Abstract
Antiretroviral therapy (ART) suppresses human immunodeficiency virus (HIV) infection. Research seeking to transform viral suppression into elimination has generated novel immune, chemical and molecular antiviral agents. However, none, to date, have excised latent integrated proviral DNA or removed infected cells from infected persons. These efforts included, but are not limited to, broadly neutralizing antibodies, “shock” and “kill” latency-reversing agents, innate immune regulators, and sequential long-acting antiretroviral nanoformulated prodrugs and CRISPR-Cas9 gene editing. While, the latter, enabled the complete excision of latent HIV-1 from the host genome success was so far limited. We contend that improvements in antiretroviral delivery, potency, agent specificity, or combinatorial therapies can provide a pathway towards complete HIV elimination.
Keywords: Antiretroviral therapy, Latency reversing agents, Broadly neutralizing antibodies, Long-acting slow-effective release antiretroviral therapy, HIV-1 tissue reservoirs, CRISPR-Cas9 gene editing
Abbreviations: ADCC, antibody dependent cellular cytotoxicity; APC, antigen-presenting cells; ART, antiretroviral therapy; ARVs, antiretroviral drugs; B2m, B2 macroglobulin; Bcl-2, B-cell lymphoma 2; bNabs, broadly neutralizing antibodies; CARs, chimeric antigen receptors; CCR5, CC chemokine receptor 5; CRISPR-Cas9, clustered regularly interspaced short palindromic repeats and CRISPR-associated protein 9; CRISPR, clustered regularly interspaced short palindromic repeats; CTL, cytotoxic T lymphocyte; CTLA4, cytotoxic T lymphocyte antigen 4; CXCR4, C-X-C chemokine receptor type 4; dCas9, deactivated or death Cas9; DCs, dendritic cells; dsb, double-stranded break; gRNA, guide RNA; HCDR3, heavy chain third complementarity-determining regions; HDDACi, histone deacetylase inhibitors
1. Introduction
The past thirty-six years has witnessed considerable progress in the understanding of the pathobiology of human immunodeficiency virus type one (HIV-1) infection. Impactful advances, in quality and longevity of life, were made by antiretroviral therapy (ART). These are undeniable. ART has demonstrated sustained abilities to suppress viral replication leading to improved immune function and reduced disease co-morbidities. However, ART still requires life-long daily administration [1,2]. Even with strict antiretroviral drug (ARV) adherence, latent integrated provirus remains operative and is perpetuated in CD4+ memory (including central memory and CD45 RA+ T cells), effector and regulatory T cells, monocyte-macrophages, microglia, and dendritic cells (DCs) [3], [4], [5], [6], [7], [8]. Thus, new efforts are fast emerging to find a “cure” strategy that extends beyond the two reported cases [9,10]. A first step underlies an immediate need to deliver ARVs and other therapeutic or viral elimination cargos to viral tissue reservoir sites. This is essential in the design of any HIV-1 therapeutic strategy seeking to eliminate the viral reservoir. Excision of HIV-1 proviral DNA and/or elimination of infected cells are required as both cannot be achieved by ARV regimens alone [10], [11], [12].
The inabilities to eliminate HIV-1 reflect the prolonged life span of infected cells [11] and the sustained proliferation of CD4+ T cells to carry integrated latent virus. Both are operative during HIV persistence unaffected by ART [12,13]. The half-life of virus-infected cells, measured in years [14,15], is a major obstacle towards viral elimination. Notably, based on available data, it would take 73 ART treatment years to complete a total cell decay [16,17]. A potential means to speed this process is by boosting innate immune responses, eliciting broadly neutralizing antiviral antibodies, and facilitating cytotoxic T lymphocyte (CTL) activation. While each and all have been tried, none so far has been successful in the total elimination of HIV-1 infected cells.
Adherence to ARV regimens, while remaining an obstacle to any cure effort, can be overcome. Transformation of daily ARV regimens to a once a month or every other month parenteral holds promise for sustained viral suppression and adherence. Moreover, long-acting (LA) ARVs may reduce systemic toxicities and ease medicine access [18], [19], [20], [21], [22]. Further refinements in current LA ART regimens are underway. They rest in the development of ARV prodrugs with both lipophilic and hydrophobic properties. These have advantages in improving ARV entry into the virus's target cells and tissue sites of infection that could diminish the infectious reservoir beyond what can be achieved by current ART. The LA ART prodrugs may also facilitate viral excision strategies by affecting reductions in total proviral DNA [23]. This idea was recently investigated using LA slow-effective release ART (LASER ART) prodrugs with the sequential administration of Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR) HIV-1 gene editing platform. In pre-clinical trials completed in infected humanized mice, the combined regimen proved successful in up to a third of virus-infected animals. LASER ART elicited maximal virus reductions shown by ARV tissue penetrance. Early efforts to validate HIV-1 excision has seen success in a range of HIV/AIDS animal models [23], [24], [25], [26], [27].
2. Boosting immune responses
2.1. Generating innate and cell-based immune antiretroviral responses
Innate immune antiretroviral responses are controlled, in part, by mononuclear phagocytes (MPs; monocytes, macrophages and DCs) serving as the first line of defense against microbial infections [28]. However and paradoxically, MPs also serve as both vehicles and reservoirs for HIV-1 infection and can speed viral dissemination [29]. While a central role of MP in viral surveillance is well accepted, its role in viral pathogenesis remains openly debated [30]. As per the former, DCs, the principal antigen-presenting cell (APC), are both widely distributed within the mucosal and active in surveillance. DCs are the first cells that encounter HIV, and the cells that are able to induce HIV-1 specific CTLs and other adaptive antiretroviral immune responses [31,32]. Following early virus-DC interactions, the cells process HIV-1 proteins and transports them to the cell surface (Fig. 1A). This facilitates antigen presentation facilitating adaptive immunity [32]. DCs can also modulate HIV-1 infection. Indeed, following activation with latency reversing agents (LRAs) for example, (histone deacetylase inhibitors (HDACi) interactions between infected effector cells and DCs have been shown to reverse latency affected by phosphoinositol-3-kinase (PI3K) pathways [33]. Programmed DCs can also drive antigen-specific elimination of infected CD4+ T cells [34].
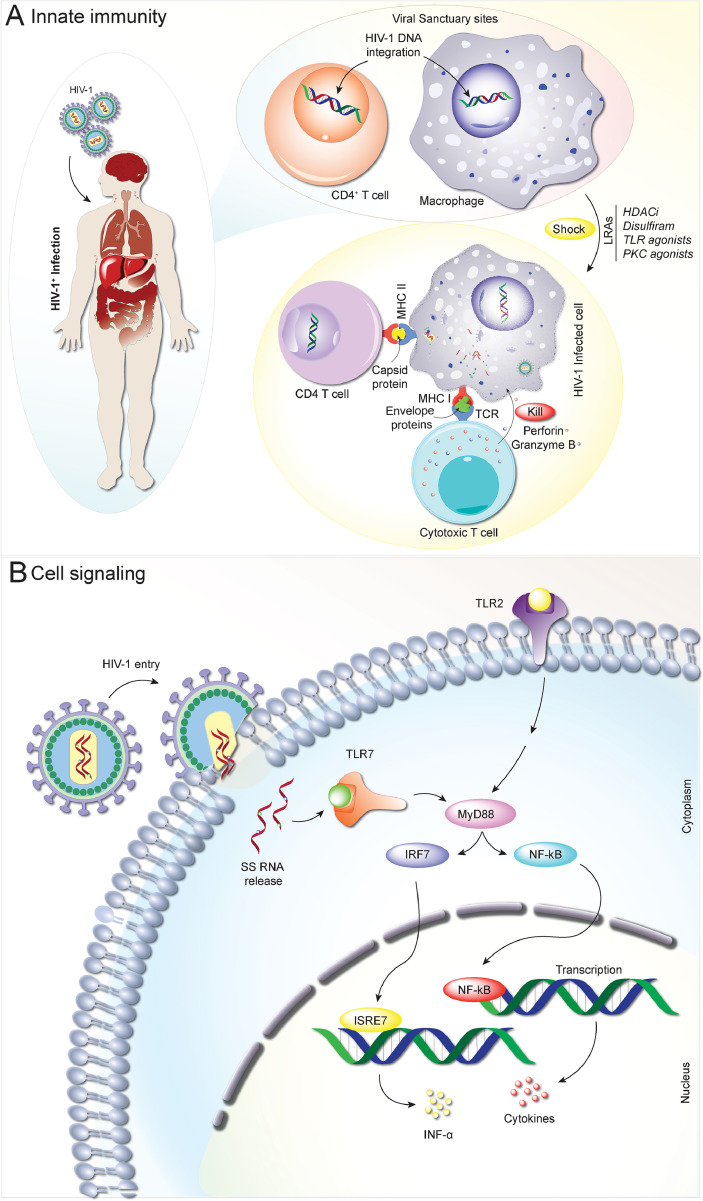
Fig. 1.
Innate immunity and antiretroviral activities. Upon HIV infection, virus dissemination occurs rapidly from viral sanctuary sites such as memory CD4+ T cells and macrophages. Early innate host immune response dictates viral load at the acute phase. While inflammation contributes critically to innate control of infection, it also recruits HIV target cells during the acute phase, impairs CD4+ T cell recovery, and promotes disease progression and viral latency. The latency can be reversed by several LRAs leading to activation of quiescent cells and exposure of the viral antigens by APCs rending the subsequent removal of infected cells by CTL mediated immunity (A). To combat HIV, the immune system has evolved complex and diverse transcriptional signatures, driving differential cellular and humoral responses. These signatures are induced by immune receptors, in this case Toll like receptors (e.g. TLR2) sensing the pathogen (HIV-1) and by the production of cytokines at the earliest onset of infection. The virus-mediated release of molecules in contact with TLR7 activate the MyD88 pathway, blocking the function of IRF7 and NF-κB response generally induced by viral signaling and ultimately secretion of cytokines like interferons leading to a restoration of immune function and viral clearance. This specific nature of immune activation is as critical to HIV-1 clearance as the induction of an adaptive immune response (B).
The innate immune system can, by itself, clear viral infection. This is realized through toll-like receptors (TLRs) that operate by their recognition of pathogen-associated molecular patterns (PAMPs) [35] (Fig. 1B). A TLR9 agonist, for example, can facilitate viral clearance through affecting adaptive immunity [36,37]. Here, TLR9 affects CTL mediated viral clearance resulting in reductions of integrated HIV-1 DNA [36,37]. Likewise, TLR7 agonists (GS-986 and GS-9620) were previously shown to control viremia in simian immunodeficiency virus (SIV) infected and ART suppressed monkeys. During ART interruption, a subset of infected animals (2/9) remained aviremic after two years, thereby supporting the idea that TLR7 agonists promote viral reservoir reductions [38]. However, subsequent studies have failed to support these initial findings [39,40]. In these studies, the administration of the TLR7 agonist (GS-9620) to SIV-infected macaques during suppressive ART led to induction of interferon (IFN)-stimulated genes, immune cell activation, and pro-inflammatory cytokines. However, no changes in levels of proviral DNA in blood or tissues were observed [39]. Prior studies tested the therapeutic efficacy of programmed cell death protein 1 (PD1) blockage with a TLR7 agonist in SIV-infected macaques that were virally suppressed for two years. ART interruption led to viral rebound bringing the SIV reservoir to preART levels [40]. Clearly, follow up studies are needed to better define the role for TLRs when used in combination with other antiviral agents in viral elimination strategies.
