Abstract
Background:
The effects of exercise on the innate/inflammatory immune responses are crucially mediated by catecholamines and adrenoreceptors; and mediations in both stimulatory and anti-inflammatory responses have been attributed to them. Obesity and metabolic syndrome are included among low-grade chronic inflammatory pathologies; particularly because patients have a dysregulation of the inflammatory and stress responses, which can lead to high levels of inflammatory cytokines that induce insulin resistance, contributing to the onset or exacerbation of type 2 diabetes. Macrophages play a crucial role in this obesity-induced inflammation. Although most of the anti-inflammatory effects of catecholamines are mediated by β adrenergic receptors (particularly β2), it is not known whether in altered homeostatic conditions, such as obesity and during exercise, innate/inflammatory responses of macrophages to β2 adrenergic stimulation are similar to those in cells of healthy organisms at baseline.
Objective:
This review aims to emphasize that there could be possible different responses to β2 adrenergic stimulation in obesity, and exercise in this condition.
Methods:
A revision of the literature based on the hypothesis that obesity affects β2 adrenergic regulation of macrophage-mediated innate/inflammatory responses, as well as the effect of exercise in this context.
Conclusion:
The inflammatory responses mediated by β2 adrenoreceptors are different in obese individuals with altered inflammatory states at baseline compared to healthy individuals, and exercise can also interfere with these responses. Nevertheless, it is clearly necessary to develop more studies that contribute to widening the knowledge of the neuroimmune regulation process in obesity, particularly in this context.
Keyword: Macrophages, monocytes, β2 adrenergic receptors, inflammation, cytokines, phagocytosis, obesity, exercise
1. INTRODUCTION
1.1. Effect of Noradrenaline on Macrophage Function, and Role of Α and Β Adrenergic Receptors
It is now well known that the sensitivity of the immune system to stress is a consequence of the reciprocal regulatory influence between the immune, endocrine and central nervous systems. For years, many experiments have evaluated the impact of catecholamines on the immune response and most of them started by observing that catecholamines modulated lymphocyte responses. Classically, it was considered that, in general, high concentrations of catecholamines inhibit the proliferative function of lymphocytes through β receptors (which constituted one of the first immunophysiological bases of immunosuppression attributed to catecholamines), while low concentrations could stimulate it through α receptors [1, 2]. In any case, an immunosuppressive effect for catecholamines was concluded overall. Today, it is considered that almost all the mechanisms involved in an immune response may be affected by noradrenergic neurotransmitters [3, 4], although without a clearly defined role as immunosuppressor or immunostimulator; particularly when considering the responses mediated by phagocytic cells, such as macrophages, which are the main cells responsible for innate/inflammatory responses [5]. Although there are not many studies of sympathoadrenergic regulation of the innate immune response via β2 adrenergic receptors, this mechanism can be considered a novel anti-inflammatory and immunomodulating target with therapeutic potential, as recently suggested by Scanzano and Cosentino [6]. Thus, activation of β2 adrenergic receptors in monocytes is usually anti-inflammatory, and β2 adrenergic receptor dysregulation in microglia and astrocytes may provoke neuroinflammation in autoimmune and neurodegenerative diseases [6].
Although each type of catecholamine can affect each type of immune cell differently, it seems that noradrenaline (NA) has the greatest capacity to modulate the immune system [6, 7]. Besedovsky and Del Rey [4] have suggested that, in addition to the affected cell subpopulation, the effects of NA also depend on the stimulus that triggers the immune response and, above all, on the activation stage at which macrophages and lymphocytes are exposed to neurotransmitters [5]. The processes directly or indirectly affected by sympathetic neurotransmitters are mainly the phagocytic process and antigenic presentation, the expression of adhesion molecules, the activation of lymphoid cells, the production of cytokines and chemokines, the Th1/Th2 balance, and the generation of cytotoxic cells [4, 6, 8-11]. NA can also play a very important role in recirculation and leukocyte trafficking between lymphoid organs and blood [1, 4, 9, 11], modulating chemotactic capacity and accumulation of phagocytes in inflammation sites as well [8, 12, 13].
In general, macrophages exposed to NA respond differently to lymphocytes, particularly during stressful situations such as physical exercise [5, 8, 12, 13]. In addition, the response of macrophages to NA does not only depend on the concentration of NA or the age of the animals, but also on the end products of their catabolism, such as HMPG (4-hydroxy-3-methoxyphenyl-glycol), which can establish a very fine-tuned physiological regulation of macrophage functions, maintaining the phagocytic functions at physiologically optimal levels [12, 14-16]. In general, it has been suggested that physiological (and even higher) concentrations of catecholamines can stimulate the innate immune response functions of macrophages, such as chemotaxis, phagocytosis, and microbicidal capacity [12]. Nevertheless, as individuals age, a higher concentration of NA in old animals is necessary to achieve thesestimulatory effects on the innate defensive response [15]. However, this NA-induced stimulation of the innate response, aimed to develop the phagocytic process against pathogens, does not correspond to the NA-induced inhibition of the production of inflammatory cytokines (IL-1β, TNF-α, and IL-6) and stimulation of the release of anti-inflammatory cytokines (IL-10); which has formed the basis for the hypothesis of the anti-inflammatory effects of catecholamines on macrophages and other immune cells [9, 17-19]. Nevertheless, this response can be the opposite in inflammatory pathologies, having been proposed as a cause in various inflammatory disorders [9, 18], also including in this context an immune-neuroendocrine dysregulation that involves inflammatory cytokines, particularly IL-6, and NA in obesity and metabolic syndrome [19, 20]. Moreover, β2 adrenergic receptors, through increased activation of immune cells and production of pro-inflammatory cytokines, have been recently proposed to be involved in functional pain syndromes associated with enhanced levels of catecholamines, by promoting neuroinflammation and nociceptor activation [21].
Then, it could be concluded that the idea that catecholamines are always immunosuppressive is not valid. Instead, they should be considered as immunomodulators, being able to favor a greater innate immune response during stress situations by sending signals to phagocytic cells in order to prevent possible infections at times when the lymphocyte-mediated adaptive response could be weakened [5, 13, 22]. In any case, inhibited immune responses are generally associated with β adrenoreceptors, particularly β2 receptors and fundamentally in relation to inflammatory activity as it will be discussed below.
