Abstract
Background
To determine whether adjunctive dronabinol, a licensed form of delta-9-tetrahydrocannabinol, reduces opioid consumption when used off-label for managing acute pain following traumatic injury.
Methods
This matched cohort study included patients who were admitted with a traumatic injury between 1 March 2017 and 30 October 2017. The hospital pharmacy database was used to identify patients who received dronabinol (cases), and they were matched 1:1 to patients who did not receive dronabinol (controls) using age, cause of injury and hospital length of stay. The primary outcome, change in opioid consumption, was calculated using morphine milligram equivalents (MME). The change in MME was calculated for cases as total MME over 48 hours with adjunctive dronabinol minus 48 hours prior to dronabinol, and for controls as total MME 48–96 hours from admission minus 0–48 hours from admission. Data are presented as mean and SE or median and IQR. Statistical analysis was performed using paired t-tests and McNemar’s tests.
Results
There were 66 patients included: 33 cases and 33 matched controls. Dronabinol was initiated 55 (28–107) hours from admission. Cases and controls were well matched. Cases had a significant reduction in opioid consumption with adjunctive dronabinol (−79 (20) MME, p<0.001), while opioid consumption was unchanged for controls (−9 (20) MME, p=0.63). This resulted in a ninefold greater reduction in opioid consumption for cases versus controls that was statistically different between pairs (p=0.02). Nineteen (58%) cases reported using marijuana; in this subset, opioid consumption was reduced with adjunctive dronabinol (−97 (24) MME, p<0.001) versus a non-significant increase in opioid consumption in matched controls (11 (29) MME, p=0.70); difference between groups, p=0.01.
Conclusions
The results of this study suggest adjunctive dronabinol reduces opioid consumption following traumatic injury. The opioid-sparing effect of dronabinol may be greater in patients who are marijuana users.
Level of evidence
III.
Introduction
Delta-9-tetrahydrocannabinol (Δ9-THC) is a cannabinoid with psychotropic properties and is the primary active pharmacological compound in cannabis (marijuana). THC and other cannabinoids responsible for marijuana’s effect, such as cannabidiol and cannabinol, bind to the G protein-coupled cannabinoid receptors CB1 and CB2. CB1 receptors are predominantly found at central and peripheral nerve terminals where they mediate transmitter release and have varied roles including inducing properties associated with analgesia. CB2 receptors are highly expressed throughout the immune system and are likely involved in cytokine release.1 Cannabinoid receptors were not discovered until 1990, thus research and development efforts into the endogenous cannabinoid (endocannabinoid) system were only recently accelerated.1 Cannabinoids affect normal inhibitory pathways that influence nociception in humans. There is growing interest in the therapeutic potential of cannabinoids, especially in treating chronic pain2–5 and neuropathic pain.6 7
There are two active Food and Drug Administration-approved synthetic cannabinoids containing Δ9-THC: dronabinol (capsule and oral solution) and nabilone. Dronabinol is available by prescription in the USA, Canada, Germany, Australia and New Zealand to treat nausea and vomiting with chemotherapy and for weight loss and appetite loss in patients with HIV. Dronabinol and other cannabinoids have been studied in painful conditions, but almost exclusively in chronic pain, cancer pain, HIV-associated sensory neuropathy and pain associated with multiple sclerosis.8–11 Results in patients with chronic pain are inconclusive; some studies show marked decreases in pain,12 13 whereas others demonstrate no significant reductions in pain compared with placebo, or suggest a questionable risk versus benefit ratio.3 11 14 Few studies have examined cannabinoid use with acute pain.15
The analgesic effects that are produced with cannabinoids are more likely to occur in hyperalgesic and inflammatory states,16 giving rise to the hope of being effective at reducing acute pain following injury. Dronabinol was approved on the hospital system formulary at our level I trauma Center without restrictions. Recently, there has been an increased use of adjunctive dronabinol for treating pain based on anecdotal evidence of a beneficial treatment effect. However, there are no studies to date that have been conducted to examine the effect of dronabinol in trauma patients. The objective of this matched cohort study is to examine the effects of adjunctive dronabinol for acute pain management following traumatic injury, hypothesizing that dronabinol reduces opiate consumption compared with matched controls.
