Abstract
Gene duplication and polyploidization are genetic mechanisms that instantly add genetic material to an organism's genome. Subsequent modification of the duplicated material leads to the evolution of neofunctionalization (new genetic functions), subfunctionalization (differential retention of genetic functions), redundancy, or a decay of duplicated genes to pseudogenes. Phytochromes are light receptors that play a large role in plant development. They are encoded by a small gene family that in tomato is comprised of five members: PHYA, PHYB1, PHYB2, PHYE, and PHYF. The most recent gene duplication within this family was in the ancestral PHYB gene. Using transcriptome profiling, co‐expression network analysis, and physiological and molecular experimentation, we show that tomato SlPHYB1 and SlPHYB2 exhibit both common and non‐redundant functions. Specifically, PHYB1 appears to be the major integrator of light and auxin responses, such as gravitropism and phototropism, while PHYB1 and PHYB2 regulate aspects of photosynthesis antagonistically to each other, suggesting that the genes have subfunctionalized since their duplication.
Keywords: auxin, gene duplication, photosynthesis, phototropism, phytochrome, subfunctionalization
1. INTRODUCTION
Gene duplication is a powerful evolutionary mechanism that creates new genetic material on which natural selection can act (Moore & Purugganan, 2005). Duplication of genes can occur during polyploidization, also known as whole genome duplication (WGD), which may be the outcome of somatic genome doubling or the result of errors during meiosis leading to unreduced gametes (Bomblies & Madlung, 2014). While WGD is the most drastic form of gene duplication, other mechanisms can also lead to the duplication of individual genes. For example, unequal crossing over can lead to the formation of chromosomes with more or fewer copies of a given gene (known as tandem duplication), or transposons can copy and move genes within or between chromosomes (Panchy, Lehti‐Shiu, & Shiu, 2016). Regardless by which mechanism they arise, duplicated genes can lead to the formation of multi‐gene families and lay the foundation for evolutionary innovations (Van de Peer, Maere, & Meyer, 2009).
One important family of plant genes are the phytochromes. Plants use both internal and external cues as signals to guide their growth and development, and to help them respond to their environment, such as to light quality and light quantity, temperature, moisture, or nutrient availability. Phytochromes (phys) are light‐absorbing chromoproteins that consist of a chromophore and an apoprotein, which together transmit light signals and regulate gene expression in response to light (Chen & Chory, 2011; Franklin & Quail, 2010). The phy apoproteins are encoded by a multi‐gene family that generally consists of a predominantly far‐red (FR) responsive phy, phyA, and one or more predominantly red light (R) responsive phys. In Arabidopsis, the R responsive phys are encoded by four genes: AtPHYB—AtPHYE. Phylogenetically, gene duplication of an ancestral phytochrome gene first separated PHYA/C from the other PHYs. Subsequently, PHYA separated from PHYC, and PHYB/D from PHYE (Li et al., 2015; Mathews & Sharrock, 1997). Eventually, after the divergence of the Brassicales, PHYB/D separated into PHYB and PHYD genes in Arabidopsis (Mathews & Sharrock, 1997).
PHYs in tomato have not undergone the same phylogenetic evolution as in Arabidopsis. For instance, SlPHYB1 and SlPHYB2 (hereafter simply called PHYB1 and PHYB2) are similar to AtPHYB and AtPHYD but these genes arose separately by a gene duplication event after the separation of the Solanales from the Brassicales about 110 Mya (Alba, Kelmenson, Cordonnier‐Pratt, & Pratt, 2000; Pratt, Cordonnier‐Pratt, Hauser, & Caboche, 1995), suggesting that any functional divergence of the duplicated genes would be unlikely to be the same in the two plant families. In contrast to Arabidopsis, mutation of phyB1 in tomato results only in temporary red light insensitivity at a young seedling stage while phyB1 adults look very similar in phenotype to WT tomato (Lazarova et al., 1998). In Arabidopsis and pea, PHYB plays a role during de‐etiolation (Neff & Chory, 1998), chlorophyll production (Foo, Ross, Davies, Reid, & Weller, 2006), photo‐reversible seed germination (Shinomura et al., 1996), timing of flowering (Khanna, Kikis, & Quail, 2003), the shade avoidance response (Keller et al., 2011), and the mediation of hormone responses (Borevitz et al., 2002), including lateral root initiation via auxin transport signaling (Salisbury, Hall, Grierson, & Halliday, 2007), polar auxin transport (Liu, Cohen, & Gardner, 2011), and seed germination via the regulation of abscisic acid (ABA) (Seo et al., 2006). Compared to Arabidopsis, much less is known about the functions of phys in the Solanales. In tomato, PHYB1 is involved in hypocotyl inhibition, de‐etiolation, and pigment production in R (Kendrick et al., 1994; Kendrick, Kerckhoffs, Tuinen, & Koornneef, 1997; van Tuinen, Kerckhoffs, Nagatani, Kendrick, & Koornneef, 1995). PHYB2 plays a role in early seedling development (Hauser, Cordonnier‐Pratt, & Pratt, 1998), and, in cooperation with PHYA and PHYB1, in the control of de‐etiolation (Weller, Schreuder, Smith, Koornneef, & Kendrick, 2000). Analysis of phyb1;phyb2 double mutants in tomato showed that a high level of redundancy exists between the two genes with respect to hypocotyl elongation during de‐etiolation in both white light and R (Weller et al., 2000). Chlorophyll and anthocyanin production, on the other hand, was only reduced in the phyB1 mutant and not in phyB2, but the phyb1;phyb2 double mutant displayed a synergistic phenotype with less of both pigments than found in the phyb1 mutant alone, suggesting that phyB2 contributes to pigment production in a significant manner (Weller et al., 2000). Subfunctionalization of the B‐class phytochromes was also shown in maize, where ZmPHYB1 was the predominant phy to regulate mesocotyl elongation in R, while ZmPHYB2 was mainly responsible for the photoperiod‐dependent transition from vegetative to floral development (Sheehan, Kennedy, Costich, & Brutnell, 2007).
To better understand to what degree subfunctionalization has occurred between tomato phyB1 and phyB2, we employed transcriptome profiling and co‐expression network analysis. We found that tomato PHYB1 and PHYB2 exhibit both common and non‐redundant functions. According to our analysis, two major areas of potential subfunctionalization are the regulation of genes involved in response to auxin and in photosynthesis. To verify the biological relevance of our genomic analyses, we tested phyB1 and phyB2 mutants for classical auxin responses, including phototropism and gravitropism, and for the rate of photosynthetic assimilation. We report here that phyB1 and phyB2 indeed differ in their involvement in some of these phenotypes, suggesting that the recent PHYB duplication in tomato has led to subfunctionalization that is different from those in maize or Arabidopsis.
2. METHODS
2.1. Plant materials and growth conditions
Solanum lycopersicum seeds of cultivar Moneymaker (Gourmet Seed, Hollister, CA, United States) and homozygous phyB1 mutants (allele tri1) and phyB2 mutants (allele 2‐1 (aka 70F), (Kerckhoffs et al., 1999; Weller et al., 2000) were used in all experiments. Both mutants used in this study were in the Moneymaker background (original source: Tomato Genome Resource Center, Davis, CA, USA). For RNAseq experiments, seeds were surface sterilized using 10% bleach for 15 min in ambient laboratory conditions and then sown on water‐saturated, sterile filter paper in light‐excluding plastic boxes. Plants were grown in a dark growth chamber at 25°C. Five‐day‐old seedlings of similar height were harvested under green safe light (522 nm LED), and flash‐frozen in liquid nitrogen. Seedling handling and harvesting at room temperature under safelight conditions was limited to a few minutes of indirect exposure. The remaining seedlings were exposed to 60 min of red light (660 nm, using a custom‐made LED display, 10 μmol m−2 s−1) and then selected, harvested, and frozen as described for the dark‐grown seedlings. Specimens were stored at −80°C until RNA was extracted. Tissue was grown in four biological replicates under the same conditions.
