Abstract
Background:
Glucocorticoid (GC)-induced osteoporosis and fractures have become a serious problem for Eastern Asians. Bisphosphonates (BPs), vitamin D and a combination treatment are effective methods to prevent and treat GC-induced osteoporosis.
Objective:
The study aimed to compare the efficacy of BPs, vitamin D and a combination treatment for preventing and managing GC-induced osteoporosis in Eastern Asians.
Methods:
A comprehensive search in the PubMed, EMBASE, Web of Science and Cochrane CENTRAL databases was undertaken for randomized controlled trials (RCTs) on the effect of BPs, vitamin D and the combination treatment on GCs-induced osteoporosis in Eastern Asian populations. Primary outcome measures were the change in bone mineral density (BMD) and bone turnover markers. The final search was performed in March 2019.
Results:
Nine RCTs were included. A total of 545 patients met the inclusion criteria. Compared with vitamin D, BPs and the combination treatment significantly alleviated osteoporosis of the spine and femoral neck in Eastern Asians with GC-induced osteoporosis. At the same time, the change in serum bone-specific alkaline phosphatase (BAP) and serum C-telopeptide of type I collagen (CTX) levels was observed to be significantly less with BPs and the combination treatment with vitamin D alone. No significant difference was found between BPs and the combination treatment in the markers mentioned above.
Conclusion:
Compared with vitamin D alone, BPs alone and the combination treatment were significantly effective on Eastern Asians with GC-induced osteoporosis. Compared with the combination treatment, BPs alone were observed to be effective enough to increase the BMDs of the spine and femoral neck on both sides and thus prevent GC-induced osteoporosis in Eastern Asians.
Keywords: Glucocorticoids, Vitamin D, osteoporosis, Eastern Asians, meta-analysis, randomized controlled trials
1. INTRODUCTION
Glucocorticoids (GCs) are widely used for various pathological conditions, such as inflammation, allergies and immunosuppressive diseases. Although GC therapy benefits an extremely large number of patients and improves their prognosis, it is universally acknowledged that long-term use of GCs in patients, even at a low dosages, can result in the loss of bone mass density (BMD), resulting in osteoporosis, and increase the risk of bone fracture, which is a serious side effect for patients [1, 2]. It is reported that 30% to 50% of patients who received long-term GC therapy suffer from fractures [3]. GC-induced fractures further cause a reduction in the quality of life for those who already have comorbid diseases. Additionally, the clinical and economic burdens of fractures in this patient group have increased over the past decade [4, 5]. As a result of a large population, a high number of GC-induced osteoporosis and fractures occur in Eastern Asia. Therefore, elucidating an effective way to prevent GC-induced osteoporosis has become important for Eastern Asians who received long-term GC therapy.
According to the guidelines on the prevention and management of GC-induced osteoporosis, two primary methods can be used for the prevention of GC-induced osteoporosis [6]. One treatment method is the administration of bisphosphonates (BPs). BPs are a group of drugs that are commonly used to prevent bone loss, and their major mechanism of action is the induction of osteoclast apoptosis, thus inhibiting bone resorption. The other treatment option is vitamin D and its analogues. These drugs can stimulate the formation and action of osteoblasts, which lead to increased bone formation. Moreover, doctors often combine BPs and vitamin D to enhance their effect on increasing BMD. However, few evidence-based conclusions regarding which method is more effective have been made. Although numerous randomized controlled trials (RCTs) have reported the efficacy of the application of BPs or vitamin D alone or the combination of the two, several meta-analyses have also demonstrated the effect of BPs on GC-induced osteoporosis [7]; however, few systematic reviews or meta-analyses have concluded which method is the best [8]. Moreover, data on their effects on Eastern Asians with GC-induced osteoporosis are scarce. It is widely known that the efficacy of vitamin D administration is different among ethnicities [9]. A recent meta-analysis also reported that the effect of BPs is different between East Asians and non-East Asians [10]. Therefore, the results of studies with other ethnicities may be not be applicable to Eastern Asians. If doctors simply treat patients based on conclusions that were suitable for other ethnicities, they may influence not only the clinical outcomes but also the well-being of patients. Consequently, it is necessary to conduct a meta-analysis to determine which treatment is the most effective for Eastern Asians.
This review compares the effect of using BPs or Vitamin D alone or the combination of the two for Eastern Asians with GC-induced osteoporosis via a meta-analysis of RCTs.
2. MATERIALS AND METHODS
2.1. Search Strategy
Two trained investigators performed an electronic literature search of major online databases, including the PubMed, EMBASE, Web of Science, and the Cochrane CENTRAL databases (all relevant studies were published in English with the date range from the inception of the database to March 23, 2019). Key terms for searching titles and abstracts were “vitamin D”, “bisphosphonates”, “Etidronate”, “Clondronate”, “Pamidronate”, “Tiludronate”, “Alendronate”, “Olpadronate”, “Neridronate”, “Ibandronate”, “Risedronate”, “Zoledronate”, “bone density”, “Japan”, “China”, “Korea”, “Mongolia”, “Japanese”, “Chinese”, “Korean”, “Mongol” and “Asian”. Our search algorithm was as follows: (“Vitamin D” OR Bisphosphonates OR “Etidronate” OR “Clondronate”, “Pamidronate” OR “Tiludronate” OR “Alendronate” OR “Olpadronate” OR “Neridronate” OR “Ibandronate”, “Risedronate” OR “Zoledronate”) AND (“bone density”) AND (“Japan” OR “China” OR “Korea” OR “Mongolia” OR “Japanese” OR “Chinese” OR “Korean” OR “Mongol” OR “Asian”) AND “randomized”.
