Abstract
Background
Inhalation of hypertonic saline improves sputum rheology, accelerates mucociliary clearance and improves clinical outcomes of people with cystic fibrosis. This is an update of a previously published Cochrane Review.
Objectives
To determine whether the timing of hypertonic saline inhalation (in relation to airway clearance techniques or in relation to time of day) has an impact on its clinical efficacy in people with cystic fibrosis.
Search methods
We identified relevant randomised and quasi‐randomised controlled trials from the Cochrane Cystic Fibrosis Trials Register, the Physiotherapy Evidence Database (PEDro), and international cystic fibrosis conference proceedings.
Date of the last search of the Cochrane Cystic Fibrosis and Genetic Disorders Group's Cystic Fibrosis Trials Register: 28 February 2019.
Selection criteria
Any trial of hypertonic saline in people with cystic fibrosis where timing of inhalation was the randomised element in the study protocol with either: inhalation up to six hours before airway clearance techniques compared to inhalation during airway clearance techniques compared to inhalation up to six hours after airway clearance techniques; or morning compared to evening inhalation with any definition provided by the author.
Data collection and analysis
Both authors independently assessed the trials identified by the search for potential inclusion in the review. The certainty of the evidence was assessed using GRADE.
Main results
The searches identified 104 trial reports which represented 51 trials, of which three cross‐over trials (providing data on 77 participants) met our inclusion criteria. We present three comparisons: inhalation before versus during airway clearance techniques; inhalation before versus after airway clearance techniques; and inhalation during versus after airway clearance techniques. One trial (50 participants), given its three‐arm design, was eligible for all three comparisons. No trials compared morning versus evening inhalation of hypertonic saline.
The evidence from the three trials was judged to be of low quality downgraded for limitations (high risk of bias due to blinding) and indirectness (all participants are adults, and therefore not applicable to children). Intervention periods ranged from one treatment to three treatments in one day. There were no clinically important differences between the timing regimens of inhaling hypertonic saline before, during or after airway clearance techniques in the mean amount of improvement in lung function or symptom scores (77 participants), with the between‐group comparisons being non‐significant (low‐certainty evidence). While there may be little or no difference in the rating of satisfaction when hypertonic saline was inhaled before versus during the airway clearance techniques (64 participants) (with the 95% confidence interval including the possibility of both a higher and lower rating of satisfaction), satisfaction may be lower on a 100‐mm scale when inhaled after the airway clearance techniques compared to before: mean difference (MD) 20.38 mm (95% confidence interval (CI) 12.10 to 28.66) and when compared to during the techniques, MD 14.80 mm (95% CI 5.70 to 23.90). Perceived effectiveness showed similar results: little or no difference for inhalation before versus during airway clearance techniques (64 participants); may be lower when inhaled after the airway clearance techniques compared to before, MD 10.62 (95% CI 2.54 to 18.70); and also when compared to during the techniques, MD 15.60 (95% CI 7.55 to 23.65). There were no quality of life or adverse events reported in any of the trials.
Authors' conclusions
Timing of hypertonic saline inhalation makes little or no difference to lung function (low‐certainty evidence). However, inhaling hypertonic saline before or during airway clearance techniques may maximise perceived efficacy and satisfaction. The long‐term efficacy of hypertonic saline has only been established for twice‐daily inhalations; however, if only one dose per day is tolerated, the time of day at which it is inhaled could be based on convenience or tolerability until evidence comparing these regimens is available.
The identified trials were all of very short intervention periods, so longer‐term research could be conducted to establish the effects arising from regular use, which would incorporate the influence of changes in adherence with long‐term use, as well as generating data on any adverse effects that occur with long‐term use.
Plain language summary
The timing of inhalation of hypertonic saline in people with cystic fibrosis
Review question
We reviewed the evidence about whether the timing (in relation to airway clearance techniques or in relation to time of day) of hypertonic saline (a strong, sterile, salt water solution) through a nebuliser improves the physical properties of sputum, stimulates cough, improves clinical outcomes (such as lung function), and improves the perceived effect of airway clearance techniques in cystic fibrosis. This is an update of a previously published Cochrane Review.
Background
Regular inhalation of hypertonic saline improves the clinical outcomes of people with cystic fibrosis. It is not certain whether it is better to inhale hypertonic saline before, during or after clearing the airways with physical techniques, nor whether it is better to inhale it in the morning or in the evening. We looked for trials that compared these different timing regimens.
Search date
The evidence is current to: 28 February 2019.
Study characteristics
The review included three studies with 77 people with cystic fibrosis aged between 18 and 64 years of age. The studies looked at the impact of the timing of hypertonic saline inhalation in relation to airway clearance techniques. The studies reported immediate outcomes after inhalation of hypertonic saline before, during or after physical airway clearance techniques. All studies were short, involving only one to three treatments of each timing regimen.
Key results
While outcomes such as lung function did not show any difference between the regimens, people with cystic fibrosis perceived that inhaling hypertonic saline before or during airway clearance techniques may be more effective and satisfying than inhaling hypertonic saline after airway clearance. No studies comparing morning and evening inhalation were found. The long‐term efficacy of hypertonic saline has only been established for twice‐daily inhalations; however, if only one dose per day is tolerated, the time of day at which it is inhaled could be based on convenience or tolerability until further evidence is available.
Quality of the evidence
Overall, the quality of the evidence was low. The only issues perhaps affecting the quality related to the fact that it was not possible for participants to be blinded to the treatment they received. However, because the studies were short‐term and most of the significant results were based on perceived efficacy, timing of administration of hypertonic saline needs further study.
Summary of findings
Background
Description of the condition
Cystic fibrosis (CF) is the most common life‐limiting autosomal recessive disorder amongst Caucasians (Cutting 2002). Mucociliary clearance is impaired in CF (Robinson 1996; Robinson 1997). Cystic fibrosis‐related pulmonary disease is the major cause of morbidity and mortality (Buzzetti 2009).
Description of the intervention
Hypertonic saline is a sterile salt‐water solution delivered as a nebulised therapy, usually at a concentration of between 3% and 10% (composition by mass). Traditionally, volumes of 3 mL to 10 mL are nebulised (Elkins 2006c; Wark 2018). Long‐term use is recommended (Button 2016).
Each dose of hypertonic saline is usually nebulised immediately prior to airway clearance, but other timing regimens are sometimes used in clinical practice. The clinical effect of hypertonic saline could be affected by the timing of its delivery in relation to physical airway clearance techniques (before, during or after) or in relation to time of day (morning or evening).
How the intervention might work
Hypertonic saline has been shown to accelerate mucociliary clearance in the CF airway (Robinson 1996; Robinson 1997). Three mechanisms are believed to contribute to this improvement in mucociliary clearance. The first is restoration of the depleted airway surface liquid volume, which peaks almost immediately after a dose, but which may be sustained for several hours (Donaldson 2006). The other mechanisms are improvement in the rheology of the mucus (King 1997; Wills 1997), and stimulation of cough (Robinson 1996; Robinson 1997). The overall effect on mucus clearance is presumably responsible for the significant clinical improvements including lung function, quality of life (QoL), and ease of expectoration with regular use of the therapy (Elkins 2006b; Eng 1996). A Cochrane Review of nebulised hypertonic saline for CF concluded that improvements in forced expiratory volume at one second (FEV1) were demonstrated over two to four weeks of therapy and QoL and pulmonary exacerbation rates were improved compared to placebo interventions over 48 weeks of treatment (Wark 2018).
In the controlled studies that established the efficacy of hypertonic saline (Elkins 2006c; Eng 1996; Robinson 1996; Robinson 1997), each dose was nebulised immediately before the administration of airway clearance techniques. However, other timing regimens may have advantages. Nebulisation of hypertonic saline during airway clearance techniques could save time. It also may capitalise on the immediate peak in the airway surface liquid volume. Nebulisation after airway clearance techniques may capitalise on the reduction in airway obstruction by mucus and therefore allow delivery of the hypertonic saline to a greater portion of the bronchial tree.
In the clinical trials that established the efficacy of the regular use of hypertonic saline (Donaldson 2006; Elkins 2006c; Eng 1996), at least two doses per day were used. However, some individuals can tolerate (or for other reasons elect to use) only one dose per day. For these people, nebulising the dose at a particular time of the day may affect its efficacy. Given that spontaneous mucociliary clearance is faster during waking hours than sleep (Bateman 1978), morning inhalation of hypertonic saline may capitalise on faster daytime mucociliary clearance and on the mucus‐clearing effects of daytime activities such as exercise (Wolff 1977). Some people with CF find evening inhalation more convenient or report that it improves ease of expectoration the following morning. Since mucociliary clearance and coughing are suppressed overnight, evening inhalation of hypertonic saline may increase its dwell time in the airways, possibly increasing its clinical efficacy.
Why it is important to do this review
Despite the theoretical rationales presented to justify the investigation of alternative timing regimens in the previous section, it is also possible that any timing regimen may have adverse effects on the efficacy, tolerability and convenience of the treatment and on the duration of the airway clearance session. For example, nebulisation of hypertonic saline during airway clearance techniques could increase the complexity of the overall session of airway clearance, perhaps requiring modified equipment. Nebulisation after airway clearance techniques presumably delivers the hypertonic saline more directly to the exposed airway epithelium, rather than an overlying mucus layer, which may reduce tolerability. Evening delivery may increase nocturnal coughing and sleep disturbance. Therefore, it is important to review well‐designed research that compares the regimens.
There is a high treatment burden associated with CF for both people with the disease and for care providers. Hypertonic saline inhalation adds to the duration of the overall treatment regimen. Therefore, even if the various timing regimens have equal clinical efficacy, it is important that the review also investigates whether the interventions differ in their effects on the duration of the overall airway clearance session, in their convenience and in their side effects.
This is an update of a previously published Cochrane Review (Elkins 2012; Elkins 2016).
Objectives
To determine whether the timing of hypertonic saline inhalation, in relation to physical airway clearance techniques or time of day, has an impact on objective and subjective measures of clinical efficacy and tolerability in people with CF.
Methods
Criteria for considering studies for this review
Types of studies
Randomised trials (published and unpublished). Both random allocation and quasi‐random allocation (e.g. where there is alternate allocation to groups) were included. Parallel and cross‐over trials were eligible.
Types of participants
People of all ages and of both sexes with CF diagnosed by genetic testing or evidence on sweat chloride or nasal potential difference, including all degrees of disease severity.
Types of interventions
Nebulised hypertonic saline, where timing of inhalation was the randomised element in the study protocol:
hypertonic saline inhalation up to six hours before airway clearance techniques, compared to inhalation during airway clearance techniques;
hypertonic saline inhalation up to six hours before airway clearance techniques, compared to up to six hours after airway clearance techniques;
hypertonic saline inhalation during airway clearance techniques, compared to up to six hours after airway clearance techniques;
morning compared to evening inhalation with any definition provided by the author. If not defined, we accepted midnight to midday as morning and midday to midnight as evening.
Note: many individuals perform two treatments with physical airway clearance techniques each day. Even if these treatments are performed as far as possible from each other (i.e. 12 hours apart), any threshold greater than six hours would mean that the hypertonic saline labelled ‘before’ would actually be closer to ‘after’ the previous treatment with physical airway clearance techniques. Therefore, six hours is the broadest threshold possible to capture all potentially relevant trials. However, some of the mechanisms by which timing may affect the outcome are short‐lived. Therefore, a sensitivity analysis is performed that only considers trials where the hypertonic saline is within 30 min of the techniques (seeSensitivity analysis).
The timing regimen could be a single treatment or could be maintained for any duration.
Hypertonic saline treatment had to be a minimum of a single dose of at least 3% concentration. If studies mentioned co‐interventions (such as bronchodilators and other inhaled medications), these were permitted provided they were the same on all trial days and taken either before or after the period during which hypertonic saline and airway clearance techniques were used.
Airway clearance techniques (ACT) had to be a minimum of 10 minutes in duration and include at least one of the following:
postural drainage with percussion and vibration (PDPV). In other reviews this has been described as conventional chest physiotherapy (CCPT) (Elkins 2006c; Main 2009; van der Schans 2009);
active cycle of breathing techniques (ACBT).This comprises relaxation or breathing control, forced expiration technique (FET), thoracic expansion exercises and may include postural drainage or percussion (Robinson 2010);
autogenic drainage (AD). This breathing technique uses high expiratory flow rates at varying lung volumes to enhance mucus clearance while avoiding airway closure;
positive expiratory pressure (PEP) (Elkins 2006a);
oral oscillatory devices that provide oscillating PEP (such as the Flutter, Cornet and Acapella) or intrapulmonary percussive ventilation, which provides continuous oscillation of the air pressure in the airways via the mouth (Morrison 2009);
thoracic oscillating devices applied via a vest to provide external chest wall oscillation (Morrison 2009);
exercise prescribed for the purpose of airway clearance either independently or as an adjunct to other techniques.
Types of outcome measures
Primary outcomes
-
Lung function, i.e., change in lung function (measured in litres or % predicted). If change values are unavailable, final post‐treatment values will be used. Values in litres and in % predicted will be analysed separately.
forced expiratory volume at one second (FEV1)
forced vital capacity (FVC)
-
Patient‐reported outcomes, using validated scales or subjective reporting measures
measures of QoL
symptom scores (including cough, tolerability, subjective ease of clearance, or treatment satisfaction)
Secondary outcomes
Measures of sputum clearance, including measures of mucociliary clearance (assessed by radioactive tracer clearance) and objective measures of sputum volume
Measures of exercise capacity (either maximal or submaximal where measured directly, or by a standard field test)
Mortality (all cause or CF‐related, analysed separately)
-
Other pulmonary parameters
forced expiratory flow between 25% and 75% of the vital capacity (FEF25‐75)
maximal instantaneous forced flow when 25% of the FVC remains to be exhaled (MEF25)
total lung capacity (TLC)
residual volume (RV)
functional residual capacity (FRC)
lung clearance index (LCI)
-
Frequency of exacerbations of respiratory infection (where a clear definition is described demonstrating an increase in symptoms or a decline in pulmonary function)
admission rates to hospital (defined as either number of inpatient hospital admissions or days as a hospital inpatient)
courses of IV antibiotics (whether received in hospital or in the home)
outpatient treatments (presentations to hospital, unscheduled visits to the doctor)
Adherence to inhaled therapies, airway clearance techniques, and other therapies
Adverse effects such as bronchospasm, cough and acute decline in pulmonary function
Search methods for identification of studies
A comprehensive search strategy was formulated in an attempt to identify all relevant trials regardless of language or publication status (published, unpublished, in press, and in progress).
