Abstract
Background:
The exact morbidity of myocarditis is unknown, as the treatment is generally delayed in virtue of misdiagnosis or missed diagnosis.
Aim:
The aim of this study was to identify prognostic factors of left-ventricular remodeling on CMRI performed in patients with pathological proven myocarditis.
Methods:
Sixty-two cases with various presentations of myocarditis (39 cases with heart failure; 23 cases with arrhythmias) were selected. All patients, who underwent coronary angiography, endomyocardial biopsy, were divided into positive-remodeling and negative-remodelling groups to analyse LGE and cardiac cine parameters at presentation and subsequent to 3 months.
Results:
Comparison of two subgroups in CMRI is as follows: positive LGE (65.6% vs. 86.7%; p<0.05), LVEF (41.3±14.8% vs. 37.6±10.1%; p=0.62), (25.7±2.0% vs. 24.0±2.5%; p=0.81), (44.5±3.9mm vs. 46.3±5.4mm; p=0.76), (129.1±8.5ml vs. 135.3±12.2ml; p=0.26), (74.8±7.3ml vs. 79.1±10.0ml; p=0.55), (52.0±5.7g vs. 49.6±6.5g; p=0.71), (34.9±3.5ml vs. 32.4±6.2ml; p=0.68), (3.8±0.7L/min vs. 3.1±0.5L/min; p=0.64), (2.9±0.6L/min*m2 vs. 2.7±0.5L/min*m2; p=0.79).
Conclusion:
LGE-MRI is rewarding as an independent predictor in left-ventricular positive and negative remodelling of myocarditis.
Keywords: Myocarditis, remodelling, heart failure, arrhythmias, magnetic resonance imaging, angiography
1. INTRODUCTION
Myocarditis is a life-threatening inflammatory heart disease characterized by myocardial inflammation, cardiac muscle cellular edema, necrosis and fibrosis in myocardial interstitium [1, 2], and disease progression and clinical symptoms are exceedingly variable. It has the potential to mimic acute myocardial infarction when the patient has various clinical symptoms of chest pain, microcirculatory disturbance, ischemia-like electrocardiographic abnormities, biochemical marker abnormities, and left ventricular dysfunction at clinical presentation [3]. Prospective postmortem data have latterly implicated myocarditis as a trigger for sudden cardiac death in up to 12% of young adults and as the causative etiology in approximately 9% of dilated cardiomyopathy [4, 5]. The various infections, systemic diseases, drugs and toxins have been associated with this disease [6]. Viruses are currently considered as the most frequent trigger of myocarditis in Europe and America. Initially, coxsackieviruses were deemed to be the most shared trigger for myocarditis due to the high antibody titres detected in patients during acute and subacute myocarditis. Afterwards, adenoviruses, was likewise identified with endomyocardial biopsies of patients with clinically suspected myocarditis [7]. It is paramount to note that the natural course of myocarditis varies, as do clinical presentation, aetiology, prognosis as well as positive and negative remodeling. As a noninvasive and comprehensive cardiac imaging technique, cardiac magnetic resonance imaging (CMRI), immense potential to identify prognostic factors in remodelling, plays an essential role in the diagnosis and follow-up of myocardial diseases, especially for the simultaneous assessment of cardiovascular anatomy, tissue characterization and cardiac functional analysis in a population of patients with myocarditis [8]. Late Gadolinium Enhancement (LGE) - MRI, recognized as the standard of reference for assessment of myocardial viability and interstitial fibrosis of myocardium, is performed 10 minutes subsequent to injecting gadolinium-based MRI contrast media, which might provide crucial prognostic information on myocarditis and nonischemic cardiomyopathy [9]. Moreover, cardiac cine MRI obtains functional parameters of left ventricular structure such as Left Ventricular Ejection Fraction (LVEF), Fraction Shortening (FS), Left Ventricular End-diastolic Dimension (LVEDD), Left Ventricular End-diastolic Volume (LVEDV), Left Ventricular End-systolic Volume (LVESV), Left Ventricular Myocardial Mass (LVMM), Left Ventricular Stroke Volume (LVSV), Cardiac Output (CO), and Cardiac Index (CI). Cardiac cine MRI, with higher specificity for the detection of left ventricular aneurysm and thrombus, allows increasingly accurate measurement of chamber volumes and ventricular function than echocardiography [10], although echocardiography is the most widely available imaging method at present [11]. The aim at this research was to evaluate the presence of positive and negative remodeling and identify prognostic factors capable of predicting improvement or progression to cardiac function, associated with various clinical presentations and follow-up data in patients with clinically suspected myocarditis subjected to cardiac biopsy.