2.2. Eligibility Criteria
Articles were included if they met the following criteria: (1) the target population was individuals who were Eastern Asians with low BMD induced by GCs; (2) the interventions were BPs, vitamin D or a combination treatment or a placebo as a control; (3) the required outcome was BMD, and a change in bone turnover markers was desirable outcomes; (4) the studies were RCTs; and (5) the full text was published in English.
2.3. Study Identification
Two investigators independently identified articles using the eligibility criteria listed above. After reading the titles and the abstracts, if they considered the articles to be eligible, they subsequently read the full text. If there existed any disagreement between the investigators, they discussed the article with a third investigator until reaching an agreement on its eligibility.
2.4. Risk of Bias and Assessment of Study Quality
The methodological quality of each eligible study was independently determined by two investigators with the Cochrane Risk of Bias tool, which is provided in the Cochrane Handbook of Systematic Reviews of Interventions (Version 5.3.0). The Cochrane Risk of Bias assessment tool evaluated the following factors: sequence generation (selection bias), allocation sequence concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other potential sources of bias. The authors categorized the studies as having “low risk”, “high risk”, or “unclear risk” of bias.
2.5. Data Extraction
The two investigators analysed the full texts of all eligible articles and then extracted the following information: author names, publishing year, the country of participants, the method of intervention, the number of participants allocated to each group, the mean age of each group and the gender composition of each group. If the two investigators disagreed with each other, they asked for the opinion of a third investigator until a consensus was reached.
2.6. Statistical Analysis
The two investigators identified and recorded the following outcomes: the change in BMD and bone turnover markers.
Statistical analyses were carried out using RevMan 5.3 software. For all comparisons, the odds ratio (OR) and 95% confidence interval (CI) were calculated as summary statistics for the dichotomous variables. The mean difference (MD) and 95% CI were calculated as summary statistics for continuous variable analyses. P < 0.05 was regarded as statistically significant. Statistical heterogeneity was quantified with the use of chi-square (χ2) and I2 tests. Pooled summary statistics were calculated with the use of a random effects model. If heterogeneity existed, determined by a statistically significant P < 0.05 and I2> 50%, subgroup analyses and sensitivity analyses were performed to identify the source of the heterogeneity.
3. RESULTS
3.1. Literature Search
By using our search strategy, 43 citations from online databases were identified, and duplicate articles were removed. Of the 43 studies, 31 articles were from PubMed, 1 was from EMBASE, 6 were from Web of Science and 5 were from Cochrane CENTRAL. (Fig. 1) shows the flowchart of the candidate and eligible articles. After reading the titles and abstracts of the 43 articles, we found that 14 studies were not RCTs, and the major fields of 6 articles were not eligible. After reading the full texts of the remaining 23 articles, we found that the comparison items of 14 articles did not meet our requirements. Finally, 9 articles were considered eligible for our study. One study reported BMD for the femoral neck on both sides [11]; we regarded the study as two different datasets and marked them as L and R for the left and right side, respectively.
Fig. (1).
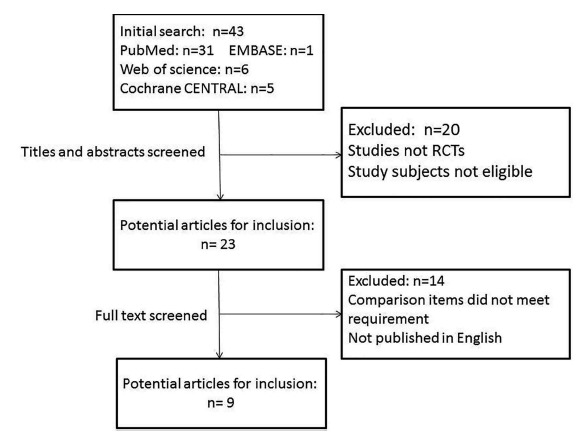
Flow diagram of the studies included in this meta-analysis.
3.2. Study characteristics
The main characteristics of the 9 eligible studies are summarized in Table 1. The earliest study was published in 2007, while the latest study was accepted in 2017. The number of participants varied from 12 to 149. A total of 545 patients met the inclusion criteria. The mean age of each group ranged from 31.4 to 72 years old. Six studies recruited only females. Intervention methods varied among the studies.
Table 1. Characteristics of the included studies.
