Abstract
Infections due to carbapenem-resistant Enterobacteriaceae (CRE) are increasingly prevalent in children and are associated with poor clinical outcomes. Optimal treatment strategies for CRE infections continue to evolve. A lack of pediatric-specific comparative effectiveness data, uncertain pediatric dosing regimens for several agents, and a relative lack of new antibiotics with pediatric indications approved by the US Food and Drug Administration (FDA) collectively present unique challenges for children. In this review, we provide a framework for antibiotic treatment of CRE infections in children, highlighting relevant microbiologic considerations and summarizing available data related to the evaluation of FDA-approved antibiotics (as of September 2019) with CRE activity, including carbapenems, ceftazidime-avibactam, meropenem-vaborbactam, imipenem/cilastatin-relebactam, polymyxins, tigecycline, eravacycline, and plazomicin.
Keywords: Klebsiella pneumoniae carbapenemase, pediatrics, multidrug-resistant organism, gram-negative resistance
We provide an overview of antibiotics with activity against carbapenem-resistant Enterobacteriaceae (CRE) and describe unique considerations for administering these agents to children with CRE infections.
Carbapenem-resistant Enterobacteriaceae (CRE) infections are increasingly being identified in children and are associated with poor clinical outcomes [1–4]. Therapeutic paradigms for CRE have evolved significantly in recent years with the availability of novel β-lactam-β-lactamase inhibitor (βL-βLI) agents (eg, ceftazidime-avibactam, meropenem-vaborbactam, and imipenem/cilastatin-relebactam [hereafter referred to as imipenem-relebactam]), which have revolutionized the treatment of CRE infections. However, optimal treatment of CRE infections in children remains challenging given limited pediatric-specific pharmacokinetic and pharmacodynamic (PK-PD) data, limited clinical experience with a number of CRE-active drugs, and few clinical trials evaluating novel antibiotics for use in this population. In this review, we summarize available CRE-active antibiotics approved by the US Food and Drug Administration (FDA) as of September 2019 and provide clinicians a framework for selecting appropriate therapy for children infected with CRE based on clinical and microbiologic characteristics.
MICROBIOLOGY
CRE are defined by the Centers for Disease Control and Prevention as any Enterobacteriaceae exhibiting carbapenem resistance, defined as an imipenem, meropenem, or doripenem minimum inhibitory concentration (MIC) ≥4 μg/mL or ertapenem MIC ≥2 μg/mL [5, 6]. Common CRE in children include Enterobacter species, Klebsiella pneumoniae, and Escherichia coli [1]. Carbapenem resistance in these organisms develops by 1 of 2 general mechanisms: by production of a carbapenemase (CP-CRE), which are encoded by genes generally found on highly transmissible mobile elements that hydrolyze the β-lactam ring of carbapenem antibiotics or by production of extended-spectrum β-lactamases (ESBLs) or AmpC β-lactamases combined with impaired membrane permeability from porin loss/mutations or efflux pumps. These latter groups are termed non–CP-CRE [7]. Several laboratory tests (including the Carba NP assay and modified carbapenem inactivation method) are available to identify the presence or absence of a carbapenemase. Molecular tests can identify specific carbapenemase genes present and are included in some commercially available rapid molecular diagnostic panels [8, 9]. Of note, since these molecular assays are only able to detect specified carbapenemase genes, “negative” results should not be interpreted as synonymous with the absence of carbapenemase genes not included in the assay or carbapenem susceptibility (as the isolate may be a non–CP-CRE).
MOLECULAR EPIDEMIOLOGY
In the United States, between one-third and one-half of CRE isolates from adults are carbapenemase-producing, and the Ambler class A serine carbapenemase K. pneumoniae carbapenemase (KPC) accounts for upwards of 90% of these CP-CRE [10–12]. Epidemiologic studies in children are limited, but available pediatric case series suggest KPC is also the most common carbapenemase identified in CP-CRE isolates infecting US children [13, 14]. The β-lactamase inhibitors avibactam, vaborbactam, and relebactam effectively protect β-lactams from hydrolysis by KPC enzymes [15, 16].
The Ambler class B carbapenemases are referred to as metallo-β-lactamases (MBLs) because they require zinc to be active. MBLs include New Delhi metallo-β-lactamases (NDM), Verona integron-encoded metallo-β-lactamases (VIM), and active on imipenem (IMP) carbapenemases. They are infrequently identified in the United States but are of particular clinical significance as they are not inhibited by avibactam, vaborbactam, or relebactam, severely limiting treatment options for infections due to these organisms [15, 16]. The possibility of an MBL-producing isolate should be considered if carbapenem resistance is detected in isolates from children with epidemiologic links to MBL-endemic regions, such as South Asia, where NDM carbapenemases are highly prevalent or in a child with a known history of infection or colonization with an MBL-producing isolate [7]. Further, there are adult and pediatric reports of sporadic domestic acquisition of VIM-, IMP-, and NDM-producing isolates in the United States [17–19].
The Ambler class D oxacillinases, which include oxacillinase (OXA)-48–like enzymes (eg, OXA-48, OXA-181, OXA-232), are increasingly being identified in Europe and sporadically in the United States [7, 20]. Avibactam and, to a lesser extent, relebactam, but not vaborbactam, inhibit OXA-48-like enzymes (Table 1) [15, 16].
Table 1.
Characteristics of Common Carbapenemases Produced by the Enterobacteriaceae
| Ambler Class | Type of Beta- Lactamase | Active Site | Example(s) | Typical β-Lactam Resistance Profile | Inhibited by Avibactam | Inhibited by Vaborbactam | Inhibited by Relebactam | Geographic Distribution |
|---|---|---|---|---|---|---|---|---|
| Class A | Penicillinase | Serine | Klebsiella pneumoniae carbapenemase | All β-lactams: Carbapenem hydrolysis can vary from low- to high-level, resulting in variable meropenem minimum inhibitory concentration and occasional isolated ertapenem resistance | Yes | Yes | Yes | Global |
| Class B | MBL | Zinc | NDM, VIM, IMP | All β-lactams: Monobactams spared from MBL hydrolysis, but frequent coproduction of ESBLs and/or AmpCs mediate monobactam resistance | No | No | No | NDM-1: India, Pakistan, Balkan states VIM: Mediterranean basin IMP: Japan, southeast Asia |
| Class D | Oxacillinase | Serine | OXA-48– like | Penicillins, carbapenems: Cephalosporins spared from OXA hydrolysis, but ESBLs are often coproduced, resulting in cephalosporin resistance | Yes | No | More data are needed | Mediterranean basin, Middle East |
Abbreviations: ESBL, extended-spectrum β-lactamase; IMP, active on imipenem; MBL, metallo-β-lactamase; NDM, New Delhi metallo-β-lactamase; OXA, oxacillinase; VIM, Verona integron-encoded metallo-β-lactamase.
Finally, it is worth noting that carbapenemase enzymes are often coproduced with elements that confer resistance to non-carbapenem antibiotics. This includes other β-lactamases (eg, the blaCTX-M-15 ESBL gene, the blaCMYampC gene), aminoglycosides (eg, aminoglycoside-modifying enzymes, ribosomal RNA methyltransferases), fluoroquinolones (eg, gyrA or parC mutations, qnr genes), and trimethoprim/sulfamethoxazole (eg, dfr, sul genes), resulting in a multidrug-resistant phenotype and often significantly limiting the utility of other antibiotic classes for CP-CRE infections [7, 21–24].
Non–CP-CRE accounts for more than half of CRE isolates from adults in the United States [10, 12]. Although no pediatric molecular epidemiologic data are available comparing the prevalence of CP-CRE vs non–CP-CRE in US children, available data suggest that non-carbapenemase–mediated resistance determinants are likely more common in children as well [13, 25]. Non–CP-CRE develop carbapenem resistance through coproduction of either Ambler class A ESBLs (eg, SHV, CTX-M) or Ambler class C cephalosporinases (AmpC enzymes) along with porin mutations (eg, OmpK35 or OmpK36) and/or the presence of efflux pumps (eg, AcrAB-TolC) [26–31].
GENERAL TREATMENT CONSIDERATIONS: CP-CRE VS NON–CP-CRE
In general, antibiotic choice should not be stratified based on the presence or absence of carbapenemase production, and the guidance provided herein should be applied similarly to patients with CP-CRE and non–CP-CRE. While it is possible that optimal treatment strategies may differ based on the presence or absence of carbapenemase production, data are currently insufficient to recommend this type of stratification.