Further dissection of the interplay between innate and adaptive immunity may provide pathways towards improving viral clearance. To this end are studies of natural killer (NK) cell-clearance responses, antibody-dependent cellular cytotoxicity (ADCC), and CTLs. Each and all may be harnessed as each alone actively participates in viral surveillance and elimination in the coordination of other immune responses. For example, β-chemokines can act as ligands for the HIV chemokine co-receptor 5 (CCR5) and may use NK cells to block HIV-1 cell entry [41]. NK and MPs together can also generate antiretroviral signals during the early stages of HIV infection resulting in cellular proliferation, cytolytic activities, cytokine production, and ADCC-mediated viral surveillance. These cell-based antiretroviral events potentiate the TLR agonists serving to activate APCs, increase B-cell maturation, cross-prime T-cells, and enhance NK cell responses [42,43]. All can promote virus infected cell elimination (Fig. 1A).
Activation of HIV gene expression followed by adaptive immune viral elimination was first tested as a therapeutic strategy with interleukin-2 (IL-2) [44]. The use of ART and IL-2 in this manner showed to be a multistage regulator for CD4+ T cells. Notably, in this context, IL-7 was shown to have two effects, one in latency reversal (induction of HIV-1 RNA) and the second in increasing the numbers of memory CD4+ T-cells (homeostatic proliferation). Both had subsequent effects on levels of HIV-1 proviral DNA [45]. IFN alpha (IFN-α) also modulates HIV-1 infection [46], [47], [48]. Short-term treatment with exogenous IFN-α lead to viral suppression and a decline in integrated and total HIV-1 DNA in ART-treated patients [46]. Indeed, some of the first immune-based therapies used to restrict viral replication came from studies of IFNs and ILs [46,47,49]. While showing no benefits in any long-term control of viral infection, both IL-2 and IL-7 restored the numbers of CD4+ T-cells [45,50]. However, IL-2 proved toxic. To date, no studies involving ILs demonstrated sustained and significant antiretroviral activities. However, they do offer pathways that could affect latent viral reservoirs [51].
2.2. Broadly neutralizing antibodies (bnAbs) and CTLs
Innate immune responses are operative immediately following viral infection. Because of its nonspecific nature, HIV-1 can circumvent innate antiretroviral activities. Thus, adaptive immunity serves as the principal clearance mechanism for virus-infected cells during disease. This arm of the immune system includes bnAbs, CTLs, and ADCC [52,53]. Persistent antigen exposure during chronic infection may lead to T cell exhaustion. This is heralded by the expression of inhibitory receptors, such as PD1, resulting in the progressive loss of effector functions. Targeting PD1 to boost the antiviral cellular immune responses has shown inconsistent results [54].
Another promising means to affect viral control is through bnAbs. They target conserved epitopes of the HIV-1 envelope, enabling them to circumvent the frequent viral mutations seen during low-level viral growth. Isolation, cloning, and single-cell antibody techniques have enabled effective bnAb discovery [55]. BnAbs are produced about two and half years after initial viral infection and are linked to slow disease progression. This delay in bnAbs production makes them highly specific as they undergo multiple iterations of somatic mutations [56,57]. Because of their longer half-lives and achievable effective therapeutic concentrations, a single infusion of bnAbs results in a rapid decline of plasma viral load (VL) that has been affirmed in long-term SIV infected macaques [58]. Moreover, modification of bnAbs by amino acid mutations has increased their half-lives even further and conferred protection against repeated viral challenges [59].
Bi-specific monoclonal antibodies (mAbs), dual-affinity re-targeting and tri-specific bnAbs have been engineered, in recent years, as antiretroviral therapies. Such antibodies recruit effector cells that are CD3- or CD16- specific binding to FcγR-bearing cells. Binding to additional viral epitopes serves to further enhance its potency against viral infection [60]. Examples include CD4-targeting bnAbs (3BNC117, VRC01, N6-LS, and VRC07-LS), V3-targeting bnAbs (10–1074 and PGT121), V1/V2-targeting bnAbs (PGDM1400, PG9, and PG16), membrane-proximal region-targeting bnAbs (10E8V-LS), HIV-1 gp120-gp41 interface-targeting bnAbs (PGT151), and finally HIV-1 gp120’s silent face can be recognized by bnAbs SF12 and VRC-PG05 [61], [62], [63], [64], [65]. Prior animal studies confirmed bnAbs effectiveness for HIV-1 suppression. Administration of 3BNC117 and 10–1074 to chronically infected rhesus macaques decreased plasma VL to undetectable levels. However, the emergence of viral mutations proved a limitation [66]. Nonetheless, administration of PGT121 and PGDM1400 antibodies to rhesus macaques during simian HIV (SHIV) challenge resulted in complete protection for up to 14 days [67].
In addition, bnAbs also offer opportunities towards a successful prophylactic HIV vaccine. Indeed, significant research efforts have been focused on the development of bNAbs to neutralize the majority of HIV-1 strains. The uses of bnAbs in vaccine research are impeded by their fast-viral escape, frequent dose requirements, potency, and rare elite neutralization responses. Nonetheless, when used for treatment, bnAbs offer novel opportunities for viral clearance with additional limitations. For example, when bnAbs are used in treatment it may be difficult to discern the origins of viral diversification and whether it is due to the natural virus evolution or responses to bnAbs themselves. This is highlighted by the presence of Env variants seen during bnAb treatments. Moreover, bnAb production may be limited in those infected for longer time periods due to the frequent loss of B precursor-producing cells. Other bnAbs limitations include the presence of long-heavy chain third complementarity-determining regions (HCDR3), high somatic mutations, and poly/autoreactivity to self-antigens. In all, bnAbs play an important role in future therapeutics; but immune intolerance may pose yet another concern [68].
The rationale of bnAbs in vaccine strategies is based on: (i) ease of infected donor isolation; (ii) ready classification into mature, intermediate, or unmutated precursors; (iii) deployment of immunogens with high affinity; and (iv) the abilities of sequential immunization to activate B-cells. These are all affected by immunogen design (Fig. 2A–C) and lead to the production of different bnAbs lineages [69,70]. The idea is to produce bnAbs using high affinity-binding immunogens to the naive B-cell receptor, followed by serial boosts to stimulate affinity maturation. Though polyvalent vaccines show promising results in rabbits and monkeys, the polyvalent HVTN505 vaccine failed to reduce VL resulting from new infections in human trials [71]. The International AIDS Vaccine Initiative (IAVI) W001 trial tested BG505 SOSIP.664 gp140 and showed that bnAbs can be produced that neutralize the virus. This consisted of glycosylated HIV-1 Env trimers resembling the native virus envelope [72].
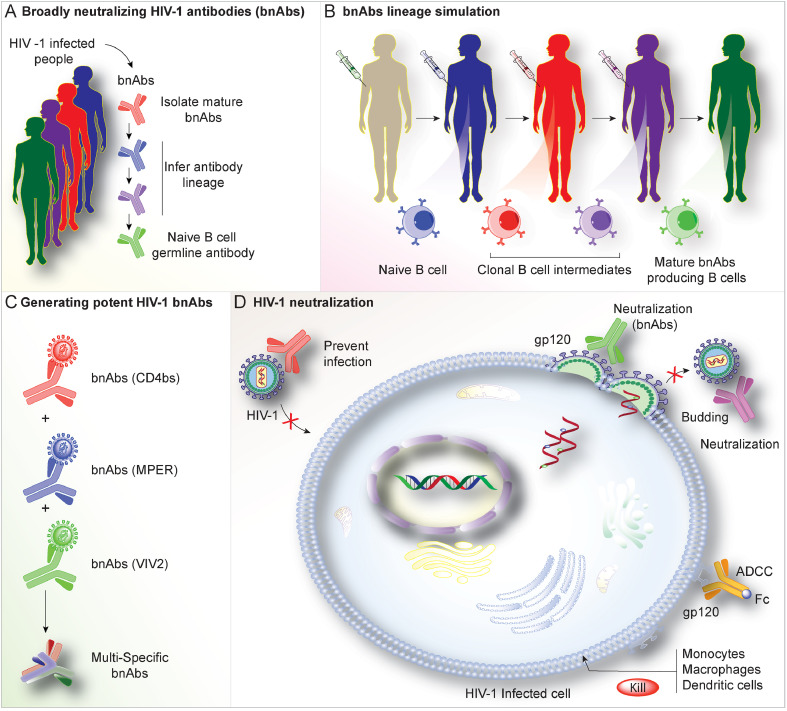
Fig. 2.
Broadly Neutralizing Antibodies (bnAbs). To overcome the limitations of using bnAbs as vaccine candidates, B-cell lineage immunogen strategies were developed (A). This is achieved by: (i) Isolation of bNAb clonal lineage antibodies from HIV-1 patients reflecting the different critical developmental stages of these bnAbs. (ii) Based on these isolated antibodies, high-affinity immunogens are designed that can optimally bind to stages of bNAb lineage antibodies. (iii) Eventually, immunization with a series of these high-affinity immunogens is followed to stimulate bNAb lineage activation. Using techniques such as antibody cloning, sequencing, and computational analyses, bnAb staged maturation is illustrated (B and C). This is critical in the design of HIV-1 Env epitopes that can engage the early precursors and elicit their development and maturation into the different bnAb lineages. Env epitopes with high binding affinity to the germline B-cell antibody precursors will enhance the lineage development that could boost the immune system to produce its bnAb-producing B cells resulting in neutralization of the virus (D).
In another phase-I clinical trial, VRC01LS remained in circulation for longer durations yielding potent neutralizing activities [73], without autoimmunity. Administering 10–1074 to healthy and HIV-1-infected volunteers showed antibody tolerance with half-lives of 12·8 - 24 days [74]. Shorter half-lives in infected individuals correlated with the presence of viral neutralizing targets and administered antibodies demonstrated rendered faster clearance [74]. In a phase II trial, four infusions of 3BNC117 succeeded in inhibiting the virus for up to 19 weeks [75]; however, viral escape was detected in 50% of cases due to the emergence of resistant strains. When combined, 3BNC117 and 10–1074 produced suppression in 80% of the participants for 15 weeks [76]. Taken together, the studies confirm bnAbs’ antiviral effects (Fig. 2D). While existing studies on bnAbs are promising in the scope of HIV-1 treatment and prevention, progress is hindered by limitations in their development and tolerance, as sometimes they elicit strong immune responses that cause their own depletion [68].
