Abstract
BACKGROUND
Despite the fact that about one third of patients with primary localized extremity soft tissue sarcoma (eSTS) will develop metastatic disease, abdominal metastases (AM) and retroperitoneal metastases (RM) constitute rare events. There is no clear consensus on how to achieve follow-up on patients with primary localized eSTS following curative resection, especially regarding the surveillance of potential AM/RM.
AIM
To systematically analyse incidence, diagnosis, treatment and outcome of AM/RM in eSTS patients.
METHODS
In this systematic review, 899 studies available in PubMed and published between 2000 and 2018 were screened, identifying 17 original articles focused on AM or RM in eSTS. Article selection was based on the PRISMA guidelines, using the search terms (abdominal metastasis AND soft tissue sarcoma) and (soft tissue sarcoma metastasis abdomen). All studies published between January 1, 2000 and December 31, 2018 were screened. Further articles were identified by cross-searching article references, with the final search date being February 18, 2019. Due to limited data and the different reporting techniques used, the present review focused on descriptive analysis of the included studies.
RESULTS
Of the 17 studies included, six original articles reported on incidence ± diagnosis, therapy and outcome in AM and RM, whilst three original and eight case reports focused on diagnostic pathway, therapeutic procedures or outcomes without allowing conclusions regarding incidence of AM and RM. According to the former six studies, incidence of AM ranged from 0.9%-5.6% in patients with miscellaneous histological subtypes, and up to 12.1% in patients with myxoid liposarcoma. The most common histological subtypes that developed AM or RM were (myxoid) liposarcoma and leiomyosarcoma, but also rare subtypes such as epithelioid sarcoma, myxofibrosarcoma, synovial sarcoma, and malignant peripheral nerve sheath tumour had been reported to develop AM/RM. Surgery for AM/RM was performed in five of eight case-reports (62.5%) and in 20.8%-100.0% of original articles. In particular, patients with hepatic metastases undergoing metastasectomy had a survival benefit compared to patients treated with chemotherapy or best supportive care (> 3 years vs < 6 mo).
CONCLUSION
Patients with eSTS should undergo surveillance with abdominal ultrasonography/computed tomography, or even whole-body-magnetic resonance imaging to detect AM/RM at an early stage.
Keywords: Extremity soft tissue sarcoma, Abdominal metastasis, Retroperitoneal metastasis, Diagnosis, Treatment, Outcome
Core tip: Incidence of abdominal (AM) and retroperitoneal metastases (RM) in patients with primary extremity soft tissue sarcoma has been reported to be as high as 12.1%, depending on the histological subtypes and screening methods used. Patients undergoing metastasectomy seem to have a clear survival benefit. Consequently, regular abdominal check-ups with abdominal computed tomography-scans, ultrasonography or even whole-body-magnetic resonance imaging are preferable in order to detect AM/RM at an early, potentially resectable stage.
INTRODUCTION
Extremity soft tissue sarcomas (eSTS) have an estimated incidence of 4-5 per 100,000 persons per year in Europe, with liposarcoma and leiomyosarcoma being the most common histological subtypes[1,2]. About one third of patients with primary localized eSTS will develop metastatic disease during follow-up, most frequently in the lungs[3]. Surveillance for eSTS patients who have undergone curative surgery follows a heuristic approach, with regular check-ups in 3-4-mo intervals for the first 3 years, biannually up to the end of the 5th year and annually thereafter[4]. Whilst computed tomography (CT)-scans or X-rays of the thorax as well as magnetic resonance imaging (MRI)-scans of the primary tumour sites are well-established, abdominal surveillance with ultrasonography or abdominal CT scans is performed inconsistently[4]. It is well-established that eSTS can metastasize to virtually any body region, including brain, bone, abdomen and retroperitoneum. The occurrence of these metastases, however, is considered a rare event. As a result, diagnostic workup via e.g., brain MRI or abdominal CT scans are often only performed if patients are symptomatic.
Thus, the main objective of this systematic literature review is to summarize the evidence concerning incidence, diagnosis, treatment and outcome of abdominal metastases (AM) and retroperitoneal metastases (RM) in patients with primary eSTS.