2.2. RNA extraction and sequencing
Tissue was flash‐frozen in liquid nitrogen and pulverized with a mortar and pestle. About 5 seedlings (~100 mg) were pooled per biological replicate for each genotype and condition. Total RNA was extracted using an RNeasy Plant Mini Kit (Qiagen) according to the manufacturer's instructions. TruSeq stranded mRNA library construction was performed by the Research Technology Support Facility at Michigan State University. Paired end 125 bp reads were obtained using an Illumina Hi‐Seq 2500 instrument. All data were uploaded for public use to NCBI’s short read archive http://www.ncbi.nlm.nih.gov/sra/SRP108371.
2.3. RNAseq differential expression analysis
RNAseq reads were mapped with HISAT2 to the SL3.0 version of the tomato genome with ITAG3.2 genome annotation from SolGenomics (http://www.solgenomics.org). First, phyB1 experiment reads and phyB2 experiment reads were mapped separately, and then they were mapped together. DESeq was used largely with default parameters to identify differentially expressed genes between wild type in the dark and wild type in R and between phyB mutants in the dark and in R, except that we used an alpha value of 0.05 for the multiple comparison adjustment. Genes identified in the phyB1 experiment alone as significantly differentially expressed (DE) by DESeq and with a abs(log2(fold change)) > 0.63, that is, changed by at least 1.5‐fold between dark and R, were then looked at in the phyB1 comparison. If the gene had a log2(FC) that was significantly different from WT, we called the gene phyB1‐regulated. To be characterized as significantly different, the difference between the log2(FC) in WT and phyB1 had to be greater than the sum of the standard errors of the log2(FC) in WT and in phyB1. The process was repeated with the phyB2 experiment alone to identify phyB2‐regulated genes.
2.4. Co‐expression analysis with WGCNA
From the data in which phyB1 and phyB2 experiment reads were mapped together, normalized read counts were obtained from DESeq. The variance of normalized expression was calculated across all samples (10 WT‐D, 10 WT‐R, 5 B1‐D, 5 B1‐R, 5 B2‐D, 5 B2‐R), and the top 8,000 most variable genes were identified. Their expression values were log transformed [log2(normalized read count + 1)] and used as input for WGCNA in R to identify co‐expression modules. Beta was set to 10 for the adjacency function. Modules were obtained based on topological overlap and eigenvectors representing average expression of each module were correlated to condition (dark = 0, 60 min R = 1) and genotype (either phyB1 = 1, phyB2 and WT = 0 or phyB2 = 1, phyB1 and WT = 0).
2.5. GO enrichment analysis
To determine which gene ontology (GO) categories were significantly enriched among the differentially regulated or co‐expressed genes, we used the R package topGO (Alexa & Rahnenfuhrer, 2010; Alexa, Rahnenführer, & Lengauer, 2006). Only categories with p‐values < 0.05 from Fisher's exact tests (weighted models) are reported. For topGO’s “gene universe,” GO annotations for S. lycopersicum were downloaded from the Panther Classification System (http://www.pantherdb.org, downloaded May 2017).
2.6. Gravitropism
Wild type, phyB1, and phyB2 seeds were sown at 12p.m., 5p.m., and 1p.m., respectively, to coordinate germination times (age‐synchronized) and assure equal developmental stages at the time of experimentation. Seeds were sterilized by stirring for 15 min in 10% bleach in the dark and sown into light‐excluding plastic boxes with saturated paper towels and filter paper under green light. Seeds were grown in the dark in a growth chamber at 25°C for 5 days. Age‐synchronized seedlings were transferred under green light to 1% agar plates, placed either in dark, under R (135 µE) from the top, or in R from opposite sides (60 µE) and allowed to grow with the same gravity vector for 1 hr. Seedlings were then gravistimulated by rotating plates 90 degrees. Photographs were taken before gravistimulation (0 hr), after 4, 8, and 24 hr.
The angle of bending was measured with ImageJ. A three‐way ANOVA (genotype, light condition, time) was performed in R followed by Tukey's post hoc test to determine statistically significant differences between groups.
2.7. Phototropism
For phototropism experiments, age‐synchronized seedlings (Moneymaker, phyB1, and phyB2) were grown in individual plastic scintillation vials filled with soil and incubated in the dark at 25°C for 5 days. Seedlings with similar hypocotyl length were then transferred to a black box illuminated with unilateral white light through a slit in the box. The plants were positioned such that their apical hook was facing away from the light source. Every hour over a time period of five hours, a set of plants was removed and scanned. The phototrophic bending angle of these plants was determined by ImageJ analysis, and data were plotted using R software. Data were analyzed by a two‐way ANOVA (genotype, time) using the software R.
For qPCR analysis of PHOT genes, tomato seedlings were grown as for the phototropic experiments. Material was harvested and flash‐frozen at the indicated times. Total RNA was extracted using an RNeasy kit (Qiagen) according to the manufacturer's instructions. Reverse transcription was performed using the iScript cDNA Synthesis kit (Bio‐Rad) with the recommended incubation times and temperatures as follows: 25°C for 5 min, 46°C for 20 min, and 95°C for 1 min. QPCR was performed on a Bio‐Rad Mastercycler C1000 using iTAQ Universal SYBR Green Supermix (Bio‐Rad) with an incubation at 95°C for 3 min, followed by 40 cycles at 95°C for 10 s, and 60°C for 30 s. SAND (Solyc03g115810) and RPL2 (Solyc10g006580) genes were used for normalization. Primer specificity was verified using the melt curves, and data were analyzed by the 2–ΔΔCt method (Livak & Schmittgen, 2001). Statistical analysis was performed using ANOVA (R version 3.4.1) on log10 normalized expression values. The primers are listed in Table S6. Three biological replicates were used with five seedlings per genotype and time point per biological replicate.
2.8. Photosynthetic analysis and chlorophyll quantification
Six‐week‐old Moneymaker, phyB1, and phyB2 plants grown in a growth chamber at 25°C under 16 hr of light were used for photosynthetic analysis and chlorophyll quantification. A LI‐COR 6400XT portable photosynthesis system (LI‐COR) with a standard leaf chamber and a LI‐COR 6400 LED light source was used for photosynthetic efficiency measurement. To ensure best uniformity, we chose for analysis the terminal leaflet of the fourth youngest, fully developed leaf. Single leaflets still attached to the plant were clamped flat into the standard leaf chamber. The conditions in the leaf chamber were set at a reference CO2 value of 400 mmol and a temperature of 21°C, and CO2 uptake was measured at two different light intensities: 100 µmol photons m−2 s−1 and 1,500 µmol photons m−2 s−1. Each leaf was placed in the standard leaf chamber before measurement and exposed to 2 min of light of the mentioned intensities in order to allow the plants to acclimate and CO2 assimilation was measured. Matching was done after every plant to minimize errors. After measuring CO2 assimilation, the leaf was photographed, and the leaf area was measured using ImageJ. Fresh weight of the respective leaf was also recorded, and chlorophyll was extracted in 5 ml of methanol for 72 hr in the dark at 4°C. Methanol extracts were analyzed by spectrophotometry and chlorophyll concentrations determined according to published procedures (Porra et al., 1989). Photosynthetic efficiency was calculated by normalizing the assimilation rate either for area or fresh weight. Three experimental replicates were performed with ~10 plants per genotype per replicate.
2.9. Analysis of regulatory sequences
To compare the upstream regulatory region of PHYB1 and PHYB2, we analyzed the 3‐kb upstream of the transcription start site of each gene using the PLANTCARE database (Lescot et al., 2002). The sequences of PhyB1 (SlyPhyB1_SL2.50ch01_68870469.0.68867470) and PhyB2 (SlyPhyB2_SL2.50ch05_63510061.0.63507062) were obtained from Solgenomics (https://solgenomics.net/).
