Chem. 2000;65(1–3):379–381. doi: 10.1016/S0925-4005(99)00353-6. [DOI] [Google Scholar]
- 71.Lang A, Fleischer M, Meixner H. Surface modifications of Ga2O3 thin film sensors with Rh, Ru and Ir clusters. Sens Actuat B Chem. 2000;66(1–3):80–84. doi: 10.1016/S0925-4005(99)00347-0. [DOI] [Google Scholar]
- 72.de Lacy CB, Ewen RJ, Ratcliffe NM, Sivanand P. Thick film organic vapour sensors based on binary mixtures of metal oxides. Sens Actuat B Chem. 2003;92(1–2):159–166. [Google Scholar]
- 73.Tan O, Zhu W, Yan Q, Kong L. Size effect and gas sensing characteristics of nanocrystalline xSnO2-(1−x) α-Fe2O3 ethanol sensors. Sens Actuat B Chem. 2000;65(1–3):361–365. doi: 10.1016/S0925-4005(99)00414-1. [DOI] [Google Scholar]
- 74.Taurino A, Capone S, Siciliano P, Toccoli T, Boschetti A, Guerini L, et al. Nanostructured TiO2 thin films prepared by supersonic beams and their application in a sensor array for the discrimination of VOC. Sens Actuat B Chem. 2003;92(3):292–302. doi: 10.1016/S0925-4005(03)00314-9. [DOI] [Google Scholar]
- 75.Comini E, Faglia G, Sberveglieri G, Li Y, Wlodarski W, Ghantasala M. Sensitivity enhancement towards ethanol and methanol of TiO2 films doped with Pt and Nb. Sens Actuat B Chem. 2000;64(1–3):169–174. doi: 10.1016/S0925-4005(99)00502-X. [DOI] [Google Scholar]
- 76.Fang G, Liu Z, Liu C, Yao KL. Room temperature H2S sensing properties and mechanism of CeO2–SnO2 sol–gel thin films. Sens Actuat B Chem. 2000;66(1–3):46–48. doi: 10.1016/S0925-4005(99)00467-0. [DOI] [Google Scholar]
- 77.Devi GS, Manorama S, Rao V. Gas sensitivity of SnO2/CuO heterocontacts. J Electrochem Soc. 1995;142(8):2754–2757. doi: 10.1149/1.2050087. [DOI] [Google Scholar]
- 78.Di Natale C, Davide F, Faglia G, Nelli P. Study of the effect of the sensor operating temperature on SnO2-based sensor-array performance. Sens Actuat B Chem. 1995;23(2–3):187–191. doi: 10.1016/0925-4005(94)01274-L. [DOI] [Google Scholar]
- 79.Tamaki J, Yamada Y, Yamamoto Y, Matsuoka M, Ota I. Sensing properties to dilute hydrogen sulfide of ZnSb2O6 thick-film prepared by dip-coating method. Sens Actuat B Chem. 2000;66(1–3):70–73. doi: 10.1016/S0925-4005(99)00408-6. [DOI] [Google Scholar]
- 80.Kimura M. Absolute-humidity sensing independent of the ambient temperature. Sens Actuat A. 1996;55(1):7–11. doi: 10.1016/S0924-4247(96)01242-3. [DOI] [Google Scholar]
- 81.Satyanarayana L, Reddy CG, Manorama S, Rao V. Liquid-petroleum-gas sensor based on a spinel semiconductor, ZnGa2O4. Sens Actuat B Chem. 1998;46(1):1–7. doi: 10.1016/S0925-4005(97)00313-4. [DOI] [Google Scholar]
- 82.Moseley P, Williams D. A selective ammonia sensor. Sens Actuat B Chem. 1990;1(1–6):113–115. doi: 10.1016/0925-4005(90)80183-Z. [DOI] [Google Scholar]
- 83.Runthala D, Gupta R, Vyas P, Eranna G, Paris R, Schipanski D. A material for room temperature FET sensor to detect ammonia and hydrocarbon gases. Indian J Eng Mater Sci (IJEMS). 2000;7(5–6).
