Abstract
Cystic echinococcosis (CE) and liver fluke infections as important zoonotic infections impose a large socioeconomic impact on societies. As an endemic region for these infections, slaughterhouse inspections should be more considered in Iran. This study aimed to analyze the 11-year record of offal condemnation due to CE, fascioliasis, dicrocoeliasis infections in sheep and goat and its economic impact at Alborz slaughterhouse, north-central Iran. The prevalence rate was calculated as the infected organs (as nominator) divided by the slaughtered cases (as the denominator) in each year and month. The annual percent changes was used to determine trends of parasitic diseases over time. The relationship between metrological indexes and the prevalence of parasitic diseases was determined by the linear regression model. Statistical analyses were done using STATA software 14. For an estimate, the economic impact, the total numbers of offal condemnation were calculated. The overall prevalence rate of fascioliasis, dicrocoeliasis, and CE was 0.95%, 2.17%, and 12.74%, respectively. There was a declining trend in the prevalence of fascioliasis and dicrocoeliasis, whereas, the prevalence of CE increased from 7.57% in 2008 to 9.53% in 2018, representing an annual change of + 0.02%. The direct economic impact was estimated at US$ 1,670,977 and US$ 25,148 for liver and lung, respectively. The number of condemned organs due to these infections is noticeable in Alborz Province, north-central, Iran. The high economic impact of these infections showed the necessity of implementing a continuously infected animal’s trace-back and disease control in the site of infection.
Keywords: Cystic echinococcosis, Fascioliasis, Dicrocoeliasis, Economic impact, Prevalence, Iran
Introduction
Cystic echinococcosis (CE)/hydatidosis and liver fluke infections as veterinary and medically important diseases impose large socioeconomic impact on societies (Arbabi et al. 2018; Budke et al. 2006). In spite some global successes in the control of these diseases, they still remain as important challenges, especially in developing countries including Iran, and the life cycle of mentioned parasites is simply completed throughout the country (Jahed Khaniki et al. 2013). Infected offal and meat of slaughtered animals are main source of infection to human. The most important infections in slaughterd animals are leptospirosis, brucellosis, anthrax, tularemia, listeriosis, salmonellosis staphylococcosis, and campylobacteriosis (Milios et al. 2014), orf, Q fever, Crimean–Congo hemorrhagic fever (CCHF) (Drolet et al. 2002). Among the parasitic infections, trichinosis, taeniasis, toxoplasmosis, sarcosporidiosis along with pentastomiasis, CE, fascioliasis, and dicrocoeliasis are most important. Due to infectious potency of four last mentioned infections with the ability to make cyst, abscessation, hepatitis, fibrosis, and etc. they may cause economic loose in meat and offal products (Addis 2017), Condemnation of livestock offal due to parasitic infections, could lead to a deficiency in these main protein sources for human. This is effective on the inherent or physiological basic need that is an important factor in the survival of life and longevity (Irani and Sharif 2016). Malnutrition of proteins, fats, carbohydrates, minerals, and vitamins could occur due to this deficiency (Sarwar et al. 2015). As the main place for providing meat, it is essential to inspect slaughtered animals in the slaughterhouse during all slaughter procedures, including pre-slaughter handling and stunning, slaughtering, skinning, evisceration, by-products collection, meat inspection, ante-mortem and post-mortem inspection, reinspections during processing and sanitation (Kadim et al. 2013; Wheatley et al. 2014). The necessity of this inspection by veterinary personnel is essential to make effective controlling and preventing zoonotic diseases and providing food security in animal protein products (Tilman and Clark 2014), All data about the number of slaughtered animals, and the number of condemnation based on organ and causative agent were recorded at the end of the working day. This records kept for later use in decision making. (Blagojevic and Antic 2014).
The high parasite load and existing of at least one parasite in wild animal is specified (Allwin et al. 2015; Gulland 1992). Close contact of livestock with wild animal predisposes them to host the parasites. In one study conducted in north-central Nigeria on the economic impact of organ condemnations, it was revealed that 10.6% of slaughtered animal had some organ condemned. The most common condemned organs were liver, intestine, and heart. Fasciolosis and CE were the most parasitic infections as causative for liver condemnation, furthermore for total condemned organ US$ 5,467,126.40 estimated in term of economic loss (Alhaji et al. 2017). In countries such as Tanzania and Ethiopia, various studies showed the meat loss of near 1 million US$ annually (Jaja et al. 2018). The rate of condemnation in animals slaughtered in Yazd Province, central Iran, showed 0.21% of the large animals and 0.02% of the small animals have condemned carcass and offal. The most common condemned organs in large slaughtered livestock were lung (21.23%), liver (5.36%), and kidney (3.68%), respectively, whereas in small slaughtered ones, the rate of condemnation was fewer and liver (4.37%) lung (5.46%), and kidney (0.51%) were most condemned organs. The most causative agent of offal condemnation was related to parasitic infections (Hajimohammadi et al. 2014).
