Abstract
Background
Treatment of Crohn's disease (CD) remains to be a challenge due to limited insights for its pathogenesis. We aimed to determine the role of O-Linked β-N-acetylglucosamine (O-GlcNAc) in the development of CD and evaluate therapeutic effects of O-GlcNAc inhibitors on CD.
Methods
O-GlcNAc in intestinal epithelial tissues of CD, adherent-invasive Escherichia coli (AIEC) LF82-infected cells and mice was determined by immunoblot and immunohistochemistry. AIEC LF82 and dextran sulfate sodium were administrated into C57BL/6 mice for estabolishing inflammatory bowel disease model and for therapeutic study.
Findings
O-GlcNAc was increased in intestinal epithelial tissues of CD patients and AIEC LF82-infected mice. Infection of AIEC LF82 up-regulated the level of UDP-GlcNAc and increased O-GlcNAc in human colon epithelial HCT116 and HT-29 cells. We identified that IKKβ and NF-κB were O-Glycosylated in AIEC LF82-treated cells. Mutations of IKKβ (S733A) and p65 (T352A) abrogated the O-GlcNAc in IKKβ and NF-κB and inhibited AIEC LF82-induced activation of NF-κB. Application of 6-diazO-5-oxO-L-norleucine, an agent that blocks the production of UDP-GlcNAc and inhibits O-GlcNAc, inactivated NF-κB in AIEC LF82-infected cells, enhanced the formation of autophagy, promoted the removal of cell-associated AIEC LF82, alleviated intestinal epithelial inflammation, and improved the survival of the colitis mice.
Interpretation
Intestinal inflammation in CD is associated with increased O-GlcNAc modification, which is required for NF-κB activation and suppression of autophagy. Targeting O-GlcNAc could be an effective therapy for inflammatory bowel disease.
Funding
National Natural Science Foundation of China (Nos. 81573087 and 81772924) and International Cooperation Foundation of Jilin Province (20190701006GH).
Keywords: Crohn's disease (CD), inflammatory bowel disease, adherent-invasive Escherichia coli (AIEC) LF82, O-Linked β-N-acetylglucosamine (O-GlcNAc), UDP-N-acetylglucosamine (UDP-GlcNAc), NF-κB
Research in context.
Evidence before study
Bacterial cell wall is organized by peptidoglycan, which contains a repetition of carbohydrates, N-acetylglucosamine (GlcNAc) and N-acetylmuramic acid cross-linked by short chains of peptides to form a lattice surrounding the entire cell. Large amounts of GlcNAc and other bacterial cell wall building blocks, such as UDP-GlcNAc, are released during bacterial growth/division and bacterial host invasion. GlcNAc is not only important for the biogenesis of bacterial cell wall but also serves as a metabolite of the hexosamine biosynthetic pathway (HBP) for host cells, one endpoint of which is the modification and regulation of proteins by O-GlcNAcylation (O-GlcNAc). O-GlcNAc is a reversible post-translational modification, attaching a sugar moiety to serine/threonine residues of cytosolic or nuclear proteins. The modification modulates cellular signaling and transcription regulatory pathways in response to metabolic cues and stresses. It is reported that O-GlcNAc is associated with both pro- and anti-inflammatory effects in various conditions. However, the role of O-GlcNAc in the development of CD remains largely unknown.
Added value of this study
We have demonstrated that O-GlcNAc was markedly increased in intestinal epithelial tissues of CD patients and adherent-invasive Escherichia coli (AIEC) LF82-infected mice. Infection of AIEC LF82, but not inactive AIEC LF82 or non-pathogenic bacteria, up-regulated the level of UDP-GlcNAc, a donor glucosamine for glycosylation, and increased O-GlcNAc in human colon epithelial HCT116 and HT-29 cells. We identified that IKKβ and NF-κB were O-Glycosylated in AIEC LF82-treated cells. Mutations of IKKβ (S733A) and p65 (T352A) abrogated the O-GlcNAc in IKKβ and NF-κB and inhibited AIEC LF82-induced activation of NF-κB. In addition, depletion of UDP-GlcNAc or inhibition of O-GlcNAc suppressed NF-κB activation, and hence promoted autophagosome/autolysosome formation, enhanced the removal of intracellular AIEC LF82, alleviated intestinal epithelial inflammation, and promoted the survival of AIEC LF82-infected mice. Taken together, our data suggest that intestinal inflammation in CD is associated with increased levels of O-GlcNAc, which is required for NF-κB activation and suppression of autophagy.
Implication of all the available evidence
Targeting O-GlcNAc could be an effective therapy for inflammatory bowel disease.
Alt-text: Unlabelled box
1. Introduction
Crohn's disease (CD) is a chronic inflammatory disease of the gastrointestinal tract whose symptoms develop in an intermittent manner of recurrence and remission. Progress of CD leads to intestinal damage and disability [1,2]. The pathogenesis of CD involves multiple factors, such as susceptibility to inheritance, environment and immunity abnormalities [3,4]. Among the environmental factors, adherent invasive E. coli (AIEC) pathogens are considered to be the major candidate pathogen bacteria [5], [6], [7], [8], [9]. These bacteria strongly adhere to and invade intestinal epithelial cells (IECs), survive within macrophages, migrate into deep tissues, and activate immune cells to induce inflammatory cytokine secretion [7,8]. Accumulated evidence shows that most of enteropathogens are equipped with a large set of specific metabolic pathways to overcome nutritional limitations in vivo, hence increasing bacterial fitness during infections [10].
Glycosylation, one of the most common modifications for proteins and lipids, is essential for maintaining physiological cell functions. With respect to protein glycosylation, two major types of modifications have been characterized, i.e., N- and O-linked glycosylation. It was reported that AIEC adhesion to ileal enterocytes of CD patients is blocked in the presence of D-mannose, indicating that bacteria–host cell interactions occur via glycosylated receptors [5]. In addition, an elevated expression of mannosylated molecule(s) on ileal enterocytes of CD patients was observed using the mannose-binding lectin ConA [5]. Accordingly, Barnich et al. identified that AIEC strain LF82 adheres to ileal enterocytes via the common type 1 pili adhesin FimH and recognizes carcinoembryonic antigen–related cell adhesion molecule 6 (CEACAM6), a glycosylated type 1 pili receptor, abnormally expressed on CD ileal epithelial cells [5,6]. Together, these findings suggest that glycosylation plays a critical role in AIEC-induced adhesion and invasion.
O-GlcNAc is an O-linked-β-N-acetylglucosamine moiety attached to the residue of serine or threonine on nuclear or cytoplasmic proteins [11]. O-GlcNAc homeostatsis is controlled by two enzymes: O-GlcNAc transferase (OGT), which adds GlcNAc to target proteins, and O-GlcNAcase (OGA), which removes the GlcNAc [12]. The hexosamine biosynthetic pathway (HBP) converts intracellular glucose to UDP-N-acetylglucosamine (UDP-GlcNAc), an essential precursor of protein O-GlcNAc, which may in turn act as a hormone and nutrient sensor to control multiple biological processes including cell signaling, metabolism, development and aging [[13], [14], [15]]. O-GlcNAc modification is associated with both pro- and anti-inflammatory effects in various conditions [15]. However, the role of O-GlcNAcylation in the development of CD remains largely unknown. In this study, we detected the O-GlcNAc modification in intestinal tissues from CD patients and experimental inflammatory bowel disease mice, determined the functional outcomes of AIEC-induced O-GlcNAc modification in inflammation, characterized the mechanisms whereby AIEC upregulates the modification and O-GlcNAc promotes inflammatory responses in intestinal epithelial cells. In addition, we evaluated the significance of O-GlcNAc inhibition in the removal of intracellular AIEC and mitigation of the inflammatory response in an experimental mouse model.
2. Materials and methods
2.1. Ethics statement
C57BL/6 mice [8-week-old males and females] were provided by and housed in the animal facility in the College of Basic Medicine, Jilin University in accordance with the University guidelines and Chinese recommendations in experimental animals. The animals were in specific pathogen-free conditions. Food and drinking water were provided ad libitum. Protocols were approved by the Jilin University Institutional Animal Care and Use Committee (IACUC) [approval ID 2018-R35] and the Ethics Committee of Jilin University [approval Y2018-R35. Protocol for the application of human tissues was approved by IRB in the University of Alabama at Birmingham (UAB). No private information of patients was accessed. Informed consent of the patients was waived (Protocol No. N120831003).