MATERIALS AND METHODS
Literature search
All studies published between January 1, 2000 and December 31, 2018 were included in the systematic review. Studies published before 2000 were excluded, given the fact that diagnostic (pathology, radiology) and treatment modalities [radiotherapy (RTX), chemotherapy (CTX)] have changed – and markedly improved – over time. Given that AM and RM in eSTS are rare events, retrospective cohort studies and case-series, as well as single case reports, were considered eligible. PubMed was used as the primary database. Further potential articles were identified by cross-searching article references as well as meeting abstracts. The final search date was February 18, 2019.
The following search terms were used in PubMed: (abdominal metastasis AND soft tissue sarcoma) and (soft tissue sarcoma metastasis abdomen). The structure of the systematic review was based on PRISMA guidelines[5]. Studies were initially screened by their title, followed by the abstract. All studies reporting on single cases of AM or RM in eSTS, case-series and cohort studies focusing on diagnosis, treatment and outcome of AM and RM in eSTS were included. Studies containing patients with STS other than those located in the limbs were excluded. However, studies describing both patients with eSTS and abdominal STS or gastrointestinal stromal tumours (GIST), leiomyosarcoma of the uterus as well as bone sarcoma were included, given that a clear differentiation between eSTS and other STS/bone sarcomas was possible.
The main goal of the present study was to identify the rate of occurrence, diagnostic pathway and potential treatment strategies, as well as outcomes in eSTS patients with AM and RM.
Analysis
Due to limited data and the use of different reporting techniques, the present systematic review focused on descriptive analysis of the results included in articles.
RESULTS
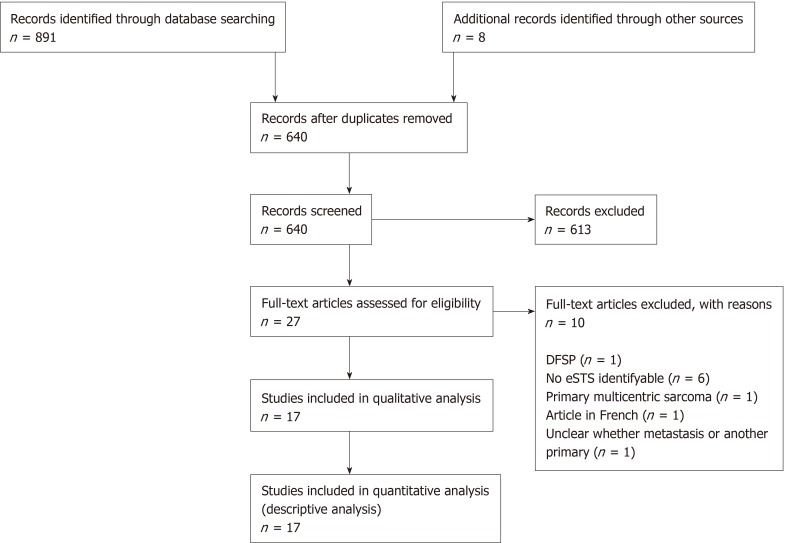
Figure 1 shows the selection process of the articles, with 17 studies being included in the final descriptive analysis. Detailed study characteristics are presented in Table 1 for original articles reporting incidence ± diagnosis, therapy and outcome (n = 6), and in Table 2 for original articles (n = 3) and case reports (n = 8) focusing on diagnostic pathway, therapeutic procedures, or outcomes without allowing conclusions about the incidence of AM/RM. There were five original articles cantered on diagnosis of AM or RM in eSTS (including one article focusing on both diagnosis and treatment) and four articles describing therapeutic procedures in eSTS patients with AM or RM. An additional eight articles described case reports of AM/RM.
Figure 1.
The selection process of the articles. eSTS: Extremity soft tissue sarcoma; DFSP: Dermatofibrosarcoma protuberans.
Table 1.
