Abstract
Background: Self-monitored blood glucose (SMBG) and real-time continuous glucose monitoring (rtCGM) are used by people living with type 1 diabetes (T1D) to assess glucose and inform decision-making. Percentage time in range (%TIR) between 3.9 and 10 mmol/L has been associated with incident microvascular complications using historical SMBG data. However, the association between %TIR calculated from rtCGM data has not been identified. This study investigates whether %TIR values generated from rtCGM and SMBG data significantly differ from each other in adults with T1D.
Materials and Methods: rtCGM and SMBG data from the REPLACE-BG study were obtained and analyzed. The dataset contained rtCGM (Dexcom G4 Platinum) and SMBG (Contour Next) values for 226 participants during a run-in phase lasting up to 10 weeks, followed by the 26-week trial. Percentages times in hypoglycemic, euglycemia and hyperglycemic ranges were generated from rtCGM and SMBG data using last observation carry forward method (zero-order hold) and linear interpolation (first-order hold).
Results: Participants had a median (interquartile range [IQR]) age of 43.0 (31.0–55.0) years, and hemoglobin A1C of 53 (49–57) mmol/mol [7.0 (6.6–7.4)%]. The median (IQR) %TIR was significantly higher with rtCGM than with SMBG; 63.0 (55.9–71.0)% versus 54.6 (45.6–63.0)%, respectively, P < 0.001. Median %times in hypoglycemia and hyperglycemia were significantly different with SMBG than rtCGM (P < 0.001). SMBG-derived data using linear interpolation significantly differed from the carry forward method (P < 0.001 for all glycemic ranges). Differences reported were greater at night than during the day (P < 0.001 for all glycemic ranges).
Conclusion: The %time in all glycemic ranges reported by SMBG and rtCGM differ significantly, suggesting relationships between times in ranges, and complication status may be different between monitoring modalities. In addition, varying methods of calculating %TIR from SMBG-derived data provide significantly differing results. %TIR targets may therefore vary by monitoring choice and methods of calculation and harmonization of TIR standards may be challenging.
Keywords: Type 1 diabetes, Real-time continuous glucose monitoring (rtCGM), Self-monitoring of blood glucose, Time in range, Hypoglycemia
Background
Real-time continuous glucose monitoring (rtCGM) has been increasingly adopted over the last few years, with technological advancements resulting in greater sensor accuracy, increased convenience, and greater ease of use.1–3 However, despite expanding reimbursement, self-monitoring of blood glucose (SMBG) using capillary samples remains the primary method of assessing glucose concentrations for most people with type 1 diabetes (T1D) worldwide.4,5
rtCGM use supports intensification of glycemia, enables meaningful improvements in hemoglobin A1C (HbA1c),6,7 while reducing exposure to, and risk of hypoglycemia8 in people using insulin pump and multiple-dose injection regimens.9 The use of rtCGM is also associated with improvements in quality of life10 and reduction in fear of hypoglycemia,11 and is cost-effective.6
Consensus guidelines identify percentage times in glucose ranges as a metric of glycemic control that provides actionable information in addition to HbA1c.6 The recommended times in ranges metrics for rtCGM include the following: time per day within target glucose range (time in range [TIR]: 3.9–10.0 mmol/L; 70–180 mg/dL), time below target glucose range (TBR: <3.0 and <3.9 mmol/L; <54 and <70 mg/dL), and time above target glucose range (TAR: >10.0 mmol/L; >180 mg/dL).
Validity of %TIR using seven-point SMBG testing has been corroborated with the demonstration of a clear association with the risk of development of, or progression of, retinopathy and development of microalbuminuria in the Diabetes Control and Complications Trial (DCCT) study data.7 The adjusted hazard ratios for developing retinopathy and microalbuminuria were 1.64 (1.51–1.78) and 1.40 (1.25–1.56), respectively, for each 10% decrease in %time spent between 3.9 and 10 mmol/L (70–180 mg/dL).
