Abstract
The interaction of the host immune system with tumor cells in the tissue microenvironment is essential in understanding tumor immunity and development of successful cancer immunotherapy. The presence of lymphocytes in tumors is highly correlated with an improved outcome. T cells have a set of cell surface receptors termed immune checkpoints that when activated suppress T cell function. Upregulation of immune checkpoint receptors such as programmed cell death 1 (PD-1) and cytotoxic T lymphocyte associated protein 4 (CTLA-4) occurs during T cell activation in an effort to prevent damage from an excessive immune response. Immune checkpoint inhibitors allow the adaptive immune system to respond to tumors more effectively. There has been clinical success in different types of cancer blocking immune checkpoint receptors such as PD-1 and CTLA. However, relapse has occurred. The innate and acquired/therapy induced resistance to treatment has been encountered. Aberrant cellular signal transduction is a major contributing factor to resistance to immunotherapy. Combination therapies with other co-inhibitory immune checkpoints such as TIM-3, LAG3 and VISTA are currently being tested to overcome resistance to cancer immunotherapy. Expression of TIM-3 has been associated with resistance to PD-1 blockade and combined blockade of TIM-3 and PD-1 has demonstrated improved responses in preclinical models. LAG3 blockade has the potential to increase the responsiveness of cytotoxic T-cells to tumors. Furthermore, tumors that were found to express VISTA had an increased rate of growth due to the T cell suppression. The growing understanding of the inhibitory immune checkpoints’ ligand biology, signaling mechanisms, and T-cell suppression in the tumor microenvironment continues to fuel preclinical and clinical advancements in design, testing, and approval of agents that block checkpoint molecules. Our review seeks to bridge fundamental regulatory mechanisms across inhibitory immune checkpoint receptors that are of great importance in resistance to cancer immunotherapy. We will summarize the biology of different checkpoint molecules, highlight the effect of individual checkpoint inhibition as anti-tumor therapies, and outline the literatures that explore mechanisms of resistance to individual checkpoint inhibition pathways.
Introduction
Cancer immunotherapy is an emerging and exciting field of cancer treatments whose main goal is to harness one’s own immune system to recognize and destroy tumor cells. Various forms of immunotherapy are being developed and are in variable stages of preclinical and clinical development. Forms of immunotherapy include, but are not limited to, monoclonal antibodies, cytokines, vaccines, and adoptive T cell transfer [1], [2], [3], [4]. Decades of scientific works, aimed at understanding the biology and regulation of T cell functions, have led to discovery of a set of cell surface receptors that, when activated, suppress the T cell functions. These receptors are collectively referred to as immune checkpoint molecules [5]. Comprehension of the inhibitory immune checkpoints’ ligand biology, signaling mechanisms, and the ensuing T-cell suppression in the tumor microenvironment (TME) fueled the preclinical and clinical advancements in design, testing, and approval of agents such as pembrolizumab, nivolumab, and ipilimumab that block checkpoint molecules. Ipilimumab is approved for the treatment of melanoma. Nivolumab and pembrolizumab were originally approved to treat melanoma, and have now also gained approval for the treatment of renal cell carcinoma, non-small cell lung cancer and more [6]. Durvalumab and avelumab have recently been developed as monoclonal antibody for the PD-L1 checkpoint receptor. Durvalumab that has shown great potential as for the treatment of urothelial carcinoma and avelumab has shown promising results in the treatment of both urothelial carcinoma and Meckel cell carcinoma, both of which currently have limited first-line chemotherapeutic treatment options [7], [8].
Checkpoint inhibition is a novel approach to cancer immunotherapy and is rapidly showing progress in both clinical and preclinical studies as an adjuvant and alternative to traditional cancer therapies. The efficacy of checkpoint inhibition results from releasing T cells from the inhibitory effects of checkpoint molecules. T cells in the TME, in response to various TME derived factors, upregulate expression of checkpoint molecules such as programmed cell death 1 (PD-1), programmed cell death 1 ligand 1 (PD-L1), and cytotoxic T lymphocyte associated protein 4 (CTLA-4). T cells may also be epigenetically reprogrammed to be poised for expression of the checkpoint molecules [9]. This upregulation, and subsequent ligand-interaction mediated downstream signaling leads to suppression of effective T cell signal transduction, proliferation, cytokine production, and effector functions such as cytotoxicity [10]. This results in T cells existing in a state of anergy where they are unable to perform their antitumor effector functions. Checkpoint inhibitors block these checkpoint molecules allowing the adaptive immune system to respond to tumors. Therefore, the presence of existing tumor specific T cells or employment of modalities that generate tumor specific T cells are required for efficacy of checkpoint inhibition [11], [12]. Checkpoint inhibition has shown tremendous potential to change the way clinicians treat cancer but not without limitations. One important limitation is innate and therapy induced resistances to checkpoint inhibitor therapy [13]. Mechanisms of innate and adaptive resistance to checkpoint blockade immunotherapy are under intense investigation. In this review, we will summarize the biology of different checkpoint molecules, highlight the effect of individual checkpoint inhibition as anti-tumor therapies, and outline the literatures that explore mechanisms of resistance to individual checkpoint inhibition pathways. Figure 1 and Table 1 summarize the signaling pathways of various checkpoint molecules discussed in this review, including PD-1, CTLA-4, LAG3, TIM-3 and VISTA.
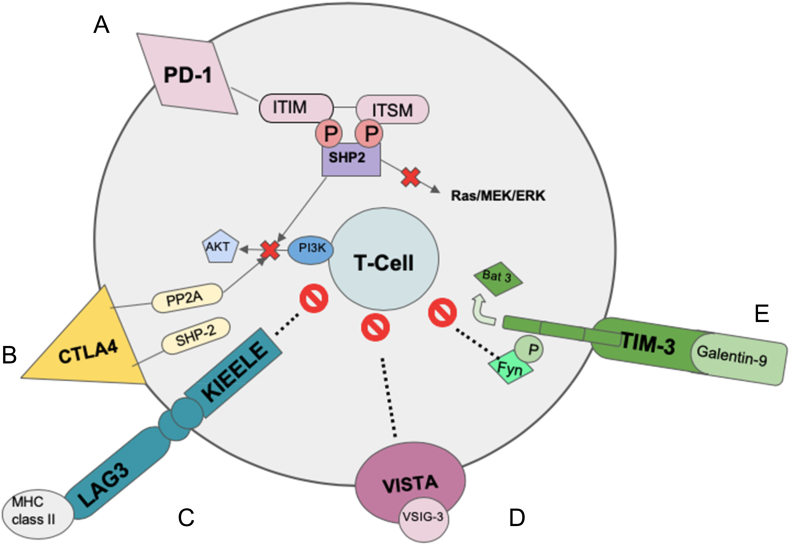
Figure 1.
Schematic representation of immune checkpoints and their signaling in T cells
A. PD-1: PD-1 ligation leads to tyrosine phosphorylation of the cytoplasmic tail of PD-1. This recruits SHP-2 to the cytoplasmic tail of PD-1. Activation of SHP-2 leads to dephosphorylation of TCR proximal kinases, causing inhibition of downstream signaling pathways [14], [15].
B. CTLA4: CTLA4 binds to B7 with a higher affinity than CD28, it then interacts with the tyrosine phosphatase SHP-2 and the serine/threonine phosphatase PP2A to inhibit T cells [16], [17].
C. LAG3: LAG3 binds MHC class II with greater affinity than does CD4. The signaling pathway for LAG3 has not been identified but the KIEELE motif has been shown to be essential for its inhibitory effects on CD4 T cells. Neither its effect on Tregs nor the intracellular proteins that bind it have yet to be identified [18].
D. VISTA: Research has shown that VISTA can function as both a receptor and a ligand, but another ligand, VSIG-3 has also been discovered. Once bound to VSIG-3, VISTA is able to inhibit T cell proliferation and cytokine production [19].
E. TIM-3: The binding of TIM-3 to its ligand Galectin-9 from tumor cells leads to phosphorylation of TIM 3 and the release of Bat-3. The release of Bat-3 allows for the interaction of TIM-3 with Fyn thereby mediating Th1 cell anergy [20].
Abbreviations: Bat3 = HLA (Human Leukocyte Antigen) B-associated transcript 3; CD = cluster of differentiation; CTLA-4 = cytotoxic T-lymphocyte-associated protein 4; ITIM = immunoreceptor tyrosine-based inhibitory motif; ITSM = immunoreceptor tyrosine-based switch motif; LAG3 = lymphocyte-activation gene 3; MHC = major histocompatibility class; PI3K = phosphoinositide 3-kinase; P = phosphate; PD-1 = programmed cell death ligand-1; PP2A = protein phosphatase 2; SHP2 = Src homology phosphatase 2; TCR = T cell receptor; Th1 = Type 1 helper T cell; TIM-3= T cell immunoglobulin and mucin domain-3; Tregs = regulatory T cells; VISTA = V domain-containing immunoglobulin suppressor of T-cell activation; VSIG-3 = V-set and immunoglobulin domain containing 3.
Table 1.