1.2. Role of α and β Adrenergic Receptors in Macrophage Stimulation: β2 Receptors
Through binding to adrenergic receptors, NA is able to induce changes in cellular activity, which in turn regulate the expression of genes that regulate the required immune responses. Innate immune response cells express both α and β adrenergic receptors [23]. Adrenaline and NA are approximately equipotent as α receptor agonists. Two types of α receptors are known: α1 and α2 receptors. β receptor responses can also be divided into two types. β1 receptors respond equally to adrenaline and NA. β2 receptors seem to respond more to adrenaline than to NA. A third type of receptor, β3, does not seem to exist in the membrane of immune cells [24]. Although the aim of this review is not to analyze the intracellular signaling of the different responses mediated by catecholamines through their receptors, it is important to remind that adrenoreceptors are coupled to G proteins. Upon interaction with its ligands, and depending on the type of adrenergic receptor, intracellular second messengers, such as cAMP, Ca2+, diacylglycerol, and inositol 1,4,5-triphostate, are activated or inhibited, thus regulating cellular functions [6]. For years, the existence of the adenylate cyclase system associated with β responses for the production of cAMP as a second messenger in immune cells has been clear [1, 25]. Activation of stimulatory G proteins (Gs) coupled to β2 receptors induces an increase in intracellular cAMP leading to the activation of protein kinase A (PKA), with the participation of the Iκβ/NF-κβ pathway to induce anti-inflammatory effects in monocytic cells [6, 26]. It has already been mentioned that stimulation of β2 adrenoreceptors by catecholamines suppresses the inflammatory response through inhibition of the production of pro-inflammatory cytokines by Th1 lymphocytes while promoting an anti-inflammatory Th2 response (IL-10 and TFG-β). These responses may be mediated by a previous inhibition of the production of the pro-inflammatory cytokine IL-12 and stimulation of the release of the anti-inflammatory cytokine IL-4 [9, 10]. Stimulation of β2 adrenergic receptors also inhibits the production of TNF-α by monocytes, microglial cells, and astrocytes [27-29]. Inflammation control in innate cells via activation of β2 adrenergic receptors by NA also occurs through rapid induction of the release of the anti-inflammatory cytokine IL-10 [30]. In fact, when α and β adrenergic receptors are blocked in macrophages, cytokine/chemokine production by these cells is significantly inhibited [31, 32]. Because of all of this, it has been considered that the inflammatory cytokine/chemokine network could be one of the most important systems that are strongly controlled by catecholamines via adrenergic receptors. On the other hand, in the context of this regulation, it remains to be determined whether the secretion of catecholamines by immune cells is an omnipresent phenomenon or a rare phenomenon, the last weapon of immune cells aimed at the strongest attacks by pathogens [33, 34]. However, blockade of α receptors (and to a lesser extent of β adrenergic receptors) prevents stimulation of chemotaxis by NA, and blocking both α and β receptors prevent stimulation of phagocytosis by NA. Thus, while stimulation of chemotaxis is mainly mediated by α adrenoreceptors, stimulation of phagocytosis needs both α and β receptor activation [8, 12]. The fact that blockade of β adrenergic receptors inhibits, on the one hand, the inflammatory response (through release of inflammatory cytokines) and, on the other hand, prevents stimulation of the innate response (through phagocytosis) by NA in macrophages, clearly suggests that this receptor plays a pivotal role in the “bioregulatory responses” in the context of physiological stress. This concept entails inhibition of excess release of inflammatory mediators without immunocompromising innate defensive responses mediated by these cells [35]. Thus, as Pires-Lapa and coworkers [36] have accurately proposed in 2018, macrophages can be autocrinally activated, through β adrenoreceptors, by locally synthesized cathecholamines and/or by NA released by sympathetic nerve endings [9, 33, 37]. Although in this context NA stimulates chemotaxis (mainly mediated by α receptors) and phagocytosis (mediated by both α and β receptors) of macrophages [12, 13], NA and stimulation of β2 adrenoceptors reduce the synthesis and release of pro-inflammatory cytokines and other products of activated macrophages, such as TNF-α, IL-6, IL-1β [19, 38, 39], metalloproteinase-12 protein, and MCP-1, [40]; and also control inflammation via the rapid induction of the anti-inflammatory cytokine IL-10 [30]. In addition, β adrenoreceptor-mediated adrenergic stimulation, cAMP, and NF-κβ have also been associated with induction of the release of other mediators of the innate/inflammatory response by macrophages, such as eHsp72, which in turn modulates the innate/inflammatory responses with the participation of β adrenoreceptors and cAMP [13, 41-43], or melatonin [36]. While the mobility of macrophages to the focus of infection (i.e. chemotaxis) is stimulated by low concentrations of NA, which can also be chemoattractant/chemokinetic for innate immune cells [13, 15], it has been also proposed that β2 adrenergic stimulation reduces macrophage mobility, favoring the predominance of “resolution phase macrophages” [36]. Thus, it seems clear that the stimulation of β2 adrenergic receptors dampens activated M1 (pro-inflammatory macrophages) polarization [44] and promotes induction of M2 anti-inflammatory/regulatory macrophages [45].
2. OBESITY, IMMUNE SYSTEM, AND INFLAMMATORY RESPONSE
According to recent estimates from the World Health Organization, the number of people suffering from obesity is increasing at a great pace: 39% of adults aged 18 or over were overweight (1900 million adults), and around 13% of the world's adult population were obese (650 million) in 2016 [46]. Therefore, it is clear that obesity is a worldwide epidemic associated with comorbidities and metabolic disorders that increase the incidence of pathologies such as atherosclerosis, type 2 diabetes mellitus (DM2) and cardiovascular diseases; but it is also associated with infectious, inflammatory, allergic, and auto-immune diseases. Numerous clinical and experimental evidences indicate that obesity is associated with alterations of the immune system and the inflammatory response [47-50]. Obese individuals and experimental obesity models have greater susceptibility to infections, exhibiting alterations in the lipopolysaccharide (LPS) response profile [51-54], as well as phenotypic and leukocyte activity changes: alterations in the expression of Toll-like receptors (TLR), in the intensity of production of pro- and anti-inflammatory mediators, in the microbicidal capacity, and in the cytotoxic capacity of these cells [52, 55-59].
Nutritional excess and physical inactivity associated with other factors (such as genetic and endocrine factors) induce hyperplasia and hypertrophy of the adipose tissue. Hypertrophic adipocytes are subjected to metabolic stress, which can result in cell death, activation of infiltrated leukocytes in the adipose tissue (mostly macrophages), and inflammation [60]. Free fatty acids (FFA), adipokines (such as leptin and resistin), and other inflammatory mediators (such as TNF-α, IL-6, and MCP-1) released by inflamed adipose tissue can reach systemic circulation in addition to participating in the local inflammatory response [47, 60, 61]. Thus, alterations induced by the energy imbalance in the circulating levels of FFA, glucose, adipokines, cytokines, chemokines, and other inflammatory mediators, lead to metabolic dysfunction in several cell types besides adipocytes, such as hepatocytes, endothelial cells, leukocytes, and neurons [47, 61]. Altogether, these phenomena contribute to the development of obesity-related low-grade systemic inflammation and the progression of metabolic syndrome [47, 59, 61- 63].
The role of monocytes and macrophages in obesity is crucial. In humans, monocytes can be classified into different subsets according to the expression of membrane glycoproteins CD14 and CD16 [64, 65]. CD14++ CD16– monocytes are classical monocytes, being the most prevalent circulating subset in healthy individuals. CD16+ monocyte population is subdivided into CD14++ CD16+ intermediate monocytes and CD14+ CD16++ non-classical monocytes [64, 65]. CD16+ cells exhibit increased antigen presentation capacity, high endothelial affinity, and increased the ability to produce pro-inflammatory cytokines, such as TNF-α, compared to CD16– cells [66]. CD16+ monocyte population increases in acute inflammatory conditions, but also in chronic inflammatory conditions, such as in obesity [67-69]. In mice, based on the expression of surface markers, monocytes are divided into two groups: Ly6C++CD43CCR2+ CD62L+CX3CR1low monocytes, which present a pro-inflammatory profile, and Ly6C-CD43+CCR2-CD62L-CX3CR1hi monocytes, which present an anti-inflammatory profile [70].
After circulating for a few days, monocytes migrate to tissues where they differentiate into macrophages and dendritic cells [71, 65]. Furthermore, during infections and tissue damage, these cells are recruited to inflammatory foci when the resident macrophage population is insufficient [65, 71]. In obesity, macrophages with M1/pro-inflammatory phenotype (expression of CD11c and inducible nitric oxide synthase, iNOS) are more prevalent than macrophages with M2/anti-inflammatory phenotype (expression of CD206 and type-1 arginase, ARG1) [60, 72, 73]. M1 macrophages are associated with the high microbicidal activity, production of pro-inflammatory mediators, and cellular immunity, while M2 macrophages are associated with tissue repair, remodeling processes, and humoral immunity [60, 72, 73]. Therefore, since an increased prevalence of monocytes and macrophages with a pro-inflammatory profile is linked to the development of insulin resistance, DM2, and renal and cardiovascular diseases, among others; it seems clear that monocytes and macrophages play a key role in the pathophysiology of obesity and its comorbidities [47, 48].