Original articles (n = 6) analysing incidence of abdominal/retroperitoneal metastases in patients with extremity soft tissue sarcoma
| Ref. | Topic |
Patients |
(Prevalent) Histological subtype(s) | Chemotherapy/ radiotherapy | Outcome (after diagnosis of metastasis) | |
| Total number of STS (n), of whom eSTS, n (%) | Of whom eSTS with AM/RM, n (%) | |||||
| Thompson et al[16], 2015 | Diagnostic pathway | 140 (100) | 7 (5.0) AM | Epithelioid sarcoma, leiomyosarcoma, liposarcoma, synovial sarcoma | N/A | N/A |
| King et al[9], 2009 | Diagnostic pathway, outcome | 124 (100) | 7 (5.6) AM | MPNST, leiomyosarcoma, myxofibrosarcoma, pleomorphic sarcoma | N/A | 21% mortality rate (n = 21/124) |
| Gorelik et al[6], 2018 | Diagnostic pathway | 33 (100) | 4 (12.1) AM | Myxoid liposarcoma | 89% of patients with metastases (including others than AM) received neoadj. RTX 22% received adj. RTX for primary tumour | N/A |
| Behranwala et al[8], 2004 | Therapeutic approach | 2127 (100) | 19 (0.9) AM | Myxoid liposarcoma, leiomyosarcoma | CTX + RTX in 3 patients, and CTX or RTX in 1 patient each following resection of AM | Median OS: 12 mo |
| Ogose et al[11], 2000 | Therapeutic approach | 282 (100) | 24 (8.5) AM | Liposarcoma | None of patients with AM received RTX or CTX | Mean OS (liver/GI metastases): 4.6 mo; Mean OS (pancreatic metastasis): 3.3 mo |
| Sheah et al[7], 2008 | Diagnostic pathway | 112 (100) | 9 (8.0) AM; 2 (1.8) RM | Myxoid liposarcoma | N/A | N/A |
AM: Abdominal metastasis; AWD: Alive with disease; DOD: Dead of disease; eSTS: Extremity soft tissue sarcoma; NED: No evidence of disease; OS: Overall survival; PFS: Progression-free survival; STS: Soft tissue sarcoma; CTX: Chemotherapy; RTX: Radiotherapy; N/A: Not applicable.
Table 2.
Original articles (n = 3) and case reports (n = 8) exclusively reporting on patients developing abdominal or retroperitoneal metastases
| Ref. | Type | Topic | Patients | (Prevalent) Histological subtype(s) | Chemotherapy/ radiotherapy | Outcome (after diagnosis of metastasis) |
| Grimme et al[15], 2019 | Original article | Therapeutic approach, outcome | 38 patients with STS developing liver metastases [of whom 5 (13.2%) had been eSTS] | Leiomyosarcoma, liposarcoma | N/A | Median PFS: 16 mo Median OS: 46 mo |
| Lev-Chelouche et al[14], 2000 | Original article | Diagnostic pathway, therapeutic approach | 10 eSTS patients with RM | Liposarcoma | Adj. CTX in 4 patients, ± adj. RTX in 7 (details on combination of CTX/RTX not provided) | Mean OS: 13.3 mo |
| Rehders et al[23], 2009 | Original article | Therapeutic approach | 5 patients with eSTS (of 45 patients with STS) developing liver metastases | N/A | CTX following hepatic metastasectomy in 5 patients (no information whether eSTS or other STS) | 44 mo (including both eSTS and other STS) |
| Sabel et al[19], 2001 | Case report | N/A | 58-yr-old male patient with small-bowel metastasis after 15 yr of follow-up | Alveolar soft part sarcoma | Adj. RTX for primary tumour, CTX for metastases (prior to development of AM) | N/A |
| Lee et al[18], 2010 | Case report | N/A | 23-yr-old male patient with gastric metastasis at 16 mo of follow-up | Alveolar soft part sarcoma | RTX for cerebral metastases, CTX declined by patient | DOD soon after metastasectomy (not specified by authors) |
| Koh et al[21], 2007 | Case report | N/A | 66-yr-old female with pancreatic metastasis soon after primary tumour diagnosis | Malignant mesenchymoma (70% osteosarcoma, 30% leiomyosarcoma) | CTX and RTX declined by patient | AWD at 9 mo |
| Mizoshiri et al[17], 2018 | Case report | N/A | 51-yr-old female with liver metastasis from leiomyosarcoma at 11 mo of follow-up | Leiomyosarcoma | Neoadj. + adj. CTX for primary tumour and CTX following hepatic metastasectomy in female patient | NED at 12 mo (male patient) |
| 60-yr-old male with liver metastasis from leiomyosarcoma at 3 yr of follow-up | Neoadj. + adj. CTX for male patient for primary tumour | |||||
| Carboni et al[13], 2006 | Case report | N/A | 66-yr-old male patient with pancreatic metastasis at 6 yr of follow-up | Myxoid liposarcoma | Adjuvant RTX for local recurrence of primary tumour | NED at 6 mo |
| Watanabe et al[12], 2001 | Case report | N/A | 20-yr-old female patient with massive AM (37 cm diameter) | Myxoid liposarcoma | N/A | DOD (after several months, not specified by authors) |
| Lin et al[10], 2015 | Case report | N/A | 53-yr-old male patient with AM after 35 mo of follow-up | Myxoid liposarcoma | CTX and RTX for metastatic disease (prior to development of AM) | DOD after 3 mo |
| Willekens et al[20], 2011 | Case report | N/A | 27-yr-old female patient with duodenal metastasis | Alveolar soft part sarcoma | RTX for primary tumour | NED at 2 mo |
AM: Abdominal metastasis; AWD: Alive with disease; DOD: Dead of disease; eSTS: Extremity soft tissue sarcoma; NED: No evidence of disease; OS: Overall survival; PFS: Progression-free survival; STS: Soft tissue sarcoma; CTX: Chemotherapy; RTX: Radiotherapy; N/A: Not applicable.
