Abstract
Objectives
This study assessed the psychometric properties of the fatigue numeric rating scale (NRS) and sought to establish values for clinically meaningful change (responder definition).
Methods
Using disease-specific clinician-reported and patient-reported data from two randomised clinical trials of patients with psoriatic arthritis (PsA), the fatigue NRS was evaluated for test–retest reliability, construct validity and responsiveness. A responder definition was also explored using anchor-based and distribution-based methods.
Results
Test–retest reliability analyses supported the reproducibility of the fatigue NRS in patients with PsA (intraclass correlation coefficient=0.829). Mean (SD) values at baseline and week 2 were 5.7 (2.2) and 5.7 (2.4), respectively. Supporting construct validity of the fatigue NRS, moderate-to-large correlations with other assessments measuring similar concepts as measured by Sackett’s conventions were demonstrated. Fatigue severity was reduced when the underlying disease activity was improved and reductions remained consistent at week 12 and 24. A 3-point improvement was identified as being optimal for demonstrating a level of clinically meaningful improvement in fatigue NRS after 12–24 weeks of treatment.
Conclusions
Fatigue NRS is a valid and responsive patient-reported outcome instrument for use in patients with PsA. The established psychometric properties from this study support the use of fatigue NRS in clinical trials and in routine clinical practice. Robust validation of reliability for use in routine clinical practice in treating patients with active PsA in less active disease states and other more diverse ethnic groups is needed.
Keywords: psoriatic arthritis, patient perspective, outcomes research
Key messages.
What is already known about this subject?
Fatigue is an important domain of disease assessment in patients with psoriatic arthritis (PsA).
What does this study add?
Using disease-specific clinician-reported and patient-reported data from two phase III randomised, double-blind, parallel-group, placebo-controlled, multicentre clinical trials of patients with PsA, Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT)-P1 (NCT01695239) and SPIRIT-P2 (NCT02349295), this analysis showed that the fatigue numeric rating scale (NRS) is a well-defined, valid and responsive patient-reported outcomes instrument for evaluating fatigue over time in a clinical trial setting.
How might this impact on clinical practice?
The established psychometric properties from this study support the use of fatigue NRS in clinical trials to evaluate treatment efficacy at group level and potentially in routine clinical practice to assess and manage PsA-related fatigue.
Introduction
Psoriatic arthritis (PsA) is a chronic musculoskeletal disease affecting ~30% of patients with psoriasis in the USA,1 with an estimated 30–100 cases per 10 000 adults.2 Manifestations of PsA are heterogeneous, and assessing domains associated with disease activity drives treatment choices.2
Fatigue is a relevant and important symptom to patients with PsA. Studies have shown that up to 50% of patients with PsA experience moderate-to-severe fatigue.3–5 Fatigue is multifactorial and related to physical disability, pain, psychological distress and poor sleep quality.6 7 Patients with PsA experience inflammation, chronic pain and reduced physical fitness.8 These symptoms, coupled with decreased self-esteem and depression, manifest as fatigue and sleep disorders, ultimately affecting a patient’s ability to work,9 as well as their social relationships and quality of life.10 11 Assessing fatigue is paramount because it is considered an important domain for both clinical practice and clinical trials in patients with PsA, second only to pain.6
The area of fatigue assessment in PsA is evolving and requires further consideration.12–14 Although up to 78% of patients with PsA consider the fatigue domain a priority, fatigue is rarely reported as a core outcome.5 7 14–16 The PsA core domain set includes peripheral joint assessment, skin assessment, pain, patient global assessment, physical function and health-related quality of life,5 17 18 and, since 2016, musculoskeletal disease activity (arthritis, enthesitis, dactylitis, spondylitis), fatigue and systemic inflammation have been included.15
Several patient-reported outcome (PRO) scales have been used to assess fatigue in patients with PsA.12 Until 2016, 10 different instruments have been used to assess fatigue in PsA randomised, controlled trials, observational studies or registries.16 Few have been validated in PsA,8 18 and no single measure is favoured to evaluate symptoms of fatigue in PsA patients.12
The fatigue numeric rating scale (NRS) is a single-item PRO measure assessing severity of fatigue. The fatigue NRS is validated for use in rheumatoid arthritis,19 and it is currently used in a PsA-specific composite score (psoriatic arthritis impact of disease, PsAID). The objective of this study was to assess the psychometric properties of the fatigue NRS, including (1) test–retest reliability, (2) construct validity and (3) responsiveness and to establish an appropriate clinically meaningful responder definition for the fatigue NRS using disease-specific clinician-reported and patient-reported data from two randomised clinical trials of patients with PsA.
Methods
Eligibility criteria
Patients were males or females at least 18 years old who had an established diagnosis of active PsA for a minimum of 6 months according to the classification criteria for psoriatic arthritis, active PsA defined as the presence of ≥3 tender and ≥3 swollen joints, and the presence of active psoriatic skin lesion or a documented history of plaque psoriasis. Patients in Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT)-P1 were naïve to biological disease-modifying antirheumatic drugs,20 and participants in SPIRIT-P2 had been treated with one or two tumour necrosis factor inhibitors and discontinued due to either an inadequate response (≥12 weeks on therapy) or intolerance.21
Study design
The dataset used for these analyses came from two phase III randomised, double-blind, parallel-group, placebo-controlled, multicentre clinical trials; SPIRIT-P1 (NCT01695239)20 and SPIRIT-P2 (NCT02349295),21 conducted to assess the efficacy and safety of ixekizumab (IXE), a high-affinity monoclonal antibody that selectively targets IL-17A, for the treatment of active PsA. Details of these studies are reported elsewhere.20 21 Patients were randomised to IXE, placebo or adalimumab in SPIRIT-P120 and IXE or placebo in SPIRIT-P2.21 Both studies involved an initial 24-week treatment period; the primary safety and efficacy endpoints of the trials focused on this 24-week treatment period. The analyses described in this report do not evaluate treatment effects.