2. MATERIALS AND METHODS
Between September 2015 and October 2017, we examined 94 consecutive patients with suspected myocarditis in accordance with a combination of clinical signs and symptoms, including chest pain, exhaustion, sweaty and palpitations, as well as 24-hour dynamic electrocardiographic abnormities and serum myocardial damage markers abnormalities, coupled with a history compatible with inflammatory disease, such as sore throat, cough, expectoration, vomiting and diarrhea. On admission, patients with acute coronary-like syndrome were assessed with coronary angiography performed as urgent proceedings, while in all other cases, as optional proceedings. Thirty-two patients were excluded due to coronary artery stenoses >50% at coronary angiography before the biopsy. The research samples consist of sixty-two patients (38 males, 24 females; mean age, 32 years; age range, 14-69 years). All selected patients underwent cardiac catheterisation with cardiac biopsy and MRI with gadolinium, which is a sort of paramagnetic contrast agent. Ultimately, all cases were followed up with LGE-MRI and cardiac cine MRI after 3 months.
3. CARDIAC MAGNETIC RESONANCE IMAGING
The CMRI examination, performed with a whole-body clinical 3.0T MRI system (Philips Achieva TX), was equipped with high-performance gradients. Images were acquired in the four-chamber and two-chamber long-axis views with cine-MRI steady-state free precession sequences and in the short axis plane with black-blood fast spin echo sequences, with double inversion recovery for suppression of the blood signal and an additional inversion recovery pulse for suppression of the fat signal to demonstrate the presence of feasible areas of high signal intensity given rise by oedema. Then a pile of continuous short-axis sections was acquired with cine-MRI sequences to assess regional and global biventricular function kinetics. Subsequent to perfusion imaging, an additional dose of 0.1 mmol/kg of gadolinium-DPTA was administered at a rate of 2.0 ml/s. LGE-MRI images were acquired after 10 minutes with breath-holding for each stack in the utilisation of an IR-prepared and segmented GRE sequence. These quintessential settings were as following: a pile of 8 contiguous slices in the short-axis view with the orientations identical to perfusion imaging, a slice thickness of 10 mm in the absence of an intersection gap, a TR/TE of 6.1/3.0 ms, an FOV of 320 mm, a matrix of 192 × 160 and a flip angle of 25°. Qualitative regional wall motion analysis was performed according to the American Heart Association (AHA) segmental model for 16 segments in short-axis orientation [12]. The outline of endocardial and epicardial contours was delineated in the short-axis planes of the cine-MRI images, for calculating regional contractile function and biventricular global systolic function of left ventricle. All the diagnoses are strictly with reference to Lake Louise Consensus Criteria proposed by the American Journal of Cardiology in 2009 as following [13]:
(A) In the setting of clinically suspected myocarditis, CMR findings are consistent with myocardial inflammation, if at least 2 of the following criteria are present: (a) Regional or global myocardial SI increase in T2-weighted images; (b) Increased global myocardial early gadolinium enhancement ratio between myocardium and skeletal muscle in gadolinium-enhanced T1-weighted images; (c) There is at least 1 focal lesion with nonischemic regional distribution in inversion recovery-prepared gadolinium-enhanced T1-weighted images (LGE).
(B) A CMR study is consistent with myocyte injury and/or scar caused by myocardial inflammation if Criterion (c) above is present.
(C) A repeat CMR study between 1 and 2 weeks after the initial CMR study is recommended if (a) None of the criteria are present, but the onset of symptoms has been very recent and there is strong clinical evidence for myocardial inflammation; (b) One of the criteria is present.
