Abstract
Background
Acute severe mitral regurgitation (MR) associated with cardiogenic shock is a life-threatening emergency. Traditional teaching has focused on the need for emergent coronary angiography and/or intra-aortic balloon counterpulsation in preparation for emergent open-heart surgery for repair/replacement. Unfortunately, emergent open-heart surgery in patients with acute MR complicated by cardiogenic shock is associated with 25–46% perioperative mortality. New devices have provided additional options for stabilization prior to emergent surgery which facilitate improved outcomes.
Case summary
We present two cases of acute severe MR resulting in cardiogenic shock and profound hypoxaemia. TandemHeart® mechanical circulatory support with an oxygenator spliced into the circuit, akin to veno-arterial extracorporeal membrane oxygenation (ECMO), facilitated haemodynamic stabilization and decongestion of the lungs facilitating successful bridge to mitral valve surgery. Successful discharge to home was achieved in both patients with good neurological outcomes and sustained long-term functional recovery at 18 and 14 months, respectively.
Discussion
Selective use of the TandemHeart®, with or without ECMO, facilitates management of the critically ill cardiogenic shock patient with acute severe MR.
Keywords: Case series, Acute mitral regurgitation, Cardiogenic shock, Mechanical circulatory support, Hypoxaemia
Learning points
Acute severe mitral regurgitation associated with cardiogenic shock is associated with high peri-procedural mortality.
TandemHeart, with or without extracorporeal membrane oxygenation, can facilitate haemodynamic stabilization and bridge to surgery with successful surgical and patient outcomes.
Introduction
Acute severe mitral regurgitation (MR) is a medical, and often surgical emergency, associated with acute pulmonary oedema and/or cardiogenic shock. While prompt surgical intervention is generally the definitive therapy, there are situations where the availability, or the risk/benefit ratio, of cardiac surgery necessitates interim stabilization with medical management and mechanical circulatory support (MCS). Studies have shown the perioperative mortality rate of patients undergoing mitral valve surgery for acute severe MR ranges from 14.8% to 27%.1 Furthermore, surgical outcomes in patients undergoing surgery with shock physiology are significantly worse compared to patients without cardiogenic shock.2–4
Traditionally, placement of an intra-aortic balloon pump (IABP) has been utilized due to its ability to augment cardiac output and decrease afterload, thereby supporting mean arterial pressure and potentially decreasing MR.5 Alternatively, newer MCS devices such as the Impella® (Abiomed, Danvers, MA, USA) and TandemHeart® (TH) (LivaNova, London, UK) left ventricular (LV) assist devices could be used. The Impella® axial flow pump can be rapidly deployed, provide haemodynamic support, and effect LV decompression. However, neither the IABP nor the Impella® can ameliorate severe refractory hypoxaemia from acute pulmonary oedema. The TH is unique in its ability to offer solutions to both hypotension and hypoxaemia due to poor forward stroke volume. Inserted percutaneously, a left atrial (LA) drainage cannula is placed by transseptal puncture across the interatrial septum (Figure 1), with an arterial return cannula placed in the femoral artery. When hypoxaemia is an additional challenge, an oxygenator can be spliced into the circuit, akin to peripheral veno-arterial (V-A) extracorporeal membrane oxygenation (ECMO). We report on two patients with acute severe MR presenting with cardiogenic shock and profound hypoxaemia bridged to surgery and recovery with use of a TH-ECMO approach.
Figure 1.
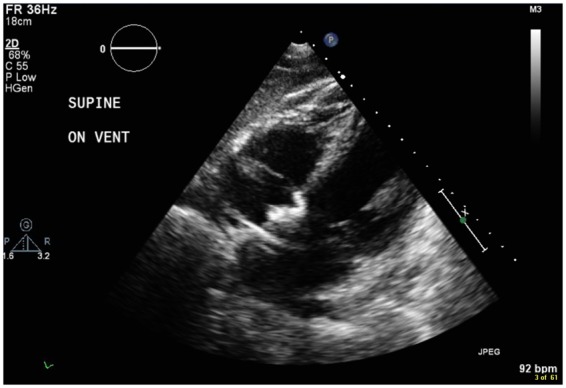
Echo image showing inflow cannula of TandemHeart® in left atrium across the interatrial septum.