The studies20 21 were conducted in accordance with the consensus ethics principles derived from international ethics guidelines, including the Declaration of Helsinki and Council for International Organizations of Medical Sciences International Ethics Guidelines, the International Conference on Harmonisation Good Clinical Practice Guidelines, and applicable laws and regulations. The protocols were reviewed and approved by the institutional ethical review board, and all participants provided informed consent.
Measures
Fatigue numeric rating scale
The fatigue NRS is a patient-administered, single-item, 11-point horizontal scale anchored at 0 and 10, with 0 representing ‘no fatigue’ and 10 representing ‘as bad as you can imagine’. Patients are asked to ‘please rate your fatigue (weariness, tiredness) by selecting the number that describes your worst level of fatigue during the past 24 hours’ (online supplementary figure 1). In the SPIRIT-P1 study, the fatigue NRS was administered at baseline and weeks 4, 12, 16 and 24. The SPIRIT-P2 study had an additional time point: week 2. The following instruments were used in the evaluation of the psychometric properties of the fatigue NRS.
rmdopen-2019-000928supp003.pdf (27.7KB, pdf)
Health assessment questionnaire-disability index
The health assessment questionnaire-disability index (HAQ-DI) is a patient-reported standardised questionnaire that is commonly used in PsA to measure disease-associated disability (assessment of physical function).22 It consists of 24 questions referring to eight domains: dressing/grooming, arising, eating, walking, hygiene, reach, grip and other daily activities. The range of scores is from 0 to 3, with higher scores reflecting higher disability.
Patient global assessment
In the patient global assessment (PGA), the patient’s overall assessment of her or his PsA activity was recorded using the 100 mm horizontal visual analogue scale (VAS), where the left (score=0) anchor represents no disease activity and the right anchor represents extremely active disease.23
Disease activity index for psoriatic arthritis
The disease activity index for psoriatic arthritis (DAPSA) is a composite sum score for joint disease activity, including patient global and pain VAS, numeric swollen and tender joint count, and C reactive protein level.13 The range is from 0 to ~160, with higher scores reflecting higher disease activity.24
Medical outcomes study short-form 36
The short-form 36 (SF-36) is a 36-item patient-administered measure designed to be a generic, multipurpose assessment of health in the areas of physical functioning, role physical, role emotional, bodily pain, vitality, social functioning, mental health and general health.25 Physical component summary (PCS) and mental component summary can be calculated using weighted SF-36 domain scores (scoring manual).26 Higher scores reflect better health status.
Psoriatic arthritis disease activity score
The psoriatic arthritis disease activity score (PASDAS) is a composite outcome measure that includes the variables of patient and physician global VAS scores, dactylitis, enthesitis, C reactive protein (CRP), swollen joint counts (SJC), SF-36 PCS and tender joint counts (TJC). The PASDAS is represented by the equation: PASDAS = (((0.18 × √physician global VAS) + (0.159 × √patient global VAS) – (0.253 × √SF36 – PCS) + (0.101×LN (swollen joint count +1)) + (0.048×LN (tender joint count +1)) + (0.23×LN (Leeds Enthesitis Index+1)) + (0.377 LN (tender dactylitis count +1)) + (0.102×LN (CRP mg/dL+1))+2)*1.5.27
Composite psoriatic disease activity index
The composite psoriatic disease activity index (CPDAI) is a measure in which disease involvement is assessed in up to five domains: peripheral joints, skin, entheseal, dactylitis and spinal manifestations.13 28 Measures used are patient self-administered, physical examination and laboratory tests, recorded on paper or electronically. Higher scores correspond to more severe disease activity. In the SPIRIT studies, a modified CPDAI (mCPDAI) was used by the exclusion of the Ankylosing Spondylitis Quality of Life (ASQoL), which was not measured in SPIRIT-P1.
Statistical analysis
Descriptive statistics (means, percentages) are presented for study participant characteristics at baseline. We assessed test–retest validity, construct validity and responsiveness of the fatigue NRS. Since SPIRIT-P1 and SPIRIT-P2 enrolled different patient populations, all the analyses were conducted separately, and presented side-by-side. Within each study, all treatment groups were pooled. Missing data were low (<3% missing fatigue NRS in both studies at baseline and <10% on or before week 16). Missing postbaseline fatigue NRS scores and other continuous measures were imputed using the last observation carried forward.
Test–retest validity
Test–retest validity was assessed using SPIRIT-P2 data from baseline and week 2 in patients with stable disease. Stable disease was defined as patients in the placebo group with <20% change in tender joint count. An intraclass correlation coefficient (ICC) was calculated between the initial and retest scores (week 2), and paired t-tests were used for differences in means. An ICC of ≥0.70 was considered acceptable.29
Construct validity
Construct validity assesses the degree to which a measure correlates with other measures that are evaluating a similar construct.30 Construct validity was determined by Pearson correlations at baseline, week 12, and week 24 between scores of the fatigue NRS and HAQ-DI, PGA, DAPSA and SF-36 role emotional, social functioning, role physical, physical functioning and vitality domains. Analyses were conducted on pooled cohort data within each trial and missing data at weeks 12 and 24 were imputed using a last observation carried forward analysis. We hypothesised a high correlation of fatigue NRS with the SF-36 vitality scale which measures a very similar concept, and moderate correlations with other measures of disease activity and impact.