Electronic searches
We identified relevant trials from the Cochrane Cystic Fibrosis and Genetic Disorders Group's Cystic Fibrosis Trials Register using the term 'hypertonic saline'.
The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work was identified by searching the abstract books of relevant conferences, including the three major cystic fibrosis conferences: the International Cystic Fibrosis Conference, the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Cochrane Cystic Fibrosis and Genetic Disorders Group's website.
Date of last search of the Cochrane Cystic Fibrosis and Genetic Disorders Group's Cystic Fibrosis Trials Register: 28 February 2019.
In addition, we searched the Physiotherapy Evidence Database (PEDro) (Michaleff 2011) and the WHO International Clinical Trials Registry Platform for all years available (Appendix 1; Appendix 2). Date of last search: 21 February 2019.
Searching other resources
We hand‐searched the biennial Australia and New Zealand CF Conference Proceedings from 1995 to 2017.
We contacted trial authors, experts and manufacturers of hypertonic saline to identify additional trials.
We searched the reference lists of the included studies.
Data collection and analysis
Selection of studies
Both authors (RD, ME) independently selected the trials to be included in the review. We performed initial screening by title and abstract, and reviewed the full text for studies remaining after the initial screening phase. We resolved disagreements by discussion. We excluded irrelevant records and have presented the details of these excluded trials and the reasons for exclusion in tabular form (Characteristics of excluded studies).
Data extraction and management
Each author independently extracted data from the included studies using a standardised data extraction form. We resolved any disagreements by discussion. Where data were absent or difficult to interpret in the presented form, the authors contacted the trial investigators to gain the information required to evaluate risk of bias in the trial and to facilitate data analysis. Data extraction was also carried out at the editorial base for the Dentice trial given we (the Cochrane Review authors) are also two of the trial authors (Dentice 2012).
Assessment of risk of bias in included studies
We independently determined the risk of bias for each trial using published and unpublished data from the trial investigators, following the domain‐based evaluation as described in the Cochrane Handbook for Systematic Reviews of Interventions 5.1 (Higgins 2011). The risk of bias was also carried out at the editorial base for the Dentice trial given we (the Cochrane Review authors) are also two of the trial authors (Dentice 2012). We assessed the following domains as either low risk, unclear risk, or high risk of bias:
sequence generation;
allocation concealment;
blinding (of participants, personnel and outcome assessors);
incomplete outcome data;
selective outcome reporting;
other sources of bias.
We resolved any disagreements by discussion. In addition, each author independently rated each trial on the PEDro Scale (Maher 2003; de Morton 2009), using published and unpublished data from the trial investigators. The PEDro Scale was chosen because it has acceptably high reliability for individual ratings and for consensus ratings (Maher 2003; Shiwa 2011). It has undergone extensive validation including convergent and construct validity and the appropriateness of the scoring system has been justified with Rasch analysis (de Morton 2009). The PEDro Scale is also widely used and understood by physiotherapists (Elkins 2013). The Cochrane Risk of Bias tool has reliability that ranges from poor to slight (Armijo‐Olivo 2012; Hartling 2009; Hartling 2013).
Measures of treatment effect
For dichotomous data we planned to use the risk ratio (RR) with 95% confidence intervals (95% CIs) as a measure of treatment effect with an intention‐to‐treat analysis. An intention‐to‐treat analysis means that, where participants did not receive treatment as allocated, and were measures of outcomes were available, the analysis was performed as if participants received the treatment to which they were allocated. We considered a trial to have analysed by intention to treat if the report explicitly states that all participants received treatment or control conditions as allocated. Mortality and adverse events were the only dichotomous outcomes.
For continuous data we planned to record the difference in mean change from baseline and standard deviation (SD) (or standard errors (SE)) for each group, or the final group means and SDs if change data are unavailable. We planned to calculate a pooled estimate of treatment effect using the mean difference (MD) with 95% CIs.
However, given that both of the included trials were cross‐over in design, we used the generic inverse variance method. Where the MD and SE of the MD were available, we entered these directly into the meta‐analysis. Where only group means and SDs were available, we calculated the MD by subtraction of one group mean from the other. We imputed the SD of the differences from that obtained from the data in a similar trial, taking into account the paired nature of the data. We then calculated SEs using the formula: imputed SD / √n.
Unit of analysis issues
We incorporated data from cross‐over trials into meta‐analysis using the generic inverse variance method, involving expression of data in terms of the paired mean differences between treatments and their SE. We calculated these values either from paired individual patient data provided by authors, or by calculation of mean differences between interventions and their SE from means, SDs and P values reported in the manuscript (Elbourne 2002). We intended to combine data from parallel‐designed trials with those from cross‐over trials in the meta‐analyses, but only cross‐over trials were obtained. If necessary, we intended to calculate the SEs in parallel trials from the MDs between treatments and their CIs, but only cross‐over trials were obtained.
Dealing with missing data
Where data were absent or difficult to interpret in the presented form, we contacted the trial investigators in an attempt to obtain the data in a form that would facilitate data analysis. Had we not been able to obtain the data, our plan was to analyse only the available data (available‐case analysis). Additional data were obtained from the authors of the van Ginderdeuren trial (Van Ginderdeuren 2011). Additional data were sought from the authors of the O'Neill trial (O'Neill 2016) and data were received for all requested outcomes.
Assessment of heterogeneity
We visually inspected forest plots for overlap of the CIs and estimated statistical heterogeneity using the I² value (Higgins 2003). Given that thresholds for the interpretation of I² can be misleading (since the importance of inconsistency depends on several factors) we chose a rough guide to interpretation as follows: moderate heterogeneity was defined as an I² value of 30% to 60%, substantial heterogeneity was defined as an I² value of 50% to 90%, and considerable heterogeneity was defined as an I² value of 75% to 100%. The P value from the chi‐squared test was also used to interpret the importance of the observed value of I². Clinical heterogeneity was also assessed by considering differences in trial designs and participants characteristics.
Assessment of reporting biases
If we had included sufficient trials in the review, we planned to assess publication bias using a funnel plot. If we suspected outcome reporting bias, we planned to contact trial investigators to clarify whether they measured and analysed certain outcomes and obtained the data. However, only two eligible trials were obtained; this was fewer than the recommended number of studies to construct a funnel plot so we were unable to assess whether outcome reporting bias was likely.
Data synthesis
We analysed the following between‐group comparisons where possible:
hypertonic saline inhalation up to six hours before airway clearance techniques, compared to inhalation during airway clearance techniques;
hypertonic saline inhalation up to six hours before airway clearance techniques, compared to up to six hours after airway clearance techniques;
hypertonic saline inhalation during airway clearance techniques, compared to up to six hours after airway clearance techniques;
morning compared to evening inhalation with any definition provided by the author. If not defined, we accepted midnight to midday as morning and midday to midnight as evening.
We performed meta‐analyses using a fixed‐effect model. However, if we had observed substantial heterogeneity, we planned to use a random‐effects model. We planned to further explore heterogeneity in the planned subgroup analyses.
Subgroup analysis and investigation of heterogeneity
We planned a subgroup analysis for different concentrations of hypertonic saline inhalation: low hypertonic concentrations (3% to 5%); medium (6% to 7%); and high (8% to 10%). We also planned to perform a subgroup analysis for intervention duration: less than two weeks; two to eight weeks; and greater than eight weeks. However, the included studies did not permit these analyses.
Sensitivity analysis
We planned to conduct sensitivity analyses to assess the robustness of the review results by repeating the analyses with the following adjustments:
exclusion of trials with unclear or inadequate sequence generation;
exclusion of trials with unclear or inadequate allocation concealment;
exclusion of trials that scored less than 5 out of 10 on the PEDro Scale (Maher 2003);
modifying the definition of 'before' and 'after' airway clearance techniques to within 30 minutes of the techniques;
with and without cross‐over trials.
However, the included studies did not permit these analyses.
Summary of findings tables
In a post hoc change, we have presented three summary of findings tables, one for each comparison (Table 1; Table 2; Table 3). We have included the following outcomes: FEV1, FVC, measures of QoL, symptom scores, measures of sputum clearance, lung clearance index and adverse events.
Summary of findings for the main comparison. Inhalation before compared with during airway clearance techniques for cystic fibrosis.
| Inhalation before compared with during airway clearance techniques for cystic fibrosis | ||||||
|
Patient or population: adults and children with cystic fibrosis Settings: outpatients Intervention: inhalation before airway clearance techniques Comparison: inhalation during airway clearance techniques | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Inhalation during airway clearance techniques | Inhalation before airway clearance techniques | |||||
|
FEV1 % predicted Follow‐up: before treatment to ˜2 hours later |
Not reported1 | The mean FEV1 (% predicted) was 0.56% higher (0.48% lower to 1.60% higher) in the inhalation before airway clearance techniques. | NA | 63 (2 studies) | ⊕⊕⊖⊖ low2,3 |
FEV1 (L) also showed no statistically significant difference between groups. Participants received both inhalation approaches as cross‐over design. |
|
FVC % predicted Follow‐up: before treatment to 2 hours later |
Not reported1 | The mean FVC (% predicted) was 2.09% higher (0.08% higher to 4.11% higher) in the inhalation before airway clearance techniques. | NA | 50 (1 study) | ⊕⊕⊖⊖ low2,3 |
FEV1 (L) showed no statistically significant difference between groups. Participants received both inhalation approaches as cross‐over design. |
| QoL | Outcome not reported. | NA | NA | NA | ||
| Symptom scores | See comments. | NA | 76 (3 studies) | ⊕⊕⊖⊖ low2,3 |
All of the symptom scores suggest no statistically significant difference between the groups. This outcome includes a range of symptom scores from many different studies but number of participants contributing to each symptom score varies. |
|
| Measures of sputum clearance: sputum wet weight immediately (g) | Not reported1 | The mean wet weight of sputum during the application of the intervention was 0.26 g lower (4.79 g lower to 4.27 g higher) in the inhalation before airway clearance techniques. | NA | 26 (2 studies) | ⊕⊕⊖⊖ low2,3 |
The mean wet weight of sputum for the 24 hours following treatment suggests no statistically significant difference between the groups. Participants received both inhalation approaches as cross‐over design. |
|
LCI Follow‐up: 90 minutes later |
Not reported1 | The mean LCI was 0.02 lower (0.63 lower to 0.59 higher) in the inhalation before airway clearance techniques. | NA | 13 (1 study) | ⊕⊕⊖⊖ low2,3 |
The mean LCI immediately after treatment suggests no statistically significant difference between the groups. Participants received both inhalation approaches as cross‐over design. |
| Adverse events | Outcome not reported. | NA | NA | NA | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FEV1: forced expiratory volume at 1 second; FVC: forced vital capacity; LCI: lung clearance index; MD: mean difference; QoL: quality of life | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
1. Only differences between inhalation before and during airway clearance techniques were presented, results within the inhalation during airway clearance techniques were not presented therefore an assumed risk cannot be calculated.
2. Downgraded once due to high risk due to lack of blinding of participants and personnel.
3. Downgraded once for lack of applicability as studies included only adults so results are not applicable to children.
Summary of findings 2. Inhalation before compared with after airway clearance techniques for cystic fibrosis.
| Inhalation before compared with after airway clearance techniques for cystic fibrosis | ||||||
|
Patient or population: adults and children with cystic fibrosis Settings: outpatients Intervention: inhalation before airway clearance techniques Comparison: inhalation after airway clearance techniques | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Inhalation after airway clearance techniques | Inhalation before airway clearance techniques | |||||
|
FEV1 % predicted Follow‐up: before treatment to 2 hours later |
Not reported1 | The mean FEV1 (% predicted) was 0.75% higher (0.45% lower to 1.95% higher) in the inhalation before airway clearance techniques. | NA | 50 (1 study) | ⊕⊕⊖⊖ low2,3 |
FEV1 (L) also showed no statistically significant difference between groups. Participants received both inhalation approaches as cross‐over design. |
|
FVC % predicted Follow‐up: before treatment to 2 hours later |
Not reported1 | The mean FVC (% predicted) was 1.66% higher (1.42% lower to 4.74% higher) in the inhalation before airway clearance techniques. | NA | 50 (1 study) | ⊕⊕⊖⊖ low2,3 |
FVC (L) showed no statistically significant difference between groups. Participants received both inhalation approaches as cross‐over design. |
| QoL | Outcome not reported. | NA | NA | NA | ||
| Symptom scores | See comments. | NA | 50 (1 study) | ⊕⊕⊖⊖ low2,3 |
Three symptom scores have been presented: Perceived efficacy shows a statistically significant difference in favour of inhalation before airway clearance techniques (MD 10.62; 95% CI 2.54 to 18.70) Tolerability showed no statistically significant difference between groups. Satisfaction shows a statistically significant difference in favour of inhalation before airway clearance techniques, MD 20.38 (95% CI 12.10 to 28.66) |
|
| Measures of sputum clearance | Outcome not reported. | NA | NA | NA | ||
| LCI | Outcome not reported | NA | NA | NA | ||
| Adverse events | Outcome not reported | NA | NA | NA | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FEV1: forced expiratory volume at 1 second; FVC: forced vital capacity; LCI: lung clearance index; MD: mean difference; QoL: quality of life | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
1. Only differences between inhalation before and during airway clearance techniques were presented, results within the inhalation during airway clearance techniques were not presented therefore an assumed risk cannot be calculated.
