Abstract
Background
A logistic European System for Cardiac Operative Risk Evaluation (logEuroSCORE) ≥20% is frequently recognised as a finite criteria for transcatheter aortic valve implantation (TAVI) reimbursement, despite guideline modifications to reflect the appropriacy of TAVI in selected lower-risk patients. The aim was to evaluate the clinical value of this threshold cut-off in TAVI patients and to identify factors associated with mortality in those below this threshold.
Methods
We analysed data from a single-centre, German, observational, TAVI-patient registry, gathered between 2008 and 2016. Patients were stratified by logEuroSCORE (≥ or <20%) for comparisons. Logistic regression was performed to identify predictors of mortality at 1 year, with this analysis used to generate a calculated (‘real’) risk value for each patient.
Results
1679 patients (logEuroSCORE <20%: n=789; logEuroSCORE ≥20%: n=890) were included. LogEuroSCORE <20% patients were significantly younger (80.1 vs 81.6 years; p<0.001) and less comorbid than logEuroSCORE ≥20% patients, with a higher rate of transfemoral TAVI (35.6% vs 26.1%; p<0.001) and predilation (70.0% vs 63.3%; p=0.004). Patients with a logEuroSCORE <20% experienced more vascular complications (3.4% vs 1.5%; p=0.010). One-year survival was 88.3% in the logEuroSCORE <20% and 81.8% in the logEuroSCORE ≥20% group (p=0.005), with the calculated mortality risk falling within 2% of the logEuroSCORE in just 12.9% of patients. In the logEuroSCORE <20% group, only coronary artery disease was significantly predictive of 1-year mortality (OR 2.408; 95% CI 1.361 to 4.262; p=0.003).
Conclusions
At our institution, patients with a logEuroSCORE <20% selected for TAVI have excellent outcomes. The decision not to reimburse TAVI in such patients may be viewed as inappropriate.
Keywords: minimally invasive, valvular disease, aortic valve disease
Key questions.
What is already known about this subject?
A logistic European System for Cardiac Operative Risk Evaluation (logEuroSCORE) ≥20% is frequently recognised as a finite criteria for transcatheter aortic valve implantation (TAVI) reimbursement, despite guideline modifications to reflect the appropriacy of TAVI in selected lower-risk patients.
What does this study add?
Aim was to evaluate the clinical value of this threshold cut-off in TAVI patients in Germany and to identify factors associated with mortality in those below this threshold.
At our institution, the outcomes of patients with a logEuroSCORE <20% selected for TAVI by our multidisciplinary Heart Team were excellent, with good periprocedural safety and high rates of medium-term survival.
How might this impact on clinical practice?
Given the overwhelming evidence that the logEuroSCORE is inaccurate for identifying TAVI-associated mortality risk, we suggest that such non-specific scores should be given less importance when evaluating an individual’s suitability for TAVI, with additional risk factors and the expert opinion of the Heart Team given greater weight.
From a clinical perspective, coronary artery disease may deserve particular attention in patients with a logEuroSCORE <20% being considered for TAVI.
Introduction
The European System for Cardiac Operative Risk Evaluation (EuroSCORE) was first introduced in 1999 as a tool for predicting the likelihood of operative mortality in cardiac surgical patients.1 The score takes into account a total of 17 objective risk factors encompassing patient-related, cardiac and operation-related variables. In Germany, it is one of the most widely used tools for preprocedural assessment of patients with severe symptomatic aortic stenosis (AS) being considered for aortic valve replacement (AVR).
The advanced age and multimorbidity of the AS population means that surgical AVR (SAVR) is typically a high-risk procedure.2 In recent years, transcatheter AVR (TAVI) has been widely adopted as a lower -risk, minimally invasive alternative. In their initial 2008 statement, European authorities proposed an arbitrary 20% logistic EuroSCORE (logEuroSCORE) cut-off as a rough guide for identifying high-risk patients that may be more suited to TAVI than SAVR.3 However, in the same consensus document, the authors emphasised the score’s modest accuracy and promoted its use in conjunction with informed clinical judgement. Current 2017 European guidelines now recommend that TAVI be considered in all patients with a logEuroSCORE ≥10%, as well as in those with a score below this threshold but with additional significant risk factors.4 As such, conventional risk-stratification tools should be supplemented by a Heart Team’s professional opinion when identifying good TAVI candidates. Despite these evidence-based guideline revisions, a logEuroSCORE of 20% continues to be recognised as the lower limit for TAVI candidates.5
The present real-world analysis aimed to evaluate the clinical value of the 20% logEuroSCORE cut-off for identifying good TAVI candidates at our site. Factors associated with post-TAVI mortality in patients with a logEuroSCORE <20% were also explored, so as to aid clinical decision-making.
Methods
The present study is a post hoc analysis of data from a single-centre, German, observational registry that was originally established as part of a quality assurance initiative. The registry’s methodology has been previously described.6 7 Briefly, patients with severe AS undergoing TAVI between 2008 and 2016 were consecutively enrolled after providing their written informed consent to participate.
Patients
All patients with a diagnosis of severe, symptomatic AS that were scheduled to undergo TAVI at the Bad Rothenfelde Heart Centre were included. For the present analysis, patients without stated values for logEuroSCORE were excluded. No further inclusion/exclusion criteria applied.
The decision to perform TAVI was taken by the resident Heart Team, independently from the registry. In line with European guidelines, this decision was based on the patient being judged to be at high risk for SAVR following a comprehensive assessment that took into account the logEuroSCORE and other important risk factors, such as frailty, porcelain aorta and sequelae of chest radiation.4 The choice of access route was governed by the ‘best for transfemoral (TF)’ principle, which has been previously described.7 Briefly, unless a patient had all of the attributes comprising an ideal candidate for TF-TAVI, transapical (TA)-TAVI was preferentially performed.
For the present analysis, patients were divided into those with a logEuroSCORE of <20% and those with a logEuroSCORE of ≥20%.