Patients and methods
Study design and population
This retrospective matched cohort study was conducted at a community-based level I trauma center over 8 months (1 March 2017 through 30 October 2017). The hospital pharmacy database was used to identify trauma patients who received dronabinol (cases). Patients who received dronabinol were matched 1:1 to trauma patients who did not receive dronabinol (controls). Matching was performed by age (±5 years), cause of injury (eg, vehicular crash, fall) and length of stay (LOS, ±5 days).
Dronabinol prescribing practice
Dronabinol has been prescribed off-label for the treatment of pain at our level I trauma center since 2015. Trauma surgeons are the main prescribers of dronabinol for our trauma population. Patients do not request adjunctive dronabinol, and it is possible some patients refused this medication. The decision to use dronabinol is provider specific and is used as part of multimodal analgesia; dronabinol is not used for withdrawal symptoms. A typical multimodal analgesia regimen begins with a scheduled non-narcotic, followed by a mild narcotic, before proceeding to an intravenous narcotic. If this regimen is unable to control the patient’s pain, then adjunctive dronabinol may be offered based on provider preference. At the time of the study, antineuropathics (gabapentin) and muscle relaxants were not commonly used for pain at our institution, although they are currently used as part of multimodal analgesia. Pet therapy and music therapy are available but are not typically used in the acute phase of pain management.
Covariates
Variables that were abstracted from the patients’ electronic medical record included dronabinol dosing and frequency, the indication for dronabinol, opioid analgesics prescribed (type, dose, frequency, route, date and time), non-opioid multimodal pain adjuncts (type, dose, frequency, route, date and time), all self-reported pain scores recorded on the pain numeric rating scale (NRS, 0–10) and current self-reported drug use (alcohol, marijuana, other drugs of abuse). Variables that were abstracted from the hospitals’ trauma registry included patient demographics (age, gender), injury characteristics (cause of injury, injury severity score, injury characteristics) and clinical outcomes (hospital LOS, intensive care unit LOS, mortality).
Outcomes
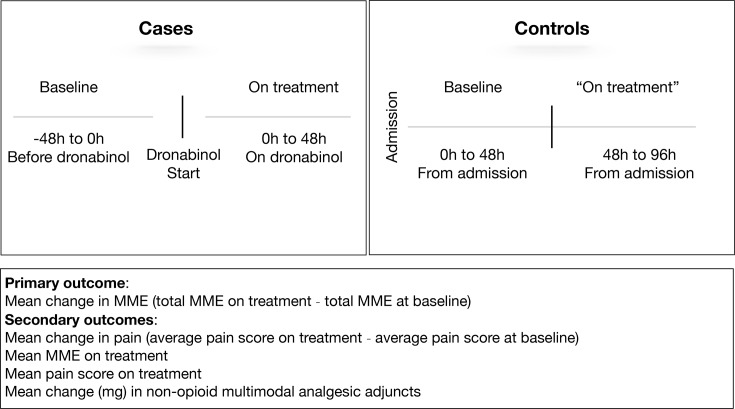
The primary outcome was change in opioid consumption, calculated as the average total morphine milligram equivalents (MME) consumed during the first 48 hours on treatment minus the average total MME consumed over a 48 hours baseline period, as defined in figure 1.
Figure 1.
Primary and secondary outcome definitions for cases and controls. MME, morphine milligram equivalents.
Secondary outcomes included: the average total MME consumed during the first 48 hours on treatment; the average pain NRS score on treatment; the average change in pain NRS score, calculated as the average pain score on treatment minus the average pain at baseline.
We also examined the change in the average total non-opioid multimodal pain adjuncts (acetaminophen, non-steroidal anti-inflammatory drugs, muscle relaxants and gabapentin) consumed on treatment minus baseline; adjuncts that were used in at least 10% of patients were tabulated.