| First Author | Publishing Year | Country | Intervention Method | Concomitant Disease | Number of Patients in BPs/Vitamin D/Combination Groups | Mean Age in BPs/Vitamin D/Combination Groups | Number of Males/Females in BPs, Vitamin D, Combination Groups |
|---|---|---|---|---|---|---|---|
| Shigeki Yamada [12] | 2007 | Japan | Risedronate (2.5 mg) or Alfacalcidol (0.5 μg) daily for 48 weeks, with 800 mg/d calcium supplement | rheumatoid arthritis | 6/6/- | 69.2/72/- | 0/6,0/6,- |
| Naohiko Fujii [13] | 2007 | Japan | Risedronate 2.5 mg/d or Vitamin D (dose unreported) or the combination for 1 year | chronic kidney disease | 23/37/40 | 41.1/42.2/40 | 12/11,16/21,15/25 |
| Yuichi Kikuchi [14] | 2007 | Japan | Risedronate 2.5 mg/d or Alfacalcidol 0.5 mg/d or the combination for 1 year | glomerular disease | 12/15/11 | 39.9/41.5/43.7 | 6/6,8/7,5/6 |
| Yosuke Okada [15] | 2008 | Japan | Alfacalcidol 1 μg/d alone or Alfacalcidol 1 μg/d with Alendronate 5 mg/d for 18 months |
autoimmune disease | -/22/25 | -/31.4/32.5 | -,0/22,0/25 |
| Seiji Takeda [16] | 2008 | Japan | Risedronate (5 mg) or Alfacalcidol(1 μg) daily for 24 months, with 800 mg/d calcium supplement | systemic autoimmune diseases | 17/16/- | 49.2/45/- | 0/17,0/16,- |
| S. Kitazaki [11] | 2009 | Japan | Alendronate (5 mg⁄ day) or Alfacalcidol (1 mg⁄ day) daily for 12 months |
ulcerative colitis |
14/18/- | 41.2/38.1/- | 10⁄6,12/8,- |
| Edmund K Li [17] | 2010 | China | Ibandronate (150 mg) or Alfacalcidol (1 μg) daily for 12 months with 500 mg/d calcium supplement | systemic lupus erythematosus | 20/20/- | 47/45.5/- | 0/20,0/20,- |
| Yoshiya Tanaka [18] | 2015 | Japan | Alendronate 35 mg/week or Alfacalcidol 1 μg/day or combination for 12 months |
systemic rheumatic diseases | 33/28/33 | 47.3/48.7/45.3 | 0/33,0/28,0/33 |
| Kichul Shin [19] | 2017 | Korea | Ibandronate 150 mg every 4 weeks or 400IU cholecalciferol daily for 48 weeks with 600 mg calcium supplement | rheumatoid arthritis and osteopenia | 76/73/- | 54.5/55.1/- | 0/76,0/73,- |
3.3. Study Quality
(Fig. 2) shows the quality assessment of the included studies. All the included studies were considered to have a low risk of bias in terms of blinding methods, incomplete outcome data and selective reporting. Two out of nine studies were considered to have a low risk of a randomized method. Four studies were considered to have a low risk of allocation concealment.
Fig. (2).
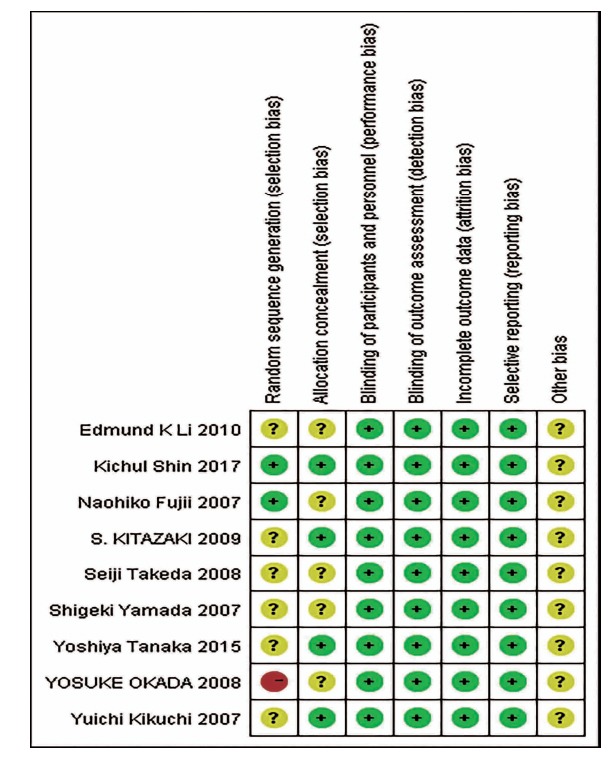
Quality of included studies.
According to Egger et al. [20], assessment for publication bias is not reliable for fewer than 10 pooled studies. Therefore, we did not evaluate the existence of publication bias by Egger’s test for funnel plot asymmetry.
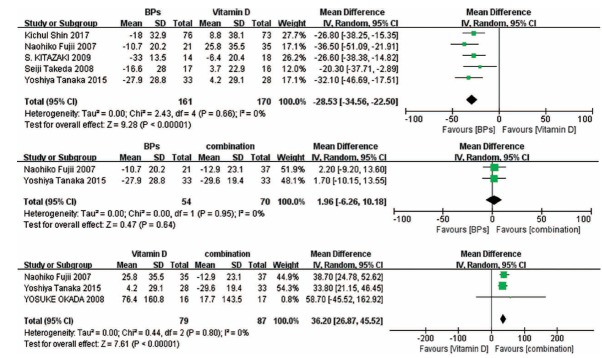
3.4. Change in BMD of the Spine
Pooled analysis indicates that the combination of the two kinds of drugs can significantly increase the BMD of the spine, while vitamin D is the least effective method (Fig. 3).
Fig. (3).