This work supports the potential use of %TIR from rtCGM data in stratifying complication risk, but the data are based on SMBG values obtained from devices over two decades old with lower accuracy than present-day devices. Despite consensus to use times in ranges to assess control and risk, equivalence between times in ranges assessed by different glucose modalities has not been clearly demonstrated, and, while harmonization may be challenging to achieve, it may be appropriate to consider %TIR targets appropriate to the glucose monitoring modality.
In a study of 161 children by the Diabetes Research in Children Network (DirecNet), mean %TIR was comparable with rtCGM and eight-point SMBG testing; 49% versus 50%, respectively.8 However, only 10% of participants completed the 8-point profile and rtCGM data were only collected for 3 days. In another study combining data from six inpatient studies, a high degree of concordance of composite outcomes based on rtCGM measurements with the corresponding outcomes based on reference blood glucose measurements was observed.9 Mean %TIR was 60% with both rtCGM and with reference blood glucose measurements,9 but this concordance may not be surprising given the very frequent Yellow Springs Instrument measurements undertaken at 15–30 min intervals.
Most recently, in a small study of 21 children over a short duration, glycemic parameters derived from intermittently scanned CGM (isCGM; also known as flash glucose monitoring) and rtCGM showed significant differences in various glycemic metrics, including %TIR.10
Given the limitations of the literature and the significant potential to use %TIR to stratify micro- and macrovascular risk in T1D, we investigate whether %time in glycemic range values generated from rtCGM and SMBG in a large dataset over a longer period differ.
Methods and Research Design
The publicly available preexisting REPLACE-BG dataset (NCT02258373) was obtained. The trial protocol has been described previously,11 but in brief, the study was conducted between May 2015 and March 2016 in adult participants with T1D of >1 year duration. All participants used an insulin pump and had an HbA1c ≤9.0% (≤75 mmol/mol), with intact awareness of hypoglycemia and no recent severe hypoglycemia. Following a 2–10-week run-in phase during which participants wore blinded rtCGM (Dexcom G4 Platinum; Dexcom, San Diego, CA), participants were randomized to make insulin dosing decisions using either rtCGM-only, or rtCGM and SMBG for 26 weeks. The Contour Next system (Ascensia, Parsipanny, NJ) was used to perform SMBG readings. CGM and SMBG data are available for all participants.
During the study, the rtCGM+SMBG group performed SMBG measurements when insulin boluses were administered in addition to treating or preventing hypoglycemia, and before going to bed. Both groups used SMBG measurements to calibrate their rtCGM devices, as per manufacturer specifications.
Percentage times in ranges analysis
%TIR (3.9–10 mmol/L) was calculated using rtCGM and SMBG measurements obtained during the run-in phase and the 26-week randomized controlled trial from all participants. %TIR for SMBG measurements was primarily calculated using a last observation carry forward method (zero-order hold; i.e., the value was carried forward to the next SMBG-value) and was additionally assessed using a linear interpolation (first-order hold) through adjacent SMBG values. Unless otherwise stated, %TIR values for SMBG data are derived from the carry forward analysis. Secondary outcomes include %TBR (<3.9 mmol/L; <70 mg/dL and <3.0 mmol/L; <54 mg/dL) and %TAR (>10 mmol/L; >180 mg/dL). Times in ranges for rtCGM and SMBG during the day and at night were also obtained. Daytime was specified as time between 07:00:00 and 22:59:59, with the remainder of the day constituting night-time.
Statistical methods
The data were tested for normal distribution using the Shapiro–Wilk test of normality. The result was P < 0.05, indicating that the data were not normally distributed. Differences between rtCGM and SMBG were tested for significance using Wilcoxon matched-pairs signed-rank tests, with the nonparametric Spearman rank tests (rs) performed for correlation. The Kruskal–Wallis test was used to analyze differences between %TIR between rtCGM and SMBG calculated values using the carry forward technique and linear interpolation. Results were considered statistically significant if P < 0.05, with statistical analyses performed using Stata version 15 (StataCorp, College Station, TX).