A potential bias was present in studies that concentrated exclusively on distinct histological subtypes (i.e., myxoid liposarcoma[6,7]). Moreover, case reports preferentially covered unusual and ”dramatic” clinical courses of eSTS patients with AM or RM.
The results of individual studies regarding patients included the prevalent histological subtypes and outcomes following diagnosis of AM or RM, and are provided in Tables 1 and 2.
Incidence
The incidence of AM following diagnosis of eSTS ranged from 0.9%[8]-5.6%[9] in patients with miscellaneous histological subtypes. In the case of eSTS with myxoid liposarcoma as the underlying histological subtype (which is known for its potential to metastasize to unusual sites), the incidence was higher, ranging from 9.8%[7]-12.1%[6].
Histological subtypes
The most prevalent histological subtypes with AM or RM were (myxoid) liposarcoma[6-8,10-15], leiomyosarcoma[8,9,15-17], and alveolar soft part sarcoma[18-20]. One case of pancreatic metastasis in malignant mesenchymoma (defined as a mesenchymal tumour composed of at least two malignant mesenchymal components) with 70% osteosarcoma and 30% leiomyosarcoma was identified as 100% derived from a leiomyosarcomatous origin[21,22]. Further rare histological subtypes presenting with AM or RM included epithelioid sarcoma[16], synovial sarcoma[16], malignant peripheral nerve sheath tumour[9], myxofibrosarcoma[9], and pleomorphic sarcoma[9].
Therapeutic approaches
In five out of eight reviewed case reports, surgery was performed for AM (62.5%)[13,17,19-21]. Among eSTS patients with AM/RM, 20.8%[11], 60%[14], 84.2%[8], and 100%[15,23] had undergone metastasectomy in the included retrospective case-series. In these studies, perioperative complication rates ranged from 20%[15]-44%[23]. Information on the administration of either RTX or CTX is provided in Tables 1 and 2.
Outcomes
Post-metastasis survival rates according to the individual studies are reported in Tables 1 and 2. In patients with hepatic metastases, overall survival (OS) appeared to be better if metastasectomies were performed (46 mo vs 4.6 mo for patients not undergoing metastasectomy)[11,15]. For patients with non-hepatic RM or AM, the reported survival rates were worse, ranging from 12-17.3 mo[8,14].
DISCUSSION
In this systematic review, the original articles that focused on AM or RM in patients with eSTS were analysed. In the following sections, results of the various included studies will be discussed in detail.
Incidence
Depending on the surveillance methods, the incidence of abdominal and RM varied: In the study by Behranwala et al[8], including over 2000 eSTS patients, the incidence of 0.9% for AM was lower than in other, smaller series, which reported rates of around 5%[9,16]. However, in the former study, abdominal CT scans were not routinely performed. Instead, these diagnostic measurements were implemented if patients were symptomatic. On the other hand, in the series of King et al[9] and Thompson et al[16], routine administration of CT scans of the thorax, abdomen and pelvis constituted the main inclusion criteria. Consequently, the study by Behranwala et al[8] may have underestimated the actual number of (asymptomatic) AM.
Moreover, in patients with myxoid liposarcoma as the underlying histological subtype, the incidence of AM/RM was significantly higher, at 9.8% and 12.1% in studies by Sheah et al[7] and Gorelik et al[6], respectively. As myxoid liposarcomas are known to metastasize to unusual locations[24], whole-body MRI has been suggested as a sensitive screening and follow-up imaging technique in patients with eSTS of this specific histological subtype[7,11]. This sensitive method may have further increased the detection rate of AM/RM during follow-up.
