Abstract
Introduction
The recent decrease in multiple organ dysfunction syndrome (MODS)-associated and adult respiratory distress syndrome (ARDS)-associated mortality could be considered a success of improvements in trauma care. However, the incidence of infections remains high in patients with polytrauma, with high morbidity and hospital resources usage. Infectious complications might be a residual effect of the decrease in MODS-related/ARDS-related mortality. This study investigated the current incidence of infectious complications in polytrauma.
Methods
A 5.5-year prospective population-based cohort study included consecutive severely injured patients (age >15) admitted to a (Level-1) trauma center intensive care unit (ICU) who survived >48 hours. Demographics, physiologic and resuscitation parameters, multiple organ failure and ARDS scores, and infectious complications (pneumonia, fracture-related infection, meningitis, infections related to blood, wound, and urinary tract) were prospectively collected. Data are presented as median (IQR), p<0.05 was considered significant.
Results
297 patients (216 (73%) men) were included with median age of 46 (27–60) years, median Injury Severity Score was 29 (22–35), 96% sustained blunt injuries. 44 patients (15%) died. One patient (2%) died of MODS and 1 died of ARDS. 134 patients (45%) developed 201 infectious complications. Pneumonia was the most common complication (50%). There was no difference in physiologic parameters on arrival in emergency department and ICU between patients with and without infectious complications. Patients who later developed infections underwent more often a laparotomy (32% vs 18%, p=0.009), had more often pelvic fractures (38% vs 25%, p=0.02), and received more blood products <8 hours. They had more often MODS (25% vs 13%, p=0.005), stayed longer on the ventilator (10 (5–15) vs 5 (2–8) days, p<0.001), longer in ICU (11 (6–17) vs 6 (3–10) days, p<0.001), and in hospital (30 (20–44) vs 16 (10–24) days, p<0.001). There was however no difference in mortality (12% vs 17%, p=0.41) between both groups.
Conclusion
45% of patients developed infectious complications. These patients had similar mortality rates, but used more hospital resources. With low MODS-related and ARDS-related mortality, infections might be a residual effect, and are one of the remaining challenges in the treatment of patients with polytrauma.
Level of evidence
Level 3.
Study type
Population-based cohort study.
Introduction
Advances in pre-hospital and in-hospital trauma care have attributed to a decrease in mortality in trauma in the past decades.1–3 Although improvement in hemorrhage control and resuscitation has been established, patients can still deteriorate at a later stage due to inflammatory (eg, infectious) complications. After injury, the innate immune system is activated resulting in an inflammatory response with a key role for neutrophils. Sometimes, however, these processes of inflammatory response become dysregulated, and immune-mediated complications may occur such as multiple organ dysfunction syndrome (MODS) and adult respiratory distress syndrome (ARDS).4
Several studies including our own have observed that MODS-related and ARDS-related deaths have decreased over the last years resulting in more severely injured patients surviving.5 6 However, we also observed that many surviving patients developed infectious complications. Since infectious complications are also an expression of the failure of the immune system of the host, we hypothesized that a decrease in death by MODS and ARDS has resulted in a residual problem of (less deleterious) infectious complications. Therefore, we conducted a prospective population-based cohort study in patients with polytrauma to investigate the current incidence of infectious complications.