Responsiveness
Responsiveness was evaluated by associating calculated changes from baseline to week 12 and 24 in scores on the fatigue NRS with American College of Rheumatology (ACR) 20% (ACR20) and 50% (ACR50) response criteria. Effect size, defined as change from baseline divided by baseline SD, is also provided to help interpret the magnitude. The association was evaluated using the analysis of covariance model, including ACR response status and baseline fatigue NRS score. The anchor was defined as disease activity at endpoint or outcome. Missing data at weeks 12 and 24 were imputed using a last observation carried forward analysis. Data from the trials were not pooled for this analysis.
Responder definition
A responder definition for the interpretation of the fatigue NRS score that corresponds with marked clinical improvement in PsA was identified using both an anchor-based and a distribution-based approach.31 Selected anchors included ACR20, ACR50, HAQ-DI minimum clinically important difference (MCID),32 minimal disease activity (MDA)33 and psoriatic arthritis response criteria (PsARC).13 Receiver operating characteristic method was utilised to identify the cut-off best representing treatment benefit. In addition to the commonly used metrics, like sensitivity and specificity, three more metrics, including positive prediction, negative prediction and phi correlation,34 were also utilised in the method.
Results
A total of 780 patients (SPIRIT-P1 (n=417) and SPIRIT-P2 (n=363)) were included in this analysis. Table 1 describes the demographics and disease characteristics, in addition to baseline fatigue NRS scores. Mean patient age was 49.5 years in SPIRIT-P1 and 51.9 in SPIRIT-P2, ≥91% were white, and mean baseline fatigue NRS scores were 5.5 and 6.0, respectively. In SPIRIT-P1 and SPIRIT-P2, mean baseline tender joint count out of 68 joints (TJC 68) was 20.1 and 23.4, respectively, and swollen joint count out of 66 joints (SJC 66) was 11.0 and 12.3, respectively.
Table 1.
Demographics and disease characteristics
| SPIRIT-P1 | SPIRIT-P2 | ||||||||
| PBO (N=106) |
IXE Q4W (N=107) |
IXE Q2W (N=103) |
ADA Q2W* (N=101) |
Total (N=417) |
PBO (N=118) |
IXE Q4W (N=122) |
IXE Q2W (N=123) |
Total (N=363) |
|
| Age, years | 50.6 (12.3) | 49.1 (10.1) | 49.8 (12.6) | 48.6 (12.4) | 49.5 (11.9) | 51.5 (10.4) | 52.6 (13.6) | 51.7 (11.9) | 51.9 (12.0) |
| Male, n (%) | 48 (45.3) | 45 (42.1) | 48 (46.6) | 51 (50.5) | 192 (46.0) | 56 (47.5) | 63 (51.6) | 50 (40.7) | 169 (46.6) |
| White, n (%) | 99 (93.4) | 102 (95.3) | 96 (93.2) | 95 (94.1) | 392 (94.0) | 108 (91.5) | 111 (91.0) | 113 (92.6) | 332 (91.7) |
| CRP, mg/L | 15.1 (23.6) | 12.8 (16.4) | 15.1 (25.9) | 13.2 (19.1) | 14.1 (21.5) | 12.1 (19.6) | 17.0 (27.5) | 13.5 (26.1) | 14.2 (24.7) |
| TJC 68 | 19.2 (13.0) | 20.5 (13.7) | 21.5 (14.1) | 19.3 (13.0) | 20.1 (13.4) | 23.0 (16.2) | 22.0 (14.1) | 25.0 (17.3) | 23.4 (15.9) |
| SJC 66 | 10.6 (7.3) | 11.4 (8.2) | 12.1 (7.2) | 9.9 (6.5) | 11.0 (7.4) | 10.3 (7.4) | 13.1 (11.2) | 13.5 (11.5) | 12.3 (10.3) |
| Time since PsO diagnosis, years | 16.0 (13.8) | 16.5 (13.8) | 17.0 (14.0) | 15.7 (12.7) | 16.3 (13.5) | 15.3 (12.6) | 15.7 (12.3) | 16.5 (13.0) | 15.8 (12.6) |
| Time since PsA diagnosis, years | 6.3 (6.9) | 6.2 (6.4) | 7.2 (8.0) | 6.9 (7.5) | 6.7 (7.2) | 9.2 (7.3) | 11.0 (9.6) | 9.9 (7.4) | 10.0 (8.2) |
| Fatigue NRS score | 5.4 (2.2) | 5.4 (2.3) | 5.8 (2.3) | 5.5 (2.4) | 5.5 (2.3) | 5.9 (2.3) | 5.9 (2.5) | 6.0 (2.5) | 6.0 (2.4) |
Data are mean (SD) unless otherwise stated.
*ADA Q2W was an active reference arm for comparison with PBO in the SPIRIT-P1 trial; the trial was not powered to test equivalence or non-inferiority of ixekizumab versus adalimumab.
ADA Q2W, adalimumab 40 mg every 2 weeks; CRP, C reactive protein; IXE Q2W, ixekizumab 80 mg every 2 weeks; IXE Q4W, ixekizumab 80 mg every 4 weeks; n, number of patients in the specified category; N, number of patients in the analysis population; NRS, numeric rating scale; PBO, placebo; PsA, psoriatic arthritis; PsO, psoriasis; SJC 66, swollen joint count out of 66 joints; SPIRIT, Standard Protocol Items: Recommendations for Interventional Trials; TJC 68, tender joint count out of 68 joints.