Histology
Myxoid liposarcoma was the most prevalent histological subtype to develop AM/RM, followed by leiomyosarcoma and alveolar soft part sarcoma; in nine of the seventeen studies, AM or RM originated from myxoid liposarcomas of the extremities[6-8,10-15].
Therapeutic approaches
Five studies specifically focused on therapeutic approaches in AM or RM[8,11,14,15,23]. In the study by Rehders et al[23], the outcome of 45 patients with STS, of whom five have had eSTS, and subsequently developed hepatic metastases, was analysed. All patients had undergone surgical resection of their metastases, most commonly wedge resections (n = 8) and hemihepatectomies (n = 7). Comparable results were reported by Grimme et al[15], including 5 eSTS patients and a further 33 patients with STS at other sites, who developed liver metastases. Most patients in their cohort (n = 24) underwent a minor resection, defined as ≤ 2 segments of the liver, whilst 13 patients were treated by major hepatic resection[15]. Three patients had intraoperative complications (i.e., small-bowel perforation, tumour rupture, bleeding), and 6 patients developed postoperative complications, resulting in a perioperative complication rate of 20%[15]. Slightly more complications were reported by Rehders et al[23], with a combined complication rate of 44%, including 7 minor complications (i.e., not requiring surgical intervention) and 5 major complications (i.e., requiring surgical interventions or proving fatal).
Of the 19 eSTS patients with AM analysed in the study by Behranwala et al[8], 16 patients (84.2%) underwent abdominal metastasectomy. Of these, three subsequently developed abdominal recurrences[8]. All 14 patients with liver metastases in the study by Ogose et al[11] were treated conservatively. Of the 10 patients with non-hepatic AM, 4 were treated conservatively, 5 patients underwent abdominal metastasectomy, and one further patient was treated by RTX[11]. The rate or type of complications were not reported in these two studies.
In the study by Lev-Chelouche et al[14], 6 out of 10 patients with RM underwent curative metastasectomy, accompanied by extensive intra-abdominal resection (colectomy, nephrectomy, splenectomy, sigmoidectomy, pancreatectomy) in 4 patients. A further two patients showed diffuse metastatic disease infiltrating the mesentery root, resulting in only diagnostic laparotomy[14]. These were treated with CTX, along with two further patients who were considered inoperable[14].
In five out of eight case reports, metastasectomy was performed for AM following eSTS diagnosis[13,17,19-21]. In a single further case, embolization was performed for bleeding gastric metastases[18]. In the remaining two cases, multiple metastases and irresectable AM lead to a conservative approach[10,12].
Outcomes
Resection of the abdominal or RM was associated with a survival benefit compared to patients treated by CTX. In the study by Ogose et al[11], none of the patients with eSTS liver metastases had undergone surgery, resulting in a mean OS of 4.6 mo. On the other hand, liver resection was performed in all 5 patients in the study by Grimme et al[15] with a median OS of 46 mo (including results from liver metastases of 33 STS patients with non-extremity primary tumours). Comparable results were observed by Rehders et al[23], with a median OS of 44 mo following metastasectomy for liver metastases. Notably, patients with RM or AM not confined to the liver had poorer post-metastasectomy survival rates. OS was limited to a median of 12 mo in the study by Behranwala et al[8], including 19 patients with AM, although 16 of them had undergone metastasectomy. Comparable rates were observed by Lev-Chelouche et al[14] in 10 patients with RM, of whom 6 had undergone surgery with a curative intent, and 4 had been treated by CTX. Mean OS was 13.3 mo for all patients and 17.3 mo for those patients treated with a curative intent, compared to 5.8 mo for the 4 patients undergoing CTX[14]. It could be argued that patients undergoing metastasectomy were generally in better condition, thereby improving their prognosis. However, according to a recently published retrospective study, even when confounding factors such as physical performance status, number of metastases and laboratory parameters (e.g., haemoglobin and albumin levels) are considered, metastasectomies result in a significant survival benefit[25].