3. ADRENERGIC SYSTEM AND INNATE/INFLAM-MATORY IMMUNE RESPONSES IN OBESITY AND IN PHYSICAL EXERCISE IN THIS CONDITION: THE ROLE OF β2 ADRENERGIC RECEPTORS
Thus far, we have tried to present the most relevant aspects of the actions that catecholamines (particularly NA) exert on the immune response through adrenergic receptors (mainly through β2 adrenoreceptors), particularly on the monocyte/macrophage-mediated innate/inflammatory immune response. In addition, it has been discussed how the inflammatory response mediated by macrophages plays a very important role in the pathophysiology of obesity and metabolic syndrome. Individuals with these conditions also have a greater susceptibility to infections due to deficiencies in the innate immune response against pathogens, among other aspects of immunity. Therefore, anti-inflammatory strategies are appropriate to deal with obesity but avoiding immunosuppressive side effects. Among the different non-pharmacological strategies in obesity management, physical activity is the most efficient and the most used. However, adherence to exercise programs of sufficient intensity to achieve immunophysiological adaptations can sometimes be difficult for obese individuals. The potential use of other anti-inflammatory strategies could help to achieve these beneficial immunophysiological changes, but could also lead to further immune deficiencies as a side-effect. In addition, intense physical activity can induce stress in these individuals, causing inflammatory dysregulations that can exacerbate the pathology [20]. In this context, the use of pharmacological anti-inflammatory strategies, such as β2 adrenoreceptor agonists, is being increasingly suggested in obesity and associated DM2. The anti-inflammatory effects of β2 adrenergic receptors on activated monocytes/macrophages have been proposed with a potential role in the macrophage regulation during inflammation and cardiovascular complications in diabetes and obesity [74- 76]. In this context, among more than 1000 compounds of the library of the US Food and Drug Administration-approved drugs screened for anti-inflammatory effects, β2 adrenergic receptor agonists were identified as the most potent inhibitors of diabetic-induced inflammatory activation (TNF-α production) of monocytes/macrophages [75]. However, we cannot forget that the response of inflammatory cells to catecholamines could stimulate the production of inflammatory mediators (including TNF-α) in inflammatory pathologies [9], such as obesity and obesity-associated diabetes. This catecholamine interaction is particularly important during physical exercise, when bioregulation of the innate/inflammatory response plays a crucial role, especially under chronic low-grade inflammation conditions [35]. Thus, it is important to address whether the response to the stimulation of β2 adrenergic receptors in macrophages may be different in obesity and during exercise in this condition. In fact, among 124 articles in PubMed with the terms “β adrenergic receptor and macrophages”, only 1 article appears when adding the term diabetes, 1 article when adding the term obesity, 5 articles when adding the term exercise, and only 1 related article by combining “β adrenergic receptor and macrophage and obesity and exercise”. This clearly shows the need for new studies in this line of research, because even different researchers defend that, although it seems clear that β2 adrenergic receptors play an anti-inflammatory role in vitro, further research is required to improve the limited applicability in vivo [77]. Therefore, we think that it is crucial to study in greater depth the influence of obesity and regular physical exercise on β2 adrenergic regulation in monocytes and macrophages, given that these cells and the molecules produced by them, apart from being important for the efficiency of the immune response, participate in the physiopathology of obesity.
Adrenergic regulation of the immune system, and particularly of the macrophage-mediated innate/inflammatory immune response, depends on the activity of the sympathetic nervous system (SNS) and the hypothalamic-pituitary-adrenal (HPA) axis, and obesity is a condition that presents immunological, sympathetic activity, and HPA axis changes [20, 47, 48, 59, 78, 79]. Catecholamines and other adrenergic agonists are important regulators of the inflammatory response [80-82]. Moreover, inflammatory conditions activate the nervous system, and one of the phenomena triggered by this activation is the secretion of catecholamines by nerve endings or by the adrenal gland, which can activate adrenergic receptors in leukocytes, resulting in the regulation of their activity [4, 5, 80, 81]. In this context, underlying inflammation in obesity and metabolic syndrome can alter the SNS-mediated feedback between inflammatory and stress responses [59]. Obesity-associated metabolic stress is also manifested in hypothalamic activity [83, 84], there being a correlation between obesity and changes in the activity of the HPA axis and SNS. These changes seem to come from neuroendocrine abnormalities in the central nervous system (CNS), including hormone secretion system alterations and intense responses to different neuropeptides or stressful events [78, 79].
It is well-known that regular physical exercise, an event that participates in neuroimmune regulation, exerts beneficial effects in obese individuals [59, 85, 86]. An exercise is a form of physical activity that requires planned, structured, and repetitive activities [87] in order to achieve both sports performance and health objectives. Physical exercise constitutes physiological stress. It activates the SNS and HPA axis, and it is an event that participates in the adrenergic regulation of the immune system, especially by modulating the innate/inflammatory immune response mediated by phagocytes, such as monocytes and macrophages, both in health and inflammatory conditions [5, 88-90]. Immune-neuroendocrine interactions involving the HPA axis, SNS, and macrophages during exercise can be different in healthy people, in people suffering from inflammatory diseases, and/or after pathogen challenge. In healthy people, exercise increases the release of catecholamines that can inhibit the production of inflammatory cytokines by lymphocytes and macrophages and, in turn, stimulates the innate function of macrophages against pathogens. These innate/inflammatory responses to exercise explain why exercise prevents, with the participation of catecholamines and β adrenergic receptors, the overproduction of inflammatory mediators without immunocompromising the organism against infectious pathogens [35]. In fact, it is currently accepted that the beneficial effects of exercise, particularly in obese individuals, can be exerted through its anti-inflammatory effects, which are mediated through a decrease of the percentage of cells with inflammatory profile and an increase in NA levels [90]. Different studies indicate that regular physical exercise alters the inflammatory profile of monocytes and macrophages in obesity [84, 90]. Thus, it has been suggested that exercise, apart from inducing an overexpression of β adrenergic receptors in immune cells [91], causes a reduction in the expression of TLR receptors, the deregulation of cytokine production, the percentage of CD14+ CD16+ monocytes, and adipose tissue inflammation (by suppression of macrophage infiltration and switch from M1 to M2 phenotype). All of these mechanisms may be involved in the reduction of chronic inflammation and other beneficial effects of physical exercise in obese individuals [86, 90, 92-95].
However, exercise-induced immuno-neuroendocrine responses can be altered in diseases involving inflammatory dysregulations, such as obesity [35]. Thus, the impact of obesity on the adrenergic regulation of the immune system, and the influence of physical activity on the mechanisms underlying this regulation in obese individuals have not yet been completely elucidated, especially considering that inadequate exercise intensity in obese individuals (even if that intensity is adequate for healthy individuals) could induce even greater dysregulation of inflammatory and stress responses, that can, in turn, induce an enhancement of the hyperglycemic state [20, 59], and an increase in macrophage infiltration and TNF-α expression in the adipose tissue [96, 97]. Then, an optimal exercise program with an appropriate intensity and duration must be aimed at achieving a decrease in “unhealthy” levels of inflammatory mediators (such as pro-inflammatory cytokines involved in the pathophysiology of obesity) accompanied by optimal phagocytic and microbicidal activities, as well as good phenotypic transitions between M1 and M2 macrophages, and between inflammatory and classical monocytes. These innate/inflammatory and stress responses, adapted to each individual's basal set-point, constitute what has been defined as the “bioregulatory effect of exercise” [35].
As pointed out above, changes in the recruitment and activation of macrophages contribute crucially to the regulation of metabolic homeostasis, with a suggested pathogenic role for M1 and a protective role for M2 in experimental models of obesity and metabolic disease [60]. Although the existence of the M1 and M2 macrophage subtypes has been established in mice [72, 98], it has not yet been fully confirmed in human adipose tissue, where macrophages appear to be a mix of the M1 and M2 phenotypes [99]. The importance of thoroughly analyzing the density and inflammatory profile of infiltrated macrophages (and their relationship with the inflammatory profile of peripheral blood monocytes) is critical when evaluating the potential SNS and β adrenergic-modulated anti-inflammatory effects of exercise. Macrophages with a pro-inflammatory profile present the membrane marker CD11c [100]. However, in humans, the pro- or anti-inflammatory effect in inflamed adipose tissue is more related to the absence or presence of the CD206 marker. CD206 is essentially considered as a marker of M2 macrophages, and it is expressed in all CD11c- macrophages (CD11c+ macrophages present low fluorescence intensity for CD206) [99]. In addition, since CD206+ M2-like macrophages are clear regulators of systemic glucose homeostasis, labelling of M2 macrophages using CD206 antibodies is considered more relevant in the context of adipose tissue macrophages (ATM). CD206- macrophages present higher levels of CD11c, TNF-α, and IL-6 than CD206+ macrophages, suggesting that CD206- macrophages can be considered M1 (pro-inflammatory) macrophages. Altogether, it has been suggested that CD206 is the most appropriate marker to identify macrophages in adipose tissue [101]. Necrotic adipocytes become surrounded by macrophages that fuse to form syncytia that sequester and scavenge the residual adipocyte lipid droplet, giving rise to characteristic crown-like structures (CLS). Finally, they will form multinucleated giant cells (MGCs), a hallmark of inflammation [102]. These macrophages present a fundamentally pro-inflammatory profile that includes the membrane marker CD11c [100]. Therefore, these CLS are a hallmark of obesity and obesity-associated chronic inflammation [103]. In fact, they have been proposed as markers of insulin resistance [99]. Since physical exercise is a strategy against inflammation by inducing potential anti-inflammatory effects, recent studies in our laboratory have observed that obese mice clearly had a greater number of CLS than lean mice, and that obese mice subjected to regular exercise presented a lower number of CLS in the adipose tissue, whereas, paradoxically, exercise seems to induce an increase in CLS in lean healthy mice1. This phenomenon of exercise-induced local anti-inflammatory responses occurring only in obese animals, but not in lean animals (which even presented a pro-inflammatory response), has also been previously observed systemically in inflammation-related pathologies, including metabolic syndrome, after single sessions of acute exercise [19, 104]. This potential anti-inflammatory responses induced after acute exercise (or in response to the β2 adrenoreceptor agonist terbutaline) in obese animals do not impair the decreased phagocytic capacity of their macrophages and monocytes, since exercise increased the phagocytic activity of both monocytes and macrophages, and exercise even increased the phagocytic capacity in response to terbutaline in monocytes [105, 106]. These recent preliminary results seem to show that physical exercise can modify the innate immune response against pathogens in phagocytes after β2 adrenergic stimulation. In this context, very recent results2,3 also seem to suggest that regular exercise prevents the β2 adrenergic-induced decrease in the phagocytic and microbicidal capacities of monocytes and macrophages, and further studies are being focused on this direction.