The test–retest reliability supported the reproducibility of the fatigue NRS in patients treated with placebo with stable PsA (n=38; ICC (95% CI 0.829 (0.697 to 0.907)). The mean (SD) values at baseline and week 2 were 5.7 (2.2) and 5.7 (2.4), respectively (p=0.815).
Construct validity was supported by correlations between fatigue NRS and other outcomes at weeks 12 and 24 (online supplementary table 1). Fatigue NRS had the lowest correlations with mCPDAI (r=0.28 (0.10, 0.45) for SPIRIT-1 and r=0.51 (0.43, 0.59) for SPIRIT-P2 at week 12; r=0.48 (0.39, 0.55) for SPIRIT-P1 and r=0.53 (0.45, 0.61) for SPIRIT-P2 at week 24) and the highest correlation with the SF-36 vitality domain (r=−0.66 (−0.71, –0.60) for SPIRIT-P1 and r=−0.75 (−0.79, –0.70) for SPIRIT-P2 at week 12; r=−0.71 (−0.75, –0.66) for SPIRIT-P1 and r=−0.76 (−0.80, –0.72) for SPIRIT-P2 at week 24), with the other outcome measures falling in the moderate range for correlation as measured by Sackett’s conventions.35 Responsiveness of fatigue NRS was associated with week 12 and 24 outcomes, ACR20 and ACR50 (figure 1). Fatigue severity was reduced when the underlying disease was improved and reductions remained consistent at week 12 and 24. Consistent results were observed in both clinical trials.
Figure 1.
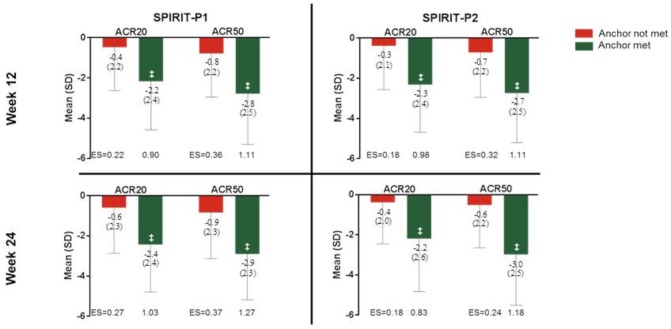
Change from baseline in fatigue numeric rating scale (NRS) score was evident in associations with week 12 and 24 ACR outcomes (NRI), intent-to-treat population, last observation carried forward. Effect size is defined as the magnitude of change from baseline divided by baseline SD. ‡p<0.001 versus anchor not met, from analysis of covariance model, including anchor status and baseline fatigue NRS score. ‘Anchor’ refers to ACR50 or 20 responder if ‘anchor met’, and non-responder if ‘anchor not met’. ACR20, American College of Rheumatology 20% response rate; ACR50, American College of Rheumatology 50% response rate; ES, effect size; NRI, non-responder imputation.
rmdopen-2019-000928supp001.pdf (42.5KB, pdf)
When using ACR20 as the anchor, the clinically meaningful change in fatigue NRS was identified by maximising the selected metrics shown in table 2A,B separately for each randomised controlled trial.
Table 2.
Receiver operating characteristic analyses of fatigue NRS—SPIRIT-P1
| Change in fatigue NRS | Sensitivity | Specificity | Positive predictive value | Negative predictive value | Phi coefficient |
| A: NRS—SPIRIT-P1 | |||||
| −8 | 0.009 | 0.995 | 0.667 | 0.464 | 0.022 |
| −7 | 0.042 | 0.995 | 0.900 | 0.472 | 0.116 |
| −6 | 0.111 | 0.995 | 0.960 | 0.491 | 0.218 |
| −5 | 0.213 | 0.973 | 0.902 | 0.516 | 0.279 |
| −4 | 0.310 | 0.914 | 0.807 | 0.533 | 0.276 |
| −3 | 0.454 | 0.828 | 0.754 | 0.566 | 0.300 |
| −2 | 0.653 | 0.634 | 0.675 | 0.611 | 0.287 |
| −1 | 0.806 | 0.478 | 0.642 | 0.679 | 0.302 |
| 0 | 0.912 | 0.290 | 0.599 | 0.740 | 0.262 |
| B: NRS—SPIRIT-P2 | |||||
| −8 | 0.007 | 1.000 | 1.000 | 0.587 | 0.063 |
| −7 | 0.062 | 1.000 | 1.000 | 0.601 | 0.192 |
| −6 | 0.123 | 1.000 | 1.000 | 0.617 | 0.276 |
| −5 | 0.226 | 0.966 | 0.825 | 0.638 | 0.298 |
| −4 | 0.342 | 0.937 | 0.794 | 0.668 | 0.359 |
| −3 | 0.425 | 0.879 | 0.713 | 0.683 | 0.346 |
| −2 | 0.541 | 0.723 | 0.581 | 0.690 | 0.268 |
| −1 | 0.678 | 0.490 | 0.485 | 0.682 | 0.168 |
| 0 | 0.870 | 0.291 | 0.465 | 0.759 | 0.190 |
Receiver operating characteristic analyses suggested –3-point fatigue NRS score improvement is the minimum clinically important difference after 24 weeks.