4. ENDOMYOCARDIAL BIOPSY, HISTOLOGY AND IMMUNOHISTOCHEMISTRY
All invasive operations were carried out after the patient’s informed consent. All patients underwent cardiac catheterisation, coronary angiography and myocardial biopsy. The biopsies, 4 to 5 for each ventricle, were obtained in the apical-septal region. Three-fourths of specimens were frozen for molecular studies, while the remaining specimens were fixed with formalin and paraffin. Five to 6 endomyocardial specimens, stained with haematoxylin and eosin, Miller’s elastic van Gieson stain and Masson’s trichrome, were obtained from each patient for histological and immunohistochemical examination and observed under light microscope. Specific stains, such as Ziehl-Nielsen and Giemsa, were also used in the event of intracellular inclusions. The Dallas criteria for histological diagnosis of myocarditis were applied. All specimens were studied by immunohistochemistry for characterization of myocardial inflammatory infiltrates.
5. STATISTICAL ANALYSIS
Statistical analysis was done with the software package SPSS 17.0 for Windows. All quantitative results are expressed in the text, figures and tables. Logarithmic transformation was used for the post hoc test for the linear trend at univariate analysis of variance. Nonparametric test applied for unpaired data (Mann-Whitney U test for comparison of two groups and Kruskal-Wallis test for comparison of more than two). Bonferroni correction applied to the Mann-Whitney U test when comparing different groups.
6. RESULTS
6.1. Clinical Presentations
Distribution of patients across the two principal patterns of clinical presentation was the following: 39 (62.9%) patients with symptoms of heart failure such as chest pain, exhaustion, sweaty and palpitations associated with abnormal marker of myocardial injury; 23 (37.1%) patients with arrhythmias including 34.8% tachycardia sinusale, 21.7% atrial fibrillation, 13.0% ventricular tachycardia, 13.0% continuous sinus tachycardia, 8.7% ventricular premature contraction and 8.7% supraventricular premature beat.
6.2. Histopathological Examinations
The pathological examinations including endomyocardial biopsy, histology and immunohistochemistry confirmed the presence of active myocarditis of all patients. The causative agent was detected exactly in 21 patients.
6.3. Cardiac Cine MRI and LGE-MRI
Patients were followed up subsequent to 3 months by clinical evaluation of cardiac cine MRI. They were divided in the light of the delta LVEF (increased compared with baseline value, cutoff 15%), while delta end-systolic volume LVESV (decreased compared with baseline, cutoff 20%), which intended as a reflection of positive remodeling [14]. Then we detected that those included in the positive-remodelling group had reduced thickness of the lateral and anterior left-ventricular wall, respectively, compared with the negative-remodeling group (lateral 8.9±1.4 mm vs. 12.7±2.8 mm; anterior 9.6±1.6 vs. 13.9±3.1 mm; p<0.05). LGE-MRI was observed in subepicardial myocardium in 75.8% (47/62) of patients (Fig. 1). Analysis of the two subgroups indicated that positive LGE was distinctly statistical difference between the positive-remodelling group and the negative-remodeling group (21/32 vs. 13/15; p<0.05) (Table 1). Comparison of positive-remodelling and negative-remodelling groups in cardiac cine MRI is (Table 1): LVEF (41.3±14.8% vs. 37.6±10.1%), FS (25.7±2.0% vs. 24.0±2.5%), LVEDD (44.5±3.9mm vs. 46.3±5.4mm), LVEDV (129.1±8.5ml vs. 135.3±12.2ml), LVESV (74.8±7.3ml vs. 79.1±10.0ml), LVMM (52.0±5.7g vs. 49.6±6.5g), LVMM (34.9±3.5ml vs. 32.4±6.2ml), CO (3.8±0.7L/min vs. 3.1±0.5L/min), CI (2.9±0.6L/min*m2 vs. 2.7±0.5L/min*m2).
Fig. (1).