3. RESULTS
3.1. PhyB1 and PhyB2 differentially affect the transcriptome during photomorphogenesis in tomato seedlings
To determine if PHYB1 and PHYB2 have acquired different functions since the divergence from their common single‐gene ancestor, we performed RNAseq analysis. We grew WT and phyB1 and phyB2 mutant seedlings for 5 days in the dark and compared them with individuals of the same genotypes and age that were also exposed to red light (R) for 60 min. We then identified genes that were differentially expressed in the mutants between dark and light (Table S1).
Using a threshold value of 1.5‐fold upregulation or downregulation, we first filtered the data from the RNAseq analysis for genes that were statistically significantly upregulated or downregulated by light treatment in the WT. Of those genes, we considered a gene to be phyB1 or phyB2 regulated if it was either (a) upregulated or downregulated by light in the WT but not differentially regulated in the mutant, (b) oppositely regulated in the mutant compared to the WT, (c) significantly less strongly regulated in the mutant compared to the WT, or (d) more strongly regulated in the mutant compared to the WT. This data filtration yielded 121 phyB1‐regulated genes, and 73 phyB2‐regulated genes. In these gene sets, we identified functional enrichment gene ontology (GO) categories (Figure 1; Table S2). To identify traits possibly subfunctionalized between PHYB1 and PHYB2 mutants, we were particularly interested in GO categories that showed significant enrichment in one phyB‐regulated gene set but not the other. GO categories significantly enriched in genes regulated by phyB1 included responses to auxin (GO: 0009733), responses to cytokinins (GO: 0009735), and protein phosphorylation (GO: 0006468). By contrast, phyB2‐regulated genes did not fall into these three GO categories, but instead into GO categories such as defense response (GO: 0006952) and processes involving aromatic amino acid metabolism and biosynthesis (GO: 0009095 and GO: 0006558) (Table S2; Figure 1).
Figure 1.
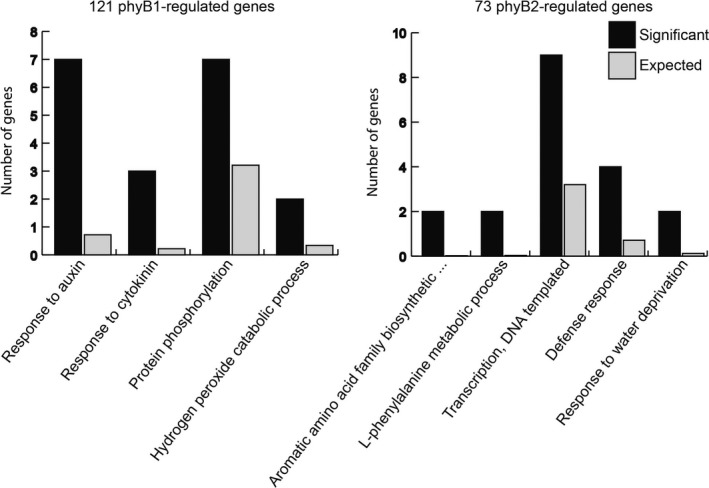
phyB1 and phyB2 regulate expression of genes involved in different biological processes. We identified 121 phyB1‐regulated genes and 73 phyB2‐regulated genes. Gene ontology functional enrichment analysis of these gene groups identified biological processes specifically regulated by phyB1 and phyB2. For all significant GO category enrichments, the black bars represent the number of genes with that annotation in that group (Significant) and the gray bars represent the expected number of genes with that annotation if representation was random (Expected)
To gain additional insight into genes that were differentially affected by their mutations in either PHYB1 or PHYB2, we employed transcriptional co‐expression analysis of the top 8,000 most variably expressed genes across all conditions and found modules containing genes that due to their co‐expression status were likely to have some degree of functional connectivity (Figure 2; Table S3). The yellow, blue, red, and light‐cyan modules contained genes positively correlated to the phyB1 mutation (i.e., they were more highly expressed in phyB1 than in WT and phyB2) but negatively or not significantly correlated to the phyB2 mutation. The opposite was true for the brown, salmon, turquoise, and green modules, which contained genes positively correlated to the phyB2 mutation (i.e., they were more highly expressed in phyB2 than in WT and phyB1) but not or negatively correlated to the phyB1 mutation. These opposite expression patterns thus indicated diversified regulation between the two PHYB genes. Such diversified regulation was also seen, albeit not significantly, in the black, green‐yellow, and cyan modules.
Figure 2.
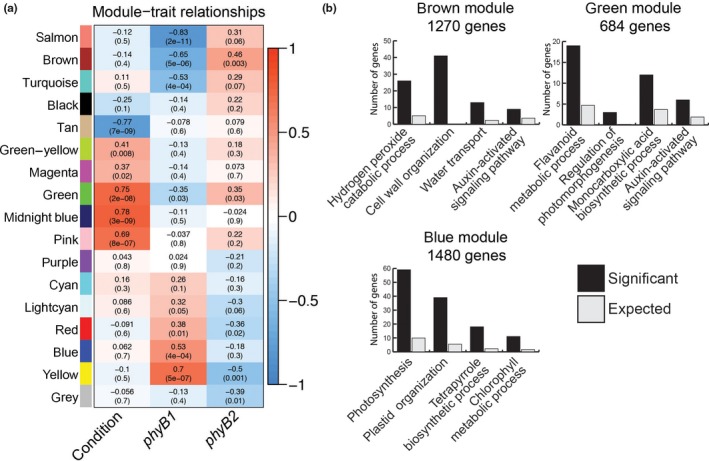
Co‐expression modules show phyB1 and phyB2 differently regulate gene networks involved in auxin and photosynthesis related biological processes among others. (a) For each co‐expression module (indicated by color) and the genes that did not fall into a co‐expression module (gray), the average expression vector (eigenvector) across conditions and genotypes was correlated to condition (dark = 0, 60 min R exposure = 1) and genotype (phyB1 column: WT and phyB2 = 0, phyB1 = 1; phyB2 column: WT and phyB1 = 0, phyB2 = 1). R 2 values from the Pearson correlations are indicated in the heatmap by color according to scale on the right as well as by their printed value in the grid with p‐values below in parentheses. (b) Gene ontology functional enrichment analysis identified biological processes central to each co‐expression module. Displayed here are four enriched GO biological processes for the brown, green, and blue modules. The black bars represent the number of genes with that annotation in that group (Significant) and the gray bars represent the expected number of genes with that annotation if random (Expected)
Modules containing genes that were regulated by light (“condition”) included the tan module (negative correlation), and the green‐yellow, magenta, green, midnight blue, and pink modules (positive correlation) (Figure 2). The green module was the only module containing genes that were significantly correlated with light (positively) and were also oppositely correlated with phyB1 and phyB2. We looked for enriched GO functions in each co‐expression module (Table S4). Among these functions were auxin‐related processes, including auxin efflux (GO: 0010329), the auxin‐regulated processes of gravitropism and phototropism (GO: 0009959, GO: 0009638), and auxin signaling (GO: 0009734), as well as photosynthesis‐related processes (GO: 0009765, GO: 0009773, and GO: 0015979), in addition to a large number of other functional categories (Figure 2b and Table S4).
To determine areas of subfunctionalization between phyB1 and phyB2 in tomato, we combined information from our differential expression, co‐expression and GO analyses to choose physiological functions for further testing and verification that transcriptomic differences had measurable effects on phenotypes. These functions were chosen based on (a) frequency of appearance in our data as being differentially regulated by phyB1 and phyB2, (b) statistical significance of our differential and co‐expression analyses data, and (c) the number of genes on which individual enrichment analyses were based. Additionally, functions for further study were chosen if they were known from the literature to be regulated, at least in part, by phyB in Arabidopsis.
