Highlights
-
•
PulseON® is a new legume ingredient containing Type 1 resistant starch.
-
•
Replacing wheat flour with PulseON™ reduces biscuit in vitro starch digestion by up to 50%.
-
•
PulseON® is a source of legume protein, dietary fibre, and potential low glycaemic starch.
Keywords: Starch digestibility, Biscuit, Legume, Wheat, Composite flour, Resistant starch, Glycaemic Index, PulseON®
Abstract
Many carbohydrate foods contain starch that is rapidly digested and elicits a high Glycaemic Index. A legume ingredient (PulseON®) rich in Type 1 resistant starch (RS1) was recently developed; however, its potential as a functional ingredient when processed into a food product required assessment. PulseON® was used to replace 0, 25, 50, 75, and 100% of the wheat flour in a savoury biscuit recipe. In vitro starch digestion kinetics of biscuits and water-holding properties of ingredients were assessed. The RS1 in PulseON® did not appear to be structurally compromised during biscuit making. Replacing 50% wheat flour with PulseON® reduced the starch hydrolysis index of biscuits by nearly 60%. This seems to result from the ingredients’ impact on water availability for starch gelatinisation. Overall, these findings highlight the potential of using biscuits as a food vehicle for PulseON® to increase consumer intakes of legume protein, dietary fibre, and potentially low glycaemic starch.
1. Introduction
There is an urgent need to increase the availability and consumption of healthy foods that appeal to consumers in order to counteract the current global epidemic of diet-related diseases such as obesity and type 2 diabetes mellitus (Augustin et al., 2015). Cereals and cereals products are a major source of available (digestible) carbohydrates, predominantly in the form of starch, worldwide. These staple foods are therefore an appealing target for dietary interventions that aim to improve public health (Lafiandra, Riccardi, & Shewry, 2014). Of particular interest is the starch component, which in foods can give rise to vastly different postprandial metabolic responses depending on its susceptibility to digestion (Englyst et al., 1992, Foster-Powell et al., 2002, Goñi et al., 1997, Jenkins et al., 1982). For instance, it is well known that foods containing slowly digestible starch attenuate glycaemic and insulinaemic responses (Jenkins et al., 1982). Moreover, the long-term consumption of low Glycaemic Index foods in the diet can potentially contribute to a reduction in the risk of non-communicable diseases such as type 2 diabetes and cardiovascular disease (Augustin et al., 2015, Livesey et al., 2019). Starch that escapes digestion in the upper gastrointestinal tract, often described as ‘Resistant Starch’ (RS), reaches the colon where its fermentation by resident microbiota is associated with health benefits similar to those reported for dietary fibre (Asp et al., 1996, Nugent, 2005). However, the majority of digestible carbohydrate is consumed in the form of more refined foods such as white and/or wholemeal bread, which are relatively poor sources of RS and tend to have a high Glycaemic Index (Foster-Powell et al., 2002).
Food processing conditions have a major impact on starch structure and thereby influence susceptibility to α-amylase digestion in the upper gastrointestinal tract (Roder et al., 2009, Wang and Copeland, 2013). In its native (raw) form, starch has a highly ordered granular structure with limited susceptibility to amylase attack (sometimes referred to as Type 2 RS) (Dhital et al., 2017, Slaughter et al., 2001). This semi-crystalline granular structure can be disrupted, with sufficient moisture, heat and mechanical energy during processing, as the starch granules swell and lose their ordered structure resulting in a gelatinised starch that is readily digested by amylase (Cooke and Gidley, 1992, Parada and Aguilera, 2009, Roder et al., 2009, Slaughter et al., 2001, Wang and Copeland, 2013). Although flexible, mobile α-glucan chains of hydrothermally-treated starch can re-associate (‘retrograde’) upon cooling (i.e., Type 3 ‘retrograded’ RS), the starch in hydrothermally treated foods is typically digested at a faster rate and to a greater extent than raw starch (Patel et al., 2017). Thus, preserving intrinsically resistant starch structures throughout the processing treatments that transform raw ingredients into safe, palatable and convenient foods presents a formidable challenge.
Controlling the food matrix that surrounds starch during processing and digestion provides an alternative means of regulating starch resistance. Interestingly, the plant cell walls (dietary fibre) that encapsulate starch in the cotyledonous tissue of pulses appear to act as physical barriers to α-amylase, and may remain intact after hydrothermal processing Pallares Pallares et al., 2018, Würsch et al., 1986, and to some extent after transit to the terminal ileum (Noah et al., 1998). Indeed, studies have demonstrated that the low glycaemic and insulinaemic responses to boiled pulses can be mainly attributed to the presence of intact plant cells, which are a source of encapsulated (Type 1) RS (Golay et al., 1986). However, these legume cells can be disrupted during milling, autoclaving and prolonged hydrothermal processing. Moreover, food products prepared from raw legumes that have been milled into flours, do not appear to retain these low glycaemic/insulinaemic properties (Edwards et al., 2014, Golay et al., 1986, Pallares Pallares et al., 2018).
We have recently developed a novel method of processing pulses into a pre-cooked powder product, PulseON® (patent pending; (Edwards, Ellis, et al., 2019)), which, unlike most conventional legume flours, contains high levels of Type 1 RS (RS1), i.e., starch that is physically inaccessible to digestive enzymes. In a compatible food matrix, displacing traditional pulse flours with this novel powder has the potential to enhance dietary intakes of RS, and to lower the glycaemic potency of food products. However, given that RS can become more digestible during processing (Pallares Pallares et al., 2018) it was crucially important to examine the effect of such an ingredient on the starch digestibility of processed food products.
The main aims of this study were to (a) determine the impact of incorporating a novel pulse ingredient (PulseON® - high in RS1 and dietary fibre) into a savoury wheat biscuit on the starch digestibility characteristics; and (b) provide a mechanistic insight of the effects of the novel ingredient on the functional properties of starch in the biscuit matrix. Biscuits, which are a low moisture food, were tested as a suitable delivery vehicle for this novel ingredient. The starch digestion kinetics of composite flour ingredients, composed of wheat and PulseON®, and the biscuits made from them were determined using an in vitro assay, which can be used for predictions of the glycaemic response (Edwards, Cochetel, Setterfield, Perez-Moral, & Warren, 2019). Physico-chemical characterisation of the ingredients and biscuit products was performed to provide understanding of how the hydration characteristics of the ingredients influence starch structure and thereby in vitro digestion kinetics within a limited moisture food system.
2. Material and methods
2.1. Food ingredients
The novel RS-enriched chickpea powder, termed PulseON®, was produced from whole Kabuli chickpeas (Cicer arietinum L.; Russian cv., supplied by AGT Poortman Ltd., London, UK) through a new specific process (patent pending, PCT/GB2019/050284; (Edwards, Ellis, et al., 2019)) by New Food Innovation Ltd., Sutton Bonington, UK. The processing used to make the PulseON® powder included a hydrothermal treatment stage at 100 °C for 1 h and 30 min; thus, the ingredient has been ‘pre-cooked’.
The properties of PulseON® were compared with chickpea flour containing mostly ruptured cotyledonary cells and free starch granules and protein (i.e., from the intracellular matrix), which was obtained from the same batch of chickpeas as PulseON®. The chickpea flour was obtained by grinding the whole chickpeas in a laboratory blender (Analysennuhle A10, Janke & Kunkel) by pulsing at maximum speed for 10 s and repeating this process until the entire sample passed through a 106 µm sieve (i.e., to achieve a similar size to commercially milled chickpea flour).