NRS, numeric rating scale; SPIRIT, Standard Protocol Items: Recommendations for Interventional Trials.
Considering the trade-off between the metrics, a 2-point to 4-point improvement (effect size 0.8–1.7) best balanced the selected metrics. Similar patterns were observed using other anchors, such as ACR50, MDA, PGA MCID and PsARC (online supplementary table 2). A 3-point improvement was chosen because corresponding effect size (1.2) was sufficiently large, and it was also greater than the SE of measurement of 1.3 suggested by distribution-based method. The distribution-based method aims to quantify a lower bound, and any change within that range can be considered random variation and hence not statistically meaningful.
rmdopen-2019-000928supp002.pdf (72.8KB, pdf)
Discussion
These analyses examined the psychometric properties of the fatigue NRS using data from two randomised clinical trials of ixekizumab in patients with PsA. These trials assessed different patient populations, one biological-naïve and one biological-experienced, thus psychometric properties were evaluated for the two trials separately. The baseline characteristics of the two SPIRIT trials are representative of a typical PsA trial population and demonstrate PsA patients are burdened by a clinically significant level of patient-reported fatigue (score of 6 on a 10 point scale, with 10=worst). This is consistent with the baseline characteristics reported from other biological or targeted synthetic disease-modifying antirheumatic drug clinical trials. While different measures of fatigue were used across studies, a trial using the fatigue assessment scale (range 0–10; higher score=greater fatigue) reported baseline scores of 5.8–6.3 across treatment groups,36 trials reporting scores from the FACIT-fatigue (0–52; higher score=less fatigue) reported scores of 24.5–30.8 across treatment groups,37–40 and a trial using a fatigue 0–100 VAS (higher score=greater fatigue) reported scores of 54.7–55.9 across treatment groups.41
Results from these two studies support the fatigue NRS as a valid and responsive PRO instrument for evaluating fatigue over time in a clinical trial setting. The test–retest reliability analyses supported the reproducibility of the measure (ICC=0.829). However, this value was lower than the ICC value of 0.95 reported by Chandran et al 18 in determining the reliability and validity of the functional assessment of chronic illness therapy-fatigue scale in PsA. This was due, in part, to limitations in how the fatigue instrument was administered in the SPIRIT trials: (1) we assessed reliability between two measurements 2 weeks apart rather than 1 week apart in the FACIT study and (2) the two assessment points were before and after randomised treatment was initiated, rather than at both measurement points prior to the start of study treatment. The latter point is the most significant limitation. We tried to circumvent this by looking at patients who had stable TJC scores; however, it did not change the fact that these patients were undergoing a clinical trial potentially affecting this reliability analysis. In addition, reliability in the current analysis was assessed only in the placebo group, and was only based on tender joint count, resulting in a low sample size of 38.
Fatigue levels correlated with disease activity. Notably, a 3-point change is optimal for demonstrating a level of clinically meaningful improvement in severity after 24 weeks of treatment, which corresponds to marked clinical improvements in PsA disease activity. This is consistent with Gudu et al 4 who found that fatigue levels were significantly high in PsA patients with more swollen (p=0.002) and tender (p=0.0005) joints. Tender joint count (OR for five extra joints 1.30 (95% CI 1.01 to 1.68)) was also an independent variable associated with high level of fatigue.
A cross-sectional correlation with other patient-reported and physician-reported outcome measures supported the construct validity with the highest correlation with the SF-36 vitality domain and substantial correlation also seen with joint pain, HAQ-DI, physical functioning SF-36 role physical and PGA at week 24. Responsiveness of the fatigue NRS was evident in correlations with week 12 and 24 outcomes. In particular, significant differences in improvement of fatigue NRS scores between ACR20 and ACR50 responders and non-responders were evident. Our results are consistent with Minnock et al 42 who confirmed responsiveness in identifying statistically significant differences in fatigue levels using NRS in PsA patients 12 weeks after initiating treatment. Contrary to what we found, data from a longitudinal study by Husted et al 5 suggest that a change in a patient’s clinical status is weakly associated with a change in fatigue. Their data suggest that the change in fatigue is more strongly associated with a 6-month change in chronic pain, depression and physical ability. This is related to the fact that fatigue has many facets associated with pain, physical disability and psychological difficulties.6
The SPIRIT trials included the fatigue NRS as the sole measurement of fatigue because at the time the trials were designed, there was no single measure favoured to assess symptoms of fatigue in PsA patients and the fatigue NRS was considered a clinically relevant (useful and easy to apply in a clinical practice setting) and acceptable endpoint. Since that time, additional publications describing the psychometric properties of the FACIT-fatigue in PsA patients are now available and the evolution of the PsAID, which includes a fatigue NRS item, has now been provisionally endorsed by Outcome Measures in Rheumatology (OMERACT) for use in PsA clinical trials, further expanding options for evaluating fatigue in this patient population.43 44
Data regarding patient perceptions of fatigue are essential in the clinic, as well as in clinical trials. Patients’ perceptions of fatigue as measured by NRS yield important information for clinicians to consider and relate to patients’ perceptions of treatment success. Such understandings have the potential to affect decisions on treatment escalation or discontinuation.45 It is recommended that clinical trials for treatment of PsA collect PRO data to supplement other efficacy and safety data.46 Our study supports previous findings37 of the feasibility of a single-item, one-dimensional scale for measuring fatigue and its potential successful use in clinical trials.