Results
Two hundred twenty-six participants were analyzed. Baseline characteristics of the combined participant groups are summarized in Table 1. The median (interquartile range [IQR]) age was 43.0 (31.0–55.0) years, with HbA1c of 53 (49–57) mmol/mol [7.0 (6.6–7.4)%]. Participants self-reported 5.0 (4.0–6.0) SMBG tests daily and had intact hypoglycemic awareness.
Table 1.
Participant Characteristics at Enrollment (n = 226)
| Participant characteristics (n = 226) | Median (IQR) or n (%) |
|---|---|
| Age (years) | 43.0 (31.0–55.0) |
| Male | 114 (50.4) |
| Female | 112 (49.6) |
| Body mass index (kg/m2) | 26.7 (24.0–30.1) |
| Race/ethnicity | |
| Caucasian | 207 (91.6) |
| Asian | 4 (1.8) |
| Hispanic/Latino | 9 (4.0) |
| Black/African American | 5 (2.2) |
| Other/unknown | 1 (0.4) |
| rtCGM use before study | |
| Never used rtCGM | 40 (17.7) |
| In past, but not currently | 79 (35.0) |
| Current Dexcom rtCGM user | 77 (34.1) |
| Current Medtronic rtCGM user | 30 (13.2) |
| HbA1c (mmol/mol) | 53 (49–57) |
| HbA1c (%) | 7.0 (6.6–7.4) |
| Self-reported SMBG testing daily frequency | 5.0 (4.0–6.0) |
| Clarke hypoglycemia unawareness survey total score | |
| 0 | 153 (67.7) |
| 1 | 48 (21.2) |
| 2 | 25 (11.1) |
| Annual household income ($)a | |
| <50,000 | 25 (11.1) |
| >50,000–100,000 | 56 (24.8) |
| ≥100,000 | 87 (38.5) |
| Education levela | |
| Less than bachelor's degree | 47 (20.8) |
| Bachelor's degree | 110 (48.7) |
| Post bachelor's degree | 64 (28.3) |
Data expressed as median (IQR) or n (%).
Missing data: annual income for 58 participants, and 5 for education level.
IQR, interquartile range; rtCGM, real-time continuous glucose monitoring; SMBG, self-monitored blood glucose.
Comparing percentage time in glycemic ranges between rtCGM and SMBG
The median (IQR) %TIR (3.9–10 mmol/L) derived from the rtCGM data was significantly higher than the calculated value from SMBG data using the carry forward method, 63.0 (55.9–71.0)% versus 61.4 (50.1–69.5)%, respectively (P < 0.001; Table 2).
Table 2.
Comparisons of %TIR Across All Glycemic Ranges Generated by Different Data Imputation Methodologies, That Is, rtCGM, SMBG Last Observation Carry Forward Method, and SMBG Using Linear Interpolation (n = 226)
| Glycemic ranges (%time) | Median %time (IQR) |
Kruskal–Wallis (P-value) | ||
|---|---|---|---|---|
| rtCGM | SMBG (carry forward method) | SMBG (linear interpolated) | ||
| TIR | ||||
| 3.9–10 mmol/L (70–180 mg/dL) | 63.0 (55.9–71.0) | 54.6 (45.3–63.0) | 61.4 (50.1–69.5) | <0.001a–c |
| TBR | ||||
| <3.9 mmol/L (70 mg/dL) | 3.5 (1.8–5.1) | 3.9 (2.0–6.3) | 1.6 (0.8–3.3) | <0.001a–c |
| <3.0 mmol/L (54 mg/dL) | 0.8 (0.3–1.3) | 0.7 (0.3–1.5) | 0.4 (0.1–0.6) | <0.001b,c |
| TAR | ||||
| >10 mmol/L (180 mg/dL) | 33.4 (25.1–41.3) | 40.1 (31.6–51.3) | 35.6 (26.5–48.2) | <0.001a–c |
| >15 mmol/L (270 mg/dL) | 5.0 (2.7–8.2) | 8.6 (4.5–13.3) | 4.5 (2.0–8.7) | <0.001a,b,d |
Significant difference between rtCGM and SMBG carry forward method (P < 0.001).