Statistical analysis
Statistical analysis was performed with SAS V.9.4 (SAS, Cary, North Carolina, USA) and significance was set at α<0.05. Data are presented as mean and SE or median and IQR range. Demographics and injury characteristics were analyzed with paired t-tests and McNemar’s tests for cases and their matched controls.
For the primary outcome, the difference between cases and controls in change in opioid consumption was analyzed with a paired t-test. We examined the primary outcome in our overall matched population, and in the subset of cases who self-reported using marijuana and their matched controls. One-sample t-tests were used to separately analyze the change in opioid consumption for cases and controls. Secondary outcomes were analyzed with paired t-tests.
Results
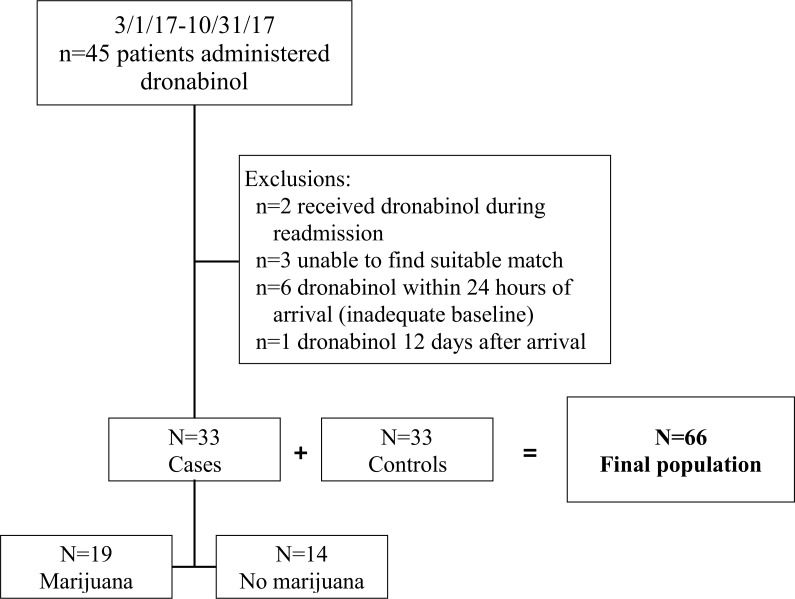
Over the 8-month study period, there were 45 patients admitted for a traumatic injury who were administered dronabinol. Twelve cases were excluded (figure 2). Thus, there were 66 patients included in the study: 33 patients received dronabinol (cases) and 33 patients did not receive dronabinol (controls). The analysis population was predominantly young (aged 27 (22–36) years) and male (73%) with injuries sustained from a vehicular crash (41%) or a fall (32%). The injury severity score was 12 (9–18), and the LOS was 8 (5–14) days. Patients were well matched for all matching variables (age, cause of injury, LOS), as well as other injury and demographic characteristics (table 1). There were significant differences between groups based on the presence of spinal cord injury and pre-injury marijuana use.
Figure 2.
Population distribution.
Table 1.