Procedures and documentation
Baseline patient demographics, cardiac history, comorbidities and echocardiographic parameters were recorded prior to TAVI. Coronary artery disease (CAD) in our dataset is a clinical diagnosis which was not revalidated in the context of this registry. A logEuroSCORE value, the components of which are outlined in online supplementary table 1, was calculated for each patient, as previously described.1
openhrt-2019-001194supp001.pdf (219.2KB, pdf)
A trans-oesophageal echocardiography, coronary angiography and a CT scan were performed to aid access route and transcatheter heart valve size selection. All TAVI procedures were performed under general anaesthesia, according to standard site protocol. Peri-procedural details and complications were documented and patients were followed up over the subsequent year. This period included outpatient visits or telephone interviews at 30 days, 6 and 12 months, during which data on vital status, complications and cardiovascular health were documented.
Statistics
Baseline and peri-procedural data were analysed using descriptive statistics, with continuous variables presented as means with SD and categorical variables presented as absolute numbers with percentages (%). Comparisons between patients with a logEuroSCORE <20% and those with a logEuroSCORE ≥20% were carried out using a Student’s t-test or Mann-Whitney U test for continuous variables and a χ2 or Fisher’s exact test for categorical variables. One-year Kaplan-Meier survival estimates were generated for each group and presented with a p value calculated by Breslow test. Multivariate logistic regression including all documented baseline variables as covariables was carried out to identify predictors of mortality within the year following TAVI in the total study population, as well as in the logEuroSCORE <20% group separately. These data are presented as ORs with 95% CIs and p values.
To retrospectively determine each patient’s ‘calculated risk’ of all-cause mortality at 1 year, output data from the regression analysis for the total population were entered into SPSS, alongside individual baseline patient data. This analysis generated a value for the ‘real’ probability of death at 1 year for each patient, expressed as a percentage (%). Appendix 1 No calculated risk was generated for patients missing data for any of the baseline variables. Each patient’s calculated risk was compared with their logEuroSCORE-predicted risk, with values within ±2% considered comparable.
All statistical analysis was carried out using IBM SPSS, V.24.0 (IBM, Armonk, New York, USA), with a p value of <0.05 considered to be statistically significant.
Results
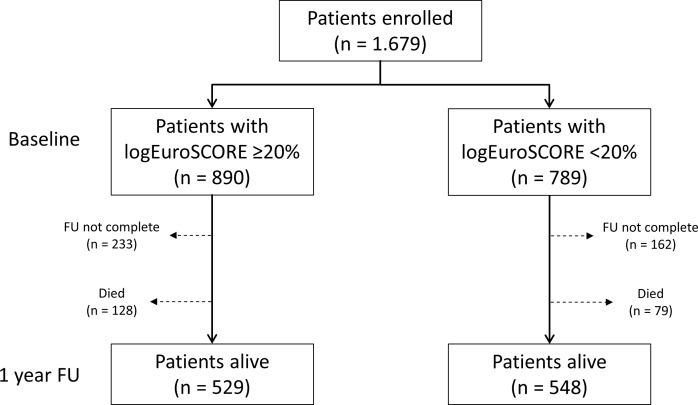
A total of 1679 patients met the study inclusion criteria and underwent TAVI at our centre (figure 1). The baseline logEuroSCORE was <20% in 789 patients (47.0%) and ≥20% in 890 patients (53.0%).
Figure 1.
Patient flow. ‘FU not complete’ comprises all patients with a follow-up of <1 year or patients lost to follow-up. FU, follow-up; logEuroSCORE, logistic European System for Cardiac Operative Risk Evaluation.
Baseline patient characteristics
Compared with patients with a logEuroSCORE ≥20%, patients with a logEuroSCORE <20% were younger (80.1%±6.3% vs 81.6%±5.4%; p<0.001), had experienced fewer prior cardiac events/surgeries and were generally less comorbid (table 1). In addition, a significantly lower proportion were in New York Heart Association (NYHA) class III/IV (78.1% vs 84.2%; p<0.001). The mean left ventricular ejection fraction (LVEF) was higher in the logEuroSCORE <20% group (57.4%±10.3% vs 49.9%±13.9%; p<0.001), as were the peak (79.2±23.5 vs 73.5±26.1 mm Hg; p<0.001) and mean (49.5±15.7 vs 45.3±17.1; p<0.001) transvalvular gradients.
Table 1.
Baseline patient characteristics
| logEuroSCORE ≥20% mean±SD or n/N (%) (n=890) |
logEuroSCORE <20% mean±SD or n/N (%) (n=789) |
P value | |
| Age (years) | 81.6±5.4 | 80.1±6.3 | <0.001 |
| Female gender | 458/890 (51.5) | 444/789 (56.3) | 0.048 |
| Cardiac history | |||
| Prior MI | 207/884 (23.4) | 82/772 (10.6) | <0.001 |
| Prior cardiac surgery* | 315/890 (35.4) | 107/788 (13.6) | <0.001 |
| Prior stroke | 210/890 (23.6) | 54/788 (6.9) | <0.001 |
| Comorbidities | |||
| AF | 414/890 (46.5) | 267/789 (33.8) | <0.001 |
| Hypertension | 844/890 (94.8) | 738/789 (93.5) | 0.256 |
| CAD | 593/883 (67.2) | 374/772 (48.4) | <0.001 |
| Mitral valve insufficiency (>II°) | 54/888 (6.1) | 19/785 (2.4) | <0.001 |
| Porcelain aorta | 266/884 (30.1) | 212/773 (27.4) | 0.232 |
| Pulmonary hypertension | 362/889 (40.7) | 158/788 (20.1) | <0.001 |
| COPD | 204/890 (22.9) | 140/789 (17.7) | 0.009 |
| Diabetes mellitus | 304/890 (34.2) | 214/789 (27.1) | 0.002 |
| Kidney insufficiency | 581/888 (65.4) | 379/789 (48.0) | <0.001 |
| Dialysis | 36/888 (4.1) | 10/789 (1.3) | <0.001 |
| NYHA class III or IV | 726/861 (84.3) | 579/741 (78.1) | 0.001 |
| LVEF (%) | 49.9±13.9 | 57.4±10.3 | <0.001 |
| Peak AV gradient (mm Hg) | 73.5±26.1 | 79.2±23.5 | <0.001 |
| Mean AV gradient (mm Hg) | 45.3±17.1 | 49.5±15.7 | <0.001 |
| STS score (%) | 11.8±10.1 | 8.0±8.3 | <0.001 |
| LogEuroSCORE (%) | 34.5±13.0 | 12.6±4.3 | <0.001 |
*Not including percutaneous coronary intervention.