Function and qualities of checkpoint inhibition molecules
| Checkpoint Inhibitor | Location | Intracellular motif | Proposed ligands | Effect on T-cell | References |
|---|---|---|---|---|---|
| PD-1 | T cells, B cells, NK cells | SHP-2 | PD-L1 (B7-H1) PD-L2 (B7-DC) |
Inhibition | Boussiotis et al., 2014 |
| CTLA-4 | Tregs | PP2A, SHP-2 | CD80 (B7-1), CD86 (B7-2) | Inhibition | Walter et al., 2002; Parry et al., 2005 |
| LAG3 | Dendritic cells, CD8 T cells, CD4 T cells, NK cells, Tregs | KIEELE | MHC class II, LSECtin, Galectin-3 | Inhibition/T cell exhaustion | Anderson et al., 2016 |
| TIM-3 | Dendritic cells, Monocytes, CD8 T cells, T helper 1 cells | FYN | Galectin -9, CEACAM1, HMGB1, and phosphatidyl serine | Inhibition | Das et al., 2017 |
| VISTA | T cells, Neutrophils, Macrophages | unknown | VSIG-3, VISTA | Inhibition | Wang et al., 2019 |
Abbreviations: CD = cluster of differentiation; CEACAM1 = carcinoembryonic antigen-related cell adhesion molecule 1; CTLA-4 = cytotoxic T-lymphocyte antigen-4; HMGB1 = high mobility group box 1 protein; LAG3 = lymphocyte activation gene-3; LSECtin = liver sinusoidal endothelial cell lectin; MHC = major histocompatibility complex; NK cells = Natural killer cells; PD-1 = programmed cell death ligand-1; PP2A = protein phosphatase 2; SHP-2 = Src homology phosphatase 2; Tim-3 = T cell immunoglobulin and mucin domain 3; Tregs = Regulatory T cells; VISTA = V domain-containing immunoglobulin suppressor of T-cell activation; VSIG-3 = V-set and immunoglobulin domain containing 3
Programmed Cell Death 1 (PD-1)
Biology
T cell mediated cellular immunity is tightly controlled by multiple stimulatory and inhibitory immune checkpoints. One of the major inhibitory checkpoint molecules, that regulate the T cell functions, is programmed cell death 1 (PD-1 or CD279), which belongs to the CD28/CTLA-4 family of the immunoglobulin superfamily. PD-1 is expressed in low levels on resting cells of the immune system, however, upon activation, PD-1 is induced on T cells, B cells, NK cells, NKT cells, DCs, and macrophages [21]. The two main ligands for PD-1 are PD-L1(B7-H1) and PD-L2 (B7-DC) [22]. PD-L1 is expressed on tumor cells, antigen presenting cells, T lymphocytes, endothelial cells, and fibroblasts, while PD-L2 is primarily induced on DCs, B-cells, macrophages, and monocytes [23]. PD-L1 binds to PD-1 or CD80 receptors on activated immune cells, which leads to T cell dysfunction, neutralization, and anergy [24]. Overexpression of PD-L1 by tumor cells help them evade cytotoxic T cell mediated cell death [25]. Ligation of PD-1 on T cells with ligands PD-L1 or PD-L2, leads to tyrosine phosphorylation of the cytoplasmic domain of PD-1 referred to as immunoreceptor tyrosine-based switch motifs (ITSM). This leads to recruitment of src-homology domain containing phosphatase 2 (SHP-2) to the cytoplasmic tail of PD-1. Activation of SHP-2 leads to dephosphorylation of TCR proximal kinases leading to inhibition of downstream signaling, cytokine release, proliferation, and cytotoxic activity while also promoting their apoptosis [14]. PD-1 signaling on other immune cells such as B cells, dendritic cells, macrophages, and NK cells has also been shown to inhibit their functions [26], [27].
The major evolutionary role of PD-1 may be to regulate T cell activation and its effector functions during an immune response in order to protect bystander tissue from damage, as well as to sustain self-tolerance [28]. Transient induction of PD-1 expression is a result of T cell activation; but sustained expression may result in response to chronic antigen stimulation [29]. T cell signal transduction and various cytokines lead to nuclear factor of activated T-cells cytoplasmic 1 (NFATC1), signal transducer and activator of transcription 3 (STAT-3), signal transducer and activator of transcription 4 (STAT-4), activator protein 1 (AP-1), and IFN-responsive factor 9 (IRF9) mediated PD-1 expression [30], [31]. PD-1 and PD-L1 engagement also leads to suppression of T-cell responses through inhibition of GATA3 and T-bet. T-bet is responsible for repression of inhibitory receptors and also for differentiation and function of CD8+ T cells and Th1 cells [32].
Resistance
Pharmacological blockade of PD-1 or PD-L1 has been at the forefront of immunotherapy for various cancers, as it facilitates efficacious anti-tumor immune responses by reinvigorating previously exhausted T cells. However, up to 50% of patients with PD-L1 positive tumors show resistance or relapse after PD-1/PD-L1 blockade [33]. After an initial response to PD-1/PD-L1 blockade, acquired resistance occurs in most patients [34]. Both innate and adaptive resistance mechanisms are involved in the heterogeneous response to immune checkpoint blockade. Effective immunotherapy is almost entirely reliant on T cell function [35]. This means that T cell generation must be intact, the T cells must be able to physically contact tumor cells, and they must be able to recognize and respond to their target cells effectively. Major factors contributing to PD-1/PD-L1 blockade resistance include constitutive PD-L1 expression in cancer cells, lack of tumor antigens, ineffective antigen presentation, activation of oncogenic pathways, mutations in IFN-γ signaling, and factors within the tumor microenvironment including exhausted T cells, Tregs, myeloid-derived suppressor cells (MDSCs), and tumor-associated macrophages (TAMs) [34]. Successes of PD-1/PD-L1 checkpoint inhibition and mechanisms of immune responses are reviewed elsewhere [36].
Lack of Neoantigens and Loss of Antigen Presentation
The efficacy of anti-PD1 therapy relies upon tumor antigen-specific T-cells within tumor tissue, therefore, tumors must express antigens, which distinguish themselves from non-transformed tissue. Absence of tumor neoantigens means that T cells recognition of tumor cannot occur, and this may contribute to failure of PD-1/PD-L1 blockade therapy. Human melanoma, renal cell carcinoma (RCC), and NSCLC are most sensitive to PD-1/PD-L1 therapy, and this finding is attributed to the fact that these are associated with highly immunogenic mutations harbored by these tumors [37]. Poorly immunogenic tumors show a lack of response to PD-1/PD-L1 blockade [38]. In pancreatic ductal adenocarcinoma (PDAC), checkpoint inhibitors are usually ineffective as monotherapy except in a small subset of patients with a mismatch repair (MMR) deficiency [39]. This may reflect the fact that microsatellite instability results in increased number of somatic mutations, which may be translated into neoantigens that can be recognized by the immune system, increasing responsiveness to checkpoint inhibitor monotherapies [40]. In fact, anti-PD-1 therapy has been approved for use in a subset of PDAC patients whose tumors harbor high microsatellite instability [41]. Loss of antigen-presenting machinery components such as beta-2-microglobulin (β2M) and HLA is another mechanism to avoid antigen processing and presentation by tumors [42]. β2M is essential for assembly of all HLA class I complexes, and for MHC presentation of tumor peptides to T cells [43]. In five cell lines derived from metastatic melanomas with functional loss of β2M expression, replacement of β2M was shown to restore antigen processing capabilities of the cells, as well as recognition of tumor by T cells [44]. In another study of β2M mutations in colorectal carcinoma (CRC), it was found that β2M mutations occur in approximately 24% of microsatellite instability-high CRCs, and were associated with β2M loss in 93% of cases. 85% of β2M-mutant CRCs demonstrated some clinical response to immune checkpoint inhibition [45].
Signaling
Aberrant cellular signal transduction is a major contributing factor to resistance to immunotherapy. Pathways involved include the PI3K/AKT pathway, WNT/ β-catenin pathway, JAK/STAT/IFN-γ pathway, and MAPK pathway [46], [47], [48], [49]. PTEN is a tumor suppressor that suppresses the activity of PI3K [50]. Loss of PTEN is correlated with increased tumor expression of immunosuppressive cytokines and decreased T cell infiltration of tumor [46]. PTEN loss leads to increased activation of the PI3K-AKT pathway, which is correlated with resistance to ICB [46], [51]. Constitutive WNT signaling pathway activity by stabilization of β-catenin is associated with increased T cell exclusion from the tumor microenvironment. Defects in this pathway causing activation of the WNT/β-catenin signaling pathway are correlated with acquired resistance to PD-1 blockade in patients with melanoma [47].
The IFN-γ pathway is another major player in resistance to ICB. Upon recognition of tumor antigens, T cells produce IFN-γ. IFN-γ acts on JAK1 and JAK2 receptors and the signal transducers and activators of transcription (STAT), which leads to upregulation of genes involved in antitumor responses, antigen presentation, and chemokine production to attract T cells to the site of tumor cells [52]. Because the IFN-γ pathway downstream of JAK1 and JAK2 controls expression of chemokines such as CXCL9, CXCL10, and CXCL11, which attract T cells, loss of function mutations in JAK1/2 may result in a lack of T-cell infiltrates, loss of interferon gamma signaling [53]. Because preexisting T cells in the tumor microenvironment are essential for anti-PD-1 therapy, a JAK1/2 mutation may result in lack of response [54].
Tumor Microenvironment
Tumor microenvironment is the cellular environment surrounding the tumor, including immune cells and inflammatory cells, fibroblasts, neuroendocrine cells, extracellular matrix and stromal cells. The interaction of the host immune system with tumor cells in the TME is essential for understanding tumor immunity and development of successful cancer immunotherapy. Some components within the TME which may be associated with primary and acquired resistance to immune checkpoint blockade include, but are not limited to, immunosuppressive cytokines, myeloid-derived suppressor cells (MDSCs), regulatory T cells (Tregs), and exhausted T cells [34].
One example of TME control of T cell migration to the tumor involves epigenetic silencing of T-helper cells (Th1) cytokines, Cxcl9 and Cxcl10 which are involved in chemotactic recruitment of tumor infiltrating lymphocytes (TILs) [55]. Increased intratumoral concentration of Cxcl9 and Cxcl10 is associated with better survival in multiple malignancies [56]. Epigenetic silencing of Th1 cell-type chemokines can enhance clinical effect of PD-L1 checkpoint blockade by increasing tumor-infiltrating lymphocytes. Two mechanisms used by cancer for epigenetic silencing of Th1 cytokines include EZH2-mediated histone modification and DNMT mediated-DNA methylation. One preclinical study of ovarian cancer showed that agents used to reprogram epigenetic pathways such as DZNep (an EZH2 inhibitor) and DNMT inhibitors alters the tumor microenvironment so that T cell infiltration is increased [57].
Chemokine receptor type 4 (CXCR4) and its ligand, CXCL12 are found to be widely expressed in tumors, and are involved in cell proliferation, migration, survival, and tumor metastasis [58]. CXCR4 stimulates tumor metastasis to sites where its ligand, CXCL12 is expressed in large quantities [59]. In an autochthonous model of pancreatic ductal adenocarcinoma, CXCL12 was shown to confer resistance to checkpoint inhibitors through exclusion of T cells, preventing T cells from exerting anti-tumor activity [60]. Blockade of the CXCL12/CXCR4 axis allowed T cell infiltration of tumor and improved efficacy of anti-PD-L1 therapy [61].