Matched cohort demographics and outcomes
| Covariate, % (n) or mean (SE) | Dronabinol cases (n=33) | Non-dronabinol controls (n=33) | P value |
| Males | 75.8 (25) | 69.7 (23) | 0.59 |
| Mean age, years | 29.9 (1.7) | 30.0 (1.7) | 0.90 |
| Mean injury severity score | 12.8 (1.3) | 15.7 (2.0) | 0.11 |
| Cause of injury | |||
| Vehicular crash | 45.5 (15) | 58.5 (16) | 1.00 |
| Fall cause | 30.3 (10) | 33.3 (11) | 1.00 |
| Other cause | 24.2 (8) | 18.2 (6) | 1.00 |
| Surgical intervention | 63.6 (21) | 63.6 (21) | 1.00 |
| Injury location/region | |||
| Head injury | 48.5 (16) | 57.6 (19) | 0.58 |
| Chest injury | 39.4 (13) | 42.4 (14) | 1.0 |
| Abdominal injury | 18.2 (6) | 30.3 (10) | 0.39 |
| Spinal injury | 27.3 (9) | 54.6 (18) | 0.01 |
| Extremity | 75.8 (25) | 54.6 (18) | 0.12 |
| Pre-injury marijuana user | 57.6 (19) | 12.1 (4) | 0.002 |
| Pre-injury opioid user | 12.1 (4) | 3.0 (1) | 0.25 |
| Pain management contract | 0 (0) | 0 (0) | NA |
| Median (SE) hours to ‘post’period | 54.5 (9.6) | 48 (0) | 0.11 |
| Mean baseline* MME | 168.8 (18.2) | 129.6 (15.7) | 0.07 |
| Mean baseline* pain | 5.8 (0.3) | 4.9 (0.3) | 0.05 |
| Mean hospital LOS, days | 9.3 (1.1) | 11.4 (1.5) | 0.17 |
| Mean ICU LOS, days | 2.2 (0.5) | 4.0 (1.1) | 0.12 |
P value: McNemar's test for categorical variables, paired t-test for continuous variables, Wilcoxon rank sum test for medians. The values in bold indicate statistical significance.
*Baseline: 48 hours before dronabinol (cases) and 0–48 hours from admission (controls).
ICU, intensive care unit; LOS, length of stay; MME, morphine milligram equivalents.
Among cases, the primary indication for dronabinol was pain (n=29, 88%), followed by anxiety (n=2) and gastrointestinal or appetite (n=2) indications. Dronabinol was administered twice daily in 88% of patients, most commonly at doses of 5 mg (n=19) and 10 mg (n=11), or 11 (8–16) mg dronabinol per day. The median number days receiving dronabinol was 3 (3–6) days.
The median time to the first dose of dronabinol was 55 hours from admission. We selected the 48 hours time point as a reasonable equivalent for our ‘pre’ and ‘post’ time periods for the control group. Thus, the postperiod interval for controls was 48–96 hours after admission, whereas for cases the median postperiod interval was 55–103 hours after admission. This difference was not statistically significant (p=0.11).
Opioid utilization
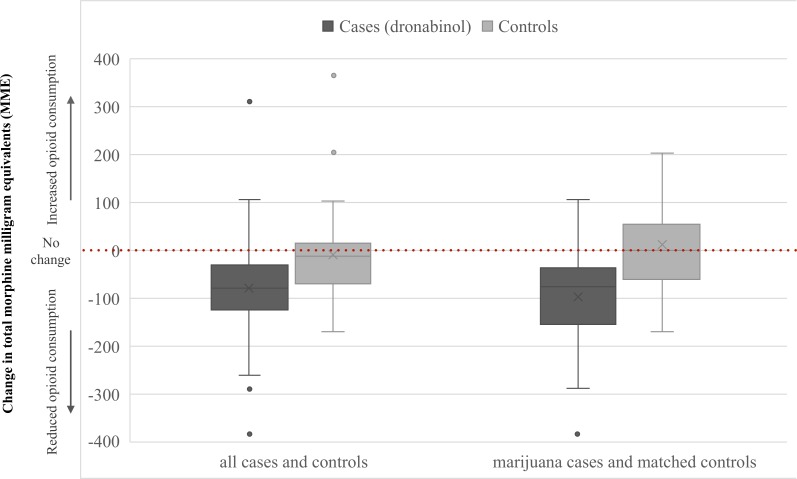
Study patients were high opioid consumers, with 68% of patients receiving ≥90 MME at baseline. The change in opioid consumption is shown in figure 3. Among cases there was a significant reduction in opioid consumption from baseline with adjunctive dronabinol (−79 (20) MME, p<0.001), while the change in opioid consumption for controls was unchanged from baseline (−9 (18) MME, p=0.63). This resulted in a ninefold greater reduction in opioid consumption for cases versus controls that was significantly different between pairs (difference: −70 MME, p=0.02) (table 2).
Figure 3.