- 84.Prasad A, Kubinski D, Gouma P. Comparison of sol–gel and ion beam deposited MoO3 thin film gas sensors for selective ammonia detection. Sens Actuat B Chem. 2003;93(1–3):25–30. doi: 10.1016/S0925-4005(03)00336-8. [DOI] [Google Scholar]
- 85.Schierbaum K-D. Engineering of oxide surfaces and metal/oxide interfaces for chemical sensors: recent trends. Sens Actuat B Chem. 1995;24(1–3):239–247. doi: 10.1016/0925-4005(95)85051-1. [DOI] [Google Scholar]
- 86.Santos J, Serrini P, O’Beirn B, Manes L. A thin film SnO2 gas sensor selective to ultra-low NO2 concentrations in air. Sens Actuat B Chem. 1997;43(1–3):154–160. doi: 10.1016/S0925-4005(97)00115-9. [DOI] [Google Scholar]
- 87.Ishihara T, Sato S, Fukushima T, Takita Y. Capacitive gas sensor of mixed oxide CoO–In2O3 to selectively detect nitrogen monoxide. J Electrochem Soc. 1996;143(6):1908–1914. doi: 10.1149/1.1836923. [DOI] [Google Scholar]
- 88.Kim S-R, Hong H-K, Kwon CH, Yun DH, Lee K, Sung YK. Ozone sensing properties of In2O3-based semiconductor thick films. Sens Actuat B Chem. 2000;66(1–3):59–62. doi: 10.1016/S0925-4005(99)00468-2. [DOI] [Google Scholar]
- 89.Zhou X, Xu Y, Cao Q, Niu S. Metal-semiconductor ohmic contact of SnO2-based ceramic gas sensors. Sens Actuat B Chem. 1997;41(1–3):163–167. doi: 10.1016/S0925-4005(97)80290-0. [DOI] [Google Scholar]
- 90.Saito S, Miyayama M, Koumoto K, Yanagida H. Gas sensing characteristics of porous ZnO and Pt/ZnO ceramics. J Am Ceram Soc. 1985;68(1):40–43. doi: 10.1111/j.1151-2916.1985.tb15248.x. [DOI] [Google Scholar]
- 91.Zhao S, Wei P, Chen S. Enhancement of trimethylamine sensitivity of MOCVD-SnO2 thin film gas sensor by thorium. Sens Actuat B Chem. 2000;62(2):117–120. doi: 10.1016/S0925-4005(99)00365-2. [DOI] [Google Scholar]
- 92.Vilanova X, Llobet E, Alcubilla R, Sueiras JE, Correig X. Analysis of the conductance transient in thick-film tin oxide gas sensors. Sens Actuat B Chem. 1996;31(3):175–180. doi: 10.1016/0925-4005(96)80063-3. [DOI] [Google Scholar]
- 93.Bhattacharyya P, Basu P, Mondal B, Saha H. A low power MEMS gas sensor based on nanocrystalline ZnO thin films for sensing methane. Microelectron Reliab. 2008;48(11–12):1772–1779. doi: 10.1016/j.microrel.2008.07.063. [DOI] [Google Scholar]
- 94.Nylander C, Armgarth M, Lundström I. An ammonia detector based on a conducting polymer. In: Seiyama T. editor. Chemical sensors: meeting proceedings. Analytical chemistry symposia series, vol. 17. Elsevier; 1983. p. 203–207.
- 95.Lu G, Qu L, Shi G. Electrochemical fabrication of neuron-type networks based on crystalline oligopyrene nanosheets. Electrochim Acta. 2005;51(2):340–346. doi: 10.1016/j.electacta.2005.04.043. [DOI] [Google Scholar]
- 96.Devaraj H, Travas-Sejdic J, Sharma R, Aydemir N, Williams D, Haemmerle E, et al. Bio-inspired flow sensor from printed PEDOT: PSS micro-hairs. Bioinspir Biomimetics. 2015;10(1):016017. doi: 10.1088/1748-3190/10/1/016017. [DOI] [PubMed] [Google Scholar]
- 97.Devaraj H, Sharma R, Haemmerle E, Aw K, editors. A portable & disposable ultra-low velocity flow sensor from bioinspired hair-like microstructures. In: Multidisciplinary digital publishing institute proceedings; 2018.