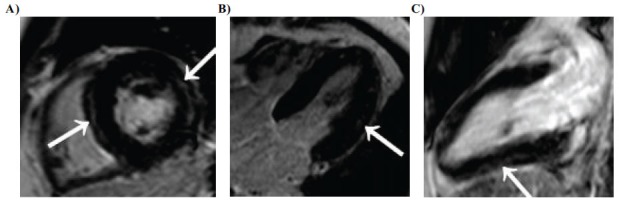
(A) Short-axis, (B) Four-chamber, and (C) Long-axis three-dimensional delayed-enhancement T1-weighted multishot gradient-echo IR MR images (6.1/3.0, 25°flip angle) of a diffuse form of positive-remodelling myocarditis in 32-year-old man. Nodular subepicardial LGE (arrows in A and C) of the left-ventricular septum, lateral and inferior wall associated with bandlike or nodular centromyocardial LGE (arrow in B) predominating in the lateral wall of the left ventricle is seen.
Table 1. Comparison of Positive/Negative-remodelling groups in CMRI.
| - | Positive-remodelling | Negative-remodelling | p value |
|---|---|---|---|
| Positive LGE(%) | 65.6 | 86.7 | <0.05 |
| LVEF(%) | 41.3±14.8 | 37.6±10.1 | 0.62 |
| FS(%) | 25.7±2.0 | 24.0±2.5 | 0.81 |
| LVEDD(mm) | 44.5±3.9 | 46.3±5.4 | 0.76 |
| LVEDV(ml) | 129.1±8.5 | 135.3±12.2 | 0.26 |
| LVESV(ml) | 74.8±7.3 | 79.1±10.0 | 0.55 |
| LVMM(g) | 52.0±5.7 | 49.6±6.5 | 0.71 |
| LVSV(ml) | 34.9±3.5 | 32.4±6.2 | 0.68 |
| CO(L/min) | 3.8±0.7 | 3.1±0.5 | 0.64 |
| CI(L/min*m2) | 2.9±0.6 | 2.7±0.5 | 0.79 |
LVEF, Left Ventricular Ejection Fraction; Fraction Shortening; LVEDD, Left Ventricular End-diastolic Dimension; LVEDV, Left Ventricular End-diastolic Volume; LVESV, Left Ventricular End-systolic Volume; LVMM, Left Ventricular Myocardial Mass; LVSV, Left Ventricular Stroke Volume; CO, Cardiac Output; CI, Cardiac Index.
7. DISCUSSION
CMRI is the most valuable imaging modality in the evaluation of left-ventricular remodeling and makes comprehensive evaluation of left-ventricular remodeling, such as establishing diagnosis of left-ventricular remodeling, measuring left-ventricular geometry, establishing aetiology, quantification, identifying prognostic factors, regular follow-up and assessment of treatment response. The latest insights regarding assessment of CMRI patterns of myocardial remodelling have led to more comprehensive imaging acquisition protocols including first-pass and LGE-MRI examinations, as well as cardiac cine MRI for evaluation of regional myocardial contractile function [15]. This research is indicative of a series of clinical presentation. The first point was represented by symptoms of acute infarction-like presentation in the absence of coronary stenoses. The interesting finding was that patients with this presentation did not reveal severely dilated ventricles and have moderate left ventricular dysfunction. Aetiological agent potentially did not affect cardiac myocyte but the vascular endothelial cell, bringing about endothelial dysfunction and exudation of inflammatory cells to the myocardial interstitium with consequent damage to the myocyte. Afterwards, focal distribution of the inflammatory process probably accounts for a fact that left ventricular function is not severely damaged. Yet the generalizability of much published research on this issue is problematic [16]. Patients invariably show palpable left ventricular remodeling at the onset and possess underdeveloped recovery. Recent evidence suggests that virus-specific immune response has the potential to explain the increasingly severe left ventricular remodeling, and the activity mediated by natural killer cells also plays a pivotal role [17]. Another finding was that patients presenting with symptoms of heart failure manifest an improvement in LVEF, which distinctly differed from the clinical manifestations of myocardial infarction [18]. Dornier et al. [19] found that an overestimation of approximately 2ml was observed for both LVEDV and LVESV with tagged CMRI and the difference between LVEF obtained by tagged CMRI and standard cine CMRI was approximately 1%, which is explained by the overestimation of both LVEDV. Progressive ventricular dilatation after cardiomyocyte necrosis is part of a complex cardiac remodeling process involving changes in mass, shape and volume [20]. Limited left ventricular dilatation is compensatory and can help to retain cardiac function. Further dilatation would yet bring about severe left ventricular dysfunction and heart failure. Patients with distinct left ventricular remodeling have worse prognosis than patients with insidious changes [21] and even insidious reductions in adverse left ventricular remodeling can reduce the risk of progression to heart failure after cardiomyocyte necrosis in myocarditis [22, 23]. Miller et al. [24] demonstrated that LVESV and LVEF are affected mainly by changes in temporal resolution, rather than by changes in spatial resolution and indicated that temporal resolution of 45ms can be deemed enough in patients with normal heart rates unless maximum ejection and filling rates are of interest. LVEF is the most commonly used method for measuring cardiac function based on its clinical importance, and reflects a combination of cardiac dilatation and myocardial systolic function, making it more challenging to explain than the simpler mathematical measurement of left ventricular enlargement over time to quantify positive-remodeling and negative-remodeling (Fig. 2).