Composite flours were made by combining PulseON® from chickpeas with Sainsbury’s British plain wheat flour in a range of different ratios such that PulseON® replaced 0, 25, 50, 75 and 100% of the wheat flour in the biscuit recipe. These composite flours were used to obtain biscuits with different ‘doses’ of PulseON®. All other food ingredients used in the biscuit recipes (see Section 2.2) were bought from a local supermarket. The nutrient composition of all ingredients is shown in Supplementary 1.
2.2. Preparation of biscuits
Biscuits were prepared in an experimental kitchen using home-cooking methods with equipment and utensils that resemble those typically used in a domestic kitchen in the UK. Savoury biscuits incorporating different doses of PulseON® in composite flours were prepared following the same recipe (Supplementary 2), such that 100 g dough contained 0.6 g sodium bicarbonate (Dr. Oetker UK Ltd.), 1.4 g of NaCl (SAXA), 13.9 g salted butter (Countrylife), 18.6 g freshly boiled tap water (100 °C and then cooled for 1 min at ambient temperature) and 65.6 g of composite flour (described in Section 2.1). For each dough preparation the dry ingredients (33.5 g flour, 0.3 g sodium bicarbonate and 0.7 g NaCl salt) were manually mixed for exactly 1 min. Butter (7.1 g), cut into chunks, was added and rubbed (using fingers) together with the dry ingredients for exactly 2 min such that the dough mixture resembled large crumbs. Water was added in two batches (3.5 mL and then 6 mL); each addition was followed by exactly 1 min of manual mixing. Biscuit cutters were then used to form the dough mixture into cylindrical shapes with a 70 mm diameter and 5 mm height. All biscuits were baked on a baking tray in a pre-heated forced-air convection oven (Hotpoint, UK) at 190 °C for 20 min.
2.3. Nutrient composition
Proximate analyses of PulseON® and chickpea flour were performed by ALS Food and Pharmaceutical, Chatteris, UK by UKAS accredited methods. In brief, samples were analysed for moisture (by weight loss on drying at 100 to 103 °C), crude protein (N × 6.25), total fat (by NMR), ash, total sugars, dietary fibre (AOAC enzymic-gravimetric method 991.43), and available carbohydrates were calculated ‘by difference’. Energy values were calculated using standard conversion factors. For the other food ingredients, the on-pack nutrition declaration was used. For the biscuits, the change in weight on baking of the dough was recorded and the moisture content of the final baked biscuit was used to express nutrient composition data on a ‘per baked biscuit’ basis.
2.4. In vitro amylolysis
Amylolysis (starch digestion) of biscuits made with composite flours (i.e., different proportions of PulseON® and wheat flour) and the original PulseON® and wheat flour ingredients was evaluated according to the methods of Edwards et al. (2014) using porcine-pancreatic α-amylase (Sigma-Aldrich Co. Ltd, UK) (A6255, EC 3.2.1.1) prepared in phosphate buffered saline (‘PBS’, from Sigma-Aldrich Co. Ltd, UK).
Samples of biscuits, wheat flour and PulseON® were prepared for testing immediately prior to the amylolysis assay. Biscuits were crushed with a pestle and mortar and sieved to obtain ground biscuit particles between 250 and 500 µm that were weighed out into tubes to achieve 100 mg starch per tube, and then suspended in 10 mL PBS (pH 7.4 at 37 °C) immediately before testing. The wheat flour, chickpea flour and PulseON® ingredients were weighed out ‘as is’ and suspended in 10 mL PBS. These were either submitted to analysis in the ‘raw’ form or after further ‘hydrothermal processing’ in a boiling water bath set at 100 °C with intermittent stirring for 10 min.
To start the assay, freshly prepared samples (i.e., ground biscuits, raw and hydrothermally processed ingredients suspended in PBS) were equilibrated at 37 ± 2 °C for 10 min on a rotary mixer and to which was added 200 μL of α-amylase working solution (200 U/mL in PBS) to achieve a final ratio of 0.4 U/mg starch in the test tube. The tube was promptly returned to the rotary mixer at 37 °C. Aliquots (200 μL) collected after 0, 10, 20, 30, 45, 60 and 90 min from each replicate sample were transferred into tubes containing 200 μL of 0.3 M Na2CO3 to inactivate the enzyme and stop the reaction. All samples were evaluated in triplicate.
2.5. Quantification of starch amylolysis products
Quantification of starch amylolysis product was achieved using the p-hydroxybenzoic acid hydrazide (‘PAHBAH’) assay (Lever, 1972) as described previously (Edwards, Maillot, Parker, & Warren, 2018). Aliquots collected at various time points throughout the amylolysis assay (Section 2.4) were centrifuged at 16,200×g at 20 °C (Heraeus Pico, Thermo Scientific, US) for 5 min and 200 μL of supernatant from each tube were transferred into new tubes. Then 20 μL of the transferred supernatants were added to 1 mL of a freshly prepared PAHBAH reagent solution (250 mg of 4-hydroxybenzoic acid hydrazide dissolved in 4.75 mL of 0.5 M HCl, and made up to 50 mL with 0.5 M NaOH) in 1.5 mL Eppendorf® safe-lock™ tubes. Similarly, 1 mL of the PAHBAH solution was added to 20 μL of maltose standard solutions from 0 to 1 mM to allow quantification of reducing sugars (mainly maltose) in the digested samples. After 5 min at ambient temperature, the sample absorbance was read at 405 nm in a spectrophotometer (Biochrom libra S50 UV/Vis). The endogenous reducing sugars values, quantified before the addition of the enzyme, were subtracted from the total sugar values in each tube to reflect only the reducing sugars (as maltose equivalents) released by amylolysis.
2.6. Kinetic analysis of the digestibility data
The rate and extent of starch amylolysis has an impact on the duration and magnitude of glycaemic responses in vivo, and various indices of in vitro starch amylolysis are of relevance to predicting relative differences in the glycaemic response (Edwards et al., 2019, Granfeldt et al., 1992). The analysis of starch digestibility curves has been described in depth elsewhere (Butterworth et al., 2012, Edwards et al., 2019, Edwards et al., 2014). In brief, the product concentration (i.e., obtained from PAHBAH assay maltose equivalents) over time was subjected to Logarithm of Slope (LOS) analysis, enabling kinetic parameters C∞ (product concentration at the reaction endpoint) and k (digestibility rate constant) to be determined, as described previously (Butterworth et al., 2012, Edwards et al., 2014). To aid interpretation, time-course data showing starch amylolysis product formation (i.e., maltose equivalent concentration at time points) were expressed as the corresponding proportion of starch digested over time, wherein the proportion of undigested starch after 90 min is considered potentially resistant starch (Goñi et al., 1997). Curves were fitted through the experimentally obtained data points using SigmaPlot 14.0 (Systat software 2017) and the area under the regression curves were obtained using the Area Under Curve (AUC) macro supplied within the software, which uses an algorithm to integrate non-linear regression curves according to the trapezoidal rule. The AUCs obtained were used to determine the Hydrolysis Index (HI) as shown in Eq. (1). HI values were calculated relative to a highly digestible reference material (plain wheat flour used for the biscuit preparation cooked in water for 10 min at 100 °C) to obtain predicted Glycaemic Index values (Edwards et al., 2019, Goñi et al., 1997).
| (1) |
Eq. (1): Calculation of HI in which boiled wheat flour, used for biscuit preparation, is the reference, and area under the curve (AUC) was determined from non-linear regression analysis fitted to the digestibility data for all flour and biscuit samples collected up to 90 min (t = 90).
