Introduction
IgA vasculitis, also called Henoch-Schönlein purpura (HSP), is a form of cutaneous small-vessel vasculitis characterized by palpable purpura favoring the lower extremities, arthritis, nephritis, and IgA deposition within postcapillary venules of the skin and mesangium. In children, respiratory infections are well known to precede HSP. In adults, infectious endocarditis is a rare but potentially fatal trigger for HSP. We report the subtle presentations of subacute endocarditis in 2 adults with cutaneous IgA vasculitis.
Case reports
Case 1
A 32-year-old woman with a history of intravenous drug use and hepatitis C was admitted with acute kidney injury, thrombocytopenia, and 2 months' history of a leg rash associated with intermittent calf tenderness. She denied fever, weight loss, abdominal pain, hematochezia, or melena. Outpatient medications included methadone and ibuprofen received for calf pain.
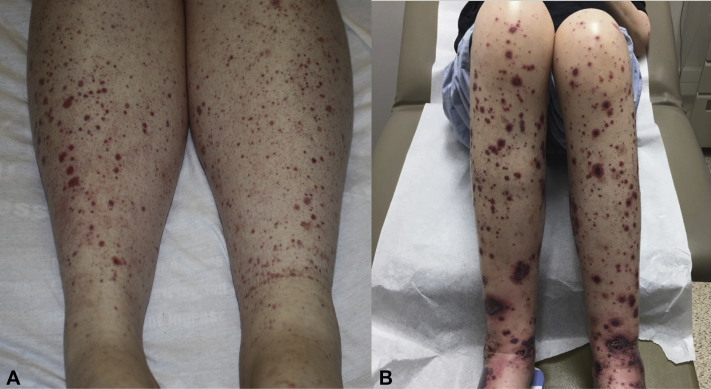
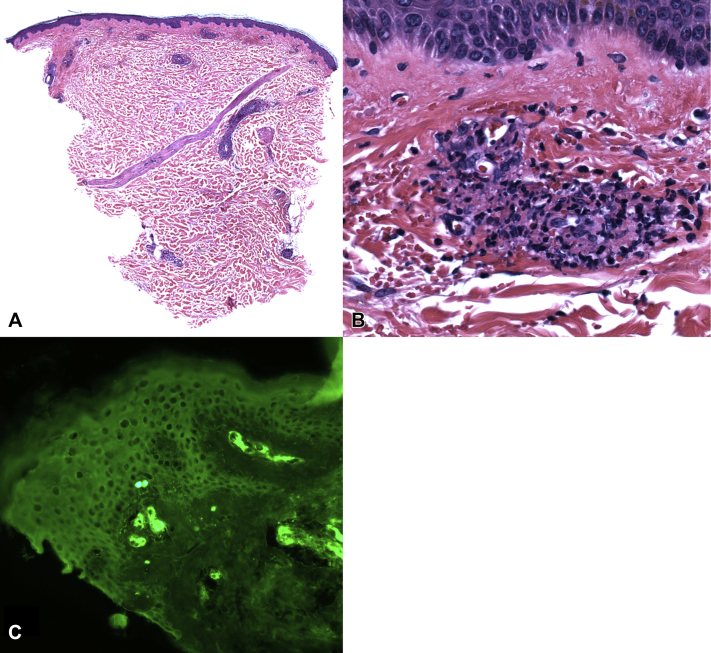
On examination, her lower legs exhibited numerous petechiae and purpuric papules (Fig 1, A). She was afebrile and had a grade I-II/VI systolic murmur. Urinalysis revealed proteinuria and hematuria. Hepatitis C viral load was undetectable. Rheumatoid factor and cryoglobulin results were negative. Skin biopsy demonstrated leukocytoclastic vasculitis (Fig 2, A and B) with IgA, immunoglobulin M, C3, and fibrinogen deposition in the vessel walls, yielding a diagnosis of IgA vasculitis. Renal biopsy showed acute interstitial nephritis (attributed to nonsteroidal anti-inflammatory drug use) and trace IgA staining on immunofluorescence.
Fig 1.
Case 1. Petechiae and palpable purpura (A). Case 2. Palpable purpura with central hemorrhagic crust of the lower extremities (B).
Fig 2.
Case 1. Tight vascular infiltration of superficial and middermal vascular walls by neutrophils and focal leukocytoclasis with fibrin and red blood cell extravasation. (A and B, Hematoxylin and eosin stain; original magnifications: A, ×40; B, ×400). Case 2. C, Direct immunofluorescence shows vascular wall accumulation of IgA. (original magnification: ×200). Similar findings were observed in both cases.