Box-and-whisker plot of the 48 hours total change in opioid consumption (MME) among all cases (n=33) and their matched controls (n=33), and patients who used marijuana and received dronabinol (n=19) and their matched controls (n=19). The box with line is the median and upper and lower quartiles, The X is the mean, and the points outside the box are outliers.
Table 2.
Matched cohort outcomes
| Mean (SE) | Dronabinol cases (n=33) | Non-dronabinol controls (n=33) | Difference | P value |
| Primary outcome | ||||
| Change in MME | −78.7 (20.2) | −8.6 (17.6) | −70.2 (28.8) | 0.02 |
| Change in MME* | −96.5 (24.5) | 11.2 (28.4) | −107.7 (39.8) | 0.01 |
| Secondary outcomes | ||||
| MME on treatment | 90.0 (17.5) | 121.0 (27.0) | −30.9 (33.2) | 0.36 |
| Pain NRS on treatment | 5.3 (0.3) | 4.3 (0.3) | 0.9 (0.5) | 0.07 |
| Change in pain NRS | −0.4 (0.3) | −0.6 (0.4) | 0.1 (0.5) | 0.78 |
| Change in non-opioid multimodal pain adjuncts | ||||
| Acetaminophen, mg | −468 (466) | −434 (268) | −34 (485) | 0.94 |
| Cyclobenzaprine, mg | −5.5 (3.3) | 0 (1.7) | −5.5 (3.5) | 0.12 |
| Methocarbamol, mg | 7.6 (68) | −136 (111) | 144 (130) | 0.28 |
*Cases using marijuana (n=19) and their matched controls (n=19).
MME, morphine milligram equivalents; NRS, numeric rating score.
Nineteen (58%) cases reported using marijuana. In this subset, opioid consumption was significantly reduced with adjunctive dronabinol (−97 (24) MME, p<0.001) versus a non-significant increase in opioid consumption in matched controls (11 (29) MME, p=0.70) (figure 2). The difference between matched pairs was statistically significant (difference: −108 MME, p=0.01) (table 2).
Change in MME: mean change in total MME consumption over 48 hours on treatment minus 48 hours at baseline, as defined for cases and controls in figure 1. On treatment: first 48 hours with adjunctive dronabinol (cases) or 48–96 hours after admission (controls).
Secondary outcomes
There was no difference in opioid use on-treatment for the dronabinol group versus the matched controls (90 MME vs 121 MME, p=0.36). The average change in pain NRS scores were similar between cases and controls (−0.4 vs −0.6, p=0.78), although there was a borderline higher pain NRS score in the dronabinol group on treatment (p=0.07) and at baseline (p=0.05) compared with the matched controls (tables 1 and 2).
Non-opioid multimodal pain adjuncts included acetaminophen (n=37, 56%), cyclobenzaprine (n=27, 41%) and methocarbamol (n=7, 11%). Fewer than 10% of patients received ketorolac (n=6), gabapentinoids (n=4) and orphenadrine (n=2). The average change in non-opioid adjuncts on treatment from the baseline period were similar for the dronabinol group versus the matched controls (table 2). Acetaminophen use was greater for cases than matched controls in the baseline and treatment time periods, although there was no difference in the reduction in acetaminophen use over the 48 hours treatment period from baseline between groups (p=0.94).
Discussion
This is the first study to examine the effect of dronabinol for acute pain management following traumatic injury. These preliminary data suggest adjunctive dronabinol used as part of a multimodal analgesia regimen may result in a marked reduction in opioid consumption. The opioid-sparing effect appears to be more pronounced in patients who are marijuana users. Adjunctive dronabinol did not lead to corresponding reductions in pain scores, although both groups experienced similar reductions in pain and the dronabinol group achieved this reduction while also significantly reducing their opioid consumption. The promising results of this study have led us to initiate a randomized controlled trial to formally evaluate the efficacy of dronabinol for reducing opioid consumption following traumatic injury (clinicaltrials.gov identifier: NCT03928015).