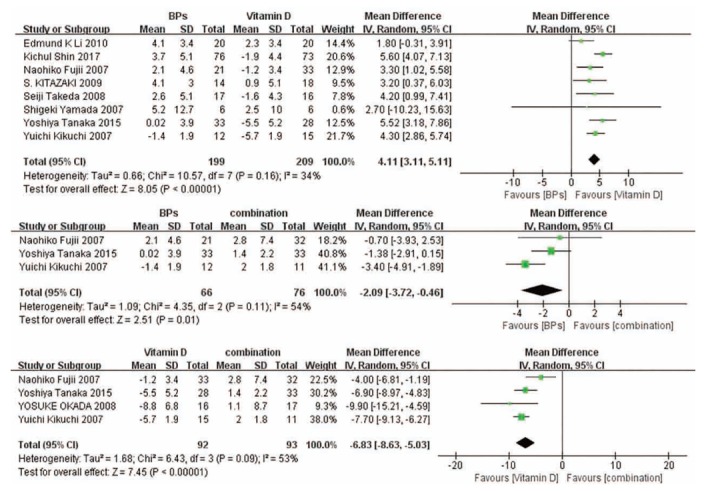
Forest plots for the change in BMD of the spine.
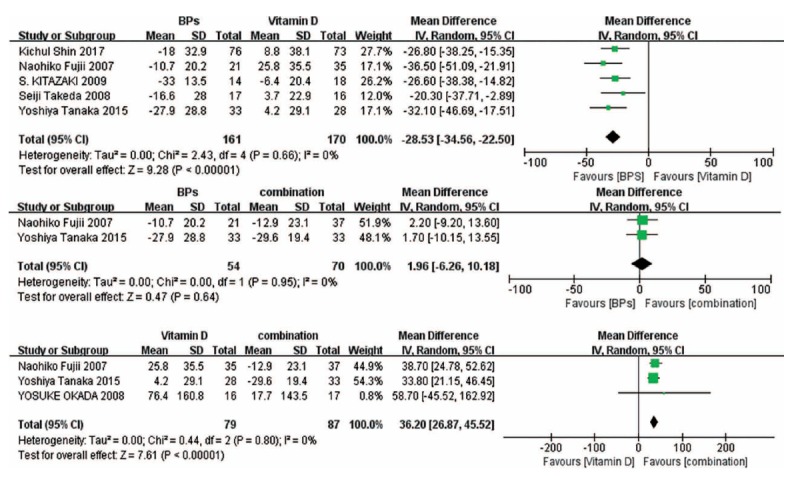
3.5. Change in BMD of the Femoral Neck
Results of forest plots show that no significant difference was found in BPs versus the combination treatment and vitamin D versus the combination treatment. Compared with vitamin D, BPs significantly increased the BMD of the femoral neck (Fig. 4).
Fig. (4).
Forest plots for the change in BMD of the femoral neck.
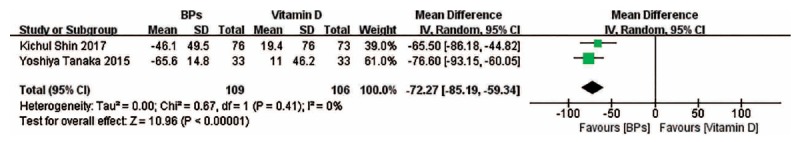
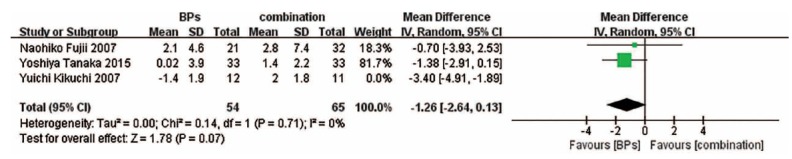
3.6. Change in Bone Turnover Markers
The forest plots show that bone-specific alkaline phosphatase (BAP) change associated with BPs and the combination treatment is obviously less than that associated with vitamin D, and there exists no significant difference between BPs versus the combination treatment (Fig. 5). The forest plots also suggest that compared with vitamin D, BPs can lead to a less significant change in serum C-telopeptide of type I collagen (CTX) concentrations (Fig. 6).
Fig. (5).
Forest plots for the changes in BAP.
Fig. (6).
Forest plots for the changes in CTX.
3.7. Sensitivity Analyses
To reduce the heterogeneity regarding BMDs of the spine associated with BPs versus the combination treatment and vitamin D versus the combination treatment, we conducted a sensitivity analysis. When we omitted the study of Yuchi Kikuchi et al. for BPs versus the combination treatment, the heterogeneity was found to be decreased significantly, and the forest plot suggested that there was no significant difference between the two treatments (Fig. 7). When we eliminated the study of Naohiko Fujii et al. for vitamin D versus the combination treatment, the heterogeneity was found to be decreased significantly, but the result of the meta-analysis did not change significantly (Fig. 8).
Fig. (7).
Result of the sensitivity analysis for BPs versus the combination treatment on BMD of the spine. When the study by Yuchi Kikuchi et al. was omitted, the conclusions from the forest plots were altered significantly; there was no significant difference between the two treatments after omission.
Fig. (8).
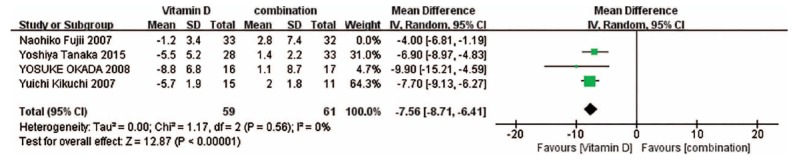
Result of the sensitivity analysis for Vitamin D versus the combination treatment on the BMD of the spine. When the study by Naohiko Fujii et al. was omitted, the conclusions from the forest plots were not significantly altered.