- 98.Li B, Sauvé G, Iovu MC, Jeffries-El M, Zhang R, Cooper J, et al. Volatile organic compound detection using nanostructured copolymers. Nano Lett. 2006;6(8):1598–1602. doi: 10.1021/nl060498o. [DOI] [PubMed] [Google Scholar]
- 99.Zhang T, Nix MB, Yoo BY, Deshusses MA, Myung NV. Electrochemically functionalized single-walled carbon nanotube gas sensor. Electroanal Int J Devot Fund Pract Aspects Electroanal. 2006;18(12):1153–1158. [Google Scholar]
- 100.Hernandez SC, Chaudhuri D, Chen W, Myung NV, Mulchandani A. Single polypyrrole nanowire ammonia gas sensor. Electroanal Int J Devot Fund Pract Aspects Electroanal. 1920;2007(19-20):2125–2130. [Google Scholar]
- 101.Dixit V, Misra S, Sharma B. Carbon monoxide sensitivity of vacuum deposited polyaniline semiconducting thin films. Sens Actuat B Chem. 2005;104(1):90–93. doi: 10.1016/j.snb.2004.05.001. [DOI] [Google Scholar]
- 102.Sadek A, Wlodarski W, Shin K, Kaner RB, Kalantar-Zadeh K. A layered surface acoustic wave gas sensor based on a polyaniline/In2O3 nanofibre composite. Nanotechnology. 2006;17(17):4488. doi: 10.1088/0957-4484/17/17/034. [DOI] [Google Scholar]
- 103.Waghuley S, Yenorkar S, Yawale S, Yawale S. Application of chemically synthesized conducting polymer-polypyrrole as a carbon dioxide gas sensor. Sens Actuat B Chem. 2008;128(2):366–373. doi: 10.1016/j.snb.2007.06.023. [DOI] [Google Scholar]
- 104.Misra S, Mathur P, Yadav M, Tiwari M, Garg S, Tripathi P. Preparation and characterization of vacuum deposited semiconducting nanocrystalline polymeric thin film sensors for detection of HCl. Polymer. 2004;45(25):8623–8628. doi: 10.1016/j.polymer.2004.10.010. [DOI] [Google Scholar]
- 105.Virji S, Fowler JD, Baker CO, Huang J, Kaner RB, Weiller BH. Polyaniline nanofiber composites with metal salts: chemical sensors for hydrogen sulfide. Small. 2005;1(6):624–627. doi: 10.1002/smll.200400155. [DOI] [PubMed] [Google Scholar]
- 106.Athawale AA, Bhagwat S, Katre PP. Nanocomposite of Pd-polyaniline as a selective methanol sensor. Sens Actuat B Chem. 2006;114(1):263–270. doi: 10.1016/j.snb.2005.05.009. [DOI] [Google Scholar]
- 107.Xie D, Jiang Y, Pan W, Li D, Wu Z, Li Y. Fabrication and characterization of polyaniline-based gas sensor by ultra-thin film technology. Sens Actuat B Chem. 2002;81(2–3):158–164. doi: 10.1016/S0925-4005(01)00946-7. [DOI] [Google Scholar]
- 108.Chyla A, Lewandowska A, Soloducho J, Gorecka-Drzazga A, Szablewski M. 4-t-butyl-CuPc-PODT molecular composite material for an effective gas sensor. IEEE Trans Dielectr Electr Insul. 2001;8(3):559–565. doi: 10.1109/94.933384. [DOI] [Google Scholar]
- 109.Li G, Martinez C, Semancik S. Controlled electrophoretic patterning of polyaniline from a colloidal suspension. J Am Chem Soc. 2005;127(13):4903–4909. doi: 10.1021/ja0441763. [DOI] [PubMed] [Google Scholar]
- 110.Kondratowicz B, Narayanaswamy R, Persaud K. An investigation into the use of electrochromic polymers in optical fibre gas sensors. Sens Actuat B Chem. 2001;74(1–3):138–144. doi: 10.1016/S0925-4005(00)00723-1. [DOI] [Google Scholar]
- 111.Im JS, Kang SC, Lee S-H, Lee Y-S. Improved gas sensing of electrospun carbon fibers based on pore structure, conductivity and surface modification. Carbon. 2010;48(9):2573–2581. doi: 10.1016/j.carbon.2010.03.045. [DOI] [Google Scholar]