Therefore, it is clear that anti-inflammatory strategies (non-pharmacological ones such as exercise, or pharmacological ones such as β2 adrenergic stimulation in individuals who may have some difficulties adhering to adequate exercise programs) are crucial in the management of obesity and its associated comorbidities. In these situations, the question we now have to ask is whether the inflammatory response (assessed through the inflammatory profile of monocytes and macrophages and their inflammatory cytokine production capacity) induced by catecholamines or other β2 adrenergic agonists is the same in obese individuals and healthy individuals, and if exercise interferes in the same way in both physiological conditions (Fig. 1). Studies conducted in an animal model of “genetic obesity” (obese Zucker rats) have observed alterations in the inflammatory cytokine production profile by macrophages and in the response of these cells to NA, thus corroborating deregulation in the local neuroimmunoendocrine response, which could be in turn reverted by regular or acute physical exercise. For example, NA induced a decrease in the release of IL-6 by macrophages from healthy lean rats but stimulated it in obese rats, and also inhibited the release of this inflammatory cytokine in macrophages from exercised obese rats [19]. NA also inhibited the release of IFN-γ by peritoneal leukocytes (macrophages and lymphocytes) from lean and obese sedentary animals but increased it in obese trained rats. In addition, after acute exercise, NA also caused a decrease in the release of IFN-γ by peritoneal macrophages from obese trained animals [107].
Fig. (1).
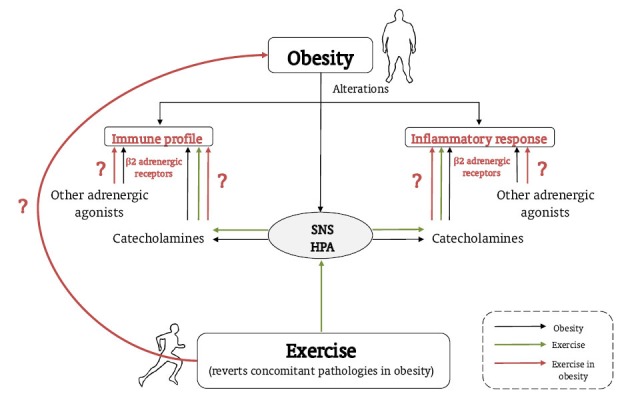
Role of obesity, and exercise in this condition, in the regulation of the innate/inflammatory responses by catecholamines and β2 adrenergic receptors. Obesity is associated with alterations in the inflammatory response, immune profile, SNS, and HPA axis (lines in black). Exercise reverts concomitant pathologies in obesity and activates the SNS and HPA axis, thus modulating the inflammatory response and immune profile via adrenergic regulation of the immune system (dashed lines). It is necessary to clarify how the effect of exercise on immune/innate responses is regulated by catecholamines and β2 adrenergic receptors in obesity (lines in grey).
Monocytes also exhibit high β2 adrenergic receptor expression and respond effectively to its activation [82]. It is well known that, in stimulated monocytes, β adrenergic agonists generally inhibit the production of pro-inflammatory mediators while increasing the secretion of IL-10, one of the cytokines with greater anti-inflammatory activity [26, 30]. However, it is not clear whether this and other inflammatory responses (both in the presence and in the absence of antigenic stimulation with LPS) may also be affected by obesity, and exercise, in turn, may interfere with inflammatory responses in obesity. In our opinion, it is crucial to focus new studies on this topic, analyzing whether the desired inflammatory responses induced by exercise or by the administration of β2 adrenergic agonists are really optimal in obese individuals, in the absence or presence of adherence to regular physical exercise programs; all of this with the purpose of attaining a better personalized prescription for both strategies for the management of obesity and its associated problems. Furthermore, although the use of adrenergic agonists as protectors against renal diabetic and cardiovascular disorders has been proposed due to the anti-inflammatory effects of these agonists on macrophages [75], it is also very important to bear in mind that these drugs are not selective for the immune system and can also exert effects on different organs of the body, with an important role in cardiac activity and glucose metabolism. In this context, β2 adrenergic specific effects on the pancreas can provoke an increase in insulin and glucagon secretions, also affecting hepatic glucose production and uptake of glucose into muscle. Thus, it has been proposed that hypoglycemia can affect β agonist sensitivity and, conversely, β agonists could play a role in the treatment or prevention of hypoglycemia [108], which can be physiologically relevant during exercise practice.
CONCLUSION AND FUTURE DIRECTIONS
Considering all the aspects put forth in this article, it clearly seems necessary to develop more studies that contribute to widening the knowledge of the neuroimmune regulation process in obesity, and how regular and adequate physical exercise, widely accepted as a non-pharmacological therapy, interferes with this regulation. Such knowledge can even contribute to the development of new combination therapeutic strategies (pharmacological and non-pharmacological ones) for the treatment of inflammatory comorbidities associated with obesity. This is especially relevant considering the current socio-economic situation of developed countries, in which, the prevalence of this pathology associated with aging and demographic change has increased dramatically. Thus, it is necessary to improve cost-benefit ratios in pharmacological treatments, and to be more effective in the prescription of physical exercise by health professionals, always taking into account that this prescription must be rigorous and appropriate for each person and physiological state in terms of intensity, duration and regularity. Indeed, it is very important to avoid potential adverse or undesired effects of inadequate physical exercise modalities, particularly if they are combined with anti-inflammatory pharmacological strategies via stimulation of β2 adrenergic receptors. As stated in this article, the response of these receptors may be different in inflammatory states at baseline, such as in obesity, compared to healthy individuals; and physical exercise can also clearly interfere with these responses. The homeostatic/homeorrhetic restoration of the “bioregulatory responses”, that is, the decrease of the excess inflammatory response together with the stimulation or non-deterioration of the defensive innate response, should be the ultimate goal in the context of the immune system; but also with particular attention in avoiding any glucose homeostasis dysregulation and any negative effect on cardiac activity.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- ARG1
Type-1 arginase
- CLS
Crown-like structures
- CNS
Central nervous system
- DM2
Diabetes mellitus type 2
- FFA
Free fatty acids
- Gs
Stimulatory G protein
- HMPG
4-hydroxy-3-methoxyphenyl-glycol
- HPA
Hypothalamus-pituitary-adrenal
- iNOS
Inducible nitric oxide synthase
- LPS
Lipopolysaccharide
- MGC
Multinucleated giant cell
- NA
Noradrenaline
- PKA
Protein kinase A
- SNS
Sympathetic nervous system
- TLR
Toll-like receptor
AUTHOR'S CONTRIBUTIONS
E. Ortega conceived, designed, and wrote the review. I. Gálvez together with E. Ortega contributed equally to the writing of the paper. L. Martín-Cordero collaborated in the writing and critically revised the paper. All the authors approved the final manuscript to be published.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This investigation was partially supported by the Ministerio de Economía y Competitividad (DEP2015-66093-R) and the Gobierno de Extremadura-FEDER. I. Gálvez is recipient of a “Formación del Profesorado Universitario (FPU)” predoctoral contract (FPU15/02395) from the Ministerio de Educación, Cultura y Deporte, Spain.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
NOTES
1 Gálvez, I.; Morán-Plata, F.J.; Martín-Cordero, L.; Hinchado, M.D.; Francisco-Morcillo, J.; Ortega, E. Reduction in macrophages forming crown-like structures in the white adipose tissue of C57BL/6J obese mice after regular exercise. Abstracts of the XXXIX Congress of the Spanish Society of Physiological Sciences (SECF), 2018, pp. 45
2 Gálvez, I.; Martín-Cordero, L.; M.D. Hinchado, M.D.; Ortega, E. Influence of obesity and habitual exercise on the β2 adrenergic regulation of phagocytic and microbicide capacity of circulating monocytes from C57BL/6J mice. Abstracts of the XXXIX Congress of the Spanish Society of Physiological Sciences (SECF), 2018, pp. 44.