2.2. Cell culture, antibodies, and reagents
The human colon epithelial cell line HCT116 and HT-29 were maintained in DMEM supplemented with 10% (vol/vol) fetal bovine serum (FBS) (Atlanta Biologicals, Inc., Flowery Branch, GA) at 37 °C in 5% CO2. HCT116/GFP-LC3 was parental cell line stably transfected with GFP-LC3 and established in the lab [16,17]. Polyclonal antibodies against OGT and OGA were purchased from Sigma-Aldrich (St Louis, MO, USA). Monoclonal antibodies against HA-tag, His-tag, and O-GlcNAc (RL2) were purchased from Fisher-Thermo Scientific (Rockford, IL). Monoclonal antibodies against IKK-β, IκBα, and NF-κB (p65) were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Anti-GAPDH monoclonal antibody was obtained from Fitzgerald Industries (Acton, MA). Anti-LC3, ATG16L1, Histon H3, β-actin, p62 polyclonal antibodies were purchased from Novus Biologicals Inc. (Littleton, CO). Rabbit recombinant monoclonal glutamine:fructose-6-phosphate amidotransferase 1 (GFAT1) antibody was purchased from Abcam (Cambridge, MA). Puromycin, 2-phenylbenzofuran, 6-diazo-5-oxo-L-norleucine (DON), and thiamet G (TG) were purchased from Sigma-Aldrich.
2.3. DNA constructs, transfections, and establishment of stable cell lines
The pcDNA3/HA-IKKβ and pcDNA3/His-NF-κB-p65 were cloned in the lab [18]. pcDNA3/HA-IKKβ S733A and pcDNA3/His-NF-κB-p65 T352A mutants were generated with Site-Directed Mutagenesis Kit from Invitrogen. Luciferase report plasmids for NF-κB p65 were previously reported [18]. Gene transfection was performed with Fugene 6 transfection reagent (Roche Diagnostics Corp., Indianapolis, IN.) according to the manufacturer's instruction.
Constructs for OGT, OGA, shRNA-Ctrl, shRNA-OGT, and shRNA-OGA were packaged with lentiviral expression system as we previously reported [19]. HCT116 cells were infected with lentiviruses expressing target genes and selected with puromycin (1.0 μg/ml) for 2 weeks, resistant stable clones were pooled and passaged. There were no deleterious effects on the viability of selected cells, as determined by trypan blue exclusion and ATP levels. Early passages of the cells were frozen and thawed for experimentation.
2.4. Measurement of UDP-GlcNAc
Measurement of UDP-GlcNAc in HCT116 and HT-29 cells with or without AIEC LF82 infection was determined as previously reported [20,21]. The content of UDP-GlcNAc was quantified by UV detection (A254nm) after calibration with UDP-GlcNAc standard.
2.5. Immunoblotting, immunoprecipitation, and succinylated wheat germ agglutinin (sWGA) pull down
Immunoblotting was performed using 30 to 50 µg of total proteins in cell lysates. GAPDH was used as a loading control. Monoclonal antibodies against O-GlcNAc (RL2) was used for the detection of O-GlcNAc-ed proteins. HA-tag, His-tag, and O-GlcNAc (RL2) antibodies were used for immunoprecipitation at 4 °C. Immunoprecipitated complexes were eluted and subjected to immunoblot analysis. For pulling down glycosylated proteins, sWGA beads were mixed with 300 µg whole cell extracts and incubased at 4 °C for overnight. The following day, the beads were washed with PBS for 4 times and then solved in 1 X loading buffer for western blot analysis.
2.6. Luciferase reporter assay
HCT116 cells were transfected with pGL3/NF-κB vector. The pRL vector expressing wild-type Renilla luciferase was used as a control reporter. Twenty-four hours later, cells were infected with AIEC LF82 in the presence or absence of O-GlcNAc inhibitor DON. Luciferase activity was assessed using Dual-Luciferase Reporter Assay System (Promega) after the treatment. Relative luciferase unit (RLU) was the ratio of NF-κB luciferase activity to Renilla activity.
2.7. Bacterial strain and invasion assay
AIEC strain LF82 was provided by Dr. Philip M Sherman [22]. HCT116 cells with or without O-GlcNAc manipulations were infected with AIEC LF82 for indicated time at a multiplicity of infection (MOI) of 10 bacteria per cell. To determine the number of cell-associated bacteria with colony forming units (CFU), infected cells were washed with PBS at least three times before detachment. Collected cells were repeatedly washed and centrifuged (200 g for 5 min) three times. Cell pellets were then lysed with 0.1% Triton X-100. Lysates were spun down at 200 g for 1 min. Supernatants were diluted and seeded on LB agar plates to determine the number of CFU.
2.8. Immunofluorescence and fluorescence microscopy
Cells were grown and treated in 6-well plates. The location and distribution of NF-κB were analyzed with an Olympus IX51 fluorescence microscope after immunostained with NF-κB antibody. GFP-LC3 punta representing autophagosome/autolysosome formation were directly examined with the fluorescence microscope [16,17].
2.9. Immunohistochemistry (IHC)
Ileal tissues from 4 healthy individuals, 14 active Crohn's disease, 6 inactive Crohn's disease, and colon tissues from 41 healthy individuals, 22 active Crohn's disease, 16 inactive Crohn's disease, and 7 chronic inflammation of colon were provided by the Tissue Procurement Facility of UAB. Avidin-biotin immunohistochemical analysis was performed as previously described [19]. Staining regions were reviewed by 3 different pathologists and categorized as negative, weak, moderate, or strong. For determining the H score, antibodies-stained tissues were scored by calculating the product of the percentage of cells staining at each intensity level and the intensity level (0, negative; 1+, weak; 2+, moderate; 3+, strong). The H score was then calculated by summing the individual intensity level scores [23].
2.10. IBD models
C57BL/6 mice (20 - 22 g) were given 3% dextran sulfate sodium (DSS) in drinking water and/or 1 - 3 × 108 AIEC LF82 per day in 200 μL PBS by gavage for 2 weeks. The mice were housed in animal barrier facility by drinking water for 3 more days before euthanized for analysis. For these treated with O-GlcNAc inhibitor, 1 mg/kg of DON dissolved in 200 µl PBS was applied by i.p. on day 1 to day 3, then administered every other day until the end of the experiment. The body weight, feeding behavior, stool, hematochezia, motor activity, and survival of mice were monitored. At the end of observation, the mice were euthanized and intestinal tissues were fixed for histological analyses.
2.11. Statistical analysis
All data presented are representative of 3 or more experiments with similar results. Quantitative data are shown as mean ± SD. The Pearson's χ2-test was used to analyze the distribution difference of O-GlcNAc and OGT staining among intestinal tissues from normal and human CD subjects. Statistical significance was determined using Student's t-test if not specially indicated (* P < .05 and ** P < .01).
3. Results
3.1. O-GlcNAc is increased in CD intestinal tissues and in AIEC LF82-infected subjects
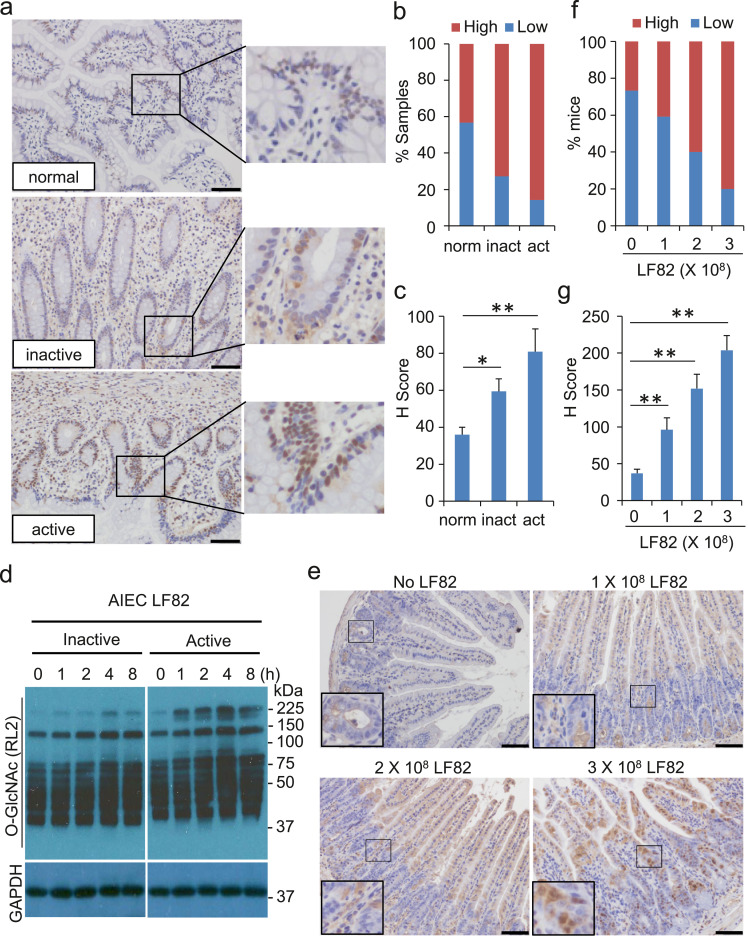
To determine the involvement of O-GlcNAc in intestinal inflammation, we detected O-GlcNAc in intestinal tissues from normal, inactive, and active CD individuals by immunohistochemistry (IHC). Normal intestinal epithelial cells bore a low level of O-GlcNAc (low H scores) in general despite a scattered pattern of relatively strong staining (Fig. 1a–c). In contrast, active CD individuals possessed a strikingly high level of O-GlcNAc with a 3-fold increase in H score in intestinal tissues as compared with those in normal controls (Fig. 1a–c). Intestinal epithelial cells in inactive CD subjects exhibited a modest increase in O-GlcNAc and an intermediate H score (Fig. 1a–c).