One limitation of the present review is that studies not clearly differentiating between STS subtypes (i.e., GIST, leiomyosarcoma of the uterus, extremity STS) had to be excluded from the present review. Furthermore, only two studies reported on eSTS patients developing RM. Considering that AM and RM are diagnosed and treated similarly, conclusions from the present systematic review are most likely applicable to both.
In conclusion, depending on the surveillance method used, up to 12.1% of eSTS patients with myxoid liposarcoma and 5.6% of eSTS patients with other histological subtypes develop AM/RM during their follow-up. Patients with myxoid liposarcoma in particular seem to benefit from regular follow-up with whole-body MRI, as metastases to sites other than the lung (including bone and abdomen/ retroperitoneum) can be detected with higher sensitivity. In cases where patients with eSTS develop metastases to the abdomen or retroperitoneum, wide resection should be attempted whenever feasible. Particularly for patients with hepatic metastases, high post-metastasectomy survival rates over a long period have been reported. However, patients with AM at other sites or RM also seem to benefit from metastasectomy in terms of survival.
Consequently, regular abdominal ultrasonography and CT scans should be performed in eSTS patients. Moreover, in myxoid liposarcoma, whole-body MRI may be administered in order to detect AM, RM or metastases at other unusual sites at an early – and potentially resectable - stage.
ARTICLE HIGHLIGHTS
Research background
Currently, there is no clear consensus on the best imaging modality or follow-up duration for patients with primary extremity soft tissue sarcoma (eSTS), which is important to detect metastases to unusual sites, including the abdomen and retroperitoneum.
Research motivation
There is limited knowledge on incidence, treatment and outcome of abdominal metastases (AM) and retroperitoneal metastases (RM) in patients with primary eSTS undergoing surgery with curative intent.
Research objectives
The objective of the present systematic review was to summarise current knowledge on incidence, diagnosis, treatment and outcome of AM and RM in eSTS patients.
Research methods
A systematic literature review was performed, screening all studies published in PubMed between January, 2000 and December, 2018 adhering to the PRISMA guidelines. Of 899 articles screened, 17 were eligible to be included in the present review.
Research results
Six original articles of the 17 studies provided information on incidence ± diagnosis, therapy and outcome, whilst three original articles and eight case reports did not allow for conclusions on the incidence of AM/RM. Incidence of AM/RM ranged between 0.9%-12.1%, depending on the underlying histological subtype. (Myxoid) liposarcoma and leiomyosarcoma were the prevalent histological subtypes, although rare entities had also been reported to develop AM/RM. Surgery was performed in 62.5% of case reports and in 20.8%-100.0% of original articles, with patients undergoing metastasectomy having an improved outcome. Especially in patients with hepatic metastases, metastasectomy was associated with improved post-metastasis survival.
Research conclusions
Abdominal ultrasonography/ computed tomography (CT) should be performed on a regular basis during follow-up in eSTS patients. In patients with a high risk of developing AM/RM– especially those with myxoid liposarcoma – even whole-body magnetic resonance imaging may be considered.
Research perspectives
Prospective studies investigating the effect of surveillance with abdominal ultrasonography or CT scans of the abdomen for reporting incidences of AM/RM as well as patient outcome are warranted.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
PRISMA 2009 Checklist statement: The PRISMA 2009 Checklist has been added at the end of the manuscript.
Manuscript source: Invited manuscript
Peer-review started: March 20, 2019
First decision: May 7, 2019
Article in press: December 6, 2019
Specialty type: Oncology
Country of origin: Austria
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Lee P, Wiemer EAC S-Editor: Tang JZ L-Editor: Filipodia E-Editor: Liu MY
Contributor Information
Maria Anna Smolle, Department of Orthopaedics and Trauma, Medical University of Graz, Graz 8036, Austria.
Andreas Leithner, Department of Orthopaedics and Trauma, Medical University of Graz, Graz 8036, Austria.
Gerwin Alexander Bernhardt, Department of Orthopaedics and Trauma, Medical University of Graz, Graz 8036, Austria. gerwin.bernhardt@medunigraz.at.