3 Martín-Cordero, L.; Gálvez, I.; Hinchado, M.D.; Ortega, E. Influence of obesity and habitual exercise on the β2 adrenergic regulation of phagocytic and microbicide capacity of macrophages from C57BL/6J mice. Abstracts of the XXXIX Congress of the Spanish Society of Physiological Sciences (SECF), 2018, pp. 44.
REFERENCES
- 1.Madden L.T., Livnat S. 2nd ed; Ader, R.; Felten, D.L.; Cohen, N., Eds.; Psychoneuroimmunology. Academic Press. 1991. Catecholamine action and immuno-logic reactivity., pp. 283–310. [DOI] [Google Scholar]
- 2.Madden K.S., Sanders V.M., Felten D.L. Catecholamine influences and sympathetic neural modulation of immune responsiveness. Annu. Rev. Pharmacol. Toxicol. 1995;35:417–448. doi: 10.1146/annurev.pa.35.040195.002221. [DOI] [PubMed] [Google Scholar]
- 3.Sanders V.M. Interdisciplinary research: noradrenergic regulation of adaptive immunity. Brain Behav. Immun. 2006;20(1):1–8. doi: 10.1016/j.bbi.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Besedovsky H.O., Rey A.D. Physiology of psychoneuroimmunology: a personal view. Brain Behav. Immun. 2007;21(1):34–44. doi: 10.1016/j.bbi.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 5.Ortega E., Giraldo E., Hinchado M.D., Martín L., García J.J., De la Fuente M. Neuroimmunomodulation during exercise: role of catecholamines as ‘stress mediator’ and/or ‘danger signal’ for the innate immune response. Neuroimmunomodulation. 2007;14(3-4):206–212. doi: 10.1159/000110648. [DOI] [PubMed] [Google Scholar]
- 6.Scanzano A., Cosentino M. Adrenergic regulation of innate immunity: a review. Front. Pharmacol. 2015;6:171. doi: 10.3389/fphar.2015.00171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Madden K.S. Catecholamines, sympathetic innervation, and immunity. Brain Behav. Immun. 2003;17(Suppl. 1):S5–S10. doi: 10.1016/S0889-1591(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 8.García J.J., del Carmen Sáez M., De la Fuente M., Ortega E. Noradrenaline and its end metabolite 3-methoxy-4-hydroxyphenylglycol inhibit lymphocyte chemotaxis: role of alpha- and beta-adrenoreceptors. Mol. Cell. Biochem. 2003;254(1-2):305–309. doi: 10.1023/A:1027349904589. [DOI] [PubMed] [Google Scholar]
- 9.Elenkov I.J., Wilder R.L., Chrousos G.P., Vizi E.S. The sympathetic nerve--an integrative interface between two supersystems: the brain and the immune system. Pharmacol. Rev. 2000;52(4):595–638. [PubMed] [Google Scholar]
- 10.Elenkov I.J. Del Rey, A.; Chrousos, G.P.; Besedovsky, O.H., Eds.; Elsevier. The Hypothalamus–Pituitary–Adrenal Axis. 2008. Effects of catecholamines on the immune response. pp. 189–206. [Google Scholar]
- 11.Cosentino M., Marino F. In: Nerve driven immunity: Noradren-aline and Adrenaline. InNerve-Driven Immunity. Levite M., editor. Wien: Springer-Verlag; 2012. pp. 47–96. [DOI] [Google Scholar]
- 12.García J.J., del Carmen Sáez M., De la Fuente M., Ortega E. Regulation of phagocytic process of macrophages by noradrenaline and its end metabolite 4-hydroxy-3-metoxyphenyl-glycol. Role of alpha- and beta-adrenoreceptors. Mol. Cell. Biochem. 2003;254(1-2):299–304. doi: 10.1023/A:1027345820519. [DOI] [PubMed] [Google Scholar]
- 13.Ortega E., Giraldo E., Hinchado M.D., Martín-Cordero L., García J.J. In: 72 kDa extracellular heat shock protein (eHsp72), norepinephrine (NE), and the innate immune response fol-lowing moderate exercise. InHeat Shock Proteins and Whole Body Physiology; Asea, A. Pedersen B.K., editor. USA: Springer; 2010. pp. 329–350. [Google Scholar]
- 14.Ortega E., García J.J., De la Fuente M. Modulation of adherence and chemotaxis of macrophages by norepinephrine. Influence of ageing. Mol. Cell. Biochem. 2000;203(1-2):113–117. doi: 10.1023/A:1007094614047. [DOI] [PubMed] [Google Scholar]
- 15.Ortega E., García J.J., Sáez M.C., De la Fuente M. Changes with aging in the modulation of macrophages by norepinephrine. Mech. Ageing Dev. 2000;118(3):103–114. doi: 10.1016/S0047-6374(00)00160-3. [DOI] [PubMed] [Google Scholar]
- 16.Saez M.C., Garcia J.J., De la Fuente M., Ortega E. Modulation of superoxide anion levels of macrophages from young-adult and old mice by the norepinephrine metabolite, 4-hydroxy-3-methoxyphenyl-glycol. Exp. Gerontol. 2002;37(2-3):395–400. doi: 10.1016/S0531-5565(01)00206-6. [DOI] [PubMed] [Google Scholar]
- 17.Elenkov I.J., Chrousos G.P. Stress hormones, Th1/Th2 patterns. Pro/anti-inflammatory cytokines and susceptibility to disease. Trends Endocrinol. Metab. 1999;10(9):359–368. doi: 10.1016/S1043-2760(99)00188-5. [DOI] [PubMed] [Google Scholar]
- 18.Elenkov I.J., Chrousos G.P. Stress hormones, proinflammatory and antiinflammatory cytokines, and autoimmunity. Ann. N. Y. Acad. Sci. 2002;966:290–303. doi: 10.1111/j.1749-6632.2002.tb04229.x. [DOI] [PubMed] [Google Scholar]
- 19.Martín-Cordero L., García J.J., Ortega E. Noradrenaline-mediated inhibition of inflammatory cytokines is altered in macrophages from obese Zucker rats: effect of habitual exercise. Endocr. Metab. Immune Disord. Drug Targets. 2013;13(3):234–239. doi: 10.2174/18715303113139990035. [DOI] [PubMed] [Google Scholar]
- 20.Martín-Cordero L., García J.J., Hinchado M.D., Ortega E. The interleukin-6 and noradrenaline mediated inflammation-stress feedback mechanism is dysregulated in metabolic syndrome: effect of exercise. Cardiovasc. Diabetol. 2011;10:42. doi: 10.1186/1475-2840-10-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang X., Hartung J.E., Bortsov A.V., Kim S., O’Buckley S.C., Kozlowski J., Nackley A.G. Sustained stimulation of β2- and β3-adrenergic receptors leads to persistent functional pain and neuroinflammation. Brain Behav. Immun. 2018;73:520–532. doi: 10.1016/j.bbi.2018.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortega Rincón E. Physiology and biochemistry: influence of exercise on phagocytosis. Int. J. Sports Med. 1994;15(Suppl. 3):S172–S178. doi: 10.1055/s-2007-1021133. [DOI] [PubMed] [Google Scholar]
- 23.Nance D.M., Sanders V.M. Autonomic innervation and regulation of the immune system (1987-2007). Brain Behav. Immun. 2007;21(6):736–745. doi: 10.1016/j.bbi.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berkowitz D.E., Nardone N.A., Smiley R.M., Price D.T., Kreutter D.K., Fremeau R.T., Schwinn D.A. Distribution of beta 3-adrenoceptor mRNA in human tissues. Eur. J. Pharmacol. 1995;289(2):223–228. doi: 10.1016/0922-4106(95)90098-5. [DOI] [PubMed] [Google Scholar]