Fig. 1.
O-GlcNAc is increased in intestinal tissues of CD individuals. Infection of AIEC LF82 leads to the increase of O-GlcNAc in intestinal epithelial cells in vitro and in vivo. (a - c) Assessment of O-GlcNAc in ileal and colon tissues from healthy (n = 45), active CD (n = 36), and inactive CD (n = 22) individuals by IHC. (a) Representative staining of O-GlcNAc for ileal tissues from each group is presented. Scale bar = 50 µm. (b) Percentage of samples with high or low expression of O-GlcNAc in (a). (c) H-scores of O-GlcNAc in (a) (see Materials and Methods for an explanation of H scores). (d) AIEC strain LF82 was used to infect HCT116 cells (MOI = 10) for the indicated time. Heat-inactivated AIEC LF82 was used in parallel. After the infection, cells were washed and lysed for the western blot analysis. Thirty micrograms of the whole cell extracts were used for the detection. GAPDH was measured for loading control. (e - g) C57BL/6 mice were given 1 - 3 × 108 AIEC LF82 per day in 200 μL PBS by gavage for 2 weeks. The mice were euthanized for the IHC analysis. (e) Ileal epithelia were fixed for the O-GlcNAc staining by IHC. Scale bar = 50 µm. (f) Percentage of samples with high or low expression of O-GlcNAc in (e). (g) H-scores of O-GlcNAc in (e). * P < .05 and ** P < .01 (Student's t-test).
Persistent infection of AIEC plays a critical role in the development of CD [22]. To determine whether AIEC infection promotes the O-GlcNAc, we exposed the intestinal epithelial HCT116 cells to inactive and active AIEC LF82, a subtype of E. coli that has been characterized in the induction of CD [22]. Cells co-cultured with heat-inactivated AIEC LF82 displayed only a marginal escalation in the level of O-GlcNAc for up to 8 h. In contrast, HCT116 cells co-cultured with active AIEC LF82 showed an elevation of O-GlcNAc as early as 1 h after the exposure (Fig. 1d), indicating that active AIEC infection may play a role in the O-GlcNAc induction in CD individuals.
To characterize the role that AIEC LF82 plays in the escalation of O-GlcNAc, we treated C57BL/6 mice with AIEC LF82 by intragastric gavage for 2 weeks and analyzed O-GlcNAc in mouse ilea, in which the CD tissue damges mostly occur. Consistent with previous reports, mice exposed to 1 - 3 × 108 LF82 for 2 weeks exhibited no marked tissue damge in ilea (Fig. 1e). However, IHC staining showed that mice exposed to AIEC LF82 had a gradual increase in O-GlcNAc staining throughout the intestinal epithelial layers (Fig. 1e-g). Taken together, our in vitro and in vivo experimental data suggest that O-GlcNAc is increased in CD intestinal tissues and in AIEC LF82-infected subjects.
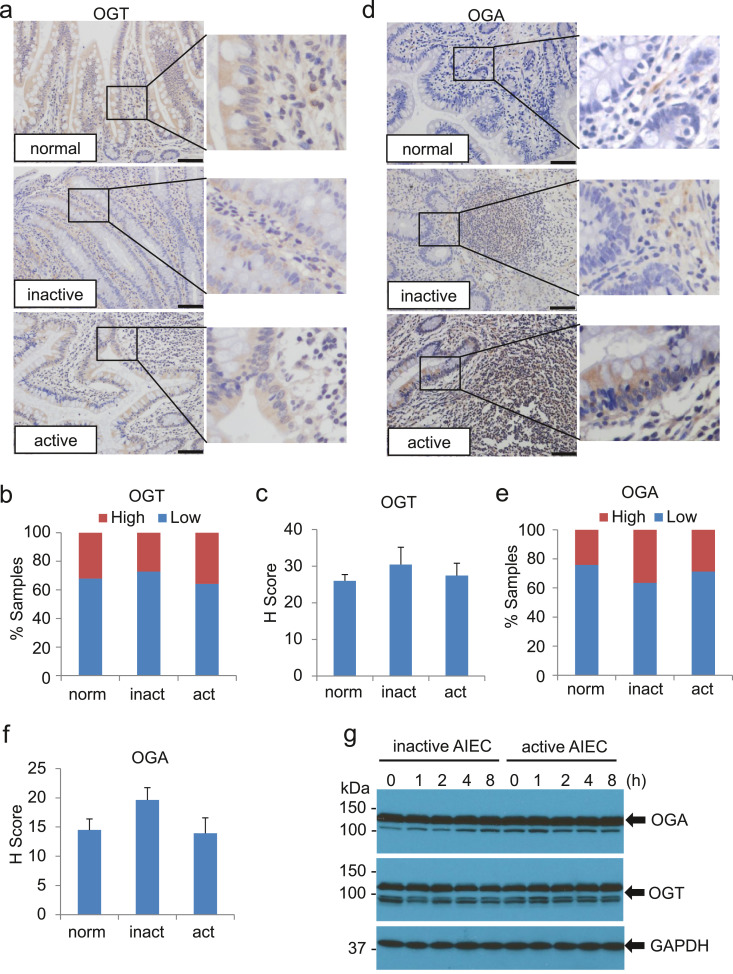
3.2. Expression of OGT and OGA in intestinal epithelial cells of CD is marginally altered
Elevated OGT, reduced OGA, or both contribute to an escalated O-GlcNAc. To interrogate these possibilities, we probed the expression of OGT and OGA in intestinal tissues from normal subjects and inactive and active CD individuals using IHC. Surprisingly, both OGT and OGA were not strikingly alterated in intestinal epithelial cells of CD as compared with those in normal intestinal tissues (Fig. 2a, d). Characterization of additional tissue specimens confirmed these initial findings (Fig. 2b, c, e, f). In addition, infection of AIEC LF82 had little effects on the protein levels of OGT and OGA in HCT116 cells (Fig. 2g). Collectively, our data indicate that alterations of OGT and/or OGA may not be sufficient to explain the high level of O-GlcNAc in intestinal tissues of CD individuals.
Fig. 2.
Expression of OGT and OGA in intestine epithelial cells of CD is marginally altered. (a - f) Assessment of OGT and OGA in intestine tissues from healthy (n = 45), active CD (n = 36), and inactive CD (n = 22) individuals by IHC. Representative staining of OGT (a) and OGA (d) for ileal tissues from each group is presented. Scale bar = 50 µm. Percentage of samples with high or low expression of OGT (b) or OGA (e) in intestinal tissues are shown. H-scores of OGT (c) and OGA (f) for the staining are also displayed. (g) HCT116 cells were treated with AIEC strain LF82 as described in Fig. 1d. Whole cell extracts from the cells were used for the detection of OGT and OGA by western blot. GAPDH was detected for loading control.
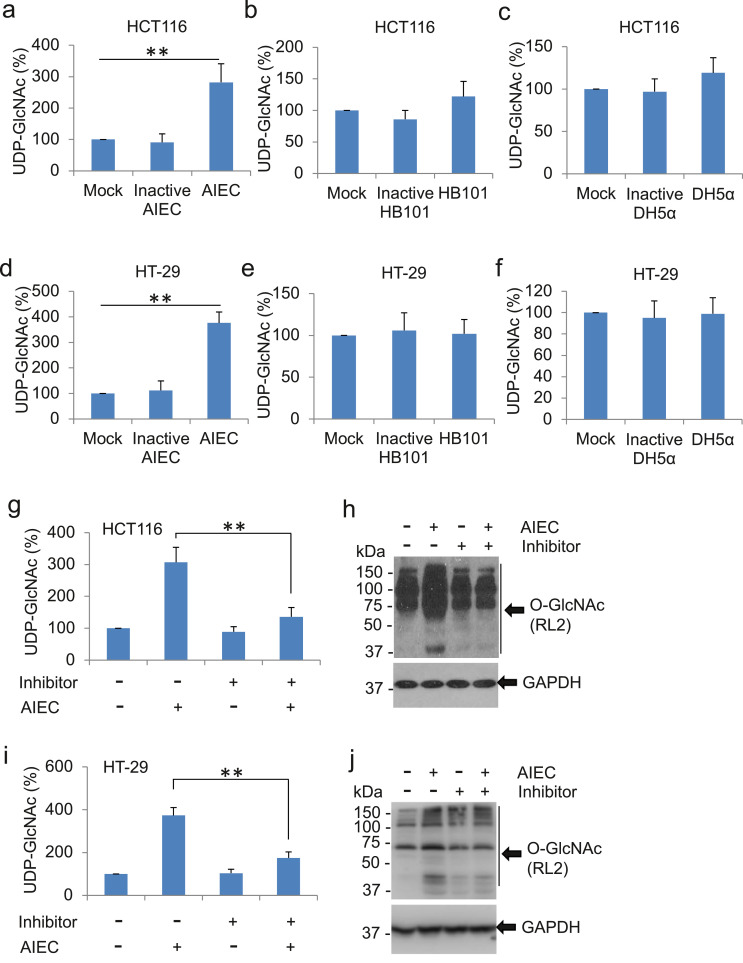
3.3. Infection of active, but not inactive AIEC LF82, leads to the increase of intracellular UDP-GlcNAc and elevation of O-GlcNAc in host cells
Large amounts of N-acetylglucosamine (GlcNAc) and other bacterial cell wall building blocks, such as UDP-GlcNAc, are released during bacterial growth/division and bacterial host invasion [24]. To ascertain whether intracellular invasion and proliferation of AIEC supply a high level of donor glucosamine to fuel in host cells, we measured intracellular UDP-GlcNAc in HCT116 cells infected with active or inactive AIEC LF82. Active AIEC infection led to a 3-fold increase in intracellular UDP-GlcNAc as compared with mock treatment (Fig. 3a). Interestingly, cells exposed to heat-inactive AIEC LF82 failed to increase in UDP-GlcNAc (Fig. 3a). In addition, there was no significant alteration in the levels of UDP-GlcNAc in cells exposed to non-pathogenic bacteria HB101 and DH5α regardless of the active or inactive status (Fig. 3b, c). Similar results were obtained in AIEC LF82-infected HT-29 cells, a cell line with characteristics of mature intestinal cells and sharing similarities with enterocytes of the small intestine (Fig. 3d–f). Together, our data suggest that infection of active AIEC LF82, but not inactive AIEC LF82 or non-pathogenic bacteria, leads to the increase of UDP-GlcNAc in host cells.