There is little research for cannabinoid use in acute pain management. A 2017 systematic review assessed the analgesic efficacy of cannabinoid medications in acute pain management in seven studies, which included a total of 116 patients.15 In this review, article acute pain was defined as ‘pain of recent onset and probably limited duration’, which aligns with our definition of acute pain following traumatic injury. Of the seven cannabinoid studies, two studies included dronabinol.17 18 Buggy et al randomized patients 1:1 to Δ9-THC 5 mg versus placebo for pain related to elective abdominal hysterectomies in 40 patients.17 A single dose was given on the second postoperative day when patients requested analgesia, with no statistical difference in pain scores at rest and movement between the groups. In a study by Seeling et al, patients were either given dronabinol 5 mg or placebo for acute pain following radical prostatectomy with regional lymphadenectomy; no differences in the resting pain score were observed between groups.18
Aligning with the above studies, our matched cohort study also demonstrated no significant difference in pain scores among patients who received dronabinol compared with their matched controls. However, pain scores are subjective and thus may not be the most appropriate measure of efficacy when examining acute pain management. The addition of dronabinol resulted in reduced opioid consumption that coincided with reduced pain scores in both groups, suggesting a beneficial opioid-sparing effect of dronabinol in acutely painful conditions.
As the USA is currently fighting an opioid epidemic, where the Centers for Disease Control and Prevention estimate 130 Americans are dying daily from opioid overdose, the use of dronabinol to decrease opioid use is an attractive option.19 Colorado was a leading state in legalizing both medical and recreational marijuana. The Colorado Department of Public Health Environment estimates that 40.4% of adults use marijuana in some form (inhalation, ingestion).20 Because our study showed that the opioid-sparing effect of dronabinol may be greatest in patients who use marijuana, use of dronabinol adjunctively may benefit nearly half of the state’s population.
Severe pain is commonly experienced following traumatic injury and needs to be treated with medication. There are several reasons we believe adding adjunctive dronabinol may be favorable to increasing narcotic dosages in patients whose pain is not well managed: (1) the addictive tendency of marijuana and the negative effects of that addiction on patient morbidity and (especially) mortality are magnitudes less for marijuana than for narcotics; (2) in the acute care setting, the effects of dronabinol on vascular neurological response and respiratory depression are not as significant as with narcotics, especially when dronabinol is used adjunctively to reduce or maintain the opioid regimen rather than increasing narcotic dosages to detrimentally high levels; (3) our providers use dronabinol only during the initial phase to get the patient through the acute trauma episode. Patients are not routinely discharged with dronabinol, and other pain medications are conservatively prescribed at discharge. This practice limits over prescription of dronabinol and narcotics.
One of our study’s objectives was to determine whether the effect of dronabinol is more pronounced in marijuana users. The gestalt is that home marijuana users would have a more profound decrease in their opioid consumption with dronabinol; this has not yet been reported in the acute setting and is one of the main findings of our study. Nearly half of patients who received dronabinol were not current marijuana users, reflecting the decision to prescribe dronabinol to be multifactorial and not based solely on marijuana use. Still, there were differences in marijuana use between cases and controls, likely reflecting clinicians’ preference to prescribe dronabinol to marijuana users. A limitation of this study is that patients were not matched by self-reported marijuana use. Our randomized controlled trial uses a stratified block randomization design that randomizes patients 1:1 based on pre-injury marijuana use; this design should elucidate whether there is an opioid-sparing effect of dronabinol, and whether it is similar for marijuana users and marijuana-naïve patients.