2.7. Swelling power and water holding tests
The swelling of starch and other polymers during hydrothermal processing is another property of relevance to food applications and has been studied under various different conditions. In this case, the swelling powers of wheat flour, PulseON®, chickpea flour (included for comparison) and the composite flours (i.e., with different ratios of PulseON®, to wheat flour) were determined at two different temperatures (23 °C and 95 °C) in triplicate according to Fu, Kovacs, and Wang (1998). In brief, 33 mg of each sample was weighed into pre-weighed 1.5 mL Eppendorf® safe-lock™ tubes and suspended in 1 mL of distilled water by vortexing for 5 s. The tubes were subsequently incubated with mixing (1,400 rpm) on a thermomixer (Eppendorf™ ThermoMixer™ C, UK) at either 23 °C for 1 min (‘ambient test’) or 95 °C for 30 min (‘hydrothermally processed’). Samples were then equilibrated in a water bath at 24 °C for 5 min and centrifuged at 15,000×g for 10 min at room temperature. The supernatant was removed and the tubes containing the remaining sediment were weighed to obtain the swelling power, then enabling the amount of water held by the sample to be calculated (Eq. (2)).
| (2) |
Eq. (2): Calculation of Swelling Power (SP) in which the wet weight of sediment gel (Wsediment) minus the original weight of dry sample (Wi) remaining after centrifugation of hydrothermally treated (95 °C for 30 min) flour samples is divided by the Wi.
2.8. Microscopy
Samples of biscuits (i.e., ground to crumbs) and flour ingredients were suspended in deionised water and examined directly using an Olympus BX60 microscope (Olympus Corporation, USA) under polarised light to display starch birefringence. Images were captured with a ProgRes® C10 plus camera (Jenoptik, Germany) and ProgRes® CapturePro software (Jenoptik, Germany). Starch was stained with 1% iodine-potassium iodide (w/w).
Heated stage polarised microscopy was used to monitor changes in ordered structure during hydrothermal treatment. Composite flours (20 mg) were suspended in excess deioinised water (400 µL), mixed by inversion, and then 50 µL of this suspension mounted on microscope slides, sealed, and then viewed under polarised light while being heated from 25 °C to 80 °C in excess water on a heated-stage (THMS600, Linkam Scientific Instruments Ltd, UK). Images were captured at regular intervals (with LINK System Software, Linkam Scientific Instruments Ltd, UK) to observe changes in starch birefringence.
2.9. Statistics
All data are represented as means (n = 3) with standard deviation (SD) unless otherwise specified. Statistical analyses were performed in IBM© SPSS© Statistics Version 22 software (IBM Corp. 2013). Analysis of Variance (ANOVA) was performed on starch digestibility data and kinetic indices, with a Tukey’s b post-hoc analysis used to identify homogenous subsets. Statistically significant differences were accepted at p < 0.05. Graphical analyses were performed using SIGMAPLOT version 14.0 (©Systat software 2017) statistical and graphical software.
3. Results
3.1. Starch digestibility analysis
3.1.1. Ingredients
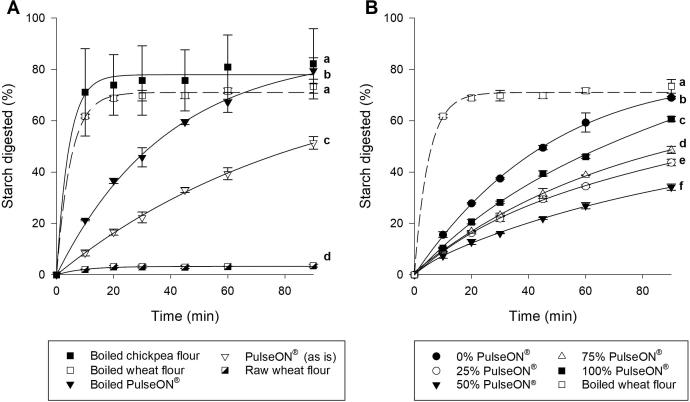
The starch digestibility of food ingredients (used to make the biscuits) and the effect of hydrothermal processing (i.e., 10 mg starch equivalent /mL suspension incubated at 100 °C for 10 min) is shown in Fig. 1A. Raw wheat flour had the lowest starch digestibility of all the ingredients, with only 4% of the starch digested after 90 min incubation with amylase, indicating that raw wheat flour is highly resistant to digestion. However, after hydrothermal processing, the digestibility of wheat flour was greatly increased in that 61% of the starch had been digested within the first 10 min of amylolysis. The starch digestibility curves of hydrothermally processed wheat and chickpea flours were not significantly different (p > 0.05) and were both seen to reach a plateau within 20 min of amylolysis. PulseON®, which has been pre-processed to enhance RS1 content (Edwards, Ellis, Butterworth, Hill, Obuchowicz, & Marson, 2019), showed an intermediate starch digestibility (51% digested after 90 min), when tested ‘as is’ (i.e., the form in which the ingredient was added to the biscuit recipe). After hydrothermal processing, the amount of starch digested from PulseON® after 90 min significantly increased to 79%; however, the digestibility profile shows that digestion of PulseON® occurred more gradually than the wheat and chickpea flours. Thus, the overall Hydrolysis Index of hydrothermally processed PulseON® remained significantly lower than the hydrothermally processed chickpea and wheat flours (p < 0.05).
Fig. 1.
Starch digestibility profiles of raw and boiled wheat and chickpea ingredients (A) and savoury biscuits prepared with different doses of PulseON® (B). Ingredients were analysed as used in the biscuit dough (i.e. raw wheat flour and PulseON® ‘as is’), and also after hydrothermal processing for 10 min at 100 °C (‘boiled’). The boiled chickpea flour was not used in the biscuit recipe but is prepared from the same chickpeas as PulseON® and was included as a comparison. Biscuits (B) were prepared using a composite flour in which PulseON® replaced 0, 25, 50, 75 or 100% of the wheat flour in the recipe. All values are means of triplicates with error bars shown as SD. Curve fitting plots were obtained through non-linear regression to a first order equation. Different superscript letters indicate significant time x treatment differences between curves shown within each graph panel and superscript letters do not apply for comparison between data in both panels (p < 0.05, ANOVA with Tukey’s b post-hoc analysis).
3.1.2. Biscuits
Starch digestibility curves obtained for biscuits made with different ratios of wheat flour and PulseON® are shown in Fig. 1B. All the biscuits incorporating PulseON® had significantly lower starch digestibility profiles compared with the original biscuit made with 100% wheat flour, regardless of the dose used (p < 0.05, time × dose, ANOVA). The relationship between the dose of PulseON® and biscuit starch digestibility is shown more clearly in Fig. 2; with reference to HI (i.e., calculated from the area under the overall starch digestibility profile) and C90 (i.e., extent of starch digested after 90 min). The HI and C90 values decreased when PulseON® was used to displace the wheat flour in the recipe. Interestingly, the lowest starch digestibility was obtained for the biscuit made with a 50–50 blend of PulseON® and wheat, for which a 58% reduction in HI and a 46% reduction in C90 was achieved relative to the control. Paradoxically, at doses of PulseON® above 50%, the starch digestibility of the biscuit increased rather than showing a continued decline. Thus, the response in starch digestibility did not follow a linear dose–response but a U-shaped relationship (Fig. 2), wherein the greatest reduction in C90, C∞ and HI is achieved with the 50–50 blend. A more pronounced U-shape dose–response relationship was observed with regard to C∞ (obtained from LOS analysis Supplementary 3), which indicates the extent of digestion at the reaction endpoint.