Fig. 3.
Infection of active, but not inactive AIEC LF82, leads to the increase of UDP-GlcNAc in HCT116 and HT-29 cells. (a - c) HCT116 cells were infected with AIEC strain LF82 (a), HB101 (b), and DH5α (c) at MOI of 10 for 2 h. Heat-inactivated respective bacteria were used in parallel. After the infections, cells were washed and cell lysates were used for the UDP-GlcNAc measurement. Levels of UDP-GlcNAc in mock-treated cells were set as 100%. The percentages of UDP-GlcNAc in infected cells to mock-treated cells were displayed. Values represent the mean ± S.D. ** p < .01 as compared with mock-treated and inactivated bacteria-treated groups, n = 3 (Student's t-test). (d - f) The levels of UDP-GlcNAc were determined in HT-29 cells with treatments and measurement as describled in a-c. (g,h) HCT116 cells were pre-treated with 10 μM 2-phenylbenzofuran for 1 h before infection with AIEC strain LF82 (MOI = 10) for 2 h. After the infection, cells were washed and cell lysates were collected. (g) Meaurement of UDP-GlcNAc in the cell lysates. Level of UDP-GlcNAc in mock-treated cells was set as 100%. The percentages of UDP-GlcNAc in AIEC LF82-treated cells with or without 2-phenylbenzofuran to mock-treated cells were displayed. Values represent the mean ± S.D. ** p < .01 as compared with infection cells without 2-phenylbenzofuran treatment, n = 3 (Student's t-test). (h) Detection of O-GlcNAc in the cell lysates by western blot. Thirty micrograms of whole cell extracts were loaded for each lane. GAPDH was detected for loading control. (i,j) UDP-GlcNAc and O-GlcNAc were determined in HT-29 cells with treatments and measurement as describled in g,h.
To verify the role of AIEC-induced UDP-GlcNAc in the upregulation of host cell O-GlcNAc, we suppressed the synthesis of bacterial UDP-GlcNAc by pretreatment of cells with 2-phenylbenzofuran, an agent that inhibits the acetyltransferase activity of bacterial GlmU that catalyzes the two final steps of UDP-GlcNAc synthesis in bacteria [25]. As expected, pre-treatment with 2-phenylbenzofuran significantly reduced the level of intracellular UDP-GlcNAc and O-GlcNAc modification in AIEC-LF82-infected HCT116 cells (Fig. 3g, h) and HT-29 cells (Fig. 3i, j), whereas the bacterial GlmU inhibitor had no effects on endogenous level of UDP-GlcNAc and O-GlcNAc modification in host cells (Fig. 3g-j). Taken together, our data indicate that invasion and proliferation of active AIEC LF82 in host cells lead to the increase of intracellular UDP-GlcNAc, which consequently provides sufficient donor glucosamine for the elevation of O-GlcNAc in host cells.
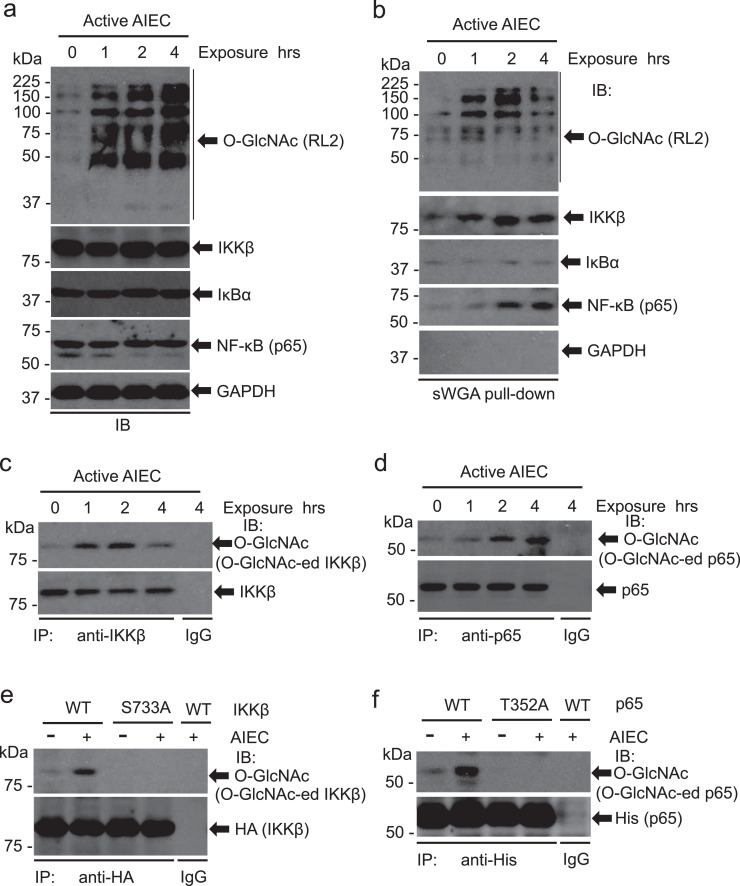
3.4. IKK and NF-kappa B are O-Glycosylated in active AIEC LF82-infected cells
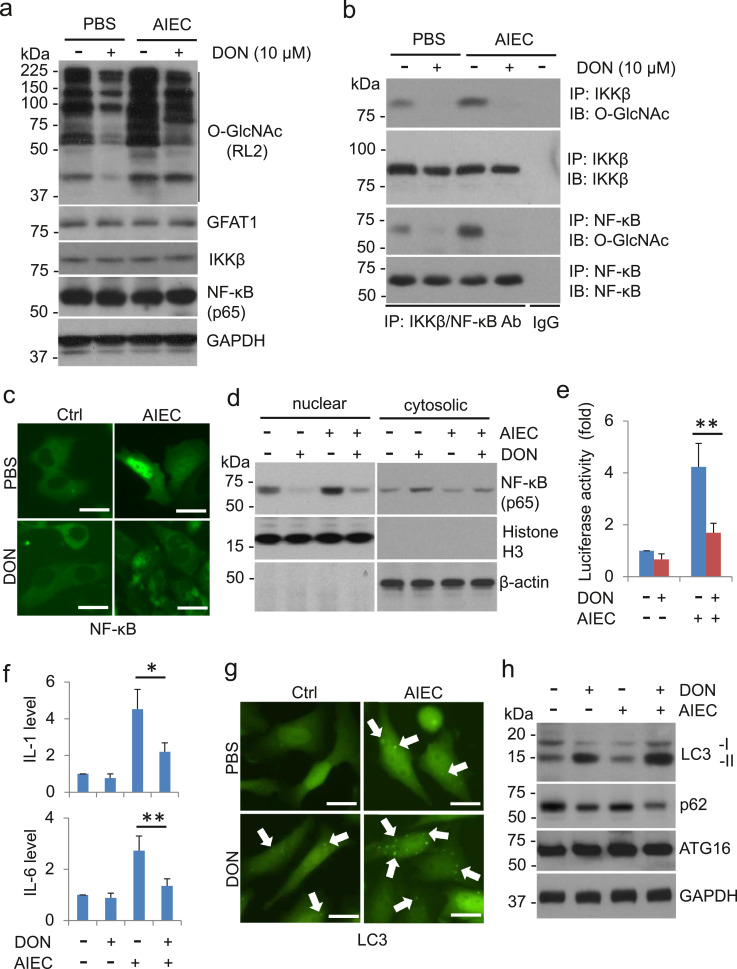
As a nutrient sensor, O-GlcNAc affects multiple cell signals to regulate physiological and pathophysiological processes of the cell. IKK-IκB-NF-κB signaling pathway is critical in inflammation and its function is fine-tuned by post-translational modifications. To determine whether the IKK-IκB-NF-κB signal nodes are O-GlcNAcylated in response to AIEC invasion, we infected HCT116 cells with active AIEC LF82 and measured O-GlcNAc of IKK / IκB / NF-κB with sWGA pull-down assay. Consistent with our previous observations, exposure to active AIEC LF82 led to markedly increases in O-GlcNAc (Fig. 4a). O-GlcNAc of IKKβ and NF-κB (p65) was stringkly increased following exposure to AIEC (Fig. 4b). However, the total levels of IKKβ, IκBα, and NF-κB (p65) and O-GlcNAc of IκBα were not altered by the treatment (Fig. 4a, b). Note that, in our experimental settings, there was no detectible O-GlcNAc of GAPDH (Fig. 4b).