Limitations
One limitation associated with this study is the lack of ethnic diversity within the cohorts, as the majority of patients were white. These findings, therefore, may not be generalised to other ethnicities. This analysis does not take into account comorbidities or psychological and cognitive aspects of fatigue in PsA. Other PROs strongly correlated with fatigue, such as pain, functional disability, sleep quality and depression, were not included in the analysis. This study does not include another multidimensional fatigue measure in the trial to allow for direct comparison but does include the vitality domain in the SF-36. Finally, although our reported ICC supports the reliability of the fatigue NRS within an acceptable range, the definition of the unchanged group using TJC 68 at week 2 and the 2-week interval between the assessments represent limitations.
In conclusion, fatigue NRS is a well-defined PRO instrument that was valid and responsive for measuring fatigue in patients with PsA in a clinical trial setting. The established psychometric properties from this study support the use of fatigue NRS in clinical trials to evaluate treatment efficacy at group level and potentially in routine clinical practice to assess and manage PsA-related fatigue; however, robust validation of reliability for use in routine clinical practice in treating individual patients with active PsA is needed.
Acknowledgments
The authors would like to thank Shannon E Gardell of Syneos Health for providing writing support.
Footnotes
Contributors: All authors have made substantial contributions to the conception or design of the work, or the acquisition, analysis or interpretation of data, have drafted the work or revised it critically for important intellectual content and have provided final approval of the manuscript.
Funding: This work was supported by Eli Lilly and Company.
Competing interests: DG received research grant support and/or consulting fees from Abbvie, Amgen, BMS, Celgene, Eli Lilly and Company, Janssen, Novartis, Pfizer and UCB. PN has received grants for research and for clinical trials and honoraria for advice and lectures from AbbVie, Amgen, BMS, Celgene, Eli Lilly and Company, Janssen, MSD, Novartis, Pfizer, Roche, Sanofi and UCB. HG has received fees for speaking and/or consulting from Abbie, Asahi Kasei, Astellas, Chugai, Eisai, Eli Lilly and Company, Janssen, Mitsubishi Tanabe, Ono, Pfizer, Santen, Takeda, and received research funding to Osaka City General Hospital from AbbVie, Chugai, Eisai, Eli Lilly and Company, Janssen, Sanofi and Santen. A-MO received research grant support and/or consulting fees from Celgene, Eli Lilly and Company, Horizon, Janssen, Novartis, Pfizer and UCB. TKK has received fees for speaking and/or consulting from AbbVie, Biogen, BMS, Boehringer Ingelheim, Celgene, Celltrion, Eli Lilly and Company, Epirus, Hospira, Merck-Serono, MSD, Mundipharma, Novartis, Oktal, Orion Pharma, Hospira/Pfizer, Roche, Sandoz, and UCB and received research funding to Diakonhjemmet Hospital from AbbVie, BMS, MSD, Pfizer, Roche and UCB. JB and C-YL are full-time employees of and hold stock/stock options in Eli Lilly and Company.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1. Villani AP, Rouzaud M, Sevrain M, et al. . Prevalence of undiagnosed psoriatic arthritis among psoriasis patients: systematic review and meta-analysis. J Am Acad Dermatol 2015;73:242–8. 10.1016/j.jaad.2015.05.001 [DOI] [PubMed] [Google Scholar]
- 2. Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med 2017;376:957–70. 10.1056/NEJMra1505557 [DOI] [PubMed] [Google Scholar]
- 3. Carneiro C, Chaves M, Verardino G, et al. . Fatigue in psoriasis with arthritis. Skinmed 2011;9:34–7. [PubMed] [Google Scholar]
- 4. Gudu T, Etcheto A, de Wit M, et al. . Fatigue in psoriatic arthritis - a cross-sectional study of 246 patients from 13 countries. Joint Bone Spine 2016;83:439–43. 10.1016/j.jbspin.2015.07.017 [DOI] [PubMed] [Google Scholar]
- 5. Husted JA, Tom BDM, Farewell VT, et al. . Longitudinal analysis of fatigue in psoriatic arthritis. J Rheumatol 2010;37:1878–84. 10.3899/jrheum.100179 [DOI] [PubMed] [Google Scholar]
- 6. Gossec L, de Wit M, Kiltz U, et al. . A patient-derived and patient-reported outcome measure for assessing psoriatic arthritis: elaboration and preliminary validation of the psoriatic arthritis impact of disease (PsAID) questionnaire, a 13-country EULAR initiative. Ann Rheum Dis 2014;73:1012–9. 10.1136/annrheumdis-2014-205207 [DOI] [PubMed] [Google Scholar]
- 7. Wong ITY, Chandran V, Li S, et al. . Sleep disturbance in psoriatic disease: prevalence and associated factors. J Rheumatol 2017;44:1369–74. 10.3899/jrheum.161330 [DOI] [PubMed] [Google Scholar]
- 8. Husted JA, Tom BD, Schentag CT, et al. . Occurrence and correlates of fatigue in psoriatic arthritis. Ann Rheum Dis 2009;68:1553–8. 10.1136/ard.2008.098202 [DOI] [PubMed] [Google Scholar]
- 9. Walsh JA, McFadden ML, Morgan MD, et al. . Work productivity loss and fatigue in psoriatic arthritis. J Rheumatol 2014;41:1670–4. 10.3899/jrheum.140259 [DOI] [PubMed] [Google Scholar]