Consequently, bnAbs production was higher in people with autoimmune diseases because of defective tolerance. In a chronic HIV-1-infected individual who had systemic lupus erythematosus (SLE), plasma from the SLE patient neutralized 41 out of 42 HIV strains tested and confirmed the presence of CD4bs-targeted bnAbs; CH98 among others [68]. CH98 was found to have undergone various mutations and deletions, which played a critical role in its affinity maturation. Immune responses against bnAbs present considerable limitations in preventative and treatment regimens demanding the use of medications to suppress the immune responses and promote bnAbs generation. Effectiveness of bnAbs requires the involvement of both immune cellular effectors and the downstream pathways triggered by the binding of Fc fragments of bnAbs to Fcγ receptors [77]. This finding was affirmed by 3BNC117 bnAb single infusion which when administered to HIV1 infected patients demonstrated strong neutralizing responses. However, this was due to the removal of free circulating viruses, prevention of new infections, and clearance of HIV infected cells [78]. These are features of NK cell mediated ADCC. Moreover, in the context of coordinated therapeutic approaches with 2nd generation bnAbs, there is a strong likelihood of clinical efficacy independent of the levels of viral infection and HIV-1 strain heterogeneity [79]. BnAbs half-life, scale-up production, and safety remain obstacles for broader use.
CTLs could overcome the limitations for bnAbs and enable viral control even without ART [80]. Several cure strategies revolve around altering or expanding CTLs. In trials where CTLs isolated from infected patients and expanded using HIV-specific peptides and then reinfused into patients failed to show permanent reductions in VL [81]. Escape mutants developed. To circumvent this problem, polyclonal CTLs were generated by exposing them to multiple HIV epitopes but still failed to affect viremia [82]. CTLs may have reduced activities against non-clade B virus variants but are limited in their abilities to be scaled-up for clinical use [83]. Nonetheless, harnessing CTLs abilities to recognize and kill infected cells could prove essential in any viral elimination strategy [84], [85], [86].
Another approach used was engineered T cells with chimeric antigen receptors (CARs) directed against the HIV envelope. However, this approach has so far failed to improve antiviral adaptive immune responses in patients [87], [88], [89], [90]. Nonetheless, CAR T cells have several advantages over ex vivo expanded CTLs, as they do not need to recognize their ligands in the context of the major histocompatibility complex (MHC). Another advantage of CAR T cells over CTLs is that they are less likely to generate escape mutants as they target highly conserved regions of the HIV envelope. Though encouraging findings were observed for CAR T cells to reduce viremia, they are limited in broader usage. The generation of CD4 ζ- or single chain variable fragment (scFv)-based chimeric protein containing CARs lacked complete viral suppression in the absence of ART [87]. The absence of antirviral CAR T cells in reservoir tissues and their inability to affect latently infected cells are additional limitations [91], [92], [93]. Newer CAR engineering and cellular manufacturing need to be addressed for safe, efficient, and specific clearance of virus from its reservoirs.
3. Pharmacological approaches to HIV-1 elimination
HIV-1 reservoirs remain latent in ART-treated individuals with minimal to no viral transcription needed to evade immune surveillance. To expose the footprint of reservoirs, an approach termed “shock and kill” was developed that implements LRAs. While sustained ART prevents newly produced virus from infecting healthy cells, these LRAs help in the reawakening of dormant virus (shock) from latently infected cells and induce viral and/or immune-mediated cell death (kill) (Fig. 3). Currently, there are over 300 chemicals identified as LRAs that target HIV-1 latency through different mechanisms (epigenetic modification, transcriptional regulation, and others) [94], [95], [96]. However, while inducing transient viral amplification, LRAs have not met meaningful clinical outcomes towards reducing HIV-1 reservoirs and delaying viral rebound. Design improvements have been proposed [97,98]. Such improvements in LRA strategies include drug dose, frequency and specificity. If achieved, the latency-reversing function would be improved with specific action on infected cells [99]. New generations of small molecules acting on alternative pathways have exhibited partial immune activation while preserving efficacy for HIV-1 reactivation. Some of these compounds synergized with current LRAs on viral reactivation and remain front-runners for clinical trials [96].
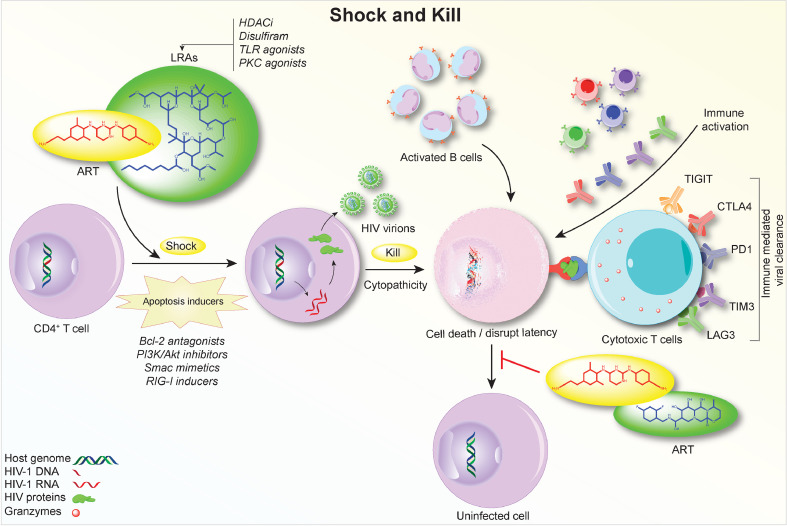
Fig. 3.
‘Shock and Kill’ Strategies for HIV-1 Elimination. The idea of ‘shock and kill’ is to induce HIV-1 transcription from latently infected cells using LRAs followed by the virus- or immune-mediated cell death. Meanwhile, ART maintenance precludes new infections. Thus far, ‘shock and kill’ trials have seen limited success for HIV-1 reactivation and less on reducing viral reservoir sizes. To address these early failures, apoptosis inducers are being employed to ‘label’ HIV-1 reservoirs that are intrinsically resistant to cellular apoptosis and are joined with LRAs on selective elimination of infected cells. A combination of LRAs, along with CTLs and ADCCs, and antiretroviral induction could enhance viral elimination that is currently limited by the results of short drug half-lives, limited tissue penetration, and complex activities of multi-regimens. It is possible that multiple LRAs could be delivered as a single dosage. By targeting immune checkpoint inhibitors, the ‘kill’ or ultimate removal of reactivated viral reservoirs can be strengthened by therapeutic vaccines, bnAbs, CAR T cell therapy, and CTLs. HIV-1 reservoirs are less stable prior to ART intervention, likely due to a pro-inflammatory environment that favors T cell activation. Instead of conventional LRAs employed during suppressive HIV-1 infection, co-delivery of LRAs and ART during early infection may further disrupt the establishment of viral latency, minimize initial reservoir size, and ease viral elimination. These immune-linked events are operative through PI3K, PKC, RIG-1 and Smac pathways.
HIV-1 reservoirs distinguish themselves from healthy cells through their apoptosis-resistant characteristics. The co-treatment with a major apoptotic inducer, the B cell lymphoma 2 (Bcl-2) antagonist venetoclax, in αCD3/αCD28-treated cells from virally suppressed patients could induce preferential killing of HIV-1 infected cells with minimal death of uninfected CD4 T cells. More importantly, cell-associated viral DNA was reduced significantly upon dual-treatment compared to αCD3/αCD28 treatment alone [100]. This suggests that priming CD4+ T cells from ART suppressed HIV-1 patients with BCL-2 antagonist, followed by HIV reactivation, can achieve a reduction in viral latency. This virus-specific elimination strategy, named “prime, shock, and kill,” needs further in vivo evaluation. Stochastic reactivation of HIV-1 latency requires repeated stimulation to shrink reservoir size [101]; the activation effect may be linked to LRA concentrations [99]. These in combination with a high dose frequency and volume of LRAs can lead to toxicities. Nanotechnology offers a solution by formulating regimens into nanosuspensions that can prolong drug half-lives and reduce dosing frequencies [102]. For example, LRAs encased in lipid nanoparticle-encapsulated bryostatin enhanced HIV-1 latency reversal from J-Lat cells compared to conventional regimens [103,104]. In addition co-delivery of ART and LRA nanoparticles could reverse HIV-1 latency while preventing viral spead [104]. Heterogeneity of HIV-1 reservoirs makes it unlikely for a single LRA to act on all hidden targets. Co-formulation and co-delivery systems through nanocarriers could also be developed to simplify treatments.
Another concern for unsuccessful “shock and kill” is suboptimal “killing” of latently infected cells [105]. Competent CTLs are prominent in HIV-1 surveillance and become functionally exhausted during chronic infection, featuring an increased expression of immune checkpoint markers such as PD-1, cytotoxic T lymphocyte antigen 4 (CTLA-4), LAG3, and T cell immunoglobulin mucin receptor 3 (Tim3). Blockages of PD-1 led to SIV specific T cell expansion and viral reduction in rhesus macaques [106]. How this approach might affect HIV-1 reservoirs and potential viral cure needs further investigation.
While efforts devoted to developing new LRAs continue, alternative timing of LRA administration is worth studying. Although HIV-1 latency is established rapidly upon infection, it is relatively unstable, as shown by a quick turnover of viral DNA before treatment initiates [107]. This is likely due to a pro-inflammatory environment, along with a highly expressed viral antigen that favors T-cell activation and hinders the formation of HIV-1 latency. One can speculate that adding LRA to ART during early HIV-1 infection may further restrain viral reservoir size compared to ART alone [108]. Notably, “early” ART intervention may not necessarily be “better” for developing effective antiretroviral immune responses. Such early intervention blunts the generation of HIV-1 specific CTLs. This may explain the failure of viral control when ART was stopped in patients treated early after viral infection [109]. To overcome this apparent limitation, adjunctive immune therapies such as bnAbs can be added to LRAs to improve “shock and kill” outcomes. Combinations of a TLR7 agonist (GS-9620) and bNAb (PGT-121) were administered to SHIV-infected ART-treated macaques. The results from 6 out of 11 dual-treated animals showed viral rebound while all singly treated infected animals produced virus by 196 days after ART discontinuation [110]. In this study, ART was started at seven days post-infection, while GS-9620 and PGT121 were administered later when viral reservoirs were firmly established. It is possible that a higher rate of viral remission could be achievable if all therapies were applied early in infection.