Significant difference between SMBG using linear interpolation and carry forward method (P < 0.001).
Significant difference between SMBG using linear interpolation and rtCGM (P < 0.001).
Significant difference between SMBG using linear interpolation and rtCGM for TAR >15 mmol/L; >270 mg/dL (P = 0.01).
TAR, time above range; TBR, time below range; TIR, time in range.
For hypoglycemia %TBR <3.9 mmol/L (<70 mg/dL), SMBG reported significantly higher TBR 3.9 (2.0–6.3)% than rtCGM at 3.5 (1.8–5.1)%; P < 0.001. No statistically significant differences were observed between the two groups for more clinically significant hypoglycemia at <3.0 mmol/L (<54 mg/dL); P = 0.08.
Median %TAR >10.0 mmol/L (>180 mg/dL) derived from SMBG data was significantly higher than the calculated value from rtCGM data, 40.1 (31.6–51.3)% versus 33.4 (25.1–41.3)%, respectively (P < 0.001; Table 2).
Mean glucose values for each glucose range was not different between the rtCGM and SMBG groups. The average glucose for the entire glycemic range was statistically different. Median average glucose for rtCGM versus BGM is 160.3 (148.3–172.5) mg/dL versus 169.8 (157.5–187.3) mg/dL, respectively (P < 0.001).
%TIR generated from SMBG-derived linear interpolation (first-order hold) compared to SMBG data imputation by a last observation carry forward method (zero-order hold) was significantly different for all times in glycemic ranges (P < 0.001; Table 2). For %TIR 3.9–10 mmol/L (70–180 mg/dL), values calculated from SMBG data by interpolation are closer to the CGM-values for TIR, although they remain significantly different (P < 0.001).
TBR calculated by linear interpolation reported significantly lower times at 1.6 (0.8–3.3)% for %TBR <3.9 mmol/L (<70 mg/dL) and 0.4 (0.1–0.6)% for %TBR <3.0 mmol/L (<54 mg/dL) than rtCGM, while the carry forward method reported higher values. Ultimately, all times in glycemic ranges generated from SMBG linear interpolation were significantly different from rtCGM (P < 0.001).
No correlation was observed between SMBG measurement frequency and the difference between rtCGM-based and SMBG-based TIR using carry forward technique (r = −0.03; P = 0.64) or using linear interpolation (r = −0.06; P = 0.37).
Correlation and bias between rtCGM and SMBG
A strong correlation for median %TIR was observed between rtCGM and SMBG, although the data were skewed toward SMBG underreporting (rs = 0.857; P < 0.001; Fig. 1A–D). Correlation for each glycemic range has been summarized in Figure 1E.
FIG. 1.
Correlation between times in glycemic range spent by participants using rtCGM and SMBG data (n = 226). (A) %TIR 3.9–10 mmol/L; 70–180 mg/dL (P < 0.001). (B) %TBR <3.9 mmol/L; <70 mg/dL (P < 0.001). (C) %TBR <3.0 mmol/L; <54 mg/dL (P < 0.001). (D) %TAR >10.0 mmol/L; >180 mg/dL (P < 0.001). (E) Summary of correlation coefficients and equations of line. rtCGM, real-time continuous glucose monitoring; SMBG, self-monitored blood glucose; TAR, time above range; TBR, time below range; TIR, time in range.