References
- 1.Stiller CA, Trama A, Serraino D, Rossi S, Navarro C, Chirlaque MD, Casali PG RARECARE Working Group. Descriptive epidemiology of sarcomas in Europe: report from the RARECARE project. Eur J Cancer. 2013;49:684–695. doi: 10.1016/j.ejca.2012.09.011. [DOI] [PubMed] [Google Scholar]
- 2.Wibmer C, Leithner A, Zielonke N, Sperl M, Windhager R. Increasing incidence rates of soft tissue sarcomas? A population-based epidemiologic study and literature review. Ann Oncol. 2010;21:1106–1111. doi: 10.1093/annonc/mdp415. [DOI] [PubMed] [Google Scholar]
- 3.Posch F, Leitner L, Bergovec M, Bezan A, Stotz M, Gerger A, Pichler M, Stöger H, Liegl-Atzwanger B, Leithner A, Szkandera J. Can Multistate Modeling of Local Recurrence, Distant Metastasis, and Death Improve the Prediction of Outcome in Patients With Soft Tissue Sarcomas? Clin Orthop Relat Res. 2017;475:1427–1435. doi: 10.1007/s11999-017-5232-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casali PG, Abecassis N, Aro HT, Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee JVMG, Brodowicz T, Broto JM, Buonadonna A, De Álava E, Dei Tos AP, Del Muro XG, Dileo P, Eriksson M, Fedenko A, Ferraresi V, Ferrari A, Ferrari S, Frezza AM, Gasperoni S, Gelderblom H, Gil T, Grignani G, Gronchi A, Haas RL, Hassan B, Hohenberger P, Issels R, Joensuu H, Jones RL, Judson I, Jutte P, Kaal S, Kasper B, Kopeckova K, Krákorová DA, Le Cesne A, Lugowska I, Merimsky O, Montemurro M, Pantaleo MA, Piana R, Picci P, Piperno-Neumann S, Pousa AL, Reichardt P, Robinson MH, Rutkowski P, Safwat AA, Schöffski P, Sleijfer S, Stacchiotti S, Sundby Hall K, Unk M, Van Coevorden F, van der Graaf WTA, Whelan J, Wardelmann E, Zaikova O, Blay JY. ESMO Guidelines Committee and EURACAN. Soft tissue and visceral sarcomas: ESMO-EURACAN Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2018;29:iv51–iv67. doi: 10.1093/annonc/mdy096. [DOI] [PubMed] [Google Scholar]
- 5.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorelik N, Reddy SMV, Turcotte RE, Goulding K, Jung S, Alcindor T, Powell TI. Early detection of metastases using whole-body MRI for initial staging and routine follow-up of myxoid liposarcoma. Skeletal Radiol. 2018;47:369–379. doi: 10.1007/s00256-017-2845-9. [DOI] [PubMed] [Google Scholar]
- 7.Sheah K, Ouellette HA, Torriani M, Nielsen GP, Kattapuram S, Bredella MA. Metastatic myxoid liposarcomas: imaging and histopathologic findings. Skeletal Radiol. 2008;37:251–258. doi: 10.1007/s00256-007-0424-1. [DOI] [PubMed] [Google Scholar]
- 8.Behranwala KA, Roy P, Giblin V, A'hern R, Fisher C, Thomas JM. Intra-abdominal metastases from soft tissue sarcoma. J Surg Oncol. 2004;87:116–120. doi: 10.1002/jso.20105. [DOI] [PubMed] [Google Scholar]
- 9.King DM, Hackbarth DA, Kilian CM, Carrera GF. Soft-tissue sarcoma metastases identified on abdomen and pelvis CT imaging. Clin Orthop Relat Res. 2009;467:2838–2844. doi: 10.1007/s11999-009-0989-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin S, Gan Z, Han K, Yao Y, Min D. Metastasis of myxoid liposarcoma to fat-bearing areas: A case report of unusual metastatic sites and a hypothesis. Oncol Lett. 2015;10:2543–2546. doi: 10.3892/ol.2015.3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ogose A, Morita T, Hotta T, Otsuka H, Imaizumi S, Kobayashi H, Hirata Y. Intra-abdominal metastases in musculoskeletal sarcomas. J Orthop Sci. 2000;5:463–469. doi: 10.1007/s007760070024. [DOI] [PubMed] [Google Scholar]
- 12.Watanabe H, Ohmori K, Kanamori M, Araki N, Yoshikawa H, Kimura T. A myxoid liposarcoma in the lower leg, with a large intra-abdominal metastasis. J Orthop Sci. 2001;6:95–97. doi: 10.1007/s007760170032. [DOI] [PubMed] [Google Scholar]