Additional limitations to our study exist. First, we did not examine adverse events, although no cases needed to be discontinued from dronabinol. Other studies have suggested that any beneficial effects of cannabis-based medicines may be offset by potential harms.3 14 It is possible that the risk versus benefit ratio may be more favorable in acutely painful conditions because the treatment period and total dosing should be less than that seen in chronic painful conditions. The median treatment period in our study was 3 days, whereas a Cochrane review of cannabis-based medicines for chronic painful neuropathy included studies with a treatment duration of 14–182 days.14 Second, controls did not receive dronabinol, so the pretreatment period was estimated to be the first 48 hours from admission. This estimate was based on the median time from admission to first administration of dronabinol among cases of 55 hours. While there were no differences in the time from admission to the start of the ‘post’period for cases and controls, some cases may have been prescribed dronabinol later in the hospital stay, whereas the ‘post’period for all controls was 48–96 hours from admission. Third, we do not know why controls were not prescribed dronabinol or if they refused dronabinol. Fourth, our results may not be generalizable to hospitals in states where marijuana is illegal because they might expect a lower prevalence of marijuana use among the trauma population. Fifth, marijuana use was based on self-report because only 13 patients had a urine toxicology screening; of those, 12 patients tested positive for drugs, including 7 cases and 5 controls. Sixth, we do not know whether patients who received dronabinol were more satisfied with their hospital pain control compared with those who received opioids without adjunctive dronabinol; satisfaction could be considered a more relevant outcome to self-reported pain NRS scores. Finally, we did not study whether other cannabinoids can be used as analgesics in acutely painful conditions because our institution does not have other cannabis-based medications on formulary.
Conclusions
The results of this matched cohort study suggest adjunctive dronabinol reduces opioid consumption in patients with acute pain following traumatic injury. The opioid-sparing effect of dronabinol may be greatest in patients who use marijuana. We are currently enrolling a prospective randomized controlled trial of approximately 120 patients to evaluate the efficacy of dronabinol for managing acute pain following traumatic injury.
Acknowledgments
The authors would like to thank Wendy Lovato at St. Anthony Hospital for assistance with data acquisition.
Footnotes
Presented at: This paper was presented at the 2018 American Society of Health-System Pharmacists meeting (Anaheim, California, USA) and the 2018 American College of Clinical Pharmacy Global Conference (Seattle, Washington, USA).
Contributors: All authors made substantial contributions to the manuscript as follows: ES-S is responsible for literature search, data acquisition and drafting the manuscript. KS is responsible for data analysis, interpretation of data and drafting the manuscript. CS is responsible for study conception, interpretation of the data and critical revisions. CM is responsible for literature search, data acquisition and manuscript revisions. RMM is responsible for interpretation of the data and critical revisions. DB is responsible for interpretation of the data and critical revisions. All authors provided final approval of the submitted manuscript.
Funding: The study was investigator initiated. Internal funding was provided by St. Anthony Hospital.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The study was approved by the Institutional Review Board of St. Anthony Hospital (Catholic Health Initiatives) with a waiver of informed consent.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available on reasonable request.
References
- 1. Pertwee RG. Cannabinoid pharmacology: the first 66 years. Br J Pharmacol 2006;147(Suppl 1):S163–71. 10.1038/sj.bjp.0706406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fine PG, Rosenfeld MJ. The endocannabinoid system, cannabinoids, and pain. Rambam Maimonides Med J 2013;4:e0022 10.5041/RMMJ.10129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martín-Sánchez E, Furukawa TA, Taylor J, Martin JLR. Systematic review and meta-analysis of cannabis treatment for chronic pain. Pain Med 2009;10:1353–68. 10.1111/j.1526-4637.2009.00703.x [DOI] [PubMed] [Google Scholar]