- 10. Liu J-T, Yeh H-M, Liu S-Y, et al. . Psoriatic arthritis: epidemiology, diagnosis, and treatment. World J Orthop 2014;5:537–43. 10.5312/wjo.v5.i4.537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gudu T, Gossec L. Quality of life in psoriatic arthritis. Expert Rev Clin Immunol 2018;14:405–17. 10.1080/1744666X.2018.1468252 [DOI] [PubMed] [Google Scholar]
- 12. Wong PCH, Leung Y-Y, Li EK, et al. . Measuring disease activity in psoriatic arthritis. Int J Rheumatol 2012;2012:839425–10. 10.1155/2012/839425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mease PJ. Measures of psoriatic arthritis: tender and swollen joint assessment, psoriasis area and severity index (PASI), nail psoriasis severity index (NAPSI), modified nail psoriasis severity index (mNAPSI), Mander/Newcastle Enthesitis index (Mei), Leeds Enthesitis index (LEI), spondyloarthritis research Consortium of Canada (SPARCC), Maastricht ankylosing spondylitis Enthesis score (MASES), Leeds Dactylitis index (LDI), patient global for psoriatic arthritis, dermatology life quality index (DLQI), psoriatic arthritis quality of life (PsAQOL), functional assessment of chronic illness Therapy-Fatigue (FACIT-F), psoriatic arthritis response criteria (PsARC), psoriatic arthritis joint activity index (PsAJAI), disease activity in psoriatic arthritis (DAPSA), and composite psoriatic disease activity index (CPDAI). Arthritis Care Res 2011;63:S64–85. 10.1002/acr.20577 [DOI] [PubMed] [Google Scholar]
- 14. Orbai A-M, Mease PJ, de Wit M, et al. . Report of the GRAPPA-OMERACT psoriatic arthritis Working group from the grappa 2015 annual meeting. J Rheumatol 2016;43:965–9. 10.3899/jrheum.160116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Orbai A-M, de Wit M, Mease P, et al. . International patient and physician consensus on a psoriatic arthritis core outcome set for clinical trials. Ann Rheum Dis 2017;76:673–80. 10.1136/annrheumdis-2016-210242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kalyoncu U, Ogdie A, Campbell W, et al. . Systematic literature review of domains assessed in psoriatic arthritis to inform the update of the psoriatic arthritis core domain set. RMD Open 2016;2:e000217 10.1136/rmdopen-2015-000217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gladman DD, Mease PJ, Strand V, et al. . Consensus on a core set of domains for psoriatic arthritis. J Rheumatol 2007;34:1167–70. [PubMed] [Google Scholar]
- 18. Chandran V, Bhella S, Schentag C, et al. . Functional assessment of chronic illness Therapy-Fatigue scale is valid in patients with psoriatic arthritis. Ann Rheum Dis 2007;66:936–9. 10.1136/ard.2006.065763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Minnock P, Kirwan J, Bresnihan B. Fatigue is a reliable, sensitive and unique outcome measure in rheumatoid arthritis. Rheumatology 2009;48:1533–6. 10.1093/rheumatology/kep287 [DOI] [PubMed] [Google Scholar]
- 20. Mease PJ, van der Heijde D, Ritchlin CT, et al. . Ixekizumab, an interleukin-17A specific monoclonal antibody, for the treatment of biologic-naive patients with active psoriatic arthritis: results from the 24-week randomised, double-blind, placebo-controlled and active (adalimumab)-controlled period of the phase III trial SPIRIT-P1. Ann Rheum Dis 2017;76:79–87. 10.1136/annrheumdis-2016-209709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nash P, Kirkham B, Okada M, et al. . Ixekizumab for the treatment of patients with active psoriatic arthritis and an inadequate response to tumour necrosis factor inhibitors: results from the 24-week randomised, double-blind, placebo-controlled period of the SPIRIT-P2 phase 3 trial. The Lancet 2017;389:2317–27. 10.1016/S0140-6736(17)31429-0 [DOI] [PubMed] [Google Scholar]
- 22. Fries JF, Spitz PW, Young DY. The dimensions of health outcomes: the health assessment questionnaire, disability, and pain scales. J Rheumatol 1982;9:789–93. [PubMed] [Google Scholar]
- 23. Felson DT, Anderson JJ, Boers M, et al. . American College of rheumatology. Preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995;38:727–35. 10.1002/art.1780380602 [DOI] [PubMed] [Google Scholar]
- 24. Aletaha D, Alasti F, Smolen JS. Disease activity states of the DAPSA, a psoriatic arthritis specific instrument, are valid against functional status and structural progression. Ann Rheum Dis 2017;76:418–21. 10.1136/annrheumdis-2016-209511 [DOI] [PubMed] [Google Scholar]
- 25. Husted JA, Gladman DD, Farewell VT, et al. . Validating the SF-36 health survey questionnaire in patients with psoriatic arthritis. J Rheumatol 1997;24:511–7. [PubMed] [Google Scholar]
- 26. Maruish ME, User’s manual for the SF-36v2 Health Survey. 3rd edn Lincoln, RI: QualityMetric Incorporated, 2011. [Google Scholar]
- 27. Coates LC, FitzGerald O, Mease PJ, et al. . Development of a disease activity and responder index for psoriatic arthritis--report of the Psoriatic Arthritis Module at OMERACT 11. J Rheumatol 2014;41:782–91. 10.3899/jrheum.131250 [DOI] [PubMed] [Google Scholar]
- 28. Mumtaz A, Gallagher P, Kirby B, et al. . Development of a preliminary composite disease activity index in psoriatic arthritis. Ann Rheum Dis 2011;70:272–7. 10.1136/ard.2010.129379 [DOI] [PubMed] [Google Scholar]
- 29. Nunnally JC, Bernstein IH. Psychometric theory. 3rd edn New York, NY: McGraw Hill, 1994. [Google Scholar]