3.2. PhyB1 and PhyB2 differentially modulate auxin responses in tomato seedlings
To determine if our gene expression analysis had predictive power on the plant's phenotype, we subjected wild type (WT) and phyB1 and phyB2 mutants to a variety of physiological experiments. Given that auxin‐related processes had been implicated as differentially regulated by phyB1 and phyB2 in both differential expression and co‐expression analyses, we tested if the auxin‐related responses phototropism and gravitropism were differentially affected between phyB1 and phyB2 mutants when compared to the WT. Phototropism, the movement of plants toward a light source, is achieved by the perception of blue light via the photoreceptors PHOT1 and PHOT2, eventually leading to unequal distribution of auxin along the hypocotyl of a seedling exposed to unilateral light (Fankhauser & Christie, 2015). Differential auxin concentrations then result in unequal growth on the light versus dark side of the stem or hypocotyl leading to curvature toward the light source (Fankhauser & Christie, 2015). Indeed, when we exposed 5‐day‐old seedlings to unilateral white light (WL) over a period of three hours, phyB1 hypocotyls displayed a significantly faster phototropic response (Figure 3) compared to the WT and phyB2 plants, indicating a differential role of phyB1 and phyB2 in the phototropic response in tomato. This suggests that phyB1, but not phyB2, normally inhibits phototropic bending.
Figure 3.
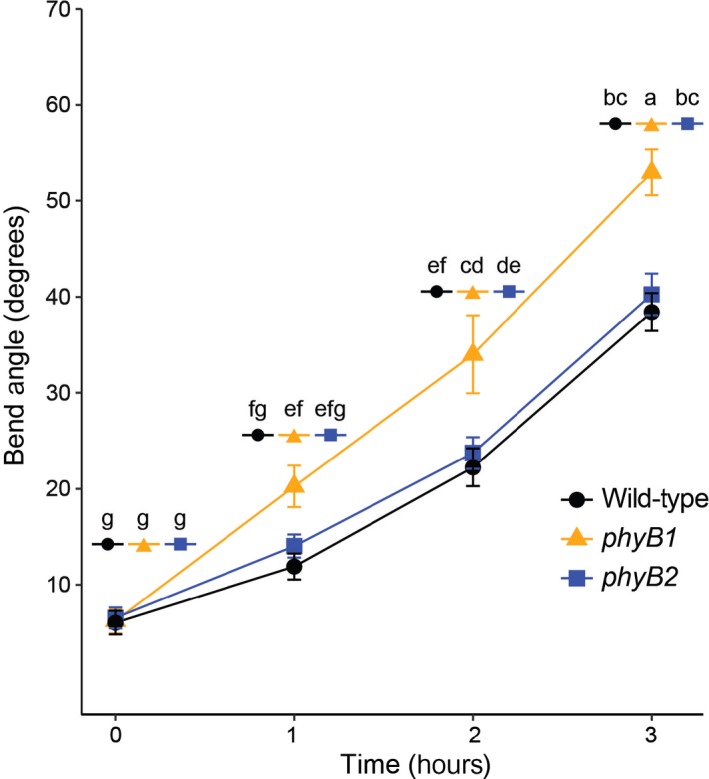
In white light, phyB1 mutants show significantly faster phototropism than wild type or phyB2 mutants. The average degree to which 5‐day‐old dark‐grown seedlings bent toward unidirectional white light (bend angle) over 3 hr is shown. Error bars represent standard error. Combined data from three biological replicates are shown, n = 5 seedlings per genotype per time point per biological replicate. A two‐way ANOVA with time and genotype was performed followed by Tukey's post hoc test using the software R. Shared letters represent no statistically significant difference
Our RNAseq differential gene expression analysis had found PHOT1 to be differentially expressed in the WT dark versus WT red light comparison but the gene was not phyB1 or phyB2 regulated. PHOT2 was not differentially expressed in either comparison. Since differences in the phototropic phenotype were recorded for seedlings grown under conditions different from those in our RNAseq experiment, we decided to check if gene expression differences of these receptors pivotal to the phototropic response might also be detectable between phyB1 and phyB2 mutants during phototropic stimulation. Testing PHOT1 and PHOT2 expression with qPCR at 0 and 3 hr of treatment with unilateral white light, we observed a decline in PHOT1 and an increase in PHOT2 expression over the 3‐hr treatment (Fig. S1), but found no significant differences of gene expression between the two phyB mutants, suggesting that regulation of the PHOT1 and PHOT2 genes does not explain the measured phenotypic differences and instead indicates that the differences are likely due to differential gene regulation downstream of PHOT1 and PHOT2 (Fig. S1).
Since gravitropism, like phototropism, is a typical auxin‐regulated response, we decided to test if gravitropism manifests itself differentially in the two phyB mutants in tomato. Five‐day‐old dark‐grown seedlings were transferred to agar plates, either exposed to R or kept in the dark, and grown upright for 1 hr immediately after the transfer. Plates were then reoriented 90 degrees to induce a gravitropic response. We observed that in R the phyB1 mutant responded statistically significantly faster to the altered gravity vector, which was especially obvious around 8 hr postgravistimulation, whereas in darkness the mutants responded to gravity at the same rate as WT (Figure 4). This experiment suggested that the differential auxin responsiveness between phyB1 and phyB2 also extends to differences in their gravitropic response. Interestingly, when we reduced the light levels from 135 to 60 µmol*m−2*s−1, the gravitropic response differences between genotypes disappeared (Fig. S2), suggesting that the phyB1‐mediated gravitropic response in tomato is also light intensity‐dependent.
Figure 4.
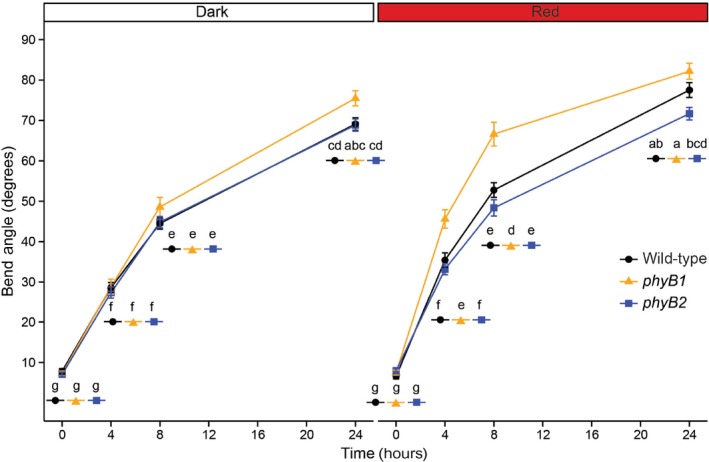
In R, phyB1 mutants show significantly faster gravitropism than wild type or phyB2 mutants. The average degree to which 5‐day‐old dark‐grown seedlings bent toward the negative gravity vector (i.e., upwards) after gravistimulation over 24 hr is shown. Seedlings were either gravistimulated in the dark (left), or with 135 µmol photons m−2 s−1 of R. Error bars represent standard error. The dark and R plots each contain data from three biological replicates. N = 20 per genotype per time point per biological replicate. A three‐way ANOVA with time, genotype, and light condition was performed followed by Tukey's post hoc test in R. Shared letters represent no statistically significant difference
Since we only observed significantly greater gravitropic curvature in the phyB1 mutants when the gravitropic experiment was done with high light intensity from the top but not with low light intensity from the side we wanted to exclude the remote possibility that in tomato phototropism can also be triggered by R alone, instead of requiring blue light. We therefore performed a series of control experiments in which we exposed seedlings to unilateral R light and measured their directional growth response over a period of three hours in a similar way to how we had performed the phototropic experiments shown in Figure 3. Not surprisingly (Fankhauser & Christie, 2015), our data showed that, like Arabidopsis, tomato does not have a red light phototropic response (data not shown), confirming that the enhancement in the gravitropic response of phyB1 could not have been due to its enhanced phototropic response.