AF, atrial fibrillation; AV, aortic valve; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; logEuroSCORE, logistic European System for Cardiac Operative Risk Evaluation; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; STS, Society of Thoracic Surgeons.
Peri-procedural details
Although TA was the most common access route in both groups (62.6% and 72.1%, respectively), a higher proportion of the logEuroSCORE <20% group underwent TAVI via the TF route compared with the logEuroSCORE ≥20% group (35.6% vs 26.1%; p<0.001) (table 2). The vast majority of patients received a SAPIEN/SAPIEN XT valve (82.3% overall). Balloon aortic valvuloplasty (BAV) predilation was more frequently performed in the logEuroSCORE <20% group than the logEuroSCORE ≥20% group (70.0% vs 63.3%; p=0.004).
Table 2.
Peri-procedural details
| logEuroSCORE ≥20% n/N (%) (n=890) |
logEuroSCORE <20% n/N (%) (n=789) |
P value | |
| Access route | <0.001 | ||
| TF | 232/890 (26.1) | 281/789 (35.6) | |
| TA | 642/890 (72.1) | 494/789 (62.6) | |
| Other | 16/890 (1.8) | 14/789 (1.8) | |
| Valve type | 0.007 | ||
| SAPIEN/SAPIEN XT | 735/890 (82.6) | 646/789 (81.9) | |
| ACURATE | 142/890 (16.0) | 142/789 (18.0) | |
| Others | 13/890 (1.5) | 1/789 (0.1) | |
| BAV predilation | 563/890 (63.3) | 552/789 (70.0) | 0.004 |
| Complications | |||
| Conversion to surgery | 4/890 (0.4) | 6/789 (0.8) | 0.530 |
| Major vascular complications | 13/871 (1.5) | 27/785 (3.4) | 0.010 |
| PPI | 66/869 (7.6) | 69/782 (8.8) | 0.363 |
| New arrhythmia | 244/871 (28.0) | 233/784 (29.7) | 0.444 |
| Moderate-to-severe PVL | 9/857 (1.1) | 9/750 (1.2) | 0.776 |
| AKI requiring dialysis | 7/858 (0.8) | 3/771 (0.4) | 0.349 |
| Postdilation | 146/889 (16.4) | 149/788 (18.9) | 0.182 |
| In-hospital mortality | 26/880 (3.0) | 21/788 (2.7) | 0.721 |
AKI, acute kidney injury; BAV, balloon aortic valvuloplasty; logEuroSCORE, logistic European System for Cardiac Operative Risk Evaluation; PPI, permanent pacemaker implantation; PVL, paravalvular leak; TA, transapical; TF, transfemoral.
The rates of conversion to surgery, permanent pacemaker implantation (PPI), new arrhythmia, moderate/severe paravalvular regurgitation (PVL), acute kidney injury (AKI) requiring dialysis and balloon post-dilation were low and statistically comparable between groups (table 2). Though rare overall, major vascular complications were significantly more common in patients with a logEuroSCORE of <20% than in those with a logEuroSCORE of ≥20% (3.4% vs 1.5%; p=0.010).
Survival
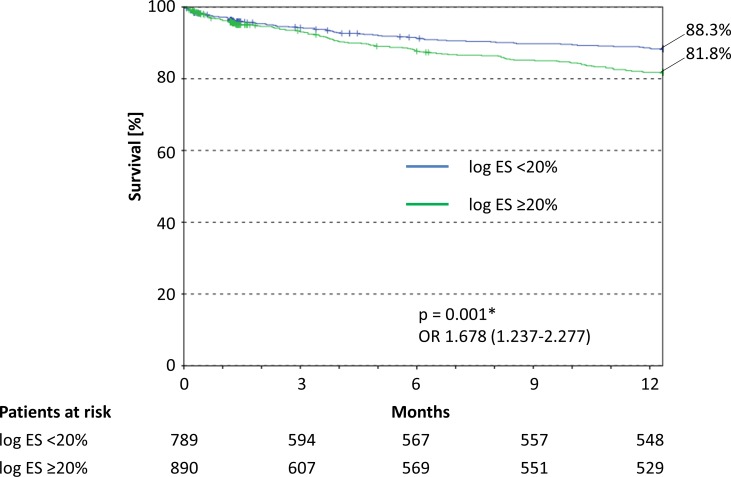
No statistical significant difference was found for in-hospital mortality between the two groups (3.0% (logEuroSCORE ≥20%) vs 2.7% (logEuroSCORE <20%); p=0.721) (table 2). At 1 year post-TAVI, the estimated survival rate was significantly higher for patients with a logEuroSCORE <20% than for those with a logEuroSCORE ≥20% (88.3% vs 81.8%; p=0.005) (figure 2). At 5 years post-TAVI, the estimated survival rate stayed significantly higher for patients with a logEuroSCORE <20% (57.1% vs 44.3%; p=0.005) (online supplementary figure 1).
Figure 2.
Kaplan-Meier survival curves for the year after TAVI. logES, logEuroSCORE.
openhrt-2019-001194supp002.pdf (609.3KB, pdf)
Predictors of mortality at 1 year
In the overall population, atrial fibrillation (AF; OR 1.758; 95% CI 1.267 to 2.439; p=0.001), CAD (OR 1.559; 95% CI 1.054 to 2.306; p=0.026), kidney insufficiency (OR 1.458; 95% CI 1.021 to 2.084; p=0.038) and NYHA class III/IV (OR 1.824; 95% CI 1.099 to 3.027; p=0.020) were independent predictors of mortality at 1 year (table 3). A higher LVEF was protective, although the effect size was small (OR 0.985; 95% CI 0.972 to 0.998; p=0.020).
Table 3.