Fig. 4.
IKKβ and NF-kappa B are O-Glycosylated in active AIEC LF82-infected cells. HCT116 cells were infected with AIEC LF82 (MOI = 10) for the indicated time. (a) Whole cell extracts were analyzed with immunoblots for O-GlcNAc, IKKβ, IκBα, and NF-κB (p65) with respective antibodies. GAPDH serves as a loading control. (b) O-GlcNAcylated proteins in HCT116 cells infected with AIEC LF82 were pulled down with sWGA beads. IKKβ, IκBα, NF-κB (p65), and O-GlcNAc in the pull-down complexes were detected with immunoblotting. (c, d) IKKβ and NF-κB (p65) are O-GlcNAcylated. IKKβ (c) and NF-κB (p65) (d) in HCT116 cells infected with AIEC LF82 were immunoprecipiated with anti-IKKβ or anti-NF-κB (p65) antibody. The O-GlcNAcylated IKKβ and NF-κB (p65) were detected with an O-GlcNAc monoclonal antibody, RL2. (e) HCT116 cells were transduced with pcDNA3/HA-IKKβ or pcDNA3/HA-IKKβ S733A for 48 h. The cells were then exposed to AIEC LF82 (MOI = 10) for 4 h before whole cell extracts were collected for the co-IP assay as describled in (c). (f) HCT116 cells were transduced with pcDNA3/His-p65 or pcDNA3/His-p65 T352A for 48 h. The cells were then exposed to AIEC LF82 (MOI = 10) for 4 h before whole cell extracts were harvested for the co-IP assay as describled in (d).
To validate the O-GlcNAc of IKKβ and NF-κB, we performed a pull-down assay using AIEC-infected HCT116 cell extracts. Immunoblots verified that O-GlcNAc of IKKβ and NF-κB was elevated following AIEC LF82 infection (Fig. 4c, d). Previous reports demonstrated that O-GlcNAc of IKKβ at S733 decreases the phosphorylation of the residue and promotes the activity of IKKβ for the phosphorylation of its downstream target, IkBα. Substitute S733 with S733A abrogated AIEC LF82-induced O-GlcNAc of IKKβ (Fig. 4e), indicating that S733 is an important residue of IKKβ in the mediation of AIEC-induced inflammation. Two p65 O-GlcNAc sites were previously mapped to T322 and T352, with the latter being a major residue for the activation of NF-κB. Thus, p65 T352 was mutated to alanine (T352A) and transfected into HCT116 cells followed by the infection of AIEC LF82. Compared to WT p65, p65 T352A mutation completely depleted the O-GlcNAc of p65 induced by AIEC LF82 (Fig. 4f). Together, these results indicate that AIEC LF82 infection promotes the O-GlcNAc of IKKβ and p65, which facilitates the activation of the IKK / IκB / NF-κB signaling in the inflammatory response.
3.5. O-GlcNAc promotes the activation of NF-κB in AIEC LF82-infected cells
To examine the role of O-GlcNAc in NF-κB activation by AIEC infection, we treated AIEC LF82-infected HCT116 cells with 10.0 μM 6-diazo-5-oxo-L-norleucine (DON), a glutamine antagonist that inhibits O-GlcNAc, and detected the activation of NF-κB in the cells. Treatment of DON decreased AIEC LF82-upregulated O-GlcNAc and the O-GlcNAc modification of IKKβ / NF-κB although the expression of IKKβ / NF-κB and GFAT1, the first rate-limited enzyme in the HBP, was not affected by either the treatment of AIEC or DON (Fig. 5a, b). NF-κB signaling relies on nuclear translocation of NF-κB subunit p65, which facilitates transcription of proinflammatory cytokines. Infection of AIEC LF82 led to nuclear translocation and accumulation of p65, which were reversed by the treatment of DON (Fig. 5c, d). Similarly, infection of AIEC LF82 strikingly increased NF-κB activation measured by the luciferase activity assay and by the secretion of proinflammatory cytokines, IL-1 and IL-6 (Fig. 5e, f). These effects were dramatically decreased with the co-treatment of DON (Fig. 5e, f). Together, our results suggest that O-GlcNAc plays a critical role in AIEC-induced IKKβ / NF-κB activation and inflmamtory response.
Fig. 5.
O-GlcNAc promotes the activation of NF-kappa B in AIEC LF82-infected cells. (a) HCT116 cells were pretreated with 10 µM DON for 6 h before exposure to AIEC LF82 (MOI = 10) for additional 4 h. Whole cell extracts were analyzed with immunoblots for O-GlcNAc, IKKβ, and NF-κB (p65) with respective antibodies. GAPDH serves as a loading control. (b) IKKβ and NF-κB (p65) in HCT116 cells treated with DON and AIEC LF82 were immunoprecipiated with anti-IKKβ or anti-NF-κB (p65) antibody. The O-GlcNAcylated IKKβ and NF-κB (p65) were detected with an O-GlcNAc monoclonal antibody, RL2. (c) HCT116 cells were transfected with pcDNA3/His-p65 for 48 h. Cells were then treated with 10 µM DON for 6 h before exposure to AIEC LF82 (MOI = 10) for additional 4 h. The cells were fixed and stained with anti-p65 antibody for the immunofluorescence analysis. (d) HCT116 cells were pretreated with 10 µM DON for 6 h before exposure to AIEC LF82 (MOI = 10) for additional 4 h. Cytosolic and nuclear components of the cells were isolated for the western blot analysis. (e) HCT116 cells were transduced with pGL3/NF-κB and pRL (Renilla A luciferase vector) for 24 h. Cells were then treated with 10 µM DON for 6 h before exposure to AIEC LF82 (MOI = 10) for additional 4 h. After the treatment, cells were harvested for luciferase activity assay using Dual-Luciferase Reporter Assay System (Promega). Relative luciferase unit (RLU) was the ratio of NF-κB luciferase activity to Renilla A activity. ** P < .01 (Student's t-test). (f) HCT116 cells were pretreated with 10 µM DON for 6 h before exposure to AIEC LF82 (MOI = 10) for additional 4 h. After the treatment, supernatants of the cell culture were collected and centrifuged for the measurement of IL-1 and IL-6. * P < .05 and ** P < .01 (Student's t-test). (g, h) HCT116/GFP-LC3 cells were treated with DON and AIEC LF82 as described above. The cells were then analyzed with an Olympus IX51 fluorescence microscope for the GFP-LC3 punta representing autophagosome/autolysosome formation (g). The cell lysates were collected for the detection of autophagy markers LC3, p62, and ATG16. GAPDH was detected as a loading control (h).
We previously reported that in human cell lines, MIR106B and MIR93 reduce levels of autophagy-related gene 16L1 (ATG16L1) and autophagy, and prevent autophagy-dependent eradication of intracellular bacteria, such as AIEC LF82 [17]. This observation is also witnessed in intestinal tissues from active CD individuals [17]. Interestingly, activation of NF-κB was reported to suppress autophagy and promote inflammation. To determine whether O-GlcNAc affects autophagy in AIEC LF82-infected cells, we transfected GFP-LC3, a vector for monitoring autophagy, into HCT116 cells [17] and infected the cells with AIEC LF82 in the presence or absence of DON. AIEC LF82 infection slightly increased basal levels of autophagosome/autolysosome by measuring GFP-LC3 puncta formation in the immunofluorescence assay and concomitantly a cytosolic form of LC3 (LC3-I) conjugated to phosphatidylethanolamine to form LC3-phosphatidylethanolamine conjugate (LC3-II) in the immunoblot assay (Fig. 5g, h). Increased autophagosome/autolysosome formation in AIEC LF82-infected cells was also verified by the reduction of p62, a well-known substrate of autophagy and an indicator for the elevation of autophagy-mediated degradation (Fig. 5g, h). DON treatment alone led to an elevation in autophagy with an average of 11–20 GFP-LC3 puncta being present in each cell (Fig. 5g) and an accumulation of LC3-II and a reduction of p62 in the immunoblot assay (Fig. 5h). Treatment of DON substantially enhanced the autophagy formation in AIEC LF82-infected cells as demonstrated by a marked increase of GFP-LC3 puncta, a striking escalation of LC3-II formation and a decrease of p62 (Fig. 5g, h). Interestingly, ATG16L1 expression was not influenced by AIEC and/or DON in the current experimental settings. Taken together, our data suggest that inhibition of O-GlcNAc promotes autophagy formation in AIEC LF82-infected cells.