The patient remained afebrile throughout the first 4 days of hospitalization. Initial transthoracic echocardiogram revealed only a bicuspid aortic valve with moderate stenosis and mild aortic regurgitation. On hospital day 5, she became febrile to 101.1°F, prompting blood cultures, which grew Candida parapsilosis. Transesophageal echocardiogram demonstrated a 1.5 × 1.0-cm aortic valve vegetation. Positive blood culture results persisted despite intravenous fluconazole, anidulafungin, and liposomal amphotericin B. After aortic valve replacement, her candidemia and rash resolved.
Case 2
A 55-year-old woman with a history of tetralogy of Fallot and bioprosthetic pulmonic valve (replaced 8 years before) developed lower extremity petechiae and palpable purpura, which waxed and waned for 2 months. Her preexisting systolic murmur was unchanged at this time. Outpatient medications included only ibuprofen as needed. Review of systems was positive for fatigue, a 10-pound weight loss in the past 1 year, and occasional chills, cough, and joint aches. She denied fevers, night sweats, or abdominal pain. She then developed new-onset hematuria and proteinuria (10-20 red blood cells/high-power field; trace protein). Skin biopsy demonstrated leukocytoclastic vasculitis and perivascular IgA, immunoglobulin M, C3, and fibrinogen deposition (Fig 2, C), yielding a diagnosis of IgA vasculitis. Colchicine treatment for 1 month produced no improvement, and her skin lesions and kidney function worsened (creatinine level 1.1 mg/dL; baseline 0.68 mg/dL), resulting in hospital admission.
On admission, her legs exhibited dozens of purpuric papules and plaques, some targetoid with central hemorrhagic crust (Fig 1, B). Her abdomen, lower back, and arms had scattered purpuric macules and papules. Her systolic murmur was louder (III/VI from baseline II/VI), and a renal biopsy demonstrated immune complex glomerulonephritis with mesangial IgA, immunoglobulin M, and C3 staining. Immunofixation electrophoresis showed a faint band suspicious for cryoglobulins, but the cryoprecipitate testing result was equivocal. The hepatitis C serology result was negative. On hospital day 2, blood cultures collected more than 60 hours before grew gram-negative rods, which speciated to Cardiobacterium hominis. A transthoracic echocardiogram revealed right ventricular outflow tract obstruction caused by endocarditis, and she underwent emergency pulmonic valve stent angioplasty. A subsequent transesophageal echocardiogram showed a residual pulmonic valve vegetation. She was treated with piperacillin-tazobactam, then ceftriaxone with clearance of blood cultures, followed by pulmonic valve replacement. One month later, her purpura resolved.
Discussion
The annual incidence of HSP in adults is estimated to be between 8 and 18 cases per million.1 Compared with children, adults less commonly develop abdominal pain and fever, but more frequently experience severe renal involvement.2 In adults, HSP has been associated with inflammatory bowel disease, systemic lupus erythematosus, Sjögren syndrome, cryoglobulinemia, infections such as upper respiratory tract infections and hepatitis viruses, solid-organ malignancies, and medications, including calcium-channel blockers and carbamazepine.2, 3, 4
Endocarditis has been rarely reported in association with HSP. Galaria et al5 described a 41-year-old woman with a history of mitral regurgitation who developed palpable purpura of the lower extremities in the setting of mitral valve subacute bacterial endocarditis caused by Streptococcus sanguis. Skin biopsy demonstrated leukocytoclastic vasculitis and IgA deposits. Another report documented leukocytoclastic vasculitis with perivascular IgA deposition in a 47-year-old man who presented with fever, asthenia, and weight loss, which resolved on treatment of S gallolyticus endocarditis of the aortic, mitral, and tricuspid valves.6 Other cases of endocarditis-associated HSP also featured high fevers up to 104°F, which prompted blood cultures that helped establish the diagnosis.7,8
Here we describe 2 initially afebrile, well-appearing patients ultimately found to have subacute endocarditis as the most likely cause for their IgA vasculitis. Neither patient received medications commonly known to cause HSP because rash onset preceded ibuprofen intake. These patients did not receive systemic corticosteroids. To our knowledge, this is the first report of C hominis and C parapsilosis endocarditis linked to HSP. We postulate that infection-induced immune complex deposition in the skin led to their similar presentations. C hominis is a member of the Haemophilus, Aggregatibacter, Cardiobacterium, Eikenella, and Kingella group of low-virulence organisms, which primarily infect structurally damaged or prosthetic cardiac valves.9 In a series of low-virulence-organism endocarditis, 58% of patients had preexisting structural cardiac disease and an additional 27% had prosthetic valves. Within fungal endocarditis (1.3%-6% of infectious endocarditis), C parapsilosis is associated with intravenous drug use (more frequently than C albicans) and high mortality (up to 41.7%).10
In most reports, fever is a prominent feature leading to the diagnosis of endocarditis. The underlying cause of our 2 patients' IgA vasculitis was obscured initially by the absence of fever, which can result in delayed diagnosis. Identification of occult infection is imperative, given the management implications. Systemic corticosteroids have traditionally been used to reduce intensity and duration of gastrointestinal and joint symptoms and severe nephritis in HSP.11 In contrast, all of the above-reported cases of endocarditis-associated HSP required antibiotics and valve replacement for resolution. This report illustrates that blood cultures to evaluate for endocarditis should be considered in patients with HSP who have risk factors such as congenital heart disease, preexisting valvular disease, and intravenous drug use, even in the absence of fever. Cutaneous findings may be the primary presenting symptom for these patients, and dermatologists can play an essential role in identifying this potentially life-threatening etiology.