- 112.Leghrib R, Llobet E. Quantitative trace analysis of benzene using an array of plasma-treated metal-decorated carbon nanotubes and fuzzy adaptive resonant theory techniques. Anal Chim Acta. 2011;708(1–2):19–27. doi: 10.1016/j.aca.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 113.Kirkpatrick S. Percolation and conduction. Rev Mod Phys. 1973;45(4):574. doi: 10.1103/RevModPhys.45.574. [DOI] [Google Scholar]
- 114.Lonergan MC, Severin EJ, Doleman BJ, Beaber SA, Grubbs RH, Lewis NS. Array-based vapor sensing using chemically sensitive, carbon black-polymer resistors. Chem Mater. 1996;8(9):2298–2312. doi: 10.1021/cm960036j. [DOI] [Google Scholar]
- 115.Zhang B, Fu R, Zhang M, Dong X, Wang L, Pittman CU., Jr Gas sensitive vapor grown carbon nanofiber/polystyrene sensors. Mater Res Bull. 2006;41(3):553–562. doi: 10.1016/j.materresbull.2005.09.009. [DOI] [Google Scholar]
- 116.Im JS, Kang SC, Lee S-H, Lee Y-S. Improved gas sensing of electrospun carbon fibers based on pore structure, conductivity and surface modification. Carbon. 2010;48(9):2573–2581. doi: 10.1016/j.carbon.2010.03.045. [DOI] [Google Scholar]
- 117.Zhang L, Wang X, Zhao Y, Zhu Z, Fong H. Electrospun carbon nano-felt surface-attached with Pd nanoparticles for hydrogen sensing application. Mater Lett. 2012;68:133–136. doi: 10.1016/j.matlet.2011.10.064. [DOI] [Google Scholar]
- 118.Lee JS, Kwon OS, Park SJ, Park EY, You SA, Yoon H, et al. Fabrication of ultrafine metal-oxide-decorated carbon nanofibers for DMMP sensor application. ACS Nano. 2011;5(10):7992–8001. doi: 10.1021/nn202471f. [DOI] [PubMed] [Google Scholar]
- 119.Tans SJ, Verschueren AR, Dekker C. Room-temperature transistor based on a single carbon nanotube. Nature. 1998;393(6680):49. doi: 10.1038/29954. [DOI] [Google Scholar]
- 120.Kong J, Franklin NR, Zhou C, Chapline MG, Peng S, Cho K, et al. Nanotube molecular wires as chemical sensors. Science. 2000;287(5453):622–625. doi: 10.1126/science.287.5453.622. [DOI] [PubMed] [Google Scholar]
- 121.Kong J, Chapline MG, Dai H. Functionalized carbon nanotubes for molecular hydrogen sensors. Adv Mater. 2001;13(18):1384–1386. doi: 10.1002/1521-4095(200109)13:18<1384::AID-ADMA1384>3.0.CO;2-8. [DOI] [Google Scholar]
- 122.Leghrib R, Llobet E. Quantitative trace analysis of benzene using an array of plasma-treated metal-decorated carbon nanotubes and fuzzy adaptive resonant theory techniques. Anal Chim Acta. 2011;708(1–2):19–27. doi: 10.1016/j.aca.2011.09.038. [DOI] [PubMed] [Google Scholar]
- 123.Schedin F, Geim A, Morozov S, Hill E, Blake P, Katsnelson M, et al. Detection of individual gas molecules adsorbed on graphene. Nat Mater. 2007;6(9):652. doi: 10.1038/nmat1967. [DOI] [PubMed] [Google Scholar]
- 124.Villalpando-Paez F, Romero A, Munoz-Sandoval E, Martınez L, Terrones H, Terrones M. Fabrication of vapor and gas sensors using films of aligned CNx nanotubes. Chem Phys Lett. 2004;386(1–3):137–143. doi: 10.1016/j.cplett.2004.01.052. [DOI] [Google Scholar]
- 125.Li W, Geng X, Guo Y, Rong J, Gong Y, Wu L, et al. Reduced graphene oxide electrically contacted graphene sensor for highly sensitive nitric oxide detection. ACS Nano. 2011;5(9):6955–6961. doi: 10.1021/nn201433r. [DOI] [PubMed] [Google Scholar]
- 126.Gottardi G, Galli E. Natural zeolites. New York: Springer; 2012. [Google Scholar]
- 127.Long JR, Yaghi OM. The pervasive chemistry of metal–organic frameworks. Chem Soc Rev. 2009;38(5):1213–1214. doi: 10.1039/b903811f. [DOI] [PubMed] [Google Scholar]