The presence of Myeloid-derived suppressor cells (MDSC) in the TME has also been shown to decrease efficacy of ICB [62]. MDSCs arise from myeloid progenitor cells that have not differentiated into dendritic cells, granulocytes, or macrophages. They have the ability to suppress T cell and natural killer cell functions [63]. Activated MDSC produce nitric oxide and upregulate expression of arginase-1, both leading to depletion of L-arginine from the TME and induction of cell cycle arrest of T cells [64]. Nitric oxide and reactive oxygen species produced by MDSCs can induce T cell apoptosis [65]. MDSC activation, arginase-1 expression, and subsequent L-arginine depletion also leads to downregulation of the T cell receptor ζ-chain through post-transcriptional mechanisms that decrease the half-life of the CD3ζ mRNA [66]. Interaction of PD-L1 on MDSC with PD-1 on the T cell leads to T cell exhaustion [67]. MDSC can also induce expansion of regulatory T cells, impairing the anti-tumor activity of effector T cells [68]. There is strong evidence highlighting MDSCs as a major driver of an immunosuppressive microenvironment [69]. Therefore, MDSCs may be a promising target in cancer immunotherapy, especially in combination with ICB. Inhibition of MDSC using PI3K inhibitors in combination with immune checkpoint inhibitors in mouse models was shown to promote tumor regression [70]. There may be a direct role for ICB in modulation of MDSCs. While checkpoint molecules were initially associated with T cell function, recent evidence shows that PD-L1 is also expressed on human granulocytic MDSCs, and therefore, targeting PD-L1 partially impairs MDSC-mediated T cell suppression [71].
Regulatory T cells (Tregs) play a role in suppression of effector T cell response by secretion of IL-10, IL-35, and TGF-β. PD-1 expression upregulates conversion of naïve CD4+ T cells to immunosuppressive Treg cells through inhibition of the mTOR-Akt signaling cascade [72]. Because Tregs upregulate expression of various immune checkpoint molecules, they are promising targets for ICB. In vitro studies show inhibition of Treg suppressive function using nivolumab; however, it is not clear whether PD-1 blockade acts directly on Tregs or via activation of effector T cells [73]. Increase in the Effector T cell (Teff) to Regulatory T cell (Treg) ratio is associated with greater clinical response to PD-1/PD-L1 blockade [74]. Clinical studies investigating the role of PD-1 blockade in suppression of Tregs is limited, but PD-1 blockade was shown to increase generation of melanoma antigen-specific CTLs by alleviating suppression of Treg [73].
Cytotoxic T Lymphocyte Associated Antigen 4 (CTLA-4)
Biology
Cytotoxic T Lymphocyte Associated Antigen-4 (CTLA-4) is a protein that is found on the surface of cells and functions as a receptor. CTLA-4 ultimately functions as a way of inhibiting immune signaling by T cells [16]. An effective T cell response is extremely important in the setting of adaptive immunity against any invading pathogen that the host needs to eliminate. For the T cell response to begin, T cells must first be activated. For T cell activation, two events must occur: antigens must be presented to a T cell and there must also be co-stimulation of a receptor on the T cell, the CD28 receptor, with proteins, usually the B7 proteins which include CD80 and CD86. The invading pathogens stimulate the expression of these proteins and their subsequent presentation on an antigen presenting cell (APC) [16]. Since it was found that CD80 and CD86 are expressed on antigen presenting cells (APCs), it is thought that CTLA-4 functions mainly within secondary lymphoid organs, which is where APCs present antigen to T cells for T cell activation [75]. (CTLA-4) is expressed on the surface of T cells following T cell activation in response to antigen recognition. CTLA-4, often expressed on the antigen specific T cells in the TME, is a negative regulator of T cell activation. It inhibits T cell activation by outcompeting CD28 ligand binding and by recruiting phosphatases in its cytoplasmic tail which leads to attenuation of T cell signaling, subsequently decreasing the immune response. Therefore, by blocking CTLA-4, a more effective and robust T cell response can occur which could confer protection against some cancers. However, since CTLA-4 also functions in the maintenance of self-tolerance, the inhibition of CTLA-4 enhances the possibility of autoimmune reactions [76].
Ipilimumab, a CTLA-4 blocking monoclonal antibody was approved by the FDA as an immune checkpoint inhibitor for use in melanoma. Normally, when the amount of CTLA-4 is upregulated in melanoma, T cell exhaustion results which leads to a decreased overall immune response and subsequent proliferation of the tumor cells [77]. When used in patients with advanced melanoma, Ipilimumab was shown to be effective both in patients that had received prior treatment and in treatment naïve patients [78]. When used in combination therapy with DTIC (Dacarbazine, a chemotherapy drug), results showed a 24% reduction in the progression of melanoma in comparison to using DTIC alone (the use of ipilimumab alone was not measured in this study). In addition to this, the use of Ipilimumab has also shown beneficial responses in patients that have metastases to the brain and in patients that have melanoma associated with BRAF kinase mutations. A trial done on 20 patients with metastases to the brain secondary to melanoma resulted in a one-year survival rate of 54.2% when Ipilimumab was used in combination with Fotemustine, an alkylating agent used in the treatment of metastatic melanoma [78]. Normally, BRAF mutations are associated with unrestrained cell proliferation and are found in 50% to 60% of melanomas. Ipilimumab was found to be effective in tumors both with and without BRAF mutations with similar response rates [78].
Studies performed in breast cancer patients show that the malignant cells in breast cancers have a higher expression of CTLA-4, suggesting that they upregulate CTLA-4. The study that was conducted was evaluating the expression of CTLA-4 in tumor cells in relation to expression in tumor infiltrating lymphocytes, or TILs (correlation between CTLA-4 and tumor type, stage, or grade was not analyzed). It was shown that the best prognosis was associated with a higher CTLA-4 expression in the TILs when compared to the tumor cells, suggesting the usage of CTLA-4 in tumor cells for avoidance of the immune response [79]. Clinical trials currently underway are looking into the use of anti CTLA-4 antibodies for the treatment of triple negative breast cancer as part of a combination therapy with either blockade of other immune checkpoint molecules, like PD-1, or with adjuvant chemotherapy [80].
Many clinical trials have been conducted in order to better understand the impact of immune checkpoint inhibition on patients with lung cancer. While immune checkpoint blockade represents a novel treatment option, outcomes are not always favorable. In a double-blind study performed on patients with non-small cell lung cancer (NSCLC), the combination of Ipilimumab and Paclitaxel/Carboplatin, which is the standard treatment, was compared to the combination of a placebo with Paclitaxel/Carboplatin. Results showed no difference with the addition of Ipilimumab to treatment [81]. Other trials have results which show definite benefit to the use of combination therapy which includes an immune checkpoint inhibitor; however, it is unknown which patients would receive benefit from the use of this combination. It was also found that combination with an immune checkpoint inhibitor for NSCLC causes more unfavorable side effects including fatigue and increased liver transaminases [82].
Resistance
While Ipilimumab has proven to be effective in the treatment of certain tumors, such as melanoma, the time necessary to achieve an effective anti-tumor response is prolonged compared to other agents currently in use. This delay in effectiveness allows for disease progression which could cause more negative consequences when compared to the use of an agent with immediate effectiveness [78]. Another drawback to the use of CTLA-4 blockade is that it could lead to side effects that occur as a result of induced autoimmunity, especially in the skin and gastrointestinal tract. The appearance of these side effects in the patient, however, ultimately leads to response to therapy and indicates a favorable outcome [83]. While anti CTLA-4 antibodies have been successful in the fight against some tumors, some tumors have been shown to have resistance. CTLA-4 blockade works to enhance T cell function and the immune response against tumors, T cells do this through interferon gamma (IFN-γ). It was found that some tumors have a lack of the genes for response to IFN-γ and those that do are more resistant to therapy with anti CTLA-4 antibodies [84]. Another mechanism of resistance found on tumor cells is the upregulation of other checkpoint inhibitors when therapy with one antibody is used. For example, when studying melanoma or prostate cancer, it was found that tumors that initially upregulated CTLA-4 and were subsequently treated with anti CTLA-4 antibodies upregulated VISTA instead, leading to a separate pathway for inhibition of T cells [36].
The CTLA-4 immune checkpoint depends on the costimulatory molecule B7 to induce its response. A separate study found that the response to anti CTLA-4 antibodies correlates to the presence of B7 on tumor cells. This signifies that tumors that have decreased immunogenicity will not respond to anti CTLA-4 therapy, such as melanoma B16-BL6 which is a melanoma that is very tumorigenic but not very immunogenic [85]. An experiment performed on mice showed that anti CTLA-4 antibodies are dependent on the Fcγ receptor which is important for the mediation of response to antibodies [86].
In order to combat many of these mechanisms to resistance the use of combinations of checkpoint blocking antibodies could be used. It was found that the combination of anti CTLA-4 and anti PD-1 antibodies had a much greater response rate compared to the use of either antibody alone, even though it also resulted in a greater amount of side effects for the patient and the possibility of relapse. Rate of side effects for anti CTLA-4 alone were 27.3%, and for anti PD-1 alone were 16.3% while the rate for combined blockade rose to 55% [36]. A trial performed on patients with melanoma showed increased survival benefits with combination therapy (58% response rate) as compared to single therapy with either anti CTLA-4 (19% response rate) or anti PD-1 (44% response rate) [87].
Combining the antibodies with other molecules that further enhance the immune response has also proven beneficial, such as the concomitant use of anti CTLA-4 antibodies and cytokines [36].
Lymphocyte Activation Gene 3 (LAG 3)
Biology
Lymphocyte Activation Gene 3 (LAG3) is an immune checkpoint receptor that suppresses T-cell activity when upregulated. LAG3 (CD223) is a transmembrane protein receptor that has been identified on activated T -cells, regulatory T-cells, B-cells, NK cells, and plasmacytoid dendritic cells [88], [89]. It is structurally similar to the CD4 protein and binds the antigen-MHC class II complex on antigen presenting cells with an even higher affinity than does CD4 [90]. Due to its ability to suppress NK cells and CD8 T-cell activities, it is proposed that LAG3 also binds other ligands, such as Galectin-3 and liver sinusoidal endothelial cell lectin (LSECtin). Galectin-3 has been suggested as an alternative ligand for LAG3 [91], [92]. Galectin-3 has been shown to moderate effector T-cell responses and LAG3 has been shown to be essential in CD8 T-cell suppression in vitro [91]. LSECtin, a receptor expressed on liver cells and many tumor cells, has been postulated to be an additional ligand for LAG3 [92]. LAG3 upregulation is required to prevent over-activation and autoimmune reaction to self by inhibiting effector T cells and promoting regulatory T-cell responses. However, persistent antigen exposure, such as in the TME, leads to persistent T-cell activation, eventually causing T-cell exhaustion [93]. Presently, it is known that LAG3 is activated when it crosslinks with CD3 [94]. The downstream effects of this association have not yet been well defined, but its cytoplasmic tail region contains a unique KIEELE motif which is responsible for its inhibitory actions in effector T-cells. It is not yet known if this is the case in regulatory T-cells [18]. As with self-antigen, continued exposure to tumor antigens causes upregulation of LAG3 and its inhibitory actions lead to T-cell exhaustion, thus rendering these cells ineffective at directing attacks towards tumors cells.