3.8. Subgroup Analyses
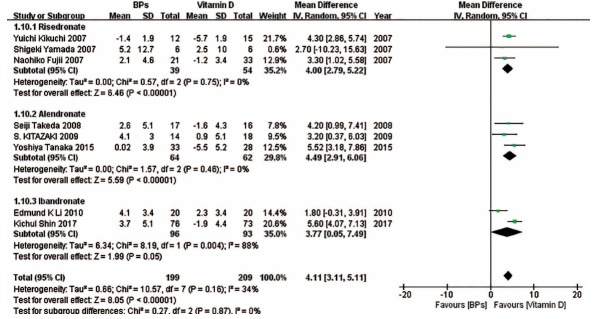
We also performed subgroup analyses on the basis of drug type in some forest plots in which enough studies were included. (Fig. 9) suggests that compared with vitamin D, risedronate and alendronate can effectively improve the BMD of the spine, while there was no significant difference between ibandronate and vitamin D in the change in the BMD of the spine. (Fig. 10) indicates that compared with vitamin D, risedronate can obviously improve the BMD of the femoral neck, while there was no significant difference between alendronate and vitamin D in the change in the BMD of the femoral neck. (Fig. 11) indicates that compared with vitamin D, the BAP levels associated with risedronate and alendronate were statistically higher than those associated with the other treatments. (Fig. 12) shows that compared with the combination treatment, the change in the BMD of the spine was significantly less with risedronate and alendronate than with vitamin D. Sensitivity analyses were not conducted for the ibandronate subgroup, as shown in Figure 9, and risedronate group, as shown in Fig. 12, because of the small sample sizes. Considering that there were only two countries besides Japan involved in this meta-analysis, we did not perform a subgroup analysis based on country.
Fig. (9).
The forest plot of the subgroup analysis for the change in the BMD of the spine between BPs and vitamin D. Compared with vitamin D, risedronate and alendronate effectively improved the BMD of the spine, while there existed no significant difference between ibandronate and vitamin D in the change in the BMD of the spine.
Fig. (10).
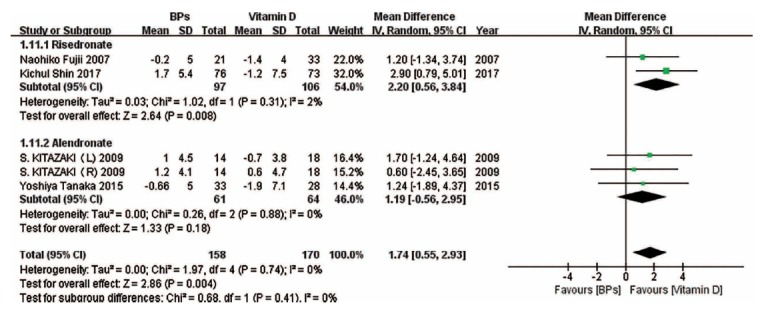
The forest plot of the subgroup analysis for the change in the BMD of the femoral neck between BPs and vitamin D. Compared with vitamin D, risedronate obviously improved the BMD in the femoral neck, while there existed no significant difference between alendronate and vitamin D in the change in the BMD of the femoral neck.
Fig. (11).
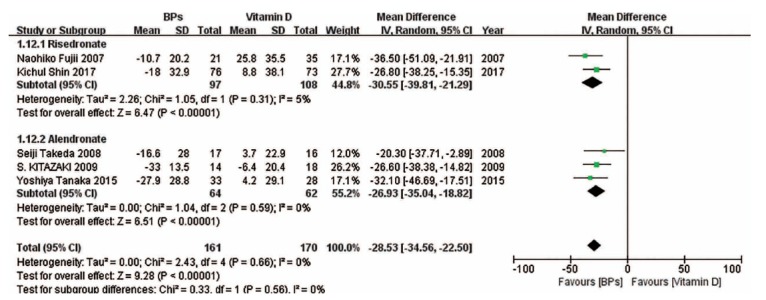
The forest plot of the subgroup analysis for the change in BAP between BPs and vitamin D. The BAP levels associated with risedronate and alendronate were statistically higher than those associated with vitamin D.
Fig. (12).
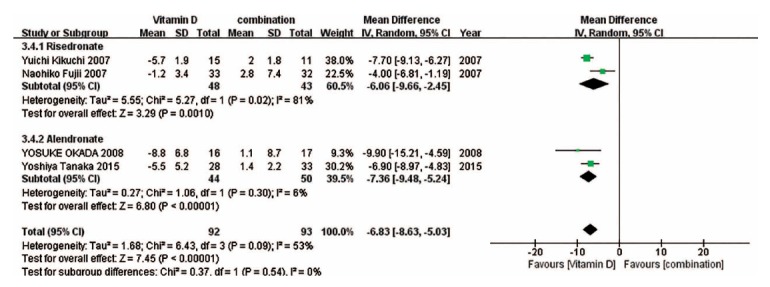
The forest plot of the subgroup analysis for the change in the BMD of the spine between vitamin D and the combination treatment. Compared with the combination treatment, the change in the BMD of the spine was significantly less with risedronate and alendronate than with vitamin D.
4. DISCUSSION
Overall, the forest plots and sensitivity analysis results suggest that compared with vitamin D, BPs and the combination treatment can significantly alleviate osteoporosis of the spine and femoral neck in Eastern Asians with GC-induced osteoporosis. At the same time, BPs and the combination treatment also caused significantly less change in serum BAP and CTX levels. No significant difference was found between BPs and the combination treatment for the markers mentioned above.