- 13.Carboni F, Ettorre GM, Lorusso R, Lepiane P, Santoro R, Mancini P, Di Matteo FM, Santoro E. Isolated pancreatic metastasis of extremity myxoid liposarcoma: Report of a case. Jpn J Clin Oncol. 2006;36:662–664. doi: 10.1093/jjco/hyl080. [DOI] [PubMed] [Google Scholar]
- 14.Lev-Chelouche D, Nakache R, Soffer D, Merimsky O, Klausner MJ, Gutman M. Metastases to the retroperitoneum in patients with extremity soft tissue sarcoma: an unusual metastatic pattern. Cancer. 2000;88:364–368. doi: 10.1002/(sici)1097-0142(20000115)88:2<364::aid-cncr17>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 15.Grimme FAB, Seesing MFJ, van Hillegersberg R, van Coevorden F, de Jong KP, Nagtegaal ID, Verhoef C, de Wilt JHW On behalf of the Dutch Liver Surgery Working Group. Liver Resection for Hepatic Metastases from Soft Tissue Sarcoma: A Nationwide Study. Dig Surg. 2019;36:479–486. doi: 10.1159/000493389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson MJ, Ross J, Domson G, Foster W. Screening and surveillance CT abdomen/pelvis for metastases in patients with soft-tissue sarcoma of the extremity. Bone Joint Res. 2015;4:45–49. doi: 10.1302/2046-3758.43.2000337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mizoshiri N, Shirai T, Terauchi R, Tsuchida S, Mori Y, Katsuyama Y, Hayashi D, Konishi E, Kubo T. Hepatic metastases from primary extremity leiomyosarcomas: Two case reports. Medicine (Baltimore) 2018;97:e0598. doi: 10.1097/MD.0000000000010598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee GW, Kim TH, Min HJ, Kim HJ, Jung WT, Lee OJ, Ko GH. Unusual gastrointestinal metastases from an alveolar soft part sarcoma. Dig Endosc. 2010;22:137–139. doi: 10.1111/j.1443-1661.2010.00937.x. [DOI] [PubMed] [Google Scholar]
- 19.Sabel MS, Gibbs JF, Litwin A, McGrath B, Kraybill WB, Brooks JJ. Alveolar soft part sarcoma metastatic to small bowel mucosa causing polyposis and intussuseption. Sarcoma. 2001;5:133–137. doi: 10.1080/13577140120048584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Willekens I, Paradisi C, Sarria L, Puertas A, Pac J, Mayayo E. Duodenal metastasis of alveolar soft part sarcoma. JBR-BTR. 2011;94:287–289. doi: 10.5334/jbr-btr.668. [DOI] [PubMed] [Google Scholar]
- 21.Koh YS, Chul J, Cho CK, Kim HJ. Pancreatic metastasis of leiomyosarcoma in the right thigh: a case report. World J Gastroenterol. 2007;13:1135–1137. doi: 10.3748/wjg.v13.i7.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stout AP. Mesenchymoma, the Mixed Tumor of Mesenchymal Derivatives. Ann Surg. 1948;127:278–290. doi: 10.1097/00000658-194802000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rehders A, Peiper M, Stoecklein NH, Alexander A, Boelke E, Knoefel WT, Rogiers X. Hepatic metastasectomy for soft-tissue sarcomas: is it justified? World J Surg. 2009;33:111–117. doi: 10.1007/s00268-008-9777-4. [DOI] [PubMed] [Google Scholar]
- 24.Schwab JH, Boland P, Guo T, Brennan MF, Singer S, Healey JH, Antonescu CR. Skeletal metastases in myxoid liposarcoma: an unusual pattern of distant spread. Ann Surg Oncol. 2007;14:1507–1514. doi: 10.1245/s10434-006-9306-3. [DOI] [PubMed] [Google Scholar]
- 25.Smolle MA, van Praag VM, Posch F, Bergovec M, Leitner L, Friesenbichler J, Heregger R, Riedl JM, Pichler M, Gerger A, Szkandera J, Stöger H, Smolle-Jüttner FM, Liegl-Atzwanger B, Fiocco M, van de Sande MA, Leithner A. Surgery for metachronous metastasis of soft tissue sarcoma - A magnitude of benefit analysis using propensity score methods. Eur J Surg Oncol. 2019;45:242–248. doi: 10.1016/j.ejso.2018.06.019. [DOI] [PubMed] [Google Scholar]