Abstract
Significance: Over the past several years, oxidative post-translational modifications of protein cysteines have been recognized for their critical roles in physiology and pathophysiology. Cells have harnessed thiol modifications involving both oxidative and reductive steps for signaling and protein processing. One of these stages requires oxidation of cysteine to sulfenic acid, followed by two reduction reactions. First, glutathione (reduced glutathione [GSH]) forms a S-glutathionylated protein, and second, enzymatic or chemical reduction removes the modification. Under physiological conditions, these steps confer redox signaling and protect cysteines from irreversible oxidation. However, oxidative stress can overwhelm protein S-glutathionylation and irreversibly modify cysteine residues, disrupting redox signaling.
Critical Issues: Glutaredoxins mainly catalyze the removal of protein-bound GSH and help maintain protein thiols in a highly reduced state without exerting direct antioxidant properties. Conversely, glutathione S-transferase (GST), peroxiredoxins, and occasionally glutaredoxins can also catalyze protein S-glutathionylation, thus promoting a dynamic redox environment.
Recent Advances: The latest studies of glutaredoxin-1 (Glrx) transgenic or knockout mice demonstrate important distinct roles of Glrx in a variety of pathologies. Endogenous Glrx is essential to maintain normal hepatic lipid homeostasis and prevent fatty liver disease. Further, in vivo deletion of Glrx protects lungs from inflammation and bacterial pneumonia-induced damage, attenuates angiotensin II-induced cardiovascular hypertrophy, and improves ischemic limb vascularization. Meanwhile, exogenous Glrx administration can reverse pathological lung fibrosis.
Future Directions: Although S-glutathionylation modifies many proteins, these studies suggest that S-glutathionylation and Glrx regulate specific pathways in vivo, and they implicate Glrx as a potential novel therapeutic target to treat diverse disease conditions.
Keywords: fatty liver disease, NAFLD, NASH, hindlimb ischemia, peripheral artery disease
Introduction
Oxidative post-translational modifications (OPTM) of protein cysteines (Cys) have emerged as a cellular signaling mechanism to regulate physiology, and they become dysregulated in pathophysiology. Reactive oxygen species (ROS) oxidize thiols (R-SH) to reversible sulfenic acid (R-SOH), and further oxidation forms sulfinic acid (R-SO2H) and irreversible sulfonic acid (R-SO3H), which may permanently alter the function of the protein. Reactive nitrogen species also interact with thiols to form reversible S-nitrosothiols (R-SNO), commonly referred to as S-nitrosylation. R-SNO and R-SOH are intermediates with a fast turnover rate known to enable redox signaling. Other thiols may react with these reversible oxidation forms and result in either an intra- or intermolecular disulfide, or a mixed disulfide with a small thiol glutathione (reduced glutathione [GSH]), a process termed S-glutathionylation (100, 103, 146, 185). The reductive cellular environment or catalysis by glutaredoxins will remove the S-glutathionylation and restore the protein cysteine. This reversible process permits redox signaling and may protect proteins from irreversible oxidation. S-glutathionylation is reported on a large number of proteins and regulates a wide variety of cellular functions, including signal transduction, transcription, cytoskeletal assembly, cell survival, and apoptosis (127, 146).
In this article, we review redox signaling by focusing on the chemical background of thiol modification, the enzymatic regulation of S-glutathionylation, and the biological roles of glutaredoxin-1 (Glrx). Thiol modifications can also occur through radical-mediated pathways, which have been extensively discussed elsewhere. Glutathione S-transferase (GST) and glutaredoxins are key enzymes that regulate the redox signaling cycle. Glrx may exhibit some overlapping function with other reducing proteins such as thioredoxins (7, 51, 66, 78, 87, 144). However, studies of Glrx transgenic or knockout mice elucidate the unique in vivo abilities of Glrx to regulate metabolism, angiogenesis, inflammation, and fibrosis (11, 17, 62, 129, 130, 163, 180).
Chemistry of Redox Signaling
Biology and chemistry of cysteines
Cysteine is a semi-essential amino acid that is structurally similar to serine (Fig. 1A). Instead of oxygen, cysteine contains sulfur in its side chain, forming a thiol. A thiol is a functional group consisting of sulfur and a hydrogen atom and, thus, is also referred to as a “sulfhydryl.” The thiol or thiolate (deprotonated negatively charged thiol) group confers unique chemical properties to cysteines, including extreme nucleophilicity, high-affinity metal binding to form zinc fingers and iron-sulfur clusters, and the ability to create structural and regulatory disulfides (149). Another closely related amino acid is selenocysteine, which harbors selenium instead of sulfur. Both proteinogenic amino acids play an indispensable role in enzyme catalysis, redox signaling, and cellular redox status.
FIG. 1.
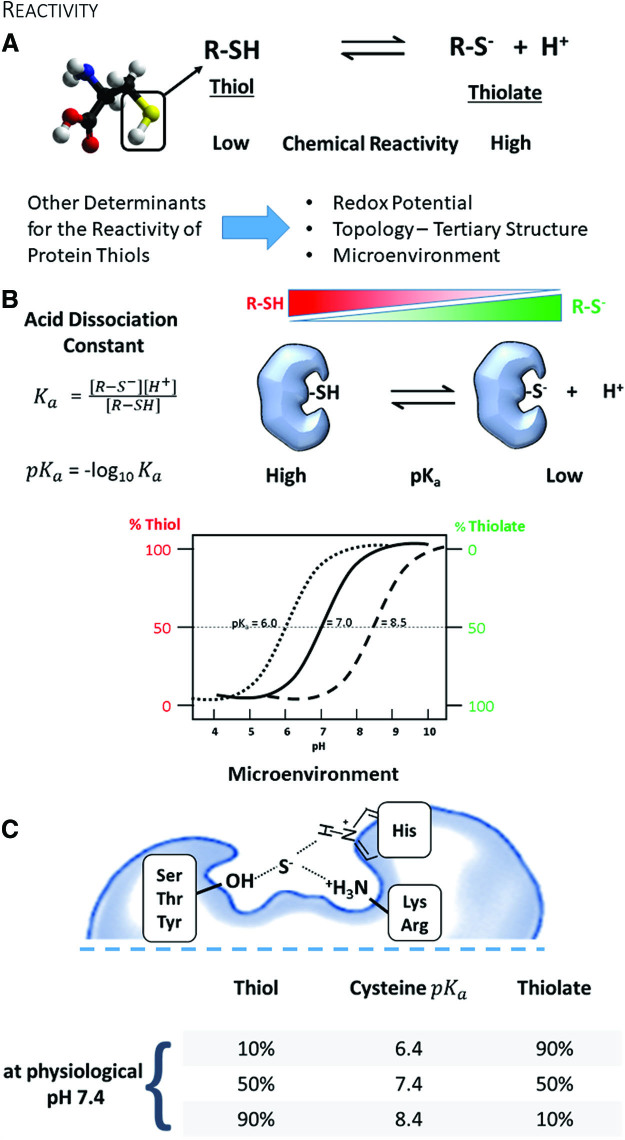
Reactivity of cysteines. (A) The protonation of a protein cysteine is a major factor that determines the chemical reactivity. A negatively charged thiolate is a much stronger nucleophile than a thiol. Also, the redox potential, the solvent exposure of the cysteine, and the surrounding amino acids influence the cysteine's reactivity. (B) The acid dissociation constant and the pka describe the chemical reactivity of a protein cysteine, with the thiolate being the more reactive form. pH influences the prevalence of a protein cysteine protonation (see qualitative titration curves). (C) Neighboring amino acids stabilize protein thiolates via hydrogen bonds and electrostatic effects and render them sensitive for oxidative post-translational modifications. Color images are available online.
Although cysteine is the least abundant amino acid in proteins, 90% of the cysteines are highly conserved within protein sequences among species (53). Surface-exposed cysteines occur less frequently than other amino acids. One explanation is that cysteines fall under a general classification of highly hydrophobic amino acids, which prevents solvent exposure (156). The basis of the hydrophobicity index of amino acids, including cysteine, is mostly derived from the analysis of three-dimensional structures. However, this classification has been recently revised, resulting in cysteine becoming grouped with polar amino acids together with serine (53). Thus, it is possible that evolution selected and preserved cysteine due to its function in redox signaling, rather than its general physicochemical properties (118). This notion is further supported by bioinformatic analysis of human genetic diseases, demonstrating that mutations of cysteine occur more frequently relative to its abundance in proteins (188). Intriguingly, cysteine appears to be a later addition to the genetic code, and it may still accumulate in the genome of present-day organisms (60).
Cysteine is an amino acid with a unique chemistry. It exists in two forms, the thiol and its deprotonated ionized form, the thiolate (Fig. 1A). Both types contain a lone pair of electrons (nonbinding) and thus are chemically nucleophiles. Although the thiol has low reactivity, conversion to a thiolate renders cysteine one of the most reactive intracellular nucleophiles that can readily undergo alkylation or redox reactions. Because the dissociation of the thiol to thiolate occurs in the context of an acid-base reaction, the negative base-10 logarithm of the acid dissociation constant—pKa—quantitatively relates to its reactivity (Fig. 1B) (149). Briefly, the pKa denotes the pH at which cysteine is equally present in the thiol and thiolate form. The pKa of the unperturbed cysteine thiol side chain is ∼8.25 (104). However, neighboring amino acids may create a milieu that perturbs the pKa of cysteine in a protein and hence changes its reactivity (31). Due to this alteration in the microenvironment, reactive cysteines can often have a lower pKa and exist predominantly in the thiolate form that is stabilized by vicinal amino acids through hydrogen bonds or positive charges (68). Thus, cysteines participating in redox processes are usually in the vicinity of either arginine (Arg, R), lysine (Lys, K), or histidine (His, H), providing an electropositive setting, or serine (Ser, S), threonine (Thr, T), or tyrosine (Tyr, Y) promoting hydrogen bonding. Often, these amino acids are organized as pairs (dyad) or triplets (triad) around the cysteine to create a particular microenvironment and enhance the thiolate stabilizing effect (Fig. 1C).
Mutating cysteines for functional redox studies
Classical consensus sequences that identify reactive cysteines are minimally helpful, and they may only apply to proteins within the same evolutionarily conserved group. Various tools and strategies (53) developed to identify reactive protein cysteines further highlight that the pKa alone is a limited predictor of reactivity, and other factors such as the solvent exposure, reduction potential, protein topology, and the microenvironment around the cysteine contribute to amino acid reactivity and redox modifications. Because of these difficulties, biological studies often involve experiments with mutant proteins to confirm the gain or loss of function regarding a particular cysteine residue.
Due to their related redox properties, the exchange of redox-active cysteine for selenocysteine, the 21st amino acid in the genetic code, occurred during evolution within groups of related enzymes (13). Fomenko et al. applied this concept to database searches and identified redox-active cysteines in several proteins selectively (53). A few studies substituted cysteine with selenocysteine, changing the enzyme's pKa and redox properties (160). Generating these mutations is technically not straightforward, and selenium supplementation can be challenging (64, 108).
On the other hand, serine and alanine are common substitutions to neutralize the thiol function of cysteine while minimizing changes in the protein's structural integrity. The role of choosing a cysteine to alanine or serine substitution should be dependent on the role that the cysteine plays in the protein's function. Due to its size, polarity, and hydrogen bonding ability, serine is the closest substitution (149). However, serine also has the potential to become a target of phosphorylation or glycosylation, which adds additional complexity to the analysis. In contrast, alanine or sometimes valine is better matched to cysteine in terms of hydrophobicity relative to serine (73), and notably, mutation to alanine enables better evaluation of any side-chain interactions.
Other strategies exchange cysteine for very polar (aspartic or glutamic acid) or aromatic amino acids (phenylalanine or tryptophan) to recapitulate the polarity of the oxidized thiol (R-SOH, R-SO2H, and RSO3H) or induced structural perturbations, respectively (179).
In summary, these approaches are not perfect, and careful biochemical and functional characterization of each mutant is required to minimize artefactual results. Although the mutants can provide insights about the biological function of cysteine, they are unsuitable for identifying the specific post-translational modification (PTM).
PTMs of cysteines
Protein cysteines, based on their nucleophilic and redox properties, can have diverse functions and undergo numerous PTMs (Fig. 2A). These functions often require ionized cysteines to act as active site catalysts such as in phosphatases, as part of zinc fingers to coordinate bivalent zinc, as components of iron-sulfur clusters for electron transport, as reactive sites for nonoxidative modifications for intracellular protein trafficking (65), or as semiconductors in the P450 enzymes to control phase I biotransformation reactions (e.g., hydrolysis, reduction, oxidation).
FIG. 2.
Common oxidative modifications and redox regulation. (A) List of common cysteine modifications involved in or that interfere with redox regulation. (B) Redox-sensitive proteins contain exposed and deprotonated reactive cysteines that exhibit a thiol peroxidase-like activity. These thiolates or “redox sensors” can react with reactive oxygen (OOx) and nitrogen (NOx) species to form labile intermediates such as sulfenic acid (S-OH) and nitrosocysteine (S-NO), respectively ①. These “activated species” quickly react with abundant intracellular glutathione (5–15 mM) ②. The resulting protein GSH adduct is a reversible, oxidative modification that can regulate enzyme activity, localization, protein interactions, and stability. For completion of the redox signaling cycle, Glrx specifically removes GSH adducts ③. Oxidative stress interrupts redox signaling by irreversible oxidation of cysteine thiolates (red arrow). Glrx, glutaredoxin-1; GSH, reduced glutathione; GSSG, oxidized glutathione. Color images are available online.
Reactive cysteines are a prerequisite for protein prenylation (thioether bond) and acylation (thioester bond) reactions catalyzed by prenyltransferases and palmitoyl acyltransferases, respectively. Both modifications anchor proteins in biological membranes and regulate trafficking between intracellular membranes such as endosomes and the plasma membrane (65). Oxidants and redox signaling, including S-glutathionylation, may compete with these modifications and interfere with membrane trafficking and activity, as demonstrated for H-Ras (33). Acylation also includes shorter modifications such as acetylation and succinylation that impact epigenetics and mitochondrial function.
Thus, oxidants and OPTMs may compete with other modifications on an affected amino acid. In the next few paragraphs, we will briefly outline these to emphasize how S-glutathionylation and glutaredoxins can impact other cysteine modifications.
Redox reactions of cysteine thiols are exceptionally dynamic processes based on complex redox chemistry. Cells can generate oxidants that preferentially react and oxidize protein thiolates (R-S−) (Fig. 2B). The most common reactions are reversible R-SOH and R-SNO, which can not only possess signaling functions but also serve as intermediates for S-glutathionylation, S-cysteinylation, and sulfhydration (R-SSH). Owing to highly abundant intracellular GSH levels, S-glutathionylation (R-SSG) is the predominant modification in the cell. Meanwhile, cysteine is the most prevalent small-molecule thiol in circulating plasma rather than glutathione, and, thus, protein S-cysteinylation is the most common thiol modification (157).
Detection of thiol modifications and S-glutathionylation
Experimentally, cysteine modifications such as S-glutathionylation can be evaluated by direct quantification with mass spectrometry (MS) (72) to more qualitative methods using antibodies, switch assays (26, 32, 72), or in situ labeling (5, 67). MS is the most direct means to identify and evaluate protein modifications and encompasses label-free analysis as well as labeling with cysteine-reactive mass tags (71). An inherent limitation to the detection of most PTMs is their low abundance. On average, the site occupancy, the percentage for which a specific amino acid residue in a protein is modified, lies within approximately 5%–20% for most modifications with a signaling function (18, 21, 131, 132, 189). Thus, MS analysis often necessitates affinity enrichment to increase sensitivity. However, in cells exposed to oxidative stress, the site occupancy of OPTMs can be much higher and occurs randomly in less reactive amino acids.
Bioinformatics presents another bottleneck for analysis, because most existing search algorithms perform poorly in identifying multiple OPTMs, including S-glutathionylation, and even fail to detect crosslinks or unknown OPTMs. Various groups have developed novel strategies to improve MS detection and analysis of OPTMs such as the “selectively excluded mass screening analysis” (SEMSA) and the search algorithms MODi (97), MODMap, MODa (133), and DBond (41). These approaches led to the discovery of additional cysteine modifications that fall into several categories: oxidation (e.g., Cys to Ser or dehydroalanine conversion, thiosulfonic acid, sulfonamide), acylation (palmitoylation, succinylation, acetylation, diacylglycerol), prenylation (e.g., geranyl-geranylation or S-farnesylation), S-ubiquitination, S-guanylation, and chemical modifications (e.g., 15d-PGJ2) (84, 137). The biological significance for some of these modifications and competition with OPTMs remains elusive.
S-guanylation is an understudied modification that is formed after peroxynitrite-mediated nitration of the guanine base in the nucleotide to 8-nitroguanosine, an electrophile that irreversibly adducts to cysteine thiols. For example, this modification inhibits KEAP1 from binding to nuclear factor (erythroid-derived 2)-like 2 (Nrf2) (55), activates the GTPase H-Ras by allowing greater translocation (135), and induces 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) opening (153). S-guanylation, however, is not enzymatically reversed and can theoretically occur at any thiolate in the presence of 8-nitroguanosine. Thus, further studies need to determine whether this modification occurs randomly or participates in directed signaling changes (95).