Fig. (2).
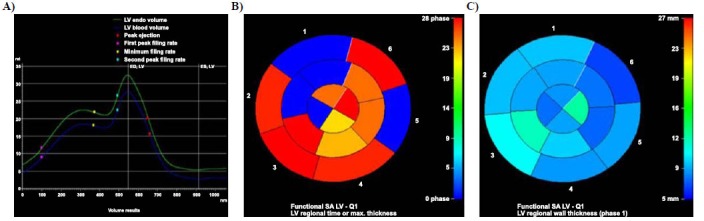
(A) Left-ventricular global volume-time curve, (B) Color-gradation map for left-ventricular regional time of thicknessmax, and (C) Color-gradation map for left-ventricular regional wall thickness of negative-remodelling myocarditis in 41-year-old woman. Arrhythmia of left ventricle was indicated in A. The longer time (B) or thicker wall (C) was displayed in the warmer color, while the shorter and thinner wall was presented as the colder color.
Previous studies have reported that areas of infarcted or scarred myocardium accumulate and retain gadolinium-based contrast material for approximately 10 minutes after the agent is administered [25, 26]. LGE-MRI was revealed to have significantly better sensitivity, specificity, predictive values, and accuracy than resting thallium-201 single-photon emission computed tomography in the prediction of regional myocardial remodelling [27]. Mahrholdt et al. [28] utilized CMRI as a guide to endocardium biopsy, reporting focus on enhancement in 88% of patients with clinically diagnosed myocarditis, with involvement being most common in the lateral wall, and as a predictor for identifying chronic ventricular dysfunction and dilation. Patients with human herpes virus 6/parvovirus B19 myocarditis presented with new onset of heart failure, had segmental LGE, and invariably progressed toward chronic remodeling [29]. The LGE-MRI technique has quickly been found to have potential utility as an essential clinical method of evaluating positive and negative in myocarditis. Blom et al. [30] utilized CMRI to demonstrate the benefits of a ventricular constraint device that provided passive mechanical diastolic support to inhibit ventricular remodeling and reduce ventricular wall stress in sheep with myocardial fibrosis. In this study, it is interesting to note that comparing the two subgroups with positive and negative remodeling, we found a significant difference in Statistics of LGE, which was evidently greater in patients with negative remodeling (Table 1). Orn et al. [31] demonstrated that scar size determined by LGE-MRI was the strongest independent predictor of LVEF and Left ventricular volumes in patients with acute cardiomyocyte necrosis and signs or symptoms of heart failure. Subacute or chronic left ventricular remodeling is predominantly triggered by myocardial injury of inflammation. Positive LGE may be seen due to fibrosis, likely due to cardiomyocyte degeneration and necrosis and high systolic pressure, as well as decreased myocardial perfusion due to lower aortic pressure, lower diastolic duration and coronary artery diastolic dysfunction [32]. It is most frequently seen in the basal segments, typically in a diffuse epicardial distribution, but occasionally in a mid-myocardial distribution in this study (Fig. 1). Myocardial interstitial fibrosis would gradually appear in the myocardium, which has the potential to result in permanent structural impair to the left ventricle, even the whole myocardial tissue. In the event that a certain threshold is exceeded, remodeling has the potential to get irreversible. A host of, if not most, studies in the literature have underlined the fundamentality of LGE, reflecting irreversible myocardial damage on a train of pathological changes in myocarditis [28, 33].