3.3. PhyB1 and PhyB2 differentially modulate photosynthetic responses in tomato seedlings
Our transcriptional co‐expression analysis had shown almost 60 photosynthesis‐related genes to be enriched in the blue module, which contains genes with expression positively correlated with the phyB1 mutation, but not significantly correlated with the phyB2 mutation (Figure 2). We therefore decided to measure a variety of photosynthesis‐related physiological parameters to test the hypothesis that gene duplication in PHYB had led to the subfunctionalization of regulation of genes involved in photosynthesis. We measured overall photosynthetic activity and related this activity to leaf size and fresh weight. Measuring overall leaf chlorophyll concentrations, we found differences between the WT and the two mutants but they were not statistically significantly different from each other (data not shown). Photosynthetic activity was not statistically significantly different between the three genotypes when the photosynthetic rate was normalized by leaf area regardless of light intensity (Figure 5a,c). However, when we normalized photosynthetic rate by fresh weight of the leaf portion used for the gas exchange analysis, we observed a statistically significant difference between the two phytochrome mutants. These differences between phyB1 and phyB2 were seen both in low and high light intensities (Figure 5b,d). Interestingly, the data suggest that phyB1 and phyB2 act antagonistically to each other and that PHYB1 and PHYB2 have subfunctionalized with respect to the role they play in regulating photosynthesis.
Figure 5.
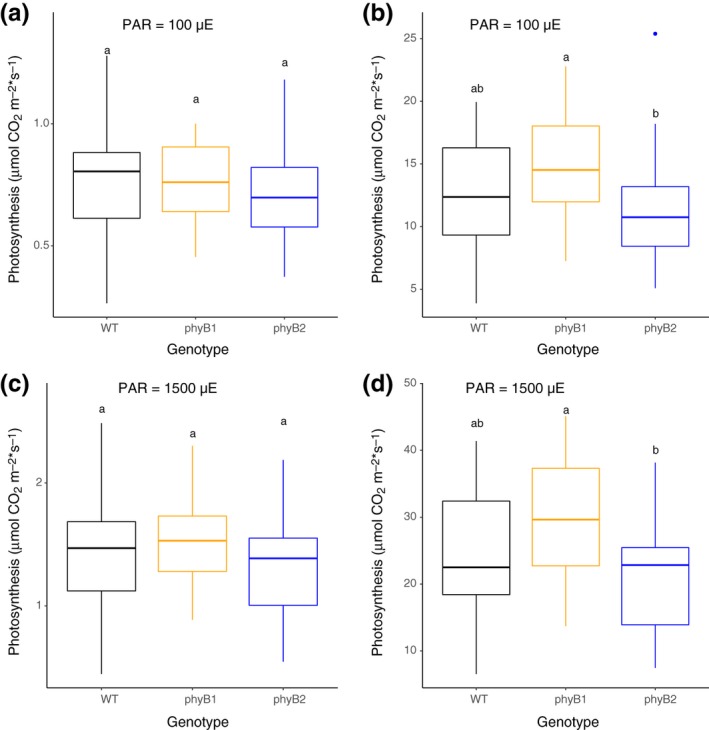
Photosynthetic activity is enhanced by phyB2 and repressed by phyB1 independent of light intensity. Photosynthetic activity was measured under varying light intensities in 6‐week‐old WT, phyB1, and phyB2 mutants grown at 25°C (16 hr day/8 hr night) using a LiCOR 6400XT. Three biological replicates were performed with 10 plants per genotype per replicate. Data were normalized in two different ways either by leaf area (a and c) or by leaf area and fresh weight of the leaf tissue that was used for photosynthetic rate measurement. Data were statistically analyzed with a one‐way ANOVA followed by a Tukey post hoc test using the software R. In each panel, data points not connected by a shared letter are statistically significantly different
3.4. Subfunctionalization of phyB1 and phyB2 is correlated with differences in the genes’ regulatory region
Using the PlantCARE database (Lescot et al., 2002), we compared the 3‐kb regulatory region immediately upstream from each gene's transcriptional start site (Table S5) and found a number of differences. Overall, PHYB1 contained 17 recognized light‐regulated cis‐acting elements, while PHYB2 only contained 7 such elements. The type of elements found in each gene's promoter region was also different. For example, the PHYB1 promoter region contained 7 G‐Box elements, which bind PHYTOCHROME INTERACTING FACOTRs (PIFs) (Pham, Kathare, & Huq, 2018), while in PHYB2, there were only 2. Several other motifs were found only in one or the other phy gene (Table S5). Overall, the differential occurrence of light regulatory sequences suggests that transcription of these duplicated genes might be differentially regulated.
4. DISCUSSION
Gene duplication is a major source of genetic material with the potential for the evolution of novel functions and the development of complexity of responses to the environment (Panchy et al., 2016). Retention of duplicated genes can either indicate that retained genes are positively selected to provide genetic redundancy (Zhang, 2012), that they are required to maintain proper dosage or genetic balance (Birchler & Veitia, 2014; Freeling & Thomas, 2006), or that duplication eventually led to the acquisition of novel or refined functions (Lynch & Conery, 2000; Ohno, 1970). PHY genes, in particular, have been estimated to be evolving at a faster rate (1.52–2.79 times) than the average plant nuclear gene, suggesting that diversification of the PHY gene family might respond either to selective pressure or to the absence of major evolutionary constraints (Alba et al., 2000).
We used differential mRNA expression and co‐expression analysis to first evaluate the degree to which the PHY genes PHYB1 and PHYB2 have functionally diversified since their separation from a common ancestor gene and then to identify and verify physiological traits for which phyB has subfunctionalized since its gene duplication event. Our analysis indicated significant differences in the transcriptome of plants mutant in either PHYB1 or PHYB2. On the other hand, after filtering, the overall number of genes that were regulated by phyB1 (121) and phyB2 (73) was relatively modest. Overall, our differential gene expression analysis showed that the group of genes regulated by phyB1 but not phyB2 was enriched in auxin response genes, and our co‐expression analysis showed that those genes found in co‐expression gene networks and that differentially correlated to phyB1 and phyB2 were enriched in auxin response and photosynthesis genes.
4.1. Regulation of auxin responses by phytochrome B
In Arabidopsis, phototropic curvature is enhanced when plants are pre‐treated with R for 2 hr before directional blue light (B) treatment (Janoudi, Konjevic, Apel, & Poff, 1992). This pre‐treatment response is phyA‐mediated, and not phyB‐mediated (Parks, Quail, & Hangarter, 1996), although it has been shown that even without R pre‐treatment, Arabidopsis phyA, phyB, and phyD promote phototropism (Whippo & Hangarter, 2004). Specifically for B intensities of greater than 1.0 µmol*m−2*s−1 of light, phyB and phyD show functional redundancy with phyA, while at fluences of B around 0.01 µmol*m−2*s−1, phyA was required for a normal phototropic response (Whippo & Hangarter, 2004). Additionally, Arabidopsis phyB has been shown to inhibit phototropism in shade‐free environments (a high R/FR ratio), while mediating the phototropic response in the shade via PHYTOCHROME INTERACTING FACTORs (PIFs) and members of the YUCCA gene family (Goyal et al., 2016). Furthermore, it was shown that the quadruple mutant for phyB, phyC, phyD, and phyE has a normal phototropic response (Strasser, Sánchez‐Lamas, Yanovsky, Casal, & Cerdán, 2010), confirming the notion that phyA is required in Arabidopsis for a normal low‐fluence B‐induced phototropic response. Direct connections between auxin signaling, phototropism and phytochrome involvement have been shown in Arabidopsis as well. Haga and colleagues (2014) used quadruple mutants in the PINOID (PID) and WAVY ROOT GROWTH (WAG) genes to show that phy upregulates the expression of PIN‐FORMED (PIN) auxin transport proteins and suggested that PIN proteins were responsible for the R pre‐treatment enhancement of phototropism.
Our data suggest that phototropism is differently regulated between tomato and Arabidopsis. Our genetic analysis shows that phyB1, but not phyB2, negatively regulates the phototropic response in tomato (Figure 3). This in turn suggests that in tomato, phyB duplication led to a defined split between phyB1 and phyB2 with respect to phototropism, while in Arabidopsis phyB and phyD share redundancy, at least for its control of phototropism in response to R pre‐treatment (Whippo & Hangarter, 2004). Additionally, while Arabidopsis work has shown phyB to be repressing phototropism in shade‐free environments (Goyal et al., 2016), we saw that phyB2 in tomato is not involved in that response. Our RNAseq analysis supports the split in function also with respect to expression differences in the PIN genes that Haga and colleagues (2014) had proposed to play a role in phy‐mediated phototropism: In tomato, our network analysis placed SlPIN4 into the brown module, which is negatively correlated with the phyB1 mutation but positively correlated with the phyB2 mutation (Figure 2). Furthermore, SlPIN4 was differentially regulated in response to R only in the phyB2 mutant, but not in the phyB1 mutant (Table S1). This differential sensitivity in auxin response signaling between the two subfunctionalized genes in tomato suggests one possible avenue for the two phy genes in tomato to differentially affect phototropic curvature.
