Abstract
Sarcocystis fusiformis is a coccidian tissue parasite that causes infection in buffalo in countries such an Egypt, China, Iraq and Iran, resulting in significant economic losses to the agricultural industry annually. There is a lack of studies examining host-parasite interactions at the level of the immune response and the present study investigates the interaction between S. fusiformis whole cyst antigens (SFWCA) and dendritic cells (DCs), cells critical to the activation of adaptive immunity. In this study bone marrow derived DCs (BMDCs) were phenotyped following treatment with SFWCA by measuring cell viability, cytokine secretion, and cell surface marker expression. While SFWCA exhibited cytotoxic effects on BMDCs at higher concentrations, lower concentrations of SFWCA activated pro-inflammatory DCs that significantly secreted interleukin (IL)-12p40, tumor necrosis factor alpha, IL-6 and IL-10. These cells also displayed enhanced expression of TLR4, CD80, CD86 and MHC II on their surface, which is indicative of full DCs maturation. Moreover, SFWCA significantly attenuated the capacity of BMDCs to suppress Th2 associated cytokines, notably IL-5 and IL-13, while simultaneously exhibiting no effects on the secretion of interferon (IFN)-γ, IL-2, IL-17, and IL-10. In conclusion, this is the first study to provide fundamental insight into the activation of DCs by SFWCA, providing us with some awareness into the interaction of the Sarcosystis parasite with its host. The pro-inflammatory inducing ability of this antigen is in keeping with studies performed in other protozoan parasites and therefore understanding these interactions is important in the development of future therapeutic strategies.
Keywords: Sarcocystis fusiformis, Dendritic cells, IL-12p40, Pro-inflammatory cytokine, Co-stimulatory marker
Introduction
The Sarcocystis species are a group of apicomplexan intracellular coccidian parasites with an obligatory two-host life cycle that causes infection in humans and livestock globally (Poulsen and Stensvold 2014; Mirzaei and Rezaei 2016; Dong et al. 2018). There are four species of Sarcosystis that infect buffalo, its intermediate host (Sarcocystis (S) fusiformis, S. buffalonis, S. levinei and S. dubeyi) in countries such as Iran, Iraq, China and Egypt. The intermediate host is infected through the ingestion of plants contaminated with oocyst or sporocyst, which then encyst in muscle and neurological tissue to form Sarcocystis. In Egypt, the highest incidence of infection is caused by S. fusiformis (Dubey et al. 2014; Huong and Uggla 1999; Morsy et al. 2018). Studies have reported the incidence as high as 89% in the Giza (Ghoneim et al. 2014), 78.9% in Beni-Suef (El-Dakhly et al. 2011) and in Assiut the highest rate was recorded at 94.4% (Metwally et al. 2014) making it an important parasitic disease of buffalo. Buffalos are a vital part of the Egyptian economy as it is an important source of leather, meat and milk in local communities. While the disease is normally asymptomatic, the parasite can cause spontaneous abortion, neurological symptoms, reduced milk production, and spoilt meat due to macroscopic cysts. The annual economic impact of the disease in Egypt remains to be calculated but it is thought to cause significant losses to the agricultural industry annually (Badawy et al. 2012; Sayed et al. 2008).
While much is known about the biology of the parasite in the intermediate host (Dubey et al. 2015), there is a dearth of knowledge on host-parasite interactions at the level of the immune response. However, studies have demonstrated that S. calchasi infection is associated with Th1 immune response at the schizogonic phase, which may facilitate evasion and suppression of host inflammatory response (Olias et al. 2013). Humans infection with S. nesbitti in the initial phase of infection express low levels of IL-12, IFNγ, RANTES, MCP-1 and IL12p70 while higher cytokines levels were detected in Phase II of infection (Tappe et al. 2015). Phase I may point towards a decrease in T cell function and DC activation and recruitment as seen in other studies (Sallusto et al. 1998).