In this meta-analysis, we compared BPs, vitamin D and the combination of the two to provide guidelines for clinical doctors. The results regarding the change in the BMDs of the spine and femoral neck suggest that the efficacy of BPs and the combination treatment is nearly the same . In the sensitivity analysis (Fig. 8), the study conducted by Naohiko Fujii et al. was omitted, and the result did not change significantly. This indicated that this study has a limited impact on the results, and this sensitivity analysis was not necessary considering that the heterogeneity was fairly low. However, when the study conducted by Yuchi Kikuchi et al. was omitted (Fig. 7), the result was clearly altered. There was nearly no significant difference among those three studies, so we could not identify the reason for the change in results when their study was omitted. The small sample size may account for the heterogeneity in the two figures. We can only conclude that compared with the combination treatment, BPs seem to be equally effective in increasing the BMD of the spine, while vitamin D is less effective. More studies are needed to confirm our hypothesis and update the results. In the subgroup analyses, the results showed that compared with vitamin D, ibandronate and risedronate did not significantly change the BMDs of the spine and femoral neck, respectively; however, this may be because of the small sample size. In addition, the results of the forest plots also indicated that there was no heterogeneity between the left femoral neck and right femoral neck. Although the results of the forest plots suggested that no significant difference was found between vitamin D and the combination treatment in the BMD of the femoral neck, we speculated that this was because there were very few studies included, and further studies are needed to confirm our speculation.
Serum BAP and CTX concentrations represent the condition of bone metabolism [21]. The lower their concentrations are, the more severe the osteoporosis. Our meta-analysis indicated that BPs alone and in combination with vitamin D are more effective than vitamin D alone in preventing bone resorption and promoting bone formation, as there exists no significant difference in the efficacy of BPs and the combination treatment. Our subgroup analyses also showed a consistent result for risedronate and alendronate. Moreover, despite the small sample size, two included studies also reported changes in urinary deoxypyridinoline levels associated with BPs versus vitamin D and vitamin D versus the combination treatment, and their results are consistent with our meta-analysis results [12,15].
The safety of BPs has been studied by many researchers [22]. Some of the included studies also provided detailed information about the safety of BPs; the most frequent adverse effects seemed to be gastrointestinal, musculoskeletal and connective tissue disorders, which were consistent with previous studies. Due to the small sample size, their results cannot be shown in the form of forest plots [11,19]. However, both the studies claimed that BPs are quite safe regarding adverse effects, such as fractures and osteonecrosis of the jaw. One study reported that the adverse effects caused by BPs were mild [11], and another study found that the incidence of serious adverse effects was higher in the control group than in the treatment group [19].
There were two major problems in the previous meta-analysis regarding the effect of BPs and other drugs on GC-induced osteoporosis. On one hand, most studies focused on BPs of one type, and only a few articles summarized their overall efficacy. On the other hand, nearly no meta-analysis or systematic review analysed the effect of BPs and other drugs on Eastern Asians [7, 8, 23, 24]. Considering the ethnic difference in the efficacy of BPs and vitamin D, previous results may lead to failure when the studied drugs are administered to Eastern Asians. Compared with previous meta-analyses, our meta-analysis demonstrates the overall efficacy of BPs on Eastern Asians with long-term GC administration. An important result found by our meta-analysis is that the efficacy of BPs and the combination treatment seems to be the same. Moreover, to prevent fractures of the spine and femoral neck, it is almost unnecessary to administer vitamin D therapy when patients are undergoing BPs therapy. In addition, although vitamin D is a necessary trace organic substance in humans, it can also lead to some adverse effects, such as hypercalcaemia and hypercalciuria [25]. For the safety of patients, it is better not to combine these two kinds of drugs. These results suggest that BPs alone are effective enough for the prevention and treatment of GC-induced osteoporosis in Eastern Asians. However, our results are different from several previous guidelines [26, 27]. Considering the small sample size, further trials are required to confirm our results.
Despite the fact that the methodological quality of nearly all included studies is relatively high, there are some limitations in this meta-analysis. First, the subjects in most of the studies were Japanese, and only two studies reported results with Chinese and Korean subjects. Second, the gender ratio was unbalanced in our meta-analysis, possibly because females are subject to several immunosuppressive diseases. Third, the patterns of BPs and vitamin D therapy varied among the included studies. Fourth, the number of included studies was slightly small, so we could not test the publication bias via a funnel plot. Last, the concomitant diseases reported in the studies varied. These limitations may have caused bias and heterogeneity, influenced our sensitivity analyses and subgroup analyses, and finally misled our results, which can affect the generalizability of the findings to Eastern Asians. Further studies are needed to resolve this limitation.
CONCLUSION
Our meta-analysis of RCTs found that compared with vitamin D alone, BPs alone and the combination treatment were significantly effective for Eastern Asians with GC-induced osteoporosis. Compared with the combination treatment, BPs alone were observed effective enough to increase the BMDs of the spine and femoral neck on both sides and thus prevent GC-induced osteoporosis in Eastern Asians. Risedronate seems to be the optimal choice because it can significantly change the BMDs of the spine and femoral neck with an obvious effect on serum bone turnover markers. In addition, the effect of BPs on GC-induced osteoporosis with concomitant chronic kidney diseases may be different from that on GC-induced osteoporosis with autoimmune diseases. Further studies are needed to confirm our results.
AUTHORS’ CONTRIBUTIONS
Wang Junjie designed research, performed research, collected data, analysed data, and wrote the paper. Li Hongzhuo designed the research.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
None.