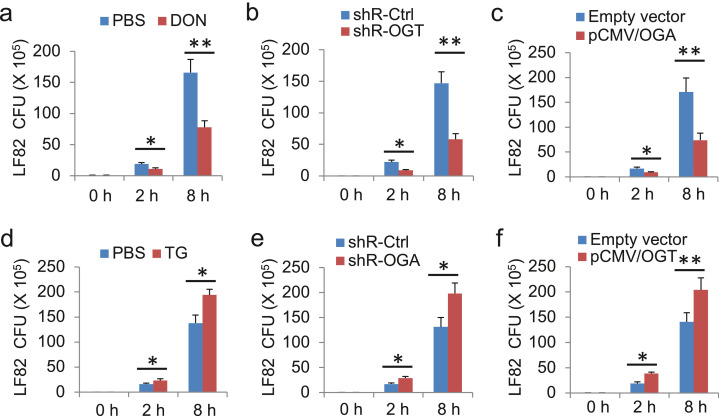
3.6. Inhibition of O-GlcNAc reduces intracellular AIEC LF82
The finding that inhibition of O-GlcNAc led to enhanced autophagy formation in AIEC-infected cells (Fig. 5g, h) prompted us to ask whether manipulation of O-GlcNAc will affect the removal of intracellular AIEC. To pursue the goal, we downregulated O-GlcNAc with O-GlcNAc inhibitor DON, shRNA-OGT, and overexpression of OGA in HCT116 cells followed by infection of the cells with AIEC LF82. The results showed that exposure to AIEC LF82 for 2–8 h led to marked increases in cell-associated bacteria with the colony forming unit (CFU) assay of the cell extracts (Fig. 6a-c). In stark contrast, suppression of O-GlcNAc by either chemical treatment or gene manipulations led to a significant reduction of cell-associated bacteria (Fig. 6a–c). Moreover, upregulation of O-GlcNAc with chemical thiamet-G (TG,10.0 μM), overexpression of OGT, or shR-OGA markedly increased intracellular AIEC accumulation (Fig. 6d–f). Taken together, these results suggest that manipulations of O-GlcNAc affect the accumulation of intracellular AIEC. Inhibition of O-GlcNAc enhances the removal of intracellular AIEC LF82.
Fig. 6.
Inhibition of O-GlcNAc reduces cell-associated AIEC LF82 accumulation. HCT116 cells in a 6-well plate were treated with 10 µM DON (a), knockdown of shR-OGT (b), or overexpression of OGA (c) for the suppression of the O-GlcNAc. Cells were exposed to 10 µM TG (d), knockdown of shR-OGA (e), or overexpression of OGT (f) for the upregulation of the O-GlcNAc. AIEC strain LF82 was used to infect the cells (MOI = 10) for the indicated time. After the infection, cells were washed and lysed for bacterial colonel formation assay. Total colony numbers per well were presented. Values represent the mean ± S.D. * P < .05 and ** P < .01 as compared with non-O-GlcNAc-manipulated groups, n = 3 (Student's t-test).
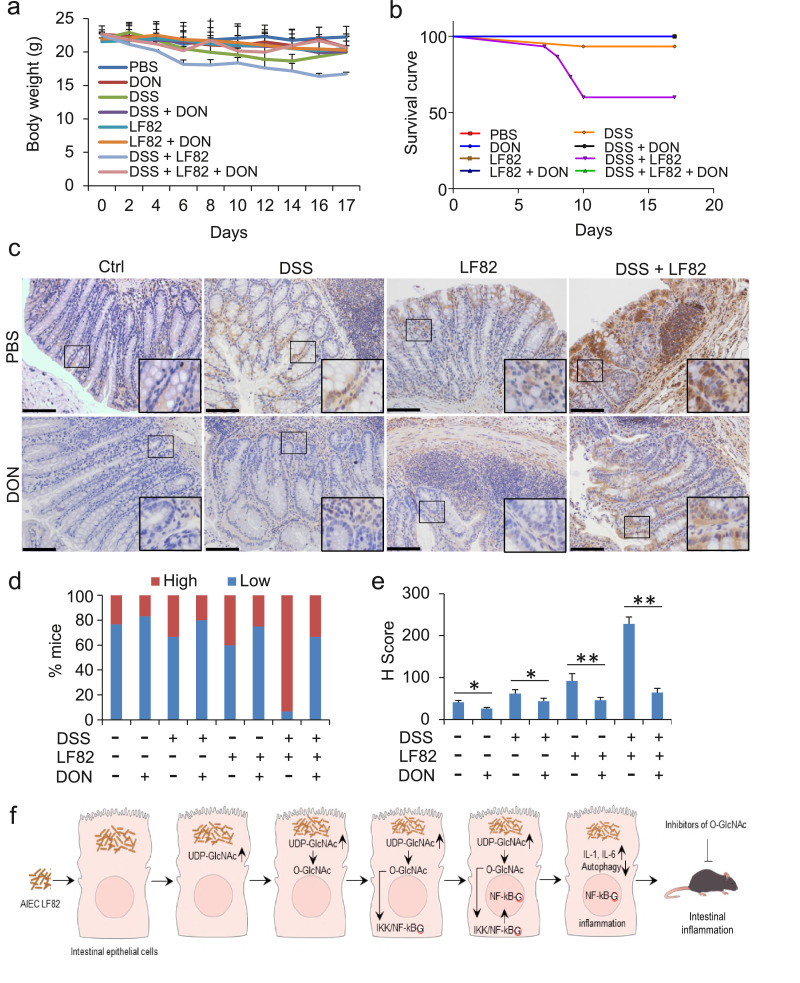
3.7. Inhibition of O-GlcNAc prevents mice from DSS and AIEC LF82-induced enteritis
To verify the role of O-GlcNAc suppression in vivo, we established an intestinal inflammatory model of mice with co-treatment of DSS and/or AIEC LF82 and treated the mice with O-GlcNAc inhibitor DON. Application of DSS and AIEC LF82 to mice for two weeks led to typical symptoms of inflammatory colitis. Mice exhibited a reduced body weight, diarrhoea, bloody stools, and decreased stool weight (Fig. 7a and data not shown). Forty percent of the mice died at the end of the experiments (Fig. 7b). Adminstration of DON to normal control mice did not produce visiable side effects in two weeks (data not shown) [19]. In the current experimental settings, DSS or AIEC LF82 alone showed only a modest effect on mice (Fig. 7a-e). The protective function of DON on these mice was also mild. DSS- and AIEC LF82-colitis mice treated with DON exhibited a marked improvement in the symptoms. Colitis mice treated with DON showed only a mild reduction in body weights (Fig. 7a). diarrhea, bloody stools, and decreased stool weight in the colitis model mice were all improved with the treatment of DON (data not shown). All the mice were survival during the experimental observation (Fig. 7b). Consistently, IHC detection demonstrated that administration of DON strikingly reduced the O-GlcNAc of intestinal epithelial cells and alleviated the intestinal impairment in the colitis mice (Fig. 7c-e). Taken together, our data suggest that inhibition of O-GlcNAc improves DSS- and AIEC LF82-induced colitis in mice, reduces the symptoms, mitigates the intestinal damage, and promotes the survival of the colitis mice.
Fig. 7.
Inhibition of O-GlcNAc prevents mice from DSS- and AIEC LF82-induced intestinal inflammation. C57BL/6 mice were given 3% DSS in drinking water and/or 2 × 108 AIEC LF82 per day in 200 μL PBS by gavage for 2 weeks. Mice were simultaneously treated with 1 mg/kg of DON dissolved in 200 µl PBS by i.p. on day 1 to day 3, then administered every other day until the end of the experiment. (a) The body weights of the mice were monitored every day. (b) Survival of the mice. (c) Colon intestine epithelia of the mice were fixed for the O-GlcNAc staining by IHC at the end of experiment. Scale bar = 50 µm. (d) Percentage of samples with high or low expression of O-GlcNAc in (c). (e) H-scores of O-GlcNAc in (c). * P < .05 and ** P < .01 (Student's t-test). (g) Illustration of O-GlcNAc in the development of intestinal inflammation induced by AIEC LF82.
4. Discussion
Large amounts of GlcNAc and other bacterial cell wall building blocks, such as UDP-GlcNAc, are released during bacterial growth / division and bacterial host invasion [24,25]. In the current study, we demonstrated that AIEC LF82 infection led to a high level of intracellular UDP-GlcNAc, which serves as a donor glucosamine for O-GlcNAc [26]. We found that intestinal epithelial tissues from CD individuals and from AIEC LF82-infected mice exhibited a marked increase in O-GlcNAc. We further identified that IKKβ and NF-κB were O-Glycosylated in AIEC LF82-treated cells. Inhibition of O-GlcNAc or mutations of IKKβ (S733A) and p65 (T352A) abrogated the O-GlcNAc of IKKβ and NF-κB, and more importantly, abolished the activation of NF-κB resulted from the infection of AIEC LF82. In addition, depletion of O-GlcNAc also enhanced the formation of autophagy, promoted the removal of intracellular AIEC LF82, and alleviated intestinal epithelial inflammation of the colitis mice. Together, our findings support the conclusion that O-GlcNAc plays a critical role in AIEC infection-induced inflammatory response in CD (Fig. 7f).