CONCLUSION
LGE, coupled with process of the clinical presentation and cardiac cine MRI, identifies cardiomyocyte damage and evaluates left ventricular positive and negative remodeling in patients with myocarditis. This study has quite a few limitations: First and foremost, the causative agent was detected in only a small percentage of patients, who are found to have pathologically proved active myocarditis. Furthermore, the follow-up was performed only with MRI to evaluate the positive or negative remodeling. The positive LGE is correlated with left ventricular remodeling, which might also be observed in patients with coronary heart disease or dilated cardiomyopathy. Further studies are accordingly a prerequisite for confirming the different argumentation.
ACKNOWLEDGEMENTS
None.
LIST OF ABBREVIATIONS
- CMRI
Cardiac Magnetic Resonance Imaging
- CO
Cardiac Output
- CI
Cardiac Index
- FS
Fraction Shortening
- LGE
Late Gadolinium Enhancement
- LVEF
Left Ventricular Ejection Fraction
- LVEDD
Left Ventricular End-diastolic Dimension
- LVEDV
Left Ventricular End-diastolic Volume
- LVESV
Left Ventricular End-Systolic Volume
- LVMM
Left Ventricular Myocardial Mass
- LVSV
Left Ventricular Stroke Volume
STANDARDS OF REPORTING
CONSORT guidelines were followed.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
The study was approved by the Medical Ethics Committee of the First Affiliated Hospital of Chengdu Medical College, China.
HUMAN AND ANIMAL RIGHTS
No animals were used in this study. The reported experiments on humans were in accordance with the ethical standards of the committee responsible for human experimentation (institutional national), and with the Helsinki Declaration of 1975, as revised in 2008 (http://www.wma.net/).
CONSENT FOR PUBLICATION
Written informed consent was taken from all the subjects.
AVAILABILITY OF DATA AND MATERIALS
The data that support the findings of this study are available from the corresponding author upon request.
FUNDING
This work was supported by following:
1. The Applied Basic Research on Projects in Yunnan Province (Grant No. 2017FE468-178).
2. The Yunnan Province Medical Subject Leaders Training Project (Grant No. D-201646).
3. The Young and Middle-aged Technical Academic Leaders Training Project in Yunnan province (Grant No. 2015HB068).
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Sagar S., Liu P.P., Cooper L.T., Jr Myocarditis. Lancet. 2012;379(9817):738–747. doi: 10.1016/S0140-6736(11)60648-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cooper L.T., Jr Myocarditis. N. Engl. J. Med. 2009;360(15):1526–1538. doi: 10.1056/NEJMra0800028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dec G.W., Jr, Waldman H., Southern J., Fallon J.T., Hutter A.M., Jr, Palacios I. Viral myocarditis mimicking acute myocardial infarction. J. Am. Coll. Cardiol. 1992;20(1):85–89. doi: 10.1016/0735-1097(92)90141-9. [DOI] [PubMed] [Google Scholar]
- 4.Fabre A., Sheppard M.N. Sudden adult death syndrome and other non-ischaemic causes of sudden cardiac death. Heart. 2006;92(3):316–320. doi: 10.1136/hrt.2004.045518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Magnani J.W., Dec G.W. Myocarditis: current trends in diagnosis and treatment. Circulation. 2006;113(6):876–890. doi: 10.1161/circulationaha.105.584532. [DOI] [PubMed] [Google Scholar]
- 6.Feldman A.M., McNamara D. Myocarditis. N. Engl. J. Med. 2000;343(19):1388–1398. doi: 10.1056/NEJM200011093431908. [DOI] [PubMed] [Google Scholar]
- 7.Bowles N.E., Ni J., Kearney D.L., et al. Detection of viruses in myocardial tissues by polymerase chain reaction. Evidence of adenovirus as a common cause of myocarditis in children and adults. J. Am. Coll. Cardiol. 2003;42(3):466–472. doi: 10.1016/S0735-1097(03)00648-X. [DOI] [PubMed] [Google Scholar]