ACKNOWLEDGEMENTS
Declared none.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Cohen D., Adachi J.D. The treatment of glucocorticoid-induced osteoporosis. J. Steroid Biochem. Mol. Biol. 2004;88(4-5):337–349. doi: 10.1016/j.jsbmb.2004.01.003. [http://dx.doi.org/10.1016/j.jsbmb.2004.01.003]. [PMID: 15145443]. [DOI] [PubMed] [Google Scholar]
- 2.Kipen Y., Buchbinder R., Forbes A., Strauss B., Littlejohn G., Morand E. Prevalence of reduced bone mineral density in systemic lupus erythematosus and the role of steroids. J. Rheumatol. 1997;24(10):1922–1929. [PMID: 9330933]. [PubMed] [Google Scholar]
- 3.van Staa T.P., Leufkens H.G., Cooper C. The epidemiology of corticosteroid-induced osteoporosis: A meta-analysis. Osteoporos. Int. 2002;13(10):777–787. doi: 10.1007/s001980200108. [http://dx.doi.org/10.1007/s001980200108]. [PMID: 12378366]. [DOI] [PubMed] [Google Scholar]
- 4.Oleksik A., Lips P., Dawson A., et al. Health-related quality of life in postmenopausal women with low BMD with or without prevalent vertebral fractures. J. Bone Miner. Res. 2000;15(7):1384–1392. doi: 10.1359/jbmr.2000.15.7.1384. [http://dx.doi.org/10.1359/jbmr.2000.15.7.1384]. [PMID: 10893688]. [DOI] [PubMed] [Google Scholar]
- 5.Johnson J.M., Franchini G. Retroviral proteins that target the major histocompatibility complex class I. Virus Res. 2002;88(1-2):119–127. doi: 10.1016/s0168-1702(02)00124-7. [http://dx.doi.org/10.1016/S0168-1702(02)00124-7]. [PMID: 12297331]. [DOI] [PubMed] [Google Scholar]
- 6.De Nijs R.N. Glucocorticoid-induced osteoporosis: A review on pathophysiology and treatment options. Minerva Med. 2008;99(1):23–43. [PMID: 18299694]. [PubMed] [Google Scholar]
- 7.Feng Z., Zeng S., Wang Y., Zheng Z., Chen Z. Bisphosphonates for the prevention and treatment of osteoporosis in patients with rheumatic diseases: a systematic review and meta-analysis. PLoS One. 2013;8(12):e80890. doi: 10.1371/journal.pone.0080890. [http://dx.doi.org/10.1371/journal.pone.0080890]. [PMID: 24324644]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Allen C.S., Yeung J.H., Vandermeer B., Homik J. Bisphosphonates for steroid-induced osteoporosis. Cochrane Database Syst. Rev. 2016;10:CD001347. doi: 10.1002/14651858.CD001347.pub2. [PMID: 27706804]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hannan M.T., Litman H.J., Araujo A.B., et al. Serum 25-hydroxyvitamin D and bone mineral density in a racially and ethnically diverse group of men. J. Clin. Endocrinol. Metab. 2008;93(1):40–46. doi: 10.1210/jc.2007-1217. [http://dx.doi.org/10.1210/jc.2007-1217]. [PMID: 17986641]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y., Jia G., Song J., Kong X., Zhang W., Meng C. Comparative efficacy of alendronate upon vertebral bone mineral density and fracture rates in east asians versus non-east asians with postmenopausal osteoporosis: A systematic review and meta-analysis. Horm. Metab. Res. 2018;50(10):738–746. doi: 10.1055/a-0741-8300. [http://dx.doi.org/10.1055/a-0741-8300]. [PMID: 30312984]. [DOI] [PubMed] [Google Scholar]
- 11.Kitazaki S., Mitsuyama K., Masuda J., et al. Clinical trial: Comparison of alendronate and alfacalcidol in glucocorticoid-associated osteoporosis in patients with ulcerative colitis. Aliment. Pharmacol. Ther. 2009;29(4):424–430. doi: 10.1111/j.1365-2036.2008.03899.x. [http://dx.doi.org/10.1111/j.1365-2036.2008.03899.x]. [PMID: 19035979]. [DOI] [PubMed] [Google Scholar]
- 12.Yamada S., Takagi H., Tsuchiya H., et al. Comparative studies on effect of risedronate and alfacalcidol against glucocorticoid-induced osteoporosis in rheumatoid arthritic patients. Yakugaku Zasshi. 2007;127(9):1491–1496. doi: 10.1248/yakushi.127.1491. [http://dx.doi.org/10.1248/yakushi.127.1491]. [PMID: 17827929]. [DOI] [PubMed] [Google Scholar]
- 13.Fujii N., Hamano T., Mikami S., et al. Risedronate, an effective treatment for glucocorticoid-induced bone loss in CKD patients with or without concomitant active vitamin D (PRIUS-CKD). Nephrol. Dial. Transplant. 2007;22(6):1601–1607. doi: 10.1093/ndt/gfl567. [http://dx.doi.org/10.1093/ndt/gfl567]. [PMID: 17124283]. [DOI] [PubMed] [Google Scholar]
- 14.Kikuchi Y., Imakiire T., Yamada M., et al. Effect of risedronate on high-dose corticosteroid-induced bone loss in patients with glomerular disease. Nephrol. Dial. Transplant. 2007;22(6):1593–1600. doi: 10.1093/ndt/gfl568. [http://dx.doi.org/10.1093/ndt/gfl568]. [PMID: 17041001]. [DOI] [PubMed] [Google Scholar]