Timeline
| Time | Event |
|---|---|
| Case 1 | |
| Day 0 | |
| 3:00 am | Presents to outside facility; echo shows severe mitral regurgitation (MR) with flail posterior leaflet and ruptured chordae |
| 7:00 pm | Transferred to our facility for consideration of V-V extracorporeal membrane oxygenation (ECMO) due to refractory hypoxaemia |
| 09:30 pm | TandemHeart (TH)-ECMO circuit initiated |
| Day 2 | Undergoes successful mitral valve replacement and removal of TH-ECMO circuit |
| Day 12 | Patient discharged home without deficits |
| Case 2 | |
| Day 0 | |
| 03:30 am | Presents to the emergency department (ED) |
| 05:30 am | Emergent echo shows severe MR with complete rupture of papillary muscle |
| 09:17 am | Initiated on TH-ECMO circuit given refractory hypoxaemia in cath lab |
| Day 5 | Undergoes successful reimplantation of ruptured papillary muscle with mitral valve annuloplasty and bypass grafting to the left circumflex artery and first obtuse marginal branch; TH-ECMO circuit removed |
| Day 6 | Found to have small subarachnoid haemorrhage and subacute cerebral ischaemic infarcts |
| Day 15 | Discharged home without neurologic deficits |
Case presentation
Case 1
A 47-year-old man without significant medical history initially presented to an outside facility with severe, progressive shortness of breath over 3 weeks with sudden onset haemoptysis requiring intubation. An emergent echocardiogram revealed severe MR with flail posterior mitral leaflet and ruptured chordae tendinae, and LV ejection fraction of 65–70% (Figures 2 and 3) (Supplementary material online, Videos S1–S5). Given refractory hypoxaemia, the patient was transferred to our facility for consideration of veno-venous (V-V) ECMO and/or IABP support as a bridge to surgery.
Figure 2.
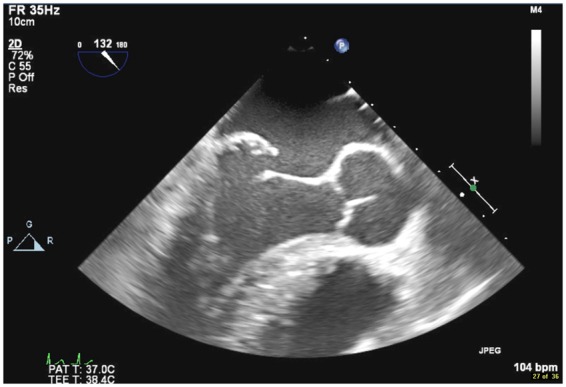
Transoesophageal echocardiogram two-dimensional mid-oesophageal four-chamber view showing flail posterior mitral valve leaflet.
Figure 3.
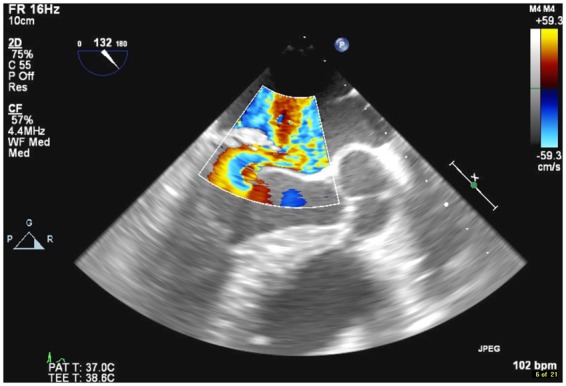
Transoesophageal echocardiogram colour Doppler mid-oesophageal view showing severe mitral regurgitation.
Upon arrival to our cath lab, heart rate was 115 b.p.m., blood pressure 94/60 mmHg (on norepinephrine 4 μg/kg/min), respiratory rate 26 breaths per min, SpO2 78% on mechanical ventilation with FiO2 100%, and positive end expiratory pressure (PEEP) 18 mmHg. Physical exam revealed three of six harsh systolic murmur at LV apex radiating to second right intercostal space with bilateral wet rales to apical lung fields.