- 8.Laraudogoitia Zaldumbide E., Pérez-David E., Larena J.A., et al. The value of cardiac magnetic resonance in patients with acute coronary syndrome and normal coronary arteries. Rev. Esp. Cardiol. 2009;62(9):976–983. doi: 10.1016/s1885-5857(09)73263-3. [DOI] [PubMed] [Google Scholar]
- 9.Cummings K.W., Bhalla S., Javidan-Nejad C., Bierhals A.J., Gutierrez F.R., Woodard P.K. A pattern-based approach to assessment of delayed enhancement in nonischemic cardiomyopathy at MR imaging. Radiographics. 2009;29(1):89–103. doi: 10.1148/rg.291085052. [DOI] [PubMed] [Google Scholar]
- 10.Mouquet F., Lions C., de Groote P., et al. Characterisation of peripartum cardiomyopathy by cardiac magnetic resonance imaging. Eur. Radiol. 2008;18(12):2765–2769. doi: 10.1007/s00330-008-1067-x. [DOI] [PubMed] [Google Scholar]
- 11.Srichai M.B., Junor C., Rodriguez L.L., et al. Clinical, imaging, and pathological characteristics of left ventricular thrombus: a comparison of contrast-enhanced magnetic resonance imaging, transthoracic echocardiography, and transesophageal echocardiography with surgical or pathological validation. Am. Heart J. 2006;152(1):75–84. doi: 10.1016/j.ahj.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 12.Cerqueira M.D., Weissman N.J., Dilsizian V., et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart: a statement for healthcare professionals from the Cardiac imaging committee of the council on clinical cardiology of the american heart association. J. Am. Soc. Echocardiogr. 2002;15(5):463–467. doi: 10.1067/mje.2002.123374. [DOI] [PubMed] [Google Scholar]
- 13.Friedrich M.G., Sechtem U., Schulz-Menger J., et al. International consensus group on cardiovascular magnetic resonance in myocarditis. cardiovascular magnetic resonance in myocarditis: a JACC white paper. J. Am. Coll. Cardiol. 2009;53(17):1475–1487. doi: 10.1016/j.jacc.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bax J.J., Abraham T., Barold S.S., et al. Cardiac resynchronization therapy: Part 2-issues during and after device implantation and unresolved questions. J. Am. Coll. Cardiol. 2005;46(12):2168–2182. doi: 10.1016/j.jacc.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 15.Sandstede J.J.W., Lipke C., Beer M., et al. Analysis of first-pass and delayed contrast-enhancement patterns of dysfunctional myocardium on MR imaging: Use in the prediction of myocardial viability. AJR Am. J. Roentgenol. 2000;174(6):1737–1740. doi: 10.2214/ajr.174.6.1741737. [DOI] [PubMed] [Google Scholar]
- 16.Baughman K.L. Diagnosis of myocarditis: death of Dallas criteria. Circulation. 2006;113(4):593–595. doi: 10.1161/CIRCULATIONAHA.105.589663. [DOI] [PubMed] [Google Scholar]
- 17.Yoshikawa T., Baba A., Nagatomo Y. Autoimmune mechanisms underlying dilated cardiomyopathy. Circ. J. 2009;73(4):602–607. doi: 10.1253/circj.CJ-08-1151. [DOI] [PubMed] [Google Scholar]
- 18.Kühl U., Pauschinger M., Schwimmbeck P.L., et al. Interferon-β treatment eliminates cardiotropic viruses and improves left ventricular function in patients with myocardial persistence of viral genomes and left ventricular dysfunction. Circulation. 2003;107(22):2793–2798. doi: 10.1161/01.CIR.0000072766.67150.51. [DOI] [PubMed] [Google Scholar]
- 19.Dornier C., Somsen G.A., Ivancevic M.K., et al. Comparison between tagged MRI and standard cine MRI for evaluation of left ventricular ejection fraction. Eur. Radiol. 2004;14(8):1348–1352. doi: 10.1007/s00330-004-2311-7. [DOI] [PubMed] [Google Scholar]