Blockade Response
LAG3 blockade has the potential to increase the responsiveness of cytotoxic T-cells to tumors. Presently, four LAG3 moderating pharmacological agents are in clinical trials as cancer treatment adjuvants [93]. The first is IMP321 (Immutep, Eftilagimod alpha), a recombinant fusion protein for the LAG3 receptor (PL1). IMP321 (PL2) consists of four immunoglobulin domains for LAG3, fused to the Fc portion of one IgG molecule [90]. Its potential as an anti-tumor agent stems from the fact that it blocks inhibitory signals in effector T-cells and stimulates dendritic cells as well as improves antigen presentation leading to T-cell activation [95]. The other three LAG3 moderating agents are monoclonal antibodies (mab) for the LAG3 receptor, and include BMS-986016 (Bristol-Myers Squibb, fully human IgG4), LAG525 (Novartis, humanized IgG4) and MK-4280 (Merck). There are additional LAG3 moderating cancer therapies being tested in preclinical trials, as well as LAG3 mabs in clinical trials for the treatment of autoimmune diseases [93]. IMP321 is currently being assessed in clinical trials as an adjuvant to the treatment of breast cancer, renal cell cancer and melanoma. 30 patients with metastatic breast carcinoma were treated with a combination of Paclitaxel chemotherapy and IMP321. Clinical benefits were seen in 90% of patients at 6 months as seen by an increased percentage of activated APCs and an increase in the percentage of NK cells and CD8+ T-cells when compared the control group who only received Paclitaxel, of which 50% saw clinical benefits [96]. 21 patients with advanced stage renal cell carcinoma were treated with IMP321 bi-weekly for six injections, with doses ranging from 0.05 mg-30 mg per subcutaneous injection. Of these patients, those treated with high dose (>6mg per injection) experienced increased activation of CD8 T-cells and were more likely to experience stable disease at 3 months than those treated with lower doses of IMP321. At the end of the trial, less tumor growth was seen in the high dose group than in the low dose group [97]. In both clinical trials described above, IMP321 was judged to be safe. Initially, ELISA detected anti-IMP321 antibodies more than 15% over baseline levels in patients receiving IMP321. Further testing by a very sensitive immunogenicity assay (MSD) analyzer for the detection of therapeutic antibodies gave a signal below the detection range, thus confirming that no anti-IMP321 antibodies were present in either of the two trials [96], [97]. LAG3 blockade is currently being assessed in murine models in combination with PD1 blockade for the treatment of Chronic Lymphocytic Leukemia (CLL). This preclinical trial demonstrated that in the treatment of CLL, blockade of both LAG3 and PD1 was superior to either anti-PD1 or anti-LAG3 antibody-based therapy alone. Mass cytometry demonstrated by a significant reduction in both the percentage and number of CCL cells in both the spleen and in the blood of mice treated with the dual therapy [98]. Separate preclinical trials have revealed that dual blockade of both LAG-3 and PD-1 with monoclonal antibodies, spartalizumab and LAG525 respectively, has increased efficacy in the treatment of neuroendocrine tumors, small cell lung cancer and diffuse large B-cell lymphoma when compared to either treatment alone [99]. Further preclinical trials with these agents are ongoing for the treatment of mesothelioma and triple-negative breast cancer [100].
Resistance
For LAG3 blocking agents to be effective at inhibiting the actions of LAG3, expression of LAG3 on various T cells, as well as other immune cells, should be assessed within the tumor microenvironment. Patients with greater expression of LAG3 may require increased doses of blocking agents, such as IMP321, whereas patients with greatly decreased expression of LAG3 may respond better to a different immunotherapy agent. Patients with greater than 1% expression of LAG3 are more likely to derive clinical benefit from receptor blockade [101]. In addition, the concentration of natural sLAG3 levels within the blood should be assessed. sLAG3 is a soluble LAG3 receptor that is not bound to any cell type. It serves a different purpose from membrane bound LAG3. sLAG3 is also capable of binding MHC class II molecules on dendritic APCs and has been shown to promote their maturation and tumor cell attack [102]. Although sLAG3 and LAG3 are functionally distinct, there may be potential for interactions between sLAG3 and IMP321, thus making IMP321 inefficient at LAG3 blockade [90]. Results from additional clinical and preclinical trials may be necessary to elucidate the mechanism of resistance to anti-LAG3 therapies. LAG3 blockade has also been shown to work synergistically with PD-1 blockade in preclinical trials, and thus is being utilized as a means to overcoming resistance to anti-PD-1 therapy [98]. This could be due to an amplifying effect of PD1 that is necessary for LAG3 blockade to be clinically effective. Presently, the first LAG3 antibody, BMS-986016, is being evaluated in twelve preclinical and clinical trials for the treatment of various malignancies. Of note, the combination of both BMS-986016 and nivolumab, an anti-PD-1 agent, has shown efficacy in the treatment of melanoma in patients who were previously refractory to anti-PD-1 therapy alone [103]. The molecular mechanism of the proposed synergy of co-blockade has not yet been fully investigated [104].
T-Cell Immunoglobulin and Mucin Domain 3 (TIM-3)
Biology
T-cell immunoglobulin and mucin-domain containing-3 (TIM-3) is a member of the TIM gene family that dampens the immune response. The family includes TIM-1, TIM-3, TIM-4 in humans and Tim 1-8 in mice. TIM-3 is a transmembrane protein expressed on Th1, Th17, CD8+ T cells-cells of myeloid lineages in mice [105], [106], [107]. TIM-3 binds to Galectin-9, carcinoembryonic antigen-related cell adhesion molecule 1 (Ceacam1), high-mobility group box 1 (HMGB1) and phosphatidylserine (PtdSer) [20]. TIM-3 has been shown to mediate immune tolerance in mouse models of infectious diseases, alloimmunity, autoimmunity, and tumor immunity.
In the absence of TIM-3 ligand binding, human leukocyte antigen B (HLA-B)-associated transcript 3 (Bat3) is bound to the cytoplasmic tail of TIM-3 and prevents inhibition of T cell signaling via recruitment of Lck [108]. Binding of TIM-3 to its ligands leads to phosphorylation of its cytoplasmic tail, release of Bat3 and possible recruitment of the protein tyrosine kinase Fyn which induces T cell anergy [109]. Some Tim-3 ligands: galectin-9 and HMBG1 are induced in inflammatory conditions [110], [111]. In macrophages binding of TIM-3 to galectin-9 induces intracellular calcium flux, aggregation and death of Th1 cells resulting in selective loss of interferon-y producing cells and suppression of Th1 autoimmunity [112]. The molecular mechanisms by which TIM-3 regulates the development of T cell exhaustion are not yet fully understood across the myeloid lineage.
Blockade Response
Increased TIM-3 and/or increased regulatory T cells (Tregs) within the tumor allow inactivation of immune system killer T cells halting tumor cells death. TIM-3 can regulate cytokine production, cell activation, and the capture of apoptotic bodies. Preclinical investigation on the effects of combined immunotherapy checkpoint inhibitors PD-1 and TIM-3 along with focal radiation on murine gliomas resulted in improved survival versus anti-TIM-3 immunotherapy alone. In the study, the median survival using anti-PD1 alone in the tumor-bearing mouse models was 33 days. When anti-TIM 3 was added to anti-PD1 the median survival days improved from 33 days to 100 days. Overall the anti-PD1 and anti-TIM 3 combination improve the overall survival from 27.8% to 57.9% a p-value of 0.103. Even though the p value was not clinically significant, when triple therapy was performed which consisted of an initial single dose of sterostatic surgery along with anti-PD1 and anti-TIM 3, the overall survival rate resulted in 100% a p-value of 0.004 [113]. This long-term survival demonstrated in this study paves the way in the development of a novel treatment of glioblastoma multiforme because positive staining for TIM-3 was detected in human glioblastoma multiforme samples. Anti-TIM-3 treated mice showed that the anti-tumor immune response was enhanced due to a reduction in CD4+, CD25+ and Foxp3+ regulatory T cells [114]. Despite a large amount of experimental data showing an immune suppressive function of TIM-3 in vivo, the exact mechanisms are not well understood.
Resistance
Expression of the co-inhibitory immune checkpoints TIM-3 have been associated with resistance to PD-1 blockade and combined TIM-3 and PD-1 blockade has demonstrated improved responses in preclinical models [115], [116]. A recent study in an immunocompetent mouse model of lung adenocarcinoma demonstrated that recurrent tumors after anti-PD-1 treatment were due to increased expression of TIM-3 on T cells. Notably, anti-PD-1 plus anti-TIM-3 led to improved responses in the tumor bearing mice. Similarly, two lung cancer patients who developed recurrent disease after anti-PD-1 treatment were found to have increased TIM-3 expression on T cells [117].
Clinical Trials
According to the National Cancer Institute, immuno-oncology two clinical trials using anti-TIM- 3 monoclonal antibodies are underway. TSR-022, monoclonal antibody against TIM-3, treatment is currently in phase 1. TRS-022 is being tested as monotherapy and in combination with an anti-PD1 antibody in patients with advanced solid tumors (Clinical Trial ID: NCT02817633). Another clinical trial underway, also in phase 1, is the testing of Sym023. Sym023 is a recombinant and fully human anti-TIM-3 monoclonal antibody. The primary purpose of the Sym023 study is to see if it is safe and tolerable in patients with locally advanced, unresectable or metastatic solid tumor malignancies or lymphomas that are refractory to available therapy or for which no standard therapy is available (Clinical Trial ID: NCT03489343). These studies will provide insight for the rational design of novel combination therapy with other checkpoint blockers like PD1 whose resistance is linked to the upregulation of TIM-3.
V domain Ig Suppressor of T Cell Activation (VISTA)
Biology and Potential
V domain immunoglobulin suppressor of T cell Activation (VISTA) suppresses the activity of T cells and works as an immune checkpoint regulator. VISTA is related to CTLA-4 and PD-L1 in that it is part of the B7 family of coreceptors used for T cell activation. VISTA has been found to be expressed on hematopoietic cells (e.g., neutrophils, macrophages, T-cells) and works as a negative regulator of immunity [75]. In murine models, tumors that were found to express VISTA had an increased rate of growth due to the T cell suppression [75]. Its newly proposed ligand, VSIG-3, has been found to be inhibitory towards T cells when combined with VISTA in the lab. Studies examining VISTA/VSIG-3 in vivo, however, were not performed [19]. In studies performed on mice, it was found that VISTA elicits a response that works against tumors and mice that were deficient in VISTA had a worse and faster progression and disease course when exposed to certain diseases, such as Systemic Lupus Erythematosus or Psoriasis [118]. In another study, it was found that VISTA is normally present in normal joint synovium as well as in the joint synovium of patients with rheumatoid arthritis. Studies show that VISTA is thus important in mounting an inflammatory response, which shows a potential use for anti-VISTA antibodies for treatment [119].