2. Downgraded once due to high risk due to lack of blinding of participants and personnel.
3. Downgraded once for lack of applicability as studies included only adults so results are not applicable to children.
Summary of findings 3. Inhalation during compared with after airway clearance techniques for cystic fibrosis.
| Inhalation during compared with after airway clearance techniques for cystic fibrosis | ||||||
|
Patient or population: adults and children with cystic fibrosis Settings: outpatients Intervention: inhalation during airway clearance techniques Comparison: Inhalation after airway clearance techniques | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Inhalation after airway clearance techniques | Inhalation during airway clearance techniques | |||||
|
FEV1 % predicted Follow‐up: before treatment to 2 hours later |
Not reported1 | The mean FEV1 (% predicted) was 0.03% lower (1.19% lower to 1.12% higher) in the inhalation during airway clearance techniques. | NA | 50 (1 study) | ⊕⊕⊖⊖ low2,3 |
FEV1 (L) also showed no statistically significant difference between groups. Participants received both inhalation approaches as cross‐over design. |
|
FVC % predicted Follow‐up: before treatment to 2 hours later |
Not reported1 | The mean FVC (% predicted) was 0.44% lower (3.34% lower to 2.46% higher) in the inhalation during airway clearance techniques. | NA | 50 (1 study) | ⊕⊕⊖⊖ low2,3 |
FVC (L) also showed no statistically significant difference between groups. Participants received both inhalation approaches as cross‐over design. |
| QoL | Outcome not reported. | NA | NA | NA | ||
| Symptom scores | See comments. | NA | 50 (1 study) | ⊕⊕⊖⊖ low2,3 |
Three symptom scores have been presented: Perceived efficacy shows a statistically significant difference in favour of inhalation during airway clearance techniques, MD 15.60 (95% CI 7.55 to 23.65) Tolerability showed no statistically significant difference between groups. Satisfaction shows a statistically significant difference in favour of inhalation during airway clearance techniques, MD 14.80 (95% CI 5.70 to 23.90) |
|
| Measures of sputum clearance | Outcome not reported. | NA | NA | NA | ||
| LCI | Outcome not reported. | NA | NA | NA | ||
| Adverse events | Outcome not reported. | NA | NA | NA | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FEV1: forced expiratory volume at 1 second; FVC: forced vital capacity; LCI: lung clearance index; MD: Mean difference; QoL: quality of life | ||||||
| GRADE Working Group grades of evidence High certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty: we are very uncertain about the estimate. | ||||||
1. Only differences between inhalation before and during airway clearance techniques were presented, results within the inhalation during airway clearance techniques were not presented therefore an assumed risk cannot be calculated.
2. Downgraded once due to high risk due to lack of blinding of participants and personnel.
3. Downgraded once for lack of applicability as studies included only adults so results are not applicable to children.
We determined the certainty of the evidence using the GRADE approach; and downgraded evidence in the presence of: a high risk of bias in at least one study; indirectness of the evidence; unexplained heterogeneity or inconsistency; imprecision of results, or a high probability of publication bias. We downgraded evidence by one level if we considered the limitation to be serious and by two levels if very serious.
Results
Description of studies
Results of the search
The searches identified 104 trial reports which represented 50 trials.
Included studies
Three trials were eligible for inclusion, which provided data for a total of 77 participants (Dentice 2012; Van Ginderdeuren 2011; O'Neill 2016) (Figure 1). One trial has not yet been published in full but has been presented as an oral presentation at a conference (although additional data has been provided by the trial authors) (Van Ginderdeuren 2011). No trial was identified regarding timing of hypertonic saline inhalation in relation to time of day.
1.
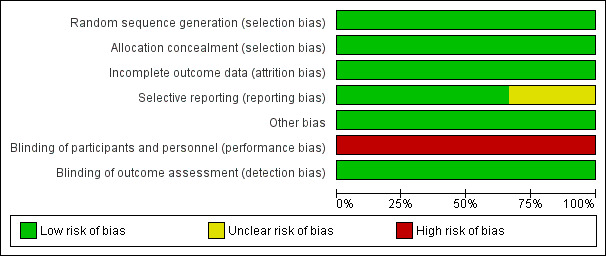
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Trial characteristics
The three included studies were randomised, cross‐over trials with concealed allocation, intention to treat analysis and blinded assessors (Dentice 2012; Van Ginderdeuren 2011; O'Neill 2016). They investigated the impact of timing of hypertonic saline inhalation in relation to airway clearance techniques. The inhalation blocks were for one day. In one trial, three treatments of the allocated timing regimen were performed on each of the three trial days (Dentice 2012). In the second trial, a single treatment of 30 minutes was undertaken on each of the three trial days (Van Ginderdeuren 2011). One day was solely autogenic drainage and did not include hypertonic saline inhalation. This arm of the trial was not relevant to the study question of this review so the data were not included. The third trial also delivered a single treatment on each trial day, but only two timing regimens (inhalation before airway clearance techniques and inhalation during airway clearance techniques) were compared, so there were only two trial days (O'Neill 2016).
Participants
Clarification of participant data was requested from the authors of the two of the three studies. One trial included 50 adults (mean (SD) age 31 (10) years, range 18 to 64 years) with a confirmed diagnosis of cystic fibrosis who were clinically stable with an FEV1 within 10% of the best recorded value in the last six months (Dentice 2012). This trial excluded people who: were hypertonic saline naïve or previously intolerant; had received a lung transplant; were colonised with Burkholderia cepacia complex; were not clinically stable; had experienced haemoptysis greater than 60 mL within the last month; or were thrombocytopaenic or pregnant. The second trial recruited 13 hospitalised participants who were over 14 years (mean age 27 years, range 18 to 37 years) (Van Ginderdeuren 2011). All were productive daily and performed autogenic drainage for their airway clearance. The lung function of participants was not stated but some were noted to be on oxygen therapy. One participant withdrew after the first arm of the trial and therefore did not provide any cross‐over data that could be included in the analysis. The third study recruited 14 adults (mean (SD) age 33 (12) years), who were nearing the end of a course of intravenous antibiotics (days 10 to 14) for a pulmonary exacerbation of cystic fibrosis (O'Neill 2016). Eligibility criteria included being productive of at least 10 g of sputum daily and demonstrated tolerance of hypertonic (7%) saline through either past or current use, The participants' mean (SD) FEV1 was 51 (22) per cent predicted.
Interventions
One trial administered 4 mL of 6% hypertonic saline via an LC Star (PARI, Germany) nebuliser three times per day with the allocated timing regimen for that day (Dentice 2012). Hypertonic saline was nebulised immediately before or after airway clearance, or during airway clearance with blocks of inhalation and pauses for airway clearance. Participants using positive expiratory pressure (PEP) devices were not permitted to administer hypertonic saline simultaneously as this alters the inhaled dose and the distribution of the deposition (Laube 2005). The airway clearance technique was optimised for each participant on recruitment to the trial and was standardised for all three trial days. The second trial administered 4 mL of 6% hypertonic saline before or during 30 minutes of autogenic drainage (Van Ginderdeuren 2011). The third trial (O'Neill 2016) administered 4mL of 7% hypertonic saline via a Portex updraft nebuliser (Smiths Medical, UK). Airway clearance involved 10 supervised cycles of oscillating PEP using the Acapella Duet device with forced expiration techniques, which in total required about 20 minutes. When hypertonic saline was allocated to be administered during airway clearance, this was achieved by attaching the updraft nebuliser to the Acapella Duet. On both days, 200 micrograms of salbutamol was administered 15 minutes before the commencement of the randomly allocation intervention for that day. The use of co‐interventions (such as bronchodilators and other inhaled medications) was not reported in the other two studies (Dentice 2012; Van Ginderdeuren 2011).
Outcomes measures
One trial measured the change in FEV1 and FVC (in litres and percentage of the predicted value) recorded prior to and two hours following the middle treatment session of each trial day (Dentice 2012). Symptom scores at the end of each intervention arm were also recorded: perceived effectiveness; tolerability; and satisfaction rated on a 100‐mm visual analogue scale. Adverse events and adherence were also recorded. At the conclusion of the three‐day trial, participants reported their preferred timing regimen.
The second trial reported the change in dyspnoea scores at the conclusion of each intervention arm, wet weight of sputum in grams produced during the treatment session, and adverse events and adherence (Van Ginderdeuren 2011).
The third trial reported FEV1 and FEF25‐75 as a percentage of the predicted value (O'Neill 2016). Ease of use of the intervention and satisfaction with the intervention were also rated by the participants and the treating physiotherapists on a 100‐mm visual analogue scale. The wet weight of sputum produced and the number of coughs occurring during the allocated treatment session were also recorded, as was the wet weight of sputum during the subsequent 24 hours. Using a multiple breath washout test, the LCI and FRC were also recorded before, immediately after, and 90 minutes after each allocated treatment regimen. The duration of treatment was also noted.
Excluded studies
Of the remaining 46 studies identified through our search strategy, two were excluded because they did not involve the administration of hypertonic saline at all and one because the comparison of timing regimens was confounded by the use of different airway clearance techniques in the two arms of the study. A further 43 studies were excluded because the interventions they compared did not differ in the timing of hypertonic saline inhalation (Excluded studies).
Risk of bias in included studies
Please refer to Figure 1; Figure 2.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Generation of sequence
All three trials reported adequate methods of random generation of the allocation sequence: computer‐generated list (Dentice 2012; O'Neill 2016) and allocations drawn from a box that had been shaken (Van Ginderdeuren 2011). Thus, the overall risk of bias for all trials due to the method of generation of the random sequence was low.
Concealment of allocation
Two trials reported using sealed opaque envelopes (Dentice 2012; Van Ginderdeuren 2011) and one reported using an independent investigator to conceal the allocation list (O'Neill 2016), so the overall risk of bias for all trials due to the non‐concealment of allocation was low.
Blinding
In all trials, participants were not blinded to the timing regimen, leading to a high risk of bias. In all trials, outcome assessors were blinded to the timing regimen, leading to a low risk of bias.
Incomplete outcome data
All trials reported all available data. One trial stated no withdrawals occurred (Dentice 2012). The second trial stated that one participant withdrew after the first arm of the trial and therefore did not provide any cross‐over data that could be included in the analysis (Van Ginderdeuren 2011) so analysis was conducted with all available data from 12 out of 13 participants (92%). The third trial ceased recruitment at 14 participants instead of the intended 31 because an interim analysis showed that the treatment was unlikely to be statistically or clinically significant (O'Neill 2016). Additionally, one of the 14 participants withdrew and data were reported on the remaining 13 participants (93%). Therefore, the risk of bias due to attrition was low across all trials.
Selective reporting
Two trials had a low risk of bias for this domain; one trial was prospectively registered and remained consistent with this protocol (Dentice 2012); one trial was prospectively registered and reported all of the registered outcome measures, but some additional unregistered outcome measures (ease, satisfaction, cough and treatment duration) were also reported (O'Neill 2016). The third trial was not registered and therefore had an unclear risk of bias (Van Ginderdeuren 2011).
Other potential sources of bias
No other potential sources of bias were identified.
All of the trials met the external validity criterion of the PEDro score by stating both the source of participants and the eligibility criteria. Both trials met eight of the ten internal validity criteria on the PEDro score, with neither trial blinding participants or therapists.
Effects of interventions
See: Table 1; Table 2; Table 3
Inhalation before versus during airway clearance techniques
Three studies with a total of 77 participants contributed data to this comparison (Dentice 2012; Van Ginderdeuren 2011; O'Neill 2016).
Primary outcomes
1. Lung function
a. FEV1
One trial (50 participants) provided data in litres for this outcome (Dentice 2012). The change in FEV1 from immediately before a treatment to two hours later was higher on average when hypertonic saline was inhaled before airway clearance techniques, but this was not statistically significant (Analysis 1.1). Two trials (63 participants) reported estimates of the effect on FEV1 using % predicted data, however, this difference was not statistically significant (low‐certainty evidence) (Analysis 1.2) (Dentice 2012; O'Neill 2016).
1.1. Analysis.
Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 1 FEV1 (L).
1.2. Analysis.
Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 2 FEV1 (% pred).
b. FVC
One trial (50 participants) provided data for this outcome (Dentice 2012). The change in FVC from immediately before a treatment to two hours later was not statistically significantly different between trial arms when analysed using data in litres (Analysis 1.3). However, change in FVC from immediately before a treatment to two hours later was higher on average when hypertonic saline was inhaled before airway clearance techniques when analysed using % predicted data, with a MD of 2.09% pred (95% CI 0.08 to 4.11) (low‐certainty evidence) (Analysis 1.4).
1.3. Analysis.
Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 3 FVC (L).
1.4. Analysis.
Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 4 FVC (% pred).
2. Patient‐reported outcomes
a. QoL
No trial reported this outcome.
b. Symptom scores
Three studies with a total of 77 participants contributed data to this comparison (low‐certainty evidence) (Dentice 2012; O'Neill 2016; Van Ginderdeuren 2011).
Cough in the 24 hours following treatment was reported for 13 participants in one trial (O'Neill 2016). There was no significant difference between the groups in the number of coughs (Analysis 1.5).
1.5. Analysis.
Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 5 Coughs (n).
The remaining symptoms were all measured on a 100‐mm visual analogue scale.
Dyspnoea was reported for 12 participants in one trial (Van Ginderdeuren 2011). There was no significant difference in the severity of dyspnoea between the groups (Analysis 1.6).
1.6. Analysis.
Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 6 Dyspnoea (mm).
Tolerability and perceived efficacy were reported for 50 participants in one trial (Dentice 2012), there were no significant differences between the groups for either outcome (Analysis 1.7; Analysis 1.8).
1.7. Analysis.
Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 7 Tolerability (mm).
1.8. Analysis.
Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 8 Perceived efficacy (mm).
Ease of sputum clearance was reported for 13 participants in one trial (O'Neill 2016). The same trial also reported data estimates of ease of sputum clearance from the treating physiotherapist. There were no significant between‐group differences for the patient ratings (Analysis 1.9), nor for the therapist ratings (Analysis 1.10).
1.9. Analysis.
Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 9 Ease (mm).
1.10. Analysis.
Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 10 Ease rated by therapist (mm).
Satisfaction with the treatment regimen was rated by the patients in two trials (64 participants) (Dentice 2012; O'Neill 2016). The pooled result of meta‐analysis indicated no significant difference between the groups (Analysis 1.11). The O'Neill trial also obtained data on satisfaction from the treating physiotherapist (O'Neill 2016). Again there was no significant difference between the groups (Analysis 1.12).
1.11. Analysis.
Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 11 Satisfaction (mm).
1.12. Analysis.
Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 12 Satisfaction rated by therapist (mm).
Secondary outcomes
1. Measures of sputum clearance
Two trials provided data from 25 participants for wet weight of sputum during the application of the intervention (O'Neill 2016; Van Ginderdeuren 2011). The pooled result of meta‐analysis indicated no significant difference between the groups (low‐certainty evidence) (Analysis 1.13). One of the trials also collected data on wet weight of sputum for the 24 hours following treatment, again there was no significant difference between the groups (O'Neill 2016) (Analysis 1.14).
1.13. Analysis.
Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 13 Sputum wet weight immediately (g).