Gravitropism, like phototropism, is an auxin‐mediated differential growth response that results in directional elongation with respect to the gravity vector (Morita, 2010). Our data showed that phyB1, but not phyB2, represses gravitropism in R (Figure 4). This response is therefore similar to the phototropic response in that it is enhanced by the phyB1 mutation. The role of phytochrome in the gravitropic response in less well understood than it is for phototropism. In Arabidopsis, but not in tomato, R perceived by both phyA and phyB results in strongly reduced shoot gravitropism (Liscum & Hangarter, 1993; Poppe, Hangarter, Sharrock, Nagy, & Schäfer, 1996) caused by PIFs that in R convert the gravity‐sensing amyloplasts in the endodermis into other, non‐gravity‐sensing types of plastids (Kim et al., 2011). Interestingly, root gravitropism in white‐light‐grown Arabidopsis is diminished in phyB but not in phyD mutants (Correll & Kiss, 2005), suggesting subfunctionalization for this trait between the two genes in Arabidopsis. Interestingly, however, in Arabidopsis roots WT phyB promotes gravitropism, whereas in tomato shoots WT phyB1 inhibits it. Since R does not inhibit shoot gravitropism in 5‐day‐old dark‐grown tomato seedlings, gravity sensing in the hypocotyl appears to follow a different signaling route than it does in Arabidopsis, but clearly phytochrome appears to play a role in both.
4.2. Regulation of photosynthesis by phytochrome B
Our co‐transcriptional analysis had suggested that photosynthesis genes were differentially affected by mutations in PHYB1 versus PHYB2 of tomato (Figure 2) and our physiological experiments had supported this finding (Figure 5). In Arabidopsis, phyB has previously been shown to increase photosynthetic rates, but only at light levels greater than 250 µmol*m−2*s−1 (Boccalandro et al., 2009). Our data show that photosynthesis is enhanced in the phyB1 mutant and reduced in the phyB2 mutant compared to the WT response (Figure 5b,d), suggesting that in tomato phyB2, apparently antagonistically to phyB1, plays the role of increasing photosynthetic rates. Interestingly, it appears that this instance of subfunctionalization did not simply split the two phyB homologs into one serving the function of the parental gene while the other largely lost its participation in the process, but instead led to opposite regulation of the same process. Another difference between the Arabidopsis and tomato responses is that, unlike in Arabidopsis, the effects of phyB1 and phyB2 on photosynthesis are not light intensity‐dependent in tomato, at least not at the two light intensities tested here. It is of note that differences in photosynthetic rates were only discernable in our analysis when we normalized carbon assimilation rates by fresh weight and leaf area as opposed to leaf area alone (Figure 5). Chlorophyll content in all genotypes was about the same but fresh weight per unit leaf area was highest in phyB2 and lowest in phyB1 among the three genotypes. This indicates that phyB1 promotes leaf thickness, water conservation or both, while phyB2 might promote transpiration (creating a net weight loss) or restrict leaf thickening. The conflict between gene functions of phyB1 and phyB2 could allow the plant to balance its photosynthetic and water needs depending on environmental conditions. More work is needed, however, to specifically assign those roles to the two phyB homologs in tomato.
4.3. In tomato, subfunctionalization of phyB has led to equally important sister genes
The relatively recent duplication of phyB into separate homologs in different species provides a window into how gene duplication can result in different evolutionary trajectories. PHYB duplications in Arabidopsis and tomato both occurred after divergence of the Solanaceae and Brassicaceae (Li et al., 2015). In Arabidopsis, comparison of the coding sequence shows 48–56% amino acid identity between PHYA, PHYB, PHYC, and PHYE, but 80% identity between PHYB and PHYD (Clack, Mathews, & Sharrock, 1994). Amino acid identities between PHYB and PHYD in Arabidopsis, and between PHYB1 and PHB2 in tomato are similarly high in the two species (Hauser, Cordonnier‐Pratt, Daniel‐Vedele, & Pratt, 1995). Functional redundancy between PHYB and PHYD in Arabidopsis is high, but mutation in PHYD enhances the phyB mutant response with respect to leaf morphology, rosette leaf number (Franklin et al., 2003) and shade avoidance (Devlin et al., 1999; Franklin et al., 2003). While single mutation of PHYD in Arabidopsis leads to an increase in hypocotyl length in continuous R and white lights, the effect of phyD on the end‐of‐day (EOD) FR response was negligible until combined with a mutation in PHYB (Aukerman et al., 1997). With respect to leaf morphology and developmental traits, mutation in Arabidopsis PHYD resulted in none or only minor consequences on the phenotype while mutation in PHYB resulted in statistically significant phenotypic change (Aukerman et al., 1997). Analysis of the phyB/D double mutant, however, showed that PHYD contributes residual function to phenotype in a manner redundant and subordinate to PHYB (Aukerman et al., 1997).
In tomato, divergence of the 5′ cis‐regulatory regions in PHYB1 and PHYB2 has resulted in variability of the number and type of light response motifs, suggesting that this variation might be part of the reason for the genes' subfunctionalization. Duplication and gene divergence in tomato, in contrast to Arabidopsis, has resulted in two genes that have taken on specialized functions for a variety of developmentally important traits. This situation is not unlike that in maize. In maize, the two homologs of ZmPHYB showed complete redundancy for involvement in several morphological traits, such as plant height and stem diameter, while regulation of photoperiod‐dependent flowering time was regulated only by ZmPHYB2 (Sheehan et al., 2007). Early work on phyB1 and phyB2 in tomato describing the mutants had already noted that phyB1 and phyB2 played different roles in early seedling development, but described the genes as largely redundant in older plants (Weller et al., 2000). Our data suggest that in tomato, phyB1 inhibits auxin responses of phototropism and gravitropism (and phyB2 does not play a role) while phyB2 promotes and phyB1 inhibits photosynthesis.
We want to caution that in the absence of multiple alleles of phyB1 and phyB2 in our analysis, it is formally possible that unknown, secondary background mutations in the material could contribute to some of the observations we made in this study.
5. CONCLUSIONS
Although phys are evolutionarily old genes and found in at least two copies, phyA and phyB, in all angiosperm species (Mathews, 2010), functional diversification is an ongoing process. PhyB is the phy homolog that has most recently duplicated again in some species (Mathews, 2010), including Arabidopsis (phyB/phyD), maize (phyB1/phyB2), and tomato (phyB1/phyB2). This latest round of duplication therefore lends itself well to analysis of variation in subfunctionalization of this important gene between species, and also provides a recent gene duplication event that plants have exploited for further specialization of their responses to light and the environment.
Conflict of Interest
The authors declare no conflict of interest associated with the work described in this manuscript.
AUTHOR CONTRIBUTIONS
A.M. conceived the original research experiment. K.C., S.B., D.A., A.Z‐M., and A.M performed the experiments and analyzed the data. Specifically, K.C. and A.M. did the bioinformatic analysis and data interpretation; S.B. performed photosynthesis, phototropism, and promoter analysis experiments; K.C. performed the gravitropic experiments; A.Z‐M. performed phototropism experiments; D.A. supervised plant growth and extracted RNA; K.C., S.B. and A.M. interpreted the data and wrote the article with contributions from all the authors. A.M. agrees to serve as corresponding author.
Supporting information
ACKNOWLEDGEMENTS
We acknowledge funding from the National Science Foundation (IOS‐1339222, to AM, PRFB 1523917 to KDC). We thank Bob Peaslee and Amy Replogle for technical help and critical discussions.