- 4. Rog DJ, Nurmikko TJ, Friede T, Young CA. Randomized, controlled trial of cannabis-based medicine in central pain in multiple sclerosis. Neurology 2005;65:812–9. 10.1212/01.wnl.0000176753.45410.8b [DOI] [PubMed] [Google Scholar]
- 5. Ware MA, Wang T, Shapiro S, Robinson A, Ducruet T, Huynh T, Gamsa A, Bennett GJ, Collet J-P. Smoked cannabis for chronic neuropathic pain: a randomized controlled trial. CMAJ 2010;182:E694–701. 10.1503/cmaj.091414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wilsey B, Marcotte T, Deutsch R, Gouaux B, Sakai S, Donaghe H. Low-dose vaporized cannabis significantly improves neuropathic pain. J Pain 2013;14:136–48. 10.1016/j.jpain.2012.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wilsey B, Marcotte T, Tsodikov A, Millman J, Bentley H, Gouaux B, Fishman S. A randomized, placebo-controlled, crossover trial of cannabis cigarettes in neuropathic pain. J Pain 2008;9:506–21. 10.1016/j.jpain.2007.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abrams DI, Jay CA, Shade SB, Vizoso H, Reda H, Press S, Kelly ME, Rowbotham MC, Petersen KL. Cannabis in painful HIV-associated sensory neuropathy: a randomized placebo-controlled trial. Neurology 2007;68:515–21. 10.1212/01.wnl.0000253187.66183.9c [DOI] [PubMed] [Google Scholar]
- 9. Svendsen KB, Jensen TS, Bach FW. Does the cannabinoid dronabinol reduce central pain in multiple sclerosis? Randomised double blind placebo controlled crossover trial. BMJ 2004;329:253 10.1136/bmj.38149.566979.AE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iskedjian M, Bereza B, Gordon A, Piwko C, Einarson TR. Meta-analysis of cannabis based treatments for neuropathic and multiple sclerosis-related pain. Curr Med Res Opin 2007;23:17–24. 10.1185/030079906X158066 [DOI] [PubMed] [Google Scholar]
- 11. Stockings E, Campbell G, Hall WD, Nielsen S, Zagic D, Rahman R, Murnion B, Farrell M, Weier M, Degenhardt L, et al. Cannabis and cannabinoids for the treatment of people with chronic noncancer pain conditions: a systematic review and meta-analysis of controlled and observational studies. Pain 2018;159:1932–54. 10.1097/j.pain.0000000000001293 [DOI] [PubMed] [Google Scholar]
- 12. Aviram J, Samuelly-Leichtag G. Efficacy of cannabis-based medicines for pain management: a systematic review and meta-analysis of randomized controlled trials. Pain Physician 2017;20:E755–96. [PubMed] [Google Scholar]
- 13. Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, Keurentjes JC, Lang S, Misso K, Ryder S, et al. Cannabinoids for medical use: a systematic review and meta-analysis. JAMA 2015;313:2456–73. 10.1001/jama.2015.6358 [DOI] [PubMed] [Google Scholar]
- 14. Mücke M, Phillips T, Radbruch L, Petzke F, Häuser W. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst Rev 2018;3:CD012182 10.1002/14651858.CD012182.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Stevens AJ, Higgins MD. A systematic review of the analgesic efficacy of cannabinoid medications in the management of acute pain. Acta Anaesthesiol Scand 2017;61:268–80. 10.1111/aas.12851 [DOI] [PubMed] [Google Scholar]
- 16. Iversen L, Chapman V. Cannabinoids: a real prospect for pain relief? Curr Opin Pharmacol 2002;2:50–5. 10.1016/S1471-4892(01)00120-5 [DOI] [PubMed] [Google Scholar]
- 17. Buggy DJ, Toogood L, Maric S, Sharpe P, Lambert DG, Rowbotham DJ. Lack of analgesic efficacy of oral delta-9-tetrahydrocannabinol in postoperative pain. Pain 2003;106:169–72. 10.1016/S0304-3959(03)00331-2 [DOI] [PubMed] [Google Scholar]
- 18. Seeling W, Kneer L, Büchele B, Gschwend JE, Maier L, Nett C, Simmet T, Steffen P, Schneider M, Rockemann M, et al. [Delta(9)-tetrahydrocannabinol and the opioid receptor agonist piritramide do not act synergistically in postoperative pain]. Anaesthesist 2006;55:391–400. 10.1007/s00101-005-0963-6 [DOI] [PubMed] [Google Scholar]
- 19. Wide-ranging online data for epidemiologic research (WONDER) Statistics NCfH, ed. Atlanta, GA: Centers for Disease Control and Prevention, 2017. [Google Scholar]
- 20. M. S. Marijuana use in Colorado rises for adults, stays the same for kids EnvironmentDoPHa, ed: Colorado Department of Public Health and Environment, 2018. [Google Scholar]