- 20.Cohn J.N., Ferrari R., Sharpe N. Cardiac remodeling-concepts and clinical implications: A consensus paper from an international forum on cardiac remodelling. J. Am. Coll. Cardiol. 2000;35(3):569–582. doi: 10.1016/S0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 21.Pfeffer M.A., Braunwald E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation. 1990;81(4):1161–1172. doi: 10.1161/01.CIR.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 22.St John Sutton M., Pfeffer M.A., Moye L., et al. Cardiovascular death and left ventricular remodeling two years after myocardial infarction: Baseline predictors and impact of long-term use of captopril: information from the Survival and Ventricular Enlargement (SAVE) trial. Circulation. 1997;96(10):3294–3299. doi: 10.1161/01.CIR.96.10.3294. [DOI] [PubMed] [Google Scholar]
- 23.Gaudron P., Eilles C., Kugler I., Ertl G. Progressive left ventricular dysfunction and remodeling after myocardial infarction. Potential mechanisms and early predictors. Circulation. 1993;87(3):755–763. doi: 10.1161/01.CIR.87.3.755. [DOI] [PubMed] [Google Scholar]
- 24.Miller S., Simonetti O.P., Carr J., Kramer U., Finn J.P. MR Imaging of the heart with cine true fast imaging with steady-state precession: Influence of spatial and temporal resolutions on left ventricular functional parameters. Radiology. 2002;223(1):263–269. doi: 10.1148/radiol.2231010235. [DOI] [PubMed] [Google Scholar]
- 25.Kim R.J. Assessment of myocardial viability by contrast enhancement. In: Higgins C.B., Roos A., editors. MRI and CT of the cardiovascular system. 2nd ed. Philadelphia: Lippincott Willams & Wilkins; 2006. pp. 233–262. [Google Scholar]
- 26.Mahrholdt H., Wagner A., Judd R.M., Sechtem U., Kim R.J. Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies. Eur. Heart J. 2005;26(15):1461–1474. doi: 10.1093/eurheartj/ehi258. [DOI] [PubMed] [Google Scholar]
- 27.Kitagawa K., Sakuma H., Hirano T., Okamoto S., Makino K., Takeda K. Acute myocardial infarction: myocardial viability assessment in patients early thereafter comparison of contrast-enhanced MR imaging with resting (201)Tl SPECT. Single photon emission computed tomography. Radiology. 2003;226(1):138–144. doi: 10.1148/radiol.2261012108. [DOI] [PubMed] [Google Scholar]
- 28.Mahrholdt H., Goedecke C., Wagner A., et al. Cardiovascular magnetic resonance assessment of human myocarditis: A comparison to histology and molecular pathology. Circulation. 2004;109(10):1250–1258. doi: 10.1161/01.CIR.0000118493.13323.81. [DOI] [PubMed] [Google Scholar]
- 29.Mahrholdt H., Wagner A., Deluigi C.C., et al. Presentation, patterns of myocardial damage, and clinical course of viral myocarditis. Circulation. 2006;114(15):1581–1590. doi: 10.1161/circulationaha.105.606509. [DOI] [PubMed] [Google Scholar]
- 30.Blom A.S., Mukherjee R., Pilla J.J., et al. Cardiac support device modifies left ventricular geometry and myocardial structure after myocardial infarction. Circulation. 2005;112(9):1274–1283. doi: 10.1161/circulationaha.104.499202. [DOI] [PubMed] [Google Scholar]
- 31.Orn S., Manhenke C., Anand I.S., et al. Effect of left ventricular scar size, location, and transmurality on left ventricular remodeling with healed myocardial infarction. Am. J. Cardiol. 2007;99(8):1109–1114. doi: 10.1016/j.amjcard.2006.11.059. [DOI] [PubMed] [Google Scholar]
- 32.Azevedo C.F., Nigri M., Higuchi M.L., et al. Prognostic significance of myocardial fibrosis quantification by histopathology and magnetic resonance imaging in patients with severe aortic valve disease. J. Am. Coll. Cardiol. 2010;56(4):278–287. doi: 10.1016/j.jacc.2009.12.074. [DOI] [PubMed] [Google Scholar]
- 33.Choudhury L., Mahrholdt H., Wagner A., et al. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2002;40(12):2156–2164. doi: 10.1016/S0735-1097(02)02602-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon request.