All cure strategies discussed so far are based upon active transcription or translation of viral products. Another promising candidate worth mentioning is the use of a recombinant IL15 or an IL-15 super-agonist (ALT-803) that have demonstrated latency-reversing properties and enhancement of CTL function [111]. Though administration of ALT-803 has shown an early reduction in plasma viremia in vivo, it was ineffective alone in controlling viral replication for a longer duration. The lack of CTL inhibition, combined with IL-15 in mediating follicular SIV control by NK cells, makes ALT-803 a promising candidate as an LRA [112]; however, it remains to be seen what effects it will have on follicular reservoirs to the cure strategy. Altogether, the use of bnAbs to control HIV-1 infection holds the promise that a cocktail containing LRA, immunotherapy, and ART can maximize viral clearance. This can facilitate the elimination of viral reservoirs in strains of virus with limited genetic diversity.
4. Viral excision CRISPR therapies for HIV-1 elimination
Over the last decade, several genome-editing methods have been developed such as transcription activator like-effector nucleases (TALENs) [113] and artificial nucleases like zinc finger nucleases (ZFNs) [113], [114], [115] with the prospect of eliminating residual viral DNA. However, the approaches lacked sensitivity and specificity and as such, led to the use of the CRISPR system [27,116]. The mechanism is based on bacterial and archaeal microorganisms that incorporates fragments of foreign DNA into a CRISPR locus present in their genetic material, enabling recognition and elimination of infections. Other applications of genome editing include, but are not limited to, targeted gene regulation, generation of knock-out and knock-in animals, cell-lines, epigenetic modulation, and chromatin manipulation [23,27,116]. However, attempts to eradicate HIV-1 reservoirs based on CRISPR strategies alone to shock then activate latent virus, knock out receptors and genes, block integration, affect transcriptional silencing, or effect excision of the proviral DNA from the host proved incomplete [117].
A first success for purging latent HIV was achieved using the Cas9 catalytic domain (deactivated or death Cas9, dCas9), working in tandem with guide RNA (gRNA). To activate the viral transcription, dCas9 proteins were targeted to the HIV-1 long terminal repeat (LTR) promoter that is known to affect chromatin modifying and transcription activating factors [118]. While this strategy does not damage DNA, it may produce off-target effects of gene activation events. Another dCas9 system, dCas-VP64 protein, enabled activation of HIV-LTR driven gene expression in latently infected cells. Prior studies identified NF-κB binding sites as the most promising target and the suitable position of the gRNA in the LTR promoter. Multiple gRNAs with non-overlapping targets of dCas9-VP64 were shown to enhance the activation of viral gene expression [119]. “Synergistic activation mediator” that uses a modified gRNA termed MS2-p65-HSF1 had shown promising results of reactivation of latent cells that include the dCas9-SunTag system and was proven successful in reactivation of latent HIV-1 [120]. The advantages of CRISPR-based reactivation are high sequence-specificity and reactivation potency to induce viral production and death of targeted cells. These approaches, nonetheless, must avoid off-target effects and should be effective in the delivery of dCas9 and gRNAs to infected and latent cells.
The working model of CRISPR-Cas9 uses a gRNA sequence in complex with the Cas9 endonuclease [27]. The gRNA has two components, the ~20 bp protospacer sequence that binds to the complementary sequence of target DNA and the scaffold component that allows binding of the gRNA to the Cas9 endonuclease. The binding of Cas9 enzyme to a protospacer adjacent motif (PAM), generally an “NGG” sequence, follows gRNA recognition of complementarity sequences, a double-stranded break (DSB) then excision of the viral DNA target. By merely changing the sequence composition of the 5′ end of gRNA [27], the target site specificity can be programmed (Fig. 4).
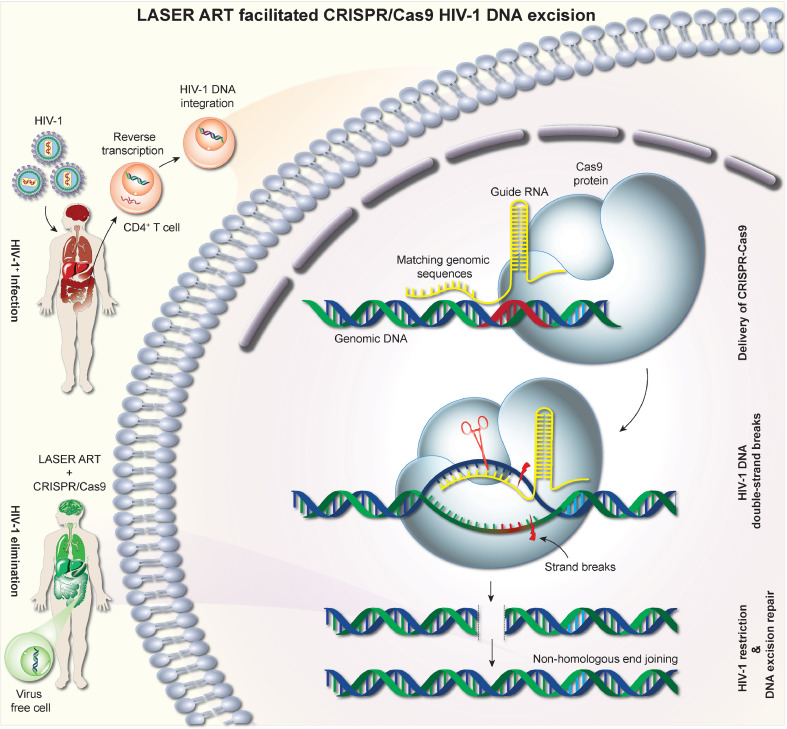
Fig. 4.
Sequential LASER ART and CRISPR-Cas9 for HIV-1 Elimination. Viral entry and fusion require CD4 receptor and CCR5 and CXCR4 coreceptors at the cell surface. Upon entering into virus susceptible CD4+ cells, viral RNA is reverse transcribed into double-stranded circular DNA and integrated into host chromosomal DNA. This provirus can remain quiescent in memory effector T cells. ART drug combinations serve to inhibit virus production at various stages of the HIV life cycle. LASER ART can improve levels of viral restriction by sustaining high drug levels at reservoir sites. Highly specific gRNA based CRISPR-Cas9 targets integrated proviral DNA. The CRISPR-Cas9 utilizes ~100 bp of gRNA to facilitate the Cas9 endonuclease activities at viral integration sites. This made complex then recognizes and cleaves a 20 bp double-stranded DNA target site (known as protospacer DNA) that is complementary to the 5′ end of the gRNA. The double-strand break excises the HIV-1 DNA from the host genome, which gets repaired by non-homologous end-joining to restore the break. This combination of LASER ART and CRISPR-Cas9 has so far achieved viral elimination from a subset of HIV infected animals.
The classic route of HIV-1 entry is considered to be through the engagement of the CD4 receptor and CCR5 and C-X-C chemokine receptor type 4 (CXCR4) coreceptors. Thus, current research efforts are targeted on two curative strategies, functional and sterilizing cures, the latter being more attractive, which entails the removal of provirus from the latent reservoir cells [121]. The two successful reported cases of HIV-1 sterilization cures targeted transplantation of CCR5Δ32 hematopoietic progenitor stem cells (HSPCs) from allogenic donors [122]. Though the results are encouraging, the question remains whether HIV-1 is being eradicated from all latent reservoirs or if both are cases of “functional cure” where the genetically modified functional immune system adequately controlled the latent reservoir.
With the requirement of single sgRNA to “program” the location of the cleavage site with improved on-target specificity, CRISPR-Cas9 technology was used to disrupt CCR5 [123]. By combining CRISPR-Cas9 with a PiggyBac transposon donor sequence to generate naturally occurring CCR5Δ32 deletions in induced pluripotent stem cells (iPSCs), the cells were found to be resistant to HIV-1 after challenge [124]. Lentiviral vectors expressing Cas9 and CCR5-targeted sgRNAs that were used to engineer CD4+ T cells showed promising CCR5 gene disruption in cell lines but resulted in toxicities in primary T cells [125], possibly due to innate immune reaction of T cells to the foreign DNA [126]. Using CRISPR-Cas9 and dual gRNAs, another study targeted CCR5 and the clinically relevant gene B2 macroglobulin (B2M) in CD4+ T cells and CD34+ progenitor cells demonstrating biallelic CCR5 disruption [127].
In spite of an allogeneic stem cell transplantation with donor cells lacking the CCR5 coreceptor, a recent study showed that the presence of a highly replication-competent CXCR4-tropic minor variant resulted in viral rebound in the absence of ART [128]. If CCR5 is lost during stem cell therapy, infection could continue by use of alternate receptors; in this scenario, targeting CCR5 may not always work in patients [128]. Nonetheless, in conjunction with long-acting ART, CRISPR-Cas9 mediated co-receptor editing could be developed as a complementary therapeutic approach in viral cure strategies [129].
In a little less than five years, CRISPR-Cas9 gene-editing technology has become a cornerstone of translational genetic research. The question now posed is whether it can be used with ART to eliminate HIV-1 [2]. Initial testing was done to determine whether CRISPR-Cas9 based genome editing could successfully excise fragments of integrated HIV-1 proviral DNA. Sequential treatment of ART in the form of LASER ART and CRISPR-Cas9 targeting of the LTR-Gag region was applied for proof-of-concept studies to infected humanized mice [130]; this resulted in the complete elimination of HIV-1 in a subset of infected humanized mice. Thus, by employing combinatorial treatments complete HIV-1 elimination could be achieved [23]. Success was affirmed by the absence of proviral DNA from viral compartments two months after cessation of ART. The result was further confirmed using multiple highly sensitive nucleic acid detection methods and viral outgrowth assays. These results open up the possibility that viral sterilization is possible.
5. Concluding remarks
Improved antiretroviral drug biodistribution into infectious reservoirs will have better treatment outcomes. To address this, pharmaceutical companies and independent research programs have invested considerable efforts to transform the current daily antiretrovirals into LA ART that can be administered monthly or at longer intervals [131,132]. Using other transforming agents into LASER ART [102] has improved reservoir targeting, adherence, and reduced dosing frequencies. LASER ARTs are characterized by poor water-solubility, slow drug-dissolution, increased bioavailability, decreased toxicities, and enhanced pharmacokinetic and pharmacodynamic profiles [102] of existing United States Food and Drug Administration approved antiretroviral drugs [102]. The advantage of nanomedicines lies in the fact that immune responses are not elicited against these drugs. However, viral resistance may develop, leading to viral escape as they cannot eliminate latently infected cells from infectious reservoirs [102]. The significant advantage of using LASER ART lies in its abilities to reach latent reservoir sites. There, native drug(s) is released at substantial levels facilitating complete suppression of plasma viremia. We posit that this underlies our abilities to effectively use CRISPR-Cas9 to effectively eliminate integrated proviral HIV-1 DNA. Neither the single LASER ART nor CRISPR-Cas9 treatments alone could totally eliminate the virus from the infected animals [23].