Predictors of mortality at 1 year post-TAVI in the overall study population
| Univariate OR (95% CI) |
P value | Multivariate OR (95% CI)* |
P value | |
| Age (years) | 0.997 (0.973 to 1.021) | 0.779 | 1.002 (0.972 to 1.033) | 0.903 |
| Female gender | 0.796 (0.591 to 1.072) | 0.134 | 1.156 (0.811 to 1.646) | 0.422 |
| Cardiac history | ||||
| Prior MI | 1.333 (0.923 to 1.924) | 0.125 | 0.979 (0.638 to 1.502) | 0.922 |
| Prior cardiac surgery† | 1.220 (0.882 to 1.687) | 0.229 | 0.881 (0.588 to 1.320) | 0.540 |
| Prior stroke | 1.062 (0.707 to 1.597) | 0.771 | 0.875 (0.561 to 1.364) | 0.556 |
| Comorbidities | ||||
| AF | 2.140 (1.584 to 2.892) | <0.001 | 1.758 (1.267 to 2.439) | 0.001 |
| Hypertension | 1.390 (0.705 to 2.743) | 0.342 | 0.879 (0.427 to 1.808) | 0.725 |
| CAD | 1.638 (1.188 to 2.258) | 0.003 | 1.559 (1.054 to 2.306) | 0.026 |
| Mitral valve insufficiency (>II°) | 1.225 (0.624 to 2.404) | 0.555 | 0.929 (0.453 to 1.905) | 0.841 |
| Porcelain aorta | 1.194 (0.864 to 1.649) | 0.283 | 1.117 (0.786 to 1.588) | 0.537 |
| Pulmonary hypertension | 1.475 (1.074 to 2.027) | 0.016 | 1.353 (0.958 to 1.909) | 0.086 |
| COPD | 1.383 (0.980 to 1.950) | 0.065 | 1.156 (0.792 to 1.687) | 0.451 |
| Diabetes mellitus | 1.861 (1.354 to 2.559) | <0.001 | 1.238 (0.876 to 1.750) | 0.226 |
| Kidney insufficiency | 1.156 (0.721 to 1.854) | 0.546 | 1.458 (1.021 to 2.084) | 0.038 |
| NYHA class III or IV | 2.233 (1.372 to 3.634) | 0.001 | 1.824 (1.099 to 3.027) | 0.020 |
| LVEF (%) | 0.976 (0.966 to 0.987) | <0.001 | 0.985 (0.972 to 0.998) | 0.020 |
| Peak AV gradient (mm Hg) | 0.989 (0.983 to 0.995) | 0.001 | 1.000 (0.982 to 1.017) | 0.965 |
| Mean AV gradient (mm Hg) | 0.983 (0.974 to 0.993) | <0.001 | 0.994 (0.969 to 1.021) | 0.676 |
*Adjusted for all other variables.
†Not including percutaneous coronary intervention.
AF, atrial fibrillation; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; STS, Society of Thoracic Surgeons; TA, transapical; TF, transfemoral.
Considering only patients with a logEuroSCORE of <20%, CAD was the only variable that remained a significant baseline predictor of 1-year mortality (OR 2.408; 95% CI 1.361 to 4.262; p=0.003) (table 4). AF also showed potential clinical, if not statistical, relevance (OR 1.620; 95% CI 0.947 to 2.770; p=0.078).
Table 4.
Predictors of mortality at 1 year post-TAVI in patients with a logEuroSCORE <20%
| Univariate OR (95% CI) |
P value | Multivariate OR (95% CI)* |
P value | |
| Age (years) | 1.001 (0.965 to 1.038) | 0.947 | 0.996 (0.950 to 1.044) | 0.868 |
| Female gender | 1.176 (0.726 to 1.905) | 0.509 | 1.482 (0.842 to 2.609) | 0.172 |
| Cardiac history | ||||
| Prior MI | 1.028 (0.488 to 2.165) | 0.942 | 0.854 (0.360 to 2.024) | 0.720 |
| Prior cardiac surgery† | 0.802 (0.397 to 1.620) | 0.539 | 0.772 (0.337 to 1.771) | 0.542 |
| Prior stroke | 0.814 (0.312 to 2.123) | 0.674 | 0.745 (0.248 to 2.236) | 0.600 |
| Comorbidities | ||||
| AF | 1.716 (1.065 to 2.766) | 0.027 | 1.620 (0.947 to 2.770) | 0.078 |
| Hypertension | 1.991 (0.601 to 6.595) | 0.260 | 1.360 (0.389 to 4.761) | 0.630 |
| CAD | 1.815 (1.112 to 2.961) | 0.017 | 2.408 (1.361 to 4.262) | 0.003 |
| Mitral valve insufficiency (>II°) | 1.757 (0.485 to 6.367) | 0.391 | 1.636 (0.403 to 6.638) | 0.491 |
| Porcelain aorta | 1.263 (0.752 to 2.121) | 0.377 | 1.115 (0.638 to 1.950) | 0.702 |
| Pulmonary hypertension | 1.628 (0.905 to 2.928) | 0.104 | 1.527 (0.797 to 2.926) | 0.202 |
| COPD | 1.169 (0.648 to 2.110) | 0.605 | 1.154 (0.605 to 2.202) | 0.664 |
| Diabetes mellitus | 1.166 (0.692 to 1.964) | 0.564 | 0.966 (0.539 to 1.733) | 0.908 |
| Kidney insufficiency | 1.156 (0.721 to 1.854) | 0.546 | 0.983 (0.580 to 1.667) | 0.949 |
| NYHA class III or IV | 1.716 (0.853 to 3.453) | 0.130 | 1.519 (0.732 to 3.152) | 0.262 |
| LVEF (%) | 0.990 (0.969 to 1.011) | 0.346 | 0.988 (0.964 to 1.013) | 0.352 |
| Peak AV gradient (mm Hg) | 1.002 (0.992 to 1.012) | 0.747 | 0.999 (0.978 to 1.021) | 0.931 |
| Mean AV gradient (mm Hg) | 1.003 (0.988 to 1.018) | 0.668 | 1.012 (0.981 to 1.045) | 0.446 |
*Adjusted for all other variables.
†Not including percutaneous coronary intervention.
AF, atrial fibrillation; COPD, chronic obstructive pulmonary disease; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NYHA, New York Heart Association; STS, Society of Thoracic Surgeons; TA, transapical; TF, transfemoral.
