Abstract
Octopamine (OCT) have an adverse effect on heart function. One of the positive effects of exercise training is improving cardiac function and cardiomyocytes signaling, which along with herbal supplements can have better effects on the heart tissue. Therefore, the aim of this study was to evaluate the effects of exercise training and OCT on changes of PGC1α and UCP1 expression in heart tissue of rat treated with deep frying oil (DFO). In this study, 45 male wistar rats were divided into 5 groups (n = 9 in each): I) control (Co), II) DFO, III) DFO + exercise, IV) DFO + OCT, and V) DFO + OCT + exercise. The quantification of apoptotic effects of DFO in heart tissue was assessed by TUNEL assay. Masson's trichrome stain applied to study cardiomyocytic fibers. Moreover, PGC1α and UCP1 genes and proteins expression in all groups were investigated using quantitative real-time PCR and immunohistochemical method. A significant increase in apoptotic cells was observed in the DFO-treated group (p < 0.05). In Masson's Trichrome stain study, more cardiomyocytic fibers were observed and some lymphocytic cells were present in some fibers. Also, the expression of PGC1α and UCP1 was significantly increase in DFO + exercise group, DFO + OCT group, and DFO + OCT + exercise group compare to DFO group (p < 0.05). Based on these findings, exercise and octopamine can be considered as factors affecting the expression of PGC1α genes and UCP1 as well as drug poisoning.
Keywords: Deep frying oil, PGC1α, UCP1, Exercise training, Heart tissue
Abbreviations: DFO, deep frying oil; OCT, octopamine; PGC-1a, Peroxisome proliferator-activated receptor gamma co-activator 1 alpha; UCP1, uncoupling protein 1
Highlights
-
•
Exercise training with octopamine can be upregulate expression of PGC1α.
-
•
Exercise training with octopamine can be upregulate expression of UCP1.
-
•
Increase the PGC1α and UCP1 can improve the mitochondria disfunction induced by DFO.
1. Introduction
Cardiovascular disease is a leading cause of death worldwide [1]. In recent years it has showed that loss of myocardial cells may be a major pathogenic factor. Cell death can occur in a destructive, uncontrolled manner via necrosis or by a highly regulated programmed cell suicide mechanism termed apoptosis [2]. There is convincing evidence that apoptosis contributes to the progression of heart failure [3,4]. Human heart failure is preceded by a process termed cardiac remodeling in which heart chambers progressively enlarge and contractile function deteriorates. Apoptosis) of cardiac muscle cells has been identified as an essential process in the progression of heart failure [5]. Recently, apoptosis in myocytes has been demonstrated experimentally after injury due to ischemia and reperfusion, myocardial infarction, cardiac aging, ventricular pacing, and coronary embolization [6]. Physiological mechanisms of cell death are used by multicellular organisms for development and morphogenesis, to control cell number, and as a defensive strategy to remove infected, mutated, or damaged cells [7]. Cell death can be classified according to its morphological appearance (which may be apoptotic, necrotic, and autophagic), enzymological criteria, functional aspects or immunological characteristics [8]. Apoptosis is a mode of cell death that is used by multicellular organisms to dispose of unwanted cells [9]. Apoptosis is a complicated process in cardiac cell distraction. The decision to cell death cannot be taken quickly, and the activity of many genes influence a cell's likelihood of activating its self-destruction schedule [10]. Autophagy is a lysosomal degradation pathway that is essential for survival, differentiation, development, and homeostasis. Autophagy principally serves an adaptive role to protect organisms against diverse pathologies, including infections, cancer, neurodegeneration, aging, and heart disease [11]. Since autophagy mediates cell death under specific circumstances, thus, damaged cardiomyocytes that show characteristics of autophagy have been observed during heart failure [12]. Autophagy occurs constitutively in the normal myocardium, and disruption of this pathway results in the development of left ventricular dilation and severe contractile dysfunction in the absence of any stress [13]. Iron is an essential element for body homeostasis, but there is no effective mechanism for elimination of an excess of this mineral. Deep frying oil or DFO is currently the treatment of choice for iron overload states from both acute iron intoxication and transfusion-dependent anemias. Octopamine is a norepinephrine analog biosynthesized from tyrosine and a sympathomimetic drug [14]. It can be found in many plant products and seafood. Octopamine stimulates lipolysis in adipocytes [15]. The roles of Octopamine in the control of diverse behaviours, such as learning, memory [16] or aggression [17], have been characterised in great detail. Peroxisome proliferator-activated receptor-γ coactivator (PGC)-1α is a member of a family of transcription coactivators that plays a central role in the regulation of cellular energy metabolism. It is strongly induced by cold exposure, linking this environmental stimulus to adaptive thermogenesis [18]. Abnormal expression of PGC1α is associated with several chronic diseases and, in recent years, it has been shown to be a critical controller of cancer development [19]. Endurance exercise has been shown to activate the PGC-1α gene in human skeletal muscle. Exercise-induced PGC-1α in skeletal muscle increases autophagy [20]. Recently, three distinct uncoupling protein isoforms, UCP-1, UCP-2, and UCP-3, have also been identified and implicated as mediators of thermogenesis. UCP-1 functions to uncouple oxidative metabolism from ATP synthesis, resulting in the generation of heat [21]. Therefore, the main objective of this study to find out if exercise and octopamine can effect on apoptotic and autophagy in heart tissue of DFO-treated and also can change the expression of the PGC1α and UCP1.
2. Materials and methods
2.1. Animals
In this experimental study, a total of 45 healthy adult male albino rats of the Wistar strain (Rattus norvegicus), 8 weeks old and weighing approximately between 180 and 220 g, were obtained from Pasteur Institute of Iran (Tehran). The animals were individually housed in plastic cages with sawdust bedding, where the relative humidity of 50% ± 10% and temperature of 23 ± 3 °C were controlled. Lighting period was maintained at 12 h light/12 h dark cycle. Rats had free access to normal diet and water, ad libitum throughout the course of study. All the procedures were approved and monitored by the ethics committee of laboratory animals of Tehran Azad University and the procedures followed the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978). In the current study, after 2 weeks of acclimatization, the animals were randomly divided into 5 groups (n = 9 in each groups): I) control (Co), II) DFO, III) DFO + exercise, IV) DFO + OCT, and V) DFO + OCT + exercise.
2.2. Chemicals
2.2.1. DFO
Deep frying oil was made by frying 5 kg catfish 3 times in 2.5 L cooking palm oil at 200 °C (measured with a cooking thermometer) for 15 min. After each frying the oil was left to cool for ±5 h at room temperature. The 3-times heated DFO preparation will subsequently be referred to as DFO-3. DFO was gavage 5 day per weeks (2 ml for each rat).
2.2.2. OCT
OCT was dissolved in distilled water and then diluted with Krebs-bicarbonate solution [22]. DFO was injected intraperitoneally 5 day per weeks [23].
2.3. Exercise protocol
Exercised rats were introduced to treadmill running for one week, during which each animal exercised on a motorized rodent treadmill at 9 m/min for 20 min per day (including 10 min at a prescribed speed, a 5-min warm-up, and 5-min cool-down). After the habituation period, rats were subjected to run at moderate exercise intensity for 5 days per week over 2 weeks. On the first day of exercise, the training began with the rats exercising at 11 m/min for 10 min per day. The speed gradually accelerated from 11 to 20 m/min over the duration of the experiment. The exercise time was also increased by 2 min per day over the same period until it reached 26 min/day at the end of the second week [24].
2.4. Apoptosis assessment by TUNEL assay
The quantification of apoptotic effects of DFO in heart tissue was assessed by TUNEL assay. Briefly, after exposure to VCM, cells were permeabilized with 70% ethanol overnight at −25 °C. The TUNEL assay was performed using the Dead End fluorometric system (Promega Corp., Madison, WI, USA) based on the manufacturer's instructions. The fluorescent cells were measured by using flow cytometer and analyzed with Cell Quest (software program) of the flow cytometer (Becton Dickinson, Frankin Lakes, NJ, USA).
2.5. Masson's trichrome staining
Masson's trichrome stain applied to differentiate necrotic myocardium (blue cytoplasm) from viable myocardium (red cytoplasm) usually with purple myocardial cytoplasm surrounding a necrotic area. Masson's Trichrome stain (Poly Scientific, Bay Shore, NY) were performed according to the manufacturer's instructions. Briefly, heart tissue slides were placed in staining jar and deparaffinized by submerging into three series of absolute xylene for 4 min. After that, the slides were washed with running tap water for 2 min. Then, slides were treated with the phosphomolybdic acid solution for another 10 min as a mordant and immediately slides were submerged into methyl blue (C.I. 42780, Merck, Germany). Next, slides were washed in running water for 2 min and lastly treated with 1% acetic acid solution for 1 min.
2.6. RNA and protein extraction
RNA and protein were extracted from the heart tissue using TRizol reagent (Invitrogen, USA) based on the manufacturer's instruction. The quality of total RNA was determined at a 260/280 nm wavelength ratio measured by a NanoDrop spectrometer (Thermo Scientific, Waltham, MA, USA).
2.7. Complementary DNA synthesis and real-time polymerase chain reaction
Complementary DNA (cDNA) was performed using a commercial kit (Fermentas, Lithuania), according to the manufacturer's instructions. Primers for PGC1α, UCP1, and GAPDH (as housekeeping gene) genes were designed by the National Center for Biotechnology Information (NCBI) website and gene runner software (Table 1). Real-time PCR reactions performed using an SYBR green master mix kit (TaKaRa, Kusatsu, Shiga Prefecture, Japan). Real-time PCR was quantified in triplicate using an ABI PRISM 7500 instrument (Applied Biosystems, USA) in a total volume of 10 μL. For real-time reactions, 5 μl of SYBR green, 0.5 μl of forward primer, 0.5 μl of reverse primer, 1 μl of cDNA and 3 μl of DEPC-treated water lifted and the master mix was built in the final volume of 10 μl. The temperature profile includes an initial denaturation at 95 °C for 10 min, followed by 40 cycles at 95 °C for 10 s, 60 °C for 20 s (annealing) and 72 °C for 25 s (extension). Also, 2−ΔΔCt method was applied to evaluate relative gene expression levels and GAPDH was considered as an internal control.
Table 1.
Sequence of primers of differente genes.
| ACCESSION Number | Reverse | Forward | Variants |
|---|---|---|---|
| NM_012682.2 | CTG ACC TTC ACC ACC TCT GT | GCC TCT ACG ATA CGG TCC AA | UCP1 |
| NM_031347.1 | TCA GCG GTC TTA GCA CCT A | TCT CTG TGG GTT TGG TGT GA | PGC1 |
| NM_017008.4 | AAG TTC AAC GGC ACA GTC AAG G | CAT ACT CAG CAC CAG CAT CAC C | GAP |
2.8. Immunohistochemical (IHC) analysis
The IHC assay for PGC1α and UCP1 protein was used. Briefly, the paraffin-embedded heart tissue blocks were cut to 3-mm sections that were then deparaffinized and heat treated for antigen retrieval. The antibody, A0485 (DAKO, Carpenteria, CA), was used as the primary antibody in an optimal dilution of 1:3500 determined in our laboratory. Biotinylated, streptavidin/biotin–labeled secondary antibody and related reagents were provided in the Level 2 USA Ultra Streptavidin Multi-Species Detection System (Signet Laboratories, Dedham, MA). All IHC assays were performed on an automated Tech Mate 1000 immunostainer (Ventana Medical Systems, Tucson, AZ).
2.9. Statistical analysis
Data are expressed as the mean ± standard error of the mean (S.E.M.) and were statistically analyzed by one-way analysis of variance, followed by Tukey-Kramer tests for multiple comparisons or by Student's t-test for comparison between two groups. The normality was evaluated by the Shapiro–Wilk test. Mann-Whitney test was used to compare the groups. Graph Pad Prism statistical software version 5.01 (Graph Pad, San Diego, CA, USA) was used for statistical analysis. For all tests, a P-value of <0.05 was considered statistically significant.
3. Results
3.1. Analysis of masson's trichrome stain and H&E
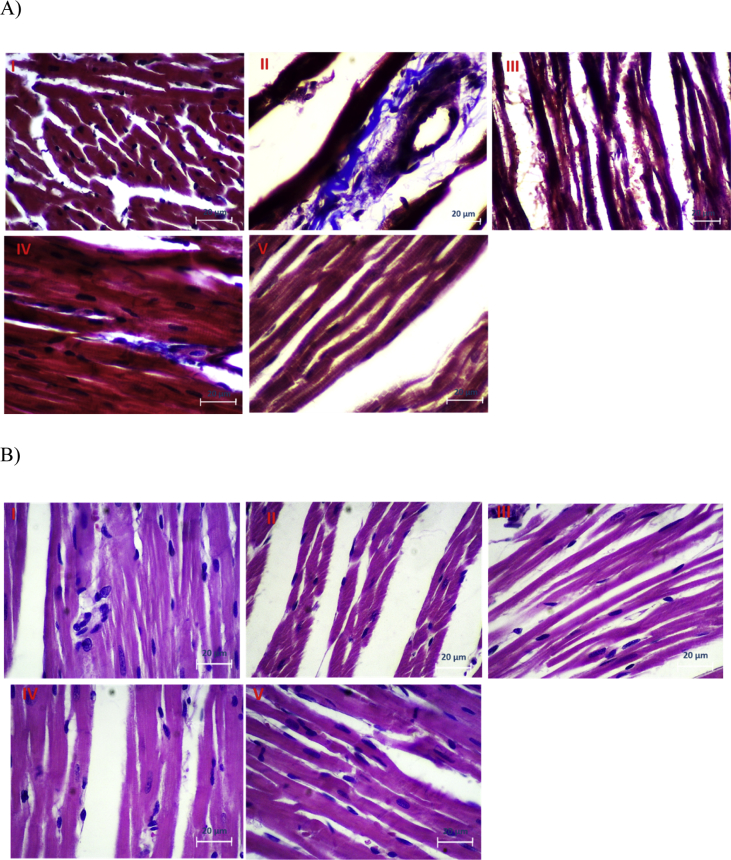
Based on the results obtained from masson's trichrome stain and H&E, the DFO group (II), have more degradation compare to other groups (Fig. 1). Also the diameter of muscle fibers in this group was significantly lower than that of other groups. However DFO + OCT + exercise group (V) had better structural and architectural integrity.
Fig. 1.
Morphology assessments by A) hematoxylin and eosin (H&E) stain, B) Massion's trichrome stain (MS) of cardiomyopathy rat hearts. In H&E stain slides, cell nuclei are stained with blue color, other intracellular or extracellular protein are stained with pink color. Normal cells are indicated by pink color in the MS assay. I) control (Co), II) DFO, III) DFO + exercise, IV) DFO + OCT, and V) DFO + OCT + exercise.
3.2. Analysis of apoptosis by TUNEL technique
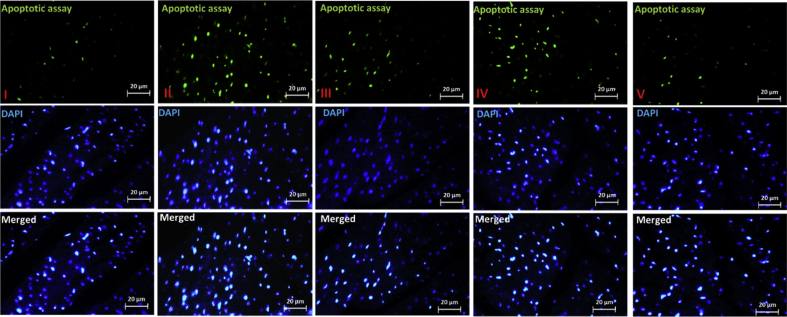
We use TUNEL technique to evaluate the apoptotic effect of DFO of heart tissue. A significant increase was seen in apoptotic cells of DFO-treated group, compared with the control group (Fig. 2). However, the DFO + OCT + exercise group significantly reduced the amount of apoptosis.
Fig. 2.
Analysis of DFO-induced apoptosis by TUNEL assay using flow cytometry. Apoptotic index was determined by analyzing five to seven randomly selected areas with 100–200 cells in each area. TUNEL assay should show a green dot if degraded DNA within a nucleus is detected, while DAPI staining will display a blue dot if a nucleus is detected. I) control (Co), II) DFO, III) DFO + exercise, IV) DFO + OCT, and V) DFO + OCT + exercise.
3.3. Gene's expression of PGC1α and UCP1
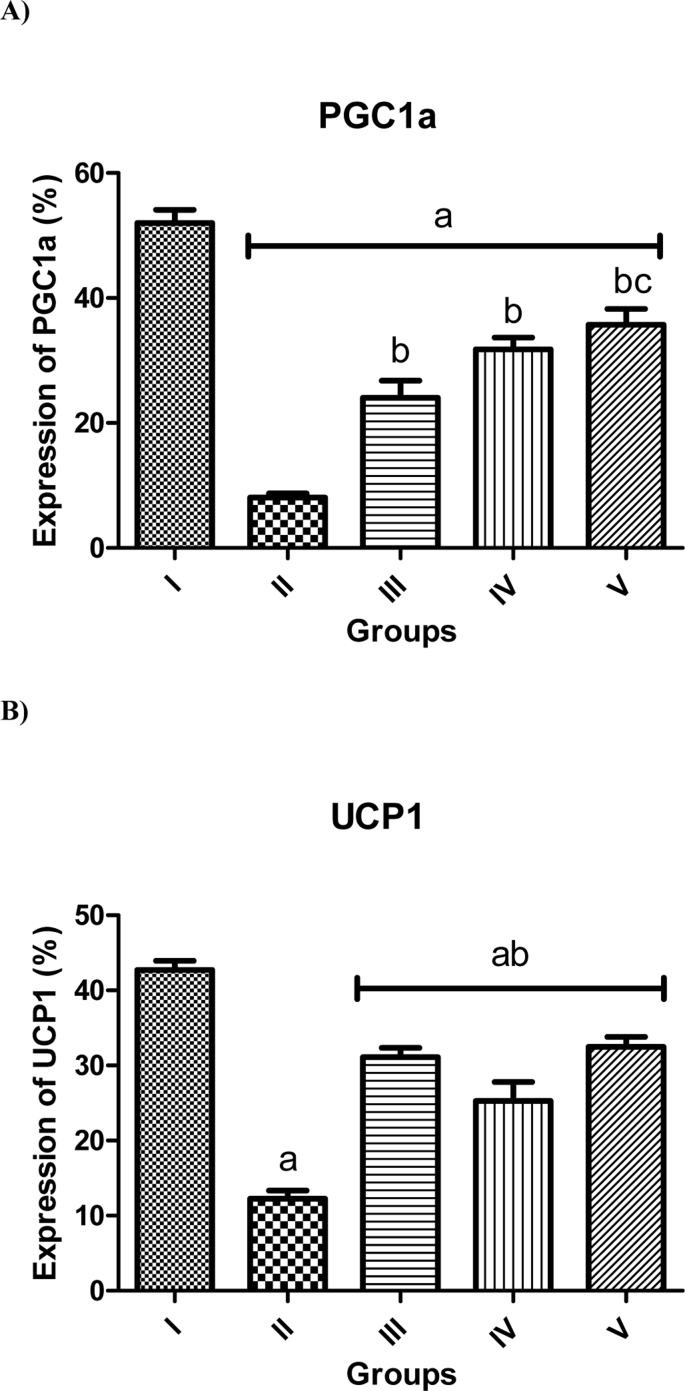
To determine the impact of DFO, OCT, and exercise (as mentioned in methods) on PGC1α and UCP1 expression in the groups studied, PGC1α and UCP1 expression was evaluated using real-time quantitative PCR. We observed various expressions of PGC1α and UCP1 in different groups studied, however, the expression of both PGC1α and UCP1 in all group was significantly lower than the control group (P < 0.05), that the largest decrease was in DFO group. In contrast, the relative expression of both genes studied in DFO + exercise, IV) DFO + OCT, and V) DFO + OCT + exercise groups were significantly increase in comparison with the DFO group (Fig. 3 A&B).
Fig. 3.
Average relative PGC1α expression in the group studied compared to control (A). Relative expression of UCP1 in the group studied compared to control (B). Data are expressed in S.E.M. a: significantly different with the control group (I) at p ˂ 0.05, b: significantly different with the DFO (II) at p ˂ 0.005, c: significantly different with the DFO + exercise (III) at p ˂ 0.05. I) control (Co), II) DFO, III) DFO + exercise, IV) DFO + OCT, and V) DFO + OCT + exercise.
3.4. Analysis of PGC1α and UCP1 proteins expression
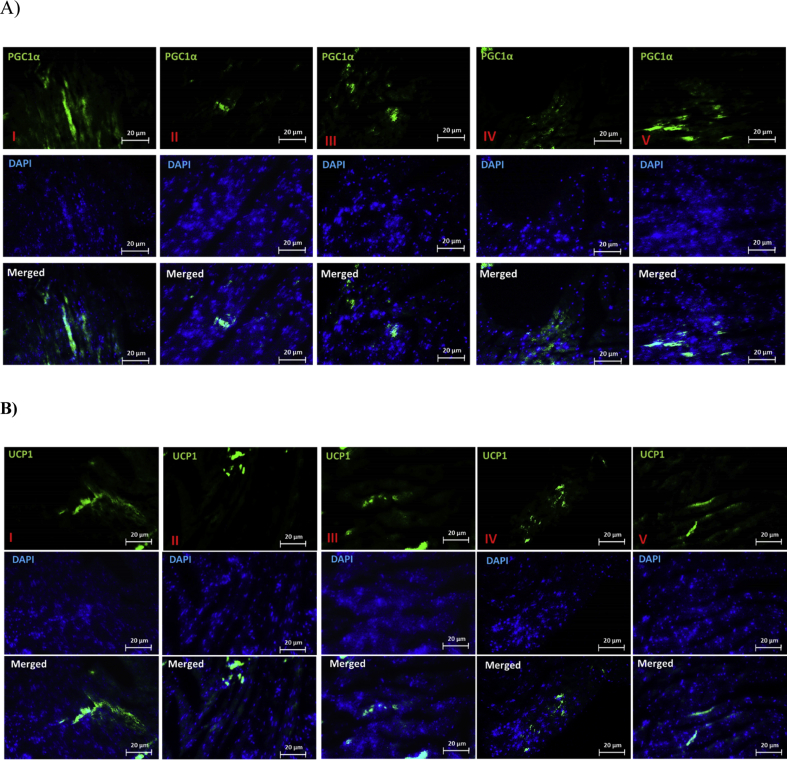
Level of PGC1α and UCP1 proteins expression were measured using IHC technique. According to the results of this study, in the group of DFO (II), the lowest expression of PGC1α and UCP1 proteins was observed. But, the highest expression of PGC1α and UCP1 protein were seen in the DFO + OCT + exercise group (V) (Fig. 4 A&B).
Fig. 4.
The level of PGC1α (A) and UCP1 (B) proteins expression by IHC technique. I) control (Co), II) DFO, III) DFO + exercise, IV) DFO + OCT, and V) DFO + OCT + exercise.
4. Discussion
The crosstalk between autophagy and apoptosis is intricate and sometimes contradictory; however, it is an important determinant of the overall fate of the cell [25]. Improvements in exercise capability following regular and effective exercise training should mainly be attributed to the overall functional enhancement of the motor and cardiopulmonary system [26,27]. Therefore, in the current study, we examined the synergic effect of exercise and octopamine on apoptotic and autophagy events in heart tissue of DFO treated male Wistar rat. Our findings showed a notable increase in apoptotic cells. Additionally, in the group I, more cardiomyocytic fibers were ruptured and some lymphocytic cells were present in some fibers. DFO has numerous clinical applications for patients presenting with iron overload in regards to the improvement in the quality of life and overall survival [28]. In a study, the effects of DFO on TNF-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in colon cancer cells were investigated. The experimental results showed that DFO treatment inhibited TRAIL-mediated cancer cell apoptosis by increasing Akt activation and decreasing caspase activation in human colon cancer cells. Furthermore, DFO treatment induced autophagy flux, and chloroquine, an autophagy inhibitor, blocked DFO-mediated inhibition of TRAIL-induced apoptosis [25]. It has proposed that high-dose DFO treatment resulted in a very pronounced reduction in wound healing and growth in breast cancer cells. High-dose DFO treatment reduces cell viability and growth and enhances apoptosis in cells [29]. Based on these results, it was hypothesized that exercise and octopamine may impact on apoptotic and autophagy events in heart tissue of DFO treated and that this may contribute to the recovery of cardiac function. On the other hand, to explore the effect of exercise and octopamine on the expression of PGC1α and UCP1 in heart tissue of DFO treated, we investigated the expression level of PGC1α and UCP1 gene and protein by qRT-PCR and IHC method, respectively. Data analysis demonstrated the lowest expression level of both genes studied in group I and also the lowest protein level was related to group I. Thus, it can be assumed that exercise and OCT have positive effect on PGC1α and UCP1 (gene and protein). It has shown that post-exercise recovery modality leads to greater expression of key mitochondrial (PGC-1α) and angiogenic (VEGF) genes than exercise alone, therefore, modulating the transcriptional adaptation towards a more oxidative phenotype [30]. In an investigation after 4 weeks of training, the Miyo-PGC-1DKOs exhibited an increase in exercise performance with a similar adaptive response compared with control animals. This increase was associated with an increase in electron transport complex (ETC) expression and activity in the absence of PGC-1α and PGC-1β expression. Taken together these data suggest that PGC-1α and PGC-1β expression are not required for training-induced exercise performance, highlighting the contribution of PGC-1-independent mechanisms [31]. UCP1 and adaptive immunity share a reciprocal relationship at the whole-transcriptome level, thereby supporting a plausible role for UCP1 in maintaining tissue homeostasis in human [23]. Based on the evidence available, regular exercise training does not represent a major stimulus of UCP1 expression in classical brown adipose tissue [32]. One putative reason for the difference between our findings and other previous investigations (about the PGC1α and UCP1 expression) may be due to the difference in exercise protocols and also various doses of OCT and DFO. This maybe suggests that the effect of the exercise and OCT is in relation to dose and exercise protocol on apoptosis, autophagy, and PGC1α and UCP1 expression. Additional researches are required to understand the apoptosis, autophagy, and PGC1α and UCP1 expression in vivo.
According to the results of this study, apoptosis and expression level of PGC1α and UCP1 was significantly difference between the groups studied. It can be concluding that exercise and octopamine can be considered as two positive factors in apoptotic and autophagy studies and also Deep frying oil. However, more studies on larger scales are required to confirm this concept.
Funding
No funding.
Compliance with ethical standards
All animal experimental protocols were reviewed and approved by the Ethics Committee of Islamic Azad University for the use of laboratory animals.
CRediT authorship contribution statement
Pantea Kianmehr: Data curation, Formal analysis, Writing - review & editing. Mohammad Ali Azarbayjani: Conceptualization, Data curation, Investigation, Methodology, Project administration,Writing - review & editing. Maghsoud Peeri: Resources, Software, Supervision, Validation, Writing - review & editing. Parvin Farzanegi: Visualization, Writing - original draft, Writing - review & editing.
Declaration of competing interest
The author declares no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2020.100735.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.McGurnaghan S. Cardiovascular disease prevalence and risk factor prevalence in Type 2 diabetes: a contemporary analysis. Diabet. Med. 2019;36(6):718–725. doi: 10.1111/dme.13825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gill C., Mestril R., Samali A. Losing heart: the role of apoptosis in heart disease—a novel therapeutic target? Faseb. J. 2002;16(2):135–146. doi: 10.1096/fj.01-0629com. [DOI] [PubMed] [Google Scholar]
- 3.Narula J. Mechanisms of disease: apoptosis in heart failure—seeing hope in death. Nat. Rev. Cardiol. 2006;3(12):681. doi: 10.1038/ncpcardio0710. [DOI] [PubMed] [Google Scholar]
- 4.Chen Z. miR-17 regulates the proliferation and apoptosis of endothelial cells in coronary heart disease via targeting insulin-like-growth factor 1. Pathol. Res. Pract. 2019:152512. doi: 10.1016/j.prp.2019.152512. [DOI] [PubMed] [Google Scholar]
- 5.van Empel V.P. Myocyte apoptosis in heart failure. Cardiovasc. Res. 2005;67(1):21–29. doi: 10.1016/j.cardiores.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Olivetti G. Apoptosis in the failing human heart. N. Engl. J. Med. 1997;336(16):1131–1141. doi: 10.1056/NEJM199704173361603. [DOI] [PubMed] [Google Scholar]
- 7.Vaux D.L., Korsmeyer S.J. Cell death in development. Cell. 1999;96(2):245–254. doi: 10.1016/s0092-8674(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 8.Kroemer G. Classification of cell death: recommendations of the nomenclature committee on cell death 2009. Cell Death Differ. 2009;16(1):3. doi: 10.1038/cdd.2008.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor R.C., Cullen S.P., Martin S.J. Apoptosis: controlled demolition at the cellular level. Nat. Rev. Mol. Cell Biol. 2008;9(3):231. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- 10.Hengartner M.O. The biochemistry of apoptosis. Nature. 2000;407(6805):770. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 11.Levine B., Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132(1):27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishida K., Yamaguchi O., Otsu K. Crosstalk between autophagy and apoptosis in heart disease. Circ. Res. 2008;103(4):343–351. doi: 10.1161/CIRCRESAHA.108.175448. [DOI] [PubMed] [Google Scholar]
- 13.Gustafsson A.s.B., Gottlieb R.A. Autophagy in ischemic heart disease. Circ. Res. 2009;104(2):150–158. doi: 10.1161/CIRCRESAHA.108.187427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Papenmeier S. Octopamine and its receptors are involved in the modulation of the immune response in Drosophila melanogaster. Pneumologie. 2019;73:A33. 02. [Google Scholar]