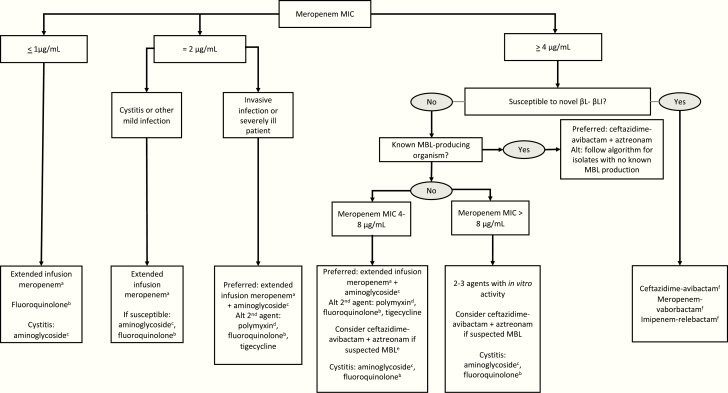
Instead, we suggest using the results of carbapenem susceptibility testing to decide whether or not a patient can be treated with an extended-infusion carbapenem (most commonly extended-infusion meropenem) vs a newer βL-βLI agent (Figure 1). For carbapenem-resistant isolates (eg, those with a meropenem MIC ≥4 μg/mL), use of the novel βL-βLI (ceftazidime-avibactam, meropenem-vaborbactam, or imipenem-relebactam) should be considered for both CP-CRE and non–CP-CRE, if susceptible in vitro, for US isolates as KPC production remains the predominant carbapenemase-producing enzyme and KPC enzymes are generally inactivated by all three of these agents. If specific carbapenemase testing is available and a carbapenemase is identified, the specific carbapenemase identified can inform selection of a βL-βLI given differential activity based on carbapenemase type. While large molecular epidemiologic studies of non–CP-CRE characterizing novel β-lactamase activity against various combinations of Ambler class A and C β-lactamases, porin mutations, and efflux pumps across Enterobacteriaceae species are limited, the available in vitro data demonstrate high rates of susceptibility among non–CP-CRE isolates to the newer βL-βLI [32–36].
Figure 1.
Suggested framework for antibiotic selection for children with carbapenemase–carbapenem-resistant Enterobacteriaceae (CP-CRE) and non–CP-CRE. aMeropenem administered over 3 hours can achieve target serum concentrations for isolates up to 8 μg/mL in healthy children. bFluoroquinolones are variably active against CRE. If susceptible, these agents can be used as part of combination regimens or as monotherapy for mild to moderate infections. cAminoglycosides are recommended as first-line agents for use in combination for systemic infections given pediatric clinical experience and familiarity with dosing. Aminoglycosides may also be used for cystitis as monotherapy. dThe polymyxin class includes colistin and polymyxin B. Polymyxin B is preferred given ease of dosing and more reliable pharmacokinetics for nonurinary sources of infection. For urinary tract infections, colistin is preferred. eConsider in children with epidemiologic link to a MBL-endemic region (eg, South Asia) or known history of MBL-producing isolate. fIf the MIC for ceftazidime-avibactam, meropenem-vaborbactam, or imipenem-relebactam is at the breakpoint, addition of a second agent could be considered, with aminoglycosides preferred. Abbreviations: Alt, alternative; βL-βLI, β-lactam/β-lactamase inhibitor; MBL, metallo-β-lactamase; MIC, minimum inhibitory concentration. (cUTIs) and (cIAIs;
Finally, as with other Enterobacteriaceae infections, aminoglycoside monotherapy should be reserved for urinary tract infections. If used for invasive infections, aminoglycosides should be a component of combination therapy. No studies have specifically evaluated fluoroquinolone use for CRE, with only sporadic reports of their use for CRE in the literature [2, 37]. However, if fluoroquinolones or trimethoprim-sulfamethoxazole remain active against a CRE in vitro, there is no evidence to indicate that they should be excluded as treatment options.
CARBAPENEMS
Because the diverse resistance mechanisms that result in in vitro carbapenem resistance result in variably elevated meropenem MICs, meropenem has an important role in the treatment of CRE in clinical scenarios where meropenem MICs are ≤2 μg/mL. Bacterial killing by carbapenems is dependent on time of free drug above the MIC, with optimal effect if time above the MIC exceeds 40% [38]. Pediatric PK data demonstrate that in healthy children, an extended infusion of meropenem over 3 hours can achieve this target for isolates with meropenem MICs of up to 8 μg/mL [39]. However, in critically ill children, target serum concentrations are only reliably achieved for isolates with meropenem MICs ≤2 μg/mL due to alterations in the volumes of distribution expected with sepsis [40]. There are limited clinical data related to the effectiveness of carbapenem monotherapy for the treatment of CRE in adults. However, consistent with available PK data, carbapenems appear most effective for isolates with carbapenem MICs ≤4 μg/mL, with clinical success reported in 69% of patients with carbapenem MICs in this range compared with 29% with carbapenem MICs >8 μg/mL [41, 42].
Based on the PK and limited clinical data, we suggest extended-infusion meropenem for nonsevere infections (eg, infections of the urinary tract) caused by CRE isolates with a meropenem MIC ≤2 μg/mL or invasive infections caused by CRE isolates with a meropenem MIC ≤1 μg/mL (ie, those isolates that met the criteria for CRE because of resistance to another carbapenem; Figure 1 and Table 2). Based on observational studies that indicate improved outcomes for patients with primarily CP-CRE treated with novel βL-βLI agents approved after 2014, these agents should preferentially be considered over carbapenems for infections with meropenem MICs ≥4 μg/mL [43–45].
Table 2.
Suggested Pediatric Dosing for Antibiotics Used to Treat Carbapenem-Resistant Enterobacteriaceae Infections
| Antibiotica | Dosing |
|---|---|
| Meropenem | 40 mg/kg/dose IV q8h infused over 3 hours (max 2000 mg/dose) |
| Meropenem-vaborbactamb | 40 mg/kg/dose meropenem and 40 mg/kg/dose vaborbactam (max 2000 mg/dose) IV q8h infused over 3 hours |
| Ceftazidime-avibactam | Aged 3 months to 6 months: 40 mg/kg/dose ceftazidime and 10 mg/kg/dose avibactam IV q8h infused over 2 hours Aged 6 months to <2 years: 50 mg/kg/dose ceftazidime and 12.5 mg/kg/dose avibactam IV q8h infused over 2 hours Aged 2 years to 18 years: 50 mg/kg/dose ceftazidime (max 2000 mg ceftazidime/dose) and 12.5 mg/kg/dose avibactam (max 500 mg avibactam/dose) IV q8h infused over 2 hours |
| Imipenem-relebactamc | Aged 3 months to < 2 years: dose not available as of September 2019 Aged 2 years to <12 years: 15 mg/kg/dose imipenem (max 500 mg) and 7.5 mg/kg/dose relebactam (max 250 mg) IV q6h infused over 30 minutes Aged 12 to <18 years: 500 mg imipenem and 250 mg relebactam IV q6h infused over 30 minutes |
| Amikacin | 7.5 mg/kg/dose IV q8h (no maximum dosed) or Extended interval: 15 to 20 mg/kg/dose IV q24h (no maximum dosed) |
| Gentamicin | 2.5 mg/kg/dose IV q8h (no maximum dosed) or Extended interval: (no maximum dosed) Aged ≥3 months to <2 years: 9.5 mg/kg/dose IV q24h Aged 2 to <8 years: 8.5 mg/kg/dose IV q24h Aged ≥8 years and adolescents: 7 mg/kg/dose IV q24h |
| Tobramycin | 2.5 mg/kg/dose IV q8h (no maximum dosed) or Extended interval: (no maximum dosed) Aged ≥3 months to <2 years: 9.5 mg/kg/dose IV q24h Aged 2 to <8 years: 8.5 mg/kg/dose IV q24h Aged ≥8 years and adolescents: 7 mg/kg/dose IV q24h |
| Ciprofloxacin | 10 mg/kg/dose IV q8h (max 400 mg/dose) or 20 mg/kg/dose PO q12h (max 1000 mg/dose) |
| Levofloxacin | Aged 6 months to <5 years: 10 mg/kg/dose IV/PO q12h (max 375 mg/dose) Aged ≥5 years: 10 mg/kg/dose IV/PO q24h (max 750 mg/dose) |
| Tigecycline | Aged ≥ 8 years: 4 mg/kg/dose IV loading dose × 1 (max 200 mg/dose) followed by 2 to 3.2 mg/kg/dose IV q12h (max 100 mg/dosee) |
| Colistin | 5 mg/kg dose IV loading dose × 1 (maximum dose 300 mg CBAf), followed by 2.5 mg/kg/dose IV q12h (maximum dose 180 mg CBAf) |
| Polymyxin B | 25 000 units/kg loading dose × 1, followed by 15 000 units/ kg/dose IV q12h (maximum dose of 2 000 000 units/day f) |
| Aztreonam | >1 month: 120 mg/kg/day IV divided q6 to 8h infused over 3 h (max 8000 mg/day) |
| Eravacycline | No pediatric dosing available |
| Plazomicin | No pediatric dosing available |
Abbreviations: CBA, colistin-base activity; h, hour; IV, intravenous; PO, per os/by mouth; q, every.
a Listed dosing and intervals assume normal renal function and apply to children aged >30 days except as noted.
b Dose used in ongoing phase 1 pharmacokinetic study (NCT02687906); interval derived from usual interval for meropenem administration as well as adult dosing interval for meropenem-vaborbactam.
c Dose used in planned phase 2/3 trial for children with complicated intraabdominal infections and complicated urinary tract infections (NCT03969901).
d Dosing should be based on adjusted body weight when actual body weight is 30% greater than ideal body weight.
e Maximum dose of 100 mg 2 times a day is based on the dose used in adult clinical trials. However, the authors have used a dose of up to 150 mg/dose for treatment of carbapenem-resistant infections.
f Maximum doses based on adult daily maximum.