DCs are antigen-presenting cells (APCs) that act as a mediator for the innate and adaptive immune response. Immature DCs are activated by foreign pathogens through Toll-like receptors (TLRs) which act as pattern recognition receptors (Kapsenberg 2003). TLRs, therefore, initiate the maturation of DCs through the recognition of endogenous and exogenous stimuli (Sheen et al. 2017). DCs are programmed to respond to certain activating stimuli, such as lipopolysaccharide (LPS), a major component of the outer membrane of gram-negative bacteria that binds specifically to TLR4 causing activation of DCs. Activated DCs secrete pro-inflammatory cytokines and have enhanced expression of major histocompatibility complex (MHC) class II, CD40, CD80 and CD86 (Banchereau and Steinman 1998), stimulatory factors that are important for the initiation and propagation of T cell responses (Janeway and Medzhitov 2002). Understanding the effect of S. fusiformis on DCs will give us some insight to host-parasite interactions that in the future open new avenues to the development of potential therapeutic drugs and vaccine candidates against the disease. In this study, we examined the immuno-stimulatory effect S. fusiformis whole cyst antigen (SFWCA) exhibits on DC maturation, cytokine secretion and capacity to elicit T-cell responses.
Materials and methods
Animals and ethical approval
Mice aged 6–8 weeks were purchased from Charles River Ltd (Kent, UK) and kept under specific pathogen free conditions at Dublin City University (DCU). All mice were housed according to the Health Products Regulatory Authority guidelines with strict adherence to standard operating procedure approved by the institutional Animal Welfare Body. Ethical permission for the use of animals was approved by the Health Products Regulatory Authority and DCU ethics committee (license number DCUREC/2010/033). All procedures involving animals were only performed by licensed personnel.
Reagents and materials
Lipopolysaccharide (LPS) from E. coli (serotype R515) was purchased from Enzo Life Sciences (Exeter, UK). All antibodies used in this investigation were obtained from eBiosciences (Hatfield, UK; CD86 (FITC) monoclonal antibody (24F), CD80 (PE) monoclonal antibody (3H5), MHCII (FITC) monoclonal antibody (MS/114.15.2) and TLR4 (PE) monoclonal antibody (UT41) or the relative isotype control. Granulocyte–macrophage colony-stimulating factor (GM‐CSF) was obtained from Sigma Aldrich. Cell culture materials were purchased from Biosciences (Dun Laoghaire, Ireland).
Parasite collection and antigen preparation
The macroscopic form of S. fusiformis was collected from fresh tissue samples dissected from the esophagus and skeletal muscles of infected buffalos and washed three times with sterile phosphate buffer saline (PBS) pH 7.2 to remove the attached skeletal tissue. The cysts were macerated using a sterile scalpel until a cream-colored suspension was formed. The suspension was then homogenized in PBS pH 7.2 using a manual glass homogenizer followed by centrifugation for 10 min at 876 g and 4 °C (Morsy et al. 1994). The resulting S. fusiformis whole cyst antigen (SFWCA) was collected in aliquots and stored at − 20 °C. Protein concentration was measured using the lowery method (Lowry et al. 1951).
Isolation and culture of bone marrow derived dendritic cells
BMDCs obtained from balb/c mice, were isolated aseptically in a Class II Laminar cabinet (ThermoElectron Corporation, USA). Bone marrow cells were extracted from the tibia and femurs of each mouse by flushing the bone cavity with sterile RPMI using a sterile 25.7 g needle and syringe. The cells were pelleted by centrifuging for 5 min at 500 g and re-suspended in 10 mL of culture medium (RPMI (Gibco, UK) supplemented with 20% heat-inactivated fetal bovine serum (FBS), 100 μg/ml penicillin/streptomycin (Invitrogen, UK), l-glutamine, and 50 ng/mL granulocyte monocyte-colony stimulating factor (GM-CSF) (Sigma Aldrich, Ireland). Cells were transferred to a petri dish and cultured in a 37 °C in a CO2 incubator. On days 3 and 6, 6 mL of media was gently removed from the petri dish and the media replenished with 10 mL of pre-warmed culture medium. On day 10 adherent cells were dislodged from the surface using a cell scraper (Sarstedt, Ireland) and then centrifuged at 500 g for 5 min prior to re-suspension in fresh media. Cell counting was performed using the trypan blue exclusion method. Harvested BMDCs were analyzed by flow cytometry and only cell preparations with a population identified as > 95% CD11C (Biolegend, No. 117317) positive were used for each experiment.