Materials and Methods
Study setting
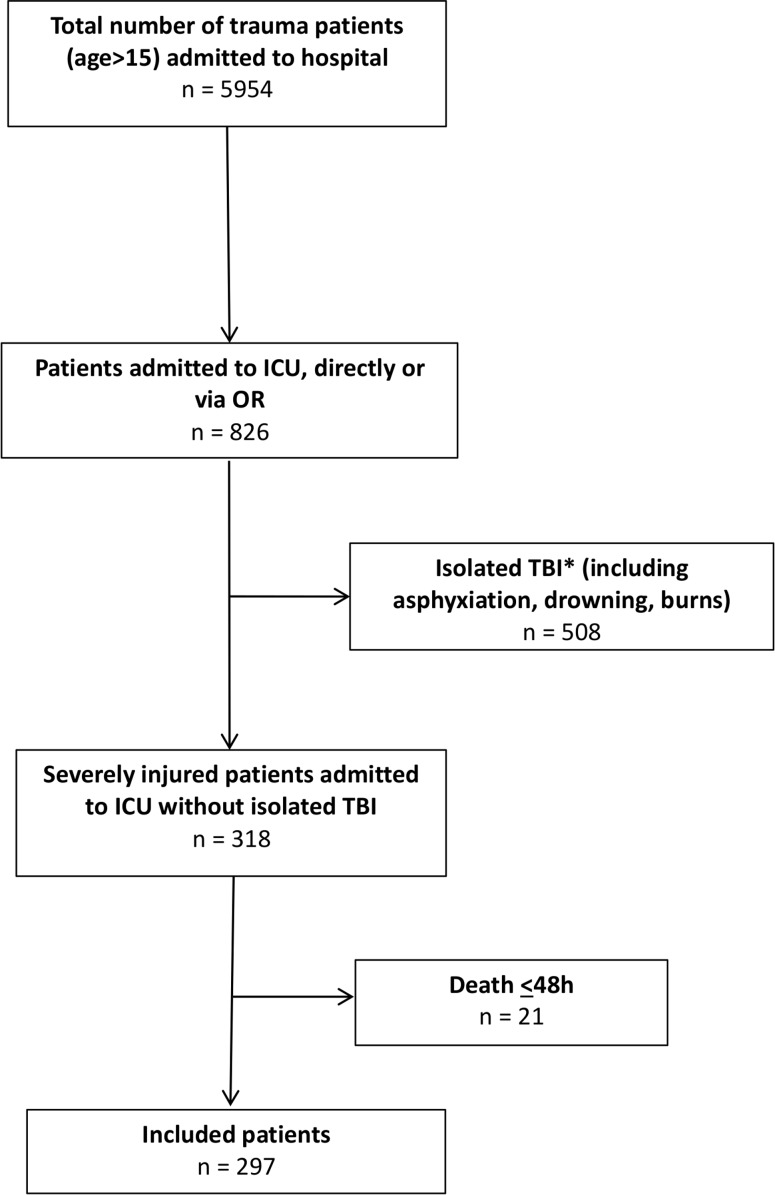
The study was conducted at an urban major (Level-1) trauma center. From November 2013, a 5.5-year prospective population-based cohort study was undertaken to investigate outcomes in severely injured patients admitted to the intensive care unit (ICU) of the University Medical Center Utrecht, the Netherlands. Detailed characteristics of the hospital and catchment area were previously described.7 All consecutive severely injured patients >15 years of age who were admitted to ICU either directly from the emergency department (ED) or postoperatively after urgent surgery and survived >48 hours were included. Patients with isolated injury to the brain (Abbreviated Injury Score (AIS) head three or more and AIS two or less in other regions), asphyxiation, drowning and burns were excluded, because of possible different physiologic response to severe trauma and a significantly different mortality and morbidity profile.8 9 A flowchart of patient inclusion is shown in figure 1.
Figure 1.
Flow diagram of included patients. *Isolated traumatic brain injury (TBI) was defined as Abbreviated Injury Score (AIS) head >3 and AIS<2 or less in other regions. ICU, intensive care unit.
Data collection
All data were prospectively collected on arrival in ED and on a daily basis in ICU by the authors (KW and LL) and included patient demographics, Injury Severity Score (ISS), shock and resuscitation parameters. Admission arterial blood gas analysis, coagulation status, and temperature measurement were performed during resuscitation in ED as part of standard procedures. Arterial blood gas analysis and temperature measurement were repeated on arrival in ICU. Urinary output was measured the first hour after arrival in ICU. Blood product (packed red blood cells (PRBC), fresh frozen plasma (FFP) and platelets (PLT)) use was recorded in the first 24 hours following admission. Infectious complications were registered by the treating physicians (surgeons and/or intensivists) and categorized as pneumonia, fracture-related infections, positive blood culture, septic shock, abdominal abscess, wound infection, thrombophlebitis, thorax empyema, urinary tract infection (UTI), secondary meningitis after traumatic brain injury, and miscellaneous complications (including, cholecystitis, otitis, pancreatitis, and pericarditis). Furthermore, both Denver multiple organ failure (MOF) scores10 and ARDS Berlin criteria11 were registered daily up until 28 days or discharge from ICU. Denver MOF score was chosen over other MOF scoring systems like Marshall MODS or sequential organ failure assessment (SOFA) to avoid difficulties by including the Glasgow Coma Scale (GCS) in the organ failure score. GCS can be challenging to obtain in patients with trauma in ICU because they are often sedated and intubated for extended periods. This could negatively influence the CNS organ failure score.9 12 13
Selective decontamination of the digestive tract (oropharynx and intestinal tract; SDD) was used in all patients admitted to ICU during the first 4 days and included administration of topical antibiotics in the gastrointestinal tract and systemic prophylaxis with an intravenous third-generation cephalosporin.14
Primary outcome was the incidence of infectious complications during hospital stay. Secondary outcomes were mortality, ARDS and MODS, ventilator days, ICU length of stay (ICU-LOS), and in-hospital length of stay (H-LOS).
Definitions
Urgent laparotomy was defined as a laparotomy that was performed in patients who were transported from ED directly (or via CT scan) to the operating room.