- 15.Okada Y., Nawata M., Nakayamada S., Saito K., Tanaka Y. Alendronate protects premenopausal women from bone loss and fracture associated with high-dose glucocorticoid therapy. J. Rheumatol. 2008;35(11):2249–2254. doi: 10.3899/jrheum.080168. [http://dx.doi.org/10.3899/jrheum.080168]. [PMID: 19031508]. [DOI] [PubMed] [Google Scholar]
- 16.Takeda S., Kaneoka H., Saito T. Effect of alendronate on glucocorticoid-induced osteoporosis in Japanese women with systemic autoimmune diseases: Versus alfacalcidol. Mod. Rheumatol. 2008;18(3):271–276. doi: 10.1007/s10165-008-0055-y. [http://dx.doi.org/10.3109/s10165-008-0055-y]. [PMID: 18427724]. [DOI] [PubMed] [Google Scholar]
- 17.Li E.K., Zhu T.Y., Hung V.Y., et al. Ibandronate increases cortical bone density in patients with systemic lupus erythematosus on long-term glucocorticoid. Arthritis Res. Ther. 2010;12(5):R198. doi: 10.1186/ar3170. [http://dx.doi.org/10.1186/ar3170]. [PMID: 20964867]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanaka Y., Mori H., Aoki T., et al. Analysis of bone metabolism during early stage and clinical benefits of early intervention with alendronate in patients with systemic rheumatic diseases treated with high-dose glucocorticoid: Early DIagnosis and Treatment of OsteopoRosis in Japan (EDITOR-J) study. J. Bone Miner. Metab. 2016;34(6):646–654. doi: 10.1007/s00774-015-0709-8. [http://dx.doi.org/10.1007/s00774-015-0709-8]. [PMID: 26308708]. [DOI] [PubMed] [Google Scholar]
- 19.Shin K., Park S.H., Park W., et al. Monthly Oral Ibandronate Reduces Bone Loss in Korean Women with Rheumatoid Arthritis and Osteopenia Receiving Long-term Glucocorticoids: A 48-week Double-blinded Randomized Placebo-controlled Investigator-initiated Trial. Clin. Ther. 2017;39(2):268–278.e2. doi: 10.1016/j.clinthera.2017.01.008. [http://dx.doi.org/10.1016/j.clinthera.2017.01.008]. [PMID: 28161119]. [DOI] [PubMed] [Google Scholar]
- 20.Egger M., Davey Smith G., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [http://dx.doi.org/10.1136/bmj.315.7109.629]. [PMID: 9310563]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hlaing T.T., Compston J.E. Biochemical markers of bone turnover - uses and limitations. Ann. Clin. Biochem. 2014;51(Pt 2):189–202. doi: 10.1177/0004563213515190. [http://dx.doi.org/10.1177/0004563213515190]. [PMID: 24399365]. [DOI] [PubMed] [Google Scholar]
- 22.Tadrous M., Mamdani M.M., Juurlink D.N., et al. Comparative gastrointestinal safety of bisphosphonates in primary osteoporosis: A network meta-analysis-reply to pazianas and abrahamsen. Osteoporos. Int. 2014;25(11):2671–2672. doi: 10.1007/s00198-014-2789-z. [http://dx.doi.org/10.1007/s00198-014-2789-z]. [DOI] [PubMed] [Google Scholar]
- 23.Kan S.L., Yuan Z.F., Li Y., et al. Alendronate prevents glucocorticoid-induced osteoporosis in patients with rheumatic diseases: A meta-analysis. Medicine. 2016;95(25):e3990. doi: 10.1097/MD.0000000000003990. [http://dx.doi.org/10.1097/MD.0000000000003990]. [PMID: 27336902]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adachi J.D., Roux C., Pitt P.I., et al. A pooled data analysis on the use of intermittent cyclical etidronate therapy for the prevention and treatment of corticosteroid induced bone loss. J. Rheumatol. 2000;27(10):2424–2431. [PMID: 11036840]. [PubMed] [Google Scholar]
- 25.Orimo H., Nakamura T., Fukunaga M., et al. Effects of alendronate plus alfacalcidol in osteoporosis patients with a high risk of fracture: The Japanese Osteoporosis Intervention Trial (JOINT) - 02. Curr. Med. Res. Opin. 2011;27(6):1273–1284. doi: 10.1185/03007995.2011.580341. [http://dx.doi.org/10.1185/03007995.2011.580341]. [PMID: 21554143]. [DOI] [PubMed] [Google Scholar]
- 26.Park S.Y., Gong H.S., Kim K.M., et al. Korean Guideline for the Prevention and Treatment of Glucocorticoid-induced Osteoporosis. J. Bone Metab. 2018;25(4):195–211. doi: 10.11005/jbm.2018.25.4.195. [http://dx.doi.org/10.11005/jbm.2018.25.4.195]. [PMID: 30574464]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buckley L., Guyatt G., Fink H.A., et al. 2017 American College of Rheumatology Guideline for the Prevention and Treatment of Glucocorticoid-Induced Osteoporosis. Arthritis Care Res. (Hoboken) 2017;69(8):1095–1110. doi: 10.1002/acr.23279. [http://dx.doi.org/10.1002/acr.23279]. [PMID: 28585410]. [DOI] [PubMed] [Google Scholar]