Glycosylation is the most common and the most dynamic protein modification in eukaryotic cells. Various protein-linked glycan structures play crucial roles in numerous cellular processes including cell-cell recognition, signal transduction, and ER protein quality control [27], [28], [29], [30], [31], [32]. Glycosylation defects have been identified as a pathomechanism for a variety of human diseases. It was reported that human adhesion molecule CEACAM6 facilitates colonization by AIEC; its gene expression in return is upregulated by inflammatory cytokines and by microbes [5,6]. CEACAM6 is a highly glycosylated protein that belongs to the large immunoglobulin superfamily. Increased serum levels of CEACAM6 serve as prognostic indicators of chronic inflammation in CD patients, given that no CEACAM6 production and mannosylation were observed in healthy ileal mucosa [5]. In addition, it has been reported that AIEC effectively binds oligomannose glycans [32]. In the current study, we characterized a new role of AIEC in the promotion of host cell O-GlcNAc by upregulaing the donor glucosamine of the modification. Together, data from other groups and us suggest that glycosylation plays a critical role in AIEC-associated pathogenesis.
We recently reported that O-GlcNAc is markedly increased in high risk HPV16/18 oncogene-transduced mouse embryonic fibroblasts, as well as in cervical cancer and precursor lesions, attributable to the ability of HPV16/18 E6 to upregulate OGT [19]. We showed that O-GlcNAc promotes transforming activities of the viral oncogenes [19]. It was reported that CD-associated Nod2 mutant 702 is O-GlcNAcylated [26]. Escalated O-GlcNAc of Nod2 increases the half-life of the protein. Maintaining the stability of proteins is one of the primary effects of the O-GlcNAc modification on proteins [27–32]. Further, increasing O-GlcNAc levels in cells treated with thiamet-G could affect the muramyl dipeptide (MDP)-induced NF-κB activity via Nod2 [33]. These data suggest that O-GlcNAc plays an important role in both virus- and bacteria-induced inflammations.
It was reported that IKKβ and NF-κB p65 are O-GlcNAcylated in response to high glucose [15,34]. Accelerated aerobic glycolysis via the exposure to high glucose promotes O-GlcNAc modification of IKKβ on S733, an inactivating phosphorylation site of the enzyme, and in that way increases the activity of IKKβ. This kind of functional cross-talk between phosphorylation and O-GlcNAc has been extensively observed [35]. O-GlcNAc of NF-κB p65 interrupts the interaction between NF-κB and IκB, leading to the release and nuclear translocation of O-GlcNAcylated NF-κB. In addition, O-GlcNAc of NF-κB was reported to be important for T and B lymphocyte activation [29]. In the current study, we demonstrated that AIEC LF82 infection promoted O-GlcNAc of IKKβ at S733 and NF-κB p65 at T352, and hence activating NF-κB and inflammatory responses in intestinal epithelial cells. Non-specific intestinal inflammation of CD is also mediated by disturbed immune processes, during which various immune cells including T cell, macrophages, and neutrophils are involved. As a regard, IKKβ and NF-κB are activated in immune cells in addition to the intestinal epithelial cells in CD [1–4]. Thus, it is possible that O-GlcNAc modification of IKKβ / NF-κB p65 may also play a role in interstitial inflammation of intestine in CD.
O-GlcNAc was increased in colonic tissues of azoxymethane (AOM)/DSS-induced colitis-associated cancer (CAC) animal models [36,37]. OGA heterozygote (OGA+/-) mice with an increased level of O-GlcNAc bear a much higher susceptibility to DSS-induced colitis and colon tumors than those in OGA+/+ mice [37]. It was believed that elevated O-GlcNAc enhances the activation of NF-κB signaling through increasing the binding of RelA/p65 to its target promoters, contributable to the development of CAC [37]. Similarly, Li et al. reported that O-GlcNAc of STAT3 possesses an inhibitory effect on STAT3 phosphorylation and IL-10 production in macrophages and promotes disease severity in chemically induced CAC model [27]. Together, these results suggest that O-GlcNAc may not only play a positive role in intestinal inflammation but also be causal to inflammation-associated tumorigenesis.
In an intestinal epithelium cell (IEC)-conditional knockout of OGT mouse model, Zhao et al. recently reported that OGT depletion leads to intestinal damage and inflammation in mice [38]. O-GlcNAc deficiency results in disruptive epithelial barrier, Paneth cell dysfunction, and microbial dysbiosis [38]. O-GlcNAc plays a variety of roles in physiology and pathophysiology. Mice with OGT knockout are lethal. Conditional knockout of OGT in IEC may influence multiple targets and functions of the cell, which may be dependent or independent of O-GlcNAc modification, and hence affecting the regeneration of IEC. In the current study, we demonstrated that escalated O-GlcNAc in host cells was due to the accumulation of UDP-GlcNAc resulted from the bacterial invasion and proliferation in the cells. These variations in the experimental settings may explain the differences in characterizing the role of O-GlcNAc in IBDs.
Genetically engineered mouse models have been established to understand the molecular mechanisms of and to develop therapeutic strategies for IBD [39,40]. However, only a handful of IEC-specific genetic models, such as ATG16L1 IECKO mice, were shown to develop spontaneous intestinal inflammation [40]. We previously reported that ATG16L1 targeted by miRNA93/25 in CDs reduces intracellular AIEC LF82 removal due to suppressed autophagy [17]. In the current study, we found that AIEC LF82 infection elevated O-GlcNAc in host cells, inhibition of which prompted the autophagy formation and AIEC LF82 removal in the cells. It is interesting in the future to determine whether ATG16L1 is O-Glycosylated in the AIEC LF82 infection and thereby affects the autophagy formation and bacterial removal.
In conclusion, in the current study, we demonstrated that infection of adherent-invasive AIEC strain LF82 up-regulated the levels of UDP-GlcNAc, a donor glucosamine for glycosylation, leading to the increase of O-GlcNAc in human intestinal epithelial HCT116 and HT-29 cells. O-GlcNAc was markedly increased in ileal tissues of CD patients and AIEC LF82-infected mice. We identified that IKKβ and NF-κB were highly O-Glycosylated in AIEC LF82-treated cells, inhibition of which suppressed the activation of NF-κB resulted from the infection. Reduction of O-GlcNAc by DON, an agent that blocks the production of UDP-GlcNAc and inhibits O-GlcNAc, enhanced the formation of autophagy, promoted the removal of cell-associated AIEC LF82, alleviated intestinal epithelial inflammation, and improved the survival of the colitis mice. Thus, we conclude that intestinal inflammation in CD is associated with increased levels of protein O-GlcNAc. O-GlcNAc inhibitors, such as DON, could be a potential therapy for CD.
Data sharing statement
No additional data are available.
Declaration of competing interest
The authors have declared that no competing of interest exists.
Acknowledgments
The study was in part supported by grants from the National Natural Science Foundation of China (Nos. 81573087 and 81772924) and International Cooperation Foundation of Jilin Province, China (20190701006GH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funders. Funders had no role in study design, data collection, data analysis, interpretation, or writing of this report.
Contributor Information
Yu-Lin Li, Email: ylli@jlu.edu.cn.
Cheng-Shi Quan, Email: quancs@jlu.edu.cn.
Zhi-Xiang Xu, Email: zhixiangxu08@gmail.com.