Cell proliferation assay
CellTiter 96® Aqueous One Solution (Pierce, UK) test was used to investigate the cytotoxic effects of SWFCA on BMDCs in vitro. BMDCs were plated in a 96-well plate (Nunc™, Ireland) with 100 μL cell suspension per well at a concentration of 1 × 106 cells/mL. SFWCA was added at increasing concentrations (10–10,000 ng/mL) or cells were plated with media, LPS (100 ng/mL) or DMSO (10% v/v). Cells were incubated overnight in a 37 °C in a CO2 incubator and then 20 μL of the CellTiter 96® Aqueous One solution added to each well. After 4 h. the colour change in the media was measured using a TECAN 96 well plate reader at 490 nm (Tecan, Männedorf, Switzerland). The cell viability of each sample was calculated by setting the absorbance value for the cells treated with media alone as a reference point and then the percentage change in absorbance for each sample was calculated.
Cytokine ELISA
BMDCS (1 × 106 cells/mL) were treated with SFWCA (10 and 100 ng/mL), LPS (100 ng/mL) and incubated for 18 h at 37 °C in a CO2 incubator. Supernatants were subsequently removed and cytokine release (IL-12p40, than, IL-6 and IL-10) measured using commercial ELISA kits in accordance with the manufacturer’s instructions (Invitrogen, UK). Each sample and standard was assayed in triplicate.
Flow cytometry
BMDCS (1 × 106 cells/mL) were treated with SFWCA (10 and 100 ng/mL), LPS (100 ng/mL) or media and then incubated at 37 °C in a CO2 incubator for 24 h. Cells were removed from the tissue culture plates and placed into a 96-well round bottom plate at a concentration of 400,000 cells/well. An equal amount of FACS Buffer (PBS suppplemented with 2% FBS was then added, to block non-specific binding for 15 min at room temperature (RT). Cells were then washed three times with FACS buffer and 1 mM EDTA (Sigma-Aldrich)) and incubated with appropriate fluorochrome-conjugated antibodies (BD Biosciences, UK) for 30 min at 4 °C whilst protected from light. Cells were subsequently washed three times to remove any unbound antibodies and analyzed using a FACSAria I (BD Biosciences, UK). All the flow cytometry data were analyzed using FlowJo software (Treestar, UK).
T-cell co-culture
Spleens from balb/c mice were harvested and splenocytes obtained by passaging of the spleen through a 40 μm filter (Sarstedt, Nümbrecht, Germany) using the plunger from a sterile 1 mL syringe (Sarstedt, Nümbrecht, Germany). CD4+ T-cells were isolated from the splenocytes using a negative selection CD4+ isolation kit (Stem cell, Vancouver, Canada) and were only used if the purity were determined to be > 95% positive for CD4+ cells by flow cytometry. Pre-stimulated BMDCs that were stimulated with 10 ng/ml SFWCA for 24 h, were washed in PBS three times and co-cultured with CD4+ T-cells at a ratio of 1:10 in culture medium, on 24 well plates (Sarstedt, Nümbrecht, Germany) that had been pre-coated overnight with anti-CD3 (1 µg/mL) (R&D systems, Minneapolis, Minnesota, USA). Supernatants were harvested after 72 h and analyzed for cytokine release (IL-5, IL-13, IFNγ, IL-2, IL-17 and IL-10) using commercial ELISA kits in accordance with the manufacturer’s instructions (Invitrogen, UK). Each sample and standard was assayed in triplicate.
Statistical analysis
Data are expressed as mean ± standard error of the mean (SEM). Statistical analyses were performed using one-way ANOVA. The statistical significance level was p < 0.05. Statistics were computed using GraphPad Prism software.
Results
Sarcocystis fusiformis whole cyst antigen at higher concentrations displays cytotoxic effects on DCs in vitro
Currently little is known about the modulatory effects of Sarcocystis on the overall immune system, much less the individual cells which are involved in the immune response such as DCs. Prior to phenotyping BMDCs treated with SFWCA we investigated its impact on cell viability using an MTS Assay. At lower concentrations (10 and 100 ng/mL) SFWCA exhibited no significant effect on the cell viability of DCs. However, at higher concentrations it significantly reduced cell viability (500 ng/mL (p < 0.05) or cell viability was comparable to cells treated with DMSO (1000 ng/mL and 10,000 ng/mL; Fig. 1). It is clear from these results that SFWCA at higher concentrations displays cytotoxic effects on DCs in vitro.