Carlson KD, Bhogale S, Anderson D, Zaragoza‐Mendoza A, Madlung A. Subfunctionalization of phytochrome B1/B2 leads to differential auxin and photosynthetic responses. Plant Direct. 2020;4:1–12. 10.1002/pld3.205
Carlson and Bhogale authors contributed equally to this work.
Reference numbers for data available in public repositories: http://www.ncbi.nlm.nih.gov/sra/SRP108371
REFERENCES
- Alba, R. , Kelmenson, P. M. , Cordonnier‐Pratt, M.‐M. , & Pratt, L. H. (2000). The phytochrome gene family in tomato and the rapid differential evolution of this family in angiosperms. Molecular Biology and Evolution, 17, 362–373. 10.1093/oxfordjournals.molbev.a026316 [DOI] [PubMed] [Google Scholar]
- Alexa, A. , & Rahnenfuhrer, J. (2010). Topgo: Enrichment Analysis for Gene Ontology.
- Alexa, A. , Rahnenführer, J. , & Lengauer, T. (2006). Improved scoring of functional groups from gene expression data by decorrelating GO graph structure. Bioinformatics, 22, 1600–1607. 10.1093/bioinformatics/btl140 [DOI] [PubMed] [Google Scholar]
- Aukerman, M. J. , Hirschfeld, M. , Wester, L. , Weaver, M. , Clack, T. , Amasino, R. M. , & Sharrock, R. A. (1997). A deletion in the PHYD gene of the Arabidopsis Wassilewskija ecotype defines a role for phytochrome D in red/far‐red light sensing. The Plant Cell, 9, 1317–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchler, J. A. , & Veitia, R. A. (2014). The gene balance hypothesis: Dosage effects in plants. Methods in Molecular Biology, 1112, 25–32. [DOI] [PubMed] [Google Scholar]
- Boccalandro, H. E. , Rugnone, M. L. , Moreno, J. E. , Ploschuk, E. L. , Serna, L. , Yanovsky, M. J. , & Casal, J. J. (2009). Phytochrome B enhances photosynthesis at the expense of water‐use efficiency in Arabidopsis. Plant Physiology, 150, 1083 10.1104/pp.109.135509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bomblies, K. , & Madlung, A. (2014). Polyploidy in the Arabidopsis genus. Chromosome Research, 22, 117–134. 10.1007/s10577-014-9416-x [DOI] [PubMed] [Google Scholar]
- Borevitz, J. O. , Maloof, J. N. , Lutes, J. , Dabi, T. , Redfern, J. L. , Trainer, G. T. , … Weigel, D. et al (2002). Quantitative trait loci controlling light and hormone response in two accessions of Arabidopsis thaliana . Genetics, 160, 683–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, M. , & Chory, J. (2011). Phytochrome signaling mechanisms and the control of plant development. Trends in Cell Biology, 21, 664–671. 10.1016/j.tcb.2011.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clack, T. , Mathews, S. , & Sharrock, R. A. (1994). The phytochrome apoprotein family in Arabidopsis is encoded by five genes: The sequences and expression ofPHYD and PHYE. Plant Molecular Biology, 25, 413–427. 10.1007/BF00043870 [DOI] [PubMed] [Google Scholar]
- Correll, M. J. , & Kiss, J. Z. (2005). The roles of phytochromes in elongation and gravitropism of roots. Plant and Cell Physiology, 46, 317–323. 10.1093/pcp/pci038 [DOI] [PubMed] [Google Scholar]
- Devlin, P. F. , Robson, P. R. H. , Patel, S. R. , Goosey, L. , Sharrock, R. A. , & Whitelam, G. C. (1999). Phytochrome D acts in the shade‐avoidance syndrome in Arabidopsis by controlling elongation growth and flowering time. Plant Physiology, 119, 909 10.1104/pp.119.3.909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fankhauser, C. , & Christie, J. M. (2015). Plant phototropic growth. Current Biology, 25, R384–R389. 10.1016/j.cub.2015.03.020 [DOI] [PubMed] [Google Scholar]
- Foo, E. , Ross, J. J. , Davies, N. W. , Reid, J. B. , & Weller, J. L. (2006). A role for ethylene in the phytochrome‐mediated control of vegetative development. The Plant Journal, 46, 911–921. 10.1111/j.1365-313X.2006.02754.x [DOI] [PubMed] [Google Scholar]
- Franklin, K. A. , Praekelt, U. , Stoddart, W. M. , Billingham, O. E. , Halliday, K. J. , & Whitelam, G. C. (2003). Phytochromes B, D, and E act redundantly to control multiple physiological responses in Arabidopsis. Plant Physiology, 131, 1340–1346. 10.1104/pp.102.015487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin, K. A. , & Quail, P. H. (2010). Phytochrome functions in Arabidopsis development. Journal of Experimental Botany, 61, 11–24. 10.1093/jxb/erp304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeling, M. , & Thomas, B. C. (2006). Gene‐balanced duplications, like tetraploidy, provide predictable drive to increase morphological complexity. Genome Research, 16, 805–814. 10.1101/gr.3681406 [DOI] [PubMed] [Google Scholar]
- Goyal, A. , Karayekov, E. , Galvão, V. C. , Ren, H. , Casal, J. J. , & Fankhauser, C. (2016). Shade promotes phototropism through phytochrome B‐controlled auxin production. Current Biology, 26, 3280–3287. 10.1016/j.cub.2016.10.001 [DOI] [PubMed] [Google Scholar]
- Hauser, B. , Cordonnier‐Pratt, M. M. , Daniel‐Vedele, F. , & Pratt, L. H. (1995). The phytochrome gene family in tomato includes a novel subfamily. Plant Molecular Biology, 29, 1143–1155. 10.1007/BF00020458 [DOI] [PubMed] [Google Scholar]
- Hauser, B. A. , Cordonnier‐Pratt, M.‐M. , & Pratt, L. H. (1998). Temporal and photoregulated expression of five tomato phytochrome genes. The Plant Journal, 14, 431–439. 10.1046/j.1365-313X.1998.00144.x [DOI] [PubMed] [Google Scholar]
- Janoudi, A. K. , Konjevic, R. , Apel, P. , & Poff, K. L. (1992). Time threshold for second positive phototropism is decreased by a preirradiation with red light. Plant Physiology, 99, 1422 10.1104/pp.99.4.1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller, M. M. , Jaillais, Y. , Pedmale, U. V. , Moreno, J. E. , Chory, J. , & Ballaré, C. L. (2011). Cryptochrome 1 and phytochrome B control shade‐avoidance responses in Arabidopsis via partially independent hormonal cascades. The Plant Journal, 67, 195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendrick, R. E. , Kerckhoffs, L. H. J. , Pundsnes, A. S. , Van Tuinen, A. , Koorneef, M. , Nagatani, A. , … Pratt, L. H. (1994). Photomorphogenic mutants of tomato. Euphytica, 79, 227–234. 10.1007/BF00022523 [DOI] [Google Scholar]
- Kendrick, R. E. , Kerckhoffs, L. H. J. , Van Tuinen, A. , & Koornneef, M. (1997). Photomorphogenic mutants of tomato. Plant, Cell & Environment, 20, 746–751. 10.1046/j.1365-3040.1997.d01-109.x [DOI] [Google Scholar]
- Kerckhoffs, L. H. J. , Kelmenson, P. M. , Schreuder, M. E. L. , Kendrick, C. I. , Kendrick, R. E. , Hanhart, C. J. , … Cordonnier‐Pratt, M. M. (1999). Characterization of the gene encoding the apoprotein of phytochrome B2 in tomato, and identification of molecular lesions in two mutant alleles. Molecular and General Genetics MGG, 261, 901–907. 10.1007/s004380051037 [DOI] [PubMed] [Google Scholar]