NOVEL β-LACTAM AGENTS
Ceftazidime-Avibactam
Ceftazidime-avibactam is a βL-βLI combination approved by the FDA in March 2019 for children ages ≥3 months [46–49]. Avibactam is a β-lactamase inhibitor that binds reversibly to serine-β-lactamases and is therefore active against most KPC and OXA-48–like carbapenemases but inactive against MBL producers [50]. However, use of the combination of ceftazidime-avibactam and aztreonam has been reported as salvage therapy for MBL-producing CRE (Figure 1 and Table 1) [51]. The mechanism that underlies this approach is that aztreonam is resistant to degradation by MBLs, but frequent coproduction of Ambler class A and D enzymes (which hydrolyze aztreonam) by most MBL-producing isolates limits use of aztreonam as monotherapy. Avibactam, however, effectively inhibits these serine carbapenemases, thus allowing aztreonam to remain active [52]. In vitro susceptibility to ceftazidime-avibactam among KPC producers is high, with 2 large series reporting approximately 98% of tested isolates susceptible at the current Clinical and Laboratory Standards Institute (CLSI) breakpoint of ≤8/4 μg/mL [6, 35, 53]. While non–CP-CRE susceptibilities were not specifically reported, susceptibility to ceftazidime-avibactam among all meropenem-nonsusceptible K. pneumoniae was 99% [35].
Several observational studies and case series have reported on clinical experiences with ceftazidime-avibactam for the treatment of CRE infections in adults, including comparative effectiveness studies that demonstrated improved outcomes with ceftazidime-avibactam compared with other treatment regimens [43–45, 54–56]. The comparator groups used in these studies were variable and heterogeneous, including patients treated with carbapenems, colistin, tigecycline, fosfomycin, and/or aminoglycosides, often in combination [43–45]. A pediatric case series that included 8 children similarly reported successful treatment of invasive CRE infections with ceftazidime-avibactam [57]. Notably, several reports have highlighted the potential for ceftazidime-avibactam resistance to develop following even a short duration of therapy, including 1 report in which resistance developed due to KPC mutations in 3 of 10 microbiologic failures [56, 58]. These findings underscore the importance of ceftazidime-avibactam antibiotic susceptibility testing for CRE isolates, which may not be routinely performed at many institutions, as well as vigilance for emerging resistance while on therapy.
Meropenem-Vaborbactam
Meropenem-vaborbactam is a βL-βLI approved by the FDA in 2017 [59]. Vaborbactam is a cyclic boronic acid–based β-lactamase inhibitor that inhibits serine carbapenemases including KPC, but not MBLs or OXA-type carbapenemases [16]. It has high in vitro activity against KPC-producing CRE [34, 60]. In a study that included 991 KPC-producing isolates, 99% were susceptible using the CLSI breakpoint of ≤4/8 μg/mL [6, 60]. Among non–CP-CRE, the addition of vaborbactam resulted in a 4-fold decrease in the meropenem MIC, with more than 95% of isolates susceptible using the CLSI breakpoint [34].
Although clinical data related to the use of this drug for the treatment of CRE are limited, a phase 3, randomized, controlled trial compared treatment with meropenem-vaborbactam monotherapy (n = 32) with the best available therapy (n = 15) for invasive CRE infections, including bacteremia (44%), (35%) complicated urinary tract infection (cUTI), pneumonia (13%), and complicated intra-abdominal infection (cIAI) (6%). Sixty-six percent of patients treated with meropenem-vaborbactam experienced clinical cure at the end of therapy compared with 33% of patients treated with the best available therapy, achieving statistical significance [61]. Development of resistance during therapy with meropenem-vaborbactam appears infrequent based on in vitro data and was not observed in the previously mentioned phase 3 trial, though 1 isolate out of 32 obtained post-randomization did exhibit a 4-fold increase in the meropenem MIC [61–63]. The extent to which resistance to meropenem-vaborbactam will occur during or following meropenem-vaborbactam therapy is unknown, but vigilance for this phenomenon is required as the drug becomes used more widely.
Pediatric data are limited to a single case report in which a 4-year-old child with KPC-producing K. pneumoniae bloodstream infection was treated successfully with meropenem-vaborbactam [64]. A phase 1 study evaluating dosing, PK, and safety of meropenem-vaborbactam in children is underway (NCT02687906) [65].
Imipenem-Relebactam
Imipenem-relebactam is a βL-βLI approved by the FDA in July 2019 [66, 67]. Like avibactam, relebactam is a β-lactamase inhibitor that is highly active against KPC-producing isolates but not MBL-producing isolates [16, 36, 68]. More data are needed for OXA-48–producing isolates. The addition of relebactam also resulted in a dose-dependent reduction in imipenem MICs in 10 non–CP-CRE isolates [36].
Clinical data related to the use of imipenem-relebactam for CRE infections are limited to a phase 3 trial that included 31 patients with hospital-acquired pneumonia (HAP), ventilator-associated pneumonia (VAP), cIAI, or cUTI caused by imipenem-nonsusceptible Pseudomonasaeruginosa (24/31) and Enterobacteriaceae. This study compared imipenem-relebactam vs the combination of imipenem and colistin. An overall favorable clinical response was demonstrated in 71% of the imipenem-relebactam group vs 70% of the imipenem-colistin group, and importantly, nephrotoxicity was significantly less frequent in the imipenem-relebactam group than in the imipenem-colistin group (10% vs 56%) [69]. Despite the small number of CRE patients included, these findings are relevant to clinicians given the unfavorable side-effect profile of colistin compared with imipenem-relebactam demonstrated in this study. Finally, a phase 3 study that evaluated imipenem-relebactam vs piperacillin-tazobactam for the treatment of VAP and HAP in adults was recently completed, with results pending [70]. A phase 1 PK study (NCT03230916) and a phase 2/3 treatment study in children with suspected or confirmed gram-negative infections (NCT03969901) are ongoing [71, 72].
Suggested Use of Novel βL-βLI
Novel βL-βLI should be strongly considered as first-line options for susceptible CRE isolates with meropenem MICs ≥4 μg/mL or isolates known to produce KPC based on rapid molecular diagnostic testing. Though pediatric data are limited, extensive clinical experience with the β-lactam component of each of the novel βL-βLI in children, efficacy data from adult studies, and significant challenges associated with other CRE active agents make these preferred agents (Figure 1 and Table 2). Formulary considerations, regional prevalence of KPC vs OXA-48-like enzymes, and the results of antibiotic susceptibility testing should be considered when selecting 1 of these 3 agents.
COMBINATION THERAPY
Because of the historical lack of antibiotics with good in vitro activity against KPC-producing CRE, the possible benefit of combining 2 or more agents (sometimes with limited in vitro activity) has been explored in several observational studies. Most of these studies focused on adults with KPC-producing bloodstream infections and demonstrated a mortality benefit with combination antibiotic therapy, in particular, with carbapenem-containing combinations [73–77]. Other studies have suggested that this benefit is limited to patients at highest risk for mortality (including patients with septic shock, rapidly fatal underlying diseases, and bacteremia from nonurinary/nonbiliary sources) and, specifically, for carbapenem-containing combinations, for isolates with carbapenem MICs ≤8 μg/mL [37, 78, 79]. No published randomized trials have sufficiently compared monotherapy with combination therapy in patients with CRE. One trial enrolled a small number of CRE patients (73/406) and compared the impact of colistin vs colistin plus meropenem in patients with infections due to carbapenem-resistant gram-negative organisms, though the majority of patients included in this study were infected with carbapenem-resistant Acinetobacter baumannii (312/406). Although no difference in a composite clinical and microbiological outcome was detected, the trial was not powered to evaluate differences in outcomes among the small number of CRE patients enrolled, limiting the generalizability of these data to patients with CRE infections [80].