Initially, VISTA expression on tumor cells was thought to be a mechanism to evade anti PD-1 and anti CTLA-4 treatments. Tumor cells that express PD-1 or CTLA-4 and treated with anti PD-1/CTLA-4 antibodies were found to upregulate VISTA instead of the former immune checkpoint inhibitors. However, VISTA was found in 99% of non-small cell lung cancers and may be used as a new target of therapy in the treatment of this cancer [120].
Potential drawbacks with the use of VISTA and anti-VISTA antibodies stem from the fact that different diseases react to VISTA in different ways. While VISTA seems to be useful in slowing the disease progression of Lupus or Psoriasis, antibodies against VISTA were shown to prevent graft vs. host disease [119].
Clinical Trial
The clinical trial currently underway involving VISTA is an evaluation of CA-170, which is a VISTA/PD-L1 antagonist. In this trial, CA-170 is given to patients with a solid tumor or lymphoma with disease progression or disease unresponsiveness to other therapies. CA-170 is an anti PDL1/L2 and anti-VISTA molecule (Clinical Trial ID: NCT02812875).
Immunotherapy Adverse Effects
Resistance to checkpoint inhibition is not the only limitation that cancer immunotherapy faces. Immune mediated toxicities following the use of immune checkpoint inhibitors have been observed involving nearly every organ system, including the colon, liver, lungs, pituitary, kidney, heart and nervous system. The side effects associated with checkpoint inhibition are termed immune-related adverse effects (irAEs). IrAEs are graded by severity on a scale of 1-4, grade 4 referring to life threatening symptoms. General guidelines regarding irEA grading and treatment are provided by Common Terminology Criteria for Adverse Events (CTCAE), however in cases of severe adverse events expert advice should be solicited [121]. The development of these irAEs is attributed to the activation of autoreactive T cells which damage host tissues [122]. A meta-analysis of fatal toxic effects associated with checkpoint inhibitors estimates approximately 613 patients have died as a result of therapy, and that fatalities tend to occur very early in therapy [122].
Monotherapy Adverse Effects
The immune-related adverse effects resulting from checkpoint inhibition therapy varies between regimens. In general, patients receiving PD-1/PD-L1 blockade therapy are at decreased risk of immune-related adverse effects than those treated with CTLA-4 blocking agents [123]. Patients treated with ipilimumab monotherapy are at increased risk of fatal colitis (70% of deaths resulting from ipilimumab were a result of colitis) [122]. PD-1/PD-L1 blocking agents that caused irAEs resulting in fatalities were commonly caused by pneumonitis, hepatitis and colitis [122]. Although fatalities as a result of irAEs do occur, they are only in approximately 1% of patients treated with checkpoint inhibition [122]. Most irAEs resolve following the administration of glucocorticoids, and at times other immunosuppressive agents such as infliximab [123].
Combination Immunotherapy
There is limited data regarding adverse effects related to dual checkpoint inhibitor therapy as it is an emerging field of clinical medicine. The combination of immune checkpoint inhibitors is largely focused on the combination of CTLA-4 inhibitors and PD-1/PD-L1 inhibitors for the treatment of metastatic melanoma and lung cancer [122]. However, there have been a variety of case reports describing these findings. One such report describes the case of a 60 year old male with metastatic melanoma being treated with a combination of ipilimumab (a CTLA-4 inhibitor) and nivolumab (a PD-1 inhibitor), in addition to palliative excision of metastatic masses [124].The combination of ipilimumab with a PD-1 inhibitor (either nivolumab or pembrolizumab) has become accepted in recent literature as superior to ipilimumab alone in cases where melanoma progression continues despite monotherapy [125], [126]. The patient in the case described began developing symptoms of nausea, constipation, weight loss, fatigue, and hypotension (seated systolic BP as low as 70 mmHg systolic) and was diagnosed with seronegative autoimmune autonomic ganglionopathy [124]. The patient was then successfully treated with high dose Solumedrol and intravenous immune globulin (IVIG). At this time, the choice was made to reintroduce the dual checkpoint inhibition therapy, resulting in a mild flare of dysautonomia for which the patient was started on a prednisone taper [124]. This instance describes a rare but ultimately mild irAE associated with ipilimumab/nivolumab. On the opposite end of the spectrum, the combination of ipilimumab and nivolumab has been shown to result in fatal myositis [127]. This paper reports the cases of two separate patients being treated with ipilimumab/nivolumab for metastatic melanoma who developed myositis with rhabdomyolysis. Despite aggressive glucocorticoid therapy, both patients ultimately suffered death. As the utilization of combined methods for checkpoint blockade become more established, the adverse effect profile of the dual regimen will become more defined. Currently, it is believed that dual therapy is associated with an increased risk of some immune related adverse events, including colitis, hepatitis, pneumonitis and thyroid disease, resulting in both hyper- and hypothyroidism [123], [128]. Additionally, irAEs that resulted in fatalities from treatment with ipilimumab/nivolumab were most commonly a result of colitis, myocarditis, hepatitis, pneumonitis and myositis, respectively [122].
Discussion and Clinical Implication
The immune checkpoints we have highlighted in our review: PD-1, CTLA-4, LAG3, TIM-3, and VISTA are naturally switched on to prevent the damaging effects of an excessive immune response. The number of lymphocytes present at the time of tumor biopsy has a positive correlation with disease control. As such, PD-L1 and PD-1 pathway blockade is heavily dependent on T cell infiltration of the tumor and T cell function in the tumor microenvironment. Therefore, the goal of cancer immunotherapy is to block these immune checkpoints in order to stop the downregulation of our adaptive immune response to tumor cells. The growing number of preclinical and clinical studies involving the use of ICB in the treatment of multiple cancers supports the immense potential of ICB in cancer therapy. The aim of this review was to research the root cause of resistance to immune checkpoint inhibition in immuno-oncology from available literature.
The introduction of PD-1 and CTLA-4 blockade as a tool against cancer has demonstrated an initial success; however, innate and acquired/therapy-induced resistance continue to be reported. Based on our literature review, a major determinant of the effectiveness of individual checkpoint inhibitors is whether the specific receptor (PD-1, LAG3, etc.) is expressed at high enough levels within the tumor microenvironment. Therefore, it is critical to screen for the levels of immune checkpoint expression when deciding which immune checkpoint inhibitors to utilize in patient-specific treatment protocols.
Another roadblock in ICB has been upregulation of a different checkpoint inhibitor upon single checkpoint blockade. The upregulation of TIM-3 in PD-1 blockade as well as upregulation of VISTA in both PD-1 and CTLA-4 single immune checkpoint inhibition blockade has been documented. Consequently, a different pathway is activated to downregulate T cell cytotoxicity against tumor cells. Combination immunotherapy with Anti-TIM 3 monoclonal antibodies treatment and PD-1 are currently in phase 1 clinical trials. Much research on VISTA is lacking. The possibility of anti-VISTA antibodies presents a novel mechanism by which to treat tumors what have grown resistant to anti CTLA-4 or anti PD-L1 antibodies. Furthermore, clinical trials have demonstrated that the recombinant fusion protein and monoclonal antibodies engineered to inhibit the LAG3 receptor have shown moderate clinical benefit when used in combination with chemotherapeutic agents or other immune checkpoint inhibitors, such as PD-1, in the treatment of various cancers. A clearer understanding of the tumor microenvironment, along with genetics and epigenetics, are essential as tumor immunotherapy research continues to evolve.
Combination immuno-therapy is an active area of research. Even though clinical trials have demonstrated improved survival, it is crucial to recognize immune-related adverse effects. Adverse effects, as mentioned, range in severity and organ involvement. They can occur at any time during treatment. Due to the paucity of immune related adverse events, general recommendations for management are updated as the use of immune-checkpoint inhibitors expands and is currently based on expert consensus.
Conclusion
The presence of lymphocytes in tumors is highly correlated with an improved outcome. Our review of different immune checkpoints provides a comprehensive and evidence-based information on where we currently stand in the treatment of cancer with immunotherapy. Published results have addressed the outcome of PD-1 and CTLA4 inhibition with initial success followed by relapse due to resistance. This necessitates illumination of cellular, genomic, and epigenomic features involved in tumor response. This initial attempt to target cancer via immunotherapy in clinical trials has opened the door to a new era in cancer treatment research. In this review, we have highlighted known and targeted mechanisms of anti-PD-1, anti-CTLA4, anti-LAG3, anti-VISTA, and anti-Tim3 antibodies that has the potential to halt resistance development. Combination immunotherapy has been associated with improved response against tumor cells and prolonged disease control in preclinical trials. This makes it likely that moving forward, the standard of care will include a more personalized and combinatory regimen. In order to continue to progress and translate these findings to a wider range of tumor types, we must continue to elucidate the biological mechanisms of resistance to these immune checkpoint inhibitors.
Contributor Information
Purushottam Lamichhane, Email: plamichhane@lecom.edu.
Rahul R. Deshmukh, Email: rdeshmukh@lecom.edu.