Calculated risk versus logEuroSCORE
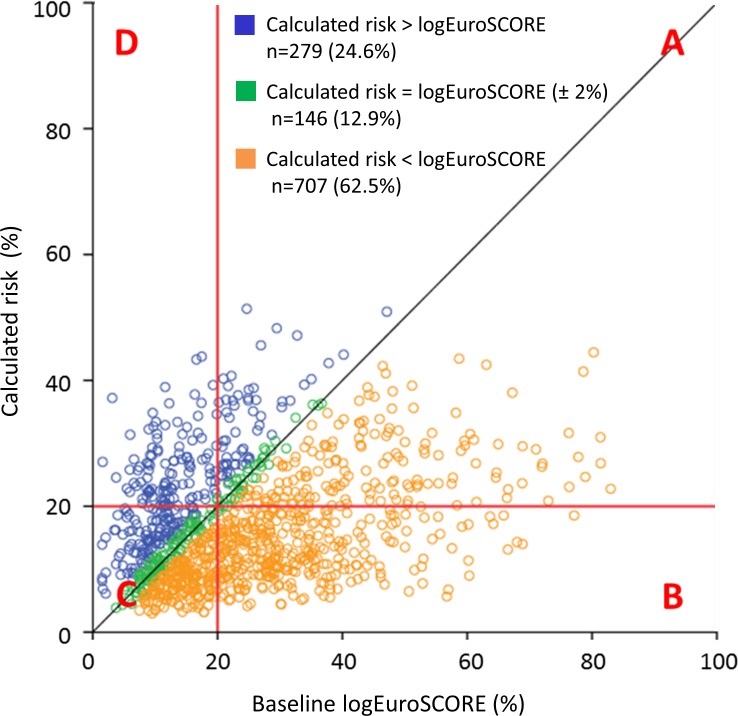
In total, 1132 patients had both a calculated risk value for mortality at 1 year and a baseline logEuroSCORE available. The calculated risk fell within 2% of the logEuroSCORE-predicted risk in just 12.9% of cases, being comparatively lower in 62.5% of patients (figure 3).
Figure 3.
Calculated risk of mortality at 1 year compared with logEuroSCORE-predicted risk. N=1132; calculated risk could not be generated for 547/1679 patients due to missing data for ≥1 baseline variables. (A) Both logEuroSCORE and actual risk ≥20% (n=256; 22.6%); (B) logEuroSCORE ≥20% and calculated risk <20% (n=334; 29.5%); (C) both logEuroSCORE and calculated risk <20% (n=432; 38.2%); (D) logEuroSCORE <20% and calculated risk ≥20% (n=110; 9.7%). logEuroSCORE, logistic European System for Cardiac Operative Risk Evaluation.
Discussion
The present real-world analysis suggests that there is little clinical value in the 20% logEuroSCORE cut-off. Indeed, despite obvious differences in baseline characteristics, patients both above and below the 20% threshold had excellent clinical outcomes, with low rates of peri-procedural complications and high survival rates at 1 year. Furthermore, the actual calculated risk of mortality following TAVI was below that predicted by the logEuroSCORE in over 60% of cases, demonstrating the score’s inaccuracy in this indication. Among other variables, CAD was associated with a greater risk of mortality in the year after TAVI, remaining the only independent predictor in patients with a logEuroSCORE <20%. Raising awareness of this association among physicians may result in more appropriate treatment decisions.
Peri-procedural outcomes
Regardless of logEuroSCORE, the peri-procedural outcomes observed in the present study were highly satisfactory, with complications being extremely rare and largely comparable between the higher-risk and lower-risk groups. This is in agreement with prior studies in low-risk/intermediate-risk patients undergoing TAVI, which have also reported low rates of AKI, new arrhythmia and re-intervention.8–10 Importantly, such studies have found the risk of these events to be significantly reduced with TAVI compared with SAVR.8–11 This was recently reinforced by the publication of two major randomised trials, showing that the procedure is at least as safe or even superior in low-risk patients.12 13 Furthermore, PPI was required by <9% of patients in each of the present logEuroSCORE groups. This value is at the lower end of the rates reported by prior TAVI studies (range: 6.5%–33.7%14–18) and only slightly higher than those reported for low-risk/intermediate-risk patients undergoing SAVR (range: 6.6%–7.3%8 9 19). While vascular complications were more common in the logEuroSCORE <20% compared with the logEuroSCORE ≥20% group, this is likely a result of TAVI being more commonly performed via the TF route in the former patients. Indeed, the greater risk of major vascular complications in TF-TAVI compared with TA-TAVI is well recognised.20 Nevertheless, the rate of 3.4% observed in the logEuroSCORE <20% group is strikingly lower than the values reported by the majority of studies in low-risk/intermediate-risk patients undergoing TAVI or SAVR.8 19 This may be partly attributable to the ‘best for TF’ approach employed at our institution, given that only patients with the most compatible access vasculature undergo TF-TAVI. Overall, our data demonstrate that patients selected for TAVI by an expert Heart Team at our site have excellent peri-procedural outcomes, regardless of whether or not they have a logEuroSCORE above or below the 20% cut-off. As such, this threshold appears to have little clinical value for discerning between good and poor candidates for TAVI.
Mortality at 1 year
The increased risk of mortality in elderly, multimorbid individuals undergoing open heart surgery is well recognised. Such characteristics feature heavily in surgical risk-stratification tools,21 the logEuroSCORE being no exception.1 Accordingly, it is appropriate for SAVR to be avoided in patients with a logEuroSCORE of ≥20% and for the minimally invasive alternative, TAVI, to be used. However, it does not necessarily follow that patients with a logEuroSCORE below this 20% threshold fail to benefit from TAVI. In the present study, 1-year survival rates were extremely high in the logEuroSCORE <20% group, being similar to those previously reported for low-risk/intermediate-risk patients undergoing SAVR.8 19 While such comparisons are hindered by between-study heterogeneity, several important clinical trials have demonstrated a lower rate of all-cause mortality after TAVI compared with SAVR in patients at intermediate surgical risk.8 9 This trend was also reiterated by a recent, high-profile propensity score-matched analysis.19
Furthermore, our in-hospital mortality rates were low (logEuroSCORE ≥20%: 3.0% and logEuroSCORE <20%: 2.7%) compared with a large-scale, real-world German study.22 This study found TF-TAVI to result in similar or lower in-hospital mortality rates compared with SAVR across all surgical-risk groups (logEuroSCORE <10%: 2.4% vs 2.0%, p=0.302; logEuroSCORE 10%–20%: 3.5% vs 5.3%, p=0.025; logEuroSCORE 20%–30%: 5.5% vs 12.2%, p<0.001; logEuroSCORE >30%: 6.5% vs 12.9%, p=0.008).22 As such, it appears that informed selection of patients with a logEuroSCORE <20% for TAVI can result in at least comparable survival compared with SAVR. As such, the use of a 20% logEuroSCORE cut-off to denote automatic ineligibility for TAVI reimbursement may require revision.