Fig. 2.
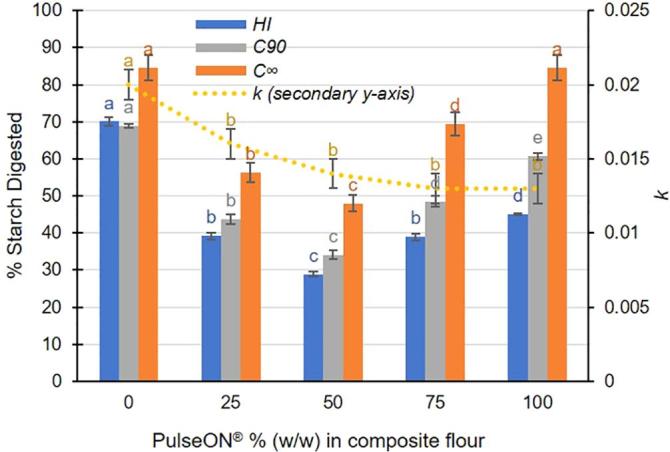
Enzyme kinetic parameters and Hydrolysis Indices of starch digestibility in savoury biscuits made with different doses of PulseON®. Values on the x-axis show the dose as % PulseON® used to replace wheat flour in each biscuit recipe. The Hydrolysis Index (HI), and the proportion of starch digested at 90 min (C90) and at the endpoint (C∞) are plotted on the y-axis and are in units of % starch digested. Values for C90, C∞ and HI all follow a similar dose-dependent trend, while the value of the rate constant, k (plotted on a secondary y-axis), gradually decreases as the dose of PulseON® in the biscuit recipe increases. Overall, there was a significant effect of the biscuit recipe on all indices (p ≤ 0.001, ANOVA), and different superscript letter of the same colour indicate significant differences (Tukey’s b post-hoc test). Values are mean of triplicate analysis with error bars as SD.
The LOS plots (Supplementary 3) showed that the digestion of biscuits followed a single-phase process characterised by a single rate constant k (r2 > 0.90). Biscuits made with PulseON® had rate constants (k range: 0.013 to 0.016) that were significantly lower (p < 0.05) than the original wheat biscuit (k = 0.0198 ± 0.0008). There seemed to be a tendency for the rate constant, k, to decrease with increasing dose of PulseON® (see Fig. 2).
The low values of C90 and C∞ for PulseON® enriched biscuits suggests that starch amylolysis occurs relatively slowly and to a limited extent, thus a high proportion of the starch in the PulseON® enriched biscuits can be considered potentially resistant starch. For instance, at the 50% dose, only 37% of the starch had been digested after 90 min (C90), and only 50% at the endpoint (C∞). Based on the starch content (55.0 g starch/100 g biscuit, see Table 1), an 18 g biscuit would contain an estimated 4.3 g of potentially resistant starch. Such starch properties have not been taken into account in the calculated nutrient composition of baked biscuits as shown in Table 1. Displacing all the wheat flour with PulseON® will result in a savoury biscuit containing 4.2 times more dietary fibre, 1.6 times more protein, 23% less starch, and based on the in vitro starch digestibility indices, it is expected that the starch component will have a greater resistance to digestion.
Table 1.
Nutrient composition (per 100 g of baked product)1 of savoury biscuits made from composite flours with different doses of PulseON®.
| PulseON® in composite flour (w/w) % | 0% | 25% | 50% | 75% | 100% |
|---|---|---|---|---|---|
| Energy (kJ) | 1818 | 1871 | 1866 | 1869 | 1906 |
| Protein (g) | 8.54 | 10.11 | 11.36 | 12.64 | 14.15 |
| Fat (g) | 15.87 | 17.26 | 18.14 | 19.02 | 20.28 |
| Carbohydrate a (g) | 62.43 | 59.74 | 55.19 | 51.08 | 47.87 |
| Starch (g) | 62.17 | 59.52 | 55.02 | 50.95 | 47.78 |
| Sugars (g) | 0.26 | 0.22 | 0.17 | 0.13 | 0.09 |
| Dietary Fibre (g) | 3.05 | 5.56 | 7.88 | 10.19 | 12.68 |
| Moisture (g) | 7.43 | 4.41 | 4.36 | 3.85 | 1.60 |
Calculated values from proximate composition of ingredients were adjusted to the measured moisture content. aCarbohydrates that are potentially available (digestible); does not include carbohydrates as dietary fibre.
3.2. Swelling power and water holding properties of ingredients
The swelling power (SP) of composite flours made with different ratios of PulseON® to wheat flour was measured in excess water before (1 min at 23 °C) and after hydrothermal processing (30 min at 95 °C) and is shown in Fig. 3. At 23 °C, SP were low (≤2.8 g water/g sample) and there were no significant differences between the composite flours tested (F4,14 = 2.255, p = 0.135, ANOVA). After hydrothermal processing in excess water, the SP values increased compared to ambient conditions. There were significant differences between SP values of the hydrothermally processed composite flours (F4,14 = 131.341, p < 0.001, ANOVA), which showed a decrease in SP with increasing proportions of PulseON®. The measured SP values are approximately equivalent to the theoretical values that would be expected from the proportions of wheat and PulseON® used in the composite blends, indicating that SP of ingredients used in the composite flours are additive.
Fig. 3.
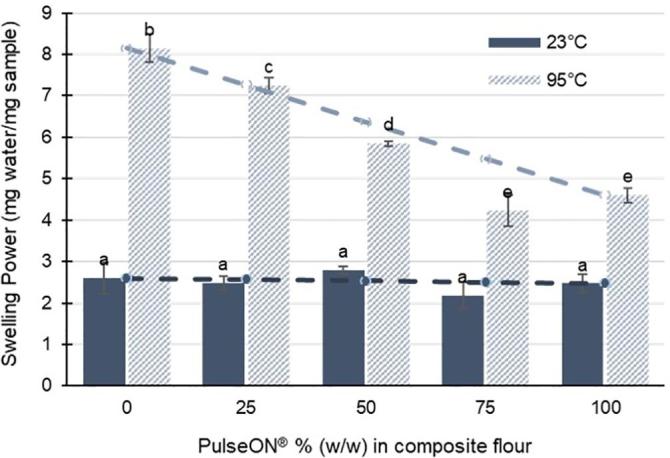
Swelling Power of composite flours made with different doses of PulseON®. Values are mean of triplicate analyses with error bars as SD. There was no significant difference between composite flours in SP measured after 1 min incubation at 23 °C (F4,14 = 2.255, p = 0.135, ANOVA). SP of composite flours was higher after samples had been hydrothermally processed for 30 min at 95 °C, and for the latter SP values decreased significantly with increasing dose of PulseON® (F4,14 = 131.341, p < 0.001, ANOVA). Different superscript letters indicate significant differences (Tukey’s b post hoc test, p < 0.05). Dashed lines represent the theoretical values that would be expected based on the swelling power and ratio of ingredients in each composite flour.