1.14. Analysis.
Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 14 Sputum wet weight next 24 hours (g).
2. Measures of exercise capacity
None of the trials reported on this outcome.
3. Mortality
No trial reported this outcome.
4. Other pulmonary parameters
a. FEF25‐75
One trial provided FEF25‐75 data as % predicted for 13 participants (O'Neill 2016) There was no significant difference between the groups (Analysis 1.15).
1.15. Analysis.
Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 15 FEF25‐75 (% pred).
e. FRC
One trial (13 participants) provided data on FRC immediately after the treatment and 90 minutes later (O'Neill 2016). There was no significant difference between the groups immediately after the treatment (Analysis 1.16) or at 90 minutes (Analysis 1.17).
1.16. Analysis.
Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 16 FRC immediately (L).
1.17. Analysis.
Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 17 FRC at 90 min (L).
f. LCI
One trial provided data on LCI immediately after the treatment and 90 minutes later (O'Neill 2016). There was no significant difference between the groups immediately after the treatment (Analysis 1.18) or at 90 minutes (low‐certainty evidence) (Analysis 1.19).
1.18. Analysis.
Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 18 LCI immediately.
1.19. Analysis.
Comparison 1 Inhalation before versus during airway clearance techniques, Outcome 19 LCI at 90 min.
No trial reported any of the other pulmonary parameters listed in the protocol.
5. Frequency of exacerbations of respiratory infection
No trial reported this outcome.
6. Adherence to inhaled therapies, airway clearance techniques, and other therapies
There was 100% compliance with all the single doses of each treatment under supervision in all trials (Dentice 2012; O'Neill 2016; Van Ginderdeuren 2011).
7. Adverse effects such as bronchospasm, cough and acute decline in pulmonary function
There were no adverse events in any trial (Dentice 2012; Van Ginderdeuren 2011; O'Neill 2016).
Inhalation before versus after airway clearance techniques
One trial with 50 participants contributed data to this comparison (Dentice 2012).
Primary outcomes
1. Lung function (absolute change and change in per cent predicted if possible, otherwise final values)
a. FEV1
The change in FEV1 from immediately before a treatment to two hours later was not significantly influenced by whether hypertonic saline was inhaled before or after airway clearance techniques, whether analysed using data in litres (Analysis 2.1) or % predicted (low‐certainty evidence) (Analysis 2.2).
2.1. Analysis.
Comparison 2 Inhalation before versus after airway clearance techniques, Outcome 1 FEV1 (L).
2.2. Analysis.
Comparison 2 Inhalation before versus after airway clearance techniques, Outcome 2 FEV1 (% pred).
b. FVC
The change in FVC from immediately before a treatment to two hours later was not significantly influenced by whether hypertonic saline was inhaled before or after airway clearance techniques, whether analysed using data in litres (Analysis 2.3) or % predicted (low‐certainty evidence) (Analysis 2.4).
2.3. Analysis.
Comparison 2 Inhalation before versus after airway clearance techniques, Outcome 3 FVC (L).
2.4. Analysis.
Comparison 2 Inhalation before versus after airway clearance techniques, Outcome 4 FVC (% pred).
2. Patient‐reported outcomes
a. QoL
The trial did not report this outcome.
b. Symptom scores
Perceived efficacy (i.e. ease of clearance) was rated statistically significantly better when hypertonic saline was inhaled before versus after airway clearance techniques, MD 10.62 mm (95% CI 2.54 to 18.70) (low‐certainty evidence) (Analysis 2.5)
2.5. Analysis.
Comparison 2 Inhalation before versus after airway clearance techniques, Outcome 5 Perceived efficacy (mm).
Tolerability was not rated significantly differently when hypertonic saline was inhaled before versus after airway clearance techniques (low‐certainty evidence) (Analysis 2.6).
2.6. Analysis.
Comparison 2 Inhalation before versus after airway clearance techniques, Outcome 6 Tolerability (mm).
Satisfaction was rated significantly better when hypertonic saline was inhaled before versus after airway clearance techniques, MD 20.38 mm (95% CI 12.10 to 28.66) (low‐certainty evidence) (Analysis 2.7).
2.7. Analysis.
Comparison 2 Inhalation before versus after airway clearance techniques, Outcome 7 Satisfaction (mm).
Secondary outcomes
1. Measures of sputum clearance
The trial did not report this outcome.
2. Measures of exercise capacity
The trial did not report this outcome.
3. Mortality
The trial did not report this outcome.
4. Other pulmonary parameters
The trial did not report this outcome.
5. Frequency of exacerbations of respiratory infection
The trial did not report this outcome.
6. Adherence to inhaled therapies, airway clearance techniques, and other therapies
There was 100% compliance with all the single doses of each treatment.
7. Adverse effects such as bronchospasm, cough and acute decline in pulmonary function
There were no adverse events in the trial.
Inhalation during versus after airway clearance techniques
One trial with 50 participants contributed data to this comparison (Dentice 2012).
Primary outcomes
1. Lung function
a. FEV1
The change in FEV1 from immediately before a treatment to two hours later was not significantly influenced by whether hypertonic saline was inhaled before or after airway clearance techniques, whether analysed using data in litres (low‐certainty evidence) (Analysis 3.1) or % predicted (Analysis 3.2).
3.1. Analysis.
Comparison 3 Inhalation during versus after airway clearance techniques, Outcome 1 FEV1 (L).
3.2. Analysis.
Comparison 3 Inhalation during versus after airway clearance techniques, Outcome 2 FEV1 (% pred).
b. FVC
The change in FVC from immediately before a treatment to two hours later was not significantly influenced by whether hypertonic saline was inhaled before or after airway clearance techniques, whether analysed using data in litres (low‐certainty evidence) (Analysis 3.3) or % predicted (Analysis 3.4).
3.3. Analysis.
Comparison 3 Inhalation during versus after airway clearance techniques, Outcome 3 FVC (L).
3.4. Analysis.
Comparison 3 Inhalation during versus after airway clearance techniques, Outcome 4 FVC (% pred).
2. Patient‐reported outcomes
a. QoL
The trial dd not report this outcome.
b. Symptom scores
Perceived efficacy (i.e. ease of clearance) was rated significantly better when hypertonic saline was inhaled during versus after airway clearance techniques, MD 15.60 mm (95% CI 17.55 to 23.65) (low‐certainty evidence) (Analysis 3.5).
3.5. Analysis.
Comparison 3 Inhalation during versus after airway clearance techniques, Outcome 5 Perceived efficacy (mm).
Tolerability was not rated significantly differently when hypertonic saline was inhaled during versus after airway clearance techniques (low‐certainty evidence) (Analysis 3.6).
3.6. Analysis.
Comparison 3 Inhalation during versus after airway clearance techniques, Outcome 6 Tolerability (mm).
Satisfaction was rated significantly better when hypertonic saline was inhaled during versus after airway clearance techniques, MD 14.80 mm (95% CI 5.70 to 23.90) (low‐certainty evidence) (Analysis 3.7).
3.7. Analysis.
Comparison 3 Inhalation during versus after airway clearance techniques, Outcome 7 Satisfaction (mm).
Secondary outcomes
1. Measures of sputum clearance
The trial did not report this outcome.
2. Measures of exercise capacity
The trial did not report this outcome.
3. Mortality
The trial did not report this outcome.
4. Other pulmonary parameters
The trial did not report this outcome.
5. Frequency of exacerbations of respiratory infection
The trial did not report this outcome.
6. Adherence to inhaled therapies, airway clearance techniques, and other therapies
There was 100% compliance with all the single doses of each treatment.
7. Adverse effects such as bronchospasm, cough and acute decline in pulmonary function
There were no adverse events in the trial.
Morning versus evening inhalation
No trial reported on this comparison.
Discussion
Summary of main results
The searches identified 51 studies, of which three studies were eligible for inclusion ‐ providing data for 77 participants. All studies used a cross‐over design. Intervention periods were short, so the overnight washout periods were probably appropriate. One trial provided three treatments in one day for each arm of the trial (Dentice 2012). The other two trials provided a single treatment for each arm of the trial on separate days (Van Ginderdeuren 2011; O'Neill 2016).
There were no clinically important differences between the timing regimens of before, during or after airway clearance techniques in the mean amount of improvement in lung function, with the between‐group comparisons being non‐significant (Dentice 2012; O'Neill 2016).
There were little or no differences in symptom scores when the timing regimens of before or during airway clearance techniques were compared. Outcomes included cough, dyspnoea, tolerability, ease of clearance and overall satisfaction (Dentice 2012; O'Neill 2016; Van Ginderdeuren 2011). Ease of sputum clearance and overall satisfaction were also estimated by the treating physiotherapist in one trial, but there were little or no differences between the timing regimens (O'Neill 2016).
Perceived efficacy was between 10 and 20 mm lower on a 100‐mm scale when hypertonic saline was inhaled after physical airway clearance techniques, as opposed to before or during the techniques (Dentice 2012). Satisfaction with the entire treatment regimen may be lower when hypertonic saline was inhaled after physical airway clearance techniques, as opposed to before or during the techniques (Dentice 2012).
Additional outcomes for the before versus during timing comparison, which included wet weight of sputum (during and 24hours following), FEF25‐75, FRC (immediately and 90 minutes post), LCI (immediately and 90 minutes post), showed there may be little or no difference between these timing regimens (Dentice 2012; O'Neill 2016; Van Ginderdeuren 2011).
One trial achieved delivery of hypertonic saline during airway clearance by using an Acapella duet device, which combines a nebuliser in series with an oscillating PEP mechanism (O'Neill 2016). Devices such as this may introduce additional considerations. For example, the Acapella duet device substantially reduces the duration of treatment (O'Neill 2016), but it also substantially reduces the dose received by the patient (Berlinski 2014). These other issues were not included in the outcome measures of this systematic review, but clinicians should nevertheless consider these issues when deciding whether to use such a device to deliver hypertonic saline during airway clearance.
Overall completeness and applicability of evidence
No trials were found comparing morning versus evening inhalation of hypertonic saline. No trials were found in paediatric populations. Recommendations of the review are therefore limited to timing of inhalation in relation to airway clearance techniques in adults.
Quality of the evidence
The evidence for each outcome was rated as low according to the GRADE system, indicating that further research is very likely to have an important impact on our confidence in the estimates of effect and is likely to change the estimates. The GRADE result reflects two limitations of the included studies. The first issue is poor applicability, because studies included only adults so results are not applicable to children. The other issue is the lack of blinding. While it is often difficult for trials of physical interventions to achieve blinding, this was achieved in most of the trials in the Cochrane Review of timing of dornase alpha (Dentice 2018) using a double‐dummy method. It is disappointing that none of the included trials in the present review used this method to achieve blinding.
Agreements and disagreements with other studies or reviews
This 2019 review update identified an additional trial (O'Neill 2016), which shared outcome measures with the previous two eligible trials, permitting meta‐analysis for the before versus during timing comparison. One of the included studies did repeat the data collection on 14 participants within one year of doing the trial (Dentice 2012), with very similar results to the original data collected on the 50 participants.
The data identified by this review support the traditional approach of inhalation of hypertonic saline before airway clearance techniques, as has been used in previous studies demonstrating the efficacy of hypertonic saline for people with cystic fibrosis (Elkins 2006c; Eng 1996; Robinson 1996; Robinson 1997). However, the data also suggest that inhalation during airway clearance may be equally effective.
When advising about inhalation of hypertonic saline in relation to time of day, what issues might be considered in the absence of direct comparisons of different timing regimens of hypertonic saline? The trials demonstrating the efficacy of regular hypertonic saline have prescribed at least two doses per day (Donaldson 2006; Elkins 2006c; Eng 1996). The effect of single daily dosing on clinical outcomes is not known. Several studies suggest that as the dose of salt received is reduced, there is a reduction in hypertonic saline's effect on sputum rheology (King 1997; Wills 1997), mucociliary clearance (Robinson 1997), and perhaps some clinical outcomes (Elkins 2006d). Therefore, individuals should be encouraged to take doses twice daily if tolerated; however, for those who only tolerate one dose per day and elect to pursue this regimen, a choice about diurnal timing must be made. In the absence of a randomised comparison of morning versus evening inhalation, individual factors such as convenience, compliance, and tolerability could be considered. For example, if an individual has a hectic morning schedule and spare time after their day's activities, evening inhalation may be more convenient. Conversely, if individuals note increased nocturnal coughing and sleep disturbance with evening inhalation, morning inhalation may be better tolerated.
Authors' conclusions
Implications for practice.
The included trials identified that the timing of hypertonic saline in relation to physical airway clearance techniques did not have a substantial effect on the change in lung function, lung clearance index, functional residual capacity, sputum wet weight, dyspnoea or cough after a single treatment session. However, participants may be more satisfied with the entire treatment session when hypertonic saline was inhaled before or during the physical airway clearance techniques – presumably because these timing regimens were perceived as more effective than inhaling hypertonic saline after the techniques. This may have important implications for long‐term adherence, which is known to be low for both hypertonic saline and physical airway clearance techniques (Abbott 2004; Elkins 2006b).
Perceived efficacy and satisfaction were lower when hypertonic saline was inhaled after physical airway clearance techniques than with the other timing regimens. Inhalation of hypertonic saline after the physical techniques may fail to capitalise on effects of hypertonic saline on mucus clearance if techniques to promote expectoration are not undertaken until four to six hours later.
On the basis of these results, we suggest that adults with cystic fibrosis who use hypertonic saline and physical airway clearance techniques to inhale the saline before or during the physical techniques. The hypertonic saline should also be inhaled after a bronchodilator as was the protocol in the three included trials, because it has previously been established that this is necessary to prevent bronchoconstriction (Bye 2007).
This review did not identify any evidence comparing the timing of hypertonic saline inhalation in relation to time of day. Until such evidence becomes available, clinicians could advise patients to inhale hypertonic saline twice daily; but if only one dose per day is tolerated, the time of day at which it is inhaled could be based on convenience or tolerability.
Implications for research.