- 128.O’Keeffe M. Design of MOFs and intellectual content in reticular chemistry: a personal view. Chem Soc Rev. 2009;38(5):1215–1217. doi: 10.1039/b802802h. [DOI] [PubMed] [Google Scholar]
- 129.Perry Iv JJ, Perman JA, Zaworotko MJ. Design and synthesis of metal–organic frameworks using metal–organic polyhedra as supermolecular building blocks. Chem Soc Rev. 2009;38(5):1400–1417. doi: 10.1039/b807086p. [DOI] [PubMed] [Google Scholar]
- 130.James SL. Metal–organic frameworks. Chem Soc Rev. 2003;32(5):276–288. doi: 10.1039/b200393g. [DOI] [PubMed] [Google Scholar]
- 131.Furukawa H, Cordova KE, O’Keeffe M, Yaghi OM. The chemistry and applications of metal–organic frameworks. Science. 2013;341(6149):1230444. doi: 10.1126/science.1230444. [DOI] [PubMed] [Google Scholar]
- 132.Lu Z-Z, Zhang R, Li Y-Z, Guo Z-J, Zheng H-G. Solvatochromic behavior of a nanotubular metal–organic framework for sensing small molecules. J Am Chem Soc. 2011;133(12):4172–4174. doi: 10.1021/ja109437d. [DOI] [PubMed] [Google Scholar]
- 133.Kreno LE, Leong K, Farha OK, Allendorf M, Van Duyne RP, Hupp JT. Metal–organic framework materials as chemical sensors. Chem Rev. 2011;112(2):1105–1125. doi: 10.1021/cr200324t. [DOI] [PubMed] [Google Scholar]
- 134.Lee J-H, Houk R, Robinson A, Greathouse J, Thornberg S, Allendorf M, et al., editors. Investigation of microcantilever array with ordered nanoporous coatings for selective chemical detection. In: Micro-and nanotechnology sensors, systems, and applications II. International Society for Optics and Photonics; 2010.
- 135.Cote AP, Benin AI, Ockwig NW, O’keeffe M, Matzger AJ, Yaghi OM. Porous, crystalline, covalent organic frameworks. Science. 2005;310(5751):1166–1170. doi: 10.1126/science.1120411. [DOI] [PubMed] [Google Scholar]
- 136.Ding S-Y, Wang W. Covalent organic frameworks (COFs): from design to applications. Chem Soc Rev. 2013;42(2):548–568. doi: 10.1039/C2CS35072F. [DOI] [PubMed] [Google Scholar]
- 137.Feng X, Ding X, Jiang D. Covalent organic frameworks. Chem Soc Rev. 2012;41(18):6010–6022. doi: 10.1039/c2cs35157a. [DOI] [PubMed] [Google Scholar]
- 138.Das G, Biswal BP, Kandambeth S, Venkatesh V, Kaur G, Addicoat M, et al. Chemical sensing in two dimensional porous covalent organic nanosheets. Chem Sci. 2015;6(7):3931–3939. doi: 10.1039/C5SC00512D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Li Z, Zhang Y, Xia H, Mu Y, Liu X. A robust and luminescent covalent organic framework as a highly sensitive and selective sensor for the detection of Cu2+ ions. Chem Commun. 2016;52(39):6613–6616. doi: 10.1039/C6CC01476C. [DOI] [PubMed] [Google Scholar]
- 140.Ding S-Y, Dong M, Wang Y-W, Chen Y-T, Wang H-Z, Su C-Y, et al. Thioether-based fluorescent covalent organic framework for selective detection and facile removal of mercury(II) J Am Chem Soc. 2016;138(9):3031–3037. doi: 10.1021/jacs.5b10754. [DOI] [PubMed] [Google Scholar]
- 141.Laftah WA, Hashim S, Ibrahim AN. Polymer hydrogels: a review. Polym Plast Technol Eng. 2011;50(14):1475–1486. doi: 10.1080/03602559.2011.593082. [DOI] [Google Scholar]
- 142.Gerlach G, Guenther M, Suchaneck G, Sorber J, Arndt KF, Richter A, editors. Application of sensitive hydrogels in chemical and pH sensors. In: Macromolecular symposia. Wiley Online Library; 2004.