Footnotes
Funding sources: Dr Little is supported by a Career Development Award from the Dermatology Foundation.
Conflicts of interest: None disclosed.
References
- 1.Piram M., Mahr A. Epidemiology of immunoglobulin A vasculitis (Henoch-Schönlein): current state of knowledge. Curr Opin Rheumatol. 2013;25(2):171–178. doi: 10.1097/BOR.0b013e32835d8e2a. [DOI] [PubMed] [Google Scholar]
- 2.Blanco R., Martinez-Taboada V.M., Rodriguez-Valverde V., Garcia-Fuentes M., Gonzalez-Gay M.A. Henoch-Schönlein purpura in adulthood and childhood: two different expressions of the same syndrome. Arthritis Rheum. 1997;40(5):859–864. doi: 10.1002/art.1780400513. [DOI] [PubMed] [Google Scholar]
- 3.Podjasek J.O., Wetter D.A., Pittelkow M.R., Wada D.A. Henoch-Schönlein purpura associated with solid-organ malignancies: three case reports and a literature review. Acta Derm Venereol. 2012;92(4):388–392. doi: 10.2340/00015555-1288. [DOI] [PubMed] [Google Scholar]
- 4.Ergin S., Sanli Erdogan B., Turgut H., Evliyaoglu D., Yalcin A.N. Relapsing Henoch-Schönlein purpura in an adult patient associated with hepatitis B virus infection. J Dermatol. 2005;32(10):839–842. doi: 10.1111/j.1346-8138.2005.tb00856.x. [DOI] [PubMed] [Google Scholar]
- 5.Galaria N.A., Lopressti N.P., Magro C.M. Henoch-Schönlein purpura secondary to subacute bacterial endocarditis. Cutis. 2002;69(4):269–273. [PubMed] [Google Scholar]
- 6.Tous-Romero F., Delgado-Marquez A.M., Gargallo-Moneva V., Zarco-Olivo C. Cutaneous vasculitis: a presentation with endocarditis to keep in mind. An Bras Dermatol. 2017;92(4):594–595. doi: 10.1590/abd1806-4841.20176317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berquist J.B., Bartels C.M. Rare association of Henoch-Schönlein purpura with recurrent endocarditis. WMJ. 2011;110(1):38–40. [PMC free article] [PubMed] [Google Scholar]
- 8.Nagayama Y., Iwasaki S., Yamaguchi H., Yoshimura A. [Infective endocarditis successfully treated by early medical therapy in a patient with Henoch-Schönlein purpura nephritis under oral steroid therapy] Nihon Jinzo Gakkai Shi. 2007;49(4):452–458. [PubMed] [Google Scholar]
- 9.Das M., Badley A.D., Cockerill F.R., Steckelberg J.M., Wilson W.R. Infective endocarditis caused by HACEK microorganisms. Annu Rev Med. 1997;48:25–33. doi: 10.1146/annurev.med.48.1.25. [DOI] [PubMed] [Google Scholar]
- 10.Garzoni C., Nobre V.A., Garbino J. Candida parapsilosis endocarditis: a comparative review of the literature. Eur J Clin Microbiol Infect Dis. 2007;26(12):915–926. doi: 10.1007/s10096-007-0386-1. [DOI] [PubMed] [Google Scholar]
- 11.Ronkainen J., Koskimies O., Ala-Houhala M. Early prednisone therapy in Henoch-Schönlein purpura: a randomized, double-blind, placebo-controlled trial. J Pediatr. 2006;149(2):241–247. doi: 10.1016/j.jpeds.2006.03.024. [DOI] [PubMed] [Google Scholar]