Any effect of the timing regimens on forced expiratory volume at one second (FEV1) was minor. The mean differences and their 95% confidence intervals (CIs) were all well below 150 mL (the a priori smallest worthwhile effect in the Dentice trial), and equated to approximately 1% of the predicted normal value (Dentice 2012). Therefore, although the mean results favoured inhalation of hypertonic saline before physical airway clearance techniques, any effects of the timing regimens on FEV1 are probably too small to be clinically important. However, unlike the narrow confidence intervals seen in the FEV1 data, some of the between‐group comparisons for forced vital capacity (FVC) had much wider 95% CIs, suggesting that further research could modify the estimate. For example, inhaling hypertonic saline before physical airway clearance techniques might increase the improvement in FVC by as much as 180 mL more than inhaling it during or after the techniques. Therefore, further data could be obtained to make the estimate of the effect on FVC more precise and then to determine whether one timing regimen has a clinically important benefit over another.
In addition, the trials were all of very short intervention periods, so longer‐term research could be conducted to establish the effects arising from regular use, which would incorporate the influence of changes in adherence with long‐term use, as well as generating data on any adverse effects that occur with long‐term use.
Devices that combine nebulisation with (oscillating) PEP have the potential to improve adherence to therapy because they may substantially reduce the overall treatment duration (O'Neill 2016). However, a benchtop study has demonstrated that the delivered dose decreases by 84% when delivered via the Acapella Duet (Berlinski 2014), presumably due to obstruction of the aerosol pathway. Several studies suggest that when the dose of salt received is reduced, there is a reduction in hypertonic saline's effect on sputum rheology (King 1997; Wills 1997), mucociliary clearance (Robinson 1997), and perhaps some clinical outcomes (Elkins 2006b). These issues warrant further investigation so that an informed recommendation can be made regarding nebulisers that incorporate PEP or oscillating PEP mechanisms that obstruct the aerosol pathway.
What's new
| Date | Event | Description |
|---|---|---|
| 24 February 2020 | New citation required but conclusions have not changed | The conclusions have not been not changed, however, one recommendation for future research has been added. |
| 24 February 2020 | New search has been performed | A search of the Cochrane Cystic Fibrosis and Genetic Disorders Cystic Fibrosis Trials Register identified seven potentially‐eligible references to five trials, one was an additional reference to a study already awaiting classification (O'Neill 2016), which has now been included in the review. |
History
Protocol first published: Issue 11, 2010 Review first published: Issue 2, 2012
| Date | Event | Description |
|---|---|---|
| 20 December 2016 | New search has been performed | Changes have been made throughout the review. Two new trials have been included in the review (Dentice 2012; Van Ginderdeuren 2011). |
| 20 December 2016 | New citation required and conclusions have changed | Previously there were no trials included in the review; however, for this update, two trials have been included (with a total of 63 participants) (Dentice 2012; Van Ginderdeuren 2011). |
Acknowledgements
We wish to thank Tracey Remmington and Nikki Jahnke for their assistance in the preparation of this review.
Appendices
Appendix 1. PEDro search strategy
1. Hypertonic saline AND cystic fibrosis (via Simple Search interface)
Appendix 2. WHO Clinical Trial Register search strategy
1. Cystic fibrosis AND Hypertonic saline
Appendix 3. ClinicalTrials.gov
1. Cystic fibrosis AND Hypertonic saline
Data and analyses
Comparison 1. Inhalation before versus during airway clearance techniques.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (L) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2 FEV1 (% pred) | 2 | Mean Difference (Fixed, 95% CI) | 0.56 [‐0.48, 1.60] | |
| 3 FVC (L) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 4 FVC (% pred) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 5 Coughs (n) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 6 Dyspnoea (mm) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 7 Tolerability (mm) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 8 Perceived efficacy (mm) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 9 Ease (mm) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 10 Ease rated by therapist (mm) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 11 Satisfaction (mm) | 2 | Mean Difference (Fixed, 95% CI) | 4.91 [‐1.58, 11.41] | |
| 12 Satisfaction rated by therapist (mm) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 13 Sputum wet weight immediately (g) | 2 | Mean Difference (Fixed, 95% CI) | ‐0.26 [‐4.79, 4.27] | |
| 14 Sputum wet weight next 24 hours (g) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 15 FEF25‐75 (% pred) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 16 FRC immediately (L) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 17 FRC at 90 min (L) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 18 LCI immediately | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 19 LCI at 90 min | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected |
Comparison 2. Inhalation before versus after airway clearance techniques.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (L) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2 FEV1 (% pred) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 3 FVC (L) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 4 FVC (% pred) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 5 Perceived efficacy (mm) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 6 Tolerability (mm) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 7 Satisfaction (mm) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected |
Comparison 3. Inhalation during versus after airway clearance techniques.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 (L) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2 FEV1 (% pred) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 3 FVC (L) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 4 FVC (% pred) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 5 Perceived efficacy (mm) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 6 Tolerability (mm) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 7 Satisfaction (mm) | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dentice 2012.
| Methods | Randomised, cross‐over trial with concealed allocation, intention‐to‐treat analysis and blinded assessors; investigating the impact of timing of hypertonic saline inhalation in relation to airway clearance techniques. The inhalation blocks were for one day. The three treatments of the allocated timing regimen were performed on each of the three trial days, with no washout day. | |
| Participants | 50 adults (mean age 31 years, SD 10, range 18 ‐ 64 years) with a confirmed diagnosis of cystic fibrosis who were clinically stable with an FEV1 within 10% of the best recorded value in the last 6 months. This trial excluded people who were hypertonic saline naïve or previously intolerant, a lung transplant recipient, colonised with Burkholderia cepacia complex, not clinically stable, haemoptysis greater than 60 mL within the last month, thrombocytopenia or pregnancy. | |
| Interventions | 4 mL of 6% hypertonic saline was nebulised via an LC Star nebuliser 3 times per day with the allocated timing regimen for that day. Hypertonic saline was nebulised immediately before or after airway clearance or during (with blocks of inhalation and pauses for airway clearance). The airway clearance technique was optimised for each participant on recruitment to the trial and was standardised for all 3 trial days. | |
| Outcomes | The primary outcome was the change in FEV1 and FVC (in litres and percentage of the predicted value) recorded prior to and two hours following the middle treatment session of each trial day. Symptom scores at the end of each intervention arm were also recorded: perceived effectiveness, tolerability and satisfaction rated on a 100 mm visual analogue scale. Adverse events and adherence were also recorded. | |
| Notes | PEDro Score: 8/10 [Eligibility criteria: yes; random allocation: yes; concealed allocation: yes; baseline comparability: yes; blind participants: no; blind therapists: no; blind assessors: yes; adequate follow up: yes; intention‐to‐treat analysis: yes; Between‐group comparisons: yes; point estimates and variability: yes. Note: eligibility criteria item does not contribute to total score]. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random allocation list. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelopes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Stated "no withdrawals" and intention‐to‐treat analysis used. |
| Selective reporting (reporting bias) | Low risk | Consistent with the prospectively registered trial protocol. |
| Other bias | Low risk | None identified. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were unblinded to the timing regimen. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
O'Neill 2016.
| Methods | Randomised cross‐over trial. The physiotherapist collecting the outcome measures was blinded. | |
| Participants | 14 adults with CF recruited, 13 completed the trial (mean (SD) age 33 years (12), FEV1% predicted 51 (22), LCI (no. turnovers) 14 (4)). | |
| Interventions | ACTs after HTS inhalation or ACTs during HTS inhalation on alternate days. Between days 10 – 14 of intravenous antibiotic course during a pulmonary exacerbation. ACT treatment consisted of 10 cycles of active cycle of breathing technique using an Acapella®. | |
| Outcomes | Participants completed a multiple breath washout (MBW) test to obtain LCI and spirometry (FEV1) at baseline and 90 min post treatment. Sputum collection during 90 min, ease of clearance and satisfaction with treatment was also recorded. Wilcoxon test was used and P < 0.05 was considered significant. | |
| Notes | PEDro Score: 8/10 [Eligibility criteria: yes; random allocation: yes; concealed allocation: yes; baseline comparability: yes; blind participants: no; blind therapists: no; blind assessors: yes; adequate follow up: yes; intention‐to‐treat analysis: yes; Between‐group comparisons: yes; point estimates and variability: yes. Note: eligibility criteria item does not contribute to total score]. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was electronically generated. |
| Allocation concealment (selection bias) | Low risk | The random allocation list was concealed by an administrator independent of the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant was lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | Consistent with the prospectively registered trial protocol. |
| Other bias | Low risk | None identified. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were unblinded to the timing regimen. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
Van Ginderdeuren 2011.
| Methods | Randomised, cross‐over trial with concealed allocation, intention to treat analysis and blinded assessors; investigating the impact of timing of hypertonic saline inhalation in relation to airway clearance techniques. The inhalation blocks were for one day. The two treatment days that contributed data to this review involved single treatment of 30 minutes with differing timing of hypertonic saline in relation to physiotherapy airway clearance techniques (i.e. hypertonic saline before or during autogenic drainage). The other day was solely autogenic drainage and did not include hypertonic saline inhalation, so it is not discussed further in this review. | |
| Participants | 13 hospitalised participants who were over 14 years (mean age 27 years, range 18 ‐ 37 years). All were productive daily and performed autogenic drainage for their airway clearance. The lung function of participants was not stated but some were noted to be on oxygen therapy. One participant withdrew and outcome data was not included in analysis. | |
| Interventions | 4 mL of 6% hypertonic saline before or during 30 minutes of autogenic drainage. The type of nebuliser and use of co‐interventions were not reported. | |
| Outcomes | Outcomes included change in dyspnoea scores at the conclusion of each intervention arm, wet weight of sputum in grams produced during the treatment session, and adverse events and adherence. | |
| Notes | PEDro Score: 8/10 [Eligibility criteria: yes; random allocation: yes; concealed allocation: yes; baseline comparability: yes; blind participants: no; blind therapists: no; blind assessors: yes; adequate follow up: yes; intention‐to‐treat analysis: yes; between‐group comparisons: yes; point estimates and variability: yes. Note: eligibility criteria item does not contribute to total score]. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocations were drawn from a box after the box had been shaken. |
| Allocation concealment (selection bias) | Low risk | Allocations were sealed in opaque envelopes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant was lost to follow up but intention to treat analysis was conducted. |
| Selective reporting (reporting bias) | Unclear risk | No registered protocol. No protocol available from the author. |
| Other bias | Low risk | None identified. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were unblinded to the timing regimen. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
FEV1: forced expiratory volume at one second FVC: forced vital capacity SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adde 2004 | Intervention not related to the timing of hypertonic saline inhalation. |
| Amin 2010 | Intervention not related to the timing of hypertonic saline inhalation. |
| Amin 2016 | Intervention not related to the timing of hypertonic saline inhalation. |
| Aquino 2012 | Intervention not related to the timing of hypertonic saline inhalation. |
| Balinotti 2015 | Intervention not related to the timing of hypertonic saline inhalation. |
| Ballmann 2002 | Intervention not related to the timing of hypertonic saline inhalation. |
| Brivio 2013 | Intervention not related to the timing of hypertonic saline inhalation. |
| Brown 2010 | Intervention not related to the timing of hypertonic saline inhalation. |
| Buonpensiero 2010 | Intervention not related to the timing of hypertonic saline inhalation. |
| Cardinale 2003 | Intervention not related to the timing of hypertonic saline inhalation. |
| Chadwick 1997 | Intervention not related to the timing of hypertonic saline inhalation. |
| Corcoran 2017 | Intervention not related to the timing of hypertonic saline inhalation. |
| De Cono 2008 | Intervention not related to the timing of hypertonic saline inhalation. |
| Dentice 2013 | Intervention not related to the timing of hypertonic saline inhalation. |
| Donaldson 2003 | Intervention not related to the timing of hypertonic saline inhalation. |
| Donaldson 2006 | Intervention not related to the timing of hypertonic saline inhalation. |
| Donaldson 2013 | Intervention not related to the timing of hypertonic saline inhalation. |
| Dwyer 2013 | Intervention not related to the timing of hypertonic saline inhalation. |
| Elkins 2006c | Intervention not related to the timing of hypertonic saline inhalation. |
| Elkins 2006d | Intervention not related to the timing of hypertonic saline inhalation. |
| Eng 1996 | Intervention not related to the timing of hypertonic saline inhalation. |
| Furnari 2012 | Intervention not related to the timing of hypertonic saline inhalation. |
| Grasemann 2005 | Did not involve administration of hypertonic saline. |
| Grasemann 2013 | Did not involve administration of hypertonic saline. |
| Grieve 2003 | Intervention not related to the timing of hypertonic saline inhalation. |
| Gupta 2012 | Intervention not related to the timing of hypertonic saline inhalation. |
| Herrero 2016 | Different airway clearance techniques in the two study arms confound the comparison of the effects of timing. |
| Hofmann 1997 | Intervention not related to the timing of hypertonic saline inhalation. |
| Homola 2013 | Intervention not related to the timing of hypertonic saline inhalation. |
| Kobylyansky 2000 | Intervention not related to the timing of hypertonic saline inhalation. |
| Laube 2009 | Intervention not related to the timing of hypertonic saline inhalation. |
| Mainz 2015 | Intervention not related to the timing of hypertonic saline inhalation. |
| Nenna 2017 | Intervention not related to the timing of hypertonic saline inhalation. |
| Palacio 2014 | Intervention not related to the timing of hypertonic saline inhalation. |
| Riedler 1996 | Intervention not related to the timing of hypertonic saline inhalation. |
| Robinson 1996 | Intervention not related to the timing of hypertonic saline inhalation. |
| Robinson 1997 | Intervention not related to the timing of hypertonic saline inhalation. |
| Robinson 1999 | Intervention not related to the timing of hypertonic saline inhalation. |
| Ros 2012 | Intervention not related to the timing of hypertonic saline inhalation. |
| Rosenfeld 2012 | Intervention not related to the timing of hypertonic saline inhalation. |
| Ruiz 2012 | Intervention not related to the timing of hypertonic saline inhalation. |
| Stahl 2017 | Intervention not related to the timing of hypertonic saline inhalation. |
| Suri 2002 | Intervention not related to the timing of hypertonic saline inhalation. |
| Suri 2007 | Intervention not related to the timing of hypertonic saline inhalation. |
| Teper 2012 | Intervention not related to the timing of hypertonic saline inhalation. |
| Vanlaethem 2008 | Intervention not related to the timing of hypertonic saline inhalation. |
Contributions of authors
RD and ME contributed to: the writing of the protocol; the conduct of the searches, the review of the search results; and the writing of the final review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
Both review authors are authors on the Dentice trial, therefore data extraction for this trial was also carried out at the editorial base (Dentice 2012).