ECG showed sinus tachycardia without significant ST-T changes. Labs showed white blood count (WBC) 19.9 k/μL [upper limit of normal (ULN) 12 k/μL], serum creatinine 1.83 mg/dL up from 1.09 mg/dL (ULN 1.3 mg/dL), lactic acid 2.3 mmol/L (normal <2 mmol/L), troponin-I <0.03 pg/dL (normal < 0.03 ng/mL), and arterial blood gas (ABG) pre-TH pH 7.14/PaCO2 58 mmHg/PaO2 43 mmHg/HCO3 20 mmol/L on FiO2 100%. Chest X-ray showed bilateral diffuse pulmonary oedema (Figure 4). Given cardiogenic shock physiology with severe refractory hypoxaemia, a decision was made to unload the left atrium with a TH device. An oxygenator was spliced onto the arterial return cannula akin to V-A ECMO with improvement in the arterial PaO2 to 599 mmHg. Subsequent coronary angiography revealed normal coronary arteries. Hypothermia was instituted for neuroprotection given severe persistent hypoxaemia. The patient remained on the TH-ECMO circuit for 48 h with a decrease in pulmonary vascular congestion (Figure 5) and improved lung compliance. Patient subsequently underwent mitral valve replacement with a 31-mm bioprosthesis, removal of the TH-ECMO circuit, and repair of the interatrial septum. The patient was successfully discharged home on Day 12 of hospitalization without any neurological deficits.
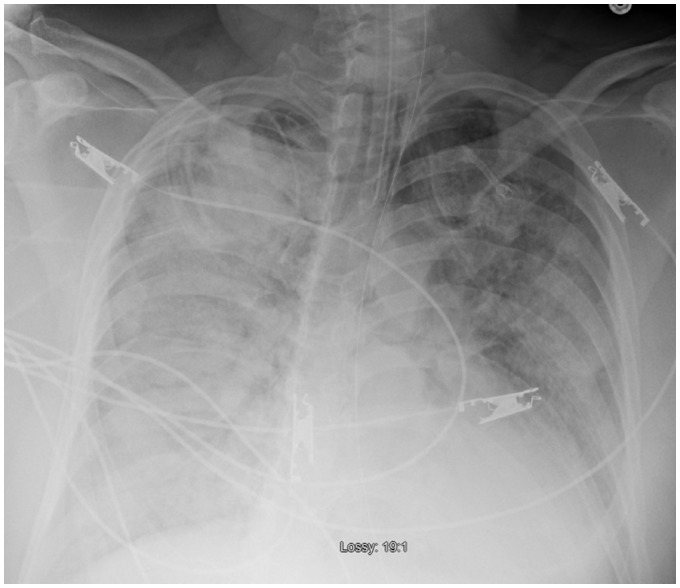
Figure 4.
Pre-TandemHeart® chest X-ray showing bilateral diffuse pulmonary oedema.
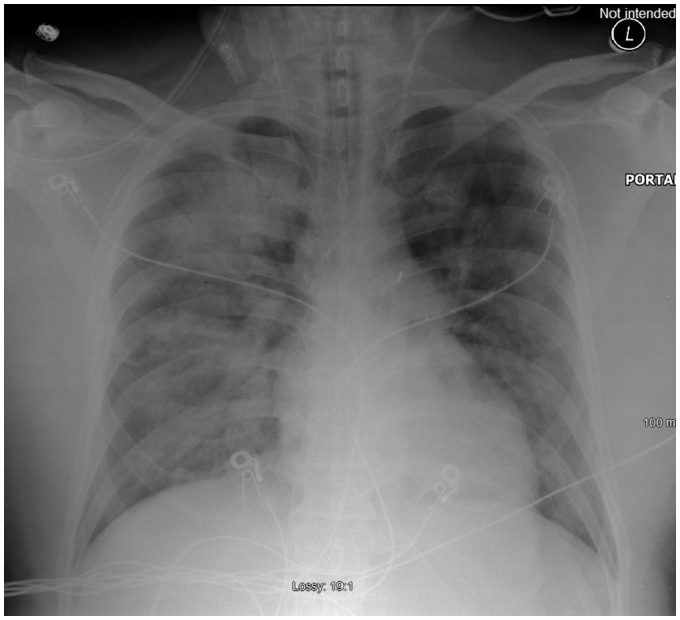
Figure 5.