- 30. Tugwell P, Boers M, D'Agostino M-A, et al. . Updating the OMERACT filter: implications of filter 2.0 to select outcome instruments through assessment of "truth": content, face, and construct validity. J Rheumatol 2014;41:1000–4. 10.3899/jrheum.131310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McLeod LD, Coon CD, Martin SA, et al. . Interpreting patient-reported outcome results: US FDA guidance and emerging methods. Expert Rev Pharmacoecon Outcomes Res 2011;11:163–9. 10.1586/erp.11.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mease PJ, Woolley JM, Bitman B, et al. . Minimally important difference of health assessment questionnaire in psoriatic arthritis: relating thresholds of improvement in functional ability to patient-rated importance and satisfaction. J Rheumatol 2011;38:2461–5. 10.3899/jrheum.110546 [DOI] [PubMed] [Google Scholar]
- 33. Coates LC, Fransen J, Helliwell PS. Defining minimal disease activity in psoriatic arthritis: a proposed objective target for treatment. Ann Rheum Dis 2010;69:48–53. 10.1136/ard.2008.102053 [DOI] [PubMed] [Google Scholar]
- 34. Warrens MJ. On association coefficients for 2x2 tables and properties that do not depend on the marginal distributions. Psychometrika 2008;73:777–89. 10.1007/s11336-008-9070-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sackett PR, Lievens F, Berry CM, et al. . A cautionary note on the effects of range restriction on predictor intercorrelations. J Appl Psychol 2007;92:538–44. 10.1037/0021-9010.92.2.538 [DOI] [PubMed] [Google Scholar]
- 36. Gladman D, Fleischmann R, Coteur G, et al. . Effect of Certolizumab pegol on multiple facets of psoriatic arthritis as reported by patients: 24-week patient-reported outcome results of a phase III, multicenter study. Arthritis Care Res 2014;66:1085–92. 10.1002/acr.22256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gladman DD, Mease PJ, Cifaldi MA, et al. . Adalimumab improves joint-related and skin-related functional impairment in patients with psoriatic arthritis: patient-reported outcomes of the adalimumab effectiveness in psoriatic arthritis trial. Ann Rheum Dis 2007;66:163–8. 10.1136/ard.2006.057901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ritchlin C, Rahman P, Kavanaugh A, et al. . Efficacy and safety of the anti-IL-12/23 p40 monoclonal antibody, ustekinumab, in patients with active psoriatic arthritis despite conventional non-biological and biological anti-tumour necrosis factor therapy: 6-month and 1-year results of the phase 3, multicentre, double-blind, placebo-controlled, randomised PSUMMIT 2 trial. Ann Rheum Dis 2014;73:990–9. 10.1136/annrheumdis-2013-204655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Strand V, Mease P, Gossec L, et al. . Secukinumab improves patient-reported outcomes in subjects with active psoriatic arthritis: results from a randomised phase III trial (future 1). Ann Rheum Dis 2017;76:203–7. 10.1136/annrheumdis-2015-209055 [DOI] [PubMed] [Google Scholar]
- 40. Strand V, de Vlam K, Covarrubias-Cobos JA, et al. . Tofacitinib or adalimumab versus placebo: patient-reported outcomes from opal Broaden-a phase III study of active psoriatic arthritis in patients with an inadequate response to conventional synthetic disease-modifying antirheumatic drugs. RMD Open 2019;5:e000806 10.1136/rmdopen-2018-000806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gniadecki R, Robertson D, Molta CT, et al. . Self-Reported health outcomes in patients with psoriasis and psoriatic arthritis randomized to two etanercept regimens. J Eur Acad Dermatol Venereol 2012;26:1436–43. 10.1111/j.1468-3083.2011.04308.x [DOI] [PubMed] [Google Scholar]
- 42. Minnock P, Kirwan J, Veale D, et al. . Fatigue is an independent outcome measure and is sensitive to change in patients with psoriatic arthritis. Clin Exp Rheumatol 2010;28:401–4. [PubMed] [Google Scholar]
- 43. Cella D, Wilson H, Shalhoub H, et al. . Retracted article: content validity and psychometric evaluation of functional assessment of chronic illness Therapy-Fatigue in patients with psoriatic arthritis. J Patient Rep Outcomes 2019;3 10.1186/s41687-019-0094-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Orbai A-M, Holland R, Leung YY, et al. . PsAID12 provisionally endorsed at OMERACT 2018 as core outcome measure to assess psoriatic arthritis-specific health-related quality of life in clinical trials. J Rheumatol 2019;46:990–5. 10.3899/jrheum.181077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dures E, Hewlett S, Lord J, et al. . Important treatment outcomes for patients with psoriatic arthritis: a multisite qualitative study. Patient 2017;10:455–62. 10.1007/s40271-017-0221-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Betteridge N, Boehncke W-H, Bundy C, et al. . Promoting patient-centred care in psoriatic arthritis: a multidisciplinary European perspective on improving the patient experience. J Eur Acad Dermatol Venereol 2016;30:576–85. 10.1111/jdv.13306 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
rmdopen-2019-000928supp003.pdf (27.7KB, pdf)
rmdopen-2019-000928supp001.pdf (42.5KB, pdf)
rmdopen-2019-000928supp002.pdf (72.8KB, pdf)