- 143.Trinh QT, Gerlach G, Sorber J, Arndt K-F. Hydrogel-based piezoresistive pH sensors: design, simulation and output characteristics. Sens Actuat B Chem. 2006;117(1):17–26. doi: 10.1016/j.snb.2005.10.041. [DOI] [Google Scholar]
- 144.Kojima J, Nakayama Y, Takenaka M, Hashimoto T. Apparatus for measuring time-resolved light scattering profiles from supercritical polymer solutions undergoing phase separation under high pressure. Rev Sci Instrum. 1995;66(8):4066–4072. doi: 10.1063/1.1145418. [DOI] [Google Scholar]
- 145.Zguris J, Pishko MV. Nitric oxide sensitive fluorescent poly(ethylene glycol) hydrogel microstructures. Sens Actuat B Chem. 2006;115(1):503–509. doi: 10.1016/j.snb.2005.10.032. [DOI] [Google Scholar]
- 146.Herber S, Olthuis W, Bergveld P, van den Berg A. Exploitation of a pH-sensitive hydrogel disk for CO2 detection. Sens Actuat B Chem. 2004;103(1–2):284–289. doi: 10.1016/j.snb.2004.04.113. [DOI] [Google Scholar]
- 147.Bai H, Sheng K, Zhang P, Li C, Shi G. Graphene oxide/conducting polymer composite hydrogels. J Mater Chem. 2011;21(46):18653–18658. doi: 10.1039/c1jm13918e. [DOI] [Google Scholar]
- 148.Wu J, Tao K, Zhang J, Guo Y, Miao J, Norford LK. Chemically functionalized 3D graphene hydrogel for high performance gas sensing. J Mater Chem A. 2016;4(21):8130–8140. doi: 10.1039/C6TA01426G. [DOI] [Google Scholar]
- 149.Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249(4968):505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- 150.Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346(6287):818. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- 151.Stoltenburg R, Reinemann C, Strehlitz B. FluMag-SELEX as an advantageous method for DNA aptamer selection. Anal Bioanal Chem. 2005;383(1):83–91. doi: 10.1007/s00216-005-3388-9. [DOI] [PubMed] [Google Scholar]
- 152.Berezovski M, Musheev M, Drabovich A, Krylov SN. Non-SELEX selection of aptamers. J Am Chem Soc. 2006;128(5):1410–1411. doi: 10.1021/ja056943j. [DOI] [PubMed] [Google Scholar]
- 153.Cao Z, Tong R, Mishra A, Xu W, Wong GC, Cheng J, et al. Reversible cell-specific drug delivery with aptamer-functionalized liposomes. Angew Chem Int Ed. 2009;48(35):6494–6498. doi: 10.1002/anie.200901452. [DOI] [PubMed] [Google Scholar]
- 154.Bagalkot V, Farokhzad OC, Langer R, Jon S. An aptamer–doxorubicin physical conjugate as a novel targeted drug-delivery platform. Angew Chem Int Ed. 2006;45(48):8149–8152. doi: 10.1002/anie.200602251. [DOI] [PubMed] [Google Scholar]
- 155.Huang YF, Shangguan D, Liu H, Phillips JA, Zhang X, Chen Y, et al. Molecular assembly of an aptamer–drug conjugate for targeted drug delivery to tumor cells. ChemBioChem. 2009;10(5):862–868. doi: 10.1002/cbic.200800805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Shangguan D, Cao Z, Meng L, Mallikaratchy P, Sefah K, Wang H, et al. Cell-specific aptamer probes for membrane protein elucidation in cancer cells. J Proteome Res. 2008;7(5):2133–2139. doi: 10.1021/pr700894d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Wang L, Zhu J, Han L, Jin L, Zhu C, Wang E, et al. Graphene-based aptamer logic gates and their application to multiplex detection. ACS Nano. 2012;6(8):6659–6666. doi: 10.1021/nn300997f. [DOI] [PubMed] [Google Scholar]