References
- 1.Gatti-Mays ME, Redman JM, Collins JM, Bilusic M. Cancer vaccines: Enhanced immunogenic modulation through therapeutic combinations. Hum Vaccin Immunother. 2017;13:2561–2574. doi: 10.1080/21645515.2017.1364322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rogers LM, Veeramani S, Weiner GJ. Complement in monoclonal antibody therapy of cancer. Immunol Res. 2014;59(0):203-210. [DOI] [PMC free article] [PubMed]
- 3.Showalter A, Limaye A, Oyer JL, Igarashi R, Kittipatarin C, Copik AJ, Khaled AR. Cytokines in immunogenic cell death: Applications for cancer immunotherapy. Cytokine. 2017;97:123–132. doi: 10.1016/j.cyto.2017.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang JC, Rosenberg SA. Adoptive T-cell therapy for cancer. Adv Immunol. 2016;130:279–294. doi: 10.1016/bs.ai.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Sousa Linhares A, Leitner J, Grabmeier-Pfistershammer K, Steinberger P. Not all immune checkpoints are created equal. Front Immunol. 2018;9:1909. doi: 10.3389/fimmu.2018.01909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fares CM, Van Allen EM, Drake CG, Allison JP, Hu-Lieskovan S. Mechanisms of resistance to immune checkpoint blockade: Why does checkpoint inhibitor immunotherapy not work for all patients? American Society of Clinical Oncology Educational Book. 2019;(39):147–164. doi: 10.1200/EDBK_240837. [DOI] [PubMed] [Google Scholar]
- 7.Teets A, Pham L, Tran EL, Hochmuth L, Deshmukh R. Avelumab: A novel anti-PD-L1 agent in the treatment of merkel cell carcinoma and urothelial cell carcinoma. Crit Rev Immunol. 2018;38(3):159–206. doi: 10.1615/CritRevImmunol.2018025204. [DOI] [PubMed] [Google Scholar]
- 8.Wills S, Hochmuth LK, Bauer KS, Jr., Deshmukh R. Durvalumab: a newly approved checkpoint inhibitor for the treatment of urothelial carcinoma. Curr Probl Cancer. 2018;43(3):181–194. doi: 10.1016/j.currproblcancer.2018.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Bally AP, Austin JW, Boss JM. Genetic and epigenetic regulation of PD-1 expression1. J Immunol. 2016;196(6):2431–2437. doi: 10.4049/jimmunol.1502643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Isakov N. Cancer immunotherapy by targeting immune checkpoint receptors. World Journal of Immunology. 2018;8(1):1–11. [Google Scholar]
- 11.Granier C, De Guillebon E, Blanc C, Roussel H, Badoual C, Colin E, Saldmann A, Gey A, Oudard S, Tartour E. Mechanisms of action and rationale for the use of checkpoint inhibitors in cancer. ESMO Open. 2017;2(2) doi: 10.1136/esmoopen-2017-000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamichhane PL, Neha PA, Manuj A, Narottam. Checkpoint inhibition: will combination with radiotherapy and nanoparticle-mediated delivery improve efficacy? Medicines. 2018;5(4):114. [DOI] [PMC free article] [PubMed]
- 13.Chen PL, Roh W, Reuben A, Cooper ZA, Spencer CN, Prieto PA, Miller JP, Bassett RL, Gopalakrishnan V, Wani K. Analysis of immune signatures in longitudinal tumor samples yields insight into biomarkers of response and mechanisms of resistance to immune checkpoint blockade. Cancer Discov. 2016;6(8):827–837. doi: 10.1158/2159-8290.CD-15-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boussiotis VA, Chatterjee P, Li L. Biochemical signaling of PD-1 on T cells and its functional implications. Cancer J. 2014;20(4):265–271. doi: 10.1097/PPO.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bardhan K, Anagnostou T, Boussiotis VA. The PD1:PD-L1/2 pathway from discovery to clinical implementation. Front Immunol. 2016;7 doi: 10.3389/fimmu.2016.00550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alberts B., Johnson A., Lewis J. Helper T Cells and Lymphocyte Activation. 4th edition. Garland Science; New York: 2002. Molecular Biology of the Cell.https://www.ncbi.nlm.nih.gov/books/NBK26827/ Available from: [Google Scholar]
- 17.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25(21):9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson AC, Joller N, Kuchroo VK. Lag-3, Tim-3, and TIGIT: Co-inhibitory receptors with specialized functions in immune regulation. Immunity. 2016;44(5):989–1004. doi: 10.1016/j.immuni.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Wu G, Manick B, Hernandez V, Renelt M, Erickson C, Guan J, Singh R, Rollins S, Solorz A. VSIG-3 as a ligand of VISTA inhibits human T-cell function. Immunology. 2019;156(1):74–85. doi: 10.1111/imm.13001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das M, Zhu C, Kuchroo VK. Tim-3 and its role in regulating anti-tumor immunity. Immunol Rev. 2017;276(1):97–111. doi: 10.1111/imr.12520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keir ME, Francisco LM, Sharpe AH. PD-1 and its ligands in T-cell immunity. Curr Opin Immunol. 2007;19(3):309–314. doi: 10.1016/j.coi.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, Fitz LJ, Malenkovich N, Okazaki T, Byrne MC. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: Mechanisms, response biomarkers, and combinations. Sci Transl Med. 2016;8(328):328rv324. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sun Z, Fourcade J, Pagliano O, Chauvin JM, Sander C, Kirkwood JM, Zarour HM. IL10 and PD-1 cooperate to limit the activity of tumor-specific CD8+ T cells. Cancer Res. 2015;75(8):1635–1644. doi: 10.1158/0008-5472.CAN-14-3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8(6):467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 26.Terme M, Ullrich E, Aymeric L, Meinhardt K, Desbois M, Delahaye N, Viaud S, Ryffel B, Yagita H, Kaplanski G. IL-18 induces PD-1–dependent immunosuppression in cancer. Cancer Res. 2011;71(16):5393–5399. doi: 10.1158/0008-5472.CAN-11-0993. [DOI] [PubMed] [Google Scholar]
- 27.Fanoni D, Tavecchio S, Recalcati S, Balice Y, Venegoni L, Fiorani R, Crosti C, Berti E. New monoclonal antibodies against B-cell antigens: possible new strategies for diagnosis of primary cutaneous B-cell lymphomas. Immunol Lett. 2011;134(2):157–160. doi: 10.1016/j.imlet.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 28.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon S, Labarriere N. PD-1 expression on tumor-specific T cells: Friend or foe for immunotherapy? Oncoimmunology. 2017;7(1) doi: 10.1080/2162402X.2017.1364828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oestreich KJ, Yoon H, Ahmed R, Boss JM. NFATc1 regulates PD-1 expression upon T cell activation. J Immunol. 2008;181(7):4832–4839. doi: 10.4049/jimmunol.181.7.4832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Terawaki S, Chikuma S, Shibayama S, Hayashi T, Yoshida T, Okazaki T, Honjo T. IFN-alpha directly promotes programmed cell death-1 transcription and limits the duration of T cell-mediated immunity. J Immunol. 2011;186(5):2772–2779. doi: 10.4049/jimmunol.1003208. [DOI] [PubMed] [Google Scholar]
- 32.Dong Y, Sun Q, Zhang X. PD-1 and its ligands are important immune checkpoints in cancer. Oncotarget. 2017;8:2171–2186. doi: 10.18632/oncotarget.13895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515(7528):563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bai J, Gao Z, Li X, Dong L, Han W, Nie J. Regulation of PD-1/PD-L1 pathway and resistance to PD-1/PD-L1 blockade. Oncotarget. 2017;8(66):110693–110707. doi: 10.18632/oncotarget.22690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang M, Yin B, Wang HY, Wang R-F. Current advances in T-cell-based cancer immunotherapy. Immunotherapy. 2014;6(12):1265–1278. doi: 10.2217/imt.14.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seidel JA, Otsuka A, Kabashima K. Anti-PD-1 and Anti-CTLA-4 therapies in cancer: mechanisms of action, efficacy, and limitations. Front Oncol. 2018;8:86. doi: 10.3389/fonc.2018.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science. 2015;348(6230):69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 39.Hilmi M, Bartholin L, Neuzillet C. Immune therapies in pancreatic ductal adenocarcinoma: Where are we now? World J Gastroenterol. 2018;24(20):2137–2151. doi: 10.3748/wjg.v24.i20.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown SD, Warren RL, Gibb EA, Martin SD, Spinelli JJ, Nelson BH, Holt RA. Neo-antigens predicted by tumor genome meta-analysis correlate with increased patient survival. Genome Res. 2014;24(5):743–750. doi: 10.1101/gr.165985.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Macherla S, Laks S, Naqash AR, Bulumulle A, Zervos E, Muzaffar M. Emerging role of immune checkpoint blockade in pancreatic cancer. Int J Mol Sci. 2018;19 doi: 10.3390/ijms19113505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marincola FM, Jaffee EM, Hicklin DJ, Ferrone S. Escape of human solid tumors from T-cell recognition: molecular mechanisms and functional significance. Adv Immunol. 2000;74:181–273. doi: 10.1016/s0065-2776(08)60911-6. [DOI] [PubMed] [Google Scholar]
- 43.Hulpke S, Tampe R. The MHC I loading complex: a multitasking machinery in adaptive immunity. Trends Biochem Sci. 2013;38(8):412–420. doi: 10.1016/j.tibs.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Restifo NP, Marincola FM, Kawakami Y, Taubenberger J, Yannelli JR, Rosenberg SA. Loss of functional beta2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. J Natl Cancer Inst. 1996;88(2):100–108. doi: 10.1093/jnci/88.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Middha S, Yaeger R, Shia J, Stadler ZK, King S, Guercio S, Paroder V, Bates DD, Rana S, Diaz LA., Jr. Majority of B2M-mutant and-deficient colorectal carcinomas achieve clinical benefit from immune checkpoint inhibitor therapy and are microsatellite instability-high. JCO precision oncology. 2019;3:1–14. doi: 10.1200/PO.18.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng W, Chen JQ, Liu C, Malu S, Creasy C, Tetzlaff MT, Xu C, McKenzie JA, Zhang C, Liang X. Loss of PTEN promotes resistance to t cell-mediated immunotherapy. Cancer Discov. 2016;6(2):202–216. doi: 10.1158/2159-8290.CD-15-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523(7559):231. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 48.Darnell JE, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 49.Liu C, Peng W, Xu C, Lou Y, Zhang M, Wargo JA, Chen JQ, Li HS, Watowich SS, Yang Y. BRAF inhibition increases tumor infiltration by T cells and enhances the antitumor activity of adoptive immunotherapy in mice. Clin Cancer Res. 2013;19(2):393–403. doi: 10.1158/1078-0432.CCR-12-1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Georgescu M-M. PTEN tumor suppressor network in PI3K-Akt pathway control. Genes Cancer. 2010;1(12):1170–1177. doi: 10.1177/1947601911407325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song MS, Salmena L, Pandolfi PP. The functions and regulation of the PTEN tumour suppressor. Nat Rev Mol Cell Biol. 2012;13(5):283–296. doi: 10.1038/nrm3330. [DOI] [PubMed] [Google Scholar]