As the methodologic quality of many of these studies was relatively low, with significant variation in antibiotics used in combination, varied definitions of carbapenem resistance, lack of sufficient statistical adjustment for confounding factors, and variable timing of exposure and outcome classification, interpretation of their findings is challenging. Perhaps reflecting these methodologic limitations, other studies have failed to demonstrate any benefit with combination therapy [81–83]. Finally, and perhaps most importantly, combination regimens have historically utilized drugs with limited in vitro activity against CRE, a paradigm that has shifted with the availability of newer β-lactam agents with excellent activity against most CRE isolates. The impact of combination therapy when one of the newer agents is utilized has not been formally evaluated, but available data do not support the need for the routine addition of a second agent.
If a second agent is added because the β-lactam prescribed (ie, a carbapenem or one of the novel β-lactams) has an MIC at the breakpoint, we preferentially prescribe an active aminoglycoside over polymixins, whenever susceptible, given availability of pediatric-specific PK-PD data to inform dosing, greater familiarity with therapeutic drug monitoring, more pediatric clinical experience with these agents, and published experience with aminoglycosides for treatment of CRE infections [37, 75–78, 81, 84] (Figure 1).
Finally, a second scenario in which treatment with 2 antibiotics is warranted is in the treatment of MBL-producing CRE, where use of both aztreonam and ceftazidime-avibactam can be considered [51]. However, the need for combination therapy in this scenario is a practical one related to the lack of a commercially available single antibiotic that contains both aztreonam and avibactam as of September 2019.
POLYMIXINS
Colistin
Colistin achieves bactericidal killing by binding to negatively charged phosphate moieties in the lipopolysaccharide layer of the cell membrane, thus disrupting the cell membrane and causing loss of intracellular products [85]. Resistance is reported in up to 27% of CRE, generally due to chromosomally mediated mechanisms [86]. Plasmid-mediated mcr genes remain a rare cause of colistin resistance [87].
Challenges to the clinical use of colistin include complex pharmacokinetics that result from colistin being administered as a prodrug (colistimethate [CMS]) with slow, unpredictable, and incomplete conversion to active drug; variable dose unit definitions depending on region (Table 3); nephrotoxicity in approximately 40–60% of adults; and controversy surrounding optimal susceptibility testing methodology [88–90]. Administration of a colistin loading dose results in more rapid serum target attainment and use of doses higher than those currently recommended by the FDA may be needed to achieve adequate serum concentrations, particularly for organisms with colistin MICs ≥1 μg/mL and in patients with normal renal function [91–97].
Table 3.
Colistin and Polymyxin B Dosage Conversions
| Colistimethate Sodium | Colistimethate Sodium | Colistin-base Activity |
|---|---|---|
| 30 000 units | 2.4 mg | 1 mg |
| 1 000 000 units | 80 mg | 34 mg |
| 4 500 000 units | 360 mg | 150 mg |
| 9 000 000 units | 720 mg | 300 mg |
| Polymyxin B | ||
| 10 000 units | 1 mg | … |
Observational clinical studies focused on comparing colistin to other CRE-active agents have suggested high mortality rates with use of colistin monotherapy for CRE [73, 74, 76, 83], including a comparative effectiveness study that demonstrated a higher probability of poor outcomes with a colistin-based regimen compared with ceftazidime-avibactam [45].
Pediatric data related to colistin are limited. Like adult studies, pediatric PK data generally suggest that the doses currently recommended by the FDA result in inadequate serum concentrations [98–100]. One report suggested that a loading dose of 150 000 units/kg followed by 75 000 units/kg every 12 hours (slightly higher than the doses recommended in the United States; Tables 2 and 3) achieved target attainment in almost all patients [101]. However, the methodology used in this study has been questioned by experts who recommend caution in interpreting the investigators’ conclusions [102]. Clinical data in children are limited to case series and have often included patients with both CRE and other carbapenem-resistant organisms [103–105]. While clinical success rates were generally >70% and nephrotoxicity reported in <20% of children in these studies, an association between colistin monotherapy and mortality has been observed [3, 103–105].
Polymyxin B
Polymyxin B has a spectrum of activity similar to colistin, differing from colistin by only a single amino acid [85]. However, unlike colistin, polymyxin B is administered in its active form, which results in more rapid and consistent achievement of therapeutic concentrations in adult PK studies, though a loading dose is still recommended [106–110]. Polymyxin B does not rely on renal excretion, so dose adjustments based on renal impairment are not necessary [106, 110]. Of note, this lack of renal tubular secretion makes polymyxin B a less favorable option for urinary sources. Few clinical studies have compared colistin and polymyxin B, but available data suggest a lower incidence of nephrotoxicity and no difference in clinical outcomes for adults treated with polymyxin B [111]. Pediatric clinical and PK data are limited to case reports and case series [112–114].
Suggested Use of Polymyxins
While newer β-lactams have largely replaced the polymyxins for susceptible CRE isolates, polymyxins remain a consideration for MBL-producing isolates or in situations where alternatives to these agents are sought [115]. We favor polymyxin B over colistin given its more favorable PK characteristics and lower rates of nephrotoxicity for nonurinary sources of infection, with colistin favored for urinary sources [110, 115]. We recommend combination therapy whenever polymixins are prescribed (Figure 1) [110]. Finally, we recommend a loading dose be administered with either polymyxin based on the PK characteristics of these agents (Table 2).
TIGECYCLINE
Tigecycline is a glycylcycline antibiotic that acts on the bacterial ribosome and has excellent in vitro activity against CRE isolates, with 89% reported susceptible [116]. Tigecycline distributes rapidly into most tissues following administration, resulting in poor achievement of bactericidal serum concentrations at standard doses, and does not concentrate well in the lung endothelium or in urine [117]. Clinical data from observational studies suggest poor outcomes when tigecycline is used at standard doses as monotherapy for CRE infections, though this finding is likely dose-dependent as several subsequent studies have demonstrated improved efficacy of tigecycline for the treatment of CRE infections in adults with higher doses of 100 mg twice daily [73, 75, 76, 78, 118–121].
Published pediatric experience with tigecycline is limited to case reports, case series, and a PK study that demonstrated that a dose of 1–1.2 mg/kg of tigecycline every 12 hours achieves a similar area under the curve as standard adult doses [122, 123]. There are no PK data related to higher doses. However, based on improved efficacy observed in adult studies, doses of up to a 4 mg/kg loading dose followed by 2–3.2 mg/kg/dose every 12 hours have been reported [123].
Newer βL-βLI should replace tigecycline in cases of KPC- or OXA-producing CRE, but tigecycline may be an option for MBL-producing isolates. If used, we suggest a loading dose and a higher standing dose of 2–3.2 mg/kg/dose every 12 hours based on published series and our own clinical experience (Table 2). We also suggest combination therapy for bloodstream infections and in severely ill patients, while we suggest monotherapy for noncritically ill children with intraabdominal infections (Figure 1). Finally, tigecycline should generally be avoided for urinary sources when other options are available.
ERAVACYCLINE
The FDA approved eravacycline in 2018 [124]. Eravacycline is a synthetic tetracycline with good in vitro activity against KPC, MBL, and OXA-48–like Enterobacteriaceae and exhibits its mechanism of action through binding to the 30S ribosome [125, 126]. No clinical studies have evaluated its use specifically for CRE or other antibiotic-resistant organisms. In 2 trials that evaluated its use for cUTI, eravacycline failed to meet the noninferiority margin when compared with levofloxacin and ertapenem [127]. Despite these findings, eravacycline likely has a role in treatment of nonurinary and nonbacteremic infections due to CRE, including those due to MBL-producing isolates. A phase 1 pediatric study evaluating the PK of eravacycline is currently ongoing (NCT03696550) [128].
PLAZOMICIN
The FDA approved plazomicin in 2018 for adults with cUTIs [129, 130]. Plazomicin is a semisynthetic aminoglycoside resistant to modification by most aminoglycoside-modifying enzymes, which results in increased activity against KPC and OXA-48–like producing CRE compared with other aminoglycosides, as well as some activity against MBL-producing isolates [131, 132]. Clinical data related to its use for CRE are limited. The single phase 3 randomized trial comparing plazomicin-containing combination therapy to colistin-containing combination therapy for CRE bloodstream infections and pneumonia was terminated for low enrollment after randomizing just 39 patients. However, the plazomicin-exposed group had lower mortality (12% vs 40%) and a lower likelihood of acute kidney injury (8% vs 38%), suggesting that plazomicin is preferred over colistin [133, 134]. Use of plazomicin in children with CRE infections is currently limited by lack of pediatric dosing information.