- 158.He Y, Xing X, Tang H, Pang D. Graphene oxide-based fluorescent biosensor for protein detection via terminal protection of small-molecule-linked DNA. Small. 2013;9(12):2097–2101. doi: 10.1002/smll.201202739. [DOI] [PubMed] [Google Scholar]
- 159.Sun W, Shi S, Yao T. Graphene oxide–Ru complex for label-free assay of DNA sequence and potassium ions via fluorescence resonance energy transfer. Anal Methods. 2011;3(11):2472–2474. doi: 10.1039/c1ay05521f. [DOI] [Google Scholar]
- 160.Wen Y, Xing F, He S, Song S, Wang L, Long Y, et al. A graphene-based fluorescent nanoprobe for silver(I) ions detection by using graphene oxide and a silver-specific oligonucleotide. Chem Commun. 2010;46(15):2596–2598. doi: 10.1039/b924832c. [DOI] [PubMed] [Google Scholar]
- 161.Zhang JR, Huang WT, Xie WY, Wen T, Luo HQ, Li NB. Highly sensitive, selective, and rapid fluorescence Hg2+ sensor based on DNA duplexes of poly(dT) and graphene oxide. Analyst. 2012;137(14):3300–3305. doi: 10.1039/c2an35528k. [DOI] [PubMed] [Google Scholar]
- 162.Staii C, Johnson AT, Chen M, Gelperin A. DNA-decorated carbon nanotubes for chemical sensing. Nano Lett. 2005;5(9):1774–1778. doi: 10.1021/nl051261f. [DOI] [PubMed] [Google Scholar]
- 163.So HM, Park DW, Jeon EK, Kim YH, Kim BS, Lee CK, et al. Detection and titer estimation of Escherichia coli using aptamer-functionalized single-walled carbon-nanotube field-effect transistors. Small. 2008;4(2):197–201. doi: 10.1002/smll.200700664. [DOI] [PubMed] [Google Scholar]
- 164.Lian Y, He F, Wang H, Tong F. A new aptamer/graphene interdigitated gold electrode piezoelectric sensor for rapid and specific detection of Staphylococcus aureus. Biosens Bioelectron. 2015;65:314–319. doi: 10.1016/j.bios.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 165.Angioy AM, Desogus A, Barbarossa IT, Anderson P, Hansson BS. Extreme sensitivity in an olfactory system. Chem Senses. 2003;28(4):279–284. doi: 10.1093/chemse/28.4.279. [DOI] [PubMed] [Google Scholar]
- 166.Kaupp UB. Olfactory signalling in vertebrates and insects: differences and commonalities. Nat Rev Neurosci. 2010;11(3):188. doi: 10.1038/nrn2789. [DOI] [PubMed] [Google Scholar]
- 167.Montagné N, de Fouchier A, Newcomb RD, Jacquin-Joly E. Advances in the identification and characterization of olfactory receptors in insects. In: Progress in molecular biology and translational science: Elsevier; 2015. p. 55–80. [DOI] [PubMed]
- 168.Khadka R, Aydemir N, Carraher C, Hamiaux C, Colbert D, Cheema J, et al. An ultrasensitive electrochemical impedance-based biosensor using insect odorant receptors to detect odorants. Biosens Bioelectron. 2019;126:207–213. doi: 10.1016/j.bios.2018.10.043. [DOI] [PubMed] [Google Scholar]
- 169.Toyokuni S. Genotoxicity and carcinogenicity risk of carbon nanotubes. Adv Drug Deliv Rev. 2013;65(15):2098–2110. doi: 10.1016/j.addr.2013.05.011. [DOI] [PubMed] [Google Scholar]