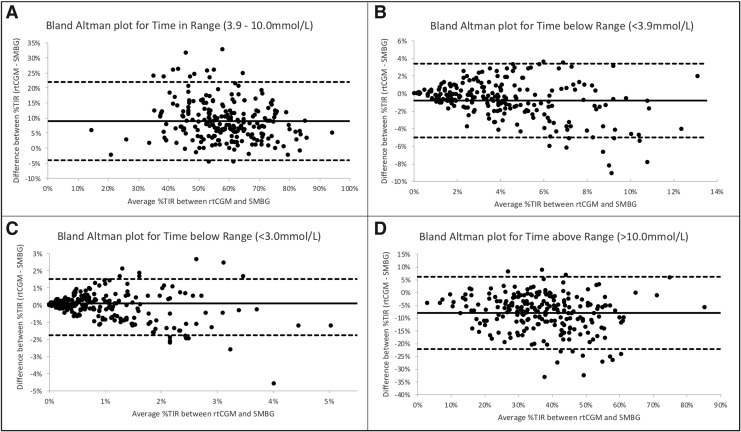
Despite high concordance observed in the correlation coefficients, Bland–Altman analyses (Fig. 2A–D) confirms bias for underreporting %TIR (3.9–10.0 mmol/l; 70–180 mg/dL) by SMBG, with wide limits of agreement. Six percent of data points lie beyond the limits of agreement. For the %TBR <3.0 mmol/L (<54 mmol/L) and <3.9 mmol/L (<70 mg/dL), as the mean difference between %TIR derived from rtCGM and SMBG increases, the greater the scatter around the bias line.
FIG. 2.
Bland–Altman plots showing the difference between rtCGM and SMBG on the y-axis and the mean of the two readings on the x-axis. The dashed line represents the 95% confidence limits of the differences between the two methods. (A) %TIR 3.9–10 mmol/L; 70–180 mg/dL. (B) %TBR <3.9 mmol/L; <70 mg/dL. (C) %TBR <3.0 mmol/L; <54 mg/dL. (D) %TAR >10.0 mmol/L; >180 mg/dL.
Day and night comparisons for rtCGM and SMBG
Disparity between reported times in glycemic ranges with rtCGM and SMBG between day and night cycles are summarized in Table 3. With SMBG, %TBR and %TAR were higher during the day than with rtCGM, except for %TBR <3.0 mmol/L (<54 mg/dL) which was not different, %TIR measured by rtCGM was higher than with SMBG. The same pattern of greater %TIR with rtCGM and higher times out of range with SMBG was noted overnight (all P < 0.001).
Table 3.
Day and Night Median (IQR) Percentage Times in Glycemic Range with rtCGM and SMBG (Carry Forward Method)
| Glycemic ranges | Median %time (IQR) during the day |
P | Median %time (IQR) during the night |
P | ||
|---|---|---|---|---|---|---|
| rtCGM | SMBG | rtCGM | SMBG | |||
| TIR | ||||||
| 3.9–10 mmol/L (70–180 mg/dL) | 65.1 (55.8–72.5) | 56.3 (46.9–64.6) | <0.001 | 60.6 (52.7–69.7) | 46.1 (36.1–58.5) | <0.001 |
| TBR | ||||||
| <3.9 mmol/L (<70 mg/dL) | 3.4 (1.6–5.1) | 3.7 (2.0–6.3) | <0.001 | 3.2 (1.8–5.2) | 4.4 (2.2–7.4) | <0.001 |
| <3.0 mmol/L (<54 mg/dL) | 0.7 (0.2–1.2) | 0.6 (0.2–1.4) | 0.30 | 0.7 (0.3–1.3) | 1.0 (0.−2.2) | <0.001 |
| TAR | ||||||
| >10 mmol/L (>180 mg/dL) | 32.0 (22.7–40.6) | 39.3 (30.9–49.9) | <0.001 | 34.9 (26.2–43.8) | 49.5 (35.8–58.6) | <0.001 |
| >15 mmol/L (>270 mg/dL) | 4.6 (2.5–7.9) | 7.7 (4.3–12.3) | <0.001 | 5.5 (2.3–9.2) | 11.4 (5.5–18.8) | <0.001 |
In the day and night analyses, 37.1% (95% confidence interval [CI]: 36.3 − 37.9) of total rtCGM readings were during the night cycle but with SMBG, only 18.4% (95% CI: 17.3 − 19.5) of total readings were overnight.