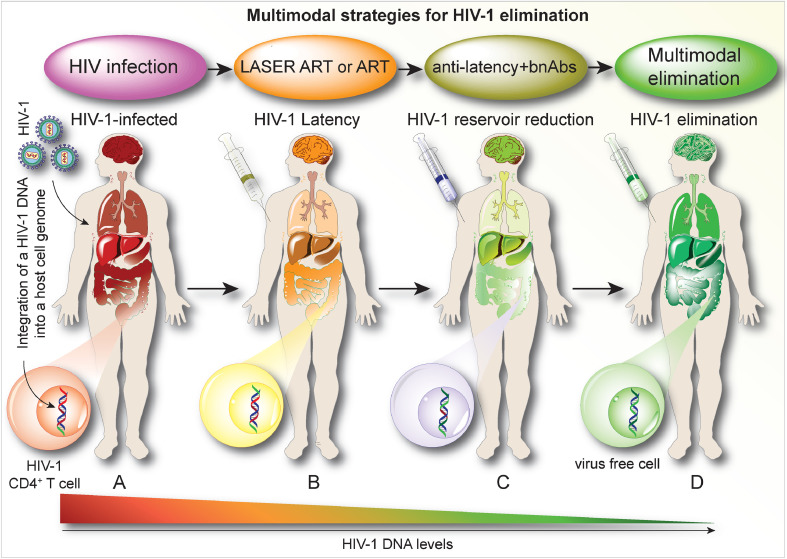
Future efforts to increase elimination rates will include better longer-acting LASER ART formulations lasting six months and beyond and will target more than one conserved region of HIV genome using CRISPR-Cas9 multiple guide RNAs at different stages of infection. With the combination of therapeutic strategies, long-term toxicities need to be assessed before testing in humans. Although no adverse responses of LASER ART formulations or CRISPR-Cas9 were observed in early animal studies, more extensive assessments again are required before these therapies can be considered for human testing. The use of multimodal therapies such as LASER ART, bnAbs, and CRISPR-Cas9, we believe, will ultimately lead to complete viral elimination. (Fig. 5). The next steps include the development of a CRISPR-Cas9 delivery system that specifically targets viral reservoirs, uncover the best combination strategies to achieve complete success for viral elimination, and limit any adverse effects of the developed strategies. The off-target effects of CRISPR-based strategies, long-term effects on tissues of long-acting drugs, and bnAbs are the most important side-effects that need to be assessed before moving into humans.
Fig. 5.
Combination Therapies Leading to an HIV Cure. CD4+ T cell activation promotes active viral growth. Systemic infection occurs in lymph nodes, spleen, genitourinary, brain, and gut tissues. While ART is administered early after viral infection, it serves only to restrict viral replication by blocking various steps of the viral life cycle that affect spreading infection. Improvements are made in the levels of viral restriction through the use of lipophilic, hydrophobic drug crystals (coined as LASER ART). These also serve to improve the biodistribution and potency of antiretroviral agents. Neither conventional nor LASER ART can eliminate proviral DNA from the human genome and as such, viral latency is easily established in infectious cell and tissue reservoirs. After ART discontinuation virus always rebounds. The elimination of HIV-1 thus requires depletion of cells harboring infectious virus and/or the removal of integrated proviral DNA. This can be achieved through multimodal immune or genetic approaches that begin with ART or LASER ART, bnAbs, boosted CTLs, and HIV-1 DNA excision mediated by CRISPR-Cas9. The process would start through the implementation of LASER ART to optimally provide effective antiretroviral drug concentrations in viral tissue reservoirs. LASER ART strategies serve to maximize viral suppression in reservoirs of infection. A putative next step needs to target the viral reservoirs by either preventing new infections or engaging adaptive antiretroviral defenses through bnAbs. Alternatively, “shock and kill” or CCR5 receptor modifications can be employed as well as other “anti-latency agents.” Any or all strategies would require a final pathway that would target latently infected cells and can be achieved through CRISPR-Cas9 based editing to excise latent proviral DNA from the host genome. The specific and efficient excision of HIV-1 DNA fragments would in a final pathway can lead to HIV-1 elimination from its infected human host.
While HIV-1 cure strategies hold significant promise, a considerable hurdle rests in the virus’ abilities to rapidly mutate and develop resistance. Thus, for any or all of the listed approaches, viral heterogeneity may be a limiting factor in ultimate success. While several of the functional cure approaches have already reached phase I and II clinical testing, none have yet to achieve the final goal of complete HIV cure. If ultimately successful that would change the landscape of the disease and end the epidemic in an ultimate dramatic fashion. With the rapid pace of the science and the multiple approaches already put to bear, one could predict that an HIV cure is seen within the next decade [129].
5.1. Search strategy and selection criteria
Data for this review were identified by searching in PubMed using the following search terms: HIV cure, Innate and MP cell-based immune response to HIV infection, ART, latency reversing agents, broadly neutralizing antibodies, long acting slow effective release antiretroviral therapy; HIV-1 tissue reservoirs; CRISPR-Cas9 gene editing. Only articles published in English were included.
5.2. Outstanding questions
Can improved ARV biodistribution into viral reservoirs affect “cure” treatment outcomes?
Can LASER ART facilitate viral gene excisions?
Can multimodal therapies enhance treatment outcomes?
If toxicities emerge from combinatorial approach, can they be reversed?
Which are the most likely schemes to elicit an HIV cure and which of these are scalable?
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
Acknowledgments
Acknowledgements
We thank Ms. Robin Taylor, Drs. JoEllyn McMillan and Benson Edagwa, University of Nebraska Medical Center, for critical review of the manuscript and Dr. Kamel Khalili, Temple University, for suggestions made on reviews’ content.
Funding sources
This work is supported by the University of Nebraska Foundation, which includes donations from the Carol Swarts, M.D., Emerging Neuroscience Research Laboratory, the Margaret R. Larson Professorship, and the Frances and Louie Blumkin, and Harriet Singer Endowments; the University of Nebraska Medical Center's Vice Chancellor for Research Core Facilities, and the National Institutes of Health grants 1R01AI145542-01A1, P01 DA028555, R01 NS36126, P01 NS31492, 2R01 NS034239, P01 MH64570, P01 NS43985, P30 MH062261, R01 AG043540, and 1 R56 AI138613-01A1.
Author contributions
PD, HEG, HS and MB provided conceptualization, shared in the writing of the orginal draft and performed review and editing support for the final manuscript. BK edited the final draft, produced the video and created and edited each of the figures and figure captions.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2020.102667.
Appendix. Supplementary materials
Video Abstract: The video abstract outlines putative cure strategies for HIV infection.
References
- 1.Volberding P.A., Deeks S.G. Antiretroviral therapy and management of HIV infection. Lancet. 2010;376(9734):49–62. doi: 10.1016/S0140-6736(10)60676-9. [DOI] [PubMed] [Google Scholar]
- 2.Lorenzo-Redondo R., Fryer H.R., Bedford T., Kim E.-.Y., Archer J., Kosakovsky Pond S.L. Persistent HIV-1 replication maintains the tissue reservoir during therapy. Nature. 2016;530(7588):51–56. doi: 10.1038/nature16933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gray J.I., Westerhof L.M., MacLeod M.K.L. The roles of resident, central and effector memory CD4 T-cells in protective immunity following infection or vaccination. Immunology. 2018 doi: 10.1111/imm.12929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong M.E., Jaworowski A., Hearps A.C. The HIV reservoir in monocytes and macrophages. Front Immunol. 2019;10:1435. doi: 10.3389/fimmu.2019.01435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pierson T., McArthur J., Siliciano R.F. Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy. Annu Rev Immunol. 2000;18:665–708. doi: 10.1146/annurev.immunol.18.1.665. [DOI] [PubMed] [Google Scholar]
- 6.Haggerty C.M., Pitt E., Siliciano R.F. The latent reservoir for HIV-1 in resting CD4+ T cells and other viral reservoirs during chronic infection: insights from treatment and treatment-interruption trials. Curr Opin HIV AIDS. 2006;1(1):62–68. doi: 10.1097/01.COH.0000191897.78309.70. [DOI] [PubMed] [Google Scholar]
- 7.Winnall W.R., Lloyd S.B., De Rose R., Alcantara S., Amarasena T.H., Hedger M.P. Simian immunodeficiency virus infection and immune responses in the pig-tailed macaque testis. J Leukoc Biol. 2015;97(3):599–609. doi: 10.1189/jlb.4A0914-438R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Castro-Gonzalez S., Colomer-Lluch M., Serra-Moreno R. Barriers for HIV cure: the latent reservoir. AIDS Res Hum Retroviruses. 2018;34(9):739–759. doi: 10.1089/aid.2018.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Siliciano J.D., Siliciano R.F. Recent developments in the effort to cure HIV infection: going beyond N = 1. J. Clin. Invest. 2016;126(2):409–414. doi: 10.1172/JCI86047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perreau M., Banga R., Pantaleo G. Targeted immune interventions for an HIV-1 cure. Trends Mol Med. 2017;23(10):945–961. doi: 10.1016/j.molmed.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Murray A.J., Kwon K.J., Farber D.L., Siliciano R.F. The latent reservoir for HIV-1: how immunologic memory and clonal expansion contribute to HIV-1 persistence. J Immunol. 2016;197(2):407–417. doi: 10.4049/jimmunol.1600343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cartwright E.K., Palesch D., Mavigner M., Paiardini M., Chahroudi A., Silvestri G. Initiation of antiretroviral therapy restores CD4+ t memory stem cell homeostasis in simian immunodeficiency virus-infected macaques. J Virol. 2016;90(15):6699–6708. doi: 10.1128/JVI.00492-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar N.A., McBrien J.B., Carnathan D.G., Mavigner M., Mattingly C., White E.R. Antibody-Mediated CD4 depletion induces homeostatic CD4(+) T cell proliferation without detectable virus reactivation in antiretroviral therapy-treated simian immunodeficiency virus-infected macaques. J Virol. 2018;92(22) doi: 10.1128/JVI.01235-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crooks A.M., Bateson R., Cope A.B., Dahl N.P., Griggs M.K., Kuruc J.D. Precise quantitation of the latent HIV-1 reservoir: implications for eradication strategies. J Infect Dis. 2015;212(9):1361–1365. doi: 10.1093/infdis/jiv218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finzi D., Blankson J., Siliciano J.D., Margolick J.B., Chadwick K., Pierson T. Latent infection of CD4+ T cells provides a mechanism for lifelong persistence of HIV-1, even in patients on effective combination therapy. Nat Med. 1999;5(5):512–517. doi: 10.1038/8394. [DOI] [PubMed] [Google Scholar]
- 16.Hill A.L., Rosenbloom D.I., Fu F., Nowak M.A., Siliciano R.F. Predicting the outcomes of treatment to eradicate the latent reservoir for HIV-1. Proc Natl Acad Sci U S A. 2014;111(37):13475–13480. doi: 10.1073/pnas.1406663111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Siliciano J.D., Kajdas J., Finzi D., Quinn T.C., Chadwick K., Margolick J.B. Long-term follow-up studies confirm the stability of the latent reservoir for HIV-1 in resting CD4+ T cells. Nat Med. 2003;9(6):727–728. doi: 10.1038/nm880. [DOI] [PubMed] [Google Scholar]