Chest X-ray 48 h post-TH-ECMO insertion showing improvement in bilateral pulmonary oedema.
Case 2
A 49-year-old man with hypertension, hypertriglyceridaemia, and moderate alcohol use presented with acute onset of chest pain and dyspnoea that awoke him from sleep. Vitals on presentation were heart rate 134 b.p.m., blood pressure 117/79 mmHg, respiratory rate 28 breaths per min, and SpO2 91% on 6 L/min O2 nasal cannula. Physical exam was significant for a blowing pan systolic murmur at LV apex and bilateral rales.
Initial troponin-I was 3.07 ng/mL (normal < 0.03 ng/mL), which trended up to 73.5 ng/mL on arrival to cath lab, and peaked at 119.6 ng/mL. Lactic acid level was 5.5 mmol/L (normal < 2 mmol/L). Serum creatinine was 1.36 mg/dL up from 0.92 mg/dL (ULN 1.3 mg/dL). Chest X-ray showed bilateral pulmonary vascular congestion. EKG showed sinus tachycardia with ST depressions in V2–V5 consistent with a posterior wall infarction. An emergent echocardiogram revealed severe MR with complete rupture of the posterior papillary muscle and flail posterior mitral leaflet (Figures 6 and 7) (Supplementary material online, Videos S6 and S7). Left ventricular ejection fraction was 50–55% with mild hypokinesis of the lateral wall. Upon arrival to the catheterization lab, patient had further deterioration with peripheral saturation of 80% on 100% non-rebreather mask necessitating endotracheal intubation and mechanical ventilation. Post-intubation, pink, frothy sputum was noted in the endotracheal tube and an ABG revealed 7.10/64/63/15.7 on FiO2 100% and PEEP 15 cmH2O. Emergent coronary angiography revealed a subtotal left circumflex artery lesion (Figure 8; Supplementary material online, Videos S8 and S9).
Figure 6.
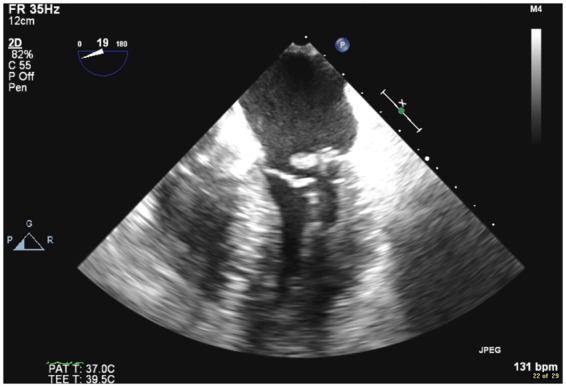
Transoesophageal echocardiogram mid-oesophageal two-dimensional view showing flail posterior leaflet with rupture papillary muscle.
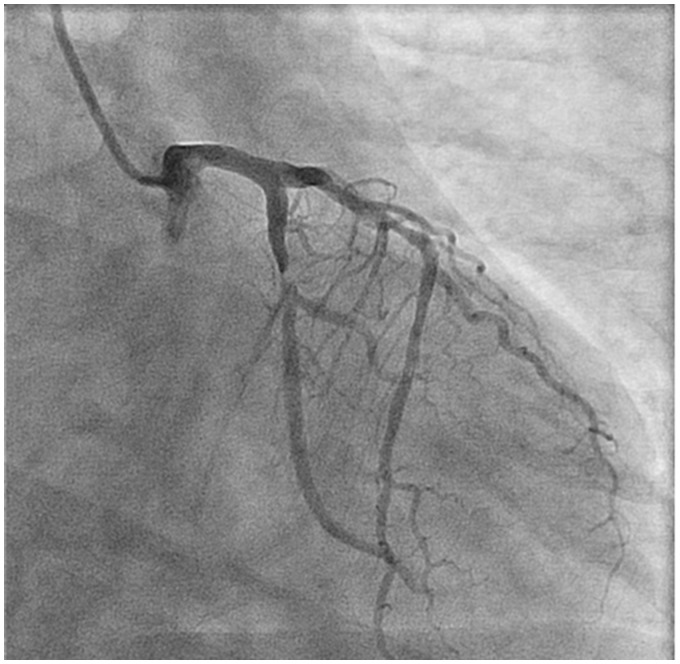
Figure 8.