Predicting TAVI-related risk
The fact that survival was greater in the logEuroSCORE <20% group compared with the logEuroSCORE ≥20% group is unsurprising considering the differences in age and comorbidities at baseline. Indeed, all of the four variables identified as independent predictors of all-cause death within the overall population (AF, CAD, kidney insufficiency and NYHA class III/IV) were more prevalent in the logEuroSCORE <20% group. However, of these, only renal insufficiency features in the logEuroSCORE algorithm. This may explain why the score was able to predict approximate trends in post-TAVI mortality but had limited accuracy for identifying actual risk, with <13% of calculated risk values falling within 2% of the logEuroSCORE. As such, the present study adds to the considerable pool of evidence demonstrating the inappropriacy of the logEuroSCORE for predicting outcomes after TAVI.4 23–27 A number of TAVI-specific risk-assessment scores have been developed, including SURTAVI, TARIS, FRANCE-2, TAVI2-SCORe, STT and OBSERVANT (contributory variables outlined in online supplementary table 2)28–33; however, while several of these have shown promise, none has yet been extensively validated, adopted or endorsed by expert consensus. In Germany specifically, the re-calibrated German AV score (GAVS) II has been presented, although still offers only modest predictive ability (C-statistic: 0.74) and has only been validated in terms of in-hospital mortality.34 Consequently, the urgent need for a TAVI-specific, accurate risk-stratification system remains.
Besides being one of several variables associated with death in the overall study population, CAD was the only independent predictor of 1-year mortality in patients with a logEuroSCORE <20%, increasing the risk more than twofold. This likely reflects the reduced capacity of patients with CAD to tolerate the ischaemic and haemodynamic burden of the procedure, which includes rapid ventricular pacing, balloon inflation and periods of hypotension. CAD does not specifically feature in the logEuroSCORE; however, it has been previously recognised as a key risk factor in TAVI patients and is included in both the SURTAVI and GAVS II TAVI-specific risk-assessment tools.33 34 CAD and AS commonly coexist due to shared risk factors and mechanisms of pathogenesis.35 36 Fortunately, the former condition may be treated through coronary artery bypass grafting or percutaneous coronary intervention (PCI).37 38 Several studies have shown that such revascularisation prior to treatment of the AS results in improved long-term outcomes39; however, these studies have mainly been performed in SAVR patients and few data exist in the context of TAVI. Nevertheless, ESC guidelines contain a class IIa recommendation for PCI to be considered in patients with a primary indication for TAVI and coronary artery diameter stenosis >70% in proximal segments.4 Our data suggest that the resolution of CAD prior to TAVI may allow better outcomes, especially in patients with a logEuroSCORE <20%, and this merits further investigation. The incorporation of CAD into future TAVI-specific risk scores should also be considered.
Interestingly, a clinically (if not statistically) significant association between AF and post-TAVI mortality risk was observed in patients with a logEuroSCORE of <20%. AF has been widely associated with poorer long-term survival and stroke in patients with AS, regardless of treatment approach or classically assessed procedural risk40; however, this effect appears to be largely related to a lack of guidelines for optimum antithrombotic therapy regimens during AVR. Development of such standardised recommendations would likely reduce the risk of thromboembolic events and bleeding complications in patients with AF undergoing TAVI, translating into a lower mortality risk and enabling more low-risk patients to safely undergo the procedure.
Limitations
Given that the present study was based on TAVI-specific data from a quality assurance initiative, SAVR data were unavailable for comparison. This limits the conclusions we are able to draw regarding the choice between TAVI and SAVR in low-risk patients. Nevertheless, our data suggest the inappropriacy of using a finite logEuroSCORE <20% cut-off to indicate that TAVI would not be beneficial. A second limitation is the proportional difference in TAVI access routes between low-risk and high-risk groups, which may have influenced outcomes. Nevertheless, route selection was based on comprehensive patient assessment by an expert Heart Team and is representative of real-world practice, with other registries also reporting a higher frequency of TF-TAVI in low-risk patients.41 Third, some patients in the present study underwent TAVI a considerable number of years ago, with the procedure’s rapid evolution in recent years having resulted in contemporary improvements in valve technology and operator experience.41 Furthermore, BAV predilation, which at the beginning of the study was a mandatory step in the TAVI procedure and is thus highly prevalent in the present dataset, is now considered largely unnecessary in the majority of patients and may even be harmful.42 As such, our data may underestimate the up-to-date safety/efficacy of the TAVI procedure. Finally, the study was confined to a single German site that adopts the ‘best for TF’ method for access route selection. As such, our findings may not be generalisable to centres in other countries or that employ alternative approaches to access decision.
Conclusions
At our institution, the outcomes of patients with a logEuroSCORE <20% selected for TAVI by our multidisciplinary Heart Team were excellent, with good periprocedural safety and high rates of medium-term survival. Given the overwhelming evidence that the logEuroSCORE is inaccurate for identifying TAVI-associated mortality risk, we suggest that such non-specific scores should be given less importance when evaluating an individual’s suitability for TAVI, with additional risk factors and the expert opinion of the Heart Team given greater weight. From a clinical perspective, CAD may deserve particular attention in patients with a logEuroSCORE <20% being considered for TAVI.
Footnotes
Contributors: All authors except PB and KB established and conducted the registry. PB and KB designed the statistical approach, analysis and interpretation. GI and PB outlined the first version of the manuscript, which all other authors revised for important intellectual content. KB performed the statistical analyses. All authors approved the final version of the manuscript to be submitted.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: PB is a consultant for Edwards Lifesciences.
Patient consent for publication: Not required.
Ethics approval: The study was approved by the responsible ethics committee in Hannover (Landesärztekammer) and was carried out in accordance with the declaration of Helsinki and its amendments.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request from the corresponding author.