- 18.Gendelman H.E., McMillan J., Bade A.N., Edagwa B., Kevadiya B.D. The promise of long-acting antiretroviral therapies: from need to manufacture. Trends Microbiol. 2019;27(7):593–606. doi: 10.1016/j.tim.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nachman S., Townsend C.L., Abrams E.J., Archary M., Capparelli E., Clayden P. Long-acting or extended-release antiretroviral products for HIV treatment and prevention in infants, children, adolescents, and pregnant and breastfeeding women: knowledge gaps and research priorities. Lancet HIV. 2019;6(8):e552–e5e8. doi: 10.1016/S2352-3018(19)30147-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Castor D., Meyers K., Allen S. The only way is up: priorities for implementing long-acting antiretrovirals for HIV prevention and treatment. Curr Opin HIV AIDS. 2020;15(1):73–80. doi: 10.1097/COH.0000000000000601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weld E.D., Flexner C. Long-acting implants to treat and prevent HIV infection. Curr Opin HIV AIDS. 2020;15(1):33–41. doi: 10.1097/COH.0000000000000591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.D'Amico R., Margolis D.A. Long-acting injectable therapy: an emerging paradigm for the treatment of HIV infection. Curr Opin HIV AIDS. 2020;15(1):13–18. doi: 10.1097/COH.0000000000000606. [DOI] [PubMed] [Google Scholar]
- 23.Dash P.K., Kaminski R., Bella R., Su H., Mathews S., Ahooyi T.M. Sequential laser art and CRISPR treatments eliminate HIV-1 in a subset of infected humanized mice. Nat Commun. 2019;10(1):2753. doi: 10.1038/s41467-019-10366-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bella R., Kaminski R., Mancuso P., Young W.B., Chen C., Sariyer R. Removal of HIV DNA by CRISPR from patient blood engrafts in humanized mice. Mol Ther Nucleic Acids. 2018;12:275–282. doi: 10.1016/j.omtn.2018.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin C., Zhang T., Qu X., Zhang Y., Putatunda R., Xiao X. In vivo excision of HIV-1 provirus by saCas9 and multiplex single-guide RNAs in animal models. Mol Ther. 2017;25(5):1168–1186. doi: 10.1016/j.ymthe.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaminski R., Chen Y., Fischer T., Tedaldi E., Napoli A., Zhang Y. Elimination of HIV-1 genomes from human T-lymphoid cells by CRISPR/Cas9 gene editing. Sci Rep. 2016;6:22555. doi: 10.1038/srep22555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sullivan N.T., Dampier W., Chung C.H., Allen A.G., Atkins A., Pirrone V. Novel gRNA design pipeline to develop broad-spectrum CRISPR/Cas9 gRNAs for safe targeting of the HIV-1 quasispecies in patients. Sci Rep. 2019;9(1):17088. doi: 10.1038/s41598-019-52353-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merad M., Sathe P., Helft J., Miller J., Mortha A. The dendritic cell lineage: ontogeny and function of dendritic cells and their subsets in the steady state and the inflamed setting. Annu Rev Immunol. 2013;31:563–604. doi: 10.1146/annurev-immunol-020711-074950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Herskovitz J., Gendelman H.E. HIV and the macrophage: from cell reservoirs to drug delivery to viral eradication. J Neuroimmune Pharmacol. 2019;14(1):52–67. doi: 10.1007/s11481-018-9785-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clayton K.L., Garcia J.V., Clements J.E., Walker B.D. HIV infection of macrophages: implications for pathogenesis and cure. Pathog Immun. 2017;2(2):179–192. doi: 10.20411/pai.v2i2.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manches O., Frleta D., Bhardwaj N. Dendritic cells in progression and pathology of HIV infection. Trends Immunol. 2014;35(3):114–122. doi: 10.1016/j.it.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodriguez-Plata M.T., Urrutia A., Cardinaud S., Buzon M.J., Izquierdo-Useros N., Prado J.G. HIV-1 capture and antigen presentation by dendritic cells: enhanced viral capture does not correlate with better T cell activation. J Immunol. 2012;188(12):6036–6045. doi: 10.4049/jimmunol.1200267. [DOI] [PubMed] [Google Scholar]
- 33.van Montfort T., van der Sluis R., Darcis G., Beaty D., Groen K., Pasternak A.O. Dendritic cells potently purge latent HIV-1 beyond TCR-stimulation, activating the PI3K-Akt-mTOR pathway. EBioMedicine. 2019;42:97–108. doi: 10.1016/j.ebiom.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kristoff J., Palma M.L., Garcia-Bates T.M., Shen C., Sluis-Cremer N., Gupta P. Type 1-programmed dendritic cells drive antigen-specific latency reversal and immune elimination of persistent HIV-1. EBioMedicine. 2019;43:295–306. doi: 10.1016/j.ebiom.2019.03.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kanzler H., Barrat F.J., Hessel E.M., Coffman R.L. Therapeutic targeting of innate immunity with toll-like receptor agonists and antagonists. Nat Med. 2007;13(5):552–559. doi: 10.1038/nm1589. [DOI] [PubMed] [Google Scholar]
- 36.Krarup A.R., Abdel-Mohsen M., Schleimann M.H., Vibholm L., Engen P.A., Dige A. The TLR9 agonist MGN1703 triggers a potent type i interferon response in the sigmoid colon. Mucosal Immunol. 2018;11(2):449–461. doi: 10.1038/mi.2017.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vibholm L., Schleimann M.H., Hojen J.F., Benfield T., Offersen R., Rasmussen K. Short-Course toll-like receptor 9 agonist treatment impacts innate immunity and plasma viremia in individuals with human immunodeficiency virus infection. Clin Infect Dis. 2017;64(12):1686–1695. doi: 10.1093/cid/cix201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lim S.Y., Osuna C.E., Hraber P.T., Hesselgesser J., Gerold J.M., Barnes T.L. TLR7 agonists induce transient viremia and reduce the viral reservoir in SIV-infected rhesus macaques on antiretroviral therapy. Sci Transl Med. 2018;10(439) doi: 10.1126/scitranslmed.aao4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Del Prete G.Q., Alvord W.G., Li Y., Deleage C., Nag M., Oswald K. TLR7 agonist administration to SIV-infected macaques receiving early initiated cART does not induce plasma viremia. JCI Insight. 2019;4(11) doi: 10.1172/jci.insight.127717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bekerman E., Hesselgesser J., Carr B., Nagel M., Hung M., Wang A. PD-1 blockade and TLR7 activation lack therapeutic benefit in chronic simian immunodeficiency virus-infected macaques on antiretroviral therapy. Antimicrob Agents Chemother. 2019;63(11) doi: 10.1128/AAC.01163-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fauci A.S., Mavilio D., Kottilil S. NK cells in HIV infection: paradigm for protection or targets for ambush. Nat Rev Immunol. 2005;5(11):835–843. doi: 10.1038/nri1711. [DOI] [PubMed] [Google Scholar]
- 42.Macedo A.B., Novis C.L., De Assis C.M., Sorensen E.S., Moszczynski P., Huang S.H. Dual TLR2 and TLR7 agonists as HIV latency-reversing agents. JCI Insight. 2018;3(19) doi: 10.1172/jci.insight.122673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Offersen R., Nissen S.K., Rasmussen T.A., Ostergaard L., Denton P.W., Sogaard O.S. A novel toll-like receptor 9 agonist, MGN1703, enhances HIV-1 transcription and NK cell-mediated inhibition of HIV-1-Infected autologous CD4+ T cells. J Virol. 2016;90(9):4441–4453. doi: 10.1128/JVI.00222-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Group I.-E.S., Committee S.S., Abrams D., Levy Y., Losso M.H., Babiker A. Interleukin-2 therapy in patients with HIV infection. N Engl J Med. 2009;361(16):1548–1559. doi: 10.1056/NEJMoa0903175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Katlama C., Lambert-Niclot S., Assoumou L., Papagno L., Lecardonnel F., Zoorob R. Treatment intensification followed by interleukin-7 reactivates HIV without reducing total HIV DNA: a randomized trial. Aids. 2016;30(2):221–230. doi: 10.1097/QAD.0000000000000894. [DOI] [PubMed] [Google Scholar]
- 46.Moron-Lopez S., Gomez-Mora E., Salgado M., Ouchi D., Puertas M.C., Urrea V. Short-term treatment with interferon alfa diminishes expression of HIV-1 and reduces CD4+ T-Cell activation in patients coinfected with HIV and Hepatitis C virus and receiving antiretroviral therapy. J Infect Dis. 2016;213(6):1008–1012. doi: 10.1093/infdis/jiv521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abdel-Mohsen M., Deng X., Liegler T., Guatelli J.C., Salama M.S., Ghanem Hel D. Effects of alpha interferon treatment on intrinsic anti-HIV-1 immunity in vivo. J Virol. 2014;88(1):763–767. doi: 10.1128/JVI.02687-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Azzoni L., Foulkes A.S., Papasavvas E., Mexas A.M., Lynn K.M., Mounzer K. Pegylated interferon alfa-2a monotherapy results in suppression of HIV type 1 replication and decreased cell-associated HIV DNA integration. J Infect Dis. 2013;207(2):213–222. doi: 10.1093/infdis/jis663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Azzoni L., Foulkes A.S., Papasavvas E., Mexas A.M., Lynn K.M., Mounzer K. Improved treatment for primary HIV infection by interferon-alfa therapy? does HCV treatment in HIV/HCV coinfected patients help us to test this hypothesis? reply to zur Wiesch and van Lunzen. J Infect Dis. 2013;208(2):363. doi: 10.1093/infdis/jit160. [DOI] [PubMed] [Google Scholar]