Coronary angiography of left coronary system showing subtotal left circumflex artery occlusion.
Figure 7.
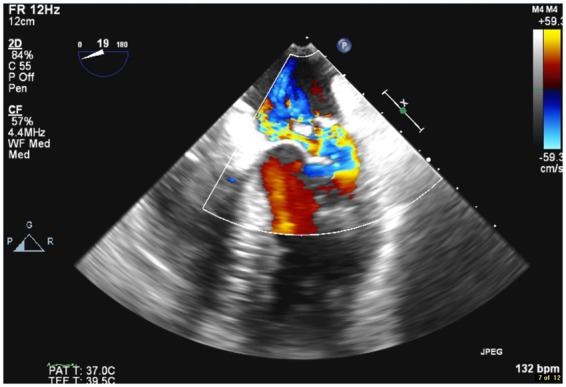
Transoesophageal echocardiogram colour Doppler mid-oesophageal view showing severe mitral regurgitation.
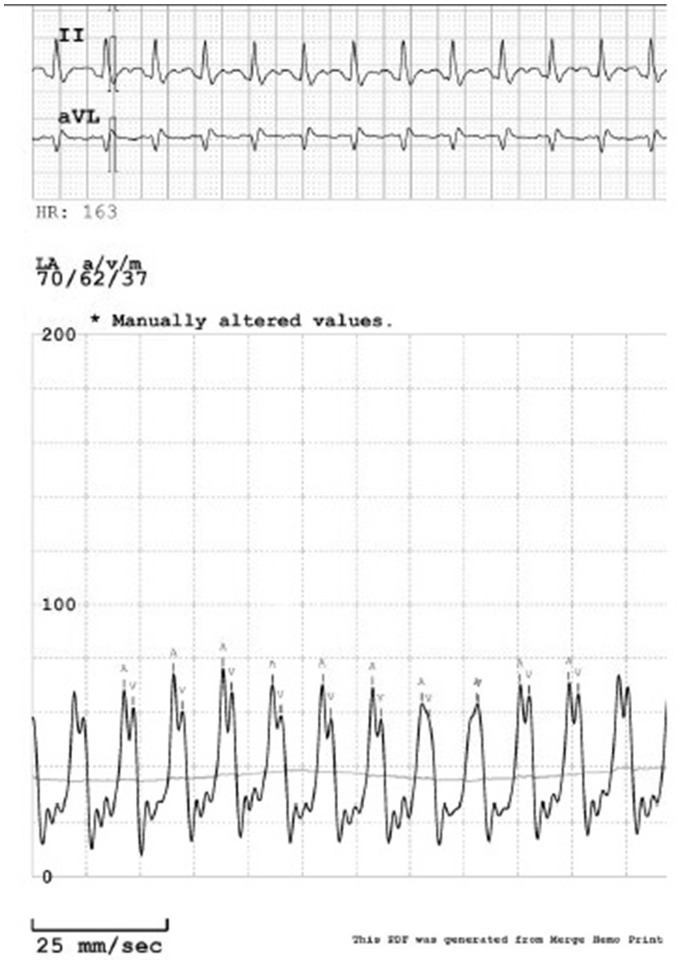
After Heart Team discussion, a decision was made to support the patient with placement of a TH LV assist device with an oxygenator spliced into the circuit post-pump for a TH-ECMO configuration. Direct LA pressures obtained immediately following transseptal puncture demonstrated single chamber physiology with ventricularization of the LA pressure with mean pressure of 37 mmHg and V wave of 70 mmHg (Figure 9). Swan-Ganz catheterization post-TH-ECMO (3.6 L/min flow) revealed RA 16 mmHg; right ventricle (RV) 45/16 mmHg; PA 45/22/33 mmHg; pulmonary capillary wedge pressure 27 mmHg; PA saturation 72.2%; and calculated cardiac output and cardiac index by Fick were 5.0 L/min and 3.1 L/min/m2, respectively. Following initiation of the TH-ECMO circuit, right radial ABG showed improvement in oxygenation to PaO2 416 mmHg. Despite continuous drainage of 3.5 L/min from the LA, there was only mild improvement in pulmonary congestion and higher ventilator support required at 24 h. In order to capture more of the native venous return, an additional right atrial (RA) 21-Fr venous drainage cannula was Y-connected to create a veno-venous-arterial (VV-A) TH-ECMO circuit, with addition of an IABP to facilitate venting of the left ventricle to allow for 1:1 aortic valve opening. This facilitated significant improvement in pulmonary vascular congestion and weaning of vasopressors. Patient was triaged to surgery on Day 5 and underwent successful reimplantation of the ruptured papillary muscle, mitral annuloplasty, and saphenous vein bypass grafting to the left circumflex artery and first obtuse marginal branch.