- 52.Bach EA, Aguet M, Schreiber RD. The IFN gamma receptor: a paradigm for cytokine receptor signaling. Annu Rev Immunol. 1997;15:563–591. doi: 10.1146/annurev.immunol.15.1.563. [DOI] [PubMed] [Google Scholar]
- 53.Fish EN, Platanias LC. Interferon receptor signaling in malignancy: a network of cellular pathways defining biological outcomes. Molecular Cancer Research. 2014;12(12):1691–1703. doi: 10.1158/1541-7786.MCR-14-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, Chmielowski B, Spasic M, Henry G, Ciobanu V. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagarsheth N, Peng D, Kryczek I, Wu K, Li W, Zhao E, Zhao L, Wei S, Frankel T, Vatan L. PRC2 epigenetically silences Th1-type chemokines to suppress effector tT-cell trafficking in colon cancer. Cancer Res. 2016;76(2):275–282. doi: 10.1158/0008-5472.CAN-15-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bronger H, Singer J, Windmüller C, Reuning U, Zech D, Delbridge C, Dorn J, Kiechle M, Schmalfeldt B, Schmitt M. CXCL9 and CXCL10 predict survival and are regulated by cyclooxygenase inhibition in advanced serous ovarian cancer. Br J Cancer. 2016;115(5):553–563. doi: 10.1038/bjc.2016.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peng D, Kryczek I, Nagarsheth N, Zhao L, Wei S, Wang W, Sun Y, Zhao E, Vatan L, Szeliga W. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature. 2015;527(7577):249–253. doi: 10.1038/nature15520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dewan M, Ahmed S, Iwasaki Y, Ohba K, Toi M, Yamamoto N. Stromal cell-derived factor-1 and CXCR4 receptor interaction in tumor growth and metastasis of breast cancer. Biomed Pharmacother. 2006;60(6):273–276. doi: 10.1016/j.biopha.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 59.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410(6824):50. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 60.Feig C, Jones JO, Kraman M, Wells RJ, Deonarine A, Chan DS, Connell CM, Roberts EW, Zhao Q, Caballero OL. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti–PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci. 2013;110(50):20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fearon DT. The carcinoma-associated fibroblast expressing fibroblast activation protein and escape from immune surveillance. Cancer immunology research. 2014;2(3):187–193. doi: 10.1158/2326-6066.CIR-14-0002. [DOI] [PubMed] [Google Scholar]
- 62.Meyer C, Cagnon L, Costa-Nunes CM, Baumgaertner P, Montandon N, Leyvraz L, Michielin O, Romano E, Speiser DE. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol Immunother. 2014;63(3):247–257. doi: 10.1007/s00262-013-1508-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Najjar YG, Finke JH. Clinical perspectives on targeting of myeloid derived suppressor cells in the treatment of cancer. Front Oncol. 2013;3:49. doi: 10.3389/fonc.2013.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rodriguez PC, Quiceno DG, Ochoa AC. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood. 2007;109(4):1568–1573. doi: 10.1182/blood-2006-06-031856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bogdan C. Nitric oxide and the immune response. Nat Immunol. 2001;2(10):907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 66.Rodriguez PC, Zea AH, Culotta KS, Zabaleta J, Ochoa JB, Ochoa AC. Regulation of T cell receptor cd3ζ chain expression byl-arginine. Journal of Biological Chemistry. 2002;277(24):21123–21129. doi: 10.1074/jbc.M110675200. [DOI] [PubMed] [Google Scholar]
- 67.Dieterich LC, Ikenberg K, Cetintas T, Kapaklikaya K, Hutmacher C, Detmar M. Tumor-associated lymphatic vessels upregulate PDL1 to inhibit T-cell activation. Front Immunol. 2017;8:66. doi: 10.3389/fimmu.2017.00066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pistoia V, Morandi F, Bianchi G, Pezzolo A, Prigione I, Raffaghello L. Immunosuppressive microenvironment in neuroblastoma. Front Oncol. 2013;3 doi: 10.3389/fonc.2013.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Parker KH, Beury DW, Ostrand-Rosenberg S. Myeloid-derived suppressor cells. Critical Cells Driving Immune Suppression in the Tumor Microenvironment. Adv Cancer Res. 2015;128:95–139. doi: 10.1016/bs.acr.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Henau O, Rausch M, Winkler D, Campesato LF, Liu C, Cymerman DH, Budhu S, Ghosh A, Pink M, Tchaicha J. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kgamma in myeloid cells. Nature. 2016;539(7629):443–447. doi: 10.1038/nature20554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ballbach M, Dannert A, Singh A, Siegmund DM, Handgretinger R, Piali L, Rieber N, Hartl D. Expression of checkpoint molecules on myeloid-derived suppressor cells. Immunol Lett. 2017;192:1–6. doi: 10.1016/j.imlet.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 72.Wang W, Lau R, Yu D, Zhu W, Korman A, Weber J. PD1 blockade reverses the suppression of melanoma antigen-specific CTL by CD4+ CD25(Hi) regulatory T cells. Int Immunol. 2009;21(9):1065–1077. doi: 10.1093/intimm/dxp072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ohaegbulam KC, Assal A, Lazar-Molnar E, Yao Y, Zang X. Human cancer immunotherapy with antibodies to the PD-1 and PD-L1 pathway. Trends Mol Med. 2015;21(1):24–33. doi: 10.1016/j.molmed.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dyck L, Wilk MM, Raverdeau M, Misiak A, Boon L, Mills KH. Anti-PD-1 inhibits Foxp3(+) Treg cell conversion and unleashes intratumoural effector T cells thereby enhancing the efficacy of a cancer vaccine in a mouse model. Cancer Immunol Immunother. 2016;65(12):1491–1498. doi: 10.1007/s00262-016-1906-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nowak EC, Lines JL, Varn FS, Deng J, Sarde A, Mabaera R, Kuta A, Le Mercier I, Cheng C, Noelle RJ. Immunoregulatory functions of VISTA. Immunol Rev. 2017;276(1):66–79. doi: 10.1111/imr.12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kavanagh B, O'Brien S, Lee D, Hou Y, Weinberg V, Rini B, Allison JP, Small EJ, Fong L. CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood. 2008;112(4):1175–1183. doi: 10.1182/blood-2007-11-125435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yu GT, Bu LL, Zhao YY, Mao L, Deng WW, Wu TF, Zhang WF, Sun ZJ. CTLA4 blockade reduces immature myeloid cells in head and neck squamous cell carcinoma. Oncoimmunology. 2016;5(6) doi: 10.1080/2162402X.2016.1151594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wolchok JD, Hodi FS, Weber JS, Allison JP, Urba WJ, Robert C, O'Day SJ, Hoos A, Humphrey R, Berman DM. Development of ipilimumab: a novel immunotherapeutic approach for the treatment of advanced melanoma. Ann N Y Acad Sci. 2013;1291:1–13. doi: 10.1111/nyas.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Voutsadakis IA. Immune blockade inhibition in breast cancer. Anticancer Res. 2016;36(11):5607–5622. doi: 10.21873/anticanres.11145. [DOI] [PubMed] [Google Scholar]
- 80.Vikas P, Borcherding N, Zhang W. The clinical promise of immunotherapy in triple-negative breast cancer. Cancer management and research. 2018;10:6823. doi: 10.2147/CMAR.S185176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Govindan R, Szczesna A, Ahn MJ, Schneider CP, Gonzalez Mella PF, Barlesi F, Han B, Ganea DE, Von Pawel J, Vladimirov V. Phase III trial of ipilimumab combined with paclitaxel and carboplatin in advanced squamous non-small-cell lung cancer. J Clin Oncol. 2017;35(30):3449–3457. doi: 10.1200/JCO.2016.71.7629. [DOI] [PubMed] [Google Scholar]
- 82.Peng M, Li X, Lei G, Weng YM, Hu MX, Song QB. The efficacy and safety of immune checkpoint inhibitor combination therapy in lung cancer: a systematic review and meta-analysis. OncoTargets and therapy. 2018;11:7369. doi: 10.2147/OTT.S177318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sandin LC, Eriksson F, Ellmark P, Loskog AS, Tötterman TH, Mangsbo SM. Local CTLA4 blockade effectively restrains experimental pancreatic adenocarcinoma growth in vivo. Oncoimmunology. 2014;3 doi: 10.4161/onci.27614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gao J, Shi LZ, Zhao H, Chen J, Xiong L, He Q, Chen T, Roszik J, Bernatchez C, Woodman SE. Loss of IFN-gamma Pathway Genes in Tumor Cells as a Mechanism of Resistance to Anti-CTLA-4 Therapy. Cell. 2016;167(2):397–404. doi: 10.1016/j.cell.2016.08.069. e399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.van Elsas A, Hurwitz AA, Allison JP. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J Exp Med. 1999;190(3):355–366. doi: 10.1084/jem.190.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ingram JR, Blomberg OS, Rashidian M, Ali L, Garforth S, Fedorov E, Fedorov AA, Bonanno JB, Le Gall C, Crowley S. Proc Natl Acad Sci U S A. Vol. 115. 2018. Anti–CTLA-4 therapy requires an Fc domain for efficacy; pp. 3912–3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chae YK, Arya A, Iams W, Cruz MR, Chandra S, Choi J, Giles F. Current landscape and future of dual anti-CTLA4 and PD-1/PD-L1 blockade immunotherapy in cancer; lessons learned from clinical trials with melanoma and non-small cell lung cancer (NSCLC) Journal for immunotherapy of cancer. 2018;6(1):39. doi: 10.1186/s40425-018-0349-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goldberg MV, Drake CG. LAG-3 in cancer immunotherapy. Curr Top Microbiol Immunol. 2011;344:269–278. doi: 10.1007/82_2010_114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Catakovic K, Klieser E, Neureiter D, Geisberger R. T cell exhaustion: from pathophysiological basics to tumor immunotherapy. Cell Commun Signal. 2017;15(1) doi: 10.1186/s12964-016-0160-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nguyen LT, Ohashi PS. Clinical blockade of PD1 and LAG3—potential mechanisms of action. Nat Rev Immunol. 2015;15(1):45–56. doi: 10.1038/nri3790. [DOI] [PubMed] [Google Scholar]