References
- 1. Nashef SA, Roques F, Michel P, et al. European system for cardiac operative risk evaluation (EuroSCORE). Eur J Cardiothorac Surg 1999;16:9–13. 10.1016/S1010-7940(99)00134-7 [DOI] [PubMed] [Google Scholar]
- 2. Osnabrugge RLJ, Mylotte D, Head SJ, et al. Aortic stenosis in the elderly: disease prevalence and number of candidates for transcatheter aortic valve replacement: a meta-analysis and modeling study. J Am Coll Cardiol 2013;62:1002–12. 10.1016/j.jacc.2013.05.015 [DOI] [PubMed] [Google Scholar]
- 3. Vahanian A, Alfieri O, Al-Attar N, et al. Transcatheter valve implantation for patients with aortic stenosis: a position statement from the European association of Cardio-Thoracic surgery (EACTS) and the European Society of cardiology (ESC), in collaboration with the European association of percutaneous cardiovascular interventions (EAPCI). Eur Heart J 2008;29:1463–70. 10.1093/eurheartj/ehn183 [DOI] [PubMed] [Google Scholar]
- 4. Baumgartner H, Falk V, Bax JJ, et al. 2017 ESC/EACTS guidelines for the management of valvular heart disease. Eur Heart J 2017;38:2739–91. 10.1093/eurheartj/ehx391 [DOI] [PubMed] [Google Scholar]
- 5. Kölbel R. Abrechnungsverstöße in Der stationären medizinischen Versorgung: Medizinische, ökonomische und juristische Perspektiven. Kohlhammer Verlag, 2013. [Google Scholar]
- 6. Imnadze G, Hofmann S, Billion M, et al. Transapical transcatheter aortic valve implantation in patients with a low ejection fraction. Interact Cardiovasc Thorac Surg 2018;26:224–9. 10.1093/icvts/ivx315 [DOI] [PubMed] [Google Scholar]
- 7. Kowalski M, Deutsch C, Hofmann S, et al. Transcatheter aortic valve implantation at a high-volume center: the bad Rothenfelde experience. Kitp 2017;4:215–24. 10.5114/kitp.2017.72224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leon MB, Smith CR, Mack MJ, et al. Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016;374:1609–20. 10.1056/NEJMoa1514616 [DOI] [PubMed] [Google Scholar]
- 9. Reardon MJ, Van Mieghem NM, Popma JJ, et al. Surgical or transcatheter aortic-valve replacement in intermediate-risk patients. N Engl J Med 2017;376:1321–31. 10.1056/NEJMoa1700456 [DOI] [PubMed] [Google Scholar]
- 10. Garg A, Rao SV, Visveswaran G, et al. Transcatheter aortic valve replacement versus surgical valve replacement in Low-Intermediate surgical risk patients: a systematic review and meta-analysis. J Invasive Cardiol 2017;29:209–16. [PubMed] [Google Scholar]
- 11. Siemieniuk RA, Agoritsas T, Manja V, et al. Transcatheter versus surgical aortic valve replacement in patients with severe aortic stenosis at low and intermediate risk: systematic review and meta-analysis. BMJ 2016;354:i5130 10.1136/bmj.i5130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mack MJ, Leon MB, Thourani VH, et al. Transcatheter aortic-valve replacement with a Balloon-Expandable valve in low-risk patients. N Engl J Med 2019;380:1695–705. 10.1056/NEJMoa1814052 [DOI] [PubMed] [Google Scholar]
- 13. Popma JJ, Deeb GM, Yakubov SJ, et al. Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med 2019;380:1706–15. 10.1056/NEJMoa1816885 [DOI] [PubMed] [Google Scholar]
- 14. Ledwoch J, Franke J, Gerckens U, et al. Incidence and predictors of permanent pacemaker implantation following transcatheter aortic valve implantation: analysis from the German transcatheter aortic valve interventions registry. Catheter Cardiovasc Interv 2013;82:n/a–77. 10.1002/ccd.24915 [DOI] [PubMed] [Google Scholar]
- 15. Erkapic D, De Rosa S, Kelava A, et al. Risk for permanent pacemaker after transcatheter aortic valve implantation: a comprehensive analysis of the literature. J Cardiovasc Electrophysiol 2012;23:391–7. 10.1111/j.1540-8167.2011.02211.x [DOI] [PubMed] [Google Scholar]
- 16. Hoyt MJ, Hathaway J, Palmer R, et al. Predictors of permanent pacemaker implantation after transcatheter aortic valve replacement. J Cardiothorac Vasc Anesth 2015;29:1162–6. 10.1053/j.jvca.2015.06.001 [DOI] [PubMed] [Google Scholar]
- 17. Maan A, Refaat MM, Heist EK, et al. Incidence and predictors of pacemaker implantation in patients undergoing transcatheter aortic valve replacement. Pacing Clin Electrophysiol 2015;38:878–86. 10.1111/pace.12653 [DOI] [PubMed] [Google Scholar]
- 18. Khatri PJ, Webb JG, Rodés-Cabau J, et al. Adverse effects associated with transcatheter aortic valve implantation: a meta-analysis of contemporary studies. Ann Intern Med 2013;158:35–46. 10.7326/0003-4819-158-1-201301010-00007 [DOI] [PubMed] [Google Scholar]
- 19. Thourani VH, Kodali S, Makkar RR, et al. Transcatheter aortic valve replacement versus surgical valve replacement in intermediate-risk patients: a propensity score analysis. The Lancet 2016;387:2218–25. 10.1016/S0140-6736(16)30073-3 [DOI] [PubMed] [Google Scholar]
- 20. Schymik G, Würth A, Bramlage P, et al. Long-Term results of transapical versus transfemoral TAVI in a real world population of 1000 patients with severe symptomatic aortic stenosis. Circulation 2015;8 10.1161/CIRCINTERVENTIONS.113.000761 [DOI] [PubMed] [Google Scholar]
- 21. Prins C, de Villiers Jonker I, Botes L, et al. Cardiac surgery risk-stratification models. Cardiovasc J Afr 2012;23:160–4. 10.5830/CVJA-2011-047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Möllmann H, Bestehorn K, Bestehorn M, et al. In-Hospital outcome of transcatheter vs. surgical aortic valve replacement in patients with aortic valve stenosis: complete dataset of patients treated in 2013 in Germany. Clin Res Cardiol 2016;105:553–9. 10.1007/s00392-016-0962-4 [DOI] [PubMed] [Google Scholar]