Given the costs of MS analysis, the development of thiol labeling using “switch techniques” has proven helpful (21, 26, 32, 194). The best-known method, the biotin switch assay, starts by blocking free cysteines with a thiol-reactive compound, unaffected by the subsequent chemical step to remove the targeted modification. In most cases, the removal step is a reduction (e.g., ascorbate for S-nitrosylation), but protocols may also use hydrolysis to cleave the thioether linkage (e.g., neutral hydroxylamine) as found in S-palmitoylation (29, 65). Recent revisions of the assay to analyze S-glutathionylation include enzymatic removal catalyzed by recombinant Glrx (170). A tagged labeling reagent then reacts with the newly liberated thiols, allowing detection with analysis techniques such as Western blot, MS, and histology. Burgoyne and Boivin (26, 32) have described the switch assays that are the most readily usable for single-protein evaluation. Switch assays are particularly useful if the modification is chemically labile, such as S-nitrosylation or S-sulfenylation, and unlikely to survive sample preparation for MS analysis. To perform proteome-wide analysis of thiol modifications, labeling with stable isotope mass tags combined with MS is ideal (21, 132, 194). However, this technique requires advanced bioinformatics, demanding sample preparation, and instrumentation, and it is often available only in specialized laboratories. A limitation of switch assays is the indirect detection of the thiol modification, and if performed incorrectly, switch assays may misrepresent cysteine modifications. Common pitfalls include insufficient degassing of buffers to limit artefactual thiol oxidation, incomplete removal of incompatible chemicals during the switch procedure, lack of specificity of the reagent to remove targeted modification (95), over-alkylation, and nonspecific labeling. Nevertheless, correctly executed and well-controlled switch assays produce meaningful results.
Other described methods to detect S-glutathionylation include specific antibodies (74, 165). Because GSH is a small peptide and universal biomolecule present in plasma, raising antibodies requires peptide- or protein-GSH conjugates (hapten). Various groups linked GSH with glutaraldehyde to bovine serum albumin and demonstrated the specific recognition of cytoplasmic and mitochondrial glutathione (74, 165). Söderdahl et al. also showed its usefulness for detecting protein S-glutathionylation in cultured cells (165). One report produced a specific GSH antibody against Cys141 of p53 (197). In collaboration with Millipore, our lab attempted to generate a site-specific antibody against Cys118 of H-Ras by using the same strategy. However, the affinity-purified antibody still recognized the Cys to Ser H-Ras mutant. Because S-glutathionylation is reversible, the modification may be reduced after injection of the hapten into the rabbit. Thus, it is not surprising that site-specific antibodies against S-glutathionylation are a rarity. We believe that the generation of site-specific antibodies is possible, but likely an antigen conjugate with a nonreducible GSH-like modification or screening against phage-display libraries (27) is necessary. In our hands, the best commercial anti-protein GSH antibody is from Virogen. However, we know from MS studies that not all S-glutathionylated proteins are recognized with equal sensitivity. Also, nonspecific binding to mouse proteins should be tested by the secondary antibody alone when a mouse monoclonal antibody is used for Western blot.
Metabolic labeling of cysteine modifications using radioactive or stable isotopes is another sensitive method for analyzing OPTMs, particularly S-glutathionylation. Biotin (171) or newer CLICK-chemistry labels (91) can be attached to the amino group of cell-permeable glutathione monoethyl ester, a cell-permeable version of glutathione, allowing direct and specific detection of S-glutathionylated proteins. The high costs and high concentrations required to load cells, however, currently make these compounds impractical for large-scale or in vivo experiments.
In summary, the available tools to detect a thiol modification or S-glutathionylation specifically are limited and slowly evolving. Better and more specific molecular tools with broader applicability are necessary to advance the field.
Glutathione
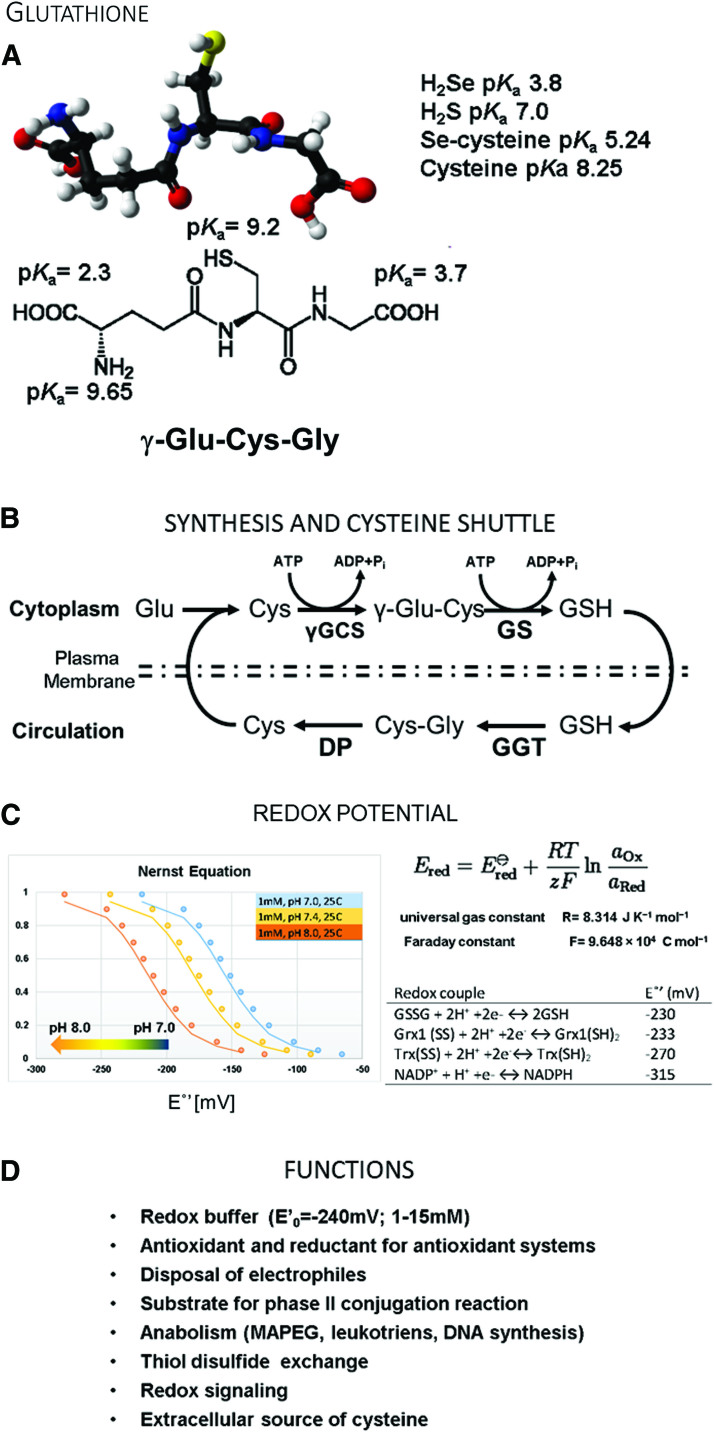
In 1888, De Rey-Pailhade originally described “philothion,” which Hopkins rediscovered in 1921 and renamed as glutathione (GSH) (8). GSH is a small tripeptide comprising glutamine, cysteine, and glycine (γ-l-glutamyl-l-cysteinylglycine) (Fig. 3A). Every cell synthesizes GSH in a two-step process catalyzed by glutamate-cysteine ligase (previously known as γ-glutamylcysteine synthase) and GSH synthetase in the cytoplasm (Fig. 3B). The unusual peptide bond between the γ-glutamyl side chain and cysteine protects GSH from breakdown by cellular and circulating serum peptidases (111). The estimated pKa of the thiol in the cellular environment is between 8.5 and 8.7 (68, 148), which confers low reactivity and poor antioxidant properties to GSH. However, due to the high intracellular concentrations (1–15 mM) that have been described in various cell types, GSH can exhibit antioxidant properties to some extent. GSTs can lower the pKa of the GSH thiol below 6 to activate the strong nucleophile for phase II bioconjugation and detoxification reactions of xenobiotics. Specialized transport systems of the phase III detoxification, including members of the multidrug resistance-associated proteins (MRPs), export these conjugates from the cell (82).
FIG. 3.
Glutathione. (A) Structure of glutathione (GSH) and pKa values for chemical systems. The pKa of the GSH thiol is lower in the intracellular environment. (B) Glutathione synthesis and distribution through the blood circulation as a source of cysteine. (C) Calculation of the redox potential for glutathione-containing buffers using the Nernst equation. The y-axis depicts the ratio of GSH:GSSG in the redox buffer system. The effects of pH on the redox potential are plotted for pH 7.0, 7.4, and 8.0 and demonstrate that increasing the pH lowers the redox potential of the GSH:GSSG buffer. Glutathione redox buffers are useful tools to investigate redox-sensitive proteins. Redox potentials for thioredoxin, glutaredoxin, glutathione, and NADPH under standard conditions are listed. (D) Biological functions of glutathione. γGCS, γ-glutamylcysteine ligase; DP, dipeptidases; GGT, γ-glutamyl transferase; GS, GSH synthase; NADPH, nicotinamide adenine dinucleotide phosphate. Color images are available online.
Antioxidant systems, including peroxiredoxins, glutaredoxins, and glutathione peroxidases, have active site thiolates or selenolates. For all these proteins, the enzyme reacts with oxidants or an oxidized target protein and becomes oxidized itself by direct or trans-S-glutathionylation. GSH serves as a reductant for the enzyme and forms GSSG (oxidized glutathione), restoring the protein's function. Glutathione reductase then recycles the oxidized GSSG back to GSH with the consumption of NADPH (nicotinamide adenine dinucleotide phosphate) and restores the cellular redox equilibrium. In situations when excess GSSG is formed, cells can also release GSSG through MRPs to restore the redox potential quickly (79).
Thus, the GSH and GSSG equilibrium constitutes the most critical cellular redox buffer, and the ratio determines the redox potential (159). The cell uses the GSH:GSSG ratio to create cellular compartments with defined redox conditions. For example, the cytoplasm has a redox potential between −220 and −260 mV; and a GSH:GSSG ratio of 30 to 100:1, which is actively reducing. Under these conditions, it is difficult to form intra- and intermolecular protein disulfides. The endoplasmic reticulum (ER), which contains the enzymatic machinery to catalyze protein disulfide formation, has a more oxidizing environment of −180 mV, established by a lower GSH:GSSG ratio. The Nernst equation allows researchers to calculate the redox potential and recreate redox buffers with defined redox potential to mimic physiological conditions (159) (Fig. 3C).
In summary, a lower pKa would render the GSH thiol too reactive and promote nonspecific reactions in the cell. The higher pKa of GSH allows it to act as the cellular redox buffer. This function, coupled with the actions of GSTs, provides a means to harness and control the chemical reactivity of the thiolate nucleophile.
The liver plays a unique role in GSH metabolism since it removes the major amount of resorbed cysteine from the portal circulation (57) and converts it into less reactive GSH, which is released back into the blood flow (168). Thus, GSH may also serve as a cysteine source for other tissues (111). The normal GSH turnover is about 40 mmol per day in a human adult and is estimated to be slightly higher than the protein cysteine turnover (168). All functions of glutathione are summarized in Figure 3D.
Cellular oxidant sources
Various isoforms of NADPH oxidases (Nox) generate oxidants at physiological levels. These enzymes predominantly release superoxide (•O2−), which is quickly converted into hydrogen peroxide (H2O2). Nox 4 is the only isoform that has been reported to release H2O2 directly (28). NADPH oxidases contribute to growth factor signaling, cell differentiation, and cell migration but have also been linked to pathology, playing roles in vascular disease and inflammation. The inflammatory system, particularly immune cells such as neutrophils, contains high amounts of Nox 2, which participates in phagocytosis and produces an oxidative burst to neutralize pathogens. Rather than a defense mechanism, other organs and tissues have utilized this system for cellular signaling. For example, vascular smooth muscle cells express large amounts of Nox in hypertrophy, contributing to oxidative stress, endothelial dysfunction, and vascular remodeling (107, 181). Most tissues, however, express Nox at much lower levels, which allow for participation in cellular redox control. The pentose phosphate pathway, the primary metabolic pathway and the source of NADPH, regulates Nox-induced oxidant generation. Lower NADPH production in glucose-6-phosphate dehydrogenase-deficient mice causes decreased oxidant formation in the vasculature (121, 122). This effect is explained by the Km of NADPH, which is markedly higher for NADPH oxidases (172) than for the reductive system (186). Thus, even at low NADPH concentrations, the antioxidant system is fully active, whereas the oxidant sources are attenuated (121, 122).
Another group of oxidant sources consists of uncoupled nitric oxide synthases (NOS). NOS usually produces nitric oxide, which regulates vascular tone, cell proliferation, platelet aggregation, immune response, and neurophysiology. When l-arginine or the cofactor tetrahydrobiopterin (BH4) becomes limiting, NOS can start producing superoxide and the highly reactive peroxynitrite (89, 190). Other uncoupling mechanisms such as zinc finger oxidation and monomerization (34, 192), protein interactions such as with HSP90 (139), and threonine phosphorylation (112) have been reported but are controversial.
Various P450 enzymes (including epoxygenases) and flavin-containing proteins (including xanthine oxidase) are additional oxidant sources that are capable of generating both superoxide and H2O2. Under physiological conditions, xanthine dehydrogenase is the principal form of the enzyme. Cysteine oxidation or proteolytic cleavage converts xanthine dehydrogenase into an oxidase that may play a role in pathobiology, as with ischemia–reperfusion injury (16, 124). Because its conversion is dependent on oxidants, xanthine oxidase may also amplify the generation of oxidants initiated via other oxidases.
Yet another source of cellular oxidants is mitochondria. Various mitochondrial flavoproteins such as the 2-oxoadipate dehydrogenase, pyruvate dehydrogenase complex, and 2-oxoglutarate dehydrogenase complex (134) can produce oxidants, but the components of the electron transport chain, especially complex I (NADH ubiquinone oxidoreductase) and complex III (coenzyme Q cytochrome c oxidoreductase), are prominent sources of superoxide. However, more recent findings have also identified complex II as a significant producer of superoxide (61). Predominant fatty acid oxidation can generate conditions of a highly reduced ubiquinone pool combined with low succinate levels (perturbed anaplerosis), leading to an electron leak at complex II (61) and oxidant generation. These conditions often occur in metabolic disease and obesity when muscle cells, such as cardiomyocytes, predominantly metabolize fatty acids through β-oxidation (143).
The ER has a low GSH:GSSG ratio to enable proper protein folding and introduction of structural protein disulfide bridges. The ER oxidoreductin 1 (ERO1) system generates H2O2, oxidizing highly conserved thiolates of ERO1 to disulfides. Oxidized ERO1 then transfers the disulfide through a thiol-disulfide exchange reaction via protein-disulfide isomerases (PDI) to the nascent target protein. Misfolded proteins and GSSG can be redirected and reoxidize PDI as well. In the final step, correctly folded proteins are transported by vesicles to the Golgi apparatus for secretion or insertion into the plasma membrane.
A second independent mechanism from the ERO1-PDI system controlling ER protein folding involves peroxiredoxin 4 (PRDX4) and an unknown H2O2 source (114). In combination with the glutathione redox buffer, the ER-resident PRDX4 harnesses oxidation and scavenges excess H2O2 to prevent irreversible cysteine oxidation. Interestingly, neither ER-produced H2O2 nor cytosolic- or mitochondrial-produced H2O2 diffuses across the ER membrane, suggesting a very tight regulation of ER-specific oxidants. Thus, oxidants produced in the ER may be limited to ER function and stress, whereas redox signaling in the cytosol or other organelles such as mitochondria proceeds unaffected (16, 114). Whether the overproduction of H2O2 by perturbations of the ERO-1 system can lead to ER stress is unclear.
Lysosomes are other cellular organelles affected by ROS and can also produce oxidants. Lysosomes and related vesicles, including phagosomes and autophagosomes, have a membrane-associated NADPH oxidase that becomes activated during the degradation process. Lysosomal degradation of proteins such as ferritin releases iron that initiates Fenton chemistry, generating highly reactive hydroxyl radicals, which, subsequently, results in ferroptosis (102). This mechanism of inducing oxidative stress is being leveraged for cancer chemotherapy with the use of FDA-approved iron nanoparticles (98, 199). Specialized phagosomes also use myeloperoxidase for generating hypochlorite, a highly reactive and bactericidal species.
Lysosomes also seem to be directly activated by external or mitochondrial ROS via a redox sensor transient receptor potential cation channel 1 (TRPML1). Activation of the channel causes lysosomal calcium release that induces autophagy to remove damaged mitochondria and, thus, limits excess ROS formation by dysfunctional mitochondria (200).