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Dentice 2012 {published data only}
- Dentice R, Elkins M, Bye P. Hypertonic saline before vs during vs after physiotherapy techniques for airway clearance in people with cystic fibrosis: A randomised trial. Physiotherapy 2011;97:eS308‐eS309. [Abstract no.: RR‐PL‐3136; CENTRAL: 1089301; CRS: 5500050000000300; EMBASE: 71882596] [Google Scholar]
- Dentice R, Elkins MR, Bye PT. A randomised trial of the effect of timing of hypertonic saline inhalation in relation to airway clearance physiotherapy in adults with cystic fibrosis. Pediatric Pulmonology 2010;Suppl 33:384. [Google Scholar]
- Dentice RL, Elkins MR, Bye PT. Adults with cystic fibrosis prefer hypertonic saline before or during airway clearance techniques: a randomised crossover trial. Journal of Physiotherapy 2012;58(1):33‐40. [] [DOI] [PubMed] [Google Scholar]
- Dentice RL, Elkins MR, Bye PT. Online Supplementary Material ‐ Table 3 : Individual outcome data to "Adults with cystic fibrosis prefer hypertonic saline before or during airway clearance techniques: a randomised crossover trial" [online]. Journal of Physiotherapy 2012;58(1):33‐40 Online. [] [DOI] [PubMed] [Google Scholar]
O'Neill 2016 {published data only}
- O'Neill K, Moran F, Bradbury I, Downey DG, Rendall J, Tunney MM, et al. Exploring the timing of hypertonic saline (HTS) and airways clearance techniques (ACT) in cystic fibrosis (CF): a crossover study. Thorax 2016;71 Suppl 3:A134. [Abstract no: P95; CENTRAL: 1253123; CFGD Register: BD231a; CRS: 5500135000001677] [Google Scholar]
- O'Neill K, Moran F, Tunney MM, Elborn JS, Bradbury I, Downey DG, et al. Timing of hypertonic saline and airway clearance techniques in adults with cystic fibrosis during pulmonary exacerbation: pilot data from a randomised crossover study. BMJ Open Respiratory Research 2017;4:e000168. [CFGD Register: BD231b] [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Ginderdeuren 2011 {published and unpublished data}
- Ginderdeuren F, Vanlaethem S, Eyns H, Schutter I, Dewachter E, Malfroot A. Influence of inhaled hypertonic saline (NaCI 6%) before or during autogenic drainage on sputum weight, oxygen saturation, heart frequency and dysponoea in cystic fibrosis. Journal of Cystic Fibrosis 2011;10 Suppl 1:S62. [CFGD Register: BD164; ] [Google Scholar]
References to studies excluded from this review
Adde 2004 {published data only}
- Adde FV, Borges KTL, Hatanaka ACF, Nakaie CMA, Cardieri JMA, Oliveira RC, et al. Hypertonic saline X recombinant human DNase: a randomised crossover study in 18 cystic fibrosis patients. Journal of Cystic Fibrosis 2004;4(Suppl 1):S66. [Google Scholar]
Amin 2010 {published data only}
- Amin R, Subbarao P, Jabar A, Balkovec S, Jensen R, Kerrigan S, et al. Hypertonic saline improves the LCI in paediatric patients with CF with normal lung function. Thorax 2010;65(5):379‐83. [DOI] [PubMed] [Google Scholar]
- Amin R, Subbarao P, Jabar A, Balkovec S, Jensen R, Kerrigan S, et al. Inhaled hypertonic saline (7%) improves LCI in paediatric CF patients with normal lung function. Pediatric Pulmonology 2009;44(Suppl 32):289. [Abstract no.: 223] [Google Scholar]
Amin 2016 {published data only}
- Amin R, Stanojevic S, Kane M, Webster H, Ratjen F. A randomized controlled trial to evaluate the lung clearance index as an outcome measure for early phase studies in patients with cystic fibrosis. Respiratory Medicine 2016;112:59‐64. [CRS: 5500135000001524; PUBMED: 26856191] [DOI] [PubMed] [Google Scholar]
Aquino 2012 {published data only}
- Aquino ES, Shimura F, Santos AS, Goto DM, Coelho CC, Fuccio MB, et al. CPAP has no effect on clearance, sputum properties, or expectorated volume in cystic fibrosis. Respiratory Care 2012;57(11):1914‐9. [; JID:: 7510357] [DOI] [PubMed] [Google Scholar]
Balinotti 2015 {published data only}
- Balinotti J, Rodriguez V, Zaragoza S, Lubovich S, Kofman C, Garcia Bournissen F. Effect of early intervention with inhaled hypertonic saline on lung function in infants and toddlers with cystic fibrosis diagnosed by neonatal screening. American Journal of Respiratory and Critical Care Medicine 2015;191(Meeting Abstracts):A3342. [CENTRAL: 1127230; CRS: 5500050000000303] [Google Scholar]
Ballmann 2002 {published data only}
- Ballmann M, Hardt H. Hypertonic saline and recombinant human DNase: a randomised cross‐over pilot study in patients with cystic fibrosis. Proceedings of the 22nd European Cystic Fibrosis Conference; 1998 June 13‐19; Berlin, Germany. 1998:80. [DOI] [PubMed]
- Ballmann M, Hardt H. Hyptertonic saline and recombinant human DNase: a randomised cross‐over pilot study in patients with cystic fibrosis. Journal of Cystic Fibrosis 2002;1(1):35‐7. [DOI] [PubMed] [Google Scholar]
Brivio 2013 {published data only}
- Brivio A, Ceruti C, Gambazza S, Colombo C. Randomized double‐blind monocentric trial on tolerability, acceptability and efficacy of two formulations of inhaled 7% hypertonic saline with and without hyaluronic acid in reducing airways inflammation in patients with cystic fibrosis ‐ Preliminary results. Journal of Cystic Fibrosis 2013;12(Suppl 1):S104. [Google Scholar]
- Brivio A, Conese M, Gambazza S, Biffi A, Tirelli AS, Russo M, et al. Pilot Randomized Controlled Trial Evaluating the Effect of Hypertonic Saline With and Without Hyaluronic Acid in Reducing Inflammation in Cystic Fibrosis. Journal of Aerosol Medicine and Pulmonary Drug Delivery 2016;29(6):482‐9. [CENTRAL: 1155400; CRS: 5500135000001523; PUBMED: 27149365] [DOI] [PubMed] [Google Scholar]
Brown 2010 {published data only}
- Brown AW, Laube BL, Zeman K, Lechtzin N, Sharpless G, Wu J, et al. Durability of hypertonic saline for enhancing mucociliary clearance in cystic fibrosis. Pediatric Pulmonology 2010;45 Suppl 33:303. [Abstract no.: 237; ] [Google Scholar]
Buonpensiero 2010 {published data only}
- Buonpensiero P, Gregorio F, Sepe A, Pasqua A, Ferri P, Siano M, et al. Hyaluronic acid improves "pleasantness" and tolerability of nebulized hypertonic saline in a cohort of patients with cystic fibrosis. Advances in Therapy 2010;27(11):870‐8. [CFGD Register: BD156c] [DOI] [PubMed] [Google Scholar]
- Buonpensiero P, Gregorio F, Sepe A, Pasqua A, Ferri P, Siano M, et al. Hyaluronic acid improves tolerability of hypertonic saline in CF patients. Journal of Cytsic Fibrosis 2010;9(Suppl 1):S63. [Abstract no: 245; CFGD Register: BD156b] [Google Scholar]
- Buonpensiero P, Gregorio F, Sepe A, Pasqua A, Ferri P, Siano M, et al. Inhaled hyaluronic acid improves pleasantness and tolerability of nebulised hypertonic saline in patients with cystic fibrosis. Pediatric Pulmonology 2009;44 Suppl 32:243. [Absract no: 93; CFGD Register: BD156a] [Google Scholar]
Cardinale 2003 {published data only}
- Cardinale F, Manca A, Mappa L, Tesse R, Cavallone R, Loffredo MS, et al. Effects on exhaled nitric oxide levels and lung function of ultrasonically nebulized hypertonic solution in cystic fibrosis. European Respiratory Journal 2003;22(Suppl 45):231s. [Google Scholar]
Chadwick 1997 {published data only}
- Chadwick SL, Moss SJ, Bott J, Geddes DM, Alton EWFW. Effect of hypertonic saline, isotonic saline and water challenges on the airways of cystic fibrosis patients. Thorax 1997;52(Suppl 6):A43. [Google Scholar]
Corcoran 2017 {published data only}
- Corcoran TE, Godovchik JE, Donn KH, Busick DR, Goralski J, Locke LW, et al. Overnight delivery of hypertonic saline by nasal cannula aerosol for cystic fibrosis. Pediatric Pulmonology 2017;52(9):1142‐9. [CFGD Register: BD207] [DOI] [PMC free article] [PubMed] [Google Scholar]
De Cono 2008 {published data only}
- Cono N, Schelstraete P, Haerynck F, daele S, Sanders N, Baets F. Hypertonic saline: effect on mucus rheology and spirometry. Journal of Cystic Fibrosis 2008;7(Suppl 2):S24. [Abstract no: 93; CFGD Regsiter: BD130] [Google Scholar]
Dentice 2013 {published data only}
- Dentice R, Elkins M, Bye P. A randomised controlled trial of hypertonic saline inhalation to enhance airway clearance physiotherapy in adults hospitalised with Cystic Fibrosis (CF). Physiotherapy 2015;101:eS356. [CENTRAL: 1126510; CRS: 5500050000000302; EMBASE: 72114064] [Google Scholar]
- Dentice R, Elkins M, Bye P. A randomised trial of hypertonic saline nebulisation during hospitalisation for pulmonary exacerbation in adults with cystic fibrosis. Pediatric Pulmonology 2012;47 Suppl:257‐8. [Abstract no.: 102; CENTRAL: 1030604; CRS: 5500050000000301; EMBASE: 70891850] [Google Scholar]
- Dentice R, Elkins M, Middleton P, Bishop J, Wark P, Dorahy D, et al. A randomised controlled trial of the effect of hypertonic saline (HS) inhalation on exacerbation resolution, hospital length of stay and time to relapse in adults with cystic fibrosis. Journal of Cystic Fibrosis 2013;12 Suppl 1:S38. [Abstract no.: WS19.4; ] [Google Scholar]
- Dentice RL, Elkins MR, Middleton PG, Bishop JR, Wark PAB, Dorahy DJ, et al. A randomised trial of hypertonic saline during hospitalisation for exacerbation of cystic fibrosis. Thorax 2016;71(2):141‐7. [CENTRAL: 1138352; CRS: 5500050000000401; EMBASE: 20160158105] [DOI] [PubMed] [Google Scholar]
Donaldson 2003 {published data only}
- Donaldson SH, Bennett W, Zeman K, Knowles MR, Boucher RC. Efficacy of amiloride and hypertonic saline in cystic fibrosis. Pediatric Pulmonology 2003;Suppl 25:251. [Google Scholar]
Donaldson 2006 {published data only}
- Donaldson SH, Bennett W, Zeman K, Knowles MR, Boucher RC. Efficacy of amiloride and hypertonic saline in cystic fibrosis. Pediatric Pulmonology 2003;Suppl 25:251. [CFGD Register: BD110a] [Google Scholar]
- Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. New England Journal of Medicine 2006;354(3):241‐50. [CFGD Register: BD110b] [DOI] [PubMed] [Google Scholar]
- Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Online Supplement to 'Mucus clearance and lung function in cystic fibrosis with hypertonic saline' [online supplement]. New England Journal of Medicine 2006; Vol. 354, issue 3:241‐50. [CFGD Register: BD110c] [DOI] [PubMed]
Donaldson 2013 {published data only}
- Donaldson SH, Samulski D, LaFave C, Wu J, Zeman K, Salazar C, Bennett WD, Davis SD. Sustained effect of hypertonic saline on mucociliary clearance in CF children with mild lung disease. Pediatric Pulmonology 2013;48(Suppl 36):210. [Google Scholar]
Dwyer 2013 {published data only}
- Dwyer T, Elkins M, Dentice R, Forbes S, McArthur M, Cooper P, et al. Saline at a lower tonicity in cystic fibrosis (SALTI‐CF) trial ‐ a randomised, controlled trial comparing 0.9% v 3% v 6% nebulised saline. Journal of Cystic Fibrosis 2013;12 Suppl 1:S19. [Abstract no.: WS9.5; ] [Google Scholar]
Elkins 2006c {published data only}
- Bye P, Elkins M, Robinson M, Moriarty C, Rose B, Harbour C, et al. Long‐term inhalation of hypertonic saline in patients with cystic fibrosis‐ a randomised controlled trial. Pediatric Pulmonology 2004;38(Suppl 27):329. [CFGD Register: BD114a] [Google Scholar]
- Elkins M, Robinson M, Moriarty C, Sercombe J, Rose B, Harbour C, et al. Three methods of monitoring adherence in a long‐term trial in Cystic Fibrosis. Journal of Cystic Fibrosis 2006;5(Suppl 1):S111. [Abstract no: 504; CFGD Register: BD114d] [Google Scholar]
- Elkins MR, Robinson M, Moriarty C, Sercombe J, Rose B, Harbour C. Three methods of monitoring adherence in the national hypertonic saline trial in cystic fibrosis. Pediatric Pulmonology 2005;40(Suppl 28):268. [CFGD Register: BD114c] [Google Scholar]
- Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, et al. A controlled trial of long‐term inhaled hypertonic saline in patients with cystic fibrosis. New England Journal of Medicine 2006;354(3):229‐40. [CFGD Register: BD114b] [DOI] [PubMed] [Google Scholar]
- Ratjen F. Inhaled hypertonic saline produces small increases in lung function in patients with cystic fibrosis. Journal of Pediatrics 2006;149(1):142. [CENTRAL: 1253122; CFGD Register: BD114e; CRS: 5500135000001675; PUBMED: 17243308] [DOI] [PubMed] [Google Scholar]
Elkins 2006d {published data only}
- Elkins MR, Tingpej P, Moriarty CP, Yozghatlian V, Rose BR, Harbour C, et al. Tolerability of hypertonic saline when delivered rapidly via the Eflow® rapid nebulizer in subjects with cystic fibrosis. Pediatric Pulmonlogy 2006;41(Suppl 29):292. [Google Scholar]
Eng 1996 {published data only}
- Button BM, Riedler J, Reade T, Eng P, Robertson CF. Inhaled hypertonic saline as an adjunct to chest physiotherapy in cystic fibrosis: The three‐year clinical experience. Pediatric Pulmonology 1996;22(Suppl 13):306. [Google Scholar]
- Eng P, Morton J, Douglass J, Riedler J, Wilson J, Robertson CF. Efficacy of short‐term ultrasonically nebulised hypertonic saline in patients with cystic fibrosis. European Respiratory Journal 1995;8(Suppl 19):510s. [Google Scholar]