- 25.Kalinichenko V.V., Mokyr M.B., Graf L.H., Jr, Cohen R.L., Chambers D.A. Norepinephrine-mediated inhibition of anti-tumor cytotoxic T lymphocyte generation involves a beta-adrenergic receptor mechanism and decreased TNF-alpha gene expression. J. Immunol. 1999;163:2492–2499. [PubMed] [Google Scholar]
- 26.Farmer P., Pugin J. beta-adrenergic agonists exert their “anti-inflammatory” effects in monocytic cells through the IkappaB/NF-kappaB pathway. Am. J. Physiol. Lung Cell. Mol. Physiol. 2000;279(4):L675–L682. doi: 10.1152/ajplung.2000.279.4.L675. [DOI] [PubMed] [Google Scholar]
- 27.Hetier E., Ayala J., Bousseau A., Prochiantz A. Modulation of interleukin-1 and tumor necrosis factor expression by beta-adrenergic agonists in mouse ameboid microglial cells. Exp. Brain Res. 1991;86(2):407–413. doi: 10.1007/BF00228965. [DOI] [PubMed] [Google Scholar]
- 28.Severn A., Rapson N.T., Hunter C.A., Liew F.Y. Regulation of tumor necrosis factor production by adrenaline and beta-adrenergic agonists. J. Immunol. 1992;148(11):3441–3445. [PubMed] [Google Scholar]
- 29.Nakamura A., Johns E.J., Imaizumi A., Abe T., Kohsaka T. Regulation of tumour necrosis factor and interleukin-6 gene transcription by beta2-adrenoceptor in the rat astrocytes. J. Neuroimmunol. 1998;88(1-2):144–153. doi: 10.1016/S0165-5728(98)00109-X. [DOI] [PubMed] [Google Scholar]
- 30.Ağaç D., Estrada L.D., Maples R., Hooper L.V., Farrar J.D. The β2-adrenergic receptor controls inflammation by driving rapid IL-10 secretion. Brain Behav. Immun. 2018;74:176–185. doi: 10.1016/j.bbi.2018.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spengler R.N., Allen R.M., Remick D.G., Strieter R.M., Kunkel S.L. Stimulation of alpha-adrenergic receptor augments the production of macrophage-derived tumor necrosis factor. J. Immunol. 1990;145(5):1430–1434. [PubMed] [Google Scholar]
- 32.Spengler R.N., Chensue S.W., Giacherio D.A., Blenk N., Kunkel S.L. Endogenous norepinephrine regulates tumor necrosis factor-alpha production from macrophages in vitro. J. Immunol. 1994;152(6):3024–3031. [PubMed] [Google Scholar]
- 33.Flierl M.A., Rittirsch D., Nadeau B.A., Chen A.J., Sarma J.V., Zetoune F.S., McGuire S.R., List R.P., Day D.E., Hoesel L.M., Gao H., Van Rooijen N., Huber-Lang M.S., Neubig R.R., Ward P.A. Phagocyte-derived catecholamines enhance acute inflammatory injury. Nature. 2007;449(7163):721–725. doi: 10.1038/nature06185. [DOI] [PubMed] [Google Scholar]
- 34.Flierl M.A., Rittirsch D., Huber-Lang M., Sarma J.V., Ward P.A. Catecholamines-crafty weapons in the inflammatory arsenal of immune/inflammatory cells or opening pandora’s box? Mol. Med. 2008;14(3-4):195–204. doi: 10.2119/2007-00105.Flierl. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ortega E. The “bioregulatory effect of exercise” on the innate/inflammatory responses. J. Physiol. Biochem. 2016;72(2):361–369. doi: 10.1007/s13105-016-0478-4. [DOI] [PubMed] [Google Scholar]
- 36.Pires-Lapa M.A., Carvalho-Sousa C.E., Cecon E., Fernandes P.A., Markus R.P. β-Adrenoceptors trigger melatonin synthesis in phagocytes. Int. J. Mol. Sci. 2018;19(8):E2182. doi: 10.3390/ijms19082182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Flierl M.A., Rittirsch D., Nadeau B.A., Sarma J.V., Day D.E., Lentsch A.B., Huber-Lang M.S., Ward P.A. Upregulation of phagocyte-derived catecholamines augments the acute inflammatory response. PLoS One. 2009;4(2):e4414. doi: 10.1371/journal.pone.0004414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bosmann M., Grailer J.J., Zhu K., Matthay M.A., Sarma J.V., Zetoune F.S., Ward P.A. Anti-inflammatory effects of β2 adrenergic receptor agonists in experimental acute lung injury. FASEB J. 2012;26(5):2137–2144. doi: 10.1096/fj.11-201640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tang S.T., Su H., Zhang Q., Tang H.Q., Wang C.J., Zhou Q., Wei W., Zhu H.Q., Wang Y. Melatonin attenuates aortic endothelial permeability and arteriosclerosis in streptozoto-cin-induced diabetic rats: Possible role of MLCK- and MLCP-dependent MLC phosphorylation. J. Cardiovasc. Pharmacol. Ther. 2016;21(1):82–92. doi: 10.1177/1074248415583090. [DOI] [PubMed] [Google Scholar]
- 40.Assis de Brito T.L., Monte-Alto-Costa A., Romana-Souza B. Propranolol impairs the closure of pressure ulcers in mice. Life Sci. 2014;100(2):138–146. doi: 10.1016/j.lfs.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 41.Giraldo E., Multhoff G., Ortega E. Noradrenaline increases the expression and release of Hsp72 by human neutrophils. Brain Behav. Immun. 2010;24(4):672–677. doi: 10.1016/j.bbi.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 42.Giraldo E., Hinchado M.D., Ortega E. Combined activity of post-exercise concentrations of NA and eHsp72 on human neutrophil function: role of cAMP. J. Cell. Physiol. 2013;228(9):1902–1906. doi: 10.1002/jcp.24354. [DOI] [PubMed] [Google Scholar]
- 43.Hinchado M.D., Giraldo E., Ortega E. Adrenoreceptors are involved in the stimulation of neutrophils by exercise-induced circulating concentrations of Hsp72: cAMP as a potential “intracellular danger signal”. J. Cell. Physiol. 2012;227(2):604–608. doi: 10.1002/jcp.22759. [DOI] [PubMed] [Google Scholar]
- 44.Bacou E., Haurogné K., Allard M., Mignot G., Bach J.M., Hervé J., Lieubeau B. β2-adrenoreceptor stimulation dampens the LPS-induced M1 polarization in pig macrophages. Dev. Comp. Immunol. 2017;76:169–176. doi: 10.1016/j.dci.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 45.Grailer J.J., Haggadone M.D., Sarma J.V., Zetoune F.S., Ward P.A. Induction of M2 regulatory macrophages through the β2-adrenergic receptor with protection during endotoxemia and acute lung injury. J. Innate Immun. 2014;6(5):607–618. doi: 10.1159/000358524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.World Health Organization (WHO) Obesity and overweight. 2017 http://www.who.int/es/news-room/fact-sheets/detail/obesity-and-overweight/ (Accessed May 2018).