- 23. Sedaghat A, Sinning J-M, Vasa-Nicotera M, et al. The revised EuroSCORE II for the prediction of mortality in patients undergoing transcatheter aortic valve implantation. Clin Res Cardiol 2013;102:821–9. 10.1007/s00392-013-0596-8 [DOI] [PubMed] [Google Scholar]
- 24. Watanabe Y, Hayashida K, Lefèvre T, et al. Is euroscore II better than EuroSCORE in predicting mortality after transcatheter aortic valve implantation? Cathet Cardiovasc Intervent 2013;81:1053–60. 10.1002/ccd.24702 [DOI] [PubMed] [Google Scholar]
- 25. Hemmann K, Sirotina M, De Rosa S, et al. The STS score is the strongest predictor of long-term survival following transcatheter aortic valve implantation, whereas access route (transapical versus transfemoral) has no predictive value beyond the periprocedural phase. Interact Cardiovasc Thorac Surg 2013;17:359–64. 10.1093/icvts/ivt132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sinning J-M, Wollert KC, Sedaghat A, et al. Risk scores and biomarkers for the prediction of 1-year outcome after transcatheter aortic valve replacement. Am Heart J 2015;170:821–9. 10.1016/j.ahj.2015.07.003 [DOI] [PubMed] [Google Scholar]
- 27. Silaschi M, Conradi L, Seiffert M, et al. Predicting risk in transcatheter aortic valve implantation: comparative analysis of EuroSCORE II and established risk stratification tools. Thorac Cardiovasc Surg 2015;63:472–8. 10.1055/s-0034-1389107 [DOI] [PubMed] [Google Scholar]
- 28. Seiffert M, Sinning J-M, Meyer A, et al. Development of a risk score for outcome after transcatheter aortic valve implantation. Clin Res Cardiol 2014;103:631–40. 10.1007/s00392-014-0692-4 [DOI] [PubMed] [Google Scholar]
- 29. Iung B, Laouénan C, Himbert D, et al. Predictive factors of early mortality after transcatheter aortic valve implantation: individual risk assessment using a simple score. Heart 2014;100:1016–23. 10.1136/heartjnl-2013-305314 [DOI] [PubMed] [Google Scholar]
- 30. Debonnaire P, Fusini L, Wolterbeek R, et al. Value of the "TAVI2-SCORe" versus surgical risk scores for prediction of one year mortality in 511 patients who underwent transcatheter aortic valve implantation. Am J Cardiol 2015;115:234–42. 10.1016/j.amjcard.2014.10.029 [DOI] [PubMed] [Google Scholar]
- 31. D'Ascenzo F, Capodanno D, Tarantini G, et al. Usefulness and validation of the survival posT TAVI score for survival after transcatheter aortic valve implantation for aortic stenosis. Am J Cardiol 2014;114:1867–74. 10.1016/j.amjcard.2014.09.031 [DOI] [PubMed] [Google Scholar]
- 32. Capodanno D, Barbanti M, Tamburino C, et al. A simple risk tool (the OBSERVANT score) for prediction of 30-day mortality after transcatheter aortic valve replacement. Am J Cardiol 2014;113:1851–8. 10.1016/j.amjcard.2014.03.014 [DOI] [PubMed] [Google Scholar]
- 33. van Mieghem NM, Head SJ, van der Boon RMA, et al. The SURTAVI model: proposal for a pragmatic risk stratification for patients with severe aortic stenosis. EuroIntervention 2012;8:258–66. 10.4244/EIJV8I2A40 [DOI] [PubMed] [Google Scholar]
- 34. Schiller W, Barnewold L, Kazmaier T, et al. The German aortic valve score II. Eur J Cardiothorac Surg 2017;52:881–7. 10.1093/ejcts/ezx282 [DOI] [PubMed] [Google Scholar]
- 35. Rapp AH, Hillis LD, Lange RA, et al. Prevalence of coronary artery disease in patients with aortic stenosis with and without angina pectoris. Am J Cardiol 2001;87:1216–7. 10.1016/S0002-9149(01)01501-6 [DOI] [PubMed] [Google Scholar]
- 36. Stewart BF, Siscovick D, Lind BK, et al. Clinical factors associated with calcific aortic valve disease. cardiovascular health study. J Am Coll Cardiol 1997;29:630–4. 10.1016/s0735-1097(96)00563-3 [DOI] [PubMed] [Google Scholar]
- 37. Virk SA, Tian DH, Liou K, et al. Systematic review of percutaneous coronary intervention and transcatheter aortic valve implantation for concomitant aortic stenosis and coronary artery disease. Int J Cardiol 2015;187:453–5. 10.1016/j.ijcard.2015.03.391 [DOI] [PubMed] [Google Scholar]
- 38. Dellis SL, Akujuo AC, Bennett EV, et al. Off-Pump coronary artery bypass grafting and Transaortic transcatheter aortic valve replacement. J Card Surg 2016;31:435–8. 10.1111/jocs.12762 [DOI] [PubMed] [Google Scholar]
- 39. Lund O, Nielsen TT, Pilegaard HK, et al. The influence of coronary artery disease and bypass grafting on early and late survival after valve replacement for aortic stenosis. J Thorac Cardiovasc Surg 1990;100:327–37. 10.1016/S0022-5223(19)35524-2 [DOI] [PubMed] [Google Scholar]
- 40. Tarantini G, Mojoli M, Urena M, et al. Atrial fibrillation in patients undergoing transcatheter aortic valve implantation: epidemiology, timing, predictors, and outcome. Eur Heart J 2017;38:1285–93. 10.1093/eurheartj/ehw456 [DOI] [PubMed] [Google Scholar]
- 41. Barbash IM, Finkelstein A, Barsheshet A, et al. Outcomes of patients at estimated low, intermediate, and high risk undergoing transcatheter aortic valve implantation for aortic stenosis. Am J Cardiol 2015;116:1916–22. 10.1016/j.amjcard.2015.09.030 [DOI] [PubMed] [Google Scholar]
- 42. Bagur R, Kwok CS, Nombela‐Franco L, et al. Transcatheter aortic valve implantation with or without preimplantation balloon aortic valvuloplasty: a systematic review and Meta‐Analysis. J Am Heart Assoc 2016;5 10.1161/JAHA.115.003191 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
openhrt-2019-001194supp001.pdf (219.2KB, pdf)
openhrt-2019-001194supp002.pdf (609.3KB, pdf)