Due to the importance of slaughterhouse as the frontline for preventing the human from infecting with these parasites, it is essential to record these infections and providing evidence-based data to authorities for the implementation of control programs. As an endemic region for CE, fascioliasis and, dicrocoeliasis, slaughterhouse inspections should be more considered in Iran. This study aimed to analyze the 11-year record of carcass and offal condemnation due to CE, fascioliasis, dicrocoeliasis infections in sheep and goat and its economic impact at Alborz slaughterhouse, north-central Iran.
Materials and methods
Data collection
For the years 2008–2018, in a retrospective study, the related data of offal condemnation due to CE, fascioliasis and dicrocoeliasis infections in slaughtered sheep and goat were evaluated. Geographical data and metrology information (i.e. average temperature, humidity, precipitation, and wind speed) of the region were collected for each year from the statistical center of Iran.
Study area
The slaughterhouse is located in Alborz Province, north-central Iran. This province covers an area of 5833 km2 and its longitude and latitude are 35.9960°N, 50.9289°E, respectively. The temperature of this region ranged from − 7° in January to 40 °C in August where its climate is varied from cold in the winter to high temperature in the summer. Annual precipitation rate in this area is 173.2 mm.
Economic impact
For estimating the economic impact in this slaughterhouse, the total number of offal condemnation due to mentioned parasitic infections were calculated. The average price of the day of liver and lungs in butchers (at least 20 local butchers opinion) were multiplied in the number of condemned offal. Finally, the result exchanged to US$. The value of each US$ 1 is equal to 130000 Iran’s currency in May 2019.
Statistical analysis
The prevalence rate was calculated as the infected organs (as nominator) divided by the slaughtered cases (as denominator) in each year and month. The annual percent changes (APC) was used to determine trends of parasitic diseases over time. We depicted the prevalence rate of parasitic diseases and the mean of metrological indexes using Graph Pad Prism 7. The relationship between metrological indexes and the prevalence of parasitic diseases was determined by the linear regression model. Statistical analyses were done at 95% confidence level using STATA software 14.
Results
A total of 1,600,225 slaughtered livestock including 1,376,895 sheep and 223,330 goats were inspected. The overall prevalence rate of fascioliasis, dicrocoeliasis, and CE was 0.95%, 2.17%, and 12.74%, respectively. The prevalence rate of fascioliasis, dicrocoeliasis, and CE in sheep was 0.83%, 2.0%, and 12.12%, respectively. The prevalence rate of fascioliasis, dicrocoeliasis, and CE in goats was 2.02%, 3.41%, and 19.07%, respectively. The overall prevalence of CE in lung and liver was 6.81% and 5.93%, respectively.
Annually trend of prevalence rate of parasitic diseases with annual percentage change and period percentage change are shown in Table 1. There was a declining trend in the prevalence of fascioliasis and dicrocoeliasis with an annual decline of 0.05% and 0.02%, respectively. Whereas, the prevalence rate of CE significantly increased from 7.57% in 2008 to 9.53% in 2018, representing an annual change of + 0.02%.
Table 1.