- 50.Levy Y., Sereti I., Tambussi G., Routy J.P., Lelievre J.D., Delfraissy J.F. Effects of recombinant human interleukin 7 on T-cell recovery and thymic output in HIV-infected patients receiving antiretroviral therapy: results of a phase I/IIa randomized, placebo-controlled, multicenter study. Clin Infect Dis. 2012;55(2):291–300. doi: 10.1093/cid/cis383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wilson E.M., Sereti I. Immune restoration after antiretroviral therapy: the pitfalls of hasty or incomplete repairs. Immunol Rev. 2013;254(1):343–354. doi: 10.1111/imr.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ali A., Kitchen S.G., Chen I.S.Y., Ng H.L., Zack J.A., Yang O.O. HIV-1-Specific chimeric antigen receptors based on broadly neutralizing antibodies. J. Virol. 2016;90(15):6999–7006. doi: 10.1128/JVI.00805-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.von Bredow B., Arias J.F., Heyer L.N., Moldt B., Le K., Robinson J.E. Comparison of antibody-dependent cell-mediated cytotoxicity and virus neutralization by HIV-1 Env-specific monoclonal antibodies. J Virol. 2016;90(13):6127–6139. doi: 10.1128/JVI.00347-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Velu V., Titanji K., Zhu B., Husain S., Pladevega A., Lai L. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2009;458(7235):206–210. doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chukwuma V.U., Kose N., Sather D.N., Sapparapu G., Falk R., King H. Increased breadth of HIV-1 neutralization achieved by diverse antibody clones each with limited neutralization breadth. PLoS ONE. 2018;13(12) doi: 10.1371/journal.pone.0209437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Boer R.J., Perelson A.S. How germinal centers evolve broadly neutralizing antibodies: the breadth of the follicular helper T cell response. J. Virol. 2017;91(22) doi: 10.1128/JVI.00983-17. e00983-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bhiman J.N., Anthony C., Doria-Rose N.A., Karimanzira O., Schramm C.A., Khoza T. Viral variants that initiate and drive maturation of V1V2-directed HIV-1 broadly neutralizing antibodies. Nat. Med. 2015;21(11):1332–1336. doi: 10.1038/nm.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu Y., Xue J., Wang C., Li W., Wang L., Chen W. Rapid elimination of broadly neutralizing antibodies correlates with treatment failure in the acute phase of simian-human immunodeficiency virus infection. J. Virol. 2019;93(20) doi: 10.1128/JVI.01077-19. e01077-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gautam R., Nishimura Y., Gaughan N., Gazumyan A., Schoofs T., Buckler-White A. A single injection of crystallizable fragment domain–modified antibodies elicits durable protection from SHIV infection. Nat. Med. 2018;24(5):610–616. doi: 10.1038/s41591-018-0001-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu L., Pegu A., Rao E., Doria-Rose N., Beninga J., McKee K. Trispecific broadly neutralizing HIV antibodies mediate potent SHIV protection in macaques. Science. 2017;358(6359):85–90. doi: 10.1126/science.aan8630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gruell H., Klein F. Opening fronts in HIV vaccine development: tracking the development of broadly neutralizing antibodies. Nat Med. 2014;20(5):478–479. doi: 10.1038/nm.3567. [DOI] [PubMed] [Google Scholar]
- 62.Ferrari G., Haynes B.F., Koenig S., Nordstrom J.L., Margolis D.M., Tomaras G.D. Envelope-specific antibodies and antibody-derived molecules for treating and curing HIV infection. Nat Rev Drug Discov. 2016;15(12):823–834. doi: 10.1038/nrd.2016.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Caskey M., Klein F., Nussenzweig M.C. Broadly neutralizing anti-HIV-1 monoclonal antibodies in the clinic. Nat. Med. 2019;25(4):547–553. doi: 10.1038/s41591-019-0412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Williams L.D., Ofek G., Schätzle S., McDaniel J.R., Lu X., Nicely N.I. Potent and broad HIV-neutralizing antibodies in memory b cells and plasma. Science Immunology. 2017;2(7) doi: 10.1126/sciimmunol.aal2200. eaal2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moody M.A., Pedroza-Pacheco I., Vandergrift N.A., Chui C., Lloyd K.E., Parks R. Immune perturbations in HIV-1–infected individuals who make broadly neutralizing antibodies. Science Immunology. 2016;1(1) doi: 10.1126/sciimmunol.aag0851. aag0851-aag. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shingai M., Nishimura Y., Klein F., Mouquet H., Donau O.K., Plishka R. Antibody-mediated immunotherapy of macaques chronically infected with SHIV suppresses viraemia. Nature. 2013;503(7475):277–280. doi: 10.1038/nature12746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Julg B., Liu P.T., Wagh K., Fischer W.M., Abbink P., Mercado N.B. Protection against a mixed SHIV challenge by a broadly neutralizing antibody cocktail. Sci Transl Med. 2017;9(408) doi: 10.1126/scitranslmed.aao4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bonsignori M., Wiehe K., Grimm S.K., Lynch R., Yang G., Kozink D.M. An autoreactive antibody from an SLE/HIV-1 individual broadly neutralizes HIV-1. J Clin Invest. 2014;124(4):1835–1843. doi: 10.1172/JCI73441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Havenar-Daughton C., Sarkar A., Kulp D.W., Toy L., Hu X., Deresa I. The human naive B cell repertoire contains distinct subclasses for a germline-targeting HIV-1 vaccine immunogen. Sci Transl Med. 2018;10(448) doi: 10.1126/scitranslmed.aat0381. eaat0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sundling C., Li Y., Huynh N., Poulsen C., Wilson R., O'Dell S. High-Resolution definition of vaccine-elicited B cell responses against the HIV primary receptor binding site. Sci Transl Med. 2012;4(142) doi: 10.1126/scitranslmed.3003752. 142ra96-ra96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahmed Y., Tian M., Gao Y. Development of an anti-HIV vaccine eliciting broadly neutralizing antibodies. AIDS Res Ther. 2017;14(1):50. doi: 10.1186/s12981-017-0178-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dey A.K., Cupo A., Ozorowski G., Sharma V.K., Behrens A.J., Go E.P. cGMP production and analysis of BG505 SOSIP.664, an extensively glycosylated, trimeric HIV-1 envelope glycoprotein vaccine candidate. Biotechnol Bioeng. 2018;115(4):885–899. doi: 10.1002/bit.26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gaudinski M.R., Coates E.E., Houser K.V., Chen G.L., Yamshchikov G., Saunders J.G. Safety and pharmacokinetics of the Fc-modified HIV-1 human monoclonal antibody VRC01LS: a phase 1 open-label clinical trial in healthy adults. PLoS Med. 2018;15(1) doi: 10.1371/journal.pmed.1002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Caskey M., Schoofs T., Gruell H., Settler A., Karagounis T., Kreider E.F. Antibody 10-1074 suppresses viremia in HIV-1-infected individuals. Nat Med. 2017;23(2):185–191. doi: 10.1038/nm.4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scheid J.F., Horwitz J.A., Bar-On Y., Kreider E.F., Lu C.-.L., Lorenzi J.C.C. HIV-1 antibody 3BNC117 suppresses viral rebound in humans during treatment interruption. Nature. 2016;535(7613):556–560. doi: 10.1038/nature18929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mendoza P., Gruell H., Nogueira L., Pai J.A., Butler A.L., Millard K. Combination therapy with anti-HIV-1 antibodies maintains viral suppression. Nature. 2018;561(7724):479–484. doi: 10.1038/s41586-018-0531-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bournazos S., Klein F., Pietzsch J., Seaman M.S., Nussenzweig M.C., Ravetch J.V. Broadly neutralizing anti-HIV-1 antibodies require fc effector functions for in vivo activity. Cell. 2014;158(6):1243–1253. doi: 10.1016/j.cell.2014.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Caskey M., Klein F., Lorenzi J.C.C., Seaman M.S., West A.P., Buckley N. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522(7557):487–491. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bruel T., Guivel-Benhassine F., Amraoui S., Malbec M., Richard L., Bourdic K. Elimination of HIV-1-infected cells by broadly neutralizing antibodies. Nat Commun. 2016;7:10844. doi: 10.1038/ncomms10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Borducchi E.N., Cabral C., Stephenson K.E., Liu J., Abbink P., Ng'ang'a D. Ad26/MVA therapeutic vaccination with TLR7 stimulation in SIV-infected rhesus monkeys. Nature. 2016;540(7632):284–287. doi: 10.1038/nature20583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Brodie S.J., Lewinsohn D.A., Patterson B.K., Jiyamapa D., Krieger J., Corey L. In vivo migration and function of transferred HIV-1-specific cytotoxic T cells. Nat Med. 1999;5(1):34–41. doi: 10.1038/4716. [DOI] [PubMed] [Google Scholar]
- 82.Leibman R.S., Riley J.L. Engineering T cells to functionally cure HIV-1 infection. Mol Ther. 2015;23(7):1149–1159. doi: 10.1038/mt.2015.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zanoni M., Palesch D., Silvestri G. Longing for HIV protection. Nat Microbiol. 2018;3(6):648–649. doi: 10.1038/s41564-018-0169-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Deng K., Pertea M., Rongvaux A., Wang L., Durand C.M., Ghiaur G. Broad CTL response is required to clear latent HIV-1 due to dominance of escape mutations. Nature. 2015;517(7534):381–385. doi: 10.1038/nature14053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sloan D.D., Lam C.-Y.K., Irrinki A., Liu L., Tsai A., Pace C.S. Targeting HIV reservoir in infected CD4 T cells by dual-affinity re-targeting molecules (DARTs) that bind HIV envelope and recruit cytotoxic T cells. PLoS Pathog. 2015;11(11) doi: 10.1371/journal.ppat.1005233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sung J.A.M., Pickeral J., Liu L., Stanfield-Oakley S.A., Lam C.-Y.K., Garrido C. Dual-Affinity re-targeting proteins direct T cell–mediated cytolysis of latently HIV-infected cells. J. Clin. Invest. 2015;125(11):4077–4090. doi: 10.1172/JCI82314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scholler J., Brady T.L., Binder-Scholl G., Hwang W.T., Plesa G., Hege K.M. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med. 2012;4(132) doi: 10.1126/scitranslmed.3003761. 132ra53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.June C.H., Riddell S.R., Schumacher T.N. Adoptive cellular therapy: a race to the finish line. Sci Transl Med. 2015;7(280) doi: 10.1126/scitranslmed.aaa3643. 280ps7. [DOI] [PubMed] [Google Scholar]
- 89.June C.H., Sadelain M. Chimeric antigen receptor therapy. N Engl J Med. 2018;379(1):64–73. doi: 10.1056/NEJMra1706169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Walker R.E., Bechtel C.M., Natarajan V., Baseler M., Hege K.M., Metcalf J.A. Long-term in vivo survival of receptor-modified syngeneic T cells in patients with human immunodeficiency virus infection. Blood. 2000;96(2):467–474. [PubMed] [Google Scholar]