3.3. Polarised microscopy
3.3.1. Ingredients
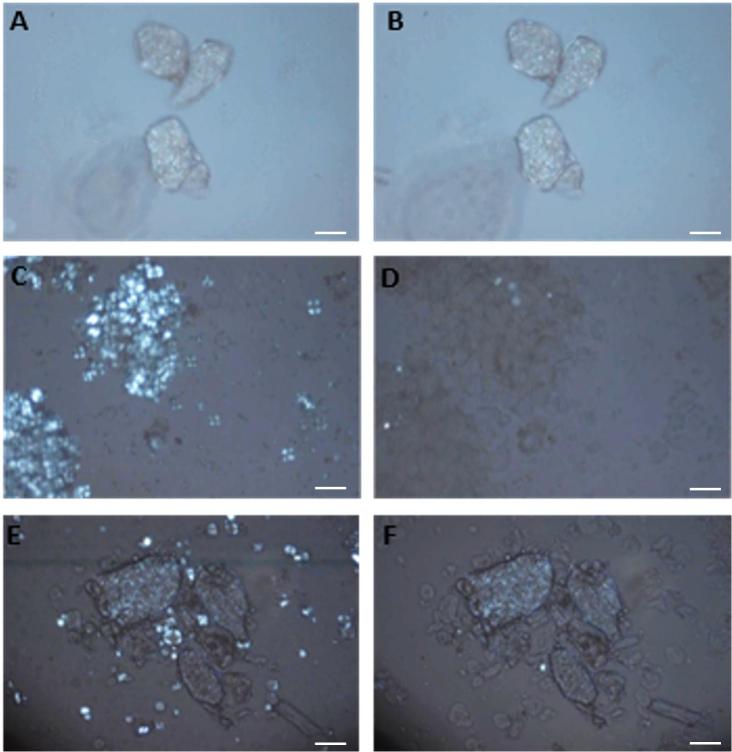
Heated-stage microscopy of ingredients suspended in excess water enabled the thermal transitions of starch to be observed in real time (videos are available in Supplementary 4–7). A selection of polarised light micrographs (shown in Fig. 4) were captured during heating of wheat flour, PulseON®, and 50–50 composite flour. In the polarised light micrograph captured at 52 °C, the PulseON® ingredient can be seen to consist of cellular material with enclosed starch (Fig. 4A). Heating PulseON® to 66 °C in excess water did not cause demonstrable changes in the size or micro structure of the ingredient (Fig. 4B), and no changes were observed when the ingredient was heated to 85 °C (video in Supplementary 5). Native wheat flour and chickpea flour both contain ordered starches that exhibit birefringence when viewed under polarised light (see Fig. 4C, and Supplementary 7 for chickpea flour). When wheat flour was heated (Supplementary 4), starch granule swelling and a loss of birefringence (indicative of starch gelatinisation due to a less ordered starch structure) occurred when the heated stage was between 50 and 60 °C (Fig. 4D). For comparison, the starch within native chickpea flour gelatinised between 60 and 70 °C, whereas no changes in birefringence were observed within the PulseON® powder over this temperature range (see Supplementary 7). When the wheat flour was combined with PulseON® (i.e., as per the 50–50 composite flour blend) and heated in excess water (Fig. 4EF and Supplementary 6), the gelatinisation of wheat starch granules occurred between 50 and 60 °C, indicating that PulseON® does not affect the gelatinisation of wheat starch in excess water conditions. These microscopy observations are consistent with the swelling power data (Section 3.2).
Fig. 4.
Polarised light micrographs captured at 52 °C (left) and 66 °C (right) during heated stage microscopy of PulseON® (AB), wheat flour (CD) and 50% PulseON® and 50% wheat flour (EF), suspended in excess water. The two temperatures represented here were selected from a series of images to depict changes before and after the key transitions that occurred between 50 and 60 °C during heating. No change in birefringence or in appearance of the cells occurred in PulseON® (AB); swelling and loss of birefringence in wheat starch granules (indicative of gelatinisation) occurred between 50 and 60 °C (C,D and E,F), while the PulseON® component appeared unchanged (E,F). Scalebar = 100 µm.
3.3.2. Biscuits
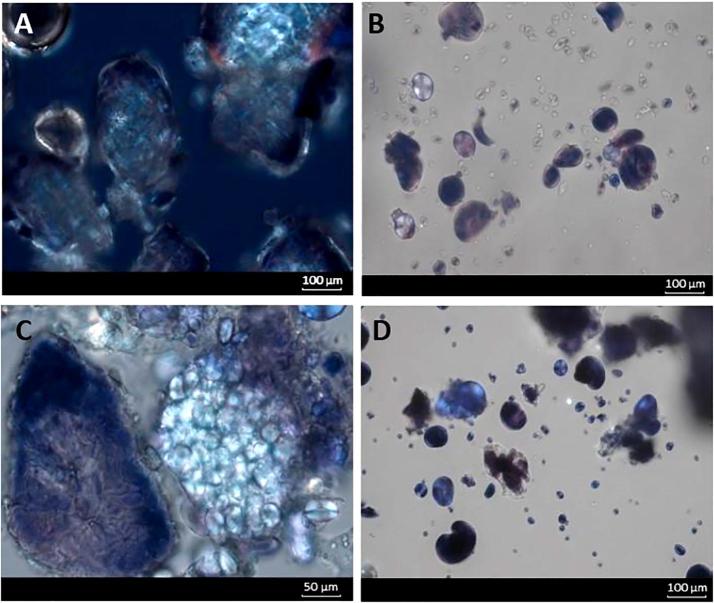
Polarised microscopy of the final biscuit products obtained from recipes with 0, 50 and 100% displacement of the wheat flour with PulseON®, are shown in Fig. 5. Comparing these micrographs to those obtained in Fig. 4 (i.e., ingredients in excess water) provides insight into the behaviour of ingredients when processed under the limited moisture conditions of a biscuit dough (25–28% moisture). Within the baked biscuits, the PulseON® particles remained intact and retained the same size as when added (as an ingredient) to the biscuits (compare Fig. 4A with 5AC). RS1 (encapsulated starch) from PulseON® was clearly evident within the 100% PulseON® biscuit, and some of the intracellular starch granules showed evidence of birefringence. In biscuits containing wheat flour (Fig. 5B and D), some of the starch from the wheat flour has become disordered, while other starch granules retained their crystalline order. The starches within the biscuits are therefore a heterogenous mixture of amorphous starch and ordered starch. Furthermore, the observation that the biscuits containing PulseON® lost more water during baking and had a lower final moisture content than those made from wheat only (Table 1) suggests a difference in internal structuring, for instance the lower cohesiveness of the PulseON® biscuit texture (as subjectively assessed by the authors) providing more voids for water loss during baking.
Fig. 5.
Micrographs of savoury biscuits examined by polarised light microscopy made with different dose of PulseON®. Biscuit made with 100% PulseON® contains chickpea cells with some birefringent starch in the intracellular matrix (A); biscuit made with 100% wheat flour consists of some gelatinised wheat starch and some birefringent granules with a Maltese cross (B); biscuit made with a 50–50 blend of PulseON® and wheat flour contains chickpea cells and aggregates of birefringent wheat starch granules (C) as well as some gelatinised and some birefringent starch granules present within the same biscuit (D). Starch was stained with 1% iodine-potassium iodide (w/w).