Different types of infections were defined according to the adapted the definitions from the Center for Disease Control and Prevention criteria,15 American College of Surgeons Wound Schema,16 Barts Health NHS Trust guidelines,17 and consensus groups for specific types of infections.18 19 Full definitions of specific infections were added as a supplemental table (online supplementary table S1).
tsaco-2019-000398supp001.pdf (61.2KB, pdf)
Statistical analysis
Data were analyzed using IBM SPSS Statistics, V.25.0 (Armonk, NY, USA). Graphs were prepared with GraphPad Prism V.8.3.0 (San Diego, CA, USA). Results are presented as median and IQR. Comparison of variables was done using Kruksal–Wallis test or Pearson’s χ2 test in dichotomous data. Variables with univariate statistical significance of less than 0.10 were included in a multivariate logistic regression analysis. These variables were analyzed with forward stepwise selection to identify independent risk factors for infectious complications and presented as ORs and 95% CIs. Statistical significance was defined as p<0.05.
Results
In total, 297 patients were included with a median age of 46 (27-60) years. Seventy-three per cent were men, 286 (96%) patients had blunt injuries, and median ISS was 29 (22-35) (table 1).
Table 1.
Patient demographics, resuscitation, and outcome parameters
| Demographics | Total cohort (n=297) |
No infectious complications (n=163) |
Infectious complications (n=134) |
P value |
| Age (years) | 46 (27–60) | 44 (24–59) | 50 (30–61) | 0.08 |
| Gender (% male) | 216 (73) | 111 (68) | 105 (78) | 0.05 |
| MOI (%blunt) | 286 (96) | 154 (94) | 132 (99) | 0.12 |
| ISS | 29 (22–35) | 29 (22–34) | 29 (22–36) | 0.48 |
| AIS head | 3 (1–4) | 3 (1–4) | 3 (1–4) | 0.72 |
| AIS face | 0 (0–2) | 0 (0–2) | 0 (0–1) | 0.15 |
| AIS chest | 3 (2–4) | 3 (2–4) | 3 (2–4) | 0.12 |
| AIS abdomen | 2 (0–3) | 2 (0–2) | 2 (0–3) | 0.75 |
| AIS extr /pelvis | 2 (1–3) | 2 (1–3) | 2 (0–3) | 0.11 |
| AIS external | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0.30 |
| Pelvic fracture | 93 (31) | 42 (25) | 51 (38) | 0.02* |
| Urgent laparotomy | 71 (24) | 29 (18) | 42 (31) | 0.009* |
| ED parameters | ||||
| SBP (mm Hg) | 120 (100–136) | 121 (100–142) | 117 (96–132) | 0.08 |
| DBP (mm Hg) | 75 (60–86) | 78 (60–90) | 74 (60–83) | 0.05 |
| Temperature (0C) | 35.5 (34.4–36.5) | 35.4 (34.3–36.5) | 35.5 (34.4–36.5) | 0.78 |
| Hb (mmol/L) | 8.0 (7.2–8.9) | 8.0 (7.4–9.1) | 8.0 (7.2–8.9) | 0.48 |
| Leukocytes (x109/L) | 15.3 (11.1–20.1) | 15.3 (11.6–19.9) | 15.3 (9.8–20.6) | 0.56 |
| Platelets(x109/L) | 235 (190–281) | 233 (190–288) | 235 (187–277) | 0.67 |
| PT | 15.2 (13.9–17.3) | 15.1 (14.0–17.0) | 15.5 (13.9–17.9) | 0.34 |
| pH | 7.31 (7.26–7.37) | 7.32 (7.26–7.38) | 7.30 (7.25–7.35) | 0.15 |
| PaCO2 (mm Hg) | 46 (41–54) | 46 (40–52) | 47 (42–54) | 0.19 |
| PaO2 (mm Hg) | 202 (105–302) | 221 (100–329) | 198 (106–277) | 0.35 |
| BD (mmol/L) | 3.0 (0.0–6.0) | 2.5 (0.0–6.0) | 3.0 (0.0–6.0) | 0.63 |
| Sat (%) | 100 (97–100) | 100 (97–100) | 100 (98–100) | 0.98 |
| ICU Parameters | ||||
| SBP (mm Hg) | 119 (105–136) | 120 (106–136) | 119 (105–137) | 0.82 |
| DBP (mm Hg) | 65 (56–74) | 66 (56–75) | 65 (55–73) | 0.38 |
| Temperature (0C) | 35.3 (34.5–36.0) | 35.4 (34.3–36.1) | 35.3 (34.5–35.9) | 0.70 |
| Hb (mmol/L) | 7.6 (6.8–8.3) | 7.6 (6.8–8.3) | 7.5 (6.8–8.2) | 0.71 |