- 47.Johnson A.R., Milner J.J., Makowski L. The inflammation highway: metabolism accelerates inflammatory traffic in obesity. Immunol. Rev. 2012;249(1):218–238. doi: 10.1111/j.1600-065X.2012.01151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lumeng C.N. Innate immune activation in obesity. Mol. Aspects Med. 2013;34(1):12–29. doi: 10.1016/j.mam.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McClean K.M., Kee F., Young I.S., Elborn J.S. Obesity and the lung: 1. Epidemiology. Thorax. 2008;63(7):649–654. doi: 10.1136/thx.2007.086801. [DOI] [PubMed] [Google Scholar]
- 50.Baumann S., Lorentz A. Obesity - a promoter of allergy? Int. Arch. Allergy Immunol. 2013;162(3):205–213. doi: 10.1159/000353972. [DOI] [PubMed] [Google Scholar]
- 51.Strandberg L., Verdrengh M., Enge M., Andersson N., Amu S., Onnheim K., Benrick A., Brisslert M., Bylund J., Bokarewa M., Nilsson S., Jansson J.O. Mice chronically fed high-fat diet have increased mortality and disturbed immune response in sepsis. PLoS One. 2009;4(10):e7605. doi: 10.1371/journal.pone.0007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin-Cordero L., Garcia J.J., Giraldo E., De la Fuente M., Manso R., Ortega E. Influence of exercise on the circulating levels and macrophage production of IL-1beta and IFNgamma affected by metabolic syndrome: an obese Zucker rat experimental animal model. Eur. J. Appl. Physiol. 2009;107(5):535–543. doi: 10.1007/s00421-009-1140-4. [DOI] [PubMed] [Google Scholar]
- 53.Martín-Cordero L., García J.J., Hinchado M.D., Bote E., Manso R., Ortega E. Habitual physical exercise improves macrophage IL-6 and TNF-α deregulated release in the obese zucker rat model of the metabolic syndrome. Neuroimmunomodulation. 2011;18(2):123–130. doi: 10.1159/000322053. [DOI] [PubMed] [Google Scholar]
- 54.Huttunen R., Syrjänen J. Obesity and the risk and outcome of infection. Int. J. Obes. 2013;37(3):333–340. doi: 10.1038/ijo.2012.62. [DOI] [PubMed] [Google Scholar]
- 55.Amar S., Zhou Q., Shaik-Dasthagirisaheb Y., Leeman S. Diet-induced obesity in mice causes changes in immune responses and bone loss manifested by bacterial challenge. Proc. Natl. Acad. Sci. USA. 2007;104(51):20466–20471. doi: 10.1073/pnas.0710335105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Trottier M.D., Naaz A., Li Y., Fraker P.J. Enhancement of hematopoiesis and lymphopoiesis in diet-induced obese mice. Proc. Natl. Acad. Sci. USA. 2012;109(20):7622–7629. doi: 10.1073/pnas.1205129109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gonzalez-Quintela A., Alonso M., Campos J., Vizcaino L., Loidi L., Gude F. Determinants of serum concentrations of lipopolysaccharide-binding protein (LBP) in the adult population: the role of obesity. PLoS One. 2013;8(1):e54600. doi: 10.1371/journal.pone.0054600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Loera-Rodriguez C.O., Delgado-Rizo V., Alvarado-Navarro A., Agraz-Cibrian J.M., Segura-Ortega J.E., Fafutis-Morris M. Over-expression of TLR4-CD14, pro-inflammatory cytokines, metabolic markers and NEFAs in obese non-diabetic Mexicans. J. Inflamm. (Lond.) 2014;11(1):39. doi: 10.1186/s12950-014-0039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ortega E., Martín-Cordero L., García-Roves P.M., Chicco A.J., González-Franquesa A., Marado D. In: Diabetes Mellitus and Metabolic Syndrome.Biomarkers of Cardiometabolic Risk, Inflammation and Disease; Palavra, F.; Reis, F.; Marado, D. Sena A., editor. Springer International Publishing Switzer-land; 2015. pp. 55–80. [Google Scholar]
- 60.Chawla A., Nguyen K.D., Goh Y.P. Macrophage-mediated inflammation in metabolic disease. Nat. Rev. Immunol. 2011;11(11):738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jin C., Henao-Mejia J., Flavell R.A. Innate immune receptors: key regulators of metabolic disease progression. Cell Metab. 2013;17(6):873–882. doi: 10.1016/j.cmet.2013.05.011. [DOI] [PubMed] [Google Scholar]
- 62.Wellen K.E., Hotamisligil G.S. Obesity-induced inflammatory changes in adipose tissue. J. Clin. Invest. 2003;112(12):1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wellen K.E., Hotamisligil G.S. Inflammation, stress, and diabetes. J. Clin. Invest. 2005;115(5):1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rogacev K.S., Ulrich C., Blömer L., Hornof F., Oster K., Ziegelin M., Cremers B., Grenner Y., Geisel J., Schlitt A., Köhler H., Fliser D., Girndt M., Heine G.H. Monocyte heterogeneity in obesity and subclinical atherosclerosis. Eur. Heart J. 2010;31(3):369–376. doi: 10.1093/eurheartj/ehp308. [DOI] [PubMed] [Google Scholar]
- 65.Shi C., Pamer E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011;11(11):762–774. doi: 10.1038/nri3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ziegler-Heitbrock L. The CD14+ CD16+ blood monocytes: their role in infection and inflammation. J. Leukoc. Biol. 2007;81(3):584–592. doi: 10.1189/jlb.0806510. [DOI] [PubMed] [Google Scholar]
- 67.Poitou C., Dalmas E., Renovato M., Benhamo V., Hajduch F., Abdennour M., Kahn J.F., Veyrie N., Rizkalla S., Fridman W.H., Sautès-Fridman C., Clément K., Cremer I. CD14dimCD16+ and CD14+CD16+ monocytes in obesity and during weight loss: relationships with fat mass and subclinical atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2011;31(10):2322–2330. doi: 10.1161/ATVBAHA.111.230979. [DOI] [PubMed] [Google Scholar]
- 68.Krinninger P., Ensenauer R., Ehlers K., Rauh K., Stoll J., Krauss-Etschmann S., Hauner H., Laumen H. Peripheral monocytes of obese women display increased chemokine receptor expression and migration capacity. J. Clin. Endocrinol. Metab. 2014;99:2500–2509. doi: 10.1210/jc.2013-2611. [DOI] [PubMed] [Google Scholar]
- 69.Devêvre E.F., Renovato-Martins M., Clément K., Sautès-Fridman C., Cremer I., Poitou C. Profiling of the three circulating monocyte subpopulations in human obesity. J. Immunol. 2015;194(8):3917–3923. doi: 10.4049/jimmunol.1402655. [DOI] [PubMed] [Google Scholar]
- 70.Rose S., Misharin A., Perlman H. A novel Ly6C/Ly6G-based strategy to analyze the mouse splenic myeloid compartment. Cytometry A. 2012;81(4):343–350. doi: 10.1002/cyto.a.22012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murray P.J., Wynn T.A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 2011;11(11):723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fujisaka S., Usui I., Bukhari A., Ikutani M., Oya T., Kanatani Y., Tsuneyama K., Nagai Y., Takatsu K., Urakaze M., Kobayashi M., Tobe K. Regulatory mechanisms for adipose tissue M1 and M2 macrophages in diet-induced obese mice. Diabetes. 2009;58(11):2574–2582. doi: 10.2337/db08-1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee B.C., Lee J. Cellular and molecular players in adipose tissue inflammation in the development of obesity-induced insulin resistance. Biochim. Biophys. Acta. 2014;1842(3):446–462. doi: 10.1016/j.bbadis.2013.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Galvan D.L., Danesh F.R. β2-adrenergic receptors in inflammation and vascular complications of diabetes. Kidney Int. 2017;92(1):14–16. doi: 10.1016/j.kint.2017.03.024. [DOI] [PubMed] [Google Scholar]
- 75.Noh H., Yu M.R., Kim H.J., Lee J.H., Park B.W., Wu I.H., Matsumoto M., King G.L. Beta 2-adrenergic receptor agonists are novel regulators of macrophage activation in diabetic renal and cardiovascular complications. Kidney Int. 2017;92(1):101–113. doi: 10.1016/j.kint.2017.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rudyk M.P., Pozur V.V., Voieikova D.O., Hurmach Y.V., Khranovska N.M., Skachkova O.V., Svyatetska V.M., Fedorchuk O.G., Skivka L.M., Berehova T.V., Ostapchenko L.I. Sex-based differences in phagocyte metabolic profile in rats with monosodium glutamate-induced obesity. Sci. Rep. 2018;8(1):5419. doi: 10.1038/s41598-018-23664-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sitkauskiene B., Sakalauskas R. The role of beta(2)-adrenergic receptors in inflammation and allergy. Curr. Drug Targets Inflamm. Allergy. 2005;4(2):157–162. doi: 10.2174/1568010053586309. [DOI] [PubMed] [Google Scholar]