- Eng P, Morton J, Douglass J, Riedler J, Wilson J, Robertson CF. Short‐term administration of ultrasonically nebulised hypertonic saline improves pulmonary function in patients with cystic fibrosis. Australian and New Zealand Journal of Medicine 1995;25:440. [Google Scholar]
- Eng PA, Morton J, Douglass JA, Riedler J, Wilson J, Robertson CF. Short‐term efficacy of ultrasonically nebulized hypertonic saline in cystic fibrosis. Pediatric Pulmonology 1996;21(2):77‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Furnari 2012 {published data only}
- Furnari ML, Termini L, Traverso G, Barrale S, Bonaccorso MR, Damiani G, et al. Nebulized hypertonic saline containing hyaluronic acid improves tolerability in patients with cystic fibrosis and lung disease compared with nebulized hypertonic saline alone: a prospective, randomized, double‐blind, controlled study. Therapeutic Advances in Respiratory Disease 2012;6(6):315‐22. [] [DOI] [PubMed] [Google Scholar]
Grasemann 2005 {published data only}
- Grasemann H, Kurtz F, Ratjen F. Inhalation of nebulized L‐Arginine increases exhaled nitric oxide and improves pulmonary function in patients with cystic fibrosis. Pediatric Pulmonology 2005;40 Suppl 28:308. [] [Google Scholar]
Grasemann 2013 {published data only}
- Grasemann H, Tullis E, Ratjen F. A randomized controlled trial of inhaled l‐Arginine in patients with cystic fibrosis. Journal of Cystic Fibrosis 2013;12(5):468‐74. [] [DOI] [PubMed] [Google Scholar]
- Grasemann H, Tullis E, Ratjen F. A randomized placebo controlled study on the effects of L‐arginine inhalation in patients with cystic fibrosis. Pediatric Pulmonology 2011;46 Suppl 34:293. [Abstract no.: 229; CENTRAL: 921615; CRS: 5500125000000391] [Google Scholar]
- Grasemann H, Tullis E, Ratjen F. Inhaled L‐arginine in patients with cystic fibrosis ‐ a randomized controlled trial]. Journal of Cystic Fibrosis 2011;10 Suppl 1:S21. [Abstract no.: 81; ] [DOI] [PubMed] [Google Scholar]
Grieve 2003 {published data only}
- Grieve R, Thompson S, Normand C, Suri R, Bush A, Wallis C. A cost‐effectiveness analysis of rhDNase in children with cystic fibrosis. International Journal of Technology Assessment in Health Care 2003;19(1):71‐9. [DOI] [PubMed] [Google Scholar]
Gupta 2012 {published data only}
- Gupta S, Ahmed F, Lodha R, Gupta YK, Kabra SK. Comparison of effects of 3 and 7% hypertonic saline nebulization on lung function in children with cystic fibrosis: a double‐blind randomized, controlled trial. Journal of Tropical Pediatrics 2012;58(5):375‐81. [] [DOI] [PubMed] [Google Scholar]
Herrero 2016 {published data only}
- Herrero Cortina B, San Miguel Pagola M, Cebria i Ranzo MA, Gomez Romero M, Diaz Gutierrez F, Reychler G. Short‐term effects of hypertonic saline nebulization combined with oscillatory positive expiratory pressure in cystic fibrosis: randomised crossover trial. Journal of Cystic Fibrosis 2016;15 Suppl 1:S33. [Abstract no.: WS21.3; CENTRAL: 1155402; CFGD Register: BD229a; CRS: 5500135000001528] [Google Scholar]
- San Miguel Pagola M, Herrero Cortina B, Cebria i Iranzo MA, Gomez Romero M, Diaz Gutierrez F, Reychler G. Hypertonic saline nebulization combined with oscillatory positive expiratory pressure accelerate sputum clearance in cystic fibrosis: a randomised crossover trial. European Respiratory Journal 2016;Suppl 60:PA1369. [CFGD Register: BD229b] [DOI] [PubMed] [Google Scholar]
Hofmann 1997 {published data only}
- Hofmann T, Senier I, Ziersch A, Geidel C, Regnis J, Lindemann H. Additive effect of amiloride and hypertonic saline on respiratory ion transport cystic fibrosis. Proceedings of the 21st European Cystic Fibrosis Conference; 1997; Davos, Switzerland. 1997:119.
Homola 2013 {published data only}
- Homola L, Holèikova A, Mikolasek P, Krbkova L. Efficacy of inhaled amiloride solution versus hypertonic saline, prospective open label single center study in children with cystic fibrosis. Journal of Cystic Fibrosis 2013;12 Suppl 1:S98. [Abstract no.: 195; ] [Google Scholar]
Kobylyansky 2000 {published data only}
- Kobylyansky VI, Gembitskaya TE. Study of the mucociliary and cough clearance in patients with mucoviscidosis and evaluation of hypertonic sodium chorlide solution influence on them. European Respiratory Journal 2000;16(Suppl 31):121S. [CFGD Register: BD111] [Google Scholar]
Laube 2009 {published data only}
- Laube BI, Sharpless GJ, Kelly AE, Mogayzel PJ. Effect of hypertonic saline on mucociliary clearance in children with cystic fibrosis. Pediatric Pulmonology 2009;44 Suppl 32:238. [Abstract no.: 77] [Google Scholar]
- Laube BL, Sharpless G, Carson KA, Kelly A, Mogayzel PJ. Acute inhalation of hypertonic saline does not improve mucociliary clearance in all children with cystic fibrosis. BMC Pulmonary Medicine 2011;11:45. [CENTRAL: 806034; CRS: 5500050000000304; PUBMED: 21896198] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mainz 2015 {published data only}
- Mainz JG, Schaedlich K, Hentschel J, Schumacher U, Koitschev C, Koitschev A, et al. Sinonasal inhalation of isotonic vs. hypertonic saline (6.0%) in CF patients with chronic rhinosinusitis ‐ results of a multicentre, double‐blind, controlled prospective trial. Journal of Cystic Fibrosis 2015;14 Suppl 1:S95. [Abstract no.: 146; CENTRAL: 1081480; CRG ID: CO59a; CRS: 5500135000000026] [DOI] [PubMed] [Google Scholar]
- Mainz JG, Schumacher U, Schadlich K, Hentschel J, Koitschev C, Koitschev A, et al. Sino nasal inhalation of isotonic versus hypertonic saline (6.0%) in CF patients with chronic rhinosinusitis ‐ Results of a multicenter, prospective, randomized, double‐blind, controlled trial. Journal of Cystic Fibrosis 2016;15(6):e57‐66. [CENTRAL: 1155399; CRG ID: CO59b; CRS: 5500135000001522; PUBMED: 27267518] [DOI] [PubMed] [Google Scholar]
Nenna 2017 {published data only}
- Nenna R, Midulla F, Castro G, Zicari AM, Indinnimeo L, Cimino G, et al. Effects of inhaled hypertonic (7%) saline on lung function test in preschool children with cystic fibrosis: results of a crossover, randomized clinical trial. Italian Journal of Pediatrics 2017;43(1):60. [CFGD Register: BD238] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palacio 2014 {published data only}
- Palacio S, Guigno H, Diaz Casaux A, Lucero B, Smith S, Giorgetti M, Bonina A, Castanos C. Inhaled 7% hypertonic saline treatment in preschool children with cystic fibrosis. Journal of Cystic Fibrosis 2014;13(Suppl. 2):S7. [Google Scholar]
Riedler 1996 {published data only}
- Button BM, Riedler J, Reade T, Eng P, Robertson CF. Inhlaed hypertonic saline as an adjunct to chest physiotherapy in cystic fibrosis; the three year clinical experience. Pediatric Pulmonology 1996;Suppl 13:306. [Google Scholar]
- Riedler J, Reade T, Button B, Robertson CF. A pilot study of inhaled hypertonic saline to increase sputum production in adolescents with cystic fibrosis. European Respiratory Journal 1994;7 Suppl 18:430s. [Google Scholar]
- Riedler J, Reade T, Button B, Robertson CF. Inhaled hypertonic saline increases sputum expectoration in cystic fibrosis. Journal of Paediatrics and Child Health 1996;32(1):48‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Robinson 1996 {published data only}
- Robinson M, Regnis JA, Bailey DL, King M, Bautovich GJ, Bye PT. Effect of hypertonic saline, amiloride, and cough on mucociliary clearance in patients with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 1996;153(5):1503‐9. [DOI] [PubMed] [Google Scholar]
Robinson 1997 {published data only}
- Robinson M, Hemming AL, Bailey DL, Mellis CM, Bautovich GJ, Bye PTP. Effect of increasing doses of hypertonic saline on mucociliary clearance in patients with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 1996;154(Suppl):A70. [DOI] [PubMed] [Google Scholar]
- Robinson M, Hemming AL, Regnis JA, Wong AG, Bailey DL, Bautovich GJ, et al. Effect of increasing doses of hypertonic saline on mucociliary clearance in patients with cystic fibrosis. Thorax 1997;52(10):900‐3. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson M, Parsons S, Hemming A, Bautovich G, Mellis CM, Bye PTP. Effect of increasing doses of hypertonic saline on mucociliary clearance in patients with cystic fibrosis. Australian and New Zealand Journal of Medicine 1997;27:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
Robinson 1999 {published data only}
- Robinson M, Daviskas E, Eberl S, Baker J, Anderson SD, Bye PT. Effect of inhaling a dry powder of mannitol on mucociliary clearance in adults with cystic fibrosis. Pediatric Pulmonology 1998;Suppl 17:280. [MEDLINE: ] [Google Scholar]
- Robinson M, Daviskas E, Eberl S, Baker J, Chan HK, Anderson SD, et al. The effect of inhaled mannitol on bronchial mucus clearance in cystic fibrosis patients: a pilot study. European Respiratory Journal 1999;14(3):678‐85. [DOI] [PubMed] [Google Scholar]
- Robinson M, Daviskas E, Eberl S, Baker J, Chan HK, Anderson SD, et al. The effect of inhaled mannitol on bronchial mucus clearance in cystic fibrosis patients: a pilot study. Pediatric Pulmonology 2000;29(6):484. [DOI] [PubMed] [Google Scholar]
Ros 2012 {published data only}
- Ros M, Casciaro R, Lucca F, Alatri F, Salonini E, Favilli F, et al. Tolerability and acceptability in patients with cystic fibrosis (CF) of two formulations of 7% hypertonic saline: a prospective multicenter clinical study. Pediatric Pulmonology 2012;47(S35):364. [Abstract no.: 390; ] [Google Scholar]
Rosenfeld 2012 {published data only}
- Alpern AN, Brumback LC, Ratjen F, Rosenfeld M, Davis SD, Quittner AL. Initial evaluation of the Parent Cystic Fibrosis Questionnaire‐‐Revised (CFQ‐R) in infants and young children. Journal of Cystic Fibrosis 2015;14(3):403‐11. [CENTRAL: 1130846; CRG ID: CRG ; CRS: 5500135000001461; PUBMED: 25443473] [DOI] [PubMed] [Google Scholar]
- Brumback LC, Baines A, Ratjen F, Davis SD, Daniel SL, Quittner AL, et al. Pulmonary exacerbations and parent‐reported outcomes in children <6 years with cystic fibrosis. Pediatric Pulmonology 2015;50:236‐43. [CENTRAL: 1046755; CRG ID: BD175g; CRS: 5500131000000349; GR:: U01 HL092931/HL/NHLBI NIH HHS/United States; JID:: 8510590; PMCID:: PMC4213320; PUBMED: 24777957; U01: HL092932/HL/NHLBI NIH HHS/United States] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SD, Ratjen F, Brumback LC, Johnson RC, Filbrun AG, Kerby GS, et al. Infant lung function tests as endpoints in the ISIS multicenter clinical trial in cystic fibrosis. Journal of Cystic Fibrosis 2016;15(3):386‐91. [CRG ID: BD175l; CRS: 5500135000001676; PUBMED: 26547590] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SD, Rosenfeld M, Brumback L, Donaldson S, Johnson R, Rowbotham R, et al. Infant PFTs as an endpoint in the infant study of inhaled saline randomized controlled trial. Pediatric Pulmonology 2012;47(S35):302. [Abstract no.: 225; CFGD Register: BD175d; ] [Google Scholar]
- Ratjen F, Rosenfeld M, Brumback L, Daniel S, Rowborham R. Efficacy of hypertonic saline in infants and young children: the ISIS study. Journal of Cystic Fibrosis 2012;11 Suppl 1:S50. [Abstract no.: WS22.1; CRG ID: BD175a; CRG ID: BD175a; ] [Google Scholar]
- Ratjen F, Salazar J, Jensen R, Amin R, Gustafsson P, Stanojevic S, et al. Alternative multiple breath washout outcomes for clinical trials in cystic fibrosis. European Respiratory Journal 2012;40 Suppl:Abstract no: 1261. [Abstract no.: 1261; CENTRAL: 1100495; CRG ID: BD175j; CRS: 5500050000000311; EMBASE: 71925430] [Google Scholar]
- Rosenfeld M. Hypertonic saline should be the first drug used in CF [summary]. Pediatric Pulmonology 2013;48 Suppl 36:157, Summary: S12.3. [CENTRAL: 921667; CRS: 5500125000000393; BD175e] [Google Scholar]
- Rosenfeld M, Ratjen F, Brumback L, Daniel S, Rowbotham R, McNamara S, et al. Inhaled hypertonic saline in infants and children younger than 6 years with cystic fibrosis: the ISIS randomized controlled trial. JAMA 2012;307(21):2269‐77. [CRG ID: BD175b; CRG ID: BD175b; ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M, Ratjen F, Brumback L, Daniel S, Rowbotham R, McNamara S, et al. Online supplement to "Inhaled hypertonic saline in infants and children younger than 6 years with cystic fibrosis: the ISIS randomized controlled trial" [online]. JAMA 2012;307(21):2269‐77. [CENTRAL: 1155401; CRS: 5500135000001526; BD175k] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M, Ratjen F, Brumback L, Kronmal R, Davis SD. Hypertonic saline in children with CF <6 years of age: Results of the ISIS trial. Pediatric Pulmonology 2012;47:172. [CENTRAL: 1030608; CRG ID: BD175h; CRS: 5500050000000298; EMBASE: 70891711] [Google Scholar]