Overall prevalence and annually trend of prevalence parasitic diseases with annual percentage change and period percentage change
| Fasciolosis | Dicrocoeliosis | Hydatidosis | |
|---|---|---|---|
| Overall | 0.95 (0.05) | 2.17 (0.14) | 12.74 (0.60) |
| 2008 | 0.84 (0.18) | 1.23 (0.21) | 7.57 (1.23) |
| 2009 | 0.94 (0.09) | 1.58 (0.69) | 6.66 (1.32) |
| 2010 | 0.85 (0.16) | 1.06 (0.10) | 11.08 (1.32) |
| 2011 | 0.90 (0.19) | 2.05 (0.50) | 11.25 (1.60) |
| 2012 | 1.10 (0.19) | 4.40 (0.60) | 17.09 (1.76) |
| 2013 | 1.18 (0.28) | 3.33 (0.38) | 15.15 (1.72) |
| 2014 | 1.14 (0.20) | 2.66 (0.27) | 18.38 (2.55) |
| 2015 | 0.83 (0.13) | 1.87 (0.30) | 14.00 (1.63) |
| 2016 | 1.09 (0.09) | 2.07 (0.19) | 12.79 (0.73) |
| 2017 | 0.98 (0.17) | 2.11 (0.30) | 15.30 (2.81) |
| 2018 | 0.39 (0.09) | 1.02 (0.19) | 9.53 (0.86) |
| Period change (%) | − 0.54 | − 0.17 | + 0.26 |
| Annual change (%) | − 0.05 | − 0.02 | + 0.02 |
The figures are reported as prevalence (standard error)
As shown in Fig. 1, there was a U-shape association between the prevalence of fascioliasis, dicrocoeliasis, and CE and month. In other word, the highest prevalence rate was found for January and December and the lowest rate was found for May and June.
Fig. 1.
Trend of monthly prevalence of hydatidosis, fasciolosis, and dicrocoeliosis in Alborz Province, Iran from 2008 to 2018
Table 2 presents the relationship between metrological indexes and the prevalence of parasitic diseases as dependent variables. Humidity had a positive relationship with fascioliasis (b = + 0.013, P = 0.004), dicrocoeliasis (b = + 0.019, P = 0.021) and CE (b = + 0.076, P = 0.067) prevalence, but it was not significant for CE. This study also showed a significant negative relationship between both temperature and wind speed with fascioliasis, dicrocoeliasis and CE prevalence. We found no significant association between precipitation and the prevalence of parasitic diseases.
Table 2.
Statistical relationship between metrological indexes and prevalence of parasitic diseases
| Coefficient (B) | SE | P value | |
|---|---|---|---|
| Fasciolosis | |||
| Humidity (mean) | 0.013 | 0.004 | 0.004 |
| Temperature (°C) | − 0.031 | 0.007 | 0.001 |
| Precipitation (mm3) | 0.002 | 0.002 | 0.236 |
| Wind speed (m/s) | − 0.191 | 0.061 | 0.003 |
| Dicrocoeliosis | |||
| Humidity (mean) | 0.019 | 0.008 | 0.021 |
| Temperature (°C) | − 0.039 | 0.014 | 0.005 |
| Precipitation (mm3) | 0.004 | 0.005 | 0.364 |
| Wind speed (m/s) | − 0.518 | 0.167 | 0.003 |
| Hydatidosis | |||
| Humidity (mean) | 0.076 | 0.040 | 0.067 |
| Temperature (°C) | − 0.195 | 0.070 | 0.014 |
| Precipitation (mm3) | 0.005 | 0.030 | 0.870 |
| Wind speed (m/s) | − 2.100 | 0.720 | 0.006 |
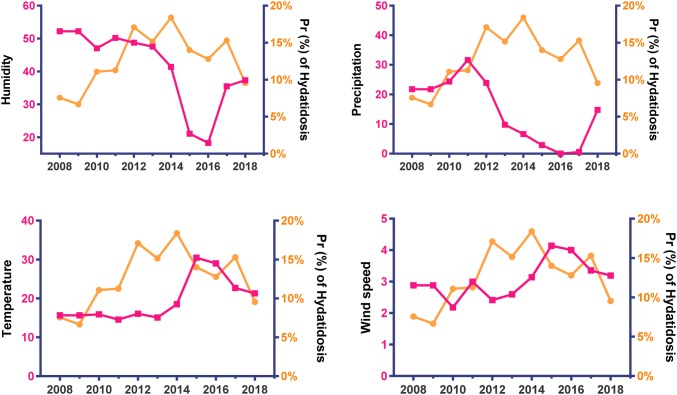
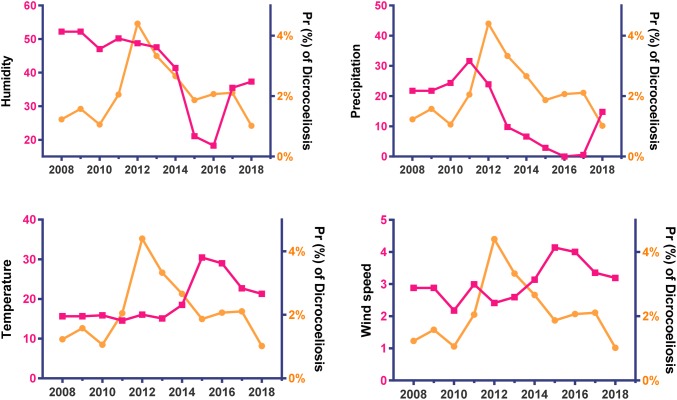
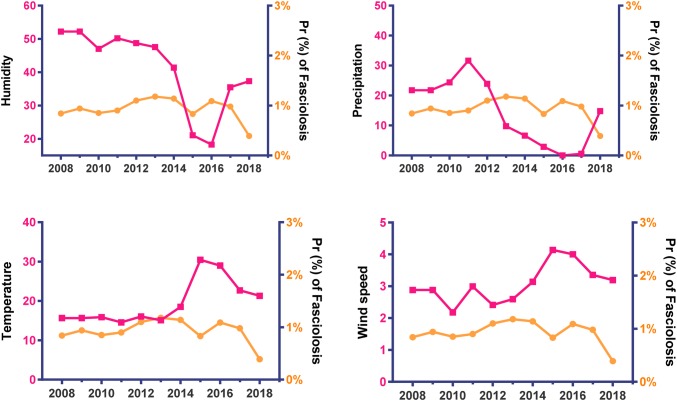
The trend of annual prevalence of parasitic diseases and metrological patterns depicted in Figs. 2, 3, and 4 in Alborz Province, Iran from 2008 to 2018. A significant declining trend was found in the mean of humidity and precipitation, but a significant increasing trend was found for the mean of temperature and wind speed during the period.
Fig. 2.
Trend of annually prevalence of hydatidosis (slope = + 0.63, P = 0.001) and metrological patterns in Alborz Province, Iran from 2008 to 2018
Fig. 3.
Trend of annually prevalence of Dicrocoeliosis (slope = + 0.01, P = 0.863) and metrological patterns in Alborz Province, Iran from 2008 to 2018
Fig. 4.
Trend of annually prevalence of Fasciolosis (slope = − 0.01, P = 0.551) and metrological patterns in Alborz Province, Iran from 2008 to 2018
A positive period change was found in the prevalence rate of fascioliasis, dicrocoeliasis, and CE in goat, whereas the prevalence rate of fascioliasis and dicrocoeliasis in sheep had a negative change from 2008 to 2018 (Fig. 5).
Fig. 5.

The period percent change in the prevalence of parasitic diseases in 2018 compared with 2008
Economic impact
Based on total prevalence, 144,819 livers and 108,975 lungs out of 1,600,225 investigated livestock condemned due to these parasitic infections. The direct economic impact was estimated at US$ 1,670,977 and US$ 25,148 for liver and lung, respectively.
Discussion
Slaughterhouses, as main centers for producing and introducing meat protein, should be more inspected by authorities concerned. For the years 2008–2018, the recorded data of CE, fascioliasis, dicrocoeliasis were evaluated in an important slaughterhouse, Alborz Province, north-central Iran.
CE, as a main zoonotic parasitic infection in the Mediterranean area, is also considered as a socioeconomic challenge in Iran. In the current study, the overall prevalence rate of CE in sheep and goat was 12.74%. The prevalence of CE was 12.12% and 19.07% in slaughtered sheep and goat, respectively. As an endemic region, the mean prevalence of CE in sheep and goats were reported 5.9% (4.9–7.1%) and 6.4% (5.6–7.7), respectively in Iran (Khalkhali et al. 2018). Compared with some previous studies, in the current study, the prevalence rate of CE was more than the mean in Iran. However, a prevalence more than the mean is reported from Fars, Sarab at East Azerbaijan, Ardabil, and Mazandaran provinces (Dalimi et al. 2002; Hosseini et al. 2018; Ziaei et al. 2011). The more infected goats in comparison with sheep are concord with the previous study in Iran (Khalkhali et al. 2018).
Infecting with CE in slaughtered livestock also frequently reported in other countries. In Jordan, the prevalence was in the high range (1.3–71.1%) in sheep and 0.1–3.6% in goats. In Syria, 2.3% of goats and 4.5% of sheep were infected with CE. Furthermore, in Kuwait, 10.4% of sheep and in Iraq 4.5–44% of sheep and 3.1–26.7% of goats were reported infected (Dalimi et al. 2002).
Fascioliasis and dicrocoeliasis as two important liver flukes infections are endemic in many countries throughout the world. Condemnation of liver organ due to these infections is considerable in slaughterhouses in the endemic countries including Iran. Prevalence of fascioliasis in sheep and goat is reported 0.1–32% and 0.2–64.3%, respectively throughout Iran (The highest and lowest prevalence rate of fascioliasis is reported from Guilan and Semnan provinces, respectively). In the current study, the prevalence of fascioliasis in sheep (0.95%) and goat (2.02%) were lower than the mean (19% for sheep and 11.50 for goat) in Iran (Ashrafi 2015; Tavakoli et al. 2008). In Iraq (Hosseini et al. 2018), fascioliasis was 0.72% for sheep and 3.30% of the goats were similar to the current study and remind that the pattern of infection in the different region followed a similar trend.
Dicrocoeliasis, a zoonotic parasitic infection is another cause of liver condemnation in slaughterhouses. In the current study, the overall prevalence of this infection was 2.17% and it showed in slaughtered sheep and goat 2.0% and 3.41%, respectively. The prevalence of dicrocoeliasis in this study was in line with previous studies in Iran. The relative prevalence of dicrocoeliasis was 3.1% (2.2–4.2) in sheep and 1.3% (0.9–1.9) in goats throughout the country (Bari et al. 2016; Khanjari et al. 2014; Majidi-Rad et al. 2018). However, the prevalence of 20% for dicrocoeliasis is reported from slaughtered sheep in Tabriz Province (Ghazani et al. 2008).
For decades, due to control programs in Iran, the prevalence of parasitic infections faced a declining trend (Ahmadi and Meshkehkar 2010; Hosseini et al. 2018).
The results of the current study showed an annual decline of 5.0% and 2.0% for fascioliasis and dicrocoeliasis, respectively. In spite of expected declining also in the annual trend of CE, but this was shown obviously an increasing trend from 2008 to 2018. This increasing trend could be alarming for the authorities on this important zoonosis infection. Recent studies in Iran showed, in spite of some declining in the rate of fascioliasis and dicrocoeliasis in both human and animal, the rate of CE remains quite high and even increased in the country (Hamzavi et al. 2011; Khalkhali et al. 2018). Although, the prevalence rate of fascioliasis and dicrocoeliasis was higher in January and December, it was without any statistically significant differences. Due to the chronicity of CE and long life of Fasciola spp. and Dicrocoelium spp. in their hosts, higher prevalence of them in some months or seasons could not be an important epidemiological index. This paternal effect on the prevalence of CE in sheep is in line with a study conducted in Kermanshah (Hashemnia and Safavi 2016) but was in contrast about goat prevalence as they revealed the highest rate in summer. This difference can be attributed to variation in climate condition which is specific for any area and also several contributing factors that further study warranted to complete understanding.
The prevalence rate of these parasitic infections is completely related to desire environmental conditions for their life cycles that led to maintain or eliminate these parasites. These conditions are including dog population, livestock exchange, grassland types, soil conditions, dogs access to infected offal, and grazing pattern beside shepherd dogs (Kaewkes 2003; Romig et al. 2017). The results showed humidity is an important metrological index that has a positive relationship with these infections. Higher humidity is more desire for stabilizing their life cycles and more resistance of eggs in the environment. There was a significant negative relationship between temperature and wind speed with the prevalence of these infections. Precipitation has no effect on the prevalence of these infections. These findings are in line with previous studies in Iran (Motazedian et al. 2019).
Economic impacts of condemned organs due to CE are evaluated in a few studies in Iran. In one study in Kermanshah Province, the total economic loss of CE in all slaughtered livestock was US$ 457,582 (Vafaei et al. 2018). In a study to estimate the monetary burden of CE in Iran by Harandi et al. (2012), the indirect and direct annual cost of CE was US$232.3 million and the livestock-associated annual cost was US$132 million. Total economic losses were estimated US$ 1,696,125 in 11 years for one abattoir in the study area which shows a remarkable human resource become useless due to parasitic infections (Harandi et al. 2012).
Economic losses of CE is estimated in other countries. Globally, the monetary burden of CE has estimated an annual loss of US$ 193,529,740 (Budke et al. 2006). Based on the market price in Turkey, the total loss estimated US$ 583 in infected animals (Umur and Kaaden 2003). The total annual economic loss of CE was estimated US$ 5869.8 in Ethiopia (Getaw et al. 2010).
The economic loss of fascioliasis and dicrocoeliasis is estimated in several studies in Iran. Arbabi et al. (2018) showed the annual economic loss due to fascioliasis and dicrocoeliasis 26698.4 and 30479.2 US$, respectively (Arbabi et al. 2018; Borji et al. 2012). These considerable economic losses of the mentioned zoonotic parasitic infections throughout the world indicated the necessity for more implementation control programs, especially in the endemic countries (Alhaji et al. 2017). Undoubtedly, the implementation of prevention and control programs in the hyper endemic areas lead to the reduction of the outbreak and will result in macroeconomic benefit. On the other hand, the use of these massive volumes of financial burden in training programs and the development of infrastructure and improving urban transport will promote health indicators and water resources that will lead to significant results in promotion health status index in these areas.
In developing countries, the life of people lived in the remote resource-poor area is mainly dependent on animal husbandry. These parasitic infections impose directly or indirectly a huge economic loss for these people. Interruption of these parasites life cycle should be more considered in the control programs of the authorities and their governments.
This study had a number of limitations; the most noticeable limitation was having access to the data of a single slaughterhouse. Having no data about sex and age of slaughtered sheep and goat was another important limitation.
Conclusion
The number of condemned organs due to CE, fascioliasis, and dicrocoeliasis in slaughtered sheep and goat is noticeable in Alborz Province, north-central, Iran. The high economic impact of these infections showed the necessity of implementation continuously infected animals trace-back and disease control in the site of infection. Quarantine of suspected livestock, reduce home slaughtering, and interruption of their life cycle should be applied to decrease the frequency of these infections. Finally, research centers should attempt to develop new vaccines and treatment protocol in order to the protection of livestock against these parasites.
Acknowledgements
This study was supported by the Kerman University of Medical Sciences. This study was reviewed and approved by the Ethics Committees of Kerman University of Medical Sciences, Kerman, Iran.
Funding
This work was supported by the Student Research Committee, Kerman University of Medical Sciences, Kerman, Iran (Grant No. 98000394).
Compliance with ethical standards
Conflict of interest
The authors declare that they have no Conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Addis AB. Causes of organ condemnation and economic loss of cattle in developing countries. Review. IJEDR. 2017;5(1):776–796. [Google Scholar]
- Ahmadi NA, Meshkehkar M. Prevalence and long term trend of liver fluke infections in sheep, goats and cattle slaughtered in Khuzestan, southwestern Iran. J Paramed Sci. 2010;1(2):26–31. [Google Scholar]
- Alhaji NB, Yatswako S, Isola TO. A survey of organs/offal condemnations and foetal losses in slaughtered trade cattle at abattoirs in north-central Nigeria: major causes and associated economic implications. Bull Anim Health Prod Africa. 2017;65(1):81–93. [Google Scholar]
- Allwin B, Balakrishnan S, Kumar VN, Jayathangaraj MG, Vedamanickam S, Gopal S. Prevalence of gastrointestinal parasites in gaur (Bos gaurus) and domestic cattle at interface zones of the Nilgiri Hills, Tamil Nadu. India. J Vet Sci Technol. 2015;7:280. doi: 10.4172/2157-7579.1000280. [DOI] [Google Scholar]
- Arbabi M, Nezami E, Hooshyar H, Delavari M. Epidemiology and economic loss of fasciolosis and dicrocoeliosis in Arak, Iran. Vet World. 2018;11:1648–1655. doi: 10.14202/vetworld.2018.1648-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafi K. The status of human and animal fascioliasis in Iran: a narrative review article. Iran J Parasitol. 2015;10(3):306. [PMC free article] [PubMed] [Google Scholar]
- Bari S, Sarvi S, Daryani A, Hezarjaribi HZ, Arbabi M, Pirestani M, Mizani A. Dicrocoelium dentriticum infection among domestic animals in Iran: a systematic review and meta-analysis. J Mazandaran Univ Med Sci. 2016;25(132):367–375. [Google Scholar]
- Blagojevic B, Antic D. Assessment of potential contribution of official meat inspection and abattoir process hygiene to biological safety assurance of final beef and pork carcasses. Food Control. 2014;36(1):174–182. doi: 10.1016/j.foodcont.2013.08.018. [DOI] [Google Scholar]
- Borji H, Azizzadeh M, Afsai A. An abattoir-based study on the prevalence of hydatidosis in livestock in Mashhad, Iran. J Helminthol. 2012;86(2):233–236. doi: 10.1017/S0022149X11000228. [DOI] [PubMed] [Google Scholar]
- Budke CM, Deplazes P, Torgerson PR. Global socioeconomic impact of cystic echinococcosis. Emerg Infect Dis. 2006;12(2):296–303. doi: 10.3201/eid1202.050499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalimi A, Motamedi GH, Hosseini M, Mohammadian B, Malaki H, Ghamari Z, Far FG. Echinococcosis/hydatidosis in western Iran. Vet Parasitol. 2002;105(2):161–171. doi: 10.1016/S0304-4017(02)00005-5. [DOI] [PubMed] [Google Scholar]
- Drolet R, D’Allaire S, Larochelle R, Magar R, Ribotta M, Higgins R. Infectious agents identified in pigs with multifocal interstitial nephritis at slaughter. Vet Rec. 2002;150(5):139–142. doi: 10.1136/vr.150.5.139. [DOI] [PubMed] [Google Scholar]
- Getaw A, Beyene D, Ayana D, Megersa B, Abunna F. Hydatidosis: prevalence and its economic importance in ruminants slaughtered at Adama municipal abattoir, Central Oromia, Ethiopia. Acta Tropica. 2010;113(3):221–225. doi: 10.1016/j.actatropica.2009.10.019. [DOI] [PubMed] [Google Scholar]
- Ghazani MHM, Valilou MR, Ahmadzadeh AR, Karami AR, Zirak K. The prevalence of sheep liver trematodes in the northwest region of Iran. Turk J Vet Anim Sci. 2008;32(4):305–307. [Google Scholar]
- Gulland FMD. The role of nematode parasites in Soay sheep (Ovis aries L.) mortality during a population crash. Parasitology. 1992;105(3):493–503. doi: 10.1017/S0031182000074679. [DOI] [PubMed] [Google Scholar]
- Hajimohammadi B, Oryan A, Zohourtabar A, Ardian M, Shokuhifar M. Rate of carcass and offal condemnation in animals slaughtered at Yazd Slaughterhouse, central Iran. Asian Pacific J Trop Biomed. 2014;4(9):736–739. doi: 10.12980/APJTB.4.2014C1201. [DOI] [Google Scholar]
- Hamzavi Y, Vejdani M, Nazari N, Mikaeili A. The trend of hydatidosis in kermanshah province, Western iran (1986–2008) Iran J Parasitol. 2011;6(4):33. [PMC free article] [PubMed] [Google Scholar]
- Harandi MF, Budke CM, Rostami S, Fasihi Harandi M, Budke CM, Rostami S. The monetary burden of cystic echinococcosis in Iran. PLOS Negl Trop Dis. 2012;6(11):e1915. doi: 10.1371/journal.pntd.0001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashemnia M, Safavi EAA. A retrospective survey of hydatidosis based on abattoir data in Kermanshah, Iran from 2008 to 2013. J Parasit Dis. 2016;40(2):459–463. doi: 10.1007/s12639-014-0526-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini SH, Fathi S, Jalousian F. Helminths of veterinary and public health importance in Iran: the great neglected parasitic diseases as important challenge for the country. Iran J Vet Med. 2018;12:2–7. [Google Scholar]
- Irani Z, Sharif AM. Sustainable food security futures: perspectives on food waste and information across the food supply chain. J Enterp Inf Manag. 2016;29(2):171–178. doi: 10.1108/JEIM-12-2015-0117. [DOI] [Google Scholar]
- Jahed Khaniki GR, Kia EB, Raei M. Liver condemnation and economic losses due to parasitic infections in slaughtered animals in Iran. J Parasit Dis. 2013;37(2):240–244. doi: 10.1007/s12639-012-0172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaja IF, Mushonga B, Green E, Muchenje V. Factors responsible for the post-slaughter loss of carcass and offal’s in abattoirs in South Africa. Acta Trop. 2018;178(2):303–310. doi: 10.1016/j.actatropica.2017.12.007. [DOI] [PubMed] [Google Scholar]
- Kadim IT, Farouk MM, Mahgoub O, Bekhit AE. Slaughtering and processing of camels. Camel meat and meat products. Wallingford: CAB International; 2013. pp. 54–72. [Google Scholar]
- Kaewkes S. Taxonomy and biology of liver flukes. Acta Trop. 2003;88(3):177–186. doi: 10.1016/j.actatropica.2003.05.001. [DOI] [PubMed] [Google Scholar]
- Khalkhali HR, Foroutan M, Khademvatan S, Majidiani H, Aryamand S, Khezri P, Aminpour A. Prevalence of cystic echinococcosis in Iran: a systematic review and meta-analysis. J Helminthol. 2018;92(3):260–268. doi: 10.1017/S0022149X17000463. [DOI] [PubMed] [Google Scholar]
- Khanjari A, Bahonar A, Fallah S, Bagheri M, Alizadeh A, Fallah M, Khanjari Z. Prevalence of fasciolosis and dicrocoeliosis in slaughtered sheep and goats in Amol Abattoir, Mazandaran, northern Iran. Asian Pacific J Trop Dis. 2014;4(2):120–124. doi: 10.1016/S2222-1808(14)60327-3. [DOI] [Google Scholar]
- Majidi-Rad M, Meshgi B, Bokaie S. The prevalence and intensity rate of Dicrocoelium dendriticum infection in ruminants of 3 provinces in coastal regions of the Caspian Sea. Iran J Vet Med. 2018;12(1):27–33. [Google Scholar]
- Milios KT, Drosinos EH, Zoiopoulos PE. Food Safety Management System validation and verification in meat industry: carcass sampling methods for microbiological hygiene criteria–A review. Food Control. 2014;43(2):74–81. doi: 10.1016/j.foodcont.2014.02.041. [DOI] [Google Scholar]
- Motazedian M, Najjari M, Zarean M, Karimi G, Karimazar M, Ebrahimipour M. An abattoir survey of hydatid and liver fluke disease in slaughtered cattle in Alborz Province, Iran. Comp Clin Pathol. 2019;28(1):99–105. doi: 10.1007/s00580-018-2800-8. [DOI] [Google Scholar]
- Romig T, Deplazes P, Jenkins D, Giraudoux P, Massolo A, Craig PS, Wassermann M, Takahashi K, De La Rue M (2017). Ecology and life cycle patterns of Echinococcus species. In: Advances in parasitology, vol 95. Elsevier, pp 213–314. https://www.mendeley.com/catalogue/epidemiology-economic-loss-fasciolosis-dicrocoeliosis-arak-iran/ [DOI] [PubMed]
- Sarwar MH, Sarwar MF, Khalid MT, Sarwar M. Effects of eating the balance food and diet to protect human health and prevent diseases. Am J Circuits Syst Signal Process. 2015;1(3):99–104. [Google Scholar]
- Tavakoli HR, Mahmoodzadeh A, Hajia M. A five years study of fascioliasis and dicrocoeliasis in Iran’s slaughterhouses. Asian Pacific J Trop Med. 2008;1(4):9–13. [Google Scholar]
- Tilman D, Clark M. Global diets link environmental sustainability and human health. Nature. 2014;515(7528):518. doi: 10.1038/nature13959. [DOI] [PubMed] [Google Scholar]
- Umur S, Kaaden O. Prevalence and economic importance of cystic echinococcosis in slaughtered ruminants in Burdur, Turkey 1. J Vet Med Ser B. 2003;50(5):247–252. doi: 10.1046/j.1439-0450.2003.00667.x. [DOI] [PubMed] [Google Scholar]
- Vafaei MR, Faridnia R, Mostafaei S, Azadi Y, Damod V, Fakhar M, Kalani H. Estimation of economic impacts due to hydatidosis among livestok in Kermanshah Province, western Iran. Comp Clin Pathol. 2018;27(2):393–398. doi: 10.1007/s00580-017-2604-2. [DOI] [Google Scholar]
- Wheatley P, Giotis ES, McKevitt AI. Effects of slaughtering operations on carcass contamination in an Irish pork production plant. Ir Vet J. 2014;67(1):1. doi: 10.1186/2046-0481-67-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziaei H, Fakhar M, Armat S. Epidemiological aspects of cystic echinococcosis in slaughtered herbivores in Sari abattoir, North of Iran. J Parasit Dis. 2011;35(2):215–218. doi: 10.1007/s12639-011-0051-6. [DOI] [PMC free article] [PubMed] [Google Scholar]