- 15.Brial F. Systems genetics of hepatic metabolome reveals octopamine as a target for non-alcoholic fatty liver disease treatment. Sci. Rep. 2019;9(1):3656. doi: 10.1038/s41598-019-40153-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwaerzel M. Dopamine and octopamine differentiate between aversive and appetitive olfactory memories in Drosophila. J. Neurosci. 2003;23(33):10495–10502. doi: 10.1523/JNEUROSCI.23-33-10495.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoyer S.C. Octopamine in male aggression of Drosophila. Curr. Biol. 2008;18(3):159–167. doi: 10.1016/j.cub.2007.12.052. [DOI] [PubMed] [Google Scholar]
- 18.Liang H., Ward W.F. PGC-1α: a key regulator of energy metabolism. Adv. Physiol. Educ. 2006;30(4):145–151. doi: 10.1152/advan.00052.2006. [DOI] [PubMed] [Google Scholar]
- 19.Tan Z. The role of PGC1α in cancer metabolism and its therapeutic implications. Mol. Canc. Therapeut. 2016;15(5):774–782. doi: 10.1158/1535-7163.MCT-15-0621. [DOI] [PubMed] [Google Scholar]
- 20.Vainshtein A. Role of PGC-1α during acute exercise-induced autophagy and mitophagy in skeletal muscle. Am. J. Physiol. Cell Physiol. 2015;308(9):C710–C719. doi: 10.1152/ajpcell.00380.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly L.J. Peroxisome proliferator-activated receptors γ and α mediate in vivo regulation of uncoupling protein (UCP-1, UCP-2, UCP-3) gene expression. Endocrinology. 1998;139(12):4920–4927. doi: 10.1210/endo.139.12.6384. [DOI] [PubMed] [Google Scholar]
- 22.Brown C. Activities of octopamine and synephrine stereoisomers on α‐adrenoceptors. Br. J. Pharmacol. 1988;93(2):417–429. doi: 10.1111/j.1476-5381.1988.tb11449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Z. Deep-fried oil consumption in rats impairs glycerolipid metabolism, gut histology and microbiota structure. Lipids Health Dis. 2016;15(1):86. doi: 10.1186/s12944-016-0252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong K.-S. Rescue effect of exercise on impaired arteriolar myogenic response with advancing age. Exerc. Sci. 2017;26(1):8–16. [Google Scholar]
- 25.Moon J.-H., Jeong J.-K., Park S.-Y. Deferoxamine inhibits TRAIL-mediated apoptosis via regulation of autophagy in human colon cancer cells. Oncol. Rep. 2015;33(3):1171–1176. doi: 10.3892/or.2014.3676. [DOI] [PubMed] [Google Scholar]
- 26.Bajbouj K., Shafarin J., Hamad M. High-dose deferoxamine treatment disrupts intracellular iron homeostasis, reduces growth, and induces apoptosis in metastatic and nonmetastatic breast cancer cell lines. Technol. Canc. Res. Treat. 2018;17 doi: 10.1177/1533033818764470. 1533033818764470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rimouche A. Cardiopulmonary limitation to exercise in systemic sclerosis: a Case-Control Study. Arch. Cardiovasc. Dis. Suppl. 2020;12(1):192–193. [Google Scholar]
- 28.Wu Y. Broadening AIEgen application: rapid and portable sensing of foodstuff hazards in deep-frying oil. Chem. Commun. 2019;55(28):4087–4090. doi: 10.1039/c9cc01172b. [DOI] [PubMed] [Google Scholar]
- 29.Lu K. Effects of high-intensity interval versus continuous moderate-intensity aerobic exercise on apoptosis, oxidative stress and metabolism of the infarcted myocardium in a rat model. Mol. Med. Rep. 2015;12(2):2374–2382. doi: 10.3892/mmr.2015.3669. [DOI] [PubMed] [Google Scholar]
- 30.Joo C. Passive and post-exercise cold-water immersion augments PGC-1α and VEGF expression in human skeletal muscle. Eur. J. Appl. Physiol. 2016;116(11-12):2315–2326. doi: 10.1007/s00421-016-3480-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ballmann C. Adult expression of PGC-1α and-1β in skeletal muscle is not required for endurance exercise-induced enhancement of exercise capacity. Am. J. Physiol. Endocrinol. Metabol. 2016;311(6):E928–E938. doi: 10.1152/ajpendo.00209.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chechi K. UCP1 expression–associated gene signatures of human epicardial adipose tissue. JCI insight. 2019;4(8) doi: 10.1172/jci.insight.123618. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.