CONCLUSIONS
Treatment of CRE infections in children is complex. Therapeutic decisions require expert consultation and an individualized approach, often based on adult data given the dearth of pediatric studies. The meropenem MIC of the infecting isolate, type of carbapenemase produced, the patient’s illness severity, and source of infection should be considered when selecting antibiotic therapy. Finally, while the treatment recommendations contained herein reflect currently available data, treatment paradigms are likely to evolve over time as agents in the antibiotic pipeline become available and pediatric experience with available agents grows.
Notes
Financial support. This work was supported by the Agency for Healthcare Research and Quality (K12-HS026393 to K. C.) and the National Institutes of Health (K23-AI127935 to P. D. T.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Logan LK, Renschler JP, Gandra S, et al. . Carbapenem-resistant Enterobacteriaceae in children, United States, 1999–2012. Emerg Infect Dis 2015; 21:2014–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chiotos K, Tamma PD, Flett KB, et al. . Increased 30-day mortality associated with carbapenem-resistant Enterobacteriaceae infections in children. Open Forum Infect Dis 2018; 5:ofy222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Montagnani C, Prato M, Scolfaro C, et al. ; Italian Society of Pediatric Infectious Diseases Carbapenem-resistant Enterobacteriaceae infections in children: an Italian retrospective multicenter study. Pediatr Infect Dis J 2016; 35:862–8. [DOI] [PubMed] [Google Scholar]
- 4. Nabarro LEB, Shankar C, Pragasam AK, et al. . Clinical and bacterial risk factors for mortality in children with carbapenem-resistant Enterobacteriaceae bloodstream infections in India. Pediatr Infect Dis J 2017; 36:e161–6. [DOI] [PubMed] [Google Scholar]
- 5. CRE Technical Information Available at: https://www.cdc.gov/hai/organisms/cre/definition.html. Accessed 3 October 2019.
- 6. Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing. M 100-S29. Wayne, PA: CLSI; 2019. [Google Scholar]
- 7. Logan LK, Weinstein RA. The epidemiology of carbapenem-resistant Enterobacteriaceae: the impact and evolution of a global menace. J Infect Dis 2017; 215:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tamma PD, Opene BN, Gluck A, et al. . Comparison of 11 phenotypic assays for accurate detection of carbapenemase-producing Enterobacteriaceae. J Clin Microbiol 2017; 55:1046–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Banerjee R, Humphries R. Clinical and laboratory considerations for the rapid detection of carbapenem-resistant Enterobacteriaceae. Virulence 2017; 8:427–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Guh AY, Bulens SN, Mu Y, et al. . Epidemiology of carbapenem-resistant Enterobacteriaceae in 7 US communities, 2012–2013. JAMA 2015; 314:1479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Duin D, Perez F, Rudin SD, et al. . Surveillance of carbapenem-resistant Klebsiella pneumoniae: tracking molecular epidemiology and outcomes through a regional network. Antimicrob Agents Chemother 2014; 58:4035–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Woodworth KR, Walters MS, Weiner LM, et al. . Vital signs: containment of novel multidrug-resistant organisms and resistance mechanisms–United States, 2006–2017. MMWR Morbid Mortal Wkly Rep 2018; 67:396–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chiotos K, Tamma PD, Flett KB, et al. . Risk factors for colonization or infection with carbapenem-resistant Enterobacteriaceae in children: a multicenter study. Antimicrob Agents Chemother 2017; 61:e01440–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pannaraj PS, Bard JD, Cerini C, Weissman SJ. Pediatric carbapenem-resistant Enterobacteriaceae in Los Angeles, California, a high-prevalence region in the United States. Pediatr Infect Dis J 2015; 34:11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pogue JM, Bonomo RA, Kaye KS. Ceftazidime/avibactam, meropenem/vaborbactam, or both? Clinical and formulary considerations. Clin Infect Dis 2019; 68:519–24. [DOI] [PubMed] [Google Scholar]
- 16. Zhanel GG, Lawrence CK, Adam H, et al. . Imipenem-relebactam and meropenem-vaborbactam: two novel carbapenem-β-lactamase inhibitor combinations. Drugs 2018; 78:65–98. [DOI] [PubMed] [Google Scholar]
- 17. Shannon DJ, Blosser S, Walters M, et al. . Notes from the field: domestically acquired Verona integron-mediated metallo-beta-lactamase-producing Enterobacteriaceae–Indiana, 2016–2017. MMWR Morbid Mort Wkly Rep 2018; 67:727–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Logan LK, Bonomo RA. Metallo-beta-lactamase (MBL)-producing Enterobacteriaceae in United States children. Open Forum Infect Dis 2016; 3:ofw090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tamma PD, Suwantarat N, Rudin SD, et al. . First report of a Verona integron-encoded metallo-β-lactamase-producing Klebsiella pneumoniae infection in a child in the United States. J Pediatric Infect Dis Soc 2016; 5:e24–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Albiger B, Glasner C, Struelens MJ, et al. . Carbapenemase-producing Enterobacteriaceae in Europe: assessment by national experts from 38 countries, May 2015. Euro Surveill 2015; 20. [DOI] [PubMed] [Google Scholar]
- 21. Doi Y, Wachino JI, Arakawa Y. Aminoglycoside resistance: the emergence of acquired 16S ribosomal RNA methyltransferases. Infect Dis Clin North Am 2016; 30:523–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Almaghrabi R, Clancy CJ, Doi Y, et al. . Carbapenem-resistant Klebsiella pneumoniae strains exhibit diversity in aminoglycoside-modifying enzymes, which exert differing effects on plazomicin and other agents. Antimicrob Agents Chemother 2014; 58:4443–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Endimiani A, Carias LL, Hujer AM, et al. . Presence of plasmid-mediated quinolone resistance in Klebsiella pneumoniae isolates possessing blaKPC in the United States. Antimicrob Agents Chemother 2008; 52:2680–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jacoby GA, Strahilevitz J, Hooper DC. Plasmid-mediated quinolone resistance. Microbiol Spectrum 2014; 2. doi: 10.1128/microbiolspec.PLAS-0006-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Little ML, Qin X, Zerr DM, Weissman SJ. Molecular diversity in mechanisms of carbapenem resistance in paediatric Enterobacteriaceae. Int J Antimicrob Agents 2012; 39:52–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tsai YK, Fung CP, Lin JC, et al. . Klebsiella pneumoniae outer membrane porins OmpK35 and OmpK36 play roles in both antimicrobial resistance and virulence. Antimicrob Agents Chemother 2011; 55:1485–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Du D, Wang Z, James NR, et al. . Structure of the AcrAB-TolC multidrug efflux pump. Nature 2014; 509:512–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hamzaoui Z, Ocampo-Sosa A, Fernandez Martinez M, et al. . Role of association of OmpK35 and OmpK36 alteration and blaESBL and/or blaAmpC genes in conferring carbapenem resistance among non-carbapenemase-producing Klebsiella pneumoniae. Int J Antimicrob Agents 2018; 52:898–905. [DOI] [PubMed] [Google Scholar]
- 29. Jacoby GA. AmpC beta-lactamases. Clin Microbiol Rev 2009; 22:161–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yang FC, Yan JJ, Hung KH, Wu JJ. Characterization of ertapenem-resistant Enterobacter cloacae in a Taiwanese university hospital. J Clin Microbiol 2012; 50:223–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Majewski P, Wieczorek P, Ojdana D, et al. . Altered outer membrane transcriptome balance with AmpC overexpression in carbapenem-resistant Enterobacter cloacae. Front Microbiol 2016; 7:2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wong D, van Duin D. Novel beta-lactamase inhibitors: unlocking their potential in therapy. Drugs 2017; 77:615–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pagès JM, Peslier S, Keating TA, et al. . Role of the outer membrane and porins in susceptibility of β-lactamase-producing Enterobacteriaceae to ceftazidime-avibactam. Antimicrob Agents Chemother 2015; 60:1349–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Castanheira M, Huband MD, Mendes RE, Flamm RK. Meropenem-vaborbactam tested against contemporary gram-negative isolates collected worldwide during 2014, including carbapenem-resistant, KPC-producing, multidrug-resistant, and extensively drug-resistant Enterobacteriaceae. Antimicrob Agents Chemother 2017; 61:e00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Castanheira M, Mills JC, Costello SE, et al. . Ceftazidime-avibactam activity tested against Enterobacteriaceae isolates from U.S. hospitals (2011 to 2013) and characterization of beta-lactamase-producing strains. Antimicrob Agents Chemother 2015; 59:3509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Livermore DM, Warner M, Mushtaq S. Activity of MK-7655 combined with imipenem against Enterobacteriaceae and Pseudomonas aeruginosa. J Antimicrob Chemother 2013; 68:2286–90. [DOI] [PubMed] [Google Scholar]