- 91.Haran K.P., Hajduczki A., Pampusch M.S., Mwakalundwa G., Vargas-Inchaustegui D.A., Rakasz E.G. Simian immunodeficiency virus (SIV)-Specific chimeric antigen receptor-T cells engineered to target B cell follicles and suppress SIV replication. Front Immunol. 2018;9:492. doi: 10.3389/fimmu.2018.00492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fukazawa Y., Lum R., Okoye A.A., Park H., Matsuda K., Bae J.Y. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med. 2015;21(2):132–139. doi: 10.1038/nm.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tjernlund A., Burgener A., Lindvall J.M., Peng T., Zhu J., Ohrmalm L. In situ staining and laser capture microdissection of lymph node residing SIV Gag-specific CD8+ T cells–A tool to interrogate a functional immune response ex vivo. PLoS ONE. 2016;11(3) doi: 10.1371/journal.pone.0149907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hashemi P., Barreto K., Bernhard W., Lomness A., Honson N., Pfeifer T.A. Compounds producing an effective combinatorial regimen for disruption of HIV-1 latency. EMBO Mol Med. 2018;10(2):160–174. doi: 10.15252/emmm.201708193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hashemi P., Sadowski I. Diversity of small molecule HIV-1 latency reversing agents identified in low- and high-throughput small molecule screens. Med Res Rev. 2018 doi: 10.1002/med.21638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kim Y., Anderson J.L., Lewin S.R. Getting the “Kill” into “Shock and Kill”: strategies to eliminate latent HIV. Cell Host Microbe. 2018;23(1):14–26. doi: 10.1016/j.chom.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Darcis G., Bouchat S., Kula A., Van Driessche B., Delacourt N., Vanhulle C. Reactivation capacity by latency-reversing agents ex vivo correlates with the size of the HIV-1 reservoir. Aids. 2017;31(2):181–189. doi: 10.1097/QAD.0000000000001290. [DOI] [PubMed] [Google Scholar]
- 98.Spivak A.M., Planelles V. Novel latency reversal agents for HIV-1 cure. Annu Rev Med. 2018;69:421–436. doi: 10.1146/annurev-med-052716-031710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gutierrez C., Serrano-Villar S., Madrid-Elena N., Perez-Elias M.J., Martin M.E., Barbas C. Bryostatin-1 for latent virus reactivation in HIV-infected patients on antiretroviral therapy. Aids. 2016;30(9):1385–1392. doi: 10.1097/QAD.0000000000001064. [DOI] [PubMed] [Google Scholar]
- 100.Cummins N.W., Sainski A.M., Dai H., Natesampillai S., Pang Y.P., Bren G.D. Prime, shock, and kill: priming CD4 T cells from HIV patients with a BCL-2 antagonist before HIV reactivation reduces HIV reservoir size. J Virol. 2016;90(8):4032–4048. doi: 10.1128/JVI.03179-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hosmane N.N., Kwon K.J., Bruner K.M., Capoferri A.A., Beg S., Rosenbloom D.I.S. Proliferation of latently infected CD4+T cells carrying replication-competent HIV-1: potential role in latent reservoir dynamics. J. Exp. Med. 2017;214(4):959–972. doi: 10.1084/jem.20170193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sillman B., Bade A.N., Dash P.K., Bhargavan B., Kocher T., Mathews S. Creation of a long-acting nanoformulated dolutegravir. Nat Commun. 2018;9(1):443. doi: 10.1038/s41467-018-02885-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Buehler D.C., Marsden M.D., Shen S., Toso D.B., Wu X., Loo J.A. Bioengineered vaults: self-assembling protein shell-lipophilic core nanoparticles for drug delivery. ACS Nano. 2014;8(8):7723–7732. doi: 10.1021/nn5002694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kovochich M., Marsden M.D., Zack J.A. Activation of latent HIV using drug-loaded nanoparticles. PLoS ONE. 2011;6(4):e18270. doi: 10.1371/journal.pone.0018270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Laird G.M., Bullen C.K., Rosenbloom D.I., Martin A.R., Hill A.L., Durand C.M. Ex vivo analysis identifies effective HIV-1 latency-reversing drug combinations. J Clin Invest. 2015;125(5):1901–1912. doi: 10.1172/JCI80142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wykes M.N., Lewin S.R. Immune checkpoint blockade in infectious diseases. Nat Rev Immunol. 2018;18(2):91–104. doi: 10.1038/nri.2017.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Petravic J., Martyushev A., Reece J.C., Kent S.J., Davenport M.P. Modeling the timing of antilatency drug administration during HIV treatment. J. Virol. 2014;88(24):14050–14056. doi: 10.1128/JVI.01701-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chomont N., Okoye A.A., Favre D., Trautmann L. Wake me up before you go: a strategy to reduce the latent HIV reservoir. AIDS. 2018;32(3):293–298. doi: 10.1097/QAD.0000000000001695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Colby D.J., Trautmann L., Pinyakorn S., Leyre L., Pagliuzza A., Kroon E. Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig I acute HIV infection. Nat Med. 2018;24(7):923–926. doi: 10.1038/s41591-018-0026-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Borducchi E.N., Liu J., Nkolola J.P., Cadena A.M., Yu W.-.H., Fischinger S. Antibody and TLR7 agonist delay viral rebound in SHIV-infected monkeys. Nature. 2018;563(7731):360–364. doi: 10.1038/s41586-018-0600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jones R.B., Mueller S., O'Connor R., Rimpel K., Sloan D.D., Karel D. A subset of latency-reversing agents expose HIV-Infected resting CD4+ T-Cells to recognition by cytotoxic T-Lymphocytes. PLoS Pathog. 2016;12(4) doi: 10.1371/journal.ppat.1005545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huot N., Jacquelin B., Garcia-Tellez T., Rascle P., Ploquin M.J., Madec Y. Natural killer cells migrate into and control simian immunodeficiency virus replication in lymph node follicles in African green monkeys. Nat Med. 2017;23(11):1277–1286. doi: 10.1038/nm.4421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Benjamin R., Berges B.K., Solis-Leal A., Igbinedion O., Strong C.L., Schiller M.R. TALEN gene editing takes aim on HIV. Hum Genet. 2016;135(9):1059–1070. doi: 10.1007/s00439-016-1678-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Urnov F.D., Rebar E.J., Holmes M.C., Zhang H.S., Gregory P.D. Genome editing with engineered zinc finger nucleases. Nat Rev Genet. 2010;11(9):636–646. doi: 10.1038/nrg2842. [DOI] [PubMed] [Google Scholar]
- 115.Owens B. Zinc-finger nucleases make the cut in HIV. Nat Rev Drug Discov. 2014;13(5):321–322. doi: 10.1038/nrd4316. [DOI] [PubMed] [Google Scholar]
- 116.Xu L., Wang J., Liu Y., Xie L., Su B., Mou D. CRISPR-Edited stem cells in a patient with HIV and acute lymphocytic leukemia. N Engl J Med. 2019;381(13):1240–1247. doi: 10.1056/NEJMoa1817426. [DOI] [PubMed] [Google Scholar]
- 117.Wang G., Zhao N., Berkhout B., Das A.T. A combinatorial CRISPR-Cas9 attack on HIV-1 DNA extinguishes all infectious provirus in infected T cell cultures. Cell Rep. 2016;17(11):2819–2826. doi: 10.1016/j.celrep.2016.11.057. [DOI] [PubMed] [Google Scholar]
- 118.Konermann S., Brigham M.D., Trevino A.E., Joung J., Abudayyeh O.O., Barcena C. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature. 2015;517(7536):583–588. doi: 10.1038/nature14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Saayman S.M., Lazar D.C., Scott T.A., Hart J.R., Takahashi M., Burnett J.C. Potent and targeted activation of latent HIV-1 using the CRISPR/dCas9 activator complex. Mol Ther. 2016;24(3):488–498. doi: 10.1038/mt.2015.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Konermann S., Brigham M.D., Trevino A.E., Joung J., Abudayyeh O.O., Barcena C. Genome-scale transcriptional activation by an engineered CRISPR-Cas9 complex. Nature. 2015;517(7536):583–588. doi: 10.1038/nature14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang Y., Yin C., Zhang T., Li F., Yang W., Kaminski R. CRISPR/gRNA-directed synergistic activation mediator (SAM) induces specific, persistent and robust reactivation of the HIV-1 latent reservoirs. Sci Rep. 2015;5(1):16277. doi: 10.1038/srep16277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Gupta R.K., Abdul-Jawad S., McCoy L.E., Mok H.P., Peppa D., Salgado M. HIV-1 remission following CCR5Delta32/Delta32 haematopoietic stem-cell transplantation. Nature. 2019;568(7751):244–248. doi: 10.1038/s41586-019-1027-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cho S.W., Kim S., Kim Y., Kweon J., Kim H.S., Bae S. Analysis of off-target effects of CRISPR/Cas-derived RNA-guided endonucleases and nickases. Genome Res. 2014;24(1):132–141. doi: 10.1101/gr.162339.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ye L., Wang J., Beyer A.I., Teque F., Cradick T.J., Qi Z. Seamless modification of wild-type induced pluripotent stem cells to the natural CCR5Delta32 mutation confers resistance to HIV infection. Proc Natl Acad Sci U S A. 2014;111(26):9591–9596. doi: 10.1073/pnas.1407473111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wang W., Ye C., Liu J., Zhang D., Kimata J.T., Zhou P. CCR5 gene disruption via lentiviral vectors expressing Cas9 and single guided RNA renders cells resistant to HIV-1 infection. PLoS ONE. 2014;9(12) doi: 10.1371/journal.pone.0115987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Monroe K.M., Yang Z., Johnson J.R., Geng X., Doitsh G., Krogan N.J. IFI16 DNA sensor is required for death of lymphoid CD4 T cells abortively infected with HIV. Science. 2014;343(6169):428–432. doi: 10.1126/science.1243640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Mandal P.K., Ferreira L.M., Collins R., Meissner T.B., Boutwell C.L., Friesen M. Efficient ablation of genes in human hematopoietic stem and effector cells using CRISPR/Cas9. Cell Stem Cell. 2014;15(5):643–652. doi: 10.1016/j.stem.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Verheyen J., Thielen A., Lubke N., Dirks M., Widera M., Dittmer U. Rapid rebound of a preexisting CXCR4-tropic human immunodeficiency virus variant after allogeneic transplantation with CCR5 Delta32 homozygous stem cells. Clin Infect Dis. 2019;68(4):684–687. doi: 10.1093/cid/ciy565. [DOI] [PubMed] [Google Scholar]
- 129.June C.H. Emerging use of CRISPR technology - Chasing the elusive HIV cure. N Engl J Med. 2019;381(13):1281–1283. doi: 10.1056/NEJMe1910754. [DOI] [PubMed] [Google Scholar]
- 130.Su H., Cheng Y., Sravanam S., Mathews S., Gorantla S., Poluektova L.Y. Immune activations and viral tissue compartmentalization during progressive HIV-1 infection of humanized mice. Front Immunol. 2019;10:340. doi: 10.3389/fimmu.2019.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Margolis D.A., Gonzalez-Garcia J., Stellbrink H.J., Eron J.J., Yazdanpanah Y., Podzamczer D. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet. 2017;390(10101):1499–1510. doi: 10.1016/S0140-6736(17)31917-7. [DOI] [PubMed] [Google Scholar]
- 132.Zhou T., Su H., Dash P., Lin Z., Dyavar Shetty B.L., Kocher T. Creation of a nanoformulated cabotegravir prodrug with improved antiretroviral profiles. Biomaterials. 2018;151:53–65. doi: 10.1016/j.biomaterials.2017.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video Abstract: The video abstract outlines putative cure strategies for HIV infection.