Fig. 1.

Sarcocystis fusiformis whole cyst antigen at high concentrations affects cell viability using MTS assay. Data was expressed as a percentage compared to LPS stimulated cells. Error bars represent mean 3 ± SEM
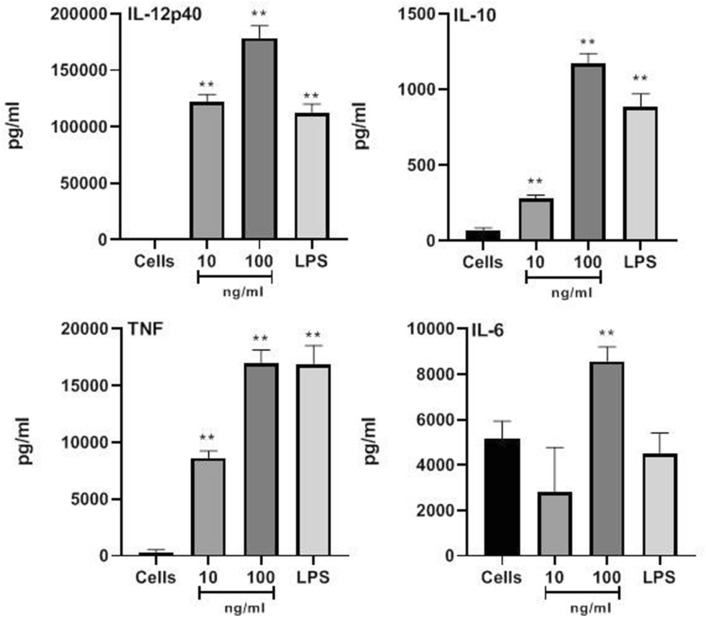
Sarcocystis fusiformis whole cyst antigen stimulates dendritic cells to produce a panel of pro-inflammatory cytokines
BMDCS treated with SFWCA (10 and 100 ng/mL) were phenotyped by measuring cytokine release after 18 h. using commercial ELISA, the gold standard method for detecting the quantities of cytokines secreted by cells. At 100 ng/mL, SFWCA induced the secretion of significant levels of the regulatory cytokine IL-10 (p < 0.01) and the Th1 inducing cytokines IL-12p40, TNFα and IL-6 (p < 0.01; Fig. 2). At 10 ng/mL, SFWCA significantly increased the secretion of IL-12p40, IL-10 and TNF-α (p < 0.01) whilst levels of IL-6 remained similar to non-stimulated cells.
Fig. 2.
Dendritic cells stimulated to produce a panel of pro-inflammatory cytokines by S. fusiformis whole cyst antigen. The cytokines IL-12p40, IL-10, TNFα and IL-6 was measured by ELISA. Statistical significance is indicated *p ≤ 0.05, and **p ≤ 0.01 compared to untreated cells
Sarcocystis fusiformis whole cyst antigen induces dendritic cells to express co-stimulatory markers important for T-cell activation
The impact of SFWCA on BMDC phenotype was also analyzed using flow cytometry by measuring MHC class II, an antigen-presenting molecule; TLR4, a cell surface molecule that plays a major role in the initiation of DCs, and the co-stimulatory molecules CD80 and CD86 that are required for the activation of T-cells by DCs. In the presence of SWFCA BMDCs expressed significantly, higher levels of MHCII (p < 0.01), TLR4 (10 ng/mL, p < 0.01); 100 ng/mL (p < 0.01), CD80 (p < 0.01) and CD86 (p < 0.01) (Fig. 3).
Fig. 3.