Figure 9.
Direct left atrial pressure recording showing ventricularization of left atrial pressure.
Post-operative course was complicated by seizures as a manifestation of a mild left Sylvian fissure subarachnoid haemorrhage and small subacute ischaemic infarcts, possibly related to the emergent TH insertion procedure. Despite this, patient made a full neurologic recovery (cerebral performance category 1) with no residual deficits and was discharged home on hospital Day 15.
Discussion
Acute severe MR was caused by myxomatous mitral valve degeneration complicated by chordal rupture in Case 1 and acute myocardial infarction complicated by complete papillary muscle rupture in Case 2. Emergent surgical intervention remains the treatment of choice for management of symptomatic acute MR but is associated with high perioperative mortality when complicated by cardiogenic shock.1,3 Both of our patients were triaged as intermediate to high surgical risk post-TH-ECMO stabilization and lung decompression, as evidenced by the Society of Thoracic Surgery (STS) scores of 4.047% and 17.6%, respectively. Specifically, in patients with post-infarction papillary muscle rupture, a high risk, as assessed by a EuroSCORE II >25%, has been shown to be an independent predictor of in-hospital mortality.2
Acute severe MR leads to a variety of haemodynamic changes primarily related to the abrupt onset without time for LA adaptation. A significant increase in the LA and pulmonary venous pressures occur from acute volume overload of a non-compliant LA resulting in severe pulmonary oedema induced hypoxaemia. Additionally, there is a drop in cardiac output due to a decrease in effective forward stroke volume across the aortic valve contributing to cardiogenic shock physiology. A compensatory systemic vasoconstrictor response increases the systemic afterload and further aggravates the MR.6
In cardiogenic shock due to acute myocardial infarction or decompensated heart failure, the TH has been shown to produce greater haemodynamic benefit than the IABP, generating a greater increase in cardiac index and mean arterial blood pressure with a significant decrease in pulmonary capillary wedge pressure, but without a mortality benefit vis-à-vis an IABP.7
The TH is a percutaneous LV assist device driven by a centrifugal pump, which works by extracting oxygenated blood from the left atrium via an outflow LA cannula placed by transseptal puncture across the interatrial septum. The blood is then circulated extracorporeally through the pump and returned via an inflow cannula into the femoral artery. By directly extracting blood from the LA and thus decompressing the LA, the TH helps to remove the increased LA volume created by acute MR. This, in turn, decreases LA pressure and subsequently decreases pulmonary venous pressure and pulmonary congestion. Additionally, because the TH returns blood directly to the systemic arterial vasculature, it augments native cardiac output and maintains organ perfusion. The TH can generate 3.5–4.5 L/min of cardiac output. Furthermore, the TH system allows for the addition of an oxygenator to be spliced into the arterial return circuit when hypoxaemia is an additional challenge as a result of poor oxygenation of blood in the pulmonary vasculature due to severe pulmonary oedema.
Use of a TH in an elective manner for acute MR with severe pulmonary hypertension to facilitate RV decompression to optimize surgical outcomes has been described.8 Our cases are unique in that both patients were in profound cardiogenic shock with severe hypoxaemia that mandated an emergent TH insertion. Use of the TH device with an oxygenator (TH-ECMO) provided prompt stabilization of the haemodynamics and refractory hypoxaemia allowing for early surgery in both patients with good outcomes. Bleeding, vascular injury, cardiac/aortic perforation, pericardial tamponade, stroke resultant to air embolism and/or thrombus formation, membrane activation from ECMO, and leg ischaemia are risks associated with TH-ECMO placement. Use of vascular ultrasound for peripheral access, intracardiac echo guidance, and/or transoesophageal echo to guide transseptal puncture, and meticulous attention to prevent air embolism help minimize complications and increase procedural safety.
Conclusion
Acute MR complicated by cardiogenic shock is a medical emergency, associated with a high mortality even after valvular and coronary artery bypass surgery. Our report demonstrates that using a TH-ECMO bridge to open-heart surgery can facilitate better surgical and patient outcomes. Future studies focusing on stabilizing such patients with an earlier application of TH-ECMO or other MCS device might help define the optimal timing for intervention in these patients.
Lead author biography

Dr Michael DiVita attended St. George's University School of Medicine. He completed his Internal Medicine residency and Cardiology fellowship at Newark Beth Israel Medical Center, Newark, NJ, USA. Dr DiVita is currently completing his Interventional Cardiology fellowship and hopes to pursue further training in Advanced Heart Failure and Transplant Cardiology.
Supplementary material
Supplementary material is available at European Heart Journal - Case Reports online.
Slide sets: A fully edited slide set detailing this case and suitable for local presentation is available online as Supplementary data.
Consent: The author/s confirm that written consent for submission and publication of this case report including image(s) and associated text has been obtained from the patient in line with COPE guidance.
Conflict of interest: none declared.
Supplementary Material
References
- 1. Lorusso R, Gelsomino S, De Cicco G, Beghi C, Russo C, De Bonis M, Colli A, Sala A.. Mitral valve surgery in emergency for severe acute regurgitation: analysis of postoperative results from a multicentre study. Eur J Cardiothorac Surg 2008;33:573–582. [DOI] [PubMed] [Google Scholar]
- 2. Bouma W, Wijdh-den Hamer IJ, Koene BM, Kuijpers M, Natour E, Erasmus ME, van der Horst ICC, Gorman Iii JH, Gorman RC, Mariani MA.. Predictors of in-hospital mortality after mitral valve surgery for post-myocardial infarction papillary muscle rupture. J Cardiothorac Surg 2014;9:171.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thompson CR, Buller CE, Sleeper LA, Antonelli TA, Webb JG, Jaber WA, Abel JG, Hochman JS.. Cardiogenic shock due to acute severe mitral regurgitation complicating acute myocardial infarction: a report from the SHOCK Trial Registry. J Am Coll Cardiol 2000;36:1104–1109. [DOI] [PubMed] [Google Scholar]
- 4. Bolooki H. [Complications of thrombolytic treatment of acute myocardial infarction]. Ugeskr Laeger 1990;152:2370–2372. [PubMed] [Google Scholar]
- 5. Dekker AL, Reesink KD, van der Veen FH, van Ommen GVA, Geskes GG, Soemers ACM, Maessen JG.. Intra-aortic balloon pumping in acute mitral regurgitation reduces aortic impedance and regurgitant fraction. Shock 2003;19:334–338. [DOI] [PubMed] [Google Scholar]
- 6. Gaasch WH, Meyer TE.. Left ventricular response to mitral regurgitation implications for management. Circulation 2008;118:2298–2303. [DOI] [PubMed] [Google Scholar]
- 7. Burkhoff D, Cohen H, Brunckhorst C, O'Neill WW.. A randomized multicenter clinical study to evaluate the safety and efficacy of the TandemHeart percutaneous ventricular assist device versus conventional therapy with intraaortic balloon pumping for treatment of cardiogenic shock. Am Heart J 2006;152:469.e1–469.e8. [DOI] [PubMed] [Google Scholar]
- 8. Hira RS, Thamwiwat A, Kar B.. TandemHeart placement for cardiogenic shock in acute severe mitral regurgitation and right ventricular failure. Catheter Cardiovasc Interv 2014;83:319–322. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.