| pH | 7.33 (7.29–7.38) | 7.34 (7.30–7.38) | 7.33 (7.28–7.38) | 0.68 |
| PaCO2 (mm Hg) | 43 (38–47) | 43 (39–47) | 43 (38–47) | 0.73 |
| PaO2 (mm Hg) | 150 (114–192) | 153 (116–190) | 144 (108–198) | 0.36 |
| BD (mmol/L) | 3.5 (1.5–5.7) | 3.2 (1.4–5.5) | 4.0 (1.5–6.1) | 0.23 |
| Sat (%) | 98 (98–99) | 98 (98–99) | 98 (97–99) | 0.10 |
| UO (ml) | 150 (80–300) | 150 (80–310) | 145 (71–295) | 0.54 |
| Resuscitation | ||||
| Crystalloids (L) | ||||
| <8 hours | 4.7 (2.4–6.3) | 4.5 (2.3–6.3) | 4.8 (2.7–6.3) | 0.31 |
| <24 hours | 7.4 (5.1–10.4) | 7.3 (4.6–10.3) | 7.9 (5.5–10.4) | 0.19 |
| PRBC (u) | ||||
| <8 hours | 1 (0–4) | 0 (0–3) | 2 (0–6) | 0.03* |
| <24 hours | 1 (0–5) | 1 (0–4) | 2 (0–7) | 0.06 |
| FFP (u) | ||||
| <8 hours | 0 (0–4) | 0 (0–3) | 1 (0–5) | 0.04* |
| <24 hours | 0 (0–5) | 0 (0–4) | 2 (0–7) | 0.06 |
| PLT (u)† | ||||
| <8 hours | 0 (0–1) | 0 (0–0) | 0 (0–1) | 0.04* |
| <24 hours | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0.03* |
| Outcome | ||||
| Ventilator days | 7 (3–11) | 5 (2–8) | 10 (5–15) | <0.001* |
| ICU-LOS (days) | 8 (4–14) | 6 (3–10) | 11 (6–17) | <0.001* |
| H-LOS (days) | 21 (13–33) | 16 (10–24) | 30 (20–44) | <0.001* |
| MODS | 56 (19) | 21 (13) | 35 (25) | 0.005* |
| ARDS | 15 (5) | 5 (3) | 10 (7) | 0.11 |
| Mortality | 44 (15) | 27 (17) | 17 (12) | 0.41 |
Data are expressed as median (IQR) or absolute numbers (%).
*Statistically significant.
†1 unit of platelets contains five donors.
AIS, Abbreviated Injury Scale; ARDS, acute respiratory dysfunction syndrome; BD, base deficit; DBP, diastolic blood pressure; FFP, fresh frozen plasma; Hb, hemoglobin; H-LOS, hospital length of stay; ICU, intensive care unit; ISS, Injury Severity Score; LOS, length of stay; MODS, multiple organ dysfunction syndrome; MOI, mechanism of injury; PaCO2, partial pressures of carbon dioxide; PaO2, partial pressures of oxygen; PLT, platelets; PRBC, packed red blood cells; PT, prothrombin time; Sat, saturation; SBP, systolic blood pressure; UO, urinary output first hour in ICU.
44 patients (15%) died; 33 due to brain injury (75%), 6 due to respiratory insufficiency (14%), 2 due to sepsis (5%), 1 due to bowel ischemia (2%), 1 died of MODS (2%), and 1 patient died of ARDS (2%). Median time to death for patients who did not die of brain injury was 15 (8–32) days.
134 patients (45%) developed 201 infectious complications. Pneumonia was the most common complication (49.8%), followed by fracture-related infection (12.4%). All other infectious complications occurred between 2% and 7% (table 2).
Table 2.
Incidence of different types of infections
| Type of infection | N (%) |
| Pneumonia | 100 (49.8) |
| Fracture-related Infection | 25 (12.4) |
| Positive blood culture | 14 (7.0) |
| Septic shock | 13 (6.5) |
| Abdominal abscess | 4 (2.0) |
| Wound infection | 9 (4.5) |
| Thrombo-phlebitis | 7 (3.5) |
| Thorax empyema | 4 (2.0) |
| Urinary tract infection | 7 (3.5) |
| Secondary meningitis | 7 (3.5) |
| Miscellaneous* | 11 (5.5) |
| Total† | 201 (100) |
*Including cholecystitis, otitis, pancreatitis, and pericarditis.
†48 patients developed more than one complication.
48 patients (36%) developed more than one infection during their hospital stay; 28 patients (21%) had two infections, 12 patients (9%) developed three infectious complications and 8 patients (6%) had more than three infectious complications.
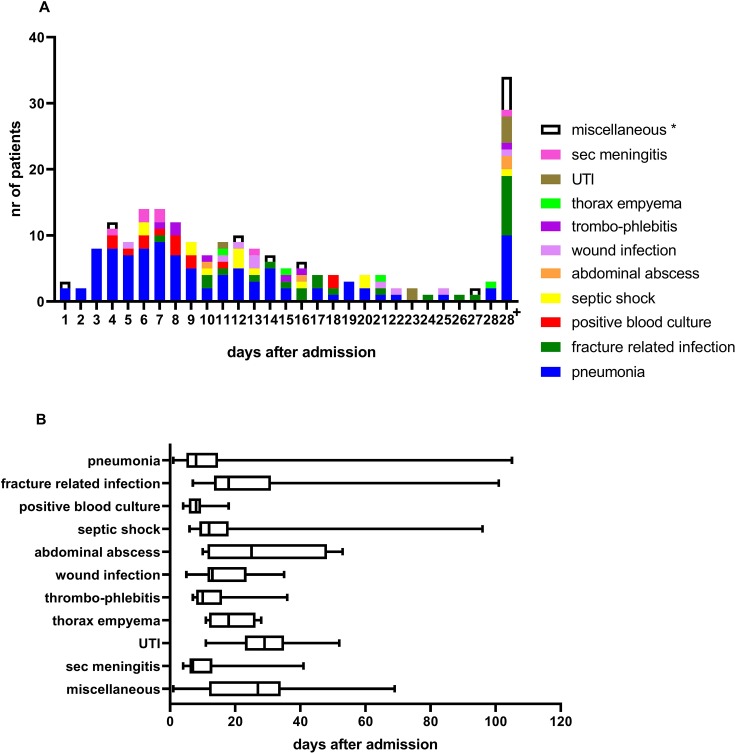
Median time to infection development was 12 (7–20) days (figure 2A). Development of pneumonia, positive blood culture, and secondary meningitis after brain injury occurred roughly within the first week after injury, whereas septic shock, wound infection, and thrombophlebitis developed in the second week after injury. Fracture-related infection and thorax empyema developed around day 18, and abdominal abscess, UTI and all miscellaneous infectious complications developed approximately 25 days after injury (figure 2B).
Figure 2.
(A) Type of infectious complications related to days after admission. (B) Occurrence of different types of infectious complications over time. UTI, urinary tract infection, Sec meningitis, secondary meningitis after traumatic brain injury. *Miscellaneous infectious complications included cholecystitis, pancreatitis, pericarditis, and otitis.
Sixteen out of 44 patients (36%), who later died, developed an infectious complication, nine of them died while having an infectious complication. Three of them died because of the infectious complication, (two sepsis and one respiratory insufficiency after Staphylococcus aureus pneumonia); 1.0% of the whole studied population died of an infection.
Comparison of patients with and without development of infectious complications
Patients who later developed infectious complications had similar injury severity (ISS for both groups 29, p=0.48) and physiologic parameters on arrival in ED and ICU compared with patients without infectious complications (table 1). However, they underwent more often an urgent laparotomy (31% vs 18%, p=0.009), had more often pelvic fractures (38% vs 25%, p=0.02), received more PRBC, FFP, and PLT<8 hours, and more FFP and PLT <24 hours (table 1). They developed more often MODS (25% vs 13%, p=0.005), but there was no difference in ARDS development (7% vs 3%, p=0.11). Furthermore, they stayed longer on the ventilator (10 (5–15) vs 5 (2–8) days, p<0.001), longer in ICU (11 (6–17) vs 6 (3–10) days, p<0.001), and in hospital (30 (20–44) vs 16 (10–24) days, p<0.001). Nevertheless, there was no difference in mortality (12% vs 17%, p=0.41) between patients who later developed infectious complications and patients who did not (table 1).
Multivariate analysis (included parameters were age, male gender, urgent laparotomy, pelvic fracture, crystalloids and blood product administration both <8 and <24 hours, MODS) showed that age, urgent laparotomy and MODS were independent predictors for development of infectious complications (table 3) Table 3 Intraoperative abdominal organ injury related to infectious complications should be table 4 and table 4 Multivariate analysis: infectious complications in patients with polytrauma should be table 3
Table 3.
Intraoperative abdominal organ injury related to infectious complications
| Type of injury* | Total (n=97) |
No infectious complications (n=41) | Infectious complications (n=56) |
| No injury† | 4 | 1 | 3 |
| Solid organ injury | 46 | 20 | 26 |
| Hollow viscus injury | 28 | 11 | 17 |
| Large abdominal vessels | 5 | 3 | 2 |
| Abdominal wall/diaphragm | 14 | 7 | 7 |
P=N.S.
*71 patients had 97 intra-abdominal injuries.
†All four patients without intra-abdominal injuries had pelvic packing for a pelvic fracture associated retroperitoneal hematoma.
Urgent laparotomy related to development of infectious complications
71 patients (24%) underwent an urgent laparotomy, of whom four had no intra-abdominal injuries, but had pelvic packing for a pelvic fracture-related retroperitoneal hematoma. There was no difference in type of abdominal injury and the development of infectious complications (table 4). The infectious complications were not directly related to the laparotomy itself. In patients who had a laparotomy pneumonia and fracture-related infections were also the most common infectious complications.
Table 4.
Multivariate analysis: infectious complications in patients with polytrauma
| Variable | β-coefficient | P value | OR | 95% CI lower upper |
|
| Step 1* | |||||
| MODS | 0.847 | 0.007 | 2333 | 1264 | 4306 |
| Constant | −0,317 | 0.020 | 0.729 | ||
| Step2† | |||||
| Urgent laparotomy | 0.650 | 0.025 | 1916 | 1085 | 3386 |
| MODS | 0.817 | 0.010 | 2263 | 1219 | 4203 |
| Constant | −0,466 | 0.002 | 0.627 | ||
| Step3† | |||||
| Age | 0.013 | 0.048 | 1013 | 1000 | 1026 |
| Urgent laparotomy | 0.747 | 0.012 | 2111 | 1178 | 3782 |
| MODS | 0.716 | 0.026 | 2045 | 1089 | 3840 |
| Constant | −1,060 | 0.002 | 0.346 | ||
*Variable(s) entered on step 1: MODS.
†Variable(s) entered on step 2: urgent laparotomy.
‡Variable(s) entered on step 3: age.
MODS, multiple organ dysfunction syndrome.
Discussion
Almost half of severely injured patients admitted to ICU who survived the first 48 hours, developed at least one infectious complication during hospital stay. Although there was no difference in ISS and physiological parameters, patients who later developed infectious complications had more often MODS, needed more blood products, and used more hospital resources (more ventilator days, longer stay in ICU and in hospital). There was however no difference in mortality between patients who later developed infectious complications and patients who did not. One per cent of the studied population died of an infectious complication.
Pneumonia was the most common infection followed by fracture-related infection. Median time to infection development was 12 days, although different infections developed during different time periods after admission.
Literature is equivocal on the relation between mortality and infectious complications. Some authors report higher mortality rates in patients who developed infectious complications,20 21 whereas others, including our own data, showed similar mortality rates.17 This discrepancy might be partly explained by the different infectious complications that have been reported; sepsis is for example more lethal than pneumonia or surgical site infections.21
Previous studies have shown comparable results regarding percentage and type of infectious complications; roughly half of severely injured patients developed infectious complications and the most common infectious complication was of respiratory origin.17 20 Different infections developed at different times during admission; Cole et al showed the same singularity in their study with early development of respiratory infections, and positive blood cultures, and later development of other infections.17 Results were however not entirely comparable, since different types of infections were registered in both studies. Several studies have shown that the neutrophil response is dependent on which bacterial species the cell is attempting to kill. It is likely that different types of infections are caused by different bacteria, and that some bacteria are better resistant to neutrophils than others, resulting in different times of development of infections.22 23 Possibly, exhaustion of the innate immune system with decreased response of neutrophils plays an important role in the selection of bacteria causing different infectious complications during different times after injury.
In our study age, the need for urgent laparotomy and MODS were independent predictors for development of infectious complications in patients with polytrauma. Other studies reported other independent predictors such as admission shock parameters,17 gender, blood transfusions, AIS chest, and GCS on scene.20 In our study, there was no difference in admission shock parameters between patients who later developed infections and patients who did not, although we did demonstrate that blood transfusion was associated with infectious complications. This association has been reported by others as well.17 20 24 25 The fact that admission shock parameters in our study were not associated with infections could be possibly explained by the fact that in our cohort parameters such as blood pressure and hemoglobin were within normal ranges on arrival in ED. This phenomenon in which severely injured patients in smaller service areas with short transport times do not have deranged physiologic parameters on arrival in the ED has been described earlier.6 7 These patients do not have the time to deteriorate because they are in the hospital before blood pressure, BD, and hemoglobin will change distinctly. One could argue that these patients were not severely injured at all. This is contradicted by the fact that patients had an ISS of 29, 31% had a pelvic fracture, 24% needed an urgent laparotomy, and they were all admitted to the ICU since that was one of the inclusion criteria. The studied cohort included the sickest patients with trauma admitted to our major trauma center. It is likely that the need for urgent laparotomy in our study reflects shock parameters in other studies. Furthermore, it is known that laparotomy as a second hit has an effect on the immune response and is associated with development of infections.20
As we have previously shown, MODS-related and ARDS-related death in trauma has decreased over the years and our previous studies have shown MODS- and ARDS-related death of less than 5%.5 6 Since both MODS/ARDS and infections are neutrophil-mediated complications, it is tempting to speculate that infectious complications are the residual (and less severe) effect of the diminishing more severe MODS and ARDS.
This theory seems to be in line with the timing of the complications. Nowadays, time to MODS development is early after injury (3 days after injury) as we have shown in previous studies,6 whereas median time to development of infectious complications was 12 (7–20) days in this study. This means that MODS precedes infectious complications. Since MODS-related mortality was very low in our cohort (2%), surviving patients may later develop infectious complications. This philosophy is confirmed by the fact that MODS was an independent predictor for infectious complications.
All patients admitted to ICU received SDD, selective oropharyngeal decontamination, and systemic prophylaxis with an intravenous third-generation cephalosporin. This policy was introduced to reduce the incidence of ICU-acquired respiratory tract infections.26 One could dispute that this could have influenced the micro-bacterial growth. However, first of all, all included patients received this regimen of antibiotic prophylaxis, so there was no difference in patients who later developed infectious complications and those who did not. Second, it has been previously shown that there was no overall ecological effect of prolonged antibiotic prophylaxis by counting resistant micro-organisms in ICU in countries where there is relatively low rates of resistant micro-organisms.27
One of the limitations of this study is that it was conducted at a single institution in which the clinical treatment and research were conducted by the same clinicians. Another limitation is that data on the type of bacteria that caused infections were not routinely collected. Furthermore, no pre-existing medical conditions were documented.
In conclusion, in this population of severely injured patients almost half of patients developed an infectious complication. With improvements of trauma and critical care death by complications such as MODS and ARDS has decreased. This success has likely resulted in a shift to less deleterious infectious complications. Future studies need to focus on the role of the immune system (eg, neutrophil function) on the development of infectious complications. Early recognition of development these complications might lead to possible prevention. This could also have a considerable beneficial impact on the use of hospital resources.
Footnotes
Contributors: KW, FH, and LL have contributed to the conception and design of the study. KW and LL have performed acquisition of data. KW has done the analysis and interpretation of data. KW and FH have drafted the article. LL has revised it critically for important intellectual content. KW, FH, and LL have given final approval of the version to be submitted.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The local ethics committee approved this prospective observational study (reference number WAG/mb/16/026664)
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: The dataset supporting the conclusions of this article are available upon reasonable request from the corresponding author.
References
- 1. Demetriades D, Kimbrell B, Salim A, Velmahos G, Rhee P, Preston C, Gruzinski G, Chan L. Trauma deaths in a mature urban trauma system: is "trimodal" distribution a valid concept? J Am Coll Surg 2005;201:343–8. 10.1016/j.jamcollsurg.2005.05.003 [DOI] [PubMed] [Google Scholar]
- 2. Lefering R, Paffrath T, Bouamra O, Coats TJ, Woodford M, Jenks T, Wafaisade A, Nienaber U, Lecky F. Epidemiology of in-hospital trauma deaths. Eur J Trauma Emerg Surg 2012;38:3–9. 10.1007/s00068-011-0168-4 [DOI] [PubMed] [Google Scholar]
- 3. Evans JA, van Wessem KJP, McDougall D, Lee KA, Lyons T, Balogh ZJ. Epidemiology of traumatic deaths: comprehensive population-based assessment. World J Surg 2010;34:158–63. 10.1007/s00268-009-0266-1 [DOI] [PubMed] [Google Scholar]
- 4. Hesselink L, Spijkerman R, van Wessem KJP, Koenderman L, Leenen LPH, Huber-Lang M, Hietbrink F. Neutrophil heterogeneity and its role in infectious complications after severe trauma. World J Emerg Surg 2019;14:24 10.1186/s13017-019-0244-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Wessem KJP, Leenen LPH. Incidence of acute respiratory distress syndrome and associated mortality in a polytrauma population. Trauma Surg Acute Care Open 2018;3:e000232 10.1136/tsaco-2018-000232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Wessem KJP, Leenen LPH. Reduction in mortality rates of Postinjury multiple organ dysfunction syndrome: a shifting paradigm? A prospective population-based cohort study. Shock 2018;49:33–8. 10.1097/SHK.0000000000000938 [DOI] [PubMed] [Google Scholar]
- 7. Gunning AC, Lansink KWW, van Wessem KJP, Balogh ZJ, Rivara FP, Maier RV, Leenen LPH. Demographic patterns and outcomes of patients in level I trauma centers in three international trauma systems. World J Surg 2015;39:2677–84. 10.1007/s00268-015-3162-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dewar DC, Tarrant SM, King KL, Balogh ZJ. Changes in the epidemiology and prediction of multiple-organ failure after injury. J Trauma Acute Care Surg 2013;74:774–9. 10.1097/TA.0b013e31827a6e69 [DOI] [PubMed] [Google Scholar]
- 9. Dewar DC, White A, Attia J, Tarrant SM, King KL, Balogh ZJ. Comparison of postinjury multiple-organ failure scoring systems: Denver versus sequential organ failure assessment. J Trauma Acute Care Surg 2014;77:624–9. 10.1097/TA.0000000000000406 [DOI] [PubMed] [Google Scholar]
- 10. Sauaia A, Moore FA, Moore EE, Haenel JB, Read RA, Lezotte DC. Early predictors of postinjury multiple organ failure. Arch Surg 1994;129:39–45. 10.1001/archsurg.1994.01420250051006 [DOI] [PubMed] [Google Scholar]
- 11. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS, ARDS Definition Task Force . Acute respiratory distress syndrome: the Berlin definition. JAMA 2012;307:2526–33. 10.1001/jama.2012.5669 [DOI] [PubMed] [Google Scholar]
- 12. Fröhlich M, Wafaisade A, Mansuri A, Koenen P, Probst C, Maegele M, Bouillon B, Sakka SG. Which score should be used for posttraumatic multiple organ failure? - Comparison of the MODS, Denver- and SOFA- Scores. Scand J Trauma Resusc Emerg Med 2016;24:130 10.1186/s13049-016-0321-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hutchings L, Watkinson P, Young JD, Willett K. Defining multiple organ failure after major trauma: a comparison of the Denver, sequential organ failure assessment, and Marshall scoring systems. J Trauma Acute Care Surg 2017;82:534–41. 10.1097/TA.0000000000001328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Oostdijk EAN, Kesecioglu J, Schultz MJ, Visser CE, de Jonge E, van Essen EHR, Bernards AT, Purmer I, Brimicombe R, Bergmans D, et al. . Effects of decontamination of the oropharynx and intestinal tract on antibiotic resistance in ICUs: a randomized clinical trial. JAMA 2014;312:1429–37. 10.1001/jama.2014.7247 [DOI] [PubMed] [Google Scholar]
- 15. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 2008;36:309–32. 10.1016/j.ajic.2008.03.002 [DOI] [PubMed] [Google Scholar]
- 16. American College of Surgeons Guide for the prevention of surgical site infection. Bull Am Coll Surg 2000;85:23–9. [PubMed] [Google Scholar]
- 17. Cole E, Davenport R, Willett K, Brohi K. The burden of infection in severely injured trauma patients and the relationship with admission shock severity. J Trauma Acute Care Surg 2014;76:730–5. 10.1097/TA.0b013e31829fdbd7 [DOI] [PubMed] [Google Scholar]
- 18. Metsemakers WJ, Morgenstern M, McNally MA, Moriarty TF, McFadyen I, Scarborough M, Athanasou NA, Ochsner PE, Kuehl R, Raschke M, et al. . Fracture-related infection: a consensus on definition from an international expert group. Injury 2018;49:505–10. 10.1016/j.injury.2017.08.040 [DOI] [PubMed] [Google Scholar]
- 19. Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent J-L, Ramsay G, et al. . 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med 2003;31:1250–6. 10.1097/01.CCM.0000050454.01978.3B [DOI] [PubMed] [Google Scholar]
- 20. Wafaisade A, Lefering R, Bouillon B, Sakka SG, Thamm OC, Paffrath T, Neugebauer E, Maegele M, Trauma Registry of the German Society for Trauma Surgery . Epidemiology and risk factors of sepsis after multiple trauma: an analysis of 29,829 patients from the trauma registry of the German Society for trauma surgery. Crit Care Med 2011;39:621–8. 10.1097/CCM.0b013e318206d3df [DOI] [PubMed] [Google Scholar]
- 21. Glance LG, Stone PW, Mukamel DB, Dick AW. Increases in mortality, length of stay, and cost associated with hospital-acquired infections in trauma patients. Arch Surg 2011;146:794–801. 10.1001/archsurg.2011.41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Taylor JV, Gordon LE, Hall H, Heinzelmann M, Polk HC. Differences between bacterial species shown by simultaneous assessment of neutrophil phagocytosis and generation of reactive oxygen intermediates in trauma patients. Arch Surg 1999;134:1222–7. discussion 1227-8 10.1001/archsurg.134.11.1222 [DOI] [PubMed] [Google Scholar]
- 23. Pechous RD. With friends like these: the complex role of neutrophils in the progression of severe pneumonia. Front Cell Infect Microbiol 2017;7:160 10.3389/fcimb.2017.00160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sadjadi J, Cureton EL, Twomey P, Victorino GP. Transfusion, not just injury severity, leads to posttrauma infection: a matched cohort study. Am Surg 2009;75:307–12. [PubMed] [Google Scholar]
- 25. Bochicchio GV, Napolitano L, Joshi M, Bochicchio K, Meyer W, Scalea TM. Outcome analysis of blood product transfusion in trauma patients: a prospective, risk-adjusted study. World J Surg 2008;32:2185–9. 10.1007/s00268-008-9655-0 [DOI] [PubMed] [Google Scholar]
- 26. de Jonge E, Schultz MJ, Spanjaard L, Bossuyt PMM, Vroom MB, Dankert J, Kesecioglu J. Effects of selective decontamination of digestive tract on mortality and acquisition of resistant bacteria in intensive care: a randomised controlled trial. Lancet 2003;362:1011–6. 10.1016/S0140-6736(03)14409-1 [DOI] [PubMed] [Google Scholar]
- 27. Buitinck S, Jansen R, Rijkenberg S, Wester JPJ, Bosman RJ, van der Meer NJM, van der Voort PHJ. The ecological effects of selective decontamination of the digestive tract (SDD) on antimicrobial resistance: a 21-year longitudinal single-centre study. Crit Care 2019;23:208 10.1186/s13054-019-2480-z [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
tsaco-2019-000398supp001.pdf (61.2KB, pdf)