- Subbarao P, Stanojevic S, Brown M, Jensen R, McDonald N, Gent K, et al. Effect of hypertonic saline on lung clearance index in infants and preschool children with CF: a pilot study. Pediatric Pulmonology 2012;47(S35):301. [Abstract no.: 223; CRG ID: BD175c; ] [Google Scholar]
- Subbarao P, Stanojevic S, Brown M, Jensen R, Rosenfeld M, Davis S, et al. Lung clearance index as an outcome measure for clinical trials in young children with cystic fibrosis. A pilot study using inhaled hypertonic saline. American Journal of Respiratory and Critical Care Medicine 2013;188(4):456‐60. [CENTRAL: 963541; CRG ID: BD15f; CRS: 5500127000000014; GR: Canadian Institutes of Health Research/Canada; JID: 9421642; OID: [Other ID]: NLM: PMC3778738; PMCID: PMC3778738; PUBMED: 23742699; SI: ClinicalTrials.gov/NCT00709280] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ruiz 2012 {published data only}
- Ruiz de Valbuena Maiz M, Lamas A, Maiz L, Giron R, Barrio M, Campo R, et al. Study of the efficacy of long‐term treatment with high volume compared to standard volume of hypertonic saline on pulmonary exacerbations in patients with cystic fibrosis. Pediatric Pulmonology 2012;47(S35):360. [Abstract no.: 379; ] [Google Scholar]
Stahl 2017 {published data only}
- Stahl M, Graeber SY, Sommerburg O, Ricklefs I, Diekmann G, Dopfer C, et al. Randomised, double‐blind, controlled pilot study on safety and efficacy of hypertonic saline as preventive inhalation therapy in infants with CF (PRESIS). Proceedings of the 40th Annual Joint Meeting of the Society for Pediatric Pulmonology, Austria 2018, issue 2:60‐1. [CFGD Register: BD249c]
- Stahl M, Graeber SY, Sommerburg O, Ricklefs I, Diekmann G, Dopfer C, et al. Randomized, double‐blind, controlled pilot study on safety and efficacy of hypertonic saline as preventive inhalation therapy in infants with CF (PRESIS). Pediatric Pulmonology 2017;Suppl 47:302. [CFGD Register: BD249b] [Google Scholar]
- Stahl M, Ricklefs I, Dopfer C, Barth S, Schlegtendal A, Graeber SY, et al. Randomised, double‐blind, controlled pilot study on safety and efficacy of hypertonic saline as preventive inhalation therapy in infants with CF (PRESIS). Journal of Cystic Fibrosis 2018;Suppl 3:S25‐26. [CFGD Register: BD249a] [Google Scholar]
Suri 2002 {published data only}
- Suri R, Grieve R, Normand C, Metcalfe C, Thompson S, Wallis C, et al. Effects of hypertonic saline, alternate day and daily rhDNase on healthcare use, costs and outcomes in children with cystic fibrosis. Thorax 2001;56(Suppl 3):iii84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri R, Grieve R, Normand C, Metcalfe C, Thompson S, Wallis C, et al. Effects of hypertonic saline, alternate day and daily rhDNase on healthcare use, costs and outcomes in children with cystic fibrosis. Thorax 2002;57(10):841‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suri R, Marshall LJ, Wallis C, Metcalfe C, Bush A, Shute JK. Effects of recombinant human DNase and hypertonic saline on airway inflammation in children with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 2002;166(3):352‐5. [DOI] [PubMed] [Google Scholar]
- Suri R, Metcalfe C, Lees B, Flather M, Normand C, Thompson S, et al. A cross‐over comparative study of hypertonic saline alternate day and daily rhDNase in children with cystic fibrosis. Thorax 2000;55:A75. [PubMed] [Google Scholar]
- Suri R, Metcalfe C, Lees B, Grieve R, Flather M, Normand C, et al. Comparison of hypertonic saline and alternate‐day or daily recombinant human deoxyribonuclease in children with cystic fibrosis: a randomised trial. Lancet 2001;358(9290):1316‐21. [DOI] [PubMed] [Google Scholar]
- Suri R, Metcalfe C, Wallis C, Bush A. Predicting response to rhDNase and hypertonic saline in children with cystic fibrosis. Pediatric Pulmonology 2004;37(4):305‐10. [DOI] [PubMed] [Google Scholar]
- Suri R, Wallis C, Bush A. In vivo use of hypertonic saline in CF. Pediatric Pulmonology 2000;Suppl 20:125‐6. [Google Scholar]
- Suri R, Wallis C, Bush A, Thompson S, Normand C, Flather M, et al. A comparative study of hypertonic saline, daily and alternate‐day rhDNase in children with cystic fibrosis. Health Technology Assessment 2002; Vol. 34, issue iii:1‐60. [PubMed]
- Suri R, Wallis C, Metcalfe C, Thompson S, Bush A, Shute J. Effects of rhDNase and hypertonic saline on airway inflammation in children with cystic fibrosis. Pediatric Pulmonology 2001;Suppl 22:281. [Google Scholar]
Suri 2007 {published data only}
- Suri R, Metcalfe C, Wallis C, Bush A. Assessing the usefulness of outcomes measured in a cystic fibrosis treatment trial. Respiratory Medicine 2007;101(2):254‐60. [DOI] [PubMed] [Google Scholar]
Teper 2012 {published data only}
- Balinotti JE, Rodriguez V, Zaragoza S, Lubovich S, Celiz M, Kofman C, et al. Inhaled 7% hypertonic saline in infants with cystic fibrosis: Is it safe and tolerable?. American Journal of Respiratory and Critical Care Medicine. American Thoracic Society, 2012; Vol. 185. [CENTRAL: 1107400; CRG ID: BD174b; CRS: 5500050000000400; EMBASE: 71989992]
- Teper A, Balinotti J, Rodriguez V, Zaragoza S Lubovich S, Kofman C, et al. Effect of early intervention with inhaled hypertonic saline on lung function in infants and toddlers with cystic fibrosis (CF) diagnosed by neonatal screening. Journal of Cystic Fibrosis 2015;14 Suppl 1:S49. [Abstract no.: ePS04.8; CRS: 5500135000001305] [Google Scholar]
- Teper A, Balinotti VA, Rodriguez VA, Zaragoza SM, Lubovich SL, Kofman C. Inhaled 7% hypertonic saline in infants with cystic fibrosis: is it safe and tolerable?. Journal of Cystic Fibrosis 2012;11 Suppl 1:S68. [Abstract no.: 48; CRG ID: BD174a; ] [Google Scholar]
Vanlaethem 2008 {published data only}
- Vanlaethem S, Ginderdeuren F, Eyns H, Malfroot A. Influence of inhaled hypertonic saline combined with airway clearance on SpO2, heart rate, dyspnoea and wet sputum weight in hospitalised CF patients. Journal of Cystic Fibrosis 2008;7(Suppl 2):S71. [Google Scholar]
Additional references
Abbott 2004
- Abbott J, Dodd ME, Webb AK. Adherence with the use of clearance techniques. In: Rubin BK, Schans CP editor(s). Therapy for Mucus‐Clearance Disorders. New York: Marcel Dekker, 2004:105‐128. [Google Scholar]
Armijo‐Olivo 2012
- Armijo‐Olivo S, Stiles CR, Hagen NA, Biondo PD, Cummings GG. Asssessment of study quality for systematic reviews: a comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: methodological research. J Eval Clin Pract 2012;18:12‐18. [DOI] [PubMed] [Google Scholar]
Bateman 1978
- Bateman JR, Pavia D, Clark SW. The retention of lung secretions during the night in normal subjects. Clinical Science and Molecular Medicine. Supplement 1978;55(6):523‐7. [DOI] [PubMed] [Google Scholar]
Berlinski 2014
- Berlinski A. In vitro evaluation of positive expiratory pressure devices attached to nebulizers. Respiratory Care 2014;59(2):216‐22. [DOI] [PubMed] [Google Scholar]
Button 2016
- Button BM, Wilson C, Dentice R, Cox NS, Middleton A, Tannenbaum E, et al. Physiotherapy for cystic fibrosis in Australia and New Zealand: A clinical practice guideline. Respirology 2016;21:656‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Buzzetti 2009
- Buzzetti R, Salvatore D, Baldo E, Forneris MP, Lucidi V, Manunza D, et al. An overview of international literature from cystic fibrosis registries: 1. Mortality and survival studies in cystic fibrosis. Journal of Cystic Fibrosis 2009;8(4):229‐37. [DOI] [PubMed] [Google Scholar]
Bye 2007
- Bye PTP, Elkins MR. Other mucoactive agents for cystic fibrosis. Paediatric Respiratory Reviews 2007;8:30‐9. [DOI] [PubMed] [Google Scholar]
Cutting 2002
- Cutting GR. Cystic fibrosis. In: Rimon DL, Connor JM, Pyeritz RE, Korf BR editor(s). Principles and Practice of Medical Genetics. 4th Edition. London: Harcourt, 2002:1561‐606. [Google Scholar]
de Morton 2009
- Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Australian Journal of Physiotherapy 2009;55(2):129‐33. [DOI] [PubMed] [Google Scholar]
Dentice 2018
- Dentice R, Elkins M. Timing of dornase alfa inhalation for cystic fibrosis. Cochrane Database of Systematic Reviews 2018, Issue 11. [DOI: 10.1002/14651858.CD007923.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Elkins 2006a
- Elkins M, Jones A, Schans CP. Positive expiratory pressure physiotherapy for airway clearance in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003147.pub3] [DOI] [PubMed] [Google Scholar]
Elkins 2006b
- Elkins MR, Bye PT. Inhaled hypertonic saline as a therapy for cystic fibrosis. Current Opinion in Pulmonary Medicine 2006;12(6):445‐52. [DOI] [PubMed] [Google Scholar]
Elkins 2013
- Elkins MR, Moseley AM, Sherrington C, Herbert RD, Maher CG. Growth in the Physiotherapy EvidenceDatabase (PEDro) and use of the PEDroscale. British Journal of Sports Medicine 2013;47(4):188‐189. [DOI] [PubMed] [Google Scholar]
Hartling 2009
- Hartling L, Ospina M, Liang Y, Dryden DNM, Hooton N, Krebs Seida J, et al. Risk of bias versus quality assessment of randomised controlled trials: cross sectional study. BMJ 2009;339:b4012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartling 2013
- Hartling L, Hamm MP, Milne A, Vandermeer B, Santaguida PL, Ansari M, et al. Testing the Risk of Bias tool showed low reliability between individual reviewers and across consensus assessments of reviewer pairs. Journal of Clinical Epidemiology 2013;66:973‐981. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins 2011: Higgins JPT, Altman DG, Sterne JAC, editor (s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org..
King 1997
- King M, Dasgupta B, Tomkiewicz RP, Brown NE. Rheology of cystic fibrosis sputum after in vitro treatment with hypertonic saline alone and in combination with recombinant human deoxyribonuclease. American Journal of Respiratory and Critical Care Medicine 1997;156(1):173‐7. [DOI] [PubMed] [Google Scholar]
Laube 2005
- Laube BL, Geller DE, Lin TC, Dalby RN, Diener‐West M, Zeitlin PL. Positive expiratory pressure changes aerosol distribution in patients with cystic fibrosis. Respiratory Care 2005;50:1438‐1444. [PubMed] [Google Scholar]
Maher 2003
- Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Physical Therapy 2003;83(8):713‐21. [PubMed] [Google Scholar]
Main 2009
- Main E, Prasad A, Schans CP. Conventional chest physiotherapy compared to other airway clearance techniques for cystic fibrosis. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD002011.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Michaleff 2011
- MIchaleff ZA, Costa LOP, Moseley AM, Maher CG, Elkins MR, Herbert RD, et al. CENTRAL, PEDro, PubMed, and EMBASE are the most comprehensive databases indexing randomized controlled trials of physical therapy interventions. Physical Therapy 2011;91(2):190‐7. [DOI] [PubMed] [Google Scholar]
Morrison 2009
- Morrison L, Agnew J. Oscillating devices for airway clearance in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD006842.pub2] [DOI] [PubMed] [Google Scholar]
Robinson 2010
- Robinson KA, Mckoy N, Saldanha I, Odelola OA. Active cycle of breathing technique for cystic fibrosis. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD007862.pub2] [DOI] [PubMed] [Google Scholar]
Shiwa 2011
- Shiwa SR, Costa LCM, Moseley AM, Lopes AD, Reggero CR, Sato TO, et al. Reproducibility of the Portuguese version of the PEDro scale. Cad Saúde Pública 2011;27:2063‐2068. [DOI] [PubMed] [Google Scholar]
van der Schans 2009
- Schans CP, Prasad A, Main E. Chest physiotherapy compared to no chest physiotherapy for cystic fibrosis. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD001401] [DOI] [PubMed] [Google Scholar]
Wark 2018
- Wark PAB, McDonald VM. Nebulised hypertonic saline for cystic fibrosis. Cochrane Database of Systematic Reviews 2018, Issue 9. [DOI: 10.1002/14651858.CD001506.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wills 1997
- Wills PJ, Hall RL, Chan W, Cole PJ. Sodium chloride increases the ciliary transportability of cystic fibrosis and bronchiectasis sputum on the mucus‐depleted bovine trachea. Journal of Clinical Investigation 1997;99(1):9‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wolff 1977
- Wolff RK, Dolovich MB, Obminski G, Newhouse MT. Effects of exercise and eucapnic hyperventilation on bronchial clearance in man. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology 1977;43(1):46‐50. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Elkins 2012
- Elkins M, Dentice R. Timing of hypertonic saline inhalation for cystic fibrosis. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD008816.pub2] [DOI] [PubMed] [Google Scholar]
Elkins 2016
- Elkins M, Dentice R. Timing of hypertonic saline inhalation for cystic fibrosis. Cochrane Database of Systematic Reviews 2016, Issue 12. [DOI: 10.1002/14651858.CD008816.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