- 37. Gutiérrez-Gutiérrez B, Salamanca E, de Cueto M, et al. ; REIPI/ESGBIS/INCREMENT Investigators Effect of appropriate combination therapy on mortality of patients with bloodstream infections due to carbapenemase-producing Enterobacteriaceae (INCREMENT): a retrospective cohort study. Lancet Infect Dis 2017; 17:726–34. [DOI] [PubMed] [Google Scholar]
- 38. Drusano GL. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug’. Nat Rev Microbiol 2004; 2:289–300. [DOI] [PubMed] [Google Scholar]
- 39. Courter JD, Kuti JL, Girotto JE, Nicolau DP. Optimizing bactericidal exposure for beta-lactams using prolonged and continuous infusions in the pediatric population. Pediatr Blood Cancer 2009; 53:379–85. [DOI] [PubMed] [Google Scholar]
- 40. Cies JJ, Moore WS 2nd, Enache A, Chopra A. Population pharmacokinetics and pharmacodynamic target attainment of meropenem in critically ill young children. J Pediatr Pharmacol Ther 2017; 22:276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Daikos GL, Markogiannakis A. Carbapenemase-producing Klebsiella pneumoniae: (when) might we still consider treating with carbapenems? Clin Microbiol Infect 2011; 17:1135–41. [DOI] [PubMed] [Google Scholar]
- 42. Tzouvelekis LS, Markogiannakis A, Psichogiou M, et al. . Carbapenemases in Klebsiella pneumoniae and other Enterobacteriaceae: an evolving crisis of global dimensions. Clin Microbiol Rev 2012; 25:682–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tumbarello M, Trecarichi EM, Corona A, et al. . Efficacy of ceftazidime-avibactam salvage therapy in patients with infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin Infect Dis 2019; 68:355–64. [DOI] [PubMed] [Google Scholar]
- 44. Shields RK, Nguyen MH, Chen L, et al. . Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother 2017; 61:e00883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. van Duin D, Lok JJ, Earley M, et al. ; Antibacterial Resistance Leadership Group Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin Infect Dis 2018; 66:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.US Food and Drug Administration. Ceftazidime-avibactam prescribing information Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/206494s005,s006lbl.pdf. Accessed 3 October 2019.
- 47. Bradley JS, Roilides E, Broadhurst H, et al. . Safety and efficacy of ceftazidime-avibactam in the treatment of children ≥3 months to <18 years with complicated urinary tract infection: results from a phase 2 randomized, controlled trial. Pediatr Infect Dis J 2019; 38:920–8. [DOI] [PubMed] [Google Scholar]
- 48. Bradley JS, Broadhurst H, Cheng K, et al. . Safety and efficacy of ceftazidime-avibactam plus metronidazole in the treatment of children ≥3 months to <18 years with complicated intra-abdominal infection: results from a phase 2, randomized, controlled trial. Pediatr Infect Dis J 2019; 38:816–24. [DOI] [PubMed] [Google Scholar]
- 49. Citron DM, Tyrrell KL, Merriam V, Goldstein EJ. In vitro activity of ceftazidime-NXL104 against 396 strains of beta-lactamase-producing anaerobes. Antimicrob Agents Chemother 2011; 55:3616–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. van Duin D, Bonomo RA. Ceftazidime/avibactam and ceftolozane/tazobactam: second-generation β-lactam/β-lactamase inhibitor combinations. Clin Infect Dis 2016; 63:234–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Davido B, Fellous L, Lawrence C, et al. . Ceftazidime-avibactam and aztreonam, an interesting strategy to overcome beta-lactam resistance conferred by metallo-beta-lactamases in Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother 2017; 61:e01008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Marshall S, Hujer AM, Rojas LJ, et al. . Can ceftazidime-avibactam and aztreonam overcome beta-lactam resistance conferred by metallo-beta-lactamases in Enterobacteriaceae? Antimicrob Agents Chemother 2017; 61:e01008–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hackel M, Kazmierczak KM, Hoban DJ, et al. . Assessment of the in vitro activity of ceftazidime-avibactam against multidrug-resistant Klebsiella spp. collected in the INFORM Global Surveillance Study, 2012 to 2014. Antimicrob Agents Chemother 2016; 60:4677–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. King M, Heil E, Kuriakose S, et al. . Multicenter study of outcomes with ceftazidime-avibactam in patients with carbapenem-resistant Enterobacteriaceae infections. Antimicrob Agents Chemother 2017; 61:e00449–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Temkin E, Torre-Cisneros J, Beovic B, et al. . Ceftazidime-avibactam as salvage therapy for infections caused by carbapenem-resistant organisms. Antimicrob Agents Chemother 2017; 61:e01964–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Shields RK, Potoski BA, Haidar G, et al. . Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis 2016; 63:1615–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Iosifidis E, Chorafa E, Agakidou E, et al. . Use of ceftazidime-avibactam for the treatment of extensively drug-resistant or pan drug-resistant Klebsiella pneumoniae in neonates and children <5 years of age. Pediatr Infect Dis J 2019; 38:812–5. [DOI] [PubMed] [Google Scholar]
- 58. Giddins MJ, Macesic N, Annavajhala MK, et al. . Successive emergence of ceftazidime-avibactam resistance through distinct genomic adaptations in blaKPC-2-harboring Klebsiella pneumoniae sequence type 307 isolates. Antimicrob Agents Chemother 2018; 62:e02101–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kaye KS, Bhowmick T, Metallidis S, et al. . Effect of meropenem-vaborbactam vs piperacillin-tazobactam on clinical cure or improvement and microbial eradication in complicated urinary tract infection: the TANGO I randomized clinical trial. JAMA 2018; 319:788–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hackel MA, Lomovskaya O, Dudley MN, Karlowsky JA, Sahm DF. In vitro activity of meropenem-vaborbactam against clinical isolates of KPC-positive Enterobacteriaceae. Antimicrob Agents Chemother 2018; 62:e01904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wunderink RG, Giamarellos-Bourboulis EJ, Rahav G, et al. . Effect and safety of meropenem-vaborbactam versus best-available therapy in patients with carbapenem-resistant Enterobacteriaceae infections: the TANGO II randomized clinical trial. Infect Dis Ther 2018; 7:439–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sun D, Rubio-Aparicio D, Nelson K, et al. . Meropenem-vaborbactam resistance selection, resistance prevention, and molecular mechanisms in mutants of KPC-producing Klebsiella pneumoniae. Antimicrob Agents Chemother 2017; 61:e01694–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lomovskaya O, Castanheira M, Vazquez J, et al. . Assessment of MIC increases with meropenem-vaborbactam and ceftazidime-avibactam in TANGO II (a phase 3 study of the treatment of CRE infections) [Abstract 1874]. In: IDWeek 2017 San Diego, CA; 4–8 October 2017. [Google Scholar]
- 64. Hanretty AM, Kaur I, Evangelista AT, et al. . Pharmacokinetics of the meropenem component of meropenem-vaborbactam in the treatment of KPC-producing Klebsiella pneumoniae bloodstream infection in a pediatric patient. Pharmacotherapy 2018; 38:e87–91. [DOI] [PubMed] [Google Scholar]
- 65. Dose-finding, pharmacokinetics, safety, and tolerability of VABOMERE (Meropenem-Vaborbactam) in pediatric subjects with serious bacterial infections (TANGOKIDS) Available at: https://www.clinicaltrials.gov/ct2/show/record/NCT02687906?term=meropenem-vaborbactam&rank=1. Accessed 3 October 2019.