- 78.Pasquali R., Vicennati V., Cacciari M., Pagotto U. The hypothalamic-pituitary-adrenal axis activity in obesity and the metabolic syndrome. Ann. N. Y. Acad. Sci. 2006;1083:111–128. doi: 10.1196/annals.1367.009. [DOI] [PubMed] [Google Scholar]
- 79.Lambert G.W., Straznicky N.E., Lambert E.A., Dixon J.B., Schlaich M.P. Sympathetic nervous activation in obesity and the metabolic syndrome--causes, consequences and therapeutic implications. Pharmacol. Ther. 2010;126(2):159–172. doi: 10.1016/j.pharmthera.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 80.Bellinger D.L., Millar B.A., Perez S., Carter J., Wood C. ThyagaRajan, S.; Molinaro, C.; Lubahn, C.; Lorton, D. Sym-pathetic modulation of immunity: relevance to disease. Cell. Immunol. 2008;252:27–56. doi: 10.1016/j.cellimm.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kumar V., Sharma A. Is neuroimmunomodulation a future therapeutic approach for sepsis? Int. Immunopharmacol. 2010;10(1):9–17. doi: 10.1016/j.intimp.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 82.Bellinger D.L., Lorton D. Autonomic regulation of cellular immune function. Auton. Neurosci. 2014;182:15–41. doi: 10.1016/j.autneu.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 83.De Souza C.T., Araujo E.P., Bordin S., Ashimine R., Zollner R.L., Boschero A.C., Saad M.J.A., Velloso L.A. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146(10):4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 84.Zhang X., Zhang G., Zhang H., Karin M., Bai H., Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135(1):61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kawanishi N., Mizokami T., Yano H., Suzuki K. Exercise attenuates M1 macrophages and CD8+ T cells in the adipose tissue of obese mice. Med. Sci. Sports Exerc. 2013;45(9):1684–1693. doi: 10.1249/MSS.0b013e31828ff9c6. [DOI] [PubMed] [Google Scholar]
- 86.You T., Arsenis N.C., Disanzo B.L., Lamonte M.J. Effects of exercise training on chronic inflammation in obesity: current evidence and potential mechanisms. Sports Med. 2013;43(4):243–256. doi: 10.1007/s40279-013-0023-3. [DOI] [PubMed] [Google Scholar]
- 87.Caspersen C.J., Powell K.E., Christenson G.M. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985;100(2):126–131. [PMC free article] [PubMed] [Google Scholar]
- 88.Ortega E. Neuroendocrine mediators in the modulation of phagocytosis by exercise: physiological implications. Exerc. Immunol. Rev. 2003;9:70–93. [PubMed] [Google Scholar]
- 89.Ortega E., García J.J., Bote M.E., Martín-Cordero L., Escalante Y., Saavedra J.M., Northoff H., Giraldo E. Exercise in fibromyalgia and related inflammatory disorders: known effects and unknown chances. Exerc. Immunol. Rev. 2009;15:42–65. [PubMed] [Google Scholar]
- 90.Gleeson M., Bishop N.C., Stensel D.J., Lindley M.R., Mastana S.S., Nimmo M.A. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011;11(9):607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- 91.Maisel A.S., Harris T., Rearden C.A., Michel M.C. Beta-adrenergic receptors in lymphocyte subsets after exercise. Alterations in normal individuals and patients with congestive heart failure. Circulation. 1990;82(6):2003–2010. doi: 10.1161/01.CIR.82.6.2003. [DOI] [PubMed] [Google Scholar]
- 92.Kawanishi N., Yano H., Yokogawa Y., Suzuki K. Exercise training inhibits inflammation in adipose tissue via both suppression of macrophage infiltration and acceleration of phenotypic switching from M1 to M2 macrophages in high-fat-diet-induced obese mice. Exerc. Immunol. Rev. 2010;16:105–118. [PubMed] [Google Scholar]
- 93.Oliveira A.G., Carvalho B.M., Tobar N., Ropelle E.R., Pauli J.R., Bagarolli R.A., Guadagnini D., Carvalheira J.B., Saad M.J. Physical exercise reduces circulating lipopolysaccharide and TLR4 activation and improves insulin signaling in tissues of DIO rats. Diabetes. 2011;60(3):784–796. doi: 10.2337/db09-1907. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 94.Carpenter K.C., Strohacker K., Breslin W.L., Lowder T.W., Agha N.H., McFarlin B.K. Effects of exercise on weight loss and monocytes in obese mice. Comp. Med. 2012;62(1):21–26. [PMC free article] [PubMed] [Google Scholar]
- 95.Huang C.J., Zourdos M.C., Jo E., Ormsbee M.J. Influence of physical activity and nutrition on obesity-related immune function. ScientificWorldJournal. 2013:2013752071. doi: 10.1155/2013/752071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Martín-Cordero L., Reis F., García J.J., Teixeira F., Ortega E. Effect of exercise without diet on functional capacity of peritoneal macrophages and TNF-a levels in blood and in adi-pose tissue in the obese Zucker rat model of the metabolic syndrome. Proc. Nutr. Soc., 2013. 72E76. [DOI]
- 97.Martín-Cordero L., Francisco-Morcillo J., Cintas R., Gálvez I., Ortega E. Increased macrophage infiltration and TNF-αlevels in the adipose tissue of obese Zucker rats after habitual exerciseinduced stress. Annals of research in sport and physical activity. 2018. pp. 245–246.
- 98.Lumeng C.N., Bodzin J.L., Saltiel A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 2007;117(1):175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wentworth J.M., Naselli G., Brown W.A., Doyle L., Phipson B., Smyth G.K., Wabitsch M., O’Brien P.E., Harrison L.C. Pro-inflammatory CD11c+CD206+ adipose tissue macrophages are associated with insulin resistance in human obesity. Diabetes. 2010;59(7):1648–1656. doi: 10.2337/db09-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lumeng C.N., DelProposto J.B., Westcott D.J., Saltiel A.R. Phenotypic switching of adipose tissue macrophages with obesity is generated by spatiotemporal differences in macrophage subtypes. Diabetes. 2008;57(12):3239–3246. doi: 10.2337/db08-0872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nawaz A., Aminuddin A., Kado T., Takikawa A., Yamamoto S., Tsuneyama K., Igarashi Y., Ikutani M., Nishida Y., Nagai Y., Takatsu K., Imura J., Sasahara M., Okazaki Y., Ueki K., Okamura T., Tokuyama K., Ando A., Matsumoto M., Mori H., Nakagawa T., Kobayashi N., Saeki K., Usui I., Fujisaka S., Tobe K. CD206+ M2-like macrophages regulate systemic glucose metabolism by inhibiting proliferation of adipocyte progenitors. Nat. Commun. 2017;8(1):286. doi: 10.1038/s41467-017-00231-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cinti S., Mitchell G., Barbatelli G., Murano I., Ceresi E., Faloia E., Wang S., Fortier M., Greenberg A.S., Obin M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005;46(11):2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 103.Murano I., Barbatelli G., Parisani V., Latini C., Muzzonigro G., Castellucci M., Cinti S. Dead adipocytes, detected as crown-like structures, are prevalent in visceral fat depots of genetically obese mice. J. Lipid Res. 2008;49(7):1562–1568. doi: 10.1194/jlr.M800019-JLR200. [DOI] [PubMed] [Google Scholar]
- 104.Bote M.E., Garcia J.J., Hinchado M.D., Ortega E. Fibromyalgia: anti-inflammatory and stress responses after acute moderate exercise. PLoS One. 2013;8(9):e74524. doi: 10.1371/journal.pone.0074524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gálvez I., Martín-Cordero L., Álvarez-Barrientos A., Ortega E. β2 adrenergic regulation of thephagocytic activity of peritonealmacrophages in obese mice: effectof an acute intense exercise. Annals of research in sport and physical activity, 2018. pp. 249–250.
- 106.Martín-Cordero L., Gálvez I., Álvarez-Barrientos A., Ortega E. β2 adrenergic regulation of the phagocytic activity of monocytes in obese mice:effect of an acute intense exercise. Annals of research in sport and physical activity, 2018. pp. 247–248.
- 107.Martín-Cordero L., García J.J., Hinchado M.D., Bote E., Ortega E. Influence of exercise on NA- and Hsp72-induced release of IFNγ by the peritoneal suspension of macrophages and lymphocytes from genetically obese Zucker rats. J. Physiol. Biochem. 2013;69(1):125–131. doi: 10.1007/s13105-012-0196-5. [DOI] [PubMed] [Google Scholar]
- 108.Philipson L.H. beta-Agonists and metabolism. J. Allergy Clin. Immunol. 2002;110(6) Suppl.:S313–S317. doi: 10.1067/mai.2002.129702. [DOI] [PubMed] [Google Scholar]


