Abstract
Background
Abnormal expression of the orphan nuclear receptor Nurr1 is a critical factor in the etiology of multiple cancers. However, its potential role in gastric cancer (GC) remains elusive. In this study, we have demonstrated that the expression of Nurr1 was elevated and had an oncogenic function in GC.
Methods
Nurr1 expression was analyzed in clinical specimens and the GEO database. Functions of Nurr1 in GC cells were analyzed using Nurr1 knockdown and overexpression. Various cell and molecular biological methods were used to explore the potential mechanisms of Nurr1 upregulation and its role in promoting GC.
Findings
Overexpression of Nurr1 was directly related to the poor prognosis of GC patients. What's more, Nurr1 was induced by Helicobacter pylori (H. pylori) via the PI3K/AKT-Sp1 pathway. Sp1 enhanced Nurr1 expression by binding to its promoter to activate the transcription. Upregulated Nurr1 then directly targeted CDK4 by binding to its promoter region to increase its expression, thereby facilitated GC cells proliferation both in vitro and in vivo.
Interpretation
We identified Nurr1 as a driving oncogenic factor in GC. In addition, Nurr1 could be used as a potential therapeutic target for the diagnosis and treatment of H. pylori-associated GC.
Funding
This work was supported by the National Natural Science Foundation of China (Nos 81801983, 81871620, 81971901, 81772151 and 81571960), and the Department of Science and Technology of Shandong Province (2018CXGC1208).
Keywords: Gastric cancer, PI3K/AKT/Sp1, Proliferation, CDK4, Helicobacter pylori
Research in context.
Evidence before this study
Orphan nuclear receptor Nurr1 has been shown to regulate specific gene expression and mediate multiple physiological and pathological effects especially in cancers. Gastric cancer is one of the most common malignancy and considered as the third leading cause of cancer-related death worldwide. Helicobacter pylori infection is one of the major causes for the occurrence of gastric cancer. Infection of Helicobacter pylori induces inflammation in the gastric mucosa and leads to tissue damage. Persistent infection promotes progression from chronic gastritis to gastric cancer. However, not much information is available on the effects of Nurr1 expression in Helicobacter pylori-associated gastric carcinogenesis.
Added value of this study
In this study, we found that Nurr1 was overexpressed in gastric cancer, and it facilitated the proliferation of gastric cancer cells both in vitro and in vivo. We also provided evidence that Nurr1 directly promoted CDK4 expression to facilitate gastric cancer cells proliferation. In addition, Nurr1 expression induced by Helicobacter pylori was dependent on the PI3K/AKT-Sp1 signaling pathway, where Sp1 directly activated Nurr1 expression at the transcription level.
Implications of all the available evidence
We identified Nurr1 as a driving oncogenic factor in gastric cancer and provided evidence that Nurr1 might be a potential therapeutic target for the treatment of Helicobacter pylori-associated gastric cancer.
Alt-text: Unlabelled box
1. Introduction
Gastric cancer (GC) is one of the most common malignancy and the third leading cause of cancer-related death worldwide [1]. The development of the intestinal type of GC involves the following steps: chronic superficial gastritis, atrophic gastritis, with or without intestinal metaplasia, hyperplasia, and then adenocarcinoma [2,3]. Late diagnoses at an advanced stage of the disease are considered as the primary reason for poor disease-free survival rates [4,5]. Therefore, identifying key molecular targets and mechanisms that can help in designing novel therapeutic approaches for GC is a critical research challenge.
The human nuclear receptor family is a group of proteins functioning as transcription factors to regulate specific gene expression and to mediate multiple physiological and pathological effects associated with cancers [6], [7], [8]. Nurr1, an orphan nuclear receptor, belongs to NR4A family and is also known as NR4A2. It has been reported that Nurr1 plays a major role in the maintenance of dopaminergic neurons and is associated with familial Parkinson's disease [9], [10], [11]. Structurally, Nurr1 cannot bind to the ligands as it's binding domain is occupied by hydrophobic amino acids [12,13]. Therefore, it is also called as an orphan nuclear receptor. Numerous studies have proven that the stimulation from growth factors, inflammation, cytokines, peptides, and hormones can induce the expression of Nurr1 [14,15]. Nurr1 can function as monomers and bind to NBRE or as homodimers binding to NURRE or as heterodimers with retinoid X receptor binding to DR5 response elements to induce gene expression [16,17]. Moreover, Nurr1 also plays an oncogenic role in multiple cancers. Nurr1 is overexpressed in prostate cancer stem/progenitor-like cells, which is strongly associated with poor prognosis and tumor progression [18]. Nurr1 is activated by I-BOP and stimulates cell proliferation through cyclinD1 in lung cancer [19]. However, the role of Nurr1 in GC has not been fully elucidated.
Helicobacter pylori (H. pylori) infection is closely linked with GC [20], [21], [22], [23]. H. pylori infection induces inflammation in the gastric mucosa and causes tissue damage. Persistent infection promotes progression from chronic gastritis to GC [24], [25], [26]. Many important signaling pathways including NF-κB, STAT1, and PI3K are triggered by H. pylori [27], [28], [29]. For example, PI3K/AKT pathway is activated by H. pylori downregulating IL-17RB expression and impairing the host defense in gastric epithelial cells [30]. Although there have been many studies on the pathogenic effects of H. pylori, the mechanisms through which GC is initiated require further exploration.
In this study, we found that Nurr1 was overexpressed in GC facilitating the proliferation of GC cells both in vitro and in vivo. We provided the first evidence that Nurr1 directly promoted CDK4 expression to promote GC cells proliferation. In addition, Nurr1 expression induced by H. pylori was dependent on the PI3K/AKT-Sp1 signaling pathway. Sp1 transcriptionally activated Nurr1 expression. In conclusion, we identified that Nurr1 as a driving oncogene and might be a potential therapeutic target of GC.
2. Materials and methods
2.1. Cell culture
Authenticated human cell lines (AGS, BGC-823, SGC-7901, GES-1) were obtained from the Zhongqiaoxinzhou Biotech (Shanghai, China). BGC-823, GES-1, and SGC-7901 cells were cultured in RPMI-1640 (Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (Gibco, Carlsbad, CA, USA). AGS cells were cultured in F12-medium containing 12% fetal bovine serum. BGC-823 cells stably expressing Nurr1 and CDK4 shRNA were selected using 2 μg/ml puromycin (Gibco, Carlsbad, CA, USA). All cells were cultured in a humidified incubator at 37 °C with 5% CO2.
2.2. Transfection
Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) was used for siRNA transfection. Sequences for these siRNAs are listed in Supplementary Table 1. Roche Transfection Reagent (Roche, Switzerland) was used for the transfection of Nurr1 plasmid (GeneChem, Shanghai, China) according to the provided protocols.
2.3. H. pylori cultures and H. pylori-infected mouse model
H. pylori strains 26695,11637 and SSI were grown in Brucella broth supplemented with 5% fetal bovine serum at 37 °C in a microaerophilic environment. Gastric epithelial cells were infected by H. pylori with different concentration and collected at different time points. In our mouse model, 48 C57BL/6 mice (6 weeks old, male) were divided into 3 groups. Control group (Group1) which contained 12 mice was given distilled water without H. pylori strains or MNU. Groups 2 and 3 were given distilled water added MNU (30 ppm) for 70 days. Then, group 3 was inoculated with the SS1 strain (1 × 109 colony-forming U/ml) every other day, for a total of three times. All mice were killed at 350 days for further study. This study was reviewed and approved by the Ethics Committee of Shandong University School of Medicine (Jinan, China).
2.4. Luciferase assay
Human CDK4 promoter fragment was cloned into the pGL3 basic reporter vector (Promega, USA). Three human Nurr1 promoter fragments were synthesized (SYE Biotech, Shandong, China). Nurr1 and Sp1 binding sites for mutated CDK4 and Nurr1 promoters were generated by KODPlus-Mutagenesis kit (Toyobo, Japan) based on the WT plasmid. Constructed dual-luciferase reporter plasmids were transfected into GC cells using Roche Transfection Reagent (Roche, Basel, Switzerland) according to the manufacturer's instructions. Luciferase reporter activity was measured with the Dual-Luciferase Assay System (Promega).
2.5. Patient samples and clinical tissue specimens
Thirty-seven GC and AG tissues were obtained from Qilu Hospital (Shandong, China). Eighty-seven AG tissues consisting of fifty-eight H. pylori-positive and twenty-nine H. pylori-negative were obtained from Jinan Central Hospital (Shandong, China). Those tissues were stored in RNAlater at −80 °C for RNA extraction. Seven GC specimens and matching adjacent control samples were obtained from Qilu Hospital (Shandong, China) and kept in liquid nitrogen for protein extraction. Fifty-one AG and eighteen GC samples were obtained from Jinan Central Hospital between 2015 and 2018 and stored in formalin for IHC. All samples were confirmed according to histological analysis. The study was approved by the Ethics Committee of Shandong University School of Medicine (Jinan, China).
2.6. RNA extraction and RT-PCR
Total RNA was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA) according to manufacturer's instructions. The mRNAs were reverse transcribed to cDNA using RT reagent Kit gDNA Eraser (Takara, Japan). cDNAs were then used for qRT-PCR analysis using SYBR Green (TaKaRa). Primer sequences are listed in Supplementary Table 2.
2.7. Tumor xenograft model
Thymus-null BALB/c nude mice (6 weeks old, male) were purchased from Mu Tu Biological (Nanjing, China). BGC-823 cells were transduced with lenti-Nurr1 shRNA, lenti-CDK4 shRNA or a lenti-negative control. Stable BGC-823 cells (3 × 105) were subcutaneously injected into the right or left flanks of nude mice. Tumor growth was examined every two days, and the mice were sacrificed after 2–3 weeks. This study was reviewed and approved by the Ethics Committee of Shandong University School of Medicine (Jinan, China).
2.8. ChIP
Chromatin immunoprecipitation (ChIP) was performed using the SimpleChIP® Enzymatic Chromatin IP Kit (Cell Signaling, Danvers, MA, USA) to detect the binding of corresponding proteins to DNA. Five ug antibodies of Nurr1 (Abcam) and Sp1 (Abcam) were used to immunoprecipitate chromatin fragments. Primer sequences are listed in Supplementary Table 3.
2.9. Colony formation and CCK8 assay
Cells (500/well) with different treatments were seeded in 6-well plates and cultivated for 1–2 weeks. Methanol was used to fix the cells followed by staining with Giemsa. The number of colonies with more than 100 cells were counted and each set of experiment was repeated three times. For CCK8 analysis approximately 1200 cells/well with different treatments were seeded in 96-well plates in triplicates and cultured for 24 h, 48 h, or 72 h. CCK8 reagent (Med Chem Express, USA) was added to condition medium for 3 h and absorbance was recorded using spectrophotometer at 450 nm.
2.10. Western blotting
Total protein was extracted from cells using protein lysis buffer supplemented with phosphatase and protease inhibitors. Equal amounts of lysates were separated on SDS-PAGE and transferred onto PVDF membranes. The PVDF membranes were blocked with 5% nonfat milk for 2 h and subsequently incubated with primary antibodies overnight at 4 °C. Then the PVDF membranes were incubated with corresponding secondary antibodies. Millipore ECL reagent was used to detect signals. Antibodies used in the study are shown in Supplementary Table 4.
2.11. IHC
FFPE (formalin-fixed, paraffin-embedded) sections on glass slides from mouse or patient samples were subjected to deparaffination and dehydration. Samples were then subjected to epitope retrieval and H2O2 treatment followed by blocking in goat serum for 30 min. Next, the samples were incubated with specific primary antibodies overnight at 4 °C. On the following day, the sections were incubated with corresponding secondary antibodies and detected using a DAB staining kit (Vector Laboratories, Burlingame, CA, USA). The IHC score was evaluated by ImageJ. The intensity of positive staining was scored as follows: 0 (no staining); 1(light brown); 2 (moderate brown) and 3 (dark brown). A scale from 0 to 3 was used to score the proportion of positively stained cells: 0 (0%), 1 (<25%), 2 (25–75%) and 3 (>75%). The results that reported as the expression score were the multiply of above two scores.
2.12. Statistical analysis
All experimental data were presented as the mean (±SEM). All experiments were repeated at least three times. Two-tailed Student's t-tests or Mann Whitney U tests were used to compare the means between two groups. Linear regression was performed for correlation analysis of the mRNA data. Repeated measures analysis of variance was used to analyze cell growth ability between two groups. GraphPad PRISM version 8 and SPSS version 23.0 were used for statistical analyses. P value < 0.05 was considered as statistically significant.
3. Results
3.1. Nurr1 is elevated in GC and increased Nurr1 level predicts poor prognosis
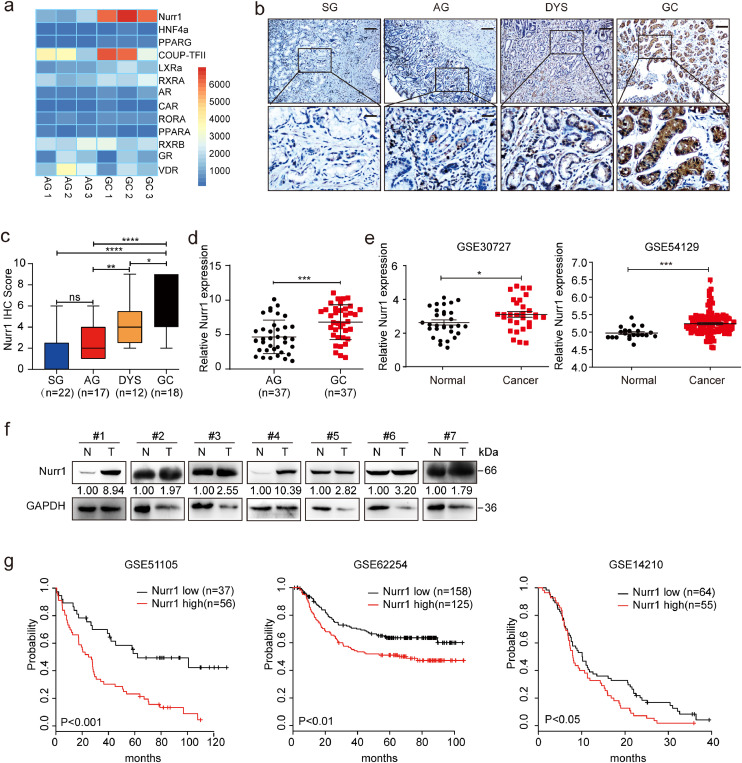
To uncover the potential function of nuclear receptors in GC, we first performed gene expression analysis on three atrophic gastritis (AG) and three GC samples. We found that the expression was upregulated for nine genes and downregulated for four genes in GC specimens with respect to the AG specimens (Fig. 1a). Among the nine upregulated genes, the change of Nurr1 expression was most obvious (Fig. 1a and Supplementary Fig. 1a). IHC staining suggested that Nurr1 expression was weak in superficial gastritis (SG), mildly upregulated in AG, moderately upregulated in dysplasia (DYS) and significantly increased in GC tissues (Fig. 1b and c). Moreover, Nurr1 mRNA expression was significantly increased in human GC samples with respect to AG samples (Fig. 1d). Next, we compared the expression of Nurr1 between GC and adjacent normal tissues and found it was overexpression in GC samples (GEO databases, GSE30727 and GSE54129) (Fig. 1e). Moreover, Nurr1 protein expression was higher in human GC samples than the adjacent normal tissues, which was consistent with the mRNA expression profile of the GEO database (Fig. 1f). The overexpression of Nurr1 predicted poor prognosis in three cohorts of GC patients (GSE51105, GSE62254, GSE14210) (Fig. 1g). Collectively, we confirmed that Nurr1 played a potential oncogenic role in gastric carcinogenesis.
Fig. 1.
Nurr1 expression is elevated in GC and its upregulation predicts poor prognosis. (a) Differential expression analysis of nuclear receptors in 3 atrophic gastritis tissues and 3 GC tissues. (b) IHC staining for Nurr1 in SG (superficial gastritis), AG (atrophic gastritis), DYS (dysplasia) and GC samples. Scale bars: 200 μm (insets 50 μm). (c) IHC score. *P < 0.05, **P < 0.01, ****P < 0.0001, by Student's t-test. (d) Nurr1 mRNA expression in 37 AG and 37 GC samples was measured by real time PCR. ***P < 0.001 by Mann-Whitney U test. (e) RNA sequencing database analysis of Nurr1 expression in human GC and paired adjacent normal tissues. The data was obtained from GSE30727 and GSE54129. (f) Western blot showed the protein levels of Nurr1 in seven pairs of human GC samples (T) and corresponding normal samples (N). (g) Kaplan–Meier analysis of Nurr1 expression in survival of GC patients (log-rank test). The data was obtained from GSE51105 (204,621_s_at), GSE62254 (204,622_x_at) and GSE14210 (204,621_s_at).
3.2. Nurr1 promotes GC cells proliferation both in vitro and in vivo
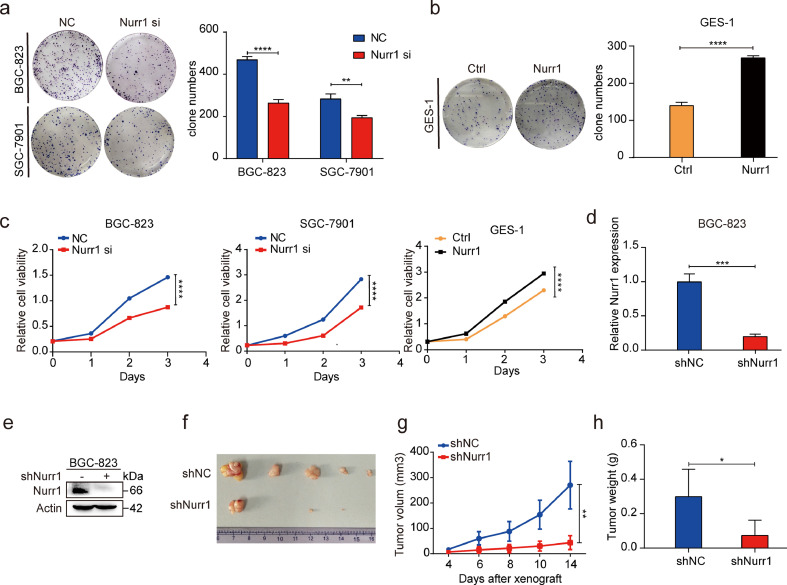
Given that Nurr1 was overexpressed in GC tissues, we explored its potential role in gastric carcinogenesis. For this objective, Nurr1 siRNA was used to inhibit its expression in BGC-823, SGC-7901 cells and Nurr1-coding plasmid was used to enhance its expression in GES-1 cells (Supplementary Fig. 1b–f). Colony formation, CCK8 and EdU assays suggested that knockdown of Nurr1 by siRNA inhibited GC cells proliferation, while overexpression of Nurr1 had the opposite effects (Fig. 2a–c, Supplementary Fig. 2) indicating the pro-proliferative function of Nurr1 in GC.
Fig. 2.
Nurr1 facilitates GC cells proliferation both in vitro and in vivo. (a and b) Colony formation assay after cells transfected with Nurr1 siRNA or Nurr1-coding plasmid. **P < 0.01, ****P < 0.0001, by Student's t-test. (c) CCK8 assay after cells transfected with Nurr1 siRNA or Nurr1-coding plasmid. (d-e) Nurr1 mRNA and protein expression in BGC-823 cells transfected with lenti-Nurr1 shRNA. ***P < 0.001, by Student's t-test. (f–h) Primary tumor formation ability in nude mice xenograft model (f), tumor growth curve (g) and tumor weight (h). * P < 0.05, **P < 0.01 by Student's t-test.
To confirm that Nurr1 could enhance GC cells proliferation in vivo as well, we generated BGC-823 cells with stable Nurr1 suppression and the matched control cells (Fig. 2d, e). These cells were injected subcutaneously into nude mice (3 × 105 cells/mouse). Tumors from the two groups had different properties. The growth rate was significantly attenuated in the group with the injection of Nurr1 suppressed cells (Fig. 2g). The harvested tumor sizes were smaller and tumor weights were lighter in the Nurr1 suppression group as compared to the controls (Fig. 2f and h). These data further supported the pro-proliferative role of Nurr1 both in vitro and in vivo.
3.3. Nurr1 enhances GC cells proliferation by directly targeting CDK4
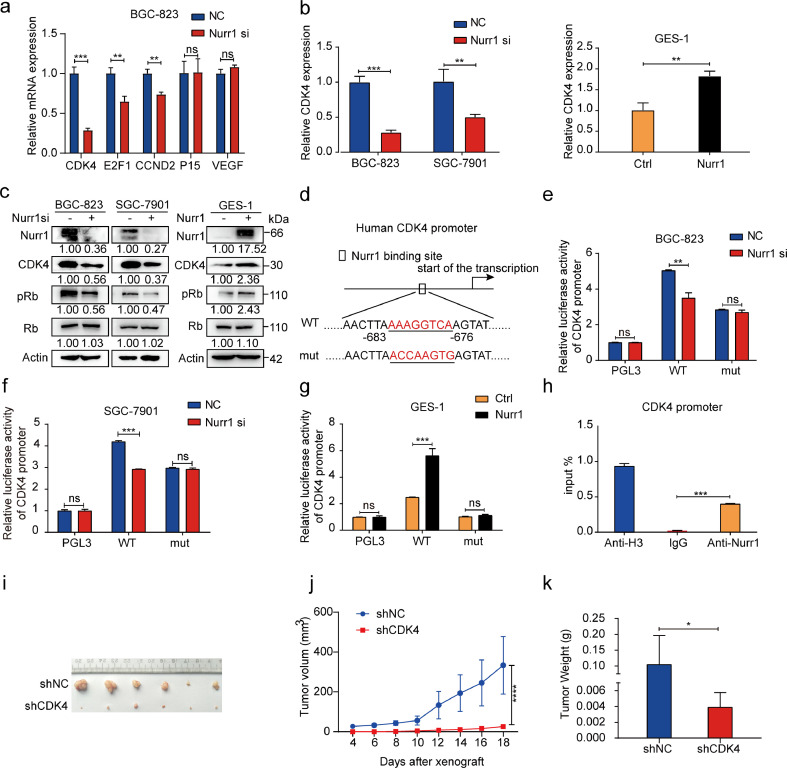
We then explored the mechanism of GC cells proliferation through Nurr1. It is well-known that Nurr1 is a transcription factor that can function as monomers binding to NBRE or as homodimers binding to NURRE to activate target genes expression [31]. Therefore, potential target genes with Nurr1 binding sites within their promoters were screened from cancer-related genes and five candidate genes were selected. Upon verification, CDK4 mRNA expression was significantly decreased with depleting Nurr1 expression (Fig. 3a). CDK4 (cyclin-dependent kinase) plays an important role in mammalian cells proliferation where CDK4/6 phosphorylates the retinoblastoma protein (Rb) tumor-suppressor protein and promotes cell cycle progression [32]. We then confirmed that CDK4 could be regulated positively by Nurr1 at both mRNA and protein levels (Fig. 3b and c). Consistently, phosphorylation of Rb protein was decreased with Nurr1 knockdown and was upregulated with overexpression of Nurr1 (Fig. 3c). These results further suggested that CDK4 was a downstream target of Nurr1. Next, we constructed luciferase reporter plasmids containing CDK4 promoter (WT, in which the Nurr1 binding site was intact) and CDK4 mutant promoter (mut, in which the Nