Abstract
Background
Reactivation of hepatitis B virus (HBV) replication is a well-recognised complication in patients receiving disease-modifying anti-rheumatic drugs (DMARDs) for rheumatoid arthritis (RA). Limited data exist on HBV reactivation among patients with RA treated with janus kinase (JAK) inhibitors. The objective of the current study was to assess HBV reactivation in clinical trials of baricitinib, an oral selective JAK1 and JAK2 inhibitor in RA.
Methods
Data were integrated from four completed Phase 3 trials and one ongoing long-term extension (data up to 1 April 2017) in patients naïve to DMARDs or who had inadequate response (IR) to DMARDs including methotrexate (MTX)-IR and/or other conventional synthetic DMARD (csDMARD)-IR, or tumour necrosis factor inhibitors-IR. Within the clinical programme, baricitinib-treated patients may have received concomitant csDMARDs including MTX, or previous treatment with active comparators including MTX or adalimumab + MTX. At screening, all patients were tested for HBV surface antigen (HBsAg), core antibody (HBcAb) and surface antibody (HBsAb). Patients were excluded if they had (1) HBsAg+, (2) HBcAb+/HBsAb− (in Japan, could enrol if HBV DNA−) or (3) HBsAb+ and HBV DNA+. HBV DNA monitoring, following randomisation in the originating Phase 3 studies, was performed in Japan for patients with HBcAb+ and/or HBsAb+ at screening, and was later instituted globally for HBcAb+ patients in accordance with evolving guidance for HBV monitoring and management with immunomodulatory therapy.
Results
In total, 2890 patients received at least one dose of baricitinib in Phase 3 (6993 patient-years exposure). Of 215 patients with baseline serology suggestive of prior HBV infection (HbcAb+) who received a post-baseline DNA test, 32 (14.9%) were HBV DNA+ at some point following treatment initiation; 8 of 215 patients (3.7%) had a single quantifiable result (≥29 IU/mL). Of these eight patients, four met the definition of reactivation of HBV (HBV DNA level ≥100 IU/mL); baricitinib was permanently discontinued in four patients, and temporarily interrupted in two patients. No patient developed clinical evidence of hepatitis and in five of eight patients, antiviral therapy was not used.
Conclusion
HBV reactivation can occur among RA patients treated with DMARDs, including baricitinib, with prior HBV exposure. Our data suggest that such patients should be monitored for HBV DNA during treatment and might be treated safely with the use of antiviral therapy as needed. The risk of HBV reactivation in patients with HBsAg treated with baricitinib is unknown.
Keywords: rheumatoid arthritis, infections, treatment
Key messages.
What is already known about this subject?
In patients with occult hepatitis B, hepatitis B virus (HBV) infection may be reactivated. An identified risk factor for HBV reactivation is type and degree of immunosuppression.
Reactivation of HBV replication is a recognised complication in patients receiving biological agents, for conditions such as rheumatoid arthritis (RA); limited data exist on HBV reactivation among patients with RA treated with biological and/or non-biological disease-modifying anti-rheumatic drugs (DMARDs), in particular those receiving janus kinase (JAK) inhibitors.
What does this study add?
The current study assessed HBV reactivation in clinical trials of baricitinib, an oral selective JAK1 and JAK2 inhibitor in RA.
In RA patients treated in baricitinib clinical trials who had serology suggestive of prior infection, reactivation was transient and did not account for any clinically relevant adverse events.
How might this impact on clinical practice?
Our data suggest that patients with prior HBV exposure should be monitored, in accordance with clinical guidelines, for HBV DNA during treatment with DMARDs, including baricitinib, and might be treated safely with the use of antiviral therapy as needed.
Introduction
Hepatitis B virus (HBV) infection is one of the most serious and prevalent global health problems, affecting more than 2 billion people worldwide.1 High-HBV prevalence is common in much of the Asia Pacific region, where it is usually acquired perinatally, and represents three-quarters of chronic HBV-positive subjects globally.2–4 In particular, the Western Pacific region (defined by the WHO as 37 countries including China, Japan, South Korea, the Philippines and Vietnam) accounts for nearly half of all chronic HBV infection globally. In Western Europe and North America, HBV infection usually presents as acute hepatitis during adolescence and adulthood. The prevalence has increased owing to population movement and migration from high-prevalence to low-prevalence countries in Europe.5 6
In acute resolving HBV infection, an adaptive immune response ultimately leads to viral clearance from infected hepatocytes and the development of neutralising antibodies that prevent viral spread. Persons who become HBV surface antigen-negative (HBsAg−) are considered to have resolved hepatitis B. Occult HBV infection is defined by detectable HBV DNA in serum and/or liver in patients who have tested negative for serum HBsAg.3 Most occult HBV infection patients have low HBV DNA levels in the serum (and liver tissues are generally not easily assessable). These low levels of HBV DNA, as well as their fluctuations, make the detection of this condition difficult. In patients with occult hepatitis B, HBV infection may be reactivated, and the key risk factors for reactivation can be broadly classified into host factors, virologic factors and type and degree of immunosuppression.7 Since curative therapy for HBV is not currently available, there is a large population at risk for HBV reactivation.
There are two components of reactivation of chronic HBV infection: reactivation of HBV replication, and subsequent flare (or exacerbation) of hepatitis. Reactivation of HBV replication is defined as a marked increase in HBV replication (≥2 log increase from baseline levels or new appearance of HBV DNA to a level of ≥100 IU/mL) in a person with previously stable or undetectable levels, respectively.3 Reactivation of HBV replication is a recognised complication in patients receiving biologic agents for conditions such as rheumatoid arthritis (RA),8 9 and can be transient and clinically silent but is sometimes severe and results in acute hepatic failure.9 10 However, data regarding the prevalence of occult infection and the incidence of its reactivation in patients with RA under treatment with biological and/or non-biological disease-modifying anti-rheumatic drugs (DMARDs) are limited, in particular among patients with RA treated with janus kinase (JAK) inhibitors. To date, JAK inhibitors have been associated with the reactivation of varicella zoster virus and, more rarely, cytomegalovirus; however, their impact on HBV is unknown.11
Baricitinib is an oral selective JAK1 and JAK2 inhibitor approved for the treatment of moderately-to-severely active RA in adults. The objective of the current study was to assess HBV reactivation in clinical trials of baricitinib in RA.
Materials and methods
Study population
Data were collected from patients with RA who received at least one dose of baricitinib in Phase 3 originating studies: RA-BEGIN (52-week study of baricitinib 4 mg, as monotherapy or combined with methotrexate (MTX), compared with MTX monotherapy in patients with active RA who had received no or minimal conventional DMARDs);12 RA-BEAM (52-week study of baricitinib 4 mg compared with adalimumab (ADA) 40 mg and placebo (switched to baricitinib after 24 weeks) in patients with active RA with an inadequate response to MTX; patients on background MTX);13 RA-BUILD (24-week study of baricitinib 2 mg or 4 mg compared with placebo in patients with RA and an inadequate response to conventional synthetic DMARDs; patients on background conventional synthetic DMARDs (csDMARDs));14 RA-BEACON (24-week study of baricitinib 2 mg or 4 mg compared with placebo in patients with RA and an inadequate response to tumour necrosis factor inhibitors (TNFi), or other biological DMARDs; patients on background csDMARDs)15 and an ongoing long-term extension study, RA-BEYOND (NCT01885078) (treatment with baricitinib 2 mg or 4 mg) up to 1 April 2017. This analysis included patients treated with baricitinib 2 mg or 4 mg concomitantly with ≥1 csDMARD including MTX, baricitinib 4 mg monotherapy, baricitinib 4 mg + MTX, ADA + MTX or MTX alone. Notably, because the studies included an option for rescue and protocol-mandated treatment switching, patients receiving baricitinib 4 mg may have previously received ADA + MTX or MTX alone.
At screening, all patients were tested for HBsAg, core antibody (HBcAb) and surface antibody (HBsAb). In Japan and elsewhere, if required by local clinical guidelines, patients had screening HBV DNA tests. Patients were excluded from treatment in these studies if they had (1) HBsAg+, (2) HBsAb−/HBcAb+ (although in Japan, patients could enrol if HBV DNA−) or (3) HBsAb+ and HBV DNA+. In all studies, concomitant stable doses of csDMARDs, non-steroidal anti-inflammatory drugs, analgesics or glucocorticoids (≤10 mg of prednisone or the equivalent per day) were permitted.
HBV DNA monitoring and evaluation of HBV reactivation
Per-protocol, routine monthly HBV DNA monitoring following treatment initiation (post-baseline) was performed in Japan for patients with HBcAb+ and/or HBsAb+ at screening. Monitoring approximately every 3 to 6 months was instituted globally (with the exception of Japan, where monthly monitoring had already been established) for patients with HBcAb+ at baseline, in accordance with evolving guidance for HBV monitoring and management with immunomodulatory therapy.3 As the guidance changed during study conduct, protocol addenda were implemented in RA-BEGIN, RA-BEAM and RA-BEYOND to retrospectively test patients with HBcAb+ at baseline; addenda were not implemented for the already completed RA-BUILD and RA-BEACON studies. Patients with post-baseline HBV DNA ≥29 IU/mL met study drug discontinuation criteria, and were referred to a hepatologist. In select cases, investigators continued study drug following an HBV DNA+ test ≥29 IU/mL in consultation with the sponsor and in accordance with HBV management guidelines. Monthly monitoring and consultation with a hepatologist were recommended for all patients with two or more results with a value of <29 IU/mL. Per investigator decision, post-baseline HBV DNA testing with or without concomitant hepatic monitoring (alanine transaminase (ALT)/aspartate transaminase (AST) evaluation) was done for patients who were HBcAb− at baseline. Reactivation of HBV replication is defined as a marked increase in HBV replication (≥2 log increase from baseline levels or new appearance of HBV DNA to a level of ≥100 IU/mL) in a person with previously stable or undetectable levels, respectively.3
Laboratory (HBV DNA monitoring)
The lower limit of quantification (LLQ) was 29 IU/mL; the lower limit of detection was 2.4 IU/mL (1 IU/mL HBV is equivalent to 5.6 copies/mL).16
Statistical analyses
Data were integrated from four completed Phase 3 trials and one ongoing long-term extension (LTE) (data up to 1 April 2017; median exposure=998 days, maximum exposure=1583 days) (all baricitinib RA Phase 3 analysis set: studies RA-BEGIN (JADZ), RA-BEAM (JADV), RA-BUILD (JADX), RA-BEACON (JADW) and RA-BEYOND (JADY; LTE)). Baseline demographic information and clinical characteristics were analysed using descriptive statistics.
Results
Patient characteristics, baseline serology and HBV DNA testing
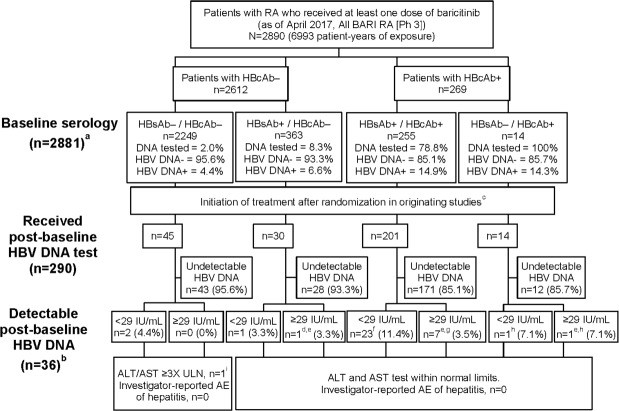
In total, this study included 2890 patients who had received at least one dose of baricitinib in Phase 3 studies, including the LTE, up to the data cut-off date (6993 patient-years exposure). The study population had a mean age of 53 years, a mean disease duration of 8 years and a mean Clinical Disease Activity Index score at baseline of 38; the majority of patients were female (79%) (table 1). There were 74% of patients taking MTX and 51.6% were taking corticosteroids at baseline. Overall, 269 patients had baseline serology suggestive of prior hepatitis B infection (HBsAg−, HBsAb+/HBcAb+, n=255; HBsAg−, HBsAb−/HBcAb+, n=14) (figure 1). HBsAb+/HBcAb+ serostatus was present in a much larger proportion of patients in Asian countries (Japan, 12%; China, 42%; Taiwan, 51%; South Korea, 37%) compared with other regions (North America, 3%; South America, 2%; European Union, 8%) (table 2). Within the 14 patients with HBsAb−/HBcAb+ at baseline, 8 patients were from Japan.
Table 1.
Baseline demographics and disease characteristics
| All BARI RA (Phase 3) (n=2890) |
|
| Age, years | 52.9 (12.3) |
| Female, n (%) | 2269 (78.5) |
| Region, n (%) | |
| Asia* (excluding Japan) | 226 (7.8) |
| South America† | 658 (22.8) |
| European Union | 670 (23.2) |
| Japan | 371 (12.8) |
| North America‡ | 606 (21.0) |
| Rest of the world | 359 (12.4) |
| Disease duration (from diagnosis), years | 7.7 (8.2) |
| Corticosteroid use at baseline, n (%) | 1492 (51.6) |
| Methotrexate use at baseline, n (%) | 2139 (74.0) |
| Tender joint counts (68 joints) | 25.1 (14.4) |
| Swollen joint counts (66 joints) | 15.3 (9.1) |
| CDAI | 38.4 (12.7) |
| DAS28-CRP | 5.8 (0.9) |
| Baseline serology,§ n | |
| HBsAg−; HBsAb−/HBcAb− | 2249 |
| HBsAg−; HBsAb+/HBcAb− | 363 |
| HBsAg−; HBsAb+/HBcAb+ | 255 |
| HBsAg−; HBsAb−/HBcAb+ | 14 |
Values are mean (SD) unless otherwise stated.
*China, Taiwan, South Korea.
†Central/South America and Mexico.
‡USA/Canada (including Puerto Rico).
§Total number of patients with baseline serology is n=2881 owing to missing data for nine patients (eight missing surface antibody and one missing core antibody data).
BARI, baricitinib; CDAI, clinical disease activity index; DAS28-CRP, Disease Activity Score 28 – C-reactive protein; HBcAb, hepatitis B virus core antibody; HBsAb, hepatitis B virus surface antibody; HBsAg, hepatitis B virus surface antibody; RA, rheumatoid arthritis.
Figure 1.
HBV serology and DNA detectability in patients treated with baricitinib in Phase 3 trials; a total number of patients with baseline serology is n=2881 owing to missing data for nine patients (eight missing surface antibody and one missing core antibody data); b patients with detectable post-baseline HBV DNA were from China (11), Japan (9), Taiwan (6), Israel (2), Greece (2), Russia (2), USA (2), Argentina (1) and South Korea (1); c initial randomised treatment assignment was placebo (concomitant csDMARDs), MTX monotherapy, adalimumab (concomitant MTX), baricitinib 2 mg (concomitant csDMARDs) or 4 mg (monotherapy or concomitant csDMARDs); d one patient (Japan, BARI 4 mg, HBcAb−/HBsAb+/HBV DNA not detected at baseline) had a single post-baseline, detectable HBV DNA result (92, not detected on retest). No antiviral treatment was administered, and there were no hepatic enzyme elevations or other relevant AEs reported; the patient continued baricitinib; e nine patients had quantifiable HBV DNA levels of 31, 36, 60, 76, 92, 256, 257, 869 and 1547 IU/mL; f baseline HBV DNA not detected (17), not tested (5), detectable <29 IU/mL (1); g baseline HBV DNA not detected (5), not tested (2); h baseline HBV DNA not detected; i one patient with ALT/AST ≥3 x ULN was discontinued from study owing to abnormal liver function test. Patient had a single detectable HBV DNA test result <29 IU/mL. At last available observation, ALT and AST were <3 x ULN and <2 x ULN, respectively. AE, adverse event; ALT, alanine transaminases;AST, aspartate transaminases; BARI, baricitinib; csDMARDs, conventional synthetic DMARDs; DMARDs, disease-modifying anti-rheumatic drugs; HBcAb, hepatitis B virus core antibody; HBsAb, hepatitis B virus surface antibody; HBV, hepatitis B virus; MTX, methotrexate; Ph, phase; RA, rheumatoid arthritis;ULN, upper limit of normal.
Table 2.
Baseline serology status and post-baseline HBV DNA detectability by geographical region
| Baseline serology status/ HBV DNA detectability |
Japan | China | Taiwan | South Korea | North America | South America | European Union | Rest of world | Total |
| Total patients* | 371 | 50 | 92 | 84 | 601 | 655 | 669 | 359 | 2881 |
| Total receiving post-baseline DNA test, n (%) | 82 (22) | 20 (40) | 46 (50) | 36 (43) | 24 (4) | 18 (3) | 31 (5) | 33 (9) | 290 |
| HBsAb−/HBcAb−, n (%) | 303 (82) | 15 (30) | 26 (28) | 18 (21) | 496 (83) | 582 (89) | 526 (79) | 283 (79) | 2249 |
| Received post-baseline DNA test | 15 | 0 | 0 | 2 | 8 | 6 | 7 | 7 | 45 |
| Undetectable HBV DNA | 15 | 0 | 0 | 2 | 7 | 6 | 6 | 7 | 43 |
| HBV DNA <29 IU/mL | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 0 | 2 |
| HBV DNA ≥29 IU/mL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| HBsAb+/HBcAb−, n (%) | 14 (4) | 13 (26) | 19 (21) | 35 (42) | 87 (14) | 61 (9) | 91 (14) | 43 (12) | 363 |
| Received post-baseline DNA test | 14 | 0 | 2 | 5 | 4 | 2 | 2 | 1 | 30 |
| Undetectable HBV DNA | 12 | 0 | 2 | 5 | 4 | 2 | 2 | 1 | 28 |
| HBV DNA <29 IU/mL | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| HBV DNA ≥29 IU/mL | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| HBsAb+/HBcAb+, n (%) | 46 (12) | 21 (42) | 47 (51) | 31 (37) | 17 (3) | 11 (2) | 52 (8) | 30 (8) | 255 |
| Received post-baseline DNA test | 45 | 19 | 44 | 29 | 11 | 9 | 22 | 22 | 201 |
| Undetectable HBV DNA | 39 | 8 | 38 | 28 | 10 | 8 | 21 | 19 | 171 |
| HBV DNA <29 IU/mL | 4 | 11 | 3 | 0 | 1 | 0 | 1 | 3 | 23 |
| HBV DNA ≥29 IU/mL | 2 | 0 | 3 | 1 | 0 | 1 | 0 | 0 | 7 |
| HBsAb−/HBcAb+, n (%) | 8 (2) | 1 (2) | 0 (0) | 0 (0) | 1 (0) | 1 (0) | 0 (0) | 3 (1) | 14 |
| Received post-baseline DNA test | 8 | 1 | 0 | 0 | 1 | 1 | 0 | 3 | 14 |
| Undetectable HBV DNA | 7 | 1 | 0 | 0 | 1 | 1 | 0 | 2 | 12 |
| HBV DNA <29 IU/mL | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| HBV DNA ≥29 IU/mL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 |
HBcAb, hepatitis B virus core antibody; HBsAb, hepatitis B virus surface antibody; HBV, hepatitis B virus.
*Total number of patients with baseline serology is n=2881 owing to missing data for nine patients (five patients from North America; three patients from South America; one patient from European Union).
HBV DNA testing at baseline was not systematically performed across the Phase 3 studies; however, testing was performed to inform study entry criteria for patients who were HBsAb+/HBcAb+ (n=255) or HBsAb−/HbcAb+ (n=14) (figure 1). In the HBsAb−/HbcAb+ cohort (n=14), eight patients were from Japan and had HBV DNA− tests at baseline (met study inclusion criteria) and six patients entered the studies from regions other than Japan (these cases were reported as protocol violations).
Post-baseline HBV DNA testing
Overall
Post-baseline (after treatment initiation) HBV DNA tests were performed for 290 patients, of whom 201 patients had HBsAb+/HBcAb+ and 14 had HBsAb–/HBcAb+ serostatus at baseline (figure 1). Fifty-four patients of 255 with HBsAb+/HBcAb+ serology at baseline did not have post-baseline HBV DNA test results, for reasons which include timing associated with implementation of the protocol addenda. Of the 290 patients who underwent post-baseline testing (including patients in the HBcAb− groups without a baseline HBV DNA result), 36 (12%) had detectable (n=27, <29 IU/mL; or n=9, ≥29 IU/mL) HBV DNA; these patients were from China (11), Japan (9), Taiwan (6), Israel (2), Greece (2), Russia (2), USA (2), Argentina (1) and South Korea (1) (table 2).
Patients with HBcAb+ at baseline
Thirty-two patients with HBcAb+ serostatus at baseline had detectable HBV DNA (<29 IU/mL or ≥29 IU/mL) at some point following treatment initiation (figure 1). Time from first baricitinib dose to first detectable HBV DNA test results in this cohort ranged from 29 to 1331 days (median 609 days). Within this group, 7 patients with HBsAb+/HBcAb+ had a single quantifiable HBV DNA+ test (≥29 IU/mL; median 256 IU/mL, range 31 to 1547 IU/mL) (table 3) and 23 had qualitative HBV DNA+ tests below the LLQ (<29 IU/mL). In the HBsAb-/HBcAb+ cohort, one patient had quantifiable HBV DNA (at 36 IU/mL; table 3) and one patient had DNA that was below the LLQ. Of 32 patients with HBcAb+ serostatus at baseline and detectable HBV DNA+ post-baseline, 30 had DNA tests that were negative at baseline. Seventeen of 32 patients were being treated concomitantly with corticosteroids at the time of the detectable DNA result.
Table 3.
Baricitinib-treated patients with HBsAg−; HBcAb+ at baseline and quantifiable post-baseline HBV DNA
| Patient | Region | Baricitinib dose at event* | Baseline HBV DNA | Relevant history | Serial HBV DNA testing† | Reported TEAE | Antiviral treatment | ALT elevation | Study drug permanently discontinued | Study drug temporarily interrupted | Available post-baseline serology |
| Baricitinib-treated patients with baseline HBsAg−; HBcAb+/HBsAb+ and quantifiable (≥29 IU/mL) post-baseline HBV DNA | |||||||||||
| 1 | Asia (excluding Japan) |
4 mg | Not detected | – | <29,<29, ND, ND, ND,<29,<29,<29,<29,<29, ND,<29,<29,<29,<29, ND,<29,<29,<29,<29, ND,<29,<29, 256,<29,<29, | – | – | No | Yes | No | – |
| 2 | Japan | 4 mg | Not detected | – | 31, ND, ND, ND | Hepatitis B DNA assay positive | Entecavir | No | Yes | No | HBsAg−, HBsAb+, HbcAb+ |
| 3 | Asia (excluding Japan) |
4 mg | Not detected | Chronic hepatitis, parenchymal liver disease, liver nodule benign, fatty liver | ND,<29, 257,<29, ND | HBV DNA polymerase increase | Telbivudine | No | Yes | No | |
| 4 | Central and South America and Mexico | 4 mg | Not done | – | <29,<29,<29,<29,<29,<29, ND,<29, ND,<29,<29,<29,<29,<29,<29, 76 | – | – | No | No | No | HBsAg−, HBsAb+, HbcAb+ HepA IgM−, HepA Ab+, HepC Ab−, HepE IgM−, HepE, IgG−, HepE PCR− |
| 5 | Asia (excluding Japan) |
4 mg | Not detected | – | ND, ND, ND, 60, ND, ND, ND, ND, ND | HBV DNA polymerase increase | – | No | No | Yes | – |
| 6 | Asia (excluding Japan) |
2 mg | Not done | – | ND, ND, ND, 869, ND, ND, ND, ND, ND, ND | – | – | No | No | Yes | HBsAg−, HBsAb+, HbcAb+ |
| 7 | Japan | 2 mg | Not detected | – | ND, ND, ND, ND, ND, ND, ND, ND, ND, ND, ND, ND, ND, ND, ND, ND, ND, ND, ND, ND, ND, ND, 1547, ND, ND, ND, ND, ND, ND, ND, ND, ND, ND, ND | – | – | No | No | No | HBsAg−, HBsAb+, HbcAb+ HepA IgM−, HepA Ab−, HepC Ab−, HepE IgM−, HepE, IgG+, HepE PCR+ |
| Baricitinib-treated patients with baseline HBsAg−; HBcAb+/HBsAb− and quantifiable (≥29 IU/mL) post-baseline HBV DNA | |||||||||||
| 8 | Rest of world | 4 mg | Not detected | – | 36, ND, ND, ND,<29, ND, ND | – | Tenofovir | No | Yes | No | HBsAg−, HBsAb−, HbcAb+ |
Results include all scheduled visits, retests and hepatic monitoring. All patients were receiving concomitant methotrexate.
*Event defined as ≥29 IU/mL HBV DNA test result.
†All available post-baseline detectable (<29 IU/mL) and quantifiable (≥29 IU/mL) results; all patients reported a single quantifiable HBV DNA result (shown in red).
ALT, alanine transaminase; HBcAb, hepatitis B virus core antibody; HBsAb, hepatitis B virus surface antibody; HBsAg, hepatitis B virus surface antigen; HBV, hepatitis B virus; Hep, hepatitis; Ig, immunoglobulin; ND, not detected; TEAE, treatment-emergent adverse event.
Eight patients (3.7%) in the HBcAb+ cohort with a post-baseline HBV DNA test (201 (HBsAb+/HBcAb+) and 14 (HBsAb−/HBcAb+)) had quantifiable HBV DNA (range from 31 to 1547 IU/mL) (six patients having HBV DNA− results and two patients not having DNA tests performed at baseline). All eight patients reported a single post-baseline quantifiable HBV DNA result, and subsequent tests were either not detectable or below the LLQ (table 3). Six patients were being treated with baricitinib 4 mg and two patients were being treated with baricitinib 2 mg at the time of the DNA+ result, with all eight patients receiving concomitant MTX. Six of the eight patients were from Asian countries. Adverse events (AE) of detectable HBV DNA resulted in discontinuation of baricitinib in four of eight patients, of whom three received antivirals; the remaining four patients continued baricitinib in the LTE and did not receive antiviral therapy. Patient 4 did not have another post-baseline HBV DNA test result after the initial quantifiable result as of the data cut-off (table 3); the most immediate result was <LLQ, but subsequent results were quantifiable. In patients 5 to 7, post-baseline test results up to the data cut-off showed undetectable HBV DNA following the single quantifiable DNA result. All patients with quantifiable HBV DNA had alanine transaminase or aspartate transaminase within normal limits, and none had an investigator-reported AE of hepatitis.
Patients with HBcAb− at baseline
Seventy-five patients had post-baseline HBV DNA testing performed despite having baseline serology (HBcAb−) that did not necessitate testing per-protocol, with 71 patients (95%) in this group having undetectable DNA (figure 1). Four patients with HBcAb− at baseline had detectable post-baseline HBV DNA (n=3,<29 IU/mL and n=1,≥29 IU/mL). One patient (HBsAb+/HBcAb− at baseline) had a single detectable result (92 IU/mL) and was undetectable on retest. This patient did not receive antiviral treatment and there were no hepatic enzyme elevations or other relevant AEs reported; the patient continued baricitinib. The second patient (HBsAb−/HBcAb− at baseline) had a single test result with detectable DNA (<29 IU/mL) and ALT/AST ≥3 times the upper limit of normal (x ULN). This patient did not receive antiviral therapy, but was discontinued from baricitinib owing to abnormal liver function test; at the last available observation, ALT and AST were <3 x ULN and <2 x ULN, respectively. The third patient (HBsAb−/HBcAb− at baseline) had three consecutive HBV DNA detectable results <29 IU/mL with no results thereafter; no hepatic enzyme elevations or relevant AEs were reported. No change was made to baricitinib administration and no antiviral treatment was administered. The patient later discontinued from the study and study drug owing to lack of efficacy. Finally, the fourth patient (HBsAb+/HBcAb− at baseline) had a single detectable HBV DNA result <29 IU/mL, with no hepatic enzyme elevations or relevant AEs reported. This patient did not receive antiviral treatment and continued baricitinib.
Discussion
In a subset of patients with RA from the baricitinib Phase 3 clinical programme with baseline serology suggestive of prior HBV infection (HBcAb+), and HBV DNA results following treatment initiation, HBV reactivation was evaluated. The data suggest that patients with prior HBV infection but without clinical indications of active infection can be treated with DMARDs, including baricitinib, with monitoring for potential reactivation as would be appropriate per current guidelines in a clinical trial setting. Additional data will be needed to fully characterise the potential risk for reactivation of HBV in the context of JAK inhibitors administered in an RA population; however, the current study provides unique and important evidence toward an evaluation of this risk outside of current standard of care and with a once-daily oral JAK inhibitor. In the current study, among patients with RA who had baseline serology suggestive of prior HBV infection, 32 baricitinib-treated patients tested HBV DNA+ (32/215 patients; 14.9%) at some point following treatment initiation up to the study data cut-off date; of these, 3.7% (8/215 patients) had quantifiable results (≥29 IU/mL). Each of the eight patients had only a single quantifiable HBV DNA result; retest results available as of the data cut-off for seven of the eight patients were either detectable, but not quantifiable (<29 IU/mL) (n=3) or not detected (n=4). Four of the eight patients met the definition of reactivation of HBV owing to having an HBV DNA level ≥100 IU/mL.3 No patient developed clinical evidence of hepatitis and in most cases (five/eight patients) antiviral therapy was not used.
With the introduction of targeted disease-modifying agents with differing mechanisms of action in RA treatment, it is becoming more important to understand the mechanisms whereby treatment with certain agents makes patients more predisposed to HBV reactivation. Among patients with occult HBV infection who are HBsAg– and HBcAb+, assessment of immunosuppressive therapies has shown that the greatest risk of HBV reactivation (≥10%), potentially leading to increased risk of HBV-reactivation related liver failure and liver related mortality, was observed with B-cell depleting therapies such as rituximab and ofatumumab.7 Similar findings are described in a review published by the American Gastroenterological Association Institute, which reported that among chemotherapeutic agents and immunosuppressants, rituximab has the highest risk of HBV reactivation in both HBsAg+ patients and patients with resolved hepatitis B, often associated with acute liver injury, whereas TNFis are classified as moderate-risk for HBV reactivation (1% to 10%) in HBsAg+ carriers.17
In recent years, increasing attention has been paid to the possibility of HBV reactivation in patients with RA undergoing treatment with biological DMARDs (bDMARDs), and HBV screening is recommended before initiating biologics.18 Among the available bDMARDs approved for RA, the risk of HBV reactivation under TNFi has been well-described in several observational studies,1 and the findings of our analyses are comparable to several previous investigations in which occurrence of HBV reactivation in patients with serological markers of past HBV infection was low (0% to 5.3%).19–22 In addition, in the majority of patients who tested HBV DNA+ at some point following treatment, HBV DNA became undetectable again within several months, and clinical outcomes were satisfactory.
Observational data for newer bDMARDs with different targets, including those that target the IL-6 (interleukin 6) receptor, are still lacking, with only a few reports published.1 Consequently, the existing practical guidelines for the prevention and management of viral reactivation are mostly targeted to the older rather than to the newer bDMARDs. JAK inhibition may counteract the suppressive effects of interferon α on viral replication; however, data on the effects of JAK inhibitors on HBV reactivation are limited. A recent retrospective analysis of 116 Taiwanese patients with RA treated with tofacitinib evaluated the risk of HBV reactivation over a 2-year period.23 Overall, 81 (69.8%) patients had a past HBV infection, based on patients being HBcAb+ with or without HBsAb. Six patients with HBsAg+ and normal ALT levels were defined as HBV carriers (patients with HBsAg+ were excluded from our analyses); in the other 75 patients, HBV infection was considered resolved (defined as prior HBV infection with normal ALT levels, but without detectable serum HBV DNA or HBsAg). Among the six carriers, only two received antiviral prophylaxis during treatment with tofacitinib. Of the four carriers without antiviral prophylaxis, two experienced HBV reactivation during treatment with tofacitinib. No HBV reactivation was observed in patients with resolved infection (using a 10-fold rise in HBV DNA to define HBV reactivation).24
There is much geographical diversity in the prevalence of HBV, which is highly endemic in Asia and other parts of the world outside North America and Western Europe, findings that are further supported by the results of this study. The frequency of resolved HBV infection in patients with rheumatic disease seems to be directly associated with the general prevalence of HBV infection within respective countries. In a recent literature review, the prevalence of resolved HBV infection in rheumatic disease was found to range from 7.3% to 66% across a number of studies.25 Rates in Western Europe (7.3% to 26.3%) were generally lower than those observed in Asian countries. HBV prevalence was similar in Japan (up to 31.5%) and South Korea (33.1%), but markedly higher in China (up to 51%) and Taiwan (66.0%).
Our study had a number of limitations. Because this analysis was retrospective, measurement of HBV DNA was not conducted according to a single protocol. Given the evolving guidance for HBV monitoring and management, HBV DNA tests were not routinely performed for all patients, possibly resulting in selection bias. In addition, risk of HBV reactivation in patients with HBsAg treated with baricitinib is unknown, as patients were excluded from the studies if they were positive for HBsAg. Further, results were descriptive, thus limiting the ability to make objective conclusions about the clinical management of patients with evidence of prior HBV infection currently being treated or who may be treated with baricitinib.
Though HBV reactivation was seen in patients with RA treated with DMARDs, including baricitinib, who had serology suggestive of prior infection, reactivation was transient even with continued baricitinib treatment and did not account for any clinically relevant AEs. Screening for viral hepatitis should be performed in accordance with clinical guidelines before starting baricitinib treatment. In patients with serology suggestive of prior infection, the level of expression of HBV DNA should be determined. If HBV DNA is detected, a liver specialist should be consulted.
Acknowledgments
Medical writing assistance was provided by John Bilbruck of ProScribe – part of the Envision Pharma Group and was funded by Eli Lilly and Company.
Footnotes
Contributors: All authors were involved in the study design, and analysis and interpretation of data and/or critically revising the manuscript. All authors agreed to be responsible for all aspects of the work and read and approved the final manuscript to be published.
Funding: This study was sponsored by Eli Lilly and Company.
Competing interests: MH has received grants/research support from: Bristol-Myers Squibb K.K, Eisai, Ono Pharmaceuticals and Takeda Pharmaceutical; and consultant fees for Eli Lilly and Company. KW has received grants/research support from: Bristol-Myers Squibb and Pfizer; and consultant fees for AbbVie, Amgen, Bristol-Myers Squibb, Eli Lilly and Company, Pfizer and UCB. TT has received consultant and/or speaker fees from: AbbVie GK, Asahi Kasei Medical K.K, Astellas Pharma Inc, Astra Zeneca K.K, Bristol-Myers Squibb K.K., Celltrion, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Eli Lilly and Company, Janssen Pharmaceutical K.K., Merck Serono, Mitsubishi Tanabe Pharma. TYH has nothing to disclose. YMC received research support from: Janssen and Pfizer, speaker fees from: AbbVie, Astellas, Bristol-Myers Squibb, Celltrion, Chugai, Eli Lilly and Company, Janssen, Novartis, Pfizer, Roche and UCB. JS has received grant/research support from AbbVie, Eli Lilly and Company, Janssen, MSD, Pfizer and Roche; and consultant fees from: AbbVie, Amgen, Astra-Zeneca, Astro, Bristol-Myers Squibb, Celgene, Celltrion, Chugai Pharmaceutical, Eli Lilly and Company, Gilead, GlaxoSmithKline, ILTOO, Janssen, Medimmune, MSD, Novartis-Sandoz, Pfizer, Roche, Samsung, Sanofi-Aventis and UCB. GB has received research support from: Eli Lilly and Company; and consultant and/or speaker fees from: Eli Lilly and Company, Janssen, Novartis and Pfizer. MG has received grant/research support and/or consultant fees from: AbbVie, Eli Lilly and Company, Galapagos and Pfizer. CW, WSW, CD, and RL are current employees and shareholders of Eli Lilly and Company.
Patient consent for publication: Not required.
Ethics approval: Trials described were conducted in accordance with the Good Clinical Practice guidelines of the International Conference on Harmonisation and with the principles of the Declaration of Helsinki. All trial protocols and documentation were approved by the institutional review board or independent ethics committee at each investigational site.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. Eli Lilly and Company provides access to all individual participant data collected during the trial, after anonymisation, with the exception of pharmacokinetic or genetic data. Data are available to request 6 months after the indication studied has been approved in the USA and European Union and after primary publication acceptance, whichever is later. No expiration date of data requests is currently set once data are made available. Access is provided after a proposal has been approved by an independent review committee identified for this purpose and after receipt of a signed data sharing agreement. Data and documents, including the study protocol, statistical analysis plan, clinical study report, blank or annotated case report forms, will be provided in a secure data sharing environment. For details on submitting a request, see the instructions provided at www.vivli.org.
References
- 1. Nard FD, Todoerti M, Grosso V, et al. Risk of hepatitis B virus reactivation in rheumatoid arthritis patients undergoing biologic treatment: extending perspective from old to newer drugs. World J Hepatol 2015;7:344–61. 10.4254/wjh.v7.i3.344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liaw Y-F. Antiviral therapy of chronic hepatitis B: opportunities and challenges in Asia. J Hepatol 2009;51:403–10. 10.1016/j.jhep.2009.04.003 [DOI] [PubMed] [Google Scholar]
- 3. Sarin SK, Kumar M, Lau GK, et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: a 2015 update. Hepatol Int 2016;10:1–98. 10.1007/s12072-015-9675-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. MacLachlan JH, Cowie BC. Hepatitis B virus epidemiology. Cold Spring Harb Perspect Med 2015;5:a021410 10.1101/cshperspect.a021410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Coppola N, Alessio L, Gualdieri L, et al. Hepatitis B virus, hepatitis C virus and human immunodeficiency virus infection in undocumented migrants and refugees in southern Italy, January 2012 to June 2013. Euro Surveill 2015;20:30009 10.2807/1560-7917.ES.2015.20.35.30009 [DOI] [PubMed] [Google Scholar]
- 6. Hampel A, Solbach P, Cornberg M, et al. [Current seroprevalence, vaccination and predictive value of liver enzymes for hepatitis B among refugees in Germany]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 2016;59:578–83. 10.1007/s00103-016-2333-8 [DOI] [PubMed] [Google Scholar]
- 7. Loomba R, Liang TJ. Hepatitis B reactivation associated with immune suppressive and biological modifier therapies: current concepts, management strategies, and future directions. Gastroenterology 2017;152:1297–309. 10.1053/j.gastro.2017.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mori S. Past hepatitis B virus infection in rheumatoid arthritis patients receiving biological and/or nonbiological disease-modifying antirheumatic drugs. Modern Rheumatology 2011;21:621–7. 10.3109/s10165-011-0458-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen Y-M, Yang S-S, Chen D-Y. Risk-Stratified management strategies for HBV reactivation in RA patients receiving biological and targeted therapy: a narrative review. J Microbiol Immunol Infect 2019;52:1–8. 10.1016/j.jmii.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 10. Hsu S-Y, CM H, Lee PH, et al. Acute hepatic failure caused by hepatitis B virus reactivation in patients receiving immunomodulatory agents for autoimmune diseases. Arch Rheumatol 2014;29:171–7. 10.5606/ArchRheumatol.2014.3822 [DOI] [Google Scholar]
- 11. Winthrop KL. Erratum: the emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol 2017;13:320 10.1038/nrrheum.2017.51 [DOI] [PubMed] [Google Scholar]
- 12. Fleischmann R, Schiff M, van der Heijde D, et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol 2017;69:506–17. 10.1002/art.39953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Taylor PC, Keystone EC, van der Heijde D, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med 2017;376:652–62. 10.1056/NEJMoa1608345 [DOI] [PubMed] [Google Scholar]
- 14. Dougados M, van der Heijde D, Chen Y-C, et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study. Ann Rheum Dis 2017;76:88–95. 10.1136/annrheumdis-2016-210094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Genovese MC, Kremer J, Zamani O, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med 2016;374:1243–52. 10.1056/NEJMoa1507247 [DOI] [PubMed] [Google Scholar]
- 16. Hepatitis B Foundation Diagnosed with chronic hepatitis B? what does your HBV DNA test tell you? 2016. Available: http://www.hepb.org/blog/diagnosed-with-chronic-hepatitis-b-what-does-your-hbv-dna-test-tell-you/ [Accessed 6 May 2019].
- 17. Perrillo RP, Gish R, Falck-Ytter YT. American Gastroenterological Association Institute technical review on prevention and treatment of hepatitis B virus reactivation during immunosuppressive drug therapy. Gastroenterology 2015;148:221–44. 10.1053/j.gastro.2014.10.038 [DOI] [PubMed] [Google Scholar]
- 18. Koutsianas C, Thomas K, Vassilopoulos D. Hepatitis B reactivation in rheumatic diseases: screening and prevention. Rheum Dis Clin North Am 2017;43:133–49. 10.1016/j.rdc.2016.09.012 [DOI] [PubMed] [Google Scholar]
- 19. Caporali R, Bobbio-Pallavicini F, Atzeni F, et al. Safety of tumor necrosis factor α blockers in hepatitis B virus occult carriers (hepatitis B surface antigen negative/anti-hepatitis B core antigen positive) with rheumatic diseases. Arthritis Care Res 2010;62:749–54. 10.1002/acr.20130 [DOI] [PubMed] [Google Scholar]
- 20. Lan J-L, Chen Y-M, Hsieh T-Y, et al. Kinetics of viral loads and risk of hepatitis B virus reactivation in hepatitis B core antibody-positive rheumatoid arthritis patients undergoing anti-tumour necrosis factor alpha therapy. Ann Rheum Dis 2011;70:1719–25. 10.1136/ard.2010.148783 [DOI] [PubMed] [Google Scholar]
- 21. Lee YH, Bae S-C, Song GG. Hepatitis B virus (HBV) reactivation in rheumatic patients with hepatitis core antigen (HBV occult carriers) undergoing anti-tumor necrosis factor therapy. Clin Exp Rheumatol 2013;31:118–21. [PubMed] [Google Scholar]
- 22. Nakamura J, Nagashima T, Nagatani K, et al. Reactivation of hepatitis B virus in rheumatoid arthritis patients treated with biological disease-modifying antirheumatic drugs. Int J Rheum Dis 2016;19:470–5. 10.1111/1756-185X.12359 [DOI] [PubMed] [Google Scholar]
- 23. Chen Y-M, Huang W-N, Wu Y-D, et al. Reactivation of hepatitis B virus infection in patients with rheumatoid arthritis receiving tofacitinib: a real-world study. Ann Rheum Dis 2018;77:780–2. 10.1136/annrheumdis-2017-211322 [DOI] [PubMed] [Google Scholar]
- 24. Hoofnagle JH. Reactivation of hepatitis B. Hepatology 2009;49:S156–65. 10.1002/hep.22945 [DOI] [PubMed] [Google Scholar]
- 25. Mori S, Fujiyama S. Hepatitis B virus reactivation associated with antirheumatic therapy: risk and prophylaxis recommendations. WJG 2015;21:10274–89. 10.3748/wjg.v21.i36.10274 [DOI] [PMC free article] [PubMed] [Google Scholar]