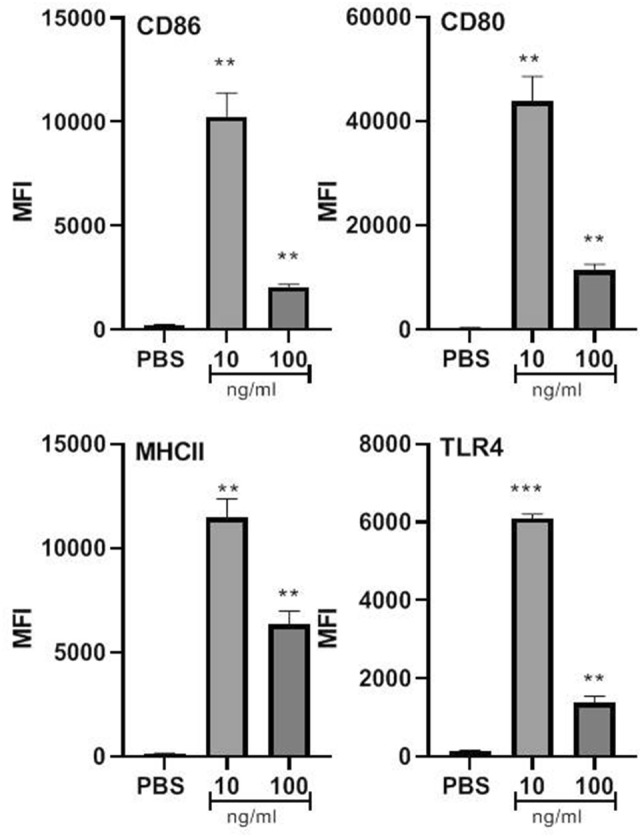
Sarcocystis fusiformis whole cyst antigen induces dendritic cells to express co-stimulatory markers important in T-cell activation. Cells were analyzed using a FACsAria. Statistical significance is indicated *p ≤ 0.05; **p ≤ 0.01 and ***p < 0.001, compared to untreated cells
Dendritic cells stimulated with S. fusiformis whole cyst antigen suppress Th2 cytokines
Considering that SFWCA can activate BMDCs by inducing cytokine secretion and enhancing the expression of co stimulatory markers, we investigated the impact these cells had on the balance between Th1, Th2 and on the inflammatory process, by examining their interaction and priming of T-cells. BMDCs stimulated with SFWCA induced significantly less IL-5 (Fig. 4*, p ≤ 0.05) and IL-13 (Fig. 4**, p ≤ 0.05) compared to control BMDCs cultured with PBS. However, no significant differences in the levels of IFNγ, IL-2, IL-17 and IL-10 were observed (Fig. 4).
Fig. 4.
Dendritic cells stimulated with S. fusiformis whole cyst antigen suppress Th2 cytokines when co-cultured with T-cells. The cytokines IFN-γ, IL-10, IL-17, IL-5, IL-13 and IL-2 was measured by ELISA. Statistical significance is indicated*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001 compared to untreated cells
Discussion
Sarcocystis fusiformis is an important veterinary parasite in Egypt causing significant loses to the agricultural community annually. It is therefore imperative that we understand its host-parasite interactions and in this study, the activation of BMDCs by SFWCA was examined. SFWCA induced a DC population that secreted a panel of cytokines indicative of a pro-inflammatory response in DCs, with cytokines associated with the induction of Th1 responses namely IL-12 (Hamza et al. 2010). It also induced the classical activation/maturation of DC characterized by the up regulation of co-stimulatory markers, CD80, CD86 and MHCII that are critical in the activation of Th1 CD4+ T cells.
In keeping with published literature, studies on Apicomplexan species closely related to Sarcosystis spp. were also shown to activate DCs in vitro, upregulating maturation markers and inducing the secretion of pro-inflammatory cytokines. Leishmania parasites activate human DCs in vitro to secrete IL-12p70, and IL-10 (McDowell et al. (2002) while infection with Neospora caninum enhanced the expression of the DC maturation markers CD80, CD86, CD40 and MHC class II (Teixeira et al. 2010). Similarly, parasite antigens can mimic the immune response observed for whole live parasites as Plasmodium-derived haemozoin induced DCs in vitro to secrete TNF, IL-12 and IL-6 (Coban et al. 2005) while Cryptosporidium parvum antigens induced DCs to secrete IL-12 p70 in vitro (Bedi and Mead 2012). Toxoplasma gondii isolated antigens activate DCs in vitro to produce CD40 and IL-12p70 (Schulz et al. 2000) where the production of IL-12 is thought to be dependent upon the expression of the CD40 co-stimulatory molecule (Reichmann et al. 2000). T. gondii is a powerful inducer of DC-induced IL-12 dependent Th1 in vitro where TLR has an important role in the activation of DCs (Sanecka and Frickel 2012).