- 66. Lucasti C, Vasile L, Sandesc D, et al. . Phase 2, dose-ranging study of relebactam with imipenem-cilastatin in subjects with complicated intra-abdominal infection. Antimicrob Agents Chemother 2016; 60:6234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sims M, Mariyanovski V, McLeroth P, et al. . Prospective, randomized, double-blind, phase 2 dose-ranging study comparing efficacy and safety of imipenem/cilastatin plus relebactam with imipenem/cilastatin alone in patients with complicated urinary tract infections. J Antimicrob Chemother 2017; 72:2616–26. [DOI] [PubMed] [Google Scholar]
- 68. Lapuebla A, Abdallah M, Olafisoye O, et al. . Activity of imipenem with relebactam against gram-negative pathogens from New York City. Antimicrob Agents Chemother 2015; 59:5029–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Motsch J, Murta de Oliveira C, Stus V, et al. . RESTORE-IMI 1: a multicenter, randomized, double-blind trial comparing efficacy and safety of imipenem/relebactam vs colistin plus imipenem in patients with imipenem-nonsusceptible bacterial infections. Clin Infect Dis 2019; epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Imipenem/relebactam/cilastatin versus piperacillin/tazobactam for treatment of participants with bacterial pneumonia (MK-7655A-014) (RESTORE-IMI 2) Available at: https://clinicaltrials.gov/ct2/show/NCT02493764. Accessed 3 October 2019.
- 71. A pharmacokinetics study of MK-7655A in pediatric participants with gram-negative infections (MK-7655A-020) Available at: https://clinicaltrials.gov/ct2/show/NCT03230916. Accessed 3 October 2019.
- 72. Safety, tolerability, efficacy and pharmacokinetics of imipenem/cilastatin/relebactam (MK-7655A) in pediatric participants with gram-negative bacterial infection (MK-7655A-021) Available at: https://clinicaltrials.gov/ct2/show/NCT03969901. Accessed 3 October 2019.
- 73. Qureshi ZA, Paterson DL, Potoski BA, et al. . Treatment outcome of bacteremia due to KPC-producing Klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrob Agents Chemother 2012; 56:2108–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zarkotou O, Pournaras S, Tselioti P, et al. . Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect 2011; 17:1798–803. [DOI] [PubMed] [Google Scholar]
- 75. Tumbarello M, Viale P, Viscoli C, et al. . Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae: importance of combination therapy. Clin Infect Dis 2012; 55:943–50. [DOI] [PubMed] [Google Scholar]
- 76. Tzouvelekis LS, Markogiannakis A, Piperaki E, et al. . Treating infections caused by carbapenemase-producing Enterobacteriaceae. Clin Microbiol Infect 2014; 20:862–72. [DOI] [PubMed] [Google Scholar]
- 77. Falagas ME, Lourida P, Poulikakos P, et al. . Antibiotic treatment of infections due to carbapenem-resistant Enterobacteriaceae: systematic evaluation of the available evidence. Antimicrob Agents Chemother 2014; 58:654–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Daikos GL, Tsaousi S, Tzouvelekis LS, et al. . Carbapenemase-producing Klebsiella pneumoniae bloodstream infections: lowering mortality by antibiotic combination schemes and the role of carbapenems. Antimicrob Agents Chemother 2014; 58:2322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Tumbarello M, Trecarichi EM, De Rosa FG, et al. ; Italian Study Group on Resistant Infections of the Società Italiana Terapia Antinfettiva Infections caused by KPC-producing Klebsiella pneumoniae: differences in therapy and mortality in a multicentre study. J Antimicrob Chemother 2015; 70:2133–43. [DOI] [PubMed] [Google Scholar]
- 80. Paul M, Daikos GL, Durante-Mangoni E, et al. . Colistin alone versus colistin plus meropenem for treatment of severe infections caused by carbapenem-resistant gram-negative bacteria: an open-label, randomised controlled trial. Lancet Infect Dis 2018; 18:391–400. [DOI] [PubMed] [Google Scholar]
- 81. Gomez-Simmonds A, Nelson B, Eiras DP, et al. . Combination regimens for treatment of carbapenem-resistant Klebsiella pneumoniae bloodstream infections. Antimicrob Agents Chemother 2016; 60:3601–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. de Maio Carrilho CM, de Oliveira LM, Gaudereto J, et al. . A prospective study of treatment of carbapenem-resistant Enterobacteriaceae infections and risk factors associated with outcome. BMC Infect Dis 2016; 16:629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. de Oliveira MS, de Assis DB, Freire MP, et al. . Treatment of KPC-producing Enterobacteriaceae: suboptimal efficacy of polymyxins. Clin Microbiol Infect 2015; 21:e1–7. [DOI] [PubMed] [Google Scholar]
- 84. Satlin MJ, Kubin CJ, Blumenthal JS, et al. . Comparative effectiveness of aminoglycosides, polymyxin B, and tigecycline for clearance of carbapenem-resistant Klebsiella pneumoniae from urine. Antimicrob Agents Chemother 2011; 55:5893–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Poirel L, Jayol A, Nordmann P. Polymyxins: antibacterial activity, susceptibility testing, and resistance mechanisms encoded by plasmids or chromosomes. Clin Microbiol Rev 2017; 30:557–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tansarli GS, Papaparaskevas J, Balaska M, et al. . Colistin resistance in carbapenemase-producing Klebsiella pneumoniae bloodstream isolates: evolution over 15 years and temporal association with colistin use by time series analysis. Int J Antimicrob Agents 2018; 52:397–403. [DOI] [PubMed] [Google Scholar]
- 87. Cannatelli A, Giani T, Antonelli A, et al. . First detection of the mcr-1 colistin resistance gene in Escherichia coli in Italy. Antimicrob Agents Chemother 2016; 60:3257–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Nation RL, Li J, Cars O, et al. . Framework for optimisation of the clinical use of colistin and polymyxin B: the Prato polymyxin consensus. Lancet Infect Dis 2015; 15:225–34. [DOI] [PubMed] [Google Scholar]
- 89. Akajagbor DS, Wilson SL, Shere-Wolfe KD, et al. . Higher incidence of acute kidney injury with intravenous colistimethate sodium compared with polymyxin B in critically ill patients at a tertiary care medical center. Clin Infect Dis 2013; 57:1300–3. [DOI] [PubMed] [Google Scholar]
- 90. Rigatto MH, Oliveira MS, Perdigão-Neto LV, et al. . Multicenter prospective cohort study of renal failure in patients treated with colistin versus polymyxin B. Antimicrob Agents Chemother 2016; 60:2443–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Karaiskos I, Friberg LE, Pontikis K, et al. . Colistin population pharmacokinetics after application of a loading dose of 9 MU colistin methanesulfonate in critically ill patients. Antimicrob Agents Chemother 2015; 59:7240–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Mohamed AF, Karaiskos I, Plachouras D, et al. . Application of a loading dose of colistin methanesulfonate in critically ill patients: population pharmacokinetics, protein binding, and prediction of bacterial kill. Antimicrob Agents Chemother 2012; 56:4241–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Nation RL, Garonzik SM, Li J, et al. . Updated US and European dose recommendations for intravenous colistin: how do they perform? Clin Infect Dis 2016; 62:552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Nation RL, Garonzik SM, Thamlikitkul V, et al. . Dosing guidance for intravenous colistin in critically-ill patients. Clin Infect Dis 2017; 64:565–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Garonzik SM, Li J, Thamlikitkul V, et al. . Population pharmacokinetics of colistin methanesulfonate and formed colistin in critically ill patients from a multicenter study provide dosing suggestions for various categories of patients. Antimicrob Agents Chemother 2011; 55:3284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Plachouras D, Karvanen M, Friberg LE, et al. . Population pharmacokinetic analysis of colistin methanesulfonate and colistin after intravenous administration in critically ill patients with infections caused by gram-negative bacteria. Antimicrob Agents Chemother 2009; 53:3430–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Perez F, El Chakhtoura NG, Yasmin M, Bonomo RA. Polymyxins: to combine or not to combine? Antibiotics 2019; 8:e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Antachopoulos C, Iosifidis E. Colistin use in neonates and children with infections due to carbapenem-resistant bacteria. Pediatr Infect Dis J 2017; 36: 905–7. [DOI] [PubMed] [Google Scholar]
- 99. Nakwan N, Usaha S, Chokephaibulkit K, et al. . Pharmacokinetics of colistin following a single dose of intravenous colistimethate sodium in critically ill neonates. Pediatr Infect Dis J 2016; 35:1211–4. [DOI] [PubMed] [Google Scholar]