Oxidative stress versus redox signaling
Oxidants at elevated levels can nonspecifically attack biological molecules, including purine bases of DNA, unsaturated lipids, or reactive side chains of proteins. Lipid peroxidation can start a chain reaction, also referred to as autoxidation, which results in various reactive end-products, including malondialdehyde and hydroxynonenal. These aldehydes preferentially modify cysteines but can also affect other amino acids. As a consequence, oxidative stress produces a broad spectrum of oxidized biological molecules and post-translational protein modifications. These oxidation reactions can damage biological macromolecules, interfere in cellular physiology, and are often observed in advanced diseases, such as heart failure, atherosclerosis, and nonalcoholic steatohepatitis (NASH), leading to cell death and tissue fibrosis.
In contrast, redox signaling is targeted and enabled by particular localized amino acids, in many cases reactive thiolates of proteins. These solvent-exposed thiolates can exhibit a thiol peroxidase-like activity to catalyze their modification (Figs. 1C and 2B).
Similar to protein phosphorylation, redox signaling requires two components: a catalysis agent (ATP vs. an oxidant source) and a reactive amino acid residue (serine, threonine, or tyrosine vs. a cysteine) that directs the specificity of the reaction. Enzymes catalyze the removal of the PTM but, unlike phosphorylation, deglutathionylation may also occur due to the reductive cellular environment.
Reversible Protein S-glutathionylation
Glutathione S-transferase
Oxidants initiate redox signaling through the reversible PTM of reactive amino acids, such as cysteine, which, in particular, leads to S-glutathionylation. In the presence of abundant cellular GSH, reactive cysteines become S-glutathionylated. This process can occur spontaneously, but it may also involve other enzymes that activate and transfer glutathione to the target protein. GSTs catalyze the conjugation of GSH to electrophilic substances in phase II detoxification reactions but may participate in S-glutathionylation as well. GSTs are dimeric and can form heteromers to adjust to different substrates and conditions. Among several classes of cytosolic GSTs, GSTπ is shown to promote S-glutathionylation both in vitro (116) and in vivo (177). GSTπ is an abundant protein in the lung and liver but is also present in tissues, not known for detoxification reactions, such as the placenta, brain, and heart. Certain tumor cells overexpress GSTπ, which may stimulate cell proliferation or confer chemoresistance (105). The expression of GSTπ markedly decreases during liver development, which might be a mechanism to minimize protein S-glutathionylation and prevent hepatic lipid accumulation (see Nonalcoholic Fatty Liver Disease section) (105).
Experiments in a clinically relevant disease model of lung fibrosis recently demonstrated the importance of GSTs for protein S-glutathionylation. GSTπ ablation in mice attenuates protein S-glutathionylation and lung fibrosis due to the mitigation of Fas ligand (CD95L) mediated cell death of lung epithelial cells (125). S-glutathionylation of the Fas receptor amplifies epithelial cell apoptosis (9, 12), and thus, using therapeutic strategies to diminish protein glutathionylation including airway delivery of Glrx (10, 11) or the GSTπ inhibitor TLK117 (125) attenuates lung fibrosis.
Interestingly, GSTπ itself is a redox-regulated enzyme. Glutathionylation of Cys47 and Cys101 inhibits GSTπ activity and binding to c-Jun N-terminal kinase (JNK) (177). Direct protein–protein interaction of GSTπ with JNK inhibits the kinase activity, attenuating JNK-induced stress responses and apoptosis. Therefore, oxidative stress may inactivate GSTπ, which, in turn, activates JNK.
In addition to GSTπ, the glyoxalase system may play a role in the enzymatic formation of glutathione adducts. The glyoxalase system removes dicarbonyl species, such as the reactive glucose-derived metabolite methylglyoxal, with high specificity by glyoxalase 1 and 2 in two concerted reactions. Glyoxalase 1 converts the spontaneously formed hemithioacetal (the product of methylglyoxal and GSH) to S-D-lactoylglutathione that is then subsequently metabolized by glyoxalase 2 to d-lactate and GSH (174). Methylglyoxal can act as a powerful glycating agent that is ∼20,000 times greater than glucose (175). Also, methylglyoxal accumulation may lead to relatively more protein impairment compared with glucose as it targets arginine residues, which are more prevalent in active sites than the lysines typically affected by glucose (152).
The glyoxalase system has been implicated in vascular disease. Deletion of glyoxalase 1 can lead to diabetic nephropathy-like pathology, whereas the overexpression of glyoxalase 1 can prevent diabetic renal disease and microcirculatory perturbations in mice (58). It appears that glyoxalase 1 knockdown in endothelial cells (ECs) results in changes that are consistent with vascular damage and dysfunction (169). In obese and overweight patients, activation of glyoxalase by trans-resveratrol (tRES) and hesperetin (HESP) improved vascular function, decreased circulating methylglyoxal levels, and induced weight loss (193). Although glyoxalase 1 seems to be only modified to a mixed disulfide with lower enzymatic activity (25), glyoxalase 2 has been shown to induce S-glutathionylation of proteins in vitro (43). Whether these observations about glutathione and the glyoxalase system hold in vivo needs further investigation.
Glutaredoxins
Dithiol glutaredoxins—GSH-dependent oxidoreductases
In an Escherichia coli mutant lacking thioredoxin, A. Holmgren initially discovered a small GSH-dependent hydrogen donor for ribonucleotide reductase, which he named glutaredoxin (78). Ribonucleotide reductase catalyzes an essential step to produce deoxyribonucleotides, the DNA precursors for DNA synthesis. Thioredoxin was an established co-factor and hydrogen donor of ribonucleotide reductase, but the functions of glutaredoxin were beyond a simple substitute for thioredoxin (51).
In parallel, Mannervik and Axelsson biochemically isolated a thioltransferase in the liver that removed or formed glutathione protein adducts depending on the GSH:GSSG ratio (14, 15, 49). Other groups confirmed this activity and improved the purification methods (56, 69). After producing specific antibodies against purified pig liver thioltransferase, Gan and Wells discovered that the calf thymus (69) and E. coli glutaredoxin (101) were identical with the liver thioltransferase. Additional biochemical characterization identified several extra activities of Glrx, including thiol-redox properties, cystine conversion, dehydroascorbate reductase activity, and transhydrogenase activity. The thioltransferase activity, however, appears to be the most critical function for physiology in vivo (51, 150).
De-glutathionylation can occur in cells spontaneously because of the reductive environment and high concentrations of GSH. Glutaredoxin, however, has a specific binding groove for GSH (191) and is the prime catalyst (Fig. 4A). Experiments in liver homogenates demonstrated very rapid GSH transfer onto or removal from proteins. More importantly, Glrx caused a marked shift in the equilibrium of glutathionylated to de-glutathionylated proteins (117). Thus, under already reducing conditions in the cell, Glrx can further decrease the levels of glutathionylated proteins.
FIG. 4.
Glrx catalysis. (A) Dithiol and monothiol catalytic mechanisms of Glrx. Glrx was modeled by using the deposited PDB NMR structure 1GRX of Escherichia coli with bound glutathione (arrow) (191) and the Java web plugin Protein workshop (128). The ball-and-stick model depicts glutathione bound to Glrx via its specific GSH-binding groove. (B) The evolutionarily highly conserved function of Glrx3 (cytoplasm) and Glrx5 (mitochondria) in the maintenance and regulation of intracellular iron homeostasis. Glrx3 forms an iron-GSH complex as an intermediate to load specific cytoplasmic proteins with iron-sulfur clusters [2Fe-2S] aided by the highly conserved BolA (DNA-binding transcriptional regulator). The interaction results in various protein-iron-sulfur complexes that may or may not involve BolA or Glrx3. These complexes serve as iron storage depots or mediate cellular stress responses. Color images are available online.
Because the catalysis occurs in both directions, Glrx can also amplify protein S-glutathionylation under oxidative stress. Also, Glrx may have a preference for targets in a specific setting. This complexity causes both favorable and unfavorable outcomes in in vivo genetic mouse models. For example, Glrx deletion shows favorable (130, 180) consequences for revascularization, and unfavorable repercussions for hepatic lipid metabolism (163), which we will discuss in more detail later.
Glutaredoxin contains the conserved Cys-X-X-Cys active site motif to reduce protein-bound GSH (R–SSG) using GSH, glutathione reductase, and NADPH. Unlike thioredoxin, Glrx does not catalyze the reduction of sulfenic acid or intra- and intermolecular disulfides (24, 42). Catalysis of deglutathionylation can occur by two distinct catalytic mechanisms: the dithiol or monothiol pathways. Monothiol catalysis depends solely on the active site thiolate and is a simple thiol exchange reaction (Fig. 4A, left). Glrx binds to the glutathionylated protein, and its active site thiol reacts with the sulfur of the GSH, which forms a new bond between GSH and Glrx. In the second step, Glrx reacts with free GSH to restore its thiolate and releases GSSG. The initial reaction of the dithiol pathway is identical to the monothiol mechanism, but the next step involves the second cysteine of Glrx, which releases GSH in the process of forming an intramolecular disulfide (Fig. 4A, right). Two molecules of GSH then reduce the oxidized Glrx and form GSSG. The biological significance of the dithiolic pathway is still unclear but may explain some of the overlapping functions with thioredoxin. Other monothiol members of the Glrx family are also involved in cellular iron homeostasis, but their participation in redox chemistry is controversial. Future in vivo studies with Glrx mutants may elucidate the biological role of the second cysteine at the active site.
Mammalian cells encode two principal dithiol isoforms of Glrx. Glrx1 mainly exists in the cytoplasm, and Glrx2 localizes to mitochondria or the nucleus depending on gene splicing. Human Glrx1 contains Cys-Pro-Tyr-Cys, and Glrx2 contains Cys-Ser-Tyr-Cys as the active site motifs. Human Glrx2 is only ∼34% homologous to Glrx1, and the specific activity is <10% of Glrx1 (59, 114). Glrx2 forms an enzymatic inactive iron-sulfur cluster with reduced GSH (two protein monomers and two glutathione molecules). During oxidative stress, the iron-sulfur cluster likely serves as a highly sensitive oxidant sensor, as disruption of the cluster activates Glrx2 (22). Glrx2 KO mice develop cardiac hypertrophy and fibrosis (88, 115). Maintenance of mitochondrial iron-sulfur clusters and protein glutathionylation are critical for the functioning of mitochondrial oxidative phosphorylation and energy fluxes in the heart.
Glrx1 is redox sensitive and is reversibly inactivated by S-glutathionylation or S-nitrosylation at its active site cysteine thiol (11, 59). Importantly, Glrx1 also catalyzes the formation of S-glutathionylation in the presence of thiyl radicals (GS•). Due to the low pKa of the active site Cys, Glrx1 may form a radical intermediate that reacts with GSH to generate Glrx-SSG, which mediates protein-SSG formation (127). Anaerobic conditions increase GAPDH-SSG formation by Glrx1 in vitro (167).
Monothiol glutaredoxins
Monothiol glutaredoxins with their active-site motif Cys-Gly-Phe-Ser are evolutionarily highly conserved from bacteria to mammals. Of the initially discovered three monothiol glutaredoxin isoforms in yeast (ScGrx3, 4, and 5), only two—Glrx3 and Glrx5—exist in mammals. Across organisms, monothiol glutaredoxins are essential for intracellular iron homeostasis and actively catalyze the assembly of iron-sulfur [2Fe-2S] clusters (Fig. 4B). Glrx3 and Glrx5 also regulate iron trafficking and gene expression of proteins involved in intracellular iron homeostasis. Other functions include the protection of proteins from oxidation, signaling, cell growth, apoptosis, and proliferation that will be explained later in more detail.
Glrx3
Several groups originally identified Glrx3 with screening assays or bioinformatics and referred to it as a human sequence similar to yeast (HUSSY-22) (166), thioredoxin-like 2 (Trxl2), or protein kinase C-interacting cousin of thioredoxin (PICOT) (184). Glrx3 can form a stable iron–glutathione complex with BolA-like proteins, whose function and structure are highly conserved among species. BolA proteins were originally identified in E. coli as stress-responsive transcriptional regulators involved in the regulation of iron homeostasis, bacterial shape, and biofilm formation. In mammalian cells, the Glrx3 and BolA-like 2 protein (BolA2) complex is essential for the assembly of iron-sulfur ([2Fe-2S]) clusters of a subset of cytoplasmic iron-containing proteins (54). Oxidative stress alters these complexes, and Glrx3 translocates to the nucleus (145), regulating iron-regulatory proteins (IRP1 and 2), cell cycle progression (40), and stress-induced DNA-damage responses (141).
Because of its conserved role in cellular iron homeostasis, the ablation of Glrx3 impairs hemoglobin maturation in zebrafish erythroid cells and upregulates molecular markers of cellular iron starvation in HeLa cells (70). Murine Glrx3 knockout (KO) is embryonic lethal after 12.5 days of gestation (40), likely due to effects on cell cycle progression and fetal hematopoiesis.
In contrast, Glrx3 overexpression protects the mouse heart from pressure overload-induced cardiac hypertrophy (83), ischemia–reperfusion injury, and angiotensin II (Ang II)-induced oxidative stress (96). Further, Glrx3 interacts with the cardiac muscle LIM protein and negatively regulates calcineurin-NFAT signaling (83), preventing cardiac hypertrophy. Mutations in the LIM protein cause cardiomyopathies in humans. The LIM protein, also known as cysteine and glycine-rich protein 3 (CSRP3), contains thiol metal-binding structures for zinc, termed LIM domains, that exist in more than 60 known proteins. Likely, Glrx3 interacts and possibly also maintains these domains that are important for protein–protein interaction and signaling in the myocardium.
Glrx3 affects various intracellular signaling cascades, including PKC isoforms, JNK, AP-1, NFAT, and nuclear factor-κB (NF-κB) (90, 136), influencing immune function, neuronal cell differentiation (30), and tumorigenesis (109, 113). However, the mechanisms involved remain to be elucidated.
Glrx5
Similar to the function of Glrx3, Glrx5 is essential for the assembly of iron-sulfur clusters in the mitochondria, but it also affects cytoplasmic iron regulation through IRP1 and 2. Deficiency of Glrx5 results in iron overload and sideroblastic anemia in zebrafish and humans (35). Glrx5 deletion is embryonic lethal in mice (183). Glrx5 is indispensable for normal regulation of hemoglobin synthesis by the iron-sulfur protein aconitase 1 (ACO1) (195), aminolevulinic acid synthase ALAS2, and ferrochelatase (46). Lipoic acid biosynthesis also depends on the iron-sulfur cluster containing enzymes, and defects in Glrx5 lead to lipoic acid deficiency (123). Mutations of Glrx5 may also cause a variant of nonketotic hyperglycinemia in humans and cause childhood-onset spastic paraplegia, spinal lesion, and optic atrophy (19). However, other direct effects on intracellular signaling are understudied.
Molecular studies of Glrx3 and Glrx5 reveal that hemoglobin biosynthesis is regulated through [2Fe-2S] cluster assembly, particularly in red blood cells.
The In Vivo Role of Glutaredoxin
Y-S. Ho at Wayne State University created genetically deleted Glrx mice to examine the role of Glrx in pathophysiology (75). Unlike the in utero lethality of thioredoxin deletion (119), mice lacking Glrx appear grossly normal. Although some reports consider Glrx as an antioxidant enzyme, global Glrx deletion in mice does not cause any apparent susceptibility to oxidative stress (75). Table 1 lists published studies using Glrx KO or transgenic mice.
Table 1.
Phenotypes of Mice with Genetically Modified Glrx Expression
| Mouse | Experimental model | The outcome of KO or TG | Major target | References | |
|---|---|---|---|---|---|
| Heart | KO | Ischemia/reperfusion in heart | No difference in infarct size | (75) | |
| KO | Angiotensin-II infusion | Attenuated cardiac and aortic hypertrophy, less oxidative stress | β-actin | (17) | |
| TG | Ischemia/reperfusion in the heart | Inhibited AR activation | AR | (182) | |
| TG | Myocardial infarction (coronary artery ligation) | Improved cardiac (LV) function, inhibited apoptotic pathway | NF-κB | (3) | |
| Glrx2 TG | Doxorubicin-induced cardiac injury | Attenuated cardiac injury | (48) | ||
| Glrx2 KO | Standard chow | Cardiac hypertrophy, fibrosis | Complex I | (115) | |
| Vascular | TG | Hindlimb ischemia (femoral artery ligation) | Impaired blood flow recovery | NF-κB | (130) |
| KO | Hindlimb ischemia (femoral artery ligation) | Improved blood flow recovery | HIF-1α | (180) | |
| TG | WD, ApoE−/− crossed | Protected endothelial barrier function | Rac1 | (67) | |
| Glrx2 TG: CD68a | HFD, LDL−/− crossed (atherogenic model) | Impaired mitochondrial function, no effects on lesions | (198) | ||
| Lung | KO | Hyperoxia-induced lung injury | No difference in lung inflammation (neutrophils) | (75) | |
| KO | LPS-induced lung inflammation | Lower levels of cytokines in the lung and lower activation of alveolar macrophages | NF-κB | (4) | |
| KO | House dust-induced allergic airway | Lower Th2 cytokines and tissue stiffness in lung | (76) | ||
| KO | Ovalbumin-induced allergic airway | Enhanced resolution of airway hypersensitivity, decreased mucus metaplasia | (77) | ||
| TG: lung epithelialb | Pseudomonas aeruginosa lung infection | Increased mortality after P. aeruginosa infection | Fas | (10) | |
| KO | P. aeruginosa lung infection | Protective from P. aeruginosa-induced pneumonia | Fas | (10) | |
| TG: lung epithelialb | Lung fibrosis induced by Bleomycin or TGFβ | Attenuated lung fibrosis | Fas | (11) | |
| KO | Lung fibrosis induced by Bleomycin or TGFβ | Worsened lung fibrosis | Fas | (11) | |
| Liver | KO | Aging (middle-age) | Fatty liver, hyperlipidemia | SirT1 | (163) |
| KO | HFHS diet | Steatohepatitis (NASH type) | (163) | ||
| Brain | TG | HFHS diet | Increased cytokines in the brain, neuronal degeneration | NF-κB | (62) |
| KO | HFHS diet | Decreased neuroinflammation | (62) |
Macrophage-specific Glrx2 TG.
Lung epithelial-specific Glrx TG.
AR, aldose reductase; Glrx, glutaredoxin-1; HFD, high-fat diet; HFHS, high-fat high sucrose; HIF, hypoxia-inducible factor; KO, knockout; LDL, low-density lipoprotein; LPS, lipopolysaccharide; LV, left ventricle; NASH, nonalcoholic steatohepatitis; NF-κB, nuclear factor-κB; Rac1, Ras-related C3 botulinum toxin substrate 1; SirT1, sirtuin-1; TG, transgenic; WD, western diet.
Cardiovascular system
Antioxidant therapy and direct inhibition of NADPH oxidase enzymes attenuate systolic cardiac dysfunction and hypertrophy caused by aortic banding and Ang II (194). In contrast, Glrx ablated mice infused with Ang II develop less cardiac and vascular hypertrophy than wild-type mice. As shown by nitrotyrosine staining in the aorta, the vasculature of Ang II-infused Glrx KO mice exhibits less oxidative stress likely due to impaired activation of NADPH oxidase (21). However, Glrx overexpression protects cardiac function after chronic ischemia in association with upregulated anti-apoptotic pathways and improved neovascularization (3). Thus, the functions of Glrx are much more far-reaching and beyond a simple cellular antioxidant.
Angiogenesis and ischemic revascularization
Peripheral artery disease (PAD) presents with clinical symptoms caused by arterial occlusion in the lower extremities. In severe cases, lasting arterial insufficiency results in critical limb ischemia associated with tissue necrosis, requiring amputation (187). PAD is associated with atherosclerosis and is potentiated by other risk factors such as age, smoking, and diabetes. The pathogenesis involving impaired angiogenesis and reduced microcirculation in the skeletal muscle accelerates to intractable limb ischemia in PAD patients (81). Various therapeutic approaches to improve vascularization with angiogenic factors, including vascular endothelial growth factor (VEGF), have shown promising effects in animal models (164) but failed in clinical trials (154). Surprisingly, patients with critical limb ischemia have elevated plasma VEGF-A levels (52), suggesting that effector molecules downstream of VEGF may impair angiogenesis in severe PAD.
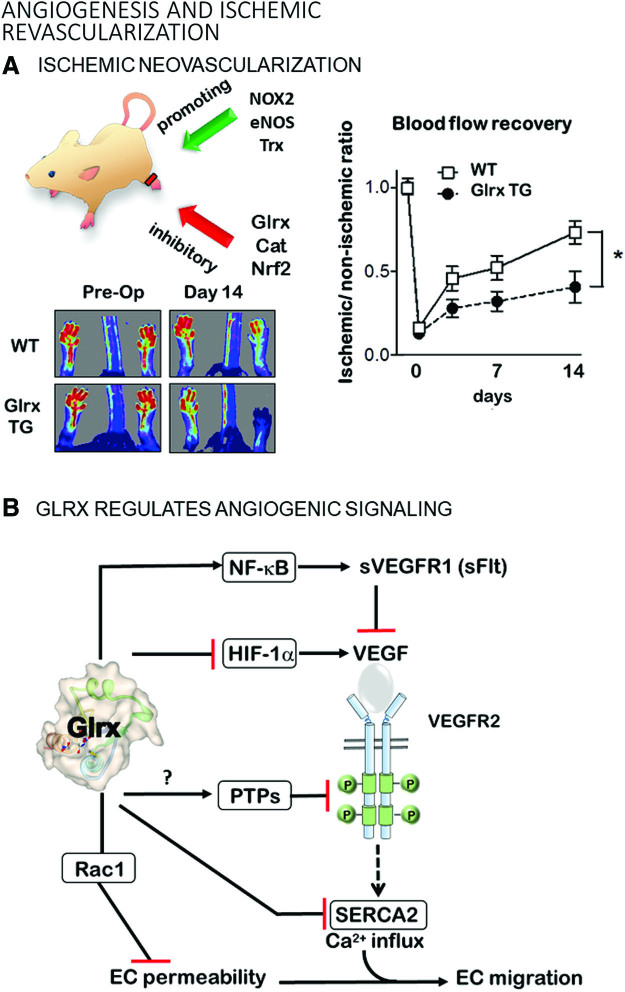
Femoral artery ligation to induce hindlimb ischemia in the mouse has been used as a PAD model to identify molecular mechanisms and develop treatments to stimulate vascularization (Fig. 5A). After femoral artery ligation, arteriogenesis (collateral formation) and angiogenesis (new blood vessel formation) gradually restore blood flow to the affected muscle. Studies using genetically modified mice demonstrated an essential role of oxidants in these processes. Nox2 deletion impaired (38) and Nox 4 overexpression improved (44) ischemic limb vascularization.
FIG. 5.
Angiogenesis and ischemic revascularization. (A) Redox regulation is an essential process that is involved in the ischemic neovascularization of the hindlimb. Oxidant sources such as NOX, eNOS, and the thioredoxin system promote angiogenesis. Antioxidant systems, including Glrx, Cat, and Nrf2, suppress angiogenesis. Glrx TG mice have impaired angiogenesis in the ischemic hindlimb after surgical-induced blockage of the femoral artery. Data were obtained by Murdoch et al. (130). *p < 0.05 (B) Angiogenic signaling cascades regulated by Glrx. ?, the direct effect of Glrx was not demonstrated. Cat, catalase; eNOS, endothelial nitric oxide synthase; NOX, NADPH oxidases; Nrf2, nuclear factor (erythroid-derived 2)-like 2; TG, transgenic. Color images are available online.
Angiogenic signaling by S-glutathionylation
S-glutathionylation occurs on a large number of proteins with redox-sensitive cysteines in vitro, but due to technical limitations, the in vivo significance is unclear (127). Oxidant-induced angiogenesis or vascularization is attributed, at least in part, to S-glutathionylation and regulation by Glrx. Thiol modifications are known to functionally control several signaling pathways in the angiogenic pathway in vivo (120), which we will explain in the next few paragraphs (Figs. 5A, B, and 6).
FIG. 6.
The NF-κB-pathway. The NF-κB inflammatory signaling pathway is regulated via S-glutathionylation at various checkpoints. S-glutathionylation inhibits TRAF6 and IKK-β as well as DNA binding of p50 and p65. Thus, Glrx promotes the inflammatory response. IκBα, an inhibitor of NF-κB; IKK-β, nuclear factor-kappa-β kinase subunit beta; IRAK1/4, interleukin-1 receptor-associated kinase 4; LPS, lipopolysaccharides; MyD88, myeloid differentiation primary response 88; NF-κB, nuclear factor-κB; TLR4, toll-like receptor 4. Color images are available online.
Tyrosine phosphatases
Growth factors such as VEGF bind to receptor tyrosine kinases, initiating signaling through phosphorylation cascades. Protein tyrosine phosphatases (PTPs) dephosphorylate the kinase substrates and cease signaling. Oxidants can target the active site Cys215 of PTP1B, an abundant PTP, and inhibit its activity via GSH adducts (20). A PTP inhibitor augmented phosphorylation of VEGF-Receptor 2 (VEGFR2) and blood flow recovery in ischemic rat limbs (37). PTP-1B (106) and other phosphatases, including low molecular weight (LMW)-PTP (80), inhibit phosphorylation of VEGFR2 in ECs. VEGF-induced S-glutathionylation of LMW-PTP also activates VEGF signaling, and reductive stress (increased GSH) inhibits this process (1), suggesting that excess GSH may potentiate the Glrx system. PTP1B ablation improves angiogenesis and cardiac function after coronary artery ligation in mice (23). EC-specific PTP1B deletion also enhances vascularization after femoral artery ligation in mice (106). Therefore, inhibiting PTPs by S-glutathionylation might be one of the targets by which oxidants promote vascularization. However, oxidized PTP1B can also form a sulfenyl-amide intermediate (158), which can be reduced by thioredoxin. The thioredoxin system can activate PTP1B (45) and inhibit VEGF-mediated angiogenesis (142). Whether Glrx can modulate the activity of PTPs remains unexplored.
Sarcoplasmic-endoplasmic reticulum calcium ATPase
S-glutathionylation of Cys674 of the sarcoplasmic-endoplasmic reticulum calcium ATPase 2 (SERCA2) activates the enzyme and triggers calcium influx (2). Overexpression of Cys674Ser mutant SERCA2 or Glrx inhibits VEGF-induced EC calcium influx and migration (50). The Cys674Ser SERCA2 mutant also impairs developmental angiogenesis in homozygous knock-in mice and is embryonically lethal (173). Similarly, the Cys674Ser SERCA2 mutation impairs blood flow recovery after hindlimb ischemia in heterozygous knock-in mice, and the mutation decreases VEGF-induced angiogenic responses in ECs isolated from these mice (126, 173). In summary, S-glutathionylation-mediated SERCA2 activation contributes to EC migration and angiogenesis.
NF-κB signaling
S-glutathionylation inhibits multiple components of the NF-κB pathway, including TRAF6 (39), IKK-β (nuclear factor-kappa-β kinase subunit beta) (155), p50 (147), and p65 (130, 151) subunits; whereas Glrx overexpression enhances NF-κB transcriptional activity (62, 130) (Fig. 6). Glrx overexpression attenuates limb revascularization after femoral artery ligation in association with elevated soluble VEGF receptor 1 levels (sVEGFR-1, also known as soluble fms-like tyrosine kinase-1s; sFlt-1) in the muscle and plasma of mice (129, 130). VEGFR-1 (Flt-1) has a higher affinity to VEGF-A, but the signal transduction is stronger through VEGFR-2. The soluble splice variant (sVEGFR-1, sFlt-1) acts as a decoy receptor competing with VEGFR2 for binding of VEGF-A (94).
Overexpression of Glrx augments NF-κB activity and induces the anti-angiogenic factor sVEGFR-1 in ECs (129, 130). Since Glrx is an NF-κB-dependent gene (6), activation of NF-κB in inflammation or diabetes (36, 62) may increase Glrx expression. Subsequently, Glrx further activates NF-κB and may positively provide feedback and amplify the inflammatory response. Inhibition of NF-κB by mutant IκBα, which serves as a repressor, increases disorganized vasculature, decreases functional arteriogenesis in the ischemic mouse muscle (176), and enhances tumor vascularization and growth (99). These data suggest that either hyper-activation or inhibition of NF-κB activity can result in dysregulated angiogenesis.
Hypoxia-inducible factor-1α
Hypoxia-inducible factor (HIF)-1 is a major transcriptional factor that responds to hypoxia by activating angiogenic and glycolytic pathways to deliver oxygen and nutrients into hypoxic tissues (Fig. 7). HIF-1 is composed of an O2-regulated HIF-1α, and a constitutively expressed HIF-1β subunit (161). Protein expression levels of HIF-1α control HIF-1 activity. Therefore, HIF-1α stability and the regulation of its stability by PTM are critical for transcriptional gene activation. Under normal oxygen levels, HIF-1α is hydroxylated at proline 402 and 564 in the oxygen-dependent degradation (ODD) domain by prolyl-hydroxylases (PHD) (138). The proline hydroxylation facilitates the ubiquitination of HIF-1α by the E3 ubiquitin ligase von Hippel Lindau protein (pVHL) and causes rapid proteasomal degradation of HIF-1α. Hypoxia limits PHD activity and HIF-1α hydroxylation and subsequent degradation, resulting in stabilized HIF-1α protein. NO (nitric oxide)-mediated S-nitrosylation at Cys533 (mouse, human Cys520 equivalent) in the ODD domain prevents degradation of HIF-1α (110). Longer lasting S-glutathionylation occurs at the same cysteine (180). Glrx inhibition or increased protein S-glutathionylation stabilizes HIF-1α in vitro by preventing its interaction with pVHL. Glrx deletion in mice enhances protein S-glutathionylation, which stabilizes and transcriptionally activates HIF-1α and increases HIF-1α and VEGF expression in the ischemic muscle, promoting revascularization and blood flow recovery of the ischemic limb after femoral artery ligation (180) (Fig. 5). Our molecular mechanism can explain the ROS-dependent HIF-1α activation (38) and may play an important role in other biological processes. In addition to the direct modification of HIF-1α, mitochondrial oxidants and various other factors may affect PHD activity (140).
FIG. 7.
The HIF-1α pathway. Oxidants cause oxidative modification and S-glutathionylation of Cys533 located in the ODD domain of HIF-1α. S-glutathionylation prevents interaction with vHL, a component of the ubiquitin ligase E3 complex, and proteasomal degradation. Thus, the S-glutathionylation of HIF-1α activates the proangiogenic program, which also includes the expression of VEGF. EC, endothelial cell; HIF, hypoxia-inducible factor; ODD, oxygen-dependent degradation; PTP, protein tyrosine phosphatase; Rac1, Ras-related C3 botulinum toxin substrate 1; SERCA, sarcoplasmic-endoplasmic reticulum calcium ATPase; sVEGFR1 (sFlt), soluble vascular endothelial growth factor receptor 1 (soluble fms-like tyrosine kinase-1s); VEGF, vascular endothelial growth factor; vHL, von Hippel–Lindau. Color images are available online.
Other targets
S-glutathionylation inactivates the small Rho GTPase Rac1 (Ras-related C3 botulinum toxin substrate 1) under metabolic stress, and Glrx overexpression maintains Rac1 activity leading to protection of the EC barrier function, lowering of vascular permeability, and inhibition of angiogenesis (67). Therefore, upregulated Glrx may protect ECs from atherogenic insults at the expense of decreasing EC migration and angiogenic processes. Aside from Rac1, many other S-glutathionylated proteins may inhibit vascularization, but endogenous Glrx mostly has antiangiogenic effects, as shown in Glrx KO mice. The mechanism by which Glrx activates specific signaling pathways likely depends on the context of the pathology.
Nonalcoholic fatty liver disease
Nonalcoholic fatty liver disease (NAFLD) starts with simple, fully reversible hepatic fat accumulation, referred to as steatosis (Fig. 8A), that is not caused by alcohol. In most cases, NAFLD is asymptomatic. Although steatosis in NAFLD is fully reversible, the disease may advance to a more severe form that involves inflammation with or without fibrosis, referred to as NASH (Fig. 8B). Glrx KO mice develop obesity, hyperlipidemia, and fatty liver by 8 months of age on standard chow, mimicking NAFLD (163). The liver disease in the Glrx KO mice follows the “two-hit hypothesis” for NAFLD (163) (Fig. 8C); the fatty liver is present, and a second event leads to liver inflammation and progression to NASH. The second hit, a high-fat diet, induces steatohepatitis and advances to NASH in the Glrx KO mice.
FIG. 8.
NAFLD and de novo lipogenesis. (A) Major pathways that control hepatic lipid content. Top right: Pathological stages of NAFLD. Liver disease progression adapted from the GB HealthWatch. Major working hypotheses explaining NAFLD disease progression (B) the “two-hit” hypothesis and (C) the “multiple parallel hit” hypothesis. (D) Effects of Glrx on the central metabolic regulator and NAD+-dependent class III histone deacetylase SirT1. (E) S-glutathionylation inhibits SirT1 activity, causing hyperacetylation and transcriptional activation of SREBP. These transcription factors increase expression of the key enzymes—fatty acid synthase and HMG-CoA reductase—for hepatic lipid synthesis. Active SirT1 inhibits de novo lipogenesis and promotes mitochondrial and peroxisomal fatty acid oxidation through the central transcription factors PPARγ and PGC1α. HMG, β-hydroxy β-methylglutaryl; NAFLD, nonalcoholic fatty liver disease; PGC1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; PPARγ, peroxisome proliferator-activated receptor gamma; SirT1, sirtuin-1; SREBP, sterol regulatory element-binding protein. Color images are available online.
However, in clinics, the exact reason or timing of NAFLD progression to NASH is often unclear and based on a complex and multifactorial etiology. Hence, the “multiple parallel hit” hypothesis (Fig. 8D) better reflects the NAFLD pathogenesis in patients.
Reconstitution of Glrx in the Glrx-deficient liver suppressed hepatic steatosis and lipid levels in the short term (163), indicating that the upregulation of Glrx might be beneficial to control lipid metabolism. Even though fibrosis is usually more difficult to produce in mice, Glrx-deficient mice fed a high-fat diet developed fibrosis, starting from the hepatic triad.
The role of Glrx and protein S-glutathionylation in the pathogenesis of NAFLD remains vastly unexplored. The high-fat diet-fed mice develop NAFLD and show increased levels of S-glutathionylated proteins. One of the target proteins is the central metabolic regulator sirtuin-1 (SirT1), an NAD+-dependent class III histone deacetylase (162). Thiol modifications of SirT1 modulated lipid metabolism via acetylation of key transcription factors. S-glutathionylation inactivates SirT1 and promotes hyperacetylation and activation of downstream target proteins such as p53 and sterol regulatory element-binding protein (SREBP) (Fig. 8E). p53 activation is a cellular response to stress that changes metabolism and halts cell cycle progression. NAFLD livers also express increased levels of p21, a downstream target gene of p53, which may interfere with liver regeneration by inhibiting hepatocyte proliferation. Hepatocytes experiencing grave oxidative stress may use p53 to initiate controlled cell death involving p53 upregulated modulator of apoptosis (PUMA), also known as Bcl-2-binding component 3. SirT1-mediated S-glutathionylation is reversed by Glrx, which can prevent the activation of p53 in hepatocytes. Consistent with this finding, overexpression of SirT1 improves NAFLD (47), whereas an inoxidizable cysteine to serine mutant SirT1 has an even stronger effect and blocks p53 activation. Conversely, SirT1 knockout mice develop NAFLD (196).
Glrx KO mice fed a standard chow diet spontaneously develop hepatic steatosis, and feeding a high-fat diet advances the disease to NASH, beginning with mild fibrosis (163). Glrx ablation increases S-glutathionylated SirT1, causing hyperacetylation and transcriptional activation of SREBP. Two isoforms of SREBP exist: SREBP-1, which regulates the de novo fatty acid synthesis, and SREBP-2, which controls cholesterol metabolism. Both of these pathways are upregulated in Glrx KO livers. Viral Glrx replenishment of the steatotic Glrx KO mouse liver attenuates de novo fatty acid synthesis and decreases hepatic lipids.
Overexpression of the cysteine to serine mutant SirT1 in steatotic Glrx KO mouse liver also reverses NAFLD (163). Active SirT1 not only attenuates hepatic lipid synthesis but also promotes fatty acid oxidation in peroxisomes and mitochondria. Clinical trials with SirT1 activators show little effect on insulin resistance and glucose metabolism, but they, undoubtedly, improve plasma lipids and likely modulate hepatic lipid homeostasis. Thus, increasing Glrx or SirT1 activity in NAFLD is an effective therapy to normalize hepatic lipid homeostasis in mice that appears to translate into clinical medicine.
In addition to lipid-lowering effects, Glrx protects endothelial barrier function (67) and inhibits monocyte chemotaxis under metabolic stress (178). These data imply that upregulation of Glrx may safeguard vessels from atherosclerotic insults.
Brain
Oxidative stress contributes to the pathogenesis of neurodegenerative diseases such as Alzheimer's and Parkinson's disease. Glutaredoxins, together with the thioredoxin system, assist in maintaining a reduced environment and protecting neuronal cells from apoptosis. The two reductive systems likely mediate amyloid neurotoxicity. Human brains affected by Alzheimer's disease increase Glrx expression levels, whereas thioredoxin expression decreases (7), indicating a distinct role of each system.
Glrx may also play a role in Parkinson's disease. The Parkinson's disease-like illness induced by the neurotoxin MPTP increases striatal Glrx expression, which is essential for the maintenance of mitochondrial complex I function in mice (93). Likewise, female mice have higher constitutive expression of Glrx than males and do not suffer the MPTP-induced loss of dopaminergic neurons. By using an estrogen receptor antagonist, Glrx activity decreases in the female mouse brain (but not in the liver), and MPTP-induced complex I dysfunction increases (92). Similarly, the loss of homologs to Glrx exacerbates neurodegeneration in Caenorhabditis elegans models of Parkinson's disease (86). Lastly, Parkinson's disease patients have decreased Glrx levels in the midbrain (86).
Therefore, the upregulation of Glrx is expected to be beneficial for Parkinson's disease. However, Glrx overexpression in microglia causes hyperactivation of NF-κB (129), increases inflammatory cytokines, and decreases tyrosine hydroxylase in the brain of Glrx-overexpressing mice fed a high-fat high-sucrose diet (85). These data indicate that higher Glrx expression in microglia induces inflammation and dopaminergic neuronal degeneration, outweighing the neuroprotective effects of overexpressed Glrx in dopaminergic neurons (63). In summary, balanced Glrx activity appears essential to maintain a healthy brain and cell-selective therapies are required to target Glrx.
Airway and lung
The respiratory system is easily exposed to environmental oxidative stress, and Glrx and S-glutathionylation significantly regulate lung pathology. In acute inflammatory states, elevated GSH adducts in Glrx KO mice suppress lung inflammation by inhibition of the NF-κB pathway. The administration of lipopolysaccharide (LPS) induces Glrx and inflammatory cytokines in the lung, but Glrx KO mice exhibit attenuated inflammatory responses (4). Glrx-deficient macrophages produce fewer oxidants and cause less phagocytosis (6, 17). Thus, Glrx induction can activate NF-κB (Fig. 5), which further induces Glrx (6) and accelerates the inflammatory responses in a feed-forward manner.
Glrx inhibits the apoptosis pathway by activating NF-κB as well as by inhibiting the pro-apoptotic receptor Fas. GSH-adducts of Fas amplify Fas-mediated apoptosis (6, 12). In Pseudomonas aeruginosa–induced pneumonia, inhibition of Glrx is protective since Fas-mediated apoptosis promotes pathogen clearance, whereas Glrx overexpression in lung epithelial cells increases mortality in infected mice (10).
In contrast, inhibiting apoptosis by Glrx overexpression in alveolar epithelial cells is protective against lung fibrosis (11). Thus, the redox regulation of Fas plays opposite roles in the lung, depending on pathological models. Interestingly, lungs from patients with idiopathic pulmonary fibrosis show lower Glrx activity and higher S-glutathionylated proteins (10). Glrx KO mice have exacerbated lung fibrosis induced by bleomycin or TGFβ, whereas Glrx overexpression in alveolar epithelial cells inhibits apoptosis and attenuates collagen accumulation and fibrosis. Intra-tracheal administration of recombinant Glrx also suppresses fibrosis in mouse lungs, suggesting the potential therapeutic use of Glrx to treat pulmonary fibrosis (11). Decreasing S-glutathionylation by inhibiting GSTπ, such as using GSTπ inhibitor TLK117, is another strategy to treat lung fibrosis (125).
Conclusions
The field of redox regulation has progressed slowly due to complex redox biochemistry and imperfect analytical tools. Recent improvements in genetically encoded redox biosensors that visualize live redox processes coupled with CRISPR-Cas9 and adeno-associated virus-mediated transduction will continue to accelerate our understanding of in vivo redox regulation.
Interconnected redox functions of antioxidant systems, such as thioredoxins and glutaredoxins, often complicate our understanding of systems. The built-in redundancies partially distract us from the real physiological importance of these enzymes. Thus, glutaredoxins are often perceived as another layer of the cellular antioxidant system and have found an entry in common protein databases as such.
However, protein cysteine modification with GSH adducts, S-glutathionylation, is controlled by oxidants and enzymes, including GSTπ and Glrx, contributing to redox regulation. In particular, the Glrx-mediated reversal of modified proteins modulates signaling cascades in vivo and controls pathological phenotypes. Upregulated Glrx inhibits ischemic vascularization of the hindlimb, whereas it improves lung fibrosis and liver steatosis in mice. This complexity in phenotypes likely derives from the hierarchical control of S-glutathionylated and Glrx-regulated proteins as well as tissue and cell type-specific regulation. Thus, cell-specific manipulation of Glrx will further elucidate Glrx-mediated redox signaling in vivo.
Further, recent experimental data suggest that redox regulation involves transglutathionylation between proteins, that is, redox relays that define novel signaling cascades between proteins. Transglutathionylation, in particular, may enable redox signaling across membranes and between cellular compartments. However, additional research is required to elucidate the significance of these redox relays further. For these reasons, Glrx can be a potential therapeutic target to modulate redox regulation and treat conditions such as fatty liver, PAD, and lung fibrosis.
Acknowledgment
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the awarding offices.
Abbreviations Used
- Ang II
angiotensin II
- Cys
cysteine
- EC
endothelial cell
- ER
endoplasmic reticulum
- ERO1
ER oxidoreductin 1
- Glrx
glutaredoxin-1
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- GST
glutathione S-transferase
- H2O2
hydrogen peroxide
- HIF-1α
hypoxia-inducible factor 1α
- IRP
iron-regulatory protein
- JNK
c-Jun N-terminal kinase
- LMW
low molecular weight
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MRP
multidrug resistance-associated proteins
- MS
mass spectrometry
- NADPH
nicotinamide adenine dinucleotide phosphate
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NF-κB
nuclear factor-κB
- NOS
nitric oxide synthase
- NOX
NADPH oxidase
- ODD
oxygen-dependent degradation
- OPTM
oxidative post-translational modification
- PAD
peripheral artery disease
- PDI
protein-disulfide isomerase
- PHD
prolyl-hydroxylases
- PRDX4
peroxiredoxin 4
- PTM
post-translational modification
- PTP
protein tyrosine phosphatases
- pVHL
von Hippel Lindau protein
- ROS
reactive oxygen species
- Ser
serine
- SERCA
sarcoplasmic-endoplasmic reticulum calcium ATPase
- SirT1
sirtuin-1
- SREBP
sterol regulatory element-binding protein
- sVEGFR-1
soluble VEGF receptor 1
- Tyr
tyrosine
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
Author Disclosure Statement
The authors have nothing to disclose.
Funding Information
This work was supported by National Institute of Health grants R01 DK103750, R01 HL133013, R21 AG058983, R21 AA026922, R01 HL137771, and R03 AG 051857, American Heart Association “Grant in Aid” 16GRNT27660006, European Cooperation in Science and Technology (COST Action BM1203/EU-ROS), and the Metabolic Clinical Research Collaborative. This work was also supported by NIH/Boston University Clinical & Translational Science Institute grant 1UL1TR001430 to M.M.B. and R.M. M.M.B. was also supported by the Evans Junior Faculty Research Award by the Department of Medicine of Boston University. B.F. was supported by NIH T32 HL007224 Multidisciplinary Training in Cardiovascular Research through the Whitaker Cardiovascular Institute at Boston University.
References
- 1. Abdelsaid MA and El-Remessy AB. S-glutathionylation of LMW-PTP regulates VEGF-mediated FAK activation and endothelial cell migration. J Cell Sci 125: 4751–4760, 2012 [DOI] [PubMed] [Google Scholar]
- 2. Adachi T, Weisbrod RM, Pimentel DR, Ying J, Sharov VS, Schöneich C, and Cohen RA. S-Glutathiolation by peroxynitrite activates SERCA during arterial relaxation by nitric oxide. Nat Med 10: 1200–1207, 2004 [DOI] [PubMed] [Google Scholar]
- 3. Adluri RS, Thirunavukkarasu M, Zhan L, Dunna NR, Akita Y, Selvaraju V, Otani H, Sanchez JA, Ho Y-S, and Maulik N. Glutaredoxin-1 overexpression enhances neovascularization and diminishes ventricular remodeling in chronic myocardial infarction. PLoS One 7: e34790, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aesif SW, Anathy V, Kuipers I, Guala AS, Reiss JN, Ho Y-S, and Janssen-Heininger YMW. Ablation of glutaredoxin-1 attenuates lipopolysaccharide-induced lung inflammation and alveolar macrophage activation. Am J Respir Cell Mol Biol 44: 491–499, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Aesif SW, Janssen-Heininger YMW, and Reynaert NL. Protocols for the detection of s-glutathionylated and s-nitrosylated proteins in situ. Methods Enzymol 474: 289–296, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aesif SW, Kuipers I, van der Velden J, Tully JE, Guala AS, Anathy V, Sheely JI, Reynaert NL, Wouters EFM, van der Vliet A, and Janssen-Heininger YMW. Activation of the glutaredoxin-1 gene by nuclear factor κB enhances signaling. Free Radic Biol Med 51: 1249–1257, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Akterin S, Cowburn RF, Miranda-Vizuete A, Jiménez A, Bogdanovic N, Winblad B, and Cedazo-Minguez A. Involvement of glutaredoxin-1 and thioredoxin-1 in beta-amyloid toxicity and Alzheimer's disease. Cell Death Differ 13: 1454–1465, 2006 [DOI] [PubMed] [Google Scholar]
- 8. Alanazi AM, Mostafa GAE, and Al-Badr AA. Glutathione. Profiles Drug Subst Excip Relat Methodol 40: 43–158, 2015 [DOI] [PubMed] [Google Scholar]
- 9. Allen EMG and Mieyal JJ. Protein-thiol oxidation and cell death: regulatory role of glutaredoxins. Antioxid Redox Signal 17: 1748–1763, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anathy V, Aesif SW, Hoffman SM, Bement JL, Guala AS, Lahue KG, Leclair LW, Suratt BT, Cool CD, Wargo MJ, and Janssen-Heininger YMW. Glutaredoxin-1 attenuates S-glutathionylation of the death receptor fas and decreases resolution of Pseudomonas aeruginosa pneumonia. Am J Respir Crit Care Med 189: 463–474, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Anathy V, Lahue KG, Chapman DG, Chia SB, Casey DT, Aboushousha R, van der Velden JLJ, Elko E, Hoffman SM, McMillan DH, Jones JT, Nolin JD, Abdalla S, Schneider R, Seward DJ, Roberson EC, Liptak MD, Cousins ME, Butnor KJ, Taatjes DJ, Budd RC, Irvin CG, Ho Y-S, Hakem R, Brown KK, Matsui R, Bachschmid MM, Gomez JL, Kaminski N, van der Vliet A, and Janssen-Heininger YMW. Reducing protein oxidation reverses lung fibrosis. Nat Med 24: 1128–1135, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Anathy V, Roberson E, Cunniff B, Nolin JD, Hoffman S, Spiess P, Guala AS, Lahue KG, Goldman D, Flemer S, van der Vliet A, Heintz NH, Budd RC, Tew KD, and Janssen-Heininger YMW. Oxidative processing of latent Fas in the endoplasmic reticulum controls the strength of apoptosis. Mol Cell Biol 32: 3464–3478, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arnér ESJ. Selenoproteins—what unique properties can arise with selenocysteine in place of cysteine? Exp Cell Res 316: 1296–1303, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Axelsson K, Eriksson S, and Mannervik B. Purification and characterization of cytoplasmic thioltransferase (glutathione:disulfide oxidoreductase) from rat liver. Biochemistry 17: 2978–2984, 1978 [DOI] [PubMed] [Google Scholar]
- 15. Axelsson K and Mannervik B. A possible role of cytoplasmic thioltransferase in the intracellular degradation of disulfide-containing proteins. Acta Chem Scand B 34: 139–140, 1980 [DOI] [PubMed] [Google Scholar]
- 16. Bachschmid M, Thurau S, Zou M-H, and Ullrich V. Endothelial cell activation by endotoxin involves superoxide/NO-mediated nitration of prostacyclin synthase and thromboxane receptor stimulation. FASEB J 17: 914–916, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Bachschmid MM, Xu S, Maitland-Toolan KA, Ho Y-S, Cohen RA, and Matsui R. Attenuated cardiovascular hypertrophy and oxidant generation in response to angiotensin II infusion in glutaredoxin-1 knockout mice. Free Radic Biol Med 49: 1221–1229, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baeza J, Dowell JA, Smallegan MJ, Fan J, Amador-Noguez D, Khan Z, and Denu JM. Stoichiometry of site-specific lysine acetylation in an entire proteome. J Biol Chem 289: 21326–21338, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Baker PR, Friederich MW, Swanson MA, Shaikh T, Bhattacharya K, Scharer GH, Aicher J, Creadon-Swindell G, Geiger E, MacLean KN, Lee W-T, Deshpande C, Freckmann M-L, Shih L-Y, Wasserstein M, Rasmussen MB, Lund AM, Procopis P, Cameron JM, Robinson BH, Brown GK, Brown RM, Compton AG, Dieckmann CL, Collard R, Coughlin CR, Spector E, Wempe MF, and Van Hove JLK. Variant non ketotic hyperglycinemia is caused by mutations in LIAS, BOLA3 and the novel gene GLRX5. Brain J Neurol 137: 366–379, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barrett WC, DeGnore JP, König S, Fales HM, Keng YF, Zhang ZY, Yim MB, and Chock PB. Regulation of PTP1B via glutathionylation of the active site cysteine 215. Biochemistry 38: 6699–6705, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Behring JB, Kumar V, Whelan SA, Chauhan P, Siwik DA, Costello CE, Colucci WS, Cohen RA, McComb ME, and Bachschmid MM. Does reversible cysteine oxidation link the Western diet to cardiac dysfunction? FASEB J 28: 1975–1987, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Berndt C, Hudemann C, Hanschmann E-M, Axelsson R, Holmgren A, and Lillig CH. How does iron-sulfur cluster coordination regulate the activity of human glutaredoxin 2? Antioxid Redox Signal 9: 151–157, 2007 [DOI] [PubMed] [Google Scholar]
- 23. Besnier M, Galaup A, Nicol L, Henry J-P, Coquerel D, Gueret A, Mulder P, Brakenhielm E, Thuillez C, Germain S, Richard V, and Ouvrard-Pascaud A. Enhanced angiogenesis and increased cardiac perfusion after myocardial infarction in protein tyrosine phosphatase 1B-deficient mice. FASEB J 28: 3351–3361, 2014 [DOI] [PubMed] [Google Scholar]
- 24. Biaglow JE, Donahue J, Tuttle S, Held K, Chrestensen C, and Mieyal J. A method for measuring disulfide reduction by cultured mammalian cells: relative contributions of glutathione-dependent and glutathione-independent mechanisms. Anal Biochem 281: 77–86, 2000 [DOI] [PubMed] [Google Scholar]
- 25. Birkenmeier G, Stegemann C, Hoffmann R, Günther R, Huse K, and Birkemeyer C. Posttranslational modification of human glyoxalase 1 indicates redox-dependent regulation. PLoS One 5: e10399, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boivin B and Tonks NK. Analysis of the redox regulation of protein tyrosine phosphatase superfamily members utilizing a cysteinyl-labeling assay. Methods Enzymol 474: 35–50, 2010 [DOI] [PubMed] [Google Scholar]
- 27. Bradbury ARM, Sidhu S, Dübel S, and McCafferty J. Beyond natural antibodies: the power of in vitro display technologies. Nat Biotechnol 29: 245–254, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brandes RP, Takac I, and Schröder K. No superoxide—no stress?: Nox4, the good NADPH oxidase! Arterioscler Thromb Vasc Biol 31: 1255–1257, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Brigidi GS and Bamji SX. Detection of protein palmitoylation in cultured hippocampal neurons by immunoprecipitation and acyl-biotin exchange (ABE). J Vis Exp 72: 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Brynczka C, Labhart P, and Merrick BA. NGF-mediated transcriptional targets of p53 in PC12 neuronal differentiation. BMC Genomics 8: 139, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bulaj G, Kortemme T, and Goldenberg DP. Ionization-reactivity relationships for cysteine thiols in polypeptides. Biochemistry 37: 8965–8972, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Burgoyne JR and Eaton P. A rapid approach for the detection, quantification, and discovery of novel sulfenic acid or S-nitrosothiol modified proteins using a biotin-switch method. Methods Enzymol 473: 281–303, 2010 [DOI] [PubMed] [Google Scholar]
- 33. Burgoyne JR, Haeussler DJ, Kumar V, Ji Y, Pimental DR, Zee RS, Costello CE, Lin C, McComb ME, Cohen RA, and Bachschmid MM. Oxidation of HRas cysteine thiols by metabolic stress prevents palmitoylation in vivo and contributes to endothelial cell apoptosis. FASEB J 26: 832–841, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cai Z, Lu Q, Ding Y, Wang Q, Xiao L, Song P, and Zou M-H. Endothelial nitric oxide synthase-derived nitric oxide prevents dihydrofolate reductase degradation via promoting S-nitrosylation. Arterioscler Thromb Vasc Biol 35: 2366–2373, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Camaschella C, Campanella A, De Falco L, Boschetto L, Merlini R, Silvestri L, Levi S, and Iolascon A. The human counterpart of zebrafish shiraz shows sideroblastic-like microcytic anemia and iron overload. Blood 110: 1353–1358, 2007 [DOI] [PubMed] [Google Scholar]
- 36. Carlsen H, Haugen F, Zadelaar S, Kleemann R, Kooistra T, Drevon CA, and Blomhoff R. Diet-induced obesity increases NF-kappaB signaling in reporter mice. Genes Nutr 4: 215–222, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Carr AN, Davis MG, Eby-Wilkens E, Howard BW, Towne BA, Dufresne TE, and Peters KG. Tyrosine phosphatase inhibition augments collateral blood flow in a rat model of peripheral vascular disease. Am J Physiol Heart Circ Physiol 287: H268–H276, 2004 [DOI] [PubMed] [Google Scholar]
- 38. Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, and Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci U S A 95: 11715–11720, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Chantzoura E, Prinarakis E, Panagopoulos D, Mosialos G, and Spyrou G. Glutaredoxin-1 regulates TRAF6 activation and the IL-1 receptor/TLR4 signalling. Biochem Biophys Res Commun 403: 335–339, 2010 [DOI] [PubMed] [Google Scholar]
- 40. Cheng N-H, Zhang W, Chen W-Q, Jin J, Cui X, Butte NF, Chan L, and Hirschi KD. A mammalian monothiol glutaredoxin, Grx3, is critical for cell cycle progression during embryogenesis. FEBS J 278: 2525–2539, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Choi S, Jeong J, Na S, Lee HS, Kim H-Y, Lee K-J, and Paek E. New algorithm for the identification of intact disulfide linkages based on fragmentation characteristics in tandem mass spectra. J Proteome Res 9: 626–635, 2010 [DOI] [PubMed] [Google Scholar]
- 42. Chrestensen CA, Starke DW, and Mieyal JJ. Acute cadmium exposure inactivates thioltransferase (Glutaredoxin), inhibits intracellular reduction of protein-glutathionyl-mixed disulfides, and initiates apoptosis. J Biol Chem 275: 26556–26565, 2000 [DOI] [PubMed] [Google Scholar]
- 43. Cianfruglia L, Galeazzi R, Massaccesi L, Spaccini R, Caniglia ML, Principato G, and Armeni T. Glyoxalase II promotes “in vitro” S-glutathionylation. Free Radic Biol Med 75(Suppl 1): S26–S27, 2014 [DOI] [PubMed] [Google Scholar]
- 44. Craige SM, Chen K, Pei Y, Li C, Huang X, Chen C, Shibata R, Sato K, Walsh K, and Keaney JF. NADPH oxidase 4 promotes endothelial angiogenesis through endothelial nitric oxide synthase activation. Circulation 124: 731–740, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dagnell M, Frijhoff J, Pader I, Augsten M, Boivin B, Xu J, Mandal PK, Tonks NK, Hellberg C, Conrad M, Arnér ESJ, and Östman A. Selective activation of oxidized PTP1B by the thioredoxin system modulates PDGF-β receptor tyrosine kinase signaling. Proc Natl Acad Sci U S A 110: 13398–13403, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Daher R, Mansouri A, Martelli A, Bayart S, Manceau H, Callebaut I, Moulouel B, Gouya L, Puy H, Kannengiesser C, and Karim Z. GLRX5 mutations impair heme biosynthetic enzymes ALA synthase 2 and ferrochelatase in Human congenital sideroblastic anemia. Mol Genet Metab 128: 342–351, 2019 [DOI] [PubMed] [Google Scholar]
- 47. Deng X-Q, Chen L-L, and Li N-X. The expression of SIRT1 in nonalcoholic fatty liver disease induced by high-fat diet in rats. Liver Int 27: 708–715, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Diotte NM, Xiong Y, Gao J, Chua BHL, and Ho Y-S. Attenuation of doxorubicin-induced cardiac injury by mitochondrial glutaredoxin 2. Biochim Biophys Acta 1793: 427–438, 2009 [DOI] [PubMed] [Google Scholar]
- 49. Eriksson S, Askelöf P, Axelsson K, and Mannervik B. Evidence for a glutathione-dependent interconversion of two forms of a thioltransferase from rat liver catalyzing thiol-disulfide interchange. Acta Chem Scand B 28: 931–936, 1974 [DOI] [PubMed] [Google Scholar]
- 50. Evangelista AM, Thompson MD, Weisbrod RM, Pimental DR, Tong X, Bolotina VM, and Cohen RA. Redox regulation of SERCA2 is required for vascular endothelial growth factor-induced signaling and endothelial cell migration. Antioxid Redox Signal 17: 1099–1108, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fernandes AP and Holmgren A. Glutaredoxins: glutathione-dependent redox enzymes with functions far beyond a simple thioredoxin backup system. Antioxid Redox Signal 6: 63–74, 2004 [DOI] [PubMed] [Google Scholar]
- 52. Findley CM, Mitchell RG, Duscha BD, Annex BH, and Kontos CD. Plasma levels of soluble Tie2 and vascular endothelial growth factor distinguish critical limb ischemia from intermittent claudication in patients with peripheral arterial disease. J Am Coll Cardiol 52: 387–393, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fomenko DE, Marino SM, and Gladyshev VN. Functional diversity of cysteine residues in proteins and unique features of catalytic redox-active cysteines in thiol oxidoreductases. Mol Cells 26: 228–235, 2008 [PMC free article] [PubMed] [Google Scholar]
- 54. Frey AG, Palenchar DJ, Wildemann JD, and Philpott CC. A glutaredoxin·BolA complex serves as an iron-sulfur cluster chaperone for the cytosolic cluster assembly machinery. J Biol Chem 291: 22344–22356, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fujii S, Sawa T, Ihara H, Tong KI, Ida T, Okamoto T, Ahtesham AK, Ishima Y, Motohashi H, Yamamoto M, and Akaike T. The critical role of nitric oxide signaling, via protein S-guanylation and nitrated cyclic GMP, in the antioxidant adaptive response. J Biol Chem 285: 23970–23984, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Gan ZR and Wells WW. Purification and properties of thioltransferase. J Biol Chem 261: 996–1001, 1986 [PubMed] [Google Scholar]
- 57. Garcia RA and Stipanuk MH. The splanchnic organs, liver and kidney have unique roles in the metabolism of sulfur amino acids and their metabolites in rats. J Nutr 122: 1693–1701, 1992 [DOI] [PubMed] [Google Scholar]
- 58. Giacco F, Du X, D'Agati VD, Milne R, Sui G, Geoffrion M, and Brownlee M. Knockdown of glyoxalase 1 mimics diabetic nephropathy in nondiabetic mice. Diabetes 63: 291–299, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Gladyshev VN, Liu A, Novoselov SV, Krysan K, Sun QA, Kryukov VM, Kryukov GV, and Lou MF. Identification and characterization of a new mammalian glutaredoxin (thioltransferase), Grx2. J Biol Chem 276: 30374–30380, 2001 [DOI] [PubMed] [Google Scholar]
- 60. Goldstein RA and Pollock DD. Observations of amino acid gain and loss during protein evolution are explained by statistical bias. Mol Biol Evol 23: 1444–1449, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Goncalves RLS, Quinlan CL, Perevoshchikova IV, Hey-Mogensen M, and Brand MD. Sites of superoxide and hydrogen peroxide production by muscle mitochondria assessed ex vivo under conditions mimicking rest and exercise. J Biol Chem 290: 209–227, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Gorelenkova Miller O, Behring JB, Siedlak SL, Jiang S, Matsui R, Bachschmid MM, Zhu X, and Mieyal JJ. Upregulation of glutaredoxin-1 activates microglia and promotes neurodegeneration: implications for Parkinson's disease. Antioxid Redox Signal 25: 967–982, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Gorelenkova Miller O and Mieyal JJ. Critical roles of glutaredoxin in brain cells-implications for Parkinson's disease. Antioxid Redox Signal 30: 1352–1368, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Guo X, Yu Y, Liu X, Zhang Y, Guan T, Xie G, and Wei J. Heterologous expression and characterization of human cellular glutathione peroxidase mutants. IUBMB Life 66: 212–219, 2014 [DOI] [PubMed] [Google Scholar]
- 65. Haeussler DJ, Pimentel DR, Hou X, Burgoyne JR, Cohen RA, and Bachschmid MM. Endomembrane H-Ras controls vascular endothelial growth factor-induced nitric-oxide synthase-mediated endothelial cell migration. J Biol Chem 288: 15380–15389, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hagihara K, Kazui M, Kurihara A, Kubota K, and Ikeda T. Glutaredoxin and thioredoxin can be involved in producing the pharmacologically active metabolite of a thienopyridine antiplatelet agent, prasugrel. Drug Metab Dispos Biol Fate Chem 39: 208–214, 2011 [DOI] [PubMed] [Google Scholar]
- 67. Han J, Weisbrod RM, Shao D, Watanabe Y, Yin X, Bachschmid MM, Seta F, Janssen-Heininger YMW, Matsui R, Zang M, Hamburg NM, and Cohen RA. The redox mechanism for vascular barrier dysfunction associated with metabolic disorders: glutathionylation of Rac1 in endothelial cells. Redox Biol 9: 306–319, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Harris TK and Turner GJ. Structural basis of perturbed pKa values of catalytic groups in enzyme active sites. IUBMB Life 53: 85–98, 2002 [DOI] [PubMed] [Google Scholar]
- 69. Hatakeyama M, Tanimoto Y, and Mizoguchi T. Purification and some properties of bovine liver cytosol thioltransferase. J Biochem (Tokyo) 95: 1811–1818, 1984 [DOI] [PubMed] [Google Scholar]
- 70. Haunhorst P, Hanschmann E-M, Bräutigam L, Stehling O, Hoffmann B, Mühlenhoff U, Lill R, Berndt C, and Lillig CH. Crucial function of vertebrate glutaredoxin 3 (PICOT) in iron homeostasis and hemoglobin maturation. Mol Biol Cell 24: 1895–1903, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Held JM, Danielson SR, Behring JB, Atsriku C, Britton DJ, Puckett RL, Schilling B, Campisi J, Benz CC, and Gibson BW. Targeted quantitation of site-specific cysteine oxidation in endogenous proteins using a differential alkylation and multiple reaction monitoring mass spectrometry approach. Mol Cell Proteomics 9: 1400–1410, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Hill BG, Ramana KV, Cai J, Bhatnagar A, and Srivastava SK. Measurement and identification of S-glutathiolated proteins. Methods Enzymol 473: 179–197, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hizi A, Shaharabany M, Tal R, and Hughes SH. The effects of cysteine mutations on the reverse transcriptases of human immunodeficiency virus types 1 and 2. J Biol Chem 267: 1293–1297, 1992 [PubMed] [Google Scholar]
- 74. Hjelle OP, Chaudhry FA, and Ottersen OP. Antisera to glutathione: characterization and immunocytochemical application to the rat cerebellum. Eur J Neurosci 6: 793–804, 1994 [DOI] [PubMed] [Google Scholar]
- 75. Ho Y-S, Xiong Y, Ho DS, Gao J, Chua BHL, Pai H, and Mieyal JJ. Targeted disruption of the glutaredoxin 1 gene does not sensitize adult mice to tissue injury induced by ischemia/reperfusion and hyperoxia. Free Radic Biol Med 43: 1299–1312, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Hoffman SM, Qian X, Nolin JD, Chapman DG, Chia SB, Lahue KG, Schneider R, Ather JL, Randall MJ, McMillan DH, Jones JT, Taatjes DJ, Aliyeva M, Daphtary N, Abdalla S, Lundblad LKA, Ho Y-S, Anathy V, Irvin CG, Wouters EFM, Reynaert NL, Dixon AE, van der Vliet A, Poynter ME, and Janssen-Heininger YMW. Ablation of glutaredoxin-1 modulates house dust mite-induced allergic airways disease in mice. Am J Respir Cell Mol Biol 55: 377–386, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Hoffman SM, Tully JE, Lahue KG, Anathy V, Nolin JD, Guala AS, van der Velden JLJ, Ho Y-S, Aliyeva M, Daphtary N, Lundblad LKA, Irvin CG, and Janssen-Heininger YMW. Genetic ablation of glutaredoxin-1 causes enhanced resolution of airways hyperresponsiveness and mucus metaplasia in mice with allergic airways disease. Am J Physiol Lung Cell Mol Physiol 303: L528–L538, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Holmgren A. Hydrogen donor system for Escherichia coli ribonucleoside-diphosphate reductase dependent upon glutathione. Proc Natl Acad Sci U S A 73: 2275–2279, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Homolya L, Váradi A, and Sarkadi B. Multidrug resistance-associated proteins: export pumps for conjugates with glutathione, glucuronate or sulfate. Biofactors 17: 103–114, 2003 [DOI] [PubMed] [Google Scholar]
- 80. Huang L, Sankar S, Lin C, Kontos CD, Schroff AD, Cha EH, Feng SM, Li SF, Yu Z, Van Etten RL, Blanar MA, and Peters KG. HCPTPA, a protein tyrosine phosphatase that regulates vascular endothelial growth factor receptor-mediated signal transduction and biological activity. J Biol Chem 274: 38183–38188, 1999 [DOI] [PubMed] [Google Scholar]
- 81. Inagaki E, Farber A, Kalish JA, Eslami MH, Siracuse JJ, Eberhardt RT, Rybin DV, Doros G, and Hamburg NM; Vascular Study Group of New England. Outcomes of peripheral vascular interventions in select patients with lower extremity acute limb ischemia. J Am Heart Assoc 7, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ishikawa T. The ATP-dependent glutathione S-conjugate export pump. Trends Biochem Sci 17: 463–468, 1992 [DOI] [PubMed] [Google Scholar]
- 83. Jeong D, Kim JM, Cha H, Oh JG, Park J, Yun S-H, Ju E-S, Jeon E-S, Hajjar RJ, and Park WJ. PICOT attenuates cardiac hypertrophy by disrupting calcineurin-NFAT signaling. Circ Res 102: 711–719, 2008 [DOI] [PubMed] [Google Scholar]
- 84. Jeong J, Jung Y, Na S, Jeong J, Lee E, Kim M-S, Choi S, Shin D-H, Paek E, Lee H-Y, and Lee K-J. Novel oxidative modifications in redox-active cysteine residues. Mol Cell Proteomics 10: M110.000513, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Johnson WM, Golczak M, Choe K, Curran PL, Miller OG, Yao C, Wang W, Lin J, Milkovic NM, Ray A, Ravindranath V, Zhu X, Wilson MA, Wilson-Delfosse AL, Chen SG, and Mieyal JJ. Regulation of DJ-1 by glutaredoxin 1 in vivo: implications for Parkinson's disease. Biochemistry 55: 4519–4532, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Johnson WM, Yao C, Siedlak SL, Wang W, Zhu X, Caldwell GA, Wilson-Delfosse AL, Mieyal JJ, and Chen SG. Glutaredoxin deficiency exacerbates neurodegeneration in C. elegans models of Parkinson's disease. Hum Mol Genet 24: 1322–1335, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Jurado J, Prieto-Alamo M-J, Madrid-Rísquez J, and Pueyo C. Absolute gene expression patterns of thioredoxin and glutaredoxin redox systems in mouse. J Biol Chem 278: 45546–45554, 2003 [DOI] [PubMed] [Google Scholar]
- 88. Kanaan GN, Ichim B, Gharibeh L, Maharsy W, Patten DA, Xuan JY, Reunov A, Marshall P, Veinot J, Menzies K, Nemer M, and Harper M-E. Glutaredoxin-2 controls cardiac mitochondrial dynamics and energetics in mice, and protects against human cardiac pathologies. Redox Biol 14: 509–521, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Karbach S, Wenzel P, Waisman A, Munzel T, and Daiber A. eNOS uncoupling in cardiovascular diseases—the role of oxidative stress and inflammation. Curr Pharm Des 20: 3579–3594, 2014 [DOI] [PubMed] [Google Scholar]
- 90. Kato N, Motohashi S, Okada T, Ozawa T, and Mashima K. PICOT, protein kinase C theta-interacting protein, is a novel regulator of FcepsilonRI-mediated mast cell activation. Cell Immunol 251: 62–67, 2008 [DOI] [PubMed] [Google Scholar]
- 91. Kekulandara DN, Samarasinghe KTG, Munkanatta Godage DNP, and Ahn Y-H. Clickable glutathione using tetrazine-alkene bioorthogonal chemistry for detecting protein glutathionylation. Org Biomol Chem 14: 10886–10893, 2016 [DOI] [PubMed] [Google Scholar]
- 92. Kenchappa RS, Diwakar L, Annepu J, and Ravindranath V. Estrogen and neuroprotection: higher constitutive expression of glutaredoxin in female mice offers protection against MPTP-mediated neurodegeneration. FASEB J 18: 1102–1104, 2004 [DOI] [PubMed] [Google Scholar]
- 93. Kenchappa RS and Ravindranath V. Glutaredoxin is essential for maintenance of brain mitochondrial complex I: studies with MPTP. FASEB J 17: 717–719, 2003 [DOI] [PubMed] [Google Scholar]
- 94. Kendall RL and Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A 90: 10705–10709, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kim H-J, Ha S, Lee HY, and Lee K-J. ROSics: chemistry and proteomics of cysteine modifications in redox biology. Mass Spectrom Rev 34: 184–208, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Kim J, Kim J, Kook H, and Park WJ. PICOT alleviates myocardial ischemia-reperfusion injury by reducing intracellular levels of reactive oxygen species. Biochem Biophys Res Commun 485: 807–813, 2017 [DOI] [PubMed] [Google Scholar]
- 97. Kim S, Na S, Sim JW, Park H, Jeong J, Kim H, Seo Y, Seo J, Lee K-J, and Paek E. MODi: a powerful and convenient web server for identifying multiple post-translational peptide modifications from tandem mass spectra. Nucleic Acids Res 34: W258–W263, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Kim SE, Zhang L, Ma K, Riegman M, Chen F, Ingold I, Conrad M, Turker MZ, Gao M, Jiang X, Monette S, Pauliah M, Gonen M, Zanzonico P, Quinn T, Wiesner U, Bradbury MS, and Overholtzer M. Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat Nanotechnol 11: 977–985, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kisseleva T, Song L, Vorontchikhina M, Feirt N, Kitajewski J, and Schindler C. NF-kappaB regulation of endothelial cell function during LPS-induced toxemia and cancer. J Clin Invest 116: 2955–2963, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Klatt P and Lamas S. Regulation of protein function by S-glutathiolation in response to oxidative and nitrosative stress. Eur J Biochem 267: 4928–4944, 2000 [DOI] [PubMed] [Google Scholar]
- 101. Klintrot IM, Höög JO, Jörnvall H, Holmgren A, and Luthman M. The primary structure of calf thymus glutaredoxin. Homology with the corresponding Escherichia coli protein but elongation at both ends and with an additional half-cystine/cysteine pair. Eur J Biochem 144: 417–423, 1984 [DOI] [PubMed] [Google Scholar]
- 102. Kubota C, Torii S, Hou N, Saito N, Yoshimoto Y, Imai H, and Takeuchi T. Constitutive reactive oxygen species generation from autophagosome/lysosome in neuronal oxidative toxicity. J Biol Chem 285: 667–674, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Kumar V, Calamaras TD, Haeussler D, Colucci WS, Cohen RA, McComb ME, Pimentel D, and Bachschmid MM. Cardiovascular redox and ox stress proteomics. Antioxid Redox Signal 17: 1528–1559, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kyte J. Structure in Protein Chemistry. 2nd ed. New York: Garland Science, 2007 [Google Scholar]
- 105. Laborde E. Glutathione transferases as mediators of signaling pathways involved in cell proliferation and cell death. Cell Death Differ 17: 1373–1380, 2010 [DOI] [PubMed] [Google Scholar]
- 106. Lanahan AA, Lech D, Dubrac A, Zhang J, Zhuang ZW, Eichmann A, and Simons M. PTP1b is a physiologic regulator of vascular endothelial growth factor signaling in endothelial cells. Circulation 130: 902–909, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Landmesser U, Cai H, Dikalov S, McCann L, Hwang J, Jo H, Holland SM, and Harrison DG. Role of p47(phox) in vascular oxidative stress and hypertension caused by angiotensin II. Hypertension 40: 511–515, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Leonard JL, Leonard DM, Shen Q, Farwell AP, and Newburger PE. Selenium-regulated translation control of heterologous gene expression: normal function of selenocysteine-substituted gene products. J Cell Biochem 61: 410–419, 1996 [DOI] [PubMed] [Google Scholar]
- 109. Li B, Chen M, Lu M, Xin-Xiang J, Meng-Xiong P, and Jun-Wu M. Glutaredoxin 3 promotes migration and invasion via the Notch signalling pathway in oral squamous cell carcinoma. Free Radic Res 52: 390–401, 2018 [DOI] [PubMed] [Google Scholar]
- 110. Li F, Sonveaux P, Rabbani ZN, Liu S, Yan B, Huang Q, Vujaskovic Z, Dewhirst MW, and Li C-Y. Regulation of HIF-1alpha stability through S-nitrosylation. Mol Cell 26: 63–74, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lieberman MW, Wiseman AL, Shi ZZ, Carter BZ, Barrios R, Ou CN, Chévez-Barrios P, Wang Y, Habib GM, Goodman JC, Huang SL, Lebovitz RM, and Matzuk MM. Growth retardation and cysteine deficiency in gamma-glutamyl transpeptidase-deficient mice. Proc Natl Acad Sci U S A 93: 7923–7926, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lin MI, Fulton D, Babbitt R, Fleming I, Busse R, Pritchard KA, and Sessa WC. Phosphorylation of threonine 497 in endothelial nitric-oxide synthase coordinates the coupling of L-arginine metabolism to efficient nitric oxide production. J Biol Chem 278: 44719–44726, 2003 [DOI] [PubMed] [Google Scholar]
- 113. Lu Y, Zhao X, Li K, Luo G, Nie Y, Shi Y, Zhou Y, Ren G, Feng B, Liu Z, Pan Y, Li T, Guo X, Wu K, Miranda-Vizuete A, Wang X, and Fan D. Thioredoxin-like protein 2 is overexpressed in colon cancer and promotes cancer cell metastasis by interaction with ran. Antioxid Redox Signal 19: 899–911, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Lundberg M, Johansson C, Chandra J, Enoksson M, Jacobsson G, Ljung J, Johansson M, and Holmgren A. Cloning and expression of a novel human glutaredoxin (Grx2) with mitochondrial and nuclear isoforms. J Biol Chem 276: 26269–26275, 2001 [DOI] [PubMed] [Google Scholar]
- 115. Mailloux RJ, Xuan JY, McBride S, Maharsy W, Thorn S, Holterman CE, Kennedy CRJ, Rippstein P, deKemp R, da Silva J, Nemer M, Lou M, and Harper M-E. Glutaredoxin-2 is required to control oxidative phosphorylation in cardiac muscle by mediating deglutathionylation reactions. J Biol Chem 289: 14812–14828, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Manevich Y, Feinstein SI, and Fisher AB. Activation of the antioxidant enzyme 1-CYS peroxiredoxin requires glutathionylation mediated by heterodimerization with pi GST. Proc Natl Acad Sci U S A 101: 3780–3785, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Mannervik B and Axelsson K. Role of cytoplasmic thioltransferase in cellular regulation by thiol-disulphide interchange. Biochem J 190: 125–130, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Marino SM and Gladyshev VN. Cysteine function governs its conservation and degeneration and restricts its utilization on protein surfaces. J Mol Biol 404: 902–916, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Matsui M, Oshima M, Oshima H, Takaku K, Maruyama T, Yodoi J, and Taketo MM. Early embryonic lethality caused by targeted disruption of the mouse thioredoxin gene. Dev Biol 178: 179–185, 1996 [DOI] [PubMed] [Google Scholar]
- 120. Matsui R, Watanabe Y, and Murdoch CE. Redox regulation of ischemic limb neovascularization—what we have learned from animal studies. Redox Biol 12: 1011–1019, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Matsui R, Xu S, Maitland KA, Hayes A, Leopold JA, Handy DE, Loscalzo J, and Cohen RA. Glucose-6 phosphate dehydrogenase deficiency decreases the vascular response to angiotensin II. Circulation 112: 257–263, 2005 [DOI] [PubMed] [Google Scholar]
- 122. Matsui R, Xu S, Maitland KA, Mastroianni R, Leopold JA, Handy DE, Loscalzo J, and Cohen RA. Glucose-6-phosphate dehydrogenase deficiency decreases vascular superoxide and atherosclerotic lesions in apolipoprotein E(−/−) mice. Arterioscler Thromb Vasc Biol 26: 910–916, 2006 [DOI] [PubMed] [Google Scholar]
- 123. Mayr JA, Feichtinger RG, Tort F, Ribes A, and Sperl W. Lipoic acid biosynthesis defects. J Inherit Metab Dis 37: 553–563, 2014 [DOI] [PubMed] [Google Scholar]
- 124. McKelvey TG, Höllwarth ME, Granger DN, Engerson TD, Landler U, and Jones HP. Mechanisms of conversion of xanthine dehydrogenase to xanthine oxidase in ischemic rat liver and kidney. Am J Physiol 254: G753–G760, 1988 [DOI] [PubMed] [Google Scholar]
- 125. McMillan DH, van der Velden JL, Lahue KG, Qian X, Schneider RW, Iberg MS, Nolin JD, Abdalla S, Casey DT, Tew KD, Townsend DM, Henderson CJ, Wolf CR, Butnor KJ, Taatjes DJ, Budd RC, Irvin CG, van der Vliet A, Flemer S, Anathy V, and Janssen-Heininger YM.. Attenuation of lung fibrosis in mice with a clinically relevant inhibitor of glutathione-S-transferase π. JCI Insight 1, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Mei Y, Thompson MD, Shiraishi Y, Cohen RA, and Tong X. Sarcoplasmic/endoplasmic reticulum Ca2+ ATPase C674 promotes ischemia- and hypoxia-induced angiogenesis via coordinated endothelial cell and macrophage function. J Mol Cell Cardiol 76: 275–282, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Mieyal JJ, Gallogly MM, Qanungo S, Sabens EA, and Shelton MD. Molecular mechanisms and clinical implications of reversible protein S-glutathionylation. Antioxid Redox Signal 10: 1941–1988, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Moreland JL, Gramada A, Buzko OV, Zhang Q, and Bourne PE. The Molecular Biology Toolkit (MBT): a modular platform for developing molecular visualization applications. BMC Bioinformatics 6: 21, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Murdoch CE, Bachschmid MM, and Matsui R. Regulation of neovascularization by S-glutathionylation via the Wnt5a/sFlt-1 pathway. Biochem Soc Trans 42: 1665–1670, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Murdoch CE, Shuler M, Haeussler DJF, Kikuchi R, Bearelly P, Han J, Watanabe Y, Fuster JJ, Walsh K, Ho Y-S, Bachschmid MM, Cohen RA, and Matsui R. Glutaredoxin-1 up-regulation induces soluble vascular endothelial growth factor receptor 1, attenuating post-ischemia limb revascularization. J Biol Chem 289: 8633–8644, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Murray CI, Uhrigshardt H, O'Meally RN, Cole RN, and Van Eyk JE. Identification and quantification of S-nitrosylation by cysteine reactive tandem mass tag switch assay. Mol Cell Proteomics 11: M111.013441, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Murray CI and Van Eyk JE. A twist on quantification: measuring the site occupancy of S-nitrosylation. Circ Res 111: 1253–1255, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Na S, Bandeira N, and Paek E. Fast multi-blind modification search through tandem mass spectrometry. Mol Cell Proteomics 11: M111.010199, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Nemeria NS, Gerfen G, Guevara E, Nareddy PR, Szostak M, and Jordan F. The human Krebs cycle 2-oxoglutarate dehydrogenase complex creates an additional source of superoxide/hydrogen peroxide from 2-oxoadipate as alternative substrate. Free Radic Biol Med 108: 644–654, 2017 [DOI] [PubMed] [Google Scholar]
- 135. Nishida M, Sawa T, Kitajima N, Ono K, Inoue H, Ihara H, Motohashi H, Yamamoto M, Suematsu M, Kurose H, van der Vliet A, Freeman BA, Shibata T, Uchida K, Kumagai Y, and Akaike T. Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration. Nat Chem Biol 8: 714–724, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Oh JG, Jeong D, Cha H, Kim JM, Lifirsu E, Kim J, Yang DK, Park CS, Kho C, Park S, Yoo YJ, Kim DH, Kim J, Hajjar RJ, and Park WJ. PICOT increases cardiac contractility by inhibiting PKCζ activity. J Mol Cell Cardiol 53: 53–63, 2012 [DOI] [PubMed] [Google Scholar]
- 137. Oh JY, Giles N, Landar A, and Darley-Usmar V. Accumulation of 15-deoxy-delta(12,14)-prostaglandin J2 adduct formation with Keap1 over time: effects on potency for intracellular antioxidant defence induction. Biochem J 411: 297–306, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Ohh M, Park CW, Ivan M, Hoffman MA, Kim TY, Huang LE, Pavletich N, Chau V, and Kaelin WG. Ubiquitination of hypoxia-inducible factor requires direct binding to the beta-domain of the von Hippel-Lindau protein. Nat Cell Biol 2: 423–427, 2000 [DOI] [PubMed] [Google Scholar]
- 139. Ou J, Ou Z, Ackerman AW, Oldham KT, and Pritchard KA. Inhibition of heat shock protein 90 (hsp90) in proliferating endothelial cells uncouples endothelial nitric oxide synthase activity. Free Radic Biol Med 34: 269–276, 2003 [DOI] [PubMed] [Google Scholar]
- 140. Pan Y, Mansfield KD, Bertozzi CC, Rudenko V, Chan DA, Giaccia AJ, and Simon MC. Multiple factors affecting cellular redox status and energy metabolism modulate hypoxia-inducible factor prolyl hydroxylase activity in vivo and in vitro. Mol Cell Biol 27: 912–925, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Pandya P, Braiman A, and Isakov N. PICOT (GLRX3) is a positive regulator of stress-induced DNA-damage response. Cell Signal 62: 109340, 2019 [DOI] [PubMed] [Google Scholar]
- 142. Park S-Y, Shi X, Pang J, Yan C, and Berk BC. Thioredoxin-interacting protein mediates sustained VEGFR2 signaling in endothelial cells required for angiogenesis. Arterioscler Thromb Vasc Biol 33: 737–743, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Perevoshchikova IV, Quinlan CL, Orr AL, Gerencser AA, and Brand MD. Sites of superoxide and hydrogen peroxide production during fatty acid oxidation in rat skeletal muscle mitochondria. Free Radic Biol Med 61: 298–309, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Peskin AV, Pace PE, Behring JB, Paton LN, Soethoudt M, Bachschmid MM, and Winterbourn CC. Glutathionylation of the active site cysteines of peroxiredoxin 2 and recycling by glutaredoxin. J Biol Chem 291: 3053–3062, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Pham K, Pal R, Qu Y, Liu X, Yu H, Shiao SL, Wang X, O'Brian Smith E, Cui X, Rodney GG, and Cheng N. Nuclear glutaredoxin 3 is critical for protection against oxidative stress-induced cell death. Free Radic Biol Med 85: 197–206, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Pimentel D, Haeussler DJ, Matsui R, Burgoyne JR, Cohen RA, and Bachschmid MM. Regulation of cell physiology and pathology by protein S-glutathionylation: lessons learned from the cardiovascular system. Antioxid Redox Signal 16: 524–542, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Pineda-Molina E, Klatt P, Vázquez J, Marina A, García de Lacoba M, Pérez-Sala D, and Lamas S. Glutathionylation of the p50 subunit of NF-kappaB: a mechanism for redox-induced inhibition of DNA binding. Biochemistry 40: 14134–14142, 2001 [DOI] [PubMed] [Google Scholar]
- 148. Pirie NW and Godwin Pinhey K. The titration curve of glutathione. J Biol Chem 84: 321–333, 1929 [Google Scholar]
- 149. Poole LB. The basics of thiols and cysteines in redox biology and chemistry. Free Radic Biol Med 80: 148–157, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Prigge JR, Coppo L, Martin SS, Ogata F, Miller CG, Bruschwein MD, Orlicky DJ, Shearn CT, Kundert JA, Lytchier J, Herr AE, Mattsson Å, Taylor MP, Gustafsson TN, Arnér ESJ, Holmgren A, and Schmidt EE. Hepatocyte hyperproliferation upon liver-specific co-disruption of thioredoxin-1, thioredoxin reductase-1, and glutathione reductase. Cell Rep 19: 2771–2781, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Qanungo S, Starke DW, Pai HV, Mieyal JJ, and Nieminen A-L. Glutathione supplementation potentiates hypoxic apoptosis by S-glutathionylation of p65-NFkappaB. J Biol Chem 282: 18427–18436, 2007 [DOI] [PubMed] [Google Scholar]
- 152. Rabbani N and Thornalley PJ. The critical role of methylglyoxal and glyoxalase 1 in diabetic nephropathy. Diabetes 63: 50–52, 2014 [DOI] [PubMed] [Google Scholar]
- 153. Rahaman MM, Sawa T, Ahtesham AK, Khan S, Inoue H, Irie A, Fujii S, and Akaike T. S-guanylation proteomics for redox-based mitochondrial signaling. Antioxid Redox Signal 20: 295–307, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Rajagopalan S, Mohler ER, Lederman RJ, Mendelsohn FO, Saucedo JF, Goldman CK, Blebea J, Macko J, Kessler PD, Rasmussen HS, and Annex BH. Regional angiogenesis with vascular endothelial growth factor in peripheral arterial disease: a phase II randomized, double-blind, controlled study of adenoviral delivery of vascular endothelial growth factor 121 in patients with disabling intermittent claudication. Circulation 108: 1933–1938, 2003 [DOI] [PubMed] [Google Scholar]
- 155. Reynaert NL, van der Vliet A, Guala AS, McGovern T, Hristova M, Pantano C, Heintz NH, Heim J, Ho Y-S, Matthews DE, Wouters EFM, and Janssen-Heininger YMW. Dynamic redox control of NF-kappaB through glutaredoxin-regulated S-glutathionylation of inhibitory kappaB kinase beta. Proc Natl Acad Sci U S A 103: 13086–13091, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Rose GD, Geselowitz AR, Lesser GJ, Lee RH, and Zehfus MH. Hydrophobicity of amino acid residues in globular proteins. Science 229: 834–838, 1985 [DOI] [PubMed] [Google Scholar]
- 157. Rossi R, Giustarini D, Milzani A, and Dalle-Donne I. Cysteinylation and homocysteinylation of plasma protein thiols during ageing of healthy human beings. J Cell Mol Med 13: 3131–3140, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Salmeen A, Andersen JN, Myers MP, Meng T-C, Hinks JA, Tonks NK, and Barford D. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature 423: 769–773, 2003 [DOI] [PubMed] [Google Scholar]
- 159. Schafer FQ and Buettner GR. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic Biol Med 30: 1191–1212, 2001 [DOI] [PubMed] [Google Scholar]
- 160. Schmohl L, Wagner FR, Schümann M, Krause E, and Schwarzer D. Semisynthesis and initial characterization of sortase A mutants containing selenocysteine and homocysteine. Bioorg Med Chem 23: 2883–2889, 2015 [DOI] [PubMed] [Google Scholar]
- 161. Semenza GL. Hypoxia-inducible factor 1 and cardiovascular disease. Annu Rev Physiol 76: 39–56, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Shao D, Fry JL, Han J, Hou X, Pimentel DR, Matsui R, Cohen RA, and Bachschmid MM. A redox-resistant sirtuin-1 mutant protects against hepatic metabolic and oxidant stress. J Biol Chem 289: 7293–7306, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Shao D, Han J, Hou X, Fry J, Behring JB, Seta F, Long MT, Roy HK, Cohen RA, Matsui R, and Bachschmid MM. Glutaredoxin-1 deficiency causes fatty liver and dyslipidemia by inhibiting sirtuin-1. Antioxid Redox Signal 27: 313–327, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Silvestre J-S, Tamarat R, Ebrahimian TG, Le-Roux A, Clergue M, Emmanuel F, Duriez M, Schwartz B, Branellec D, and Lévy BI. Vascular endothelial growth factor-B promotes in vivo angiogenesis. Circ Res 93: 114–123, 2003 [DOI] [PubMed] [Google Scholar]
- 165. Söderdahl T, Enoksson M, Lundberg M, Holmgren A, Ottersen OP, Orrenius S, Bolcsfoldi G, and Cotgreave IA. Visualization of the compartmentalization of glutathione and protein-glutathione mixed disulfides in cultured cells. FASEB J 17: 124–126, 2003 [DOI] [PubMed] [Google Scholar]
- 166. Stanchi F, Bertocco E, Toppo S, Dioguardi R, Simionati B, Cannata N, Zimbello R, Lanfranchi G, and Valle G. Characterization of 16 novel human genes showing high similarity to yeast sequences. Yeast 18: 69–80, 2001 [DOI] [PubMed] [Google Scholar]
- 167. Starke DW, Chock PB, and Mieyal JJ. Glutathione-thiyl radical scavenging and transferase properties of human glutaredoxin (thioltransferase). Potential role in redox signal transduction. J Biol Chem 278: 14607–14613, 2003 [DOI] [PubMed] [Google Scholar]
- 168. Stipanuk MH, Dominy JE, Lee J-I, and Coloso RM. Mammalian cysteine metabolism: new insights into regulation of cysteine metabolism. J Nutr 136: 1652S–1659S, 2006 [DOI] [PubMed] [Google Scholar]
- 169. Stratmann B, Engelbrecht B, Espelage BC, Klusmeier N, Tiemann J, Gawlowski T, Mattern Y, Eisenacher M, Meyer HE, Rabbani N, Thornalley PJ, Tschoepe D, Poschmann G, and Stühler K. Glyoxalase 1-knockdown in human aortic endothelial cells—effect on the proteome and endothelial function estimates. Sci Rep 6: 37737, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Su D, Gaffrey MJ, Guo J, Hatchell KE, Chu RK, Clauss TRW, Aldrich JT, Wu S, Purvine S, Camp DG, Smith RD, Thrall BD, and Qian W-J. Proteomic identification and quantification of S-glutathionylation in mouse macrophages using resin-assisted enrichment and isobaric labeling. Free Radic Biol Med 67: 460–470, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Sullivan DM, Levine RL, and Finkel T. Detection and affinity purification of oxidant-sensitive proteins using biotinylated glutathione ethyl ester. Methods Enzymol 353: 101–113, 2002 [DOI] [PubMed] [Google Scholar]
- 172. Suzuki Y and Lehrer RI. NAD(P)H oxidase activity in human neutrophils stimulated by phorbol myristate acetate. J Clin Invest 66: 1409–1418, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Thompson MD, Mei Y, Weisbrod RM, Silver M, Shukla PC, Bolotina VM, Cohen RA, and Tong X. Glutathione adducts on sarcoplasmic/endoplasmic reticulum Ca2+ ATPase Cys-674 regulate endothelial cell calcium stores and angiogenic function as well as promote ischemic blood flow recovery. J Biol Chem 289: 19907–19916, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Thornalley PJ. The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem J 269: 1–11, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Thornalley PJ. Dicarbonyl intermediates in the maillard reaction. Ann N Y Acad Sci 1043: 111–117, 2005 [DOI] [PubMed] [Google Scholar]
- 176. Tirziu D, Jaba IM, Yu P, Larrivée B, Coon BG, Cristofaro B, Zhuang ZW, Lanahan AA, Schwartz MA, Eichmann A, and Simons M. Endothelial nuclear factor-κB-dependent regulation of arteriogenesis and branching. Circulation 126: 2589–2600, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Townsend DM, Manevich Y, He L, Hutchens S, Pazoles CJ, and Tew KD. Novel role for glutathione S-transferase pi. Regulator of protein S-Glutathionylation following oxidative and nitrosative stress. J Biol Chem 284: 436–445, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Ullevig S, Zhao Q, Lee CF, Seok Kim H, Zamora D, and Asmis R. NADPH oxidase 4 mediates monocyte priming and accelerated chemotaxis induced by metabolic stress. Arterioscler Thromb Vasc Biol 32: 415–426, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Wang J, Pareja KA, Kaiser CA, and Sevier CS. Redox signaling via the molecular chaperone BiP protects cells against endoplasmic reticulum-derived oxidative stress. eLife 3: e03496, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Watanabe Y, Murdoch CE, Sano S, Ido Y, Bachschmid MM, Cohen RA, and Matsui R. Glutathione adducts induced by ischemia and deletion of glutaredoxin-1 stabilize HIF-1α and improve limb revascularization. Proc Natl Acad Sci U S A 113: 6011–6016, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Wenzel P, Kossmann S, Münzel T, and Daiber A. Redox regulation of cardiovascular inflammation—immunomodulatory function of mitochondrial and Nox-derived reactive oxygen and nitrogen species. Free Radic Biol Med 109: 48–60, 2017 [DOI] [PubMed] [Google Scholar]
- 182. Wetzelberger K, Baba SP, Thirunavukkarasu M, Ho Y-S, Maulik N, Barski OA, Conklin DJ, and Bhatnagar A. Postischemic deactivation of cardiac aldose reductase: role of glutathione S-transferase P and glutaredoxin in regeneration of reduced thiols from sulfenic acids. J Biol Chem 285: 26135–26148, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Wingert RA, Galloway JL, Barut B, Foott H, Fraenkel P, Axe JL, Weber GJ, Dooley K, Davidson AJ, Schmid B, Schmidt B, Paw BH, Shaw GC, Kingsley P, Palis J, Schubert H, Chen O, Kaplan J, and Zon LI; Tübingen 2000 Screen Consortium. Deficiency of glutaredoxin 5 reveals Fe-S clusters are required for vertebrate haem synthesis. Nature 436: 1035–1039, 2005 [DOI] [PubMed] [Google Scholar]
- 184. Witte S, Villalba M, Bi K, Liu Y, Isakov N, and Altman A. Inhibition of the c-Jun N-terminal kinase/AP-1 and NF-kappaB pathways by PICOT, a novel protein kinase C-interacting protein with a thioredoxin homology domain. J Biol Chem 275: 1902–1909, 2000 [DOI] [PubMed] [Google Scholar]
- 185. Wolhuter K and Eaton P. How widespread is stable protein S-nitrosylation as an end-effector of protein regulation? Free Radic Biol Med 109: 156–166, 2017 [DOI] [PubMed] [Google Scholar]
- 186. Worthington DJ and Rosemeyer MA. Glutathione reductase from human erythrocytes. Catalytic properties and aggregation. Eur J Biochem 67: 231–238, 1976 [DOI] [PubMed] [Google Scholar]
- 187. Writing Committee Members, Gerhard-Herman MD, Gornik HL, Barrett C, Barshes NR, Corriere MA, Drachman DE, Fleisher LA, Fowkes FGR, Hamburg NM, Kinlay S, Lookstein R, Misra S, Mureebe L, Olin JW, Patel RAG, Regensteiner JG, Schanzer A, Shishehbor MH, Stewart KJ, Treat-Jacobson D, and Walsh ME; ACC/AHA Task Force Members, Halperin JL, Levine GN, Al-Khatib SM, Birtcher KK, Bozkurt B, Brindis RG, Cigarroa JE, Curtis LH, Fleisher LA, Gentile F, Gidding S, Hlatky MA, Ikonomidis J, Joglar J, Pressler SJ, and Wijeysundera DN. 2016 AHA/ACC guideline on the management of patients with lower extremity peripheral artery disease: executive summary. Vasc Med 22: NP1–NP43, 2017 [DOI] [PubMed] [Google Scholar]
- 188. Wu H, Ma B-G, Zhao J-T, and Zhang H-Y. How similar are amino acid mutations in human genetic diseases and evolution. Biochem Biophys Res Commun 362: 233–237, 2007 [DOI] [PubMed] [Google Scholar]
- 189. Wu R, Haas W, Dephoure N, Huttlin EL, Zhai B, Sowa ME, and Gygi SP. A large-scale method to measure absolute protein phosphorylation stoichiometries. Nat Methods 8: 677–683, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Xia N, Horke S, Habermeier A, Closs EI, Reifenberg G, Gericke A, Mikhed Y, Münzel T, Daiber A, Förstermann U, and Li H. Uncoupling of endothelial nitric oxide synthase in perivascular adipose tissue of diet-induced obese mice. Arterioscler Thromb Vasc Biol 36: 78–85, 2016 [DOI] [PubMed] [Google Scholar]
- 191. Xia TH, Bushweller JH, Sodano P, Billeter M, Björnberg O, Holmgren A, and Wüthrich K. NMR structure of oxidized Escherichia coli glutaredoxin: comparison with reduced E. coli glutaredoxin and functionally related proteins. Protein Sci 1: 310–321, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Xu J, Xie Z, Reece R, Pimental D, and Zou M-H. Uncoupling of endothelial nitric oxidase synthase by hypochlorous acid: role of NAD(P)H oxidase-derived superoxide and peroxynitrite. Arterioscler Thromb Vasc Biol 26: 2688–2695, 2006 [DOI] [PubMed] [Google Scholar]
- 193. Xue M, Weickert MO, Qureshi S, Kandala N-B, Anwar A, Waldron M, Shafie A, Messenger D, Fowler M, Jenkins G, Rabbani N, and Thornalley PJ. Improved glycemic control and vascular function in overweight and obese subjects by glyoxalase 1 inducer formulation. Diabetes 65: 2282–2294, 2016 [DOI] [PubMed] [Google Scholar]
- 194. Yao C, Behring JB, Shao D, Sverdlov AL, Whelan SA, Elezaby A, Yin X, Siwik DA, Seta F, Costello CE, Cohen RA, Matsui R, Colucci WS, McComb ME, and Bachschmid MM. Overexpression of catalase diminishes oxidative cysteine modifications of cardiac proteins. PLoS One 10: e0144025, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Ye H, Jeong SY, Ghosh MC, Kovtunovych G, Silvestri L, Ortillo D, Uchida N, Tisdale J, Camaschella C, and Rouault TA. Glutaredoxin 5 deficiency causes sideroblastic anemia by specifically impairing heme biosynthesis and depleting cytosolic iron in human erythroblasts. J Clin Invest 120: 1749–1761, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Yin H, Hu M, Liang X, Ajmo JM, Li X, Bataller R, Odena G, Stevens SM, and You M. Deletion of SIRT1 from hepatocytes in mice disrupts lipin-1 signaling and aggravates alcoholic fatty liver. Gastroenterology 146: 801–811, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Yusuf MA, Chuang T, Bhat GJ, and Srivenugopal KS. Cys-141 glutathionylation of human p53: studies using specific polyclonal antibodies in cancer samples and cell lines. Free Radic Biol Med 49: 908–917, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Zamora DA, Downs KP, Ullevig SL, Tavakoli S, Kim HS, Qiao M, Greaves DR, and Asmis R. Glutaredoxin 2a overexpression in macrophages promotes mitochondrial dysfunction but has little or no effect on atherogenesis in LDL-receptor null mice. Atherosclerosis 241: 69–78, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A, Pajarinen JS, Nejadnik H, Goodman S, Moseley M, Coussens LM, and Daldrup-Link HE. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol 11: 986–994, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Zhang X, Cheng X, Yu L, Yang J, Calvo R, Patnaik S, Hu X, Gao Q, Yang M, Lawas M, Delling M, Marugan J, Ferrer M, and Xu H. MCOLN1 is a ROS sensor in lysosomes that regulates autophagy. Nat Commun 7: 12109, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]