Generally, the response against the apicomplexa species is mainly associated with Th1 immune responses that are important in controlling parasite replication during infection (Gazzinelli et al. 1993; Hayashi et al. 1996). Suppression of the immune response, especially inflammatory responses, prolongs the parasite survival within the host and thus increases its chances of completing the life cycle (Manicassamy and Pulendran 2011). In apicomplexan infection where Th1 immune responses are protective, pathogens have developed mechanisms to suppress protective immune responses,for example, T. gondii suppresses DC activation, as these cells were unable to secrete IL-12 and IFN-γ, or activate T-helper lymphocytes (McKee et al. 2004). Similarly, Plasmodium falciparum can inhibit DCs maturation (Bettiol et al. 2010). However, SWFCA does not exhibit these properties.
DCs have an important role in the induction of Th1-dependent immune responses that are thought to be protective against Sarcosystis infection (Olias et al. 2013; Spencer et al. 2005). A recent study by El Shanawany et al. (2019) demonstrated the reduced circulation of IFN-γ and enhanced levels of IL-5 in buffaloes infected with S. fusiformis compared to uninfected controls. Infected animals also expressed higher levels of circulating IgE which coincided with the infiltration of eosinophils and lymphocytes in infected tissue. There is a dearth of studies examining natural infection in the definitive host, however in vivo, experimental infection with S. neurona and S. calchasi suggest a suppression of the IFN-γ signaling pathway (Spencer et al. 2005; Olias et al. 2013). However, SWFCA did not suppress DC activation, but induced a mature pro-inflammatory DC phenotype that preferentially elicited Th1/Th17 responses, immune responses associated with S. fusiformis clearance (Gazzinelli et al. 1993; Hayashi et al. 1996; Manicassamy and Pulendran 2011). There is some supporting evidence which may explain the dichotomy observed between our findings and the reports from El Shanawany et al. in which a study reported biphasic immune responses in infected humans, with a Th1 biased response being observed during the initial phase of infection, while during the muscle stage a Th2 response was observed which correlate with hyper-eosinophilia in invasive muscle tissue (Tappe et al. 2015).
In conclusion, this is the first study to investigate the interaction between SFWCA and DCs. SFWCA was shown to induce a mature DCs phenotype which exhibited enhanced co-stimulatory marker expression and produced pro-inflammatory Th1 associated cytokines. These DCs primed T-cells response toward Th1/Th17, the main protective response against pathogens. However the response induced by SFWCA does not mimic the reported reposes observed during the later stages of natural infection in buffalo and therefore would merit further studies as SFWCA could potentially make a good vaccine candidate given that Th1 immune responses are required for protective immunity. Further studies are required in order to fully understand the relationship between host and parasite and without doubt, a full understanding of the mechanism important to protective immunity will assist us in the development of rational approaches to control disease.
Acknowledgements
The authors are grateful to the Erasmus Staff mobility for Teaching and training assignments To/From Partner Countries for supporting this work.