- 100. Antachopoulos C, Karvanen M, Iosifidis E, et al. . Serum and cerebrospinal fluid levels of colistin in pediatric patients. Antimicrob Agents Chemother 2010; 54:3985–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mesini A, Loy A, Gattorno M, et al. . Colistin area under the time-concentration in children treated with intravenous loading dose and maintenance therapy. Clin Infect Dis 2018; 66:808–9. [DOI] [PubMed] [Google Scholar]
- 102. Nation RL. Dose suggestions for intravenous colistin in pediatric patients: caution required. Clin Infect Dis 2018; 66:810–1. [DOI] [PubMed] [Google Scholar]
- 103. Karbuz A, Özdemir H, Yaman A, et al. . The use of colistin in critically ill children in a pediatric intensive care unit. Pediatr Infect Dis J 2014; 33:e19–24. [DOI] [PubMed] [Google Scholar]
- 104. Kapoor K, Jajoo M, Dublish S, et al. . Intravenous colistin for multidrug-resistant gram-negative infections in critically ill pediatric patients. Pediatr Crit Care Med 2013; 14:e268–72. [DOI] [PubMed] [Google Scholar]
- 105. Tamma PD, Newland JG, Pannaraj PS, et al. . The use of intravenous colistin among children in the United States: results from a multicenter, case series. Pediatr Infect Dis J 2013; 32:17–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Sandri AM, Landersdorfer CB, Jacob J, et al. . Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin Infect Dis 2013; 57:524–31. [DOI] [PubMed] [Google Scholar]
- 107. Manchandani P, Thamlikitkul V, Dubrovskaya Y, et al. . Population pharmacokinetics of polymyxin B. Clin Pharmacol Ther 2018; 104:534–8. [DOI] [PubMed] [Google Scholar]
- 108. Zavascki AP, Goldani LZ, Cao G, et al. . Pharmacokinetics of intravenous polymyxin B in critically ill patients. Clin Infect Dis 2008; 47:1298–304. [DOI] [PubMed] [Google Scholar]
- 109. Miglis C, Rhodes NJ, Avedissian SN, et al. . Population pharmacokinetics of polymyxin B in acutely ill adult patients. Antimicrob Agents Chemother 2018; 62:e01475–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tsuji BT, Pogue JM, Zavascki AP, et al. . International consensus guidelines for the optimal use of the polymyxins: endorsed by the American College of Clinical Pharmacy (ACCP), European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Infectious Diseases Society of America (IDSA), International Society for Anti-infective Pharmacology (ISAP), Society of Critical Care Medicine (SCCM), and Society of Infectious Diseases Pharmacists (SIDP). Pharmacotherapy 2019; 39:10–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Vardakas KZ, Falagas ME. Colistin versus polymyxin B for the treatment of patients with multidrug-resistant gram-negative infections: a systematic review and meta-analysis. Int J Antimicrob Agents 2017; 49:233–8. [DOI] [PubMed] [Google Scholar]
- 112. Siddiqui NU, Qamar FN, Jurair H, Haque A. Multi-drug resistant gram negative infections and use of intravenous polymyxin B in critically ill children of developing country: retrospective cohort study. BMC Infect Dis 2014; 14:626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Dara JS, Chen L, Levi MH, et al. . Microbiological and genetic characterization of carbapenem-resistant Klebsiella pneumoniae isolated from pediatric patients. J Pediatric Infect Dis Soc 2014; 3:e10–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Salvatore CM, Abdelraouf K, Hsing DD, Tam VH. Pharmacokinetics of polymyxin B in an infant with multidrug-resistant Klebsiella pneumoniae bacteremia. Pediatr Infect Dis J 2011; 30:537–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Thomas R, Velaphi S, Ellis S, et al. . The use of polymyxins to treat carbapenem resistant infections in neonates and children. Expert Opin Pharmacother 2019; 20:415–22. [DOI] [PubMed] [Google Scholar]
- 116. Sader HS, Castanheira M, Flamm RK, et al. . Tigecycline activity tested against carbapenem-resistant Enterobacteriaceae from 18 European nations: results from the SENTRY surveillance program (2010–2013). Diagn Microbiol Infect Dis 2015; 83:183–6. [DOI] [PubMed] [Google Scholar]
- 117. Falagas ME, Karageorgopoulos DE, Dimopoulos G. Clinical significance of the pharmacokinetic and pharmacodynamic characteristics of tigecycline. Curr Drug Metab 2009; 10:13–21. [DOI] [PubMed] [Google Scholar]
- 118. Falagas ME, Vardakas KZ, Tsiveriotis KP, et al. . Effectiveness and safety of high-dose tigecycline-containing regimens for the treatment of severe bacterial infections. Int J Antimicrob Agents 2014; 44:1–7. [DOI] [PubMed] [Google Scholar]
- 119. De Pascale G, Montini L, Pennisi M, et al. . High dose tigecycline in critically ill patients with severe infections due to multidrug-resistant bacteria. Crit Care 2014; 18:R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sbrana F, Malacarne P, Viaggi B, et al. . Carbapenem-sparing antibiotic regimens for infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae in intensive care unit. Clin Infect Dis 2013; 56:697–700. [DOI] [PubMed] [Google Scholar]
- 121. Ramirez J, Dartois N, Gandjini H, et al. . Randomized phase 2 trial to evaluate the clinical efficacy of two high-dosage tigecycline regimens versus imipenem-cilastatin for treatment of hospital-acquired pneumonia. Antimicrob Agents Chemother 2013; 57:1756–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Purdy J, Jouve S, Yan JL, et al. . Pharmacokinetics and safety profile of tigecycline in children aged 8 to 11 years with selected serious infections: a multicenter, open-label, ascending-dose study. Clin Ther 2012; 34:496–507.e1. [DOI] [PubMed] [Google Scholar]
- 123. Iosifidis E, Violaki A, Michalopoulou E, et al. . Use of tigecycline in pediatric patients with infections predominantly due to extensively drug-resistant gram-negative bacteria. J Pediatric Infect Dis Soc 2017; 6:123–8. [DOI] [PubMed] [Google Scholar]
- 124. Solomkin J, Evans D, Slepavicius A, et al. . Assessing the efficacy and safety of eravacycline vs ertapenem in complicated intra-abdominal infections in the investigating gram-negative infections treated with eravacycline (IGNITE 1) trial: a randomized clinical trial. JAMA Surg 2017; 152:224–32. [DOI] [PubMed] [Google Scholar]
- 125. Zhanel GG, Cheung D, Adam H, et al. . Review of eravacycline, a novel fluorocycline antibacterial agent. Drugs 2016; 76:567–88. [DOI] [PubMed] [Google Scholar]
- 126. Livermore DM, Mushtaq S, Warner M, Woodford N. In vitro activity of eravacycline against carbapenem-resistant Enterobacteriaceae and Acinetobacter baumannii. Antimicrob Agents Chemother 2016; 60:3840–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Tetraphase announces top line results from IGNITE3 phase 3 clinical trial of eravacycline in complicated urinary tract infections (cUTI) Available at: https://ir.tphase.com/news-releases/news-release-details/tetraphase-announces-top-line-results-ignite3-phase-3-clinical. Accessed 3 October 2019.
- 128. A safety and PK study of IV eravacycline Available at: https://clinicaltrials.gov/ct2/show/NCT03696550?term=eravacycline&rank=6. Accessed 5 June 2019.
- 129. Connolly LE, Riddle V, Cebrik D, et al. . A multicenter, randomized, double-blind, phase 2 study of the efficacy and safety of plazomicin compared with levofloxacin in the treatment of complicated urinary tract infection and acute pyelonephritis. Antimicrob Agents Chemother 2018; 62:e01989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Wagenlehner FME, Cloutier DJ, Komirenko AS, et al. ; EPIC Study Group Once-daily plazomicin for complicated urinary tract infections. N Engl J Med 2019; 380:729–40. [DOI] [PubMed] [Google Scholar]
- 131. Castanheira M, Deshpande LM, Woosley LN, et al. . Activity of plazomicin compared with other aminoglycosides against isolates from European and adjacent countries, including Enterobacteriaceae molecularly characterized for aminoglycoside-modifying enzymes and other resistance mechanisms. J Antimicrob Chemother 2018; 73:2246–54. [DOI] [PubMed] [Google Scholar]
- 132. Galani I, Nafplioti K, Adamou P, et al. ; Study Collaborators Nationwide epidemiology of carbapenem resistant Klebsiella pneumoniae isolates from Greek hospitals, with regards to plazomicin and aminoglycoside resistance. BMC Infect Dis 2019; 19:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. McKinnell JA, Dwyer JP, Talbot GH, et al. ; CARE Study Group Plazomicin for infections caused by carbapenem-resistant Enterobacteriaceae. N Engl J Med 2019; 380:791–3. [DOI] [PubMed] [Google Scholar]
- 134. Connolly LE, Jubb AM, O’Keeffe B, et al. . Plazomicin is associated with improved survival and safety compared with colistin in the treatment of serious infections due to carbapenem-resistant Enterobacteriaceae: results of the CARE study. In: 27th European Congress of Clinical Microbiology and Infectious Diseases Vienna, Austria; April 22–25, 2017. [Google Scholar]