- Khanna, R. , Kikis, E. A. , & Quail, P. H. (2003). EARLY FLOWERING 4 functions in phytochrome B‐regulated seedling de‐etiolation. Plant Physiology, 133, 1530–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, K. , Shin, J. , Lee, S.‐H. , Kweon, H.‐S. , Maloof, J. N. , & Choi, G. (2011). Phytochromes inhibit hypocotyl negative gravitropism by regulating the development of endodermal amyloplasts through phytochrome‐interacting factors. Proceedings of the National Academy of Sciences, 108, 1729–1734. 10.1073/pnas.1011066108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarova, G. I. , Kubota, T. , Frances, S. , Peters, J. L. , Hughes, M. J. G. , Brandstädter, J. , … Pratt, L.H. (1998). Characterization of tomato PHYB1 and identification of molecular defects in four mutant alleles. Plant Molecular Biology, 38, 1137–1146. [DOI] [PubMed] [Google Scholar]
- Lescot, M. , Déhais, P. , Thijs, G. , Marchal, K. , Moreau, Y. , Van de Peer, Y. , … Rombauts, S. (2002). PlantCARE, a database of plant cis‐acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Research, 30, 325–327. 10.1093/nar/30.1.325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, F.‐W. , Melkonian, M. , Rothfels, C. J. , Villarreal, J. C. , Stevenson, D. W. , Graham, S. W. , … Mathews, S. (2015). Phytochrome diversity in green plants and the origin of canonical plant phytochromes. Nature Communications, 6, 7852 10.1038/ncomms8852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum, E. , & Hangarter, R. P. (1993). Genetic evidence that the red‐absorbing form of phytochrome B modulates gravitropism in Arabidopsis thaliana . Plant Physiology, 103, 15–19. 10.1104/pp.103.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Cohen, J. D. , & Gardner, G. (2011). Low‐fluence red light increases the transport and biosynthesis of auxin. Plant Physiology, 157, 891–904. 10.1104/pp.111.181388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak, K. J. , & Schmittgen, T. D. (2001). Analysis of relative gene expression data using real‐time quantitative PCR and the 2(‐Delta Delta C(T)) Method. Methods, 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Lynch, M. , & Conery, J. S. (2000). The evolutionary fate and consequences of duplicate genes. Science, 290, 1151–1155. 10.1126/science.290.5494.1151 [DOI] [PubMed] [Google Scholar]
- Mathews, S. (2010). Evolutionary studies illuminate the structural‐functional model of plant phytochromes. The Plant Cell, 22, 4–16. 10.1105/tpc.109.072280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews, S. , & Sharrock, R. A. (1997). Phytochrome gene diversity. Plant, Cell & Environment, 20, 666–671. 10.1046/j.1365-3040.1997.d01-117.x [DOI] [Google Scholar]
- Moore, R. C. , & Purugganan, M. D. (2005). The evolutionary dynamics of plant duplicate genes. Current Opinion in Plant Biology, 8, 122–128. 10.1016/j.pbi.2004.12.001 [DOI] [PubMed] [Google Scholar]
- Morita, M. T. (2010). Directional gravity sensing in gravitropism. Annual Review of Plant Biology, 61, 705–720. 10.1146/annurev.arplant.043008.092042 [DOI] [PubMed] [Google Scholar]
- Neff, M. M. , & Chory, J. (1998). Genetic interactions between phytochrome A, phytochrome B, and cryptochrome 1 during Arabidopsis development. Plant Physiology, 118, 27–35. 10.1104/pp.118.1.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno, S. (1970). Evolution by gene duplication. New York: Springer Verlag. [Google Scholar]
- Panchy, N. , Lehti‐Shiu, M. , & Shiu, S.‐H. (2016). Evolution of gene duplication in plants. Plant Physiology, 171, 2294 10.1104/pp.16.00523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks, B. M. , Quail, P. H. , & Hangarter, R. P. (1996). Phytochrome A regulates red‐light induction of phototropic enhancement in Arabidopsis. Plant Physiology, 110, 155 10.1104/pp.110.1.155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pham, V. N. , Kathare, P. K. , & Huq, E. (2018). Phytochromes and phytochrome interacting factors. Plant Physiology, 176, 1025 10.1104/pp.17.01384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe, C. , Hangarter, R. P. , Sharrock, R. A. , Nagy, F. , & Schäfer, E. (1996). The light‐induced reduction of the gravitropic growth‐orientation of seedlings of Arabidopsis thaliana (L.) Heynh. is a photomorphogenic response mediated synergistically by the far‐red‐absorbing forms of phytochromes A and B. Planta, 199, 511–514. 10.1007/BF00195180 [DOI] [PubMed] [Google Scholar]
- Pratt, L. H. , Cordonnier‐Pratt, M.‐M. , Hauser, B. , & Caboche, M. (1995). Tomato contains two differentially expressed genes encoding B‐type phytochromes, neither of which can be considered an ortholog of Arabidopsis phytochrome B. Planta, 197, 203–206. 10.1007/BF00239958 [DOI] [PubMed] [Google Scholar]
- Salisbury, F. J. , Hall, A. , Grierson, C. S. , & Halliday, K. J. (2007). Phytochrome coordinates Arabidopsis shoot and root development. The Plant Journal, 50, 429–438. 10.1111/j.1365-313X.2007.03059.x [DOI] [PubMed] [Google Scholar]
- Seo, M. , Hanada, A. , Kuwahara, A. , Endo, A. , Okamoto, M. , Yamauchi, Y. , … Nambara, E. (2006). Regulation of hormone metabolism in Arabidopsis seeds: Phytochrome regulation of abscisic acid metabolism and abscisic acid regulation of gibberellin metabolism. The Plant Journal, 48, 354–366. 10.1111/j.1365-313X.2006.02881.x [DOI] [PubMed] [Google Scholar]
- Sheehan, M. J. , Kennedy, L. M. , Costich, D. E. , & Brutnell, T. P. (2007). Subfunctionalization of PhyB1 and PhyB2 in the control of seedling and mature plant traits in maize. The Plant Journal, 49, 338–353. 10.1111/j.1365-313X.2006.02962.x [DOI] [PubMed] [Google Scholar]
- Shinomura, T. , Nagatani, A. , Hanzawa, H. , Kubota, M. , Watanabe, M. , & Furuya, M. (1996). Action spectra for phytochrome A‐ and B‐specific photoinduction of seed germination in Arabidopsis thaliana. Proceedings of the National Academy of Sciences, 93, 8129–8133. 10.1073/pnas.93.15.8129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser, B. , Sánchez‐Lamas, M. , Yanovsky, M. J. , Casal, J. J. , & Cerdán, P. D. (2010). Arabidopsis thaliana life without phytochromes. Proceedings of the National Academy of Sciences of the United States of America, 107, 4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van de Peer, Y. , Maere, S. , & Meyer, A. (2009). The evolutionary significance of ancient genome duplications. Nature Reviews Genetics, 10, 725 10.1038/nrg2600 [DOI] [PubMed] [Google Scholar]
- van Tuinen, A. , Kerckhoffs, L. H. J. , Nagatani, A. , Kendrick, R. E. , & Koornneef, M. (1995). A temporarily red light‐insensitive mutant of tomato lacks a light‐stable, B‐like phytochrome. Plant Physiology, 108, 939–947. 10.1104/pp.108.3.939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller, J. L. , Schreuder, M. E. L. , Smith, H. , Koornneef, M. , & Kendrick, R. E. (2000). Physiological interactions of phytochromes A, B1 and B2 in the control of development in tomato. The Plant Journal, 24, 345–356. 10.1046/j.1365-313x.2000.00879.x [DOI] [PubMed] [Google Scholar]
- Whippo, C. W. , & Hangarter, R. P. (2004). Phytochrome modulation of blue‐light‐induced phototropism. Plant, Cell & Environment, 27, 1223–1228. 10.1111/j.1365-3040.2004.01227.x [DOI] [Google Scholar]
- Zhang, J. (2012). Genetic redundancies and their evolutionary maintenance In Soyer O. S. (Ed.), Evolutionary Systems Biology (pp. 279–300). New York, NY: Springer. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