4. Discussion
A novel leguminous ingredient (PulseON®) with high levels of structurally-intact dietary fibre (i.e., intact cell walls) and Type 1 resistant starch has been recently developed. However, its functional properties in a food product in relation to starch digestion had not yet been examined. Also, as far as we are aware, this is the first time such a unique food ingredient has been evaluated for use in the design of a functional food. In the present study, we incorporated different doses of PulseON® into a savoury biscuit recipe and examined the effects of this new ingredient on the digestion kinetics of starch in the final biscuit product. This study demonstrates, for the first time, that incorporating PulseON® into savoury biscuits enhances their nutritional value by retaining the structural integrity of the fibre, increasing levels of resistant starch and lowering the rate and extent of starch digestion. These results highlight therefore the potential of using savoury biscuits as a food vehicle for PulseON® to increase consumer intakes of RS, dietary fibre, legume protein and potentially carbohydrate foods with a low Glycaemic Index.
We found that incorporating even relatively low doses of PulseON® reduces the starch digestibility of the biscuits. The starch digestibility of the biscuit made with only PulseON® was similar to the starch digestibility of the original ingredient, which confirmed, for the first time, that the resistant starch properties of the PulseON® were preserved to some extent throughout secondary processing. Moreover, when combined in a biscuit dough with wheat flour, the chickpea ingredient seemed to inhibit the gelatinisation of many wheat starch granules, resulting in a biscuit with even lower starch digestibility than expected. This inhibitory effect appears to be due to the high capacity of the PulseON® to hold water, coupled with the high water requirement of wheat flour for starch swelling and gelatinisation (8 mg water per mg of hydrothermally processed wheat flour, in excess water). Under conditions of limited water (~25–28% moisture in a biscuit dough), the PulseON® is likely, therefore, to compete with wheat flour for water within the dough, thereby limiting availability of water for hydration during heating and subsequent starch gelatinisation. This proposed mechanism is supported by evidence from polarised microscopy images, which showed aggregates of birefringent (i.e., not gelatinised) wheat starch granules in biscuits made with PulseON® (Fig. 5C,D), compared to biscuits prepared from wheat flour alone. (Fig. 5B). Indeed, biscuits are known to contain birefringent starch granules, particularly on the biscuit surface where rapid water loss occurs during baking (Burt & Fearn, 1983).
Our findings are consistent with previous studies that have reported limited gelatinisation of wheat starch in biscuit doughs (Chevallier et al., 2000, Wootton and Chaudry, 1980). The availability of water has been shown to impact the gelatinisation temperature of starch; when starch is heated under conditions of excess water, the starch granules swell which facilitates melting of crystallites at the gelatinisation temperature range of 49–69 °C for wheat starch and 63–83 °C for chickpea starch (Edwards et al., 2015). However, at lower moisture levels, there is no free water available for swelling, and individual crystallites are disrupted over a higher temperature range (Bogracheva, Wang, Wang, & Hedley, 2002). Roder et al demonstrated that limited crystallite disruption occurred when starch samples were hydrothermally processed under limited water conditions of 12–30% moisture (Roder et al., 2009). Thus, the biscuit doughs used within the present study had a moisture content within this range, and therefore represented limited water conditions.
Furthermore, the presence of other hydrophilic components, specifically non-starch polysaccharides (dietary fibre) and protein which have a strong affinity for water, have been shown to increase the onset, peak and concluding temperatures of starch gelatinisation. This occurred both under excess water conditions, so that starches in durum wheat and chickpea flours gelatinised at ~2–4 °C higher onset temperatures than the equivalent extracted starches (Edwards et al., 2015), and also in dough systems, where increasing the level of wheat gluten or dietary fibre (an oat bran source) was shown to increase the temperature required for onset of starch gelatinisation (Pareyt et al., 2008, Villemejane et al., 2016). In addition to binding water, gluten proteins may also interact directly with the starch granule to limit swelling of wheat starch (Debet & Gidley, 2006). Thus, the higher proportion of fibre and protein within the biscuits made with PulseON® would have introduced further competition for water, increasing the temperature required for gelatinisation of wheat starch. The phenomenon of heat and mass transfer within biscuits during baking are certainly complex and future studies may benefit from combining the in vitro techniques used within the present study with modelling approaches (Broyart & Trystram, 2003).
The proposed role of PulseON® in limiting the gelatinisation of wheat starch provides a plausible explanation of the effect of different doses of PulseON® on biscuit starch digestibility. In the raw state, wheat starch is highly resistant to digestion, more so than the chickpea ingredient. When hydrothermally processed under conditions of excess water (i.e., 10 min at 100 °C), starch digestibility of wheat flour increased 19-fold, thus becoming more digestible than the PulseON®, which was more thermally stable. These comparisons of the digestion kinetics of raw and cooked starch are consistent with findings of previous digestibility studies (Roder et al., 2009, Slaughter et al., 2001). However, under the limited moisture conditions of the biscuit dough, which is subsequently baked, complete starch gelatinisation was not achieved. Thus, starch digestibility profiles of biscuits made from the composite flours of wheat and PulseON® were lower than biscuits produced from the individual ingredients (i.e., hydrothermally treated 100% wheat flour or PulseON®). When the wheat biscuit samples were baked from recipes containing increasing amounts of chickpea ingredient (i.e., composite flours with 25, 50, 75% PulseON®), their starch digestibility values were significantly lower than the corresponding biscuit made with 100% wheat flour, probably because in addition to its inherent low digestibility, the PulseON® component hinders wheat starch swelling and gelatinisation by restricting water availability. Thus, the susceptibility of wheat starch to amylolysis, and its contribution to overall biscuit digestibility in our experiments were shown to depend on the dose of chickpea ingredient present, but not in a manner that is easy to predict. Moreover, the overall starch digestibility of the biscuit is the sum of two types of starch digested from wheat flour and the chickpea ingredients, although the relative contributions of these starches to amylolysis cannot be delineated. These complex interactions explain why starch digestibility does not decrease proportionally when the dose of PulseON® in the biscuit dough is increased.
Differences in starch digestibility are of known importance to the glycaemic response in vivo, and the low glycaemic impact of slowly digested starches is likely to be of benefit in the dietary prevention and management of type 2 diabetes (Augustin et al., 2015, Sievenpiper et al., 2009). Starch that resists digestion in the upper gastrointestinal tract has been reported to also increase satiety and reduce food intake (Wanders et al., 2011), promote the growth of beneficial gut microbiota and support colonic health (Asp et al., 1996, Nugent, 2005). However, much of the starch consumed in typical Western diets is often highly digestible and glycaemic, and strongly linked to increased risks of cardiometabolic diseases (Augustin et al., 2015). The PulseON® ingredient used in the present study provides an opportunity to improve intakes of Type 1 RS, which can be found in minimally-processed starchy foods containing intact plant tissues (wholegrains) and/or intact cells (e.g., boiled pulses), but less common in many proprietary food products (Asp et al., 1996, Nugent, 2005). For instance, displacing 50% of the wheat flour with PulseON® left 50% of starch undigested at the endpoint (C∞) of amylolysis, leaving an estimated 4.3 g of potentially resistant starch undigested per 18 g biscuit. The amount of starch that resists amylolysis in vitro is likely of relevance to the colonic delivery of starch in vivo, although the agreement between in vitro analytical estimates and the extent of upper-gastrointestinal starch digestion under physiological conditions still remains unclear.