Compliance with ethical standard
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical permission for the use of animals was approved by the Department of Health or Health Products Regulatory Authority and Dublin City University ethics committee (License Numbers B100/2833, DCUREC/2010/033).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Badawy AII, Abouzaid NZ, Ahmed HA. Sarcocystis hominis and other Sarcocystis species infecting cattle at Sharkia Province, Egypt. Egypt J Am Sci. 2012;8:271–275. [Google Scholar]
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Bedi B, Mead JR. Cryptosporidium parvum antigens induce mouse and human dendritic cells to generate Th1-enhancing cytokines. Parasite Immunol. 2012;34:473–485. doi: 10.1111/j.1365-3024.2012.01382.x. [DOI] [PubMed] [Google Scholar]
- Bettiol E, Carapau D, Galan-Rodriguez C, Ocaña-Morgner C, Rodriguez A. Dual effect of Plasmodium-infected erythrocytes on dendritic cell maturation. Malar J. 2010;9:64. doi: 10.1186/1475-2875-9-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coban C, Ishii KJ, Kawai T, Hemmi H, Sato S, Uematsu S, Yamamoto M, Takeuchi O, Itagaki S, Kumar N, Horii T, Akira S. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. J Exp Med. 2005;201:19–25. doi: 10.1084/jem.20041836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Su R, Wang Y, Tong Z, Zhang L, Yang Y, Hu J. Sarcocystis species in wild and domestic sheep (Ovis ammon and Ovis aries) from China. BMC Vet Res. 2018;14:377. doi: 10.1186/s12917-018-1712-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey JP, Fayer R, Rosenthal BM, Calero-Bernal R, Uggla A. Identity of Sarcocystis species of the water buffalo (Bubalus bubalis) and cattle (Bos taurus) and the suppression of Sarcocystis sinensis as a nomen nudum. Vet Parasitol. 2014;205:1–6. doi: 10.1016/j.vetpar.2014.06.020. [DOI] [PubMed] [Google Scholar]
- Dubey JP, Calero-Bernal R, Rosenthal BM, Speer CA, Fayer R. Sarcocystosis of animals and humans. Boca Raton: CRC Press; 2015. pp. 1–481. [Google Scholar]
- El Shanawany EE, Nassar SA, Ata EB. Detection of humoral and cellular immune responses in buffaloes naturally infected with sarcocystosis with risk factor assessment. Acta Vet. 2019;69:275–289. doi: 10.2478/acve-2019-0023. [DOI] [Google Scholar]
- El-Dakhly KM, El-Nesr KA, El-Nahass E-S, Hirata A, Sakai H, Yanai T. Prevalence and distribution patterns of Sarcocystis spp. in buffaloes in Beni-Suef, Egypt. Trop Anim Health Prod. 2011;43:1549–1554. doi: 10.1007/s11250-011-9840-2. [DOI] [PubMed] [Google Scholar]
- Gazzinelli RT, Eltoum I, Wynn TA, Sher A. Acute cerebral toxoplasmosis is induced by in vivo neutralization of TNF-alpha and correlates with the down-regulated expression of inducible nitric oxide synthase and other markers of macrophage activation. J Immunol. 1993;151:3672–3681. [PubMed] [Google Scholar]
- Ghoneim NH, Reda WMW, Nader MS. Occurrence of Zoonotic Sarcosporidiosis in slaughtered Cattle and Buffaloes in different abattoirs in Egypt. Glob Vet. 2014;13:809–813. doi: 10.5829/idosi.gv.2014.13.05.86211. [DOI] [Google Scholar]
- Hamza T, Barnett JB, Li B. Interleukin 12 a key immunoregulatory cytokine in infection applications. Int J Mol. 2010;11:789–806. doi: 10.3390/ijms11030789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi S, Chan CC, Gazzinelli R, Roberge FG. Contribution of nitric oxide to the host parasite equilibrium in toxoplasmosis. J Immunol. 1996;156:1476–1481. [PubMed] [Google Scholar]
- Huong LT, Uggla A. Sarcocystis dubeyi n. sp. (Protozoa: sarcocystidae) in the water buffalo (Bubalus bubalis) J Parasitol. 1999;85:102–104. doi: 10.2307/3285709. [DOI] [PubMed] [Google Scholar]
- Janeway CA, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immuno. [DOI] [PubMed] [Google Scholar]
- Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984. doi: 10.1038/nri1246. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Estimation of protein with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Manicassamy S, Pulendran B. Dendritic cell control of tolerogenic responses. Immunol Rev. 2011;241:206–227. doi: 10.1111/j.1600-065X.2011.01015.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell MA, Marovich M, Lira R, Braun M, Sacks D. Leishmania priming of human dendritic cells for CD40 ligand-induced interleukin-12p70 secretion is strain and species dependent. Infect Immun. 2002;70:3994–4001. doi: 10.1128/IAI.70.8.3994-4001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AS, Dzierszinski F, Boes M, Roos DS, Pearce EJ. Functional inactivation of immature dendritic cells by the intracellular parasite Toxoplasma gondii. J Immunol. 2004;173:2632–2640. doi: 10.4049/jimmunol.173.4.2632. [DOI] [PubMed] [Google Scholar]