- 91.Kouo T, Huang L, Pucsek AB, Cao M, Solt S, Armstrong T, Jaffee E. Galectin-3 shapes antitumor immune responses by suppressing CD8+ T cells via LAG-3 and inhibiting expansion of plasmacytoid dendritic cells. Cancer Immunol Res. 2015;3(4):412–423. doi: 10.1158/2326-6066.CIR-14-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xu F, Liu J, Liu D, Liu B, Wang M, Hu Z, Du X, Tang L, He F. LSECtin expressed on melanoma cells promotes tumor progression by inhibiting antitumor T-cell responses. Cancer Res. 2014;74(13):3418–3428. doi: 10.1158/0008-5472.CAN-13-2690. [DOI] [PubMed] [Google Scholar]
- 93.Andrews LP, Marciscano AE, Drake CG, Vignali DA. LAG3 (CD223) as a cancer immunotherapy target. Immunol Rev. 2017;276(1):80–96. doi: 10.1111/imr.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hannier S, Tournier M, Bismuth G, Triebel F. CD3/TCR complex-associated lymphocyte activation gene-3 molecules inhibit CD3/TCR signaling. J Immunol. 1998;161(8):4058–4065. [PubMed] [Google Scholar]
- 95.Legat A, Maby-El Hajjami H, Baumgaertner P, Cagnon L, Maillard SA, Geldhof C, Iancu EM, Lebon L, Guillaume P, Dojcinovic D. Vaccination with LAG-3Ig (IMP321) and peptides induces specific CD4 and CD8 T-cell responses in metastatic melanoma patients--—Report of a Phase I/IIa clinical trial. Clin Cancer Res. 2016;22(6):1330–1340. doi: 10.1158/1078-0432.CCR-15-1212. [DOI] [PubMed] [Google Scholar]
- 96.Brignone C, Gutierrez M, Mefti F, Brain E, Jarcau R, Cvitkovic F, Bousetta N, Medioni J, Gligorov J, Grygar C. First-line chemoimmunotherapy in metastatic breast carcinoma: combination of paclitaxel and IMP321 (LAG-3Ig) enhances immune responses and antitumor activity. J Transl Med. 2010;8:71. doi: 10.1186/1479-5876-8-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brignone C, Escudier B, Grygar C, Marcu M, Triebel F. A phase I pharmacokinetic and biological correlative study of IMP321, a novel MHC class II agonist, in patients with advanced renal cell carcinoma. Clin Cancer Res. 2009;15(19):6225–6231. doi: 10.1158/1078-0432.CCR-09-0068. [DOI] [PubMed] [Google Scholar]
- 98.Wierz M, Pierson S, Guyonnet L, Viry E, Lequeux A, Oudin A, Niclou SP, Ollert M, Berchem G, Janji B. Blood. Vol 131. 2018. Dual PD1/LAG3 immune checkpoint blockade limits tumor development in a murine model of chronic lymphocytic leukemia; pp. 1617–1621. United States. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Uboha NV, Milhem MM, Kovacs C, Amin A, Magley A, Purkayastha DD, Piha-Paul SA. Phase II study of spartalizumab (PDR001) and LAG525 in advanced solid tumors and hematologic malignancies, In: American Society of Clinical Oncology 2019. J Clin Oncol. May 20, 2019;37(15_suppl):2553. [Google Scholar]
- 100.Hong DS, Schoffski P, Calvo A, Sarantopoulos J, Ochoa De Olza M, Carvajal RD, Prawira A, Kyi C, Esaki T, Akerley WL. Phase I/II study of LAG525±spartalizumab (PDR001) in patients (pts) with advanced malignancies, American Society of Clinical Oncology 2018. J Clin Oncol. May 20, 2018;36(15_suppl):3012. [Google Scholar]
- 101.Ascierto P, Bono P, Bhatia S. LBA18Efficacy of BMS-986016, a monoclonal antibody that targets lymphocyte activation gene-3 (LAG-3), in combination with nivolumab in pts with melanoma who progressed during prior anti–PD-1/PD-L1 therapy (mel prior IO) in all-comer and biomarker-enriched populations. Annals of Oncology. 2017;28(suppl_5) [Google Scholar]
- 102.He Y, Rivard CJ, Rozeboom L, Yu H, Ellison K, Kowalewski A, Zhou C, Hirsch FR. Lymphocyte-activation gene-3, an important immune checkpoint in cancer. Cancer Sci. 2016;107(9):1193–1197. doi: 10.1111/cas.12986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Long L, Zhang X, Chen F, Pan Q, Phiphatwatchara P, Zeng Y, Chen H. The promising immune checkpoint LAG-3: from tumor microenvironment to cancer immunotherapy. Genes & cancer. 2018;9(5-6):176. doi: 10.18632/genesandcancer.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Haines BB, Javaid S, Cui L, Hirsch H, Cemerski S, McClanahan T, Sathe M, Zhang S, Rosenzweig M, Long B. AACR; 2017. Blockade of LAG-3 amplifies immune activation signatures and augments curative antitumor responses to anti-PD-1 therapy in immune competent mouse models of cancer. [Google Scholar]
- 105.Anderson AC, Anderson DE, Bregoli L, Hastings WD, Kassam N, Lei C, Chandwaskar R, Karman J, Su EW, Hirashima M. Promotion of tissue inflammation by the immune receptor Tim-3 expressed on innate immune cells. Science. 2007;318(5853):1141–1143. doi: 10.1126/science.1148536. [DOI] [PubMed] [Google Scholar]
- 106.Hastings WD, Anderson DE, Kassam N, Koguchi K, Greenfield EA, Kent SC, Zheng XX, Strom TB, Hafler DA, Kuchroo VK. TIM-3 is expressed on activated human CD4+ T cells and regulates Th1 and Th17 cytokines. Eur J Immunol. 2009;39(9):2492–2501. doi: 10.1002/eji.200939274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Monney L, Sabatos CA, Gaglia JL, Ryu A, Waldner H, Chernova T, Manning S, Greenfield EA, Coyle AJ, Sobel RA. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature. 2002;415(6871):536–541. doi: 10.1038/415536a. [DOI] [PubMed] [Google Scholar]
- 108.Rangachari M, Zhu C, Sakuishi K, Xiao S, Karman J, Chen A, Angin M, Wakeham A, Greenfield EA, Sobel RA. Bat3 promotes T cell responses and autoimmunity by repressing Tim-3-mediated cell death and exhaustion. Nat Med. 2012;18(9):1394–1400. doi: 10.1038/nm.2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Davidson D, Schraven B, Veillette A. PAG-associated FynT regulates calcium signaling and promotes anergy in T lymphocytes. In: Mol Cell Biol. 2007;27:1960–1973. doi: 10.1128/MCB.01983-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li J, Ni L, Dong C. Immune checkpoint receptors in cancer: redundant by design? Curr Opin Immunol. 2017;45:37–42. doi: 10.1016/j.coi.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 111.Zhu C, Sakuishi K, Xiao S, Sun Z, Zaghouani S, Gu G, Wang C, Tan DJ, Wu C, Rangachari M. An IL-27/NFIL3 signalling axis drives Tim-3 and IL-10 expression and T-cell dysfunction. Nat Commun. 2015;6:6072. doi: 10.1038/ncomms7072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhu C, Anderson AC, Schubart A, Xiong H, Imitola J, Khoury SJ, Zheng XX, Strom TB, Kuchroo VK. The Tim-3 ligand galectin-9 negatively regulates T helper type 1 immunity. Nat Immunol. 2005;6(12):1245–1252. doi: 10.1038/ni1271. [DOI] [PubMed] [Google Scholar]
- 113.Kim JE, Patel MA, Mangraviti A, Kim ES, Theodros D, Velarde E, Liu A, Sankey EW, Tam A, Xu H. Combination therapy with anti-PD-1, anti-TIM-3, and focal radiation results in regression of murine gliomas. Clin Cancer Res. 2017;23(1):124–136. doi: 10.1158/1078-0432.CCR-15-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Liu JF, Wu L, Yang LL, Deng WW, Mao L, Wu H, Zhang WF, Sun ZJ. Blockade of TIM3 relieves immunosuppression through reducing regulatory T cells in head and neck cancer. J Exp Clin Cancer Res. 2018;37 doi: 10.1186/s13046-018-0713-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sakuishi K, Apetoh L, Sullivan JM, Blazar BR, Kuchroo VK, Anderson AC. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J Exp Med. 2010;207(10):2187–2194. doi: 10.1084/jem.20100643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Thommen DS, Schreiner J, Müller P, Herzig P, Roller A, Belousov A, Umana P, Pisa P, Klein C, Bacac M. Progression of lung cancer is associated with increased dysfunction of t cells defined by coexpression of multiple inhibitory receptors. Cancer Immunol Res. 2015;3(12):1344–1355. doi: 10.1158/2326-6066.CIR-15-0097. [DOI] [PubMed] [Google Scholar]
- 117.Koyama S, Akbay EA, Li YY, Herter-Sprie GS, Buczkowski KA, Richards WG, Gandhi L, Redig AJ, Rodig SJ, Asahina H. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun. 2016;7:10501. doi: 10.1038/ncomms10501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Prodeus A, Abdul-Wahid A, Sparkes A, Fischer NW, Cydzik M, Chiang N, Alwash M, Ferzoco A, Vacaresse N, Julius M. VISTA.COMP - an engineered checkpoint receptor agonist that potently suppresses T cell-mediated immune responses. JCI Insight. 2017;2(18) doi: 10.1172/jci.insight.94308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ceeraz S, Eszterhas SK, Sergent PA, Armstrong DA, Ashare A, Broughton T, Wang L, Pechenick D, Burns CM, Noelle RJ. VISTA deficiency attenuates antibody-induced arthritis and alters macrophage gene expression in response to simulated immune complexes. Arthritis Res Ther. 2017;19(1):270. doi: 10.1186/s13075-017-1474-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Villarroel-Espindola F, Yu X, Datar I, Mani N, Sanmamed M, Velcheti V, Syrigos K, Toki M, Zhao H, Chen L. Spatially resolved and quantitative analysis of VISTA/PD-1H as a novel immunotherapy target in human non-small cell lung cancer. Clin Cancer Res. 2018;24(7):1562–1573. doi: 10.1158/1078-0432.CCR-17-2542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Myers G. Immune-related adverse events of immune checkpoint inhibitors: a brief review. Current Oncology. 2018;25(5):342. doi: 10.3747/co.25.4235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Wang DY, Salem JE, Cohen JV, Chandra S, Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L. Fatal toxic effects associated with immune checkpoint inhibitors: a systematic review and meta-analysis. JAMA oncology. 2018;4(12):1721–1728. doi: 10.1001/jamaoncol.2018.3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Friedman CF, Proverbs-Singh TA, Postow MA. Treatment of the immune-related adverse effects of immune checkpoint inhibitors: a review. JAMA oncology. 2016;2(10):1346–1353. doi: 10.1001/jamaoncol.2016.1051. [DOI] [PubMed] [Google Scholar]
- 124.Gao CA, Weber UM, Peixoto AJ, Weiss SA. Seronegative autoimmune autonomic ganglionopathy from dual immune checkpoint inhibition in a patient with metastatic melanoma. Journal for ImmunoTherapy of Cancer. 2019;7(1) doi: 10.1186/s40425-019-0748-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, Schadendorf D, Dummer R, Smylie M, Rutkowski P. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372(21):2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Johnson DB, Balko JM, Compton ML, Chalkias S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL. Fulminant myocarditis with combination immune checkpoint blockade. N Engl J Med. 2016;375(18):1749–1755. doi: 10.1056/NEJMoa1609214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang B, Wu Q, Zhou YL, Guo X, Ge J, Fu J. Immune-related adverse events from combination immunotherapy in cancer patients: A comprehensive meta-analysis of randomized controlled trials. International immunopharmacology. 2018;63:292–298. doi: 10.1016/j.intimp.2018.08.014. [DOI] [PubMed] [Google Scholar]