Although the specific effects of RS1 on health are not fully understood, diets rich in pulses containing RS1 have been associated with positive metabolic outcomes (Sievenpiper et al., 2009). Biscuits have potential for delivering nutraceuticals on a regular basis and in a palatable form. For instance, wheat biscuits containing guar galactomannan (‘soluble fibre’) have previously been shown to achieve a significant plasma insulin-sparing effect in healthy human subjects, without adverse effects on palatability (Ellis, Kamalanathan, Dawoud, Strange, & Coultate, 1988).
Despite the fact that many studies report good agreement between starch digestibility data observed in vitro, and the in vivo glycaemic responses (Edwards et al., 2019, Goñi et al., 1997, Jenkins et al., 1982), it should be noted that in vitro digestion methods only suggest likely differences in Glycaemic Index. Further in vivo human studies are therefore needed to establish the postprandial metabolic effects. Such trials should be conducted using biscuits prepared on a commercial pilot scale, to obtain a more representative sample than the biscuits produced under controlled conditions in this mechanistic study. To be commercially attractive to customers, sensory analysis is required to ensure that texture and flavour expectations are met. Future studies will assess how this ingredient performs in other product categories, particularly products with a higher Glycaemic Index.
5. Conclusions
The use of PulseON® – a RS1-enriched chickpea powder, as a wheat flour substitute was shown to effectively decrease starch digestibility of biscuits in vitro, which can be attributed to the intrinsically higher proportions of encapsulated starch in the chickpea cells. In addition, the PulseON® appears to hinder water uptake by wheat starch granules during the biscuit-making process, thus reducing wheat starch gelatinisation and subsequent digestion. New food products enriched in PulseON® could provide consumers with multiple health benefits related to lower starch digestibility and lower Glycaemic Index. Such food products could help in the dietary management and/or prevention of cardiometabolic diseases including type 2 diabetes.
Author contributions
Gael Y.F. Delamare: Investigation, Data curation, Formal analysis, Writing - original draft. Peter J. Butterworth: Methodology, Writing - review & editing. Peter R. Ellis: Writing - review & editing. Sandra Hill: Resources, Supervision. Frederick J. Warren: Resources, Project administration. Cathrina H. Edwards: Conceptualization, Supervision, Project administration, Visualization, Writing - review & editing, Funding acquisition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors gratefully acknowledge the support of the Biotechnology and Biological Sciences Research Council (BBSRC), United Kingdom; this research was funded by the BBSRC Follow-On Fund (BB/M021076/1) and BBSRC Super Follow On Fund (BB/PO23770/1) awards and the BBSRC Institute Strategic Programme Food Innovation and Health BB/R012512/1 and its constituent project BBS/E/F/000PR10343 (Theme 1, Food Innovation), as well as the BBSRC Institute Strategic Programme Food and Health BBS/E/F/00044427. Thanks are also extended to AGT Poortman for providing the raw materials and New Food Innovation Ltd. for producing the ingredients as well as for their ongoing support and helpful discussions.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.fochx.2020.100078.
Contributor Information
Gael Y.F. Delamare, Email: gael.delamare@outlook.com.
Peter J. Butterworth, Email: peter.butterworth@kcl.ac.uk.
Peter R. Ellis, Email: peter.r.ellis@kcl.ac.uk.
Sandra Hill, Email: sandra.hill@biopolymersolutions.co.uk.
Frederick J. Warren, Email: fred.warren@quadram.ac.uk.
Cathrina H. Edwards, Email: cathrina.edwards@quadram.ac.uk.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Asp N.G., van Amelsvoort J.M.M., Hautvast J.G.A.J. Nutritional Implications Of Resistant Starch. Nutrition Research Reviews. 1996;9(1):1–31. doi: 10.1079/NRR19960004. [DOI] [PubMed] [Google Scholar]
- Augustin L.S.A., Kendall C.W.C., Jenkins D.J.A., Willett W.C., Astrup A., Barclay A.W.…Poli A. Glycemic index, glycemic load and glycemic response: An International Scientific Consensus Summit from the International Carbohydrate Quality Consortium (ICQC) Nutrition, Metabolism and Cardiovascular Diseases. 2015;25(9):795–815. doi: 10.1016/j.numecd.2015.05.005. [DOI] [PubMed] [Google Scholar]
- Bogracheva T.Y., Wang Y.L., Wang T.L., Hedley C.L. Structural studies of starches with different water contents. Biopolymers. 2002;64(5):268–281. doi: 10.1002/bip.10190. [DOI] [PubMed] [Google Scholar]
- Broyart B., Trystram G. Modelling of heat and mass transfer phenomena and quality changes during continuous biscuit baking using both deductive and inductive (Neural Network) modelling principles. Food and Bioproducts Processing. 2003;81(4):316–326. [Google Scholar]
- Burt D.J., Fearn T. A quantitative study of biscuit microstructure. Starch - Stärke. 1983;35(10):351–354. [Google Scholar]
- Butterworth P.J., Warren F.J., Grassby T., Patel H., Ellis P.R. Analysis of starch amylolysis using plots for first-order kinetics. Carbohydrate Polymers. 2012;87(3):2189–2197. [Google Scholar]
- Chevallier S., Colonna P., Della Valle G., Lourdin D. Contribution of major ingredients during baking of biscuit dough systems. Journal of Cereal Science. 2000;31(3):241–252. [Google Scholar]
- Cooke D., Gidley M.J. Loss of crystalline and molecular order during starch gelatinisation: Origin of the enthalpic transition. Carbohydrate Research. 1992;227:103–112. [Google Scholar]
- Debet M.R., Gidley M.J. Three classes of starch granule swelling: Influence of surface proteins and lipids. Carbohydrate Polymers. 2006;64(3):452–465. [Google Scholar]
- Dhital S., Warren F.J., Butterworth P.J., Ellis P.R., Gidley M.J. Mechanisms of starch digestion by α-amylase—Structural basis for kinetic properties. Critical Reviews in Food Science and Nutrition. 2017;57(5):875–892. doi: 10.1080/10408398.2014.922043. [DOI] [PubMed] [Google Scholar]
- Würsch P., Del Vedovo S., Koellreutter B. Cell structure and starch nature as key determinants of the digestion rate of starch in legume. American Journal of Clinical Nutrition. 1986;43(1):25–29. doi: 10.1093/ajcn/43.1.25. [DOI] [PubMed] [Google Scholar]
- Edwards, C. H., Ellis, P. R., Butterworth, P. J., Hill, S., Obuchowicz, J., & Marson, A. (2019). PCT/GB2019/050284.