- Metwally AM, Ellah MRA, AL-Hosary AA, Omar MA. Microscopical and serological studies on Sarcocystis infection with first report of S. cruzi in buffaloes (Bubalus bubalis) in Assiut, Egypt. JOPD. 2014;38:378–382. doi: 10.1007/s12639-013-0257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzaei M, Rezaei H. A survey on Sarcocystis spp. infection in cattle of Tabriz city, Iran. J Parasit Dis. 2016;40:648–651. doi: 10.1007/s12639-014-0551-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morsy T, Abdel MM, Salama MM, Hamdi KN. Assessment of intact Sarcocystis cystozoites as an ELISA antigen. J Egypt Soc Parasitol. 1994;24:85–91. [PubMed] [Google Scholar]
- Morsy K, Abdel-Ghaffar F, Dajem SB, Abdel-Gaber R, El Gazar F. First molecular characterization and morphological aspects of Sarcocystis fusiformis infecting water buffalo Bubalus bubalis in Egypt. Acta Parasitol. 2018;63:333–345. doi: 10.1515/ap-2018-0038. [DOI] [PubMed] [Google Scholar]
- Olias P, Meyer A, Klopfleisch R, Lierz M, Kaspers B, Gruber AD. Modulation of the host Th1 immune response in pigeon protozoal encephalitis caused by Sarcocystis calchasi. Vet Res. 2013;44:10. doi: 10.1186/1297-9716-44-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen CS, Stensvold CR. Current status of epidemiology and diagnosis of human sarcocystosis. JCM. 2014;52:3524–3530. doi: 10.1128/jcm.00955-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichmann G, Walker W, Villegas EN, Craig L, Cai G, Alexander J, Hunter CA. The CD40/CD40 ligand interaction is required for resistance to toxoplasmic encephalitis. Infect Immun. 2000;68:1312–1318. doi: 10.1128/iai.68.3.1312-1318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Schaerli P, Loetscher P, Schaniel C, Lenig D, Mackay CR, Qin S, Lanzavecchia A. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol. 1998;28:2760–2769. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Sanecka A, Frickel EM. Use and abuse of dendritic cells by Toxoplasma gondii. Virulence. 2012;3:678–689. doi: 10.4161/viru.22833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayed FG, Shaheen MSI, Arafa MI, Koraa HM. Sarcocystis infection in cattle at Assiut abattoir: microscopical and serological studies. Ass Univ Bull Environ Res. 2008;11:47–58. [Google Scholar]
- Schulz O, Edwards AD, Schito M, Aliberti J, Manickasingham S, Sher A, Reis e Sousa S. CD40 triggering of heterodimeric IL-12 p70 production by dendritic cells in vivo requires a microbial priming signal. Immunity. 2000;13:453–462. doi: 10.1016/s1074-7613(00)00045-5. [DOI] [PubMed] [Google Scholar]
- Sheen JH, Strainic MG, Liu J, Zhang W, Yi Z, Medof ME, Heeger PS. TLR-induced murine dendritic cell (DC) activation requires DC-intrinsic complement. J Immunol. 2017;199(1):278–291. doi: 10.4049/jimmunol.1700339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer JA, Deinnocentes P, Moyana EM, Guarino AJ, Ellison SE, Bird RC, Blagburn BL. Cytokine gene expression in response to SnSAG1 in horses with equine protozoal myeloencephalitis. Clin Diagn Lab Immunol. 2005;12:644–646. doi: 10.1128/CDLI.12.5.644-646.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tappe D, Slesak G, Pérez-Girón JV, Schäfer J, Langeheinecke A, Just-Nübling G, Muñoz-Fontela C, Püllmann K. Human invasive muscular Sarcocystosis Induces Th2 cytokine polarization and biphasic cytokine changes, based on an investigation among travelers returning from Tioman Island, Malaysia. Clin Vaccine Immunol. 2015;22:674–677. doi: 10.1128/CVI.00042-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira L, Botelho AS, Mesquita SD, Correia A, Cerca F, Costa R, Sampaio P, Castro AG, Vilanova M. Plasmacytoid and conventional dendritic cells are early producers of IL-12 in Neospora caninum-infected mice. Immunol Cell Biol. 2010;88:79–86. doi: 10.1038/icb.2009.65. [DOI] [PubMed] [Google Scholar]