References
- 1.Thia K.T., Sandborn W.J., Harmsen W.S., Loftus E.V., Jr, Loftus E.V., Jr. Risk factors associated with progression to intestinal complications of Crohn's disease in a population-based cohort. Gastroenterology. 2010;139:1147–1155. doi: 10.1053/j.gastro.2010.06.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torres J., Mehandru S., Colombel J.F., Peyrin-Biroulet L. Crohn's disease. Lancet. 2017;389(10080):1741–1755. doi: 10.1016/S0140-6736(16)31711-1. [DOI] [PubMed] [Google Scholar]
- 3.Podolsky D.K. Inflammatory bowel disease. N EngI J Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 4.Xavier R.J., Podolsky D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 5.Barnich N., Carvalho F.A., Glasser A.L. CEACAM6 acts as a receptor for adherent-invasive E. coli, supporting ileal mucosa colonization in Crohn disease. J Clin Invest. 2007;117(6):1566–1574. doi: 10.1172/JCI30504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dreux N., Denizot J., Martinez-Medina M. Point mutations in Fimh adhesin of Crohn's disease-associated adherent-invasive Escherichia coli enhance intestinal inflammatory response. PLoS Pathog. 2013;9 doi: 10.1371/journal.ppat.1003141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darfeuille-Micheaud A., Boudeau J., Bulois P. High prevalence of adherent-invasive escherichia coli associated with ileal mucosa in Crohn's disease. Gastroenterology. 2004;127:412–421. doi: 10.1053/j.gastro.2004.04.061. [DOI] [PubMed] [Google Scholar]
- 8.Simpson K.W., Dogan B., Rishniw M. Adherent and invasive Escherichia coli is associated with granulomatous colitis in boxer dogs. Infect Immun. 2006;74:4778–4792. doi: 10.1128/IAI.00067-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Conte M.P., Longhi C., Marazzato M. Adherent-invasive Escherichia coli (AIEC) in pediatric Crohn's disease patients: phenotypic and genetic pathogenic features. BMC Res Notes. 2014;7:748. doi: 10.1186/1756-0500-7-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Staib L., Fuchs T.M. From food to cell: nutrient exploitation strategies of enteropathogens. Microbiology. 2014;160:1020–1039. doi: 10.1099/mic.0.078105-0. [DOI] [PubMed] [Google Scholar]
- 11.Haltiwanger R.S., Grove K., Philipsberg G.A. Modulation of O-linked N-acetylglucosamine levels on nuclear and cytoplasmic proteins in vivo using the peptide O-GlcNAc-beta-N-acetylglucosaminidase inhibitor O - (2-acetamido -2- deoxy-D-glucopyranosylidene)amino-N-phenylcarbamate. J Biol Chem. 1998;273:3611–3617. doi: 10.1074/jbc.273.6.3611. [DOI] [PubMed] [Google Scholar]
- 12.Drake W.R., Hou C.W., Zachara N.E., Grimes C.L. New use for CETSA: monitoring innate immune receptor stability via post-translational modification by OGT. J Bioenerg Biomember. 2018;50:231–240. doi: 10.1007/s10863-018-9754-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanover J.A., Krause M.W., Love D.C. Bittersweet memories: linking metabolism to epigenetics through O-GlcNAcylation. Nat Rev Mol Cell Biol. 2012;13:312–321. doi: 10.1038/nrm3334. [DOI] [PubMed] [Google Scholar]
- 14.Ruan H.B., Dietrich M.O., Liu Z.W. O-GlcNAc transferase enables AGRP neurons to suppress browning of white fat. Cell. 2014;159:306–317. doi: 10.1016/j.cell.2014.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang X.Y., Qian K.V. Protein O-GlcNAcylation: emerging mechanisms and functions. Nat Rev Mol Cell Biol. 2017;18:452–465. doi: 10.1038/nrm.2017.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liang J., Shao S.H., Xu Z.X. The energy sensing LKB1-AMPK pathway regulates p27(kip1) phosphorylation mediating the decision to enter autophagy or apoptosis. Nat Cell Biol. 2007;9:218–224. doi: 10.1038/ncb1537. [DOI] [PubMed] [Google Scholar]
- 17.Lu C.M., Chen J.F., Xu H.G. MIR106B and MIR93 prevent removal of bacteria from epithelial cells by disrupting ATG16L1-Mediated autophagy. Gastroenterology. 2014;146:188–199. doi: 10.1053/j.gastro.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu W.S., Xu Z.X., Hittelman W.N., Salomoni P., Pandolfi P.P., Chang K.S. Promyelocytic leukemia protein sensitizes tumor necrosis factor alpha-induced apoptosis by inhibiting the NF-kappaB survival pathway. J Biol Chem. 2003;278:12294–12304. doi: 10.1074/jbc.M211849200. [DOI] [PubMed] [Google Scholar]
- 19.Zeng Q., Zhao R.X., Chen J. O-linked Glcnacylation elevated by HPV E6 mediates viral oncogenesis. Proc Natl Acad Sci U S A. 2016;113:9333–9338. doi: 10.1073/pnas.1606801113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wice B.M., Trugnan G., Pinto M. The intracellular accumulation of UDP-N-acetylhexosamines is concomitant with the inability of human colon cancer cells to differentiate. J Biol Chem. 1985;260:139–146. [PubMed] [Google Scholar]
- 21.Robinson K.A., Weinstein M.L., Lindenmayer G.E., Buse M.G. Effects of diabetes and hyperglycemia on the hexosamine synthesis pathway in rat muscle and liver. Diabetes. 1995;44:1438–1446. doi: 10.2337/diab.44.12.1438. [DOI] [PubMed] [Google Scholar]
- 22.Wine E., Ossa J.C., Gray-Owen S.D., Sherman P.M. Adherent-invasive Escherichia coli, strain LF82 disrupts apical junctional complexes in polarized epithelia. BMC Microbiol. 2009;9:180. doi: 10.1186/1471-2180-9-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Balko J.M., Cook R.S., Vaught D.B. Profiling of residual breast cancers after neoadjuvant chemotherapy identifies DUSP4 deficiency as a mechanism of drug resistance. Nat Med. 2012;18:1052–1059. doi: 10.1038/nm.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lovering A.L., Safadi S.S., Strynadka N.C. Structural perspective of peptidoglycan biosynthesis and assembly. Annu Rev Biochem. 2012;81:451–478. doi: 10.1146/annurev-biochem-061809-112742. [DOI] [PubMed] [Google Scholar]
- 25.Pereira M.P., Blanchard J.E., Murphy C., Roderick S.L., Brown E.D. High-throughput screening identifies novel inhibitors of the acetyltransferase activity of Escherichia coli GlmU. Antimicrob Agents Chemother. 2009;53:2306–2311. doi: 10.1128/AAC.01572-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou C.W., Mohanan V., Zachara N.E., Grimes C.L. Identification and biological consequences of the O-GlcNAc modification of the human innate immune receptor, Nod2. Glycobiology. 2016;26:13–18. doi: 10.1093/glycob/cwv076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X., Zhang Z., Li L. Myeloid-derived cullin 3 promotes STAT3 phosphorylation by inhibiting OGT expression and protects against intestinal inflammation. J Exp Med. 2017;214:1093–1109. doi: 10.1084/jem.20161105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thi Do T., Phoomak C., Champattanachai V., Silsrivanit A., Chaiyarit P. New evidence of connections between increased O-GlcNAcylation and inflammasome in the oral mucosa of patients with oral lichen planus. Clin Exp Immunol. 2018;192:129–137. doi: 10.1111/cei.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramakrishnan P., Clark P.M., Mason D.E., Peters E.C., Hsieh-Wilson L.C., Baltimore D. Activation of the transcriptional function of the NF-κB protein c-Rel by O-GlcNAc glycosylation. Sci Signal. 2013;6:ra75. doi: 10.1126/scisignal.2004097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Allison D.F., Wamsley J.J., Kumar M. Modification of RelA by O-linked N-acetylglucosamine links glucose metabolism to NF-κB acetylation and transcription. Proc Natl Acad Sci USA. 2012;109:16888–16893. doi: 10.1073/pnas.1208468109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li T., Li X., Attri K.S. O-GlcNAc transferase links glucose metabolism to MAVS-Mediated antiviral innate. Cell Host Microbe. 2018;24:791–803. doi: 10.1016/j.chom.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loibl M., Strahl S. Protein O-mannosylation: what we have learned from baker's yeast. Biochim Biophys Acta. 2013;1833:2438–2446. doi: 10.1016/j.bbamcr.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 33.Huang F.C. Regulation of Salmonella flagellin-induced interleukin-8 in intestinal epithelial cells by muramyl dipeptide. Cell Immunol. 2012;278:1–9. doi: 10.1016/j.cellimm.2012.06.013. [DOI] [PubMed] [Google Scholar]
- 34.Kawauchi K., Araki K., Tobiume K., Tanaka N. Loss of p53 enhances catalytic activity of IKKbeta through O-linked beta-N-acetyl glucosamine modification. Proc Natl Acad Sci USA. 2009;106:3431–3436. doi: 10.1073/pnas.0813210106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hart G.W. Nutrient regulation of signaling and transcription. J Biol Chem. 2019;294:2211–2231. doi: 10.1074/jbc.AW119.003226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Viennois E., Chen F., Merlin D. NF-κB pathway in colitis-associated cancers. Transl Gastrointest Cancer. 2013;2:21–29. doi: 10.3978/j.issn.2224-4778.2012.11.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y.R., Kim D.H., Seo Y.K. Elevated O-GlcNAcylation promotes colonic inflammation and tumorigenesis by modulating NF-κB signaling. Oncotarget. 2015;6:12529–12542. doi: 10.18632/oncotarget.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao M., Xiong X.W., Ren K. Deficiency in intestinal epithelial O‐GlcNAcylation predisposes to gut inflammation. EMBO Mol Med. 2018;10:e8736. doi: 10.15252/emmm.201708736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiesler P., Fuss I., Strober W. Experimental models of inflammatory bowel diseases. Cell Mol Gastroenterol Hepatol. 2015;1:154–170. doi: 10.1016/j.jcmgh.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tschurtschenthaler M., Adolph T.E., Ashcroft J.W. Defective ATG16L1-mediated removal of IRE1α drives Crohn's disease-like ileitis. J Exp Med. 2017;214:401–422. doi: 10.1084/jem.20160791. [DOI] [PMC free article] [PubMed] [Google Scholar]