- Edwards C.H., Cochetel N., Setterfield L., Perez-Moral N., Warren F.J. A single-enzyme system for starch digestibility screening and its relevance to understanding and predicting the glycaemic index of food products. Food and Function. 2019;10:4751–4760. doi: 10.1039/c9fo00603f. [DOI] [PubMed] [Google Scholar]
- Edwards C.H., Maillot M., Parker R., Warren F.J. A comparison of the kinetics of in vitro starch digestion in smooth and wrinkled peas by porcine pancreatic alpha-amylase. Food Chemistry. 2018;244:386–393. doi: 10.1016/j.foodchem.2017.10.042. [DOI] [PubMed] [Google Scholar]
- Edwards C.H., Warren F.J., Campbell G.M., Gaisford S., Royall P.G., Butterworth P.J., Ellis P.R. A study of starch gelatinisation behaviour in hydrothermally-processed plant food tissues and implications for in vitro digestibility. Food and Function. 2015;6(12):3634–3641. doi: 10.1039/c5fo00754b. [DOI] [PubMed] [Google Scholar]
- Edwards C.H., Warren F.J., Milligan P.J., Butterworth P.J., Ellis P.R. A novel method for classifying starch digestion by modelling the amylolysis of plant foods using first-order enzyme kinetic principles. Food and Function. 2014;5(11):2751–2758. doi: 10.1039/c4fo00115j. [DOI] [PubMed] [Google Scholar]
- Ellis P.R., Kamalanathan T., Dawoud F.M., Strange R.N., Coultate T.P. Evaluation of guar biscuits for use in the management of diabetes: Tests of physiological effects and palatability in non-diabetic volunteers. European Journal of Clinical Nutrition. 1988;42(5):425–435. [PubMed] [Google Scholar]
- Englyst H.N., Kingman S.M., Cummings J.H. Classification and measurement of nutritionally important starch fractions. European Journal of Clinical Nutrition. 1992;46(Suppl 2):S33–50. [PubMed] [Google Scholar]
- Foster-Powell K., Holt S.H., Brand-Miller J.C. International table of glycemic index and glycemic load values: 2002. American Journal of Clinical Nutrition. 2002;76(1):5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- Fu X., Kovacs M.I.P., Wang C. A simple wheat flour swelling test. Cereal Chemistry. 1998;75(4):556–567. [Google Scholar]
- Golay A., Coulston A.M., Hollenbeck C.B., Kaiser L.L., Würsch P., Reaven G.M. Comparison of metabolic effects of white beans processed into 2 different physical forms. Diabetes Care. 1986;9(3):260–266. doi: 10.2337/diacare.9.3.260. [DOI] [PubMed] [Google Scholar]
- Goñi I., Garcia-Alonso A., Saura-Calixto F. A starch hydrolysis procedure to estimate glycemic index. Nutrition Research. 1997;17(3):427–437. [Google Scholar]
- Granfeldt Y., Björck I., Drews A., Tovar J. An in vitro procedure based on chewing to predict metabolic response to starch in cereal and legume products. European Journal of Clinical Nutrition. 1992;46:649–660. [PubMed] [Google Scholar]
- Jenkins D.J.A., Ghafari H., Wolever T.M.S., Taylor R.H., Jenkins A.L., Barker H.M.…Bowling A.C. Relationship between rate of digestion of foods and post-prandial glycaemia. Diabetologia. 1982;22(6):450–455. doi: 10.1007/BF00282589. [DOI] [PubMed] [Google Scholar]
- Lafiandra D., Riccardi G., Shewry P.R. Improving cereal grain carbohydrates for diet and health. Journal of Cereal Science. 2014;59(3):312–326. doi: 10.1016/j.jcs.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever M. A new reaction for colorimetric determination of carbohydrates. Analytical Biochemistry. 1972;47(1):273–279. doi: 10.1016/0003-2697(72)90301-6. [DOI] [PubMed] [Google Scholar]
- Livesey G., Taylor R., Livesey H.F., Buyken A.E., Jenkins D.J.A., Augustin L.S.A.…Brand-Miller J.C. Dietary glycemic index and load and the risk of type 2 diabetes: Assessment of causal relations. Nutrients. 2019;11(6):1436. doi: 10.3390/nu11061436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noah L., Guillon F., Bouchet B., Buleon A., Molis C., Gratas M., Champ M. Digestion of carbohydrate from white beans (Phaseolus vulgaris L.) in healthy humans. Journal of Nutrition. 1998;128(6):977–985. doi: 10.1093/jn/128.6.977. [DOI] [PubMed] [Google Scholar]
- Nugent A.P. Health properties of resistant starch. Nutrition Bulletin. 2005;30(1):27–54. [Google Scholar]
- Pallares Pallares A., Alvarez Miranda B., Truong N.Q.A., Kyomugasho C., Chigwedere C.M., Hendrickx M., Grauwet T. Process-induced cell wall permeability modulates the in vitro starch digestion kinetics of common bean cotyledon cells. Food and Function. 2018;9(12):6544–6554. doi: 10.1039/c8fo01619d. [DOI] [PubMed] [Google Scholar]
- Parada J., Aguilera J.M. In vitro digestibility and glycemic response of potato starch is related to granule size and degree of gelatinization. Journal of Food Science. 2009;74(1):E34–38. doi: 10.1111/j.1750-3841.2008.01016.x. [DOI] [PubMed] [Google Scholar]
- Pareyt B., Wilderjans E., Goesaert H., Brijs K., Delcour J.A. The role of gluten in a sugar-snap cookie system: A model approach based on gluten–starch blends. Journal of Cereal Science. 2008;48(3):863–869. doi: 10.1016/j.foodchem.2008.02.079. [DOI] [PubMed] [Google Scholar]
- Patel H., Royall P.G., Gaisford S., Williams G.R., Edwards C.H., Warren F.J.…Butterworth P.J. Structural and enzyme kinetic studies of retrograded starch: Inhibition of α-amylase and consequences for intestinal digestion of starch. Carbohydrate Polymers. 2017;164:154–161. doi: 10.1016/j.carbpol.2017.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roder N., Gerard C., Verel A., Bogracheva T.Y., Hedley C.L., Ellis P.R., Butterworth P.J. Factors affecting the action of α-amylase on wheat starch: Effects of water availability. An enzymic and structural study. Food Chemistry. 2009;113(2):471–478. [Google Scholar]
- Sievenpiper J.L., Kendall C.W.C., Esfahani A., Wong J.M.W., Carleton A.J., Jiang H.Y.…Jenkins D.J.A. Effect of non-oil-seed pulses on glycaemic control: A systematic review and meta-analysis of randomised controlled experimental trials in people with and without diabetes. Diabetologia. 2009;52(8):1479. doi: 10.1007/s00125-009-1395-7. [DOI] [PubMed] [Google Scholar]
- Slaughter S.L., Ellis P.R., Butterworth P.J. An investigation of the action of porcine pancreatic alpha-amylase on native and gelatinised starches. Biochimica et Biophysica Acta. 2001;1525(1–2):29–36. doi: 10.1016/s0304-4165(00)00162-8. [DOI] [PubMed] [Google Scholar]
- Villemejane C., Denis S., Marsset-Baglieri A., Alric M., Aymard P., Michon C. In vitro digestion of short-dough biscuits enriched in proteins and/or fibres using a multi-compartmental and dynamic system (2): Protein and starch hydrolyses. Food Chemistry. 2016;190:164–172. doi: 10.1016/j.foodchem.2015.05.050. [DOI] [PubMed] [Google Scholar]
- Wanders A.J., van den Borne J.J., de Graaf C., Hulshof T., Jonathan M.C., Kristensen M.…Feskens E.J. Effects of dietary fibre on subjective appetite, energy intake and body weight: A systematic review of randomized controlled trials. Obesity Reviews. 2011;12(9):724–739. doi: 10.1111/j.1467-789X.2011.00895.x. [DOI] [PubMed] [Google Scholar]
- Wang S., Copeland L. Molecular disassembly of starch granules during gelatinization and its effect on starch digestibility: A review. Food and Function. 2013;4:1564–1580. doi: 10.1039/c3fo60258c. [DOI] [PubMed] [Google Scholar]
- Wootton M., Chaudry M.A. Gelatinization and in vitro digestibility of starch in baked products. Journal of Food Science. 1980;45(6):1783–1784. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.