Discussion
The presented analysis demonstrates clinically and statistically significant differences in assessed times in ranges between SMBG and rtCGM data, collected simultaneously in a large cohort with T1D over 26 weeks.
In addition, varying methods of calculating %TIR from SMBG-derived data provide significantly divergent results. To summarize, SMBG-derived %TIR was significantly lower with both methods of calculation (i.e., linear interpolation and last observation carry forward) compared to rtCGM. For TBR, in comparison to rtCGM, linear interpolation calculated a decreased %TBR, while the carry forward method increased %TBR. Both methods to assess SMBG-derived data showed increased %TAR >10 mmol/L (>180 mg/dL), however, %TAR >15 mmol/L (>270 mg/dL) was reduced through linear interpolation and increased using a carry forward method.
With an established consensus to increasingly use %times in ranges to assess glycemia and complication risk in research and clinical practice,6 the modality used to calculate the %TIR is of paramount importance. The harmonization of HbA1c to ensure comparable values across international laboratories ensures wide and reproducible data can be interpreted and, while this may not at present be possible for times in ranges, the presented data suggest that targets for times in ranges may depend on the glucose data source and cannot be used interchangeably.
The different %times in glycemic ranges between rtCGM and SMBG are likely to be driven by acquisition bias and imprecision of calculation, either through the effect of a SMBG value being carried forward until the next SMBG value is encountered or through linear interpolation, which makes assumptions about the glucose trajectory between SMBG values. Acquisition bias may occur when participants have symptoms of hypo- and hyperglycemia, with a resulting SMBG value out of range. This value will then be carried forward until the next SMBG value is encountered which may be hours later, relatively increasing time in that range. The alternative approach of linear interpolation between consecutive SMBG values may address overestimation of times in ranges with the carry forward approach. However, imprecision may be particularly noticeable for times out of ranges.
Differences in accuracy between the Dexcom G4 rtCGM system and the SMBG device,12–14 which adheres to ISO 15197 (2013), may also be responsible for some of the differences observed in the %time values reported by rtCGM and SMBG. Sensor lag15 may have an impact on discrepancies between SMBG and rtCGM over a short time period but should not contribute to differences over 26 weeks. It has also been previously shown that glycemic risk (Low and High Blood glucose Indices) derived from SMBG data is valuable in predicting events, but is different to risk from rtCGM and needs adaptation.16 These issues with TIR calculated by SMBG limit its usefulness for the future, but the association with microvascular outcomes holds promise for TIR from rtCGM devices.
Differing performance may mean times in ranges with different CGM devices require the development of an HbA1c-standardized TIR, based on data from clinical studies of each new device, or it is possible that the integrated CGM (Class II 510 (K))standard ensures acceptable agreement between values. Assessment of paired data between rtCGM and isCGM devices should be considered to assess the differences between reported times in ranges.
The limitations of this study include selection of insulin pump-treated participants with T1D and HbA1c values close to target in the REPLACE-BG trial. Further studies in people using multiple daily injections and those with HbA1c values well above target may suggest a different relationship between times in ranges.
In conclusion, we have demonstrated that clinically and statistically significantly different estimations of times in range from simultaneously collected rtCGM and SMBG data, using two different data imputation methodologies. SMBG-reported %TIR was significantly lower than rtCGM, with these differences most marked at night. Interpretation of times in ranges should take account of the glucose sensing modality and differing methodologies for calculations, with future guidelines for targets requiring standardization of the times in ranges metric.
Acknowledgments
The authors thank all the researchers and participants of the REPLACE-BG study for their valuable time and commitment. This research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Imperial College London. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Author Disclosure Statement
M.R. has received honoraria for advisory board participation from Dexcom and Roche Diabetes. N.O. has received honoraria for speaking and advisory board participation from Abbott Diabetes, Dexcom, Medtronic Diabetes, and Roche Diabetes.
Funding Information
No funding received.
References
- 1. Foster NC, Beck RW, Miller KM, et al. : State of type 1 diabetes management and outcomes from the T1D exchange in 2016–2018. Diabetes Technol Ther 2019;21:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Avari P, Reddy M, Oliver N: Is it possible to constantly and accurately monitor blood sugar levels, in people with type 1 diabetes, with a discrete device (non-invasive or invasive)? Diabet Med 2019. [Epub ahead of print]; DOI: 10.1111/dme.13942 [DOI] [PubMed] [Google Scholar]
- 3. Edelman SV, Argento NB, Pettus J, Hirsch IB: Clinical implications of real-time and intermittently scanned continuous glucose monitoring. Diabetes Care 2018;41:2265–2274 [DOI] [PubMed] [Google Scholar]
- 4. Oliver N: Continuous glucose monitoring adoption in the United Kingdom—an economic and policy perspective. Eur Endocrinol 2017;13:73–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tanenbaum ML, Hanes SJ, Miller KM, et al. : Diabetes device use in adults with type 1 diabetes: barriers to uptake and potential intervention targets. Diabetes Care 2017;40:181–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Battelino T, Danne T, Bergenstal RM, et al. : Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019;42:1593–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Beck RW, Bergenstal RM, Riddlesworth TD, et al. : Validation of time in range as an outcome measure for diabetes clinical trials. Diabetes Care 2019;42:400–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fiallo-Scharer R: Eight-point glucose testing versus the continuous glucose monitoring system in evaluation of glycemic control in type 1 diabetes. J Clin Endocrinol Metab 2005;90:3387–3391 [DOI] [PubMed] [Google Scholar]
- 9. Beck RW, Calhoun P, Kollman C: Use of continuous glucose monitoring as an outcome measure in clinical trials. Diabetes Technol Ther 2012;14:877–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Michalak A, Pagacz K, Mlynarski W, et al. : Discrepancies between methods of continuous glucose monitoring in key metrics of glucose control in children with type 1 diabetes. Pediatr Diabetes 2019;20:604–612 [DOI] [PubMed] [Google Scholar]
- 11. Aleppo G, Ruedy KJ, Riddlesworth TD, et al. : REPLACE-BG: a randomized trial comparing continuous glucose monitoring with and without routine blood glucose monitoring in adults with well-controlled type 1 diabetes. Diabetes Care 2017;40:538–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bailey TS, Chang A, Christiansen M: Clinical accuracy of a continuous glucose monitoring system with an advanced algorithm. J Diabetes Sci Technol 2015;9:209–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nakamura K, Balo A: The accuracy and efficacy of the Dexcom G4 platinum continuous glucose monitoring system. J Diabetes Sci Technol 2015;9:1021–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Matuleviciene V, Joseph JI, Andelin M, et al. : A clinical trial of the accuracy and treatment experience of the Dexcom G4 sensor (Dexcom G4 system) and Enlite sensor (guardian REAL-time system) tested simultaneously in ambulatory patients with type 1 diabetes. Diabetes Technol Ther 2014;16:759–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kovatchev BP, Patek SD, Ortiz EA, Breton MD: Assessing sensor accuracy for non-adjunct use of continuous glucose monitoring. Diabetes Technol Ther 2015;17:177–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fabris C, Patek SD, Breton MD: Are risk indices derived from CGM interchangeable with SMBG-based indices? J Diabetes Sci Technol 2015;10:50–59 [DOI] [PMC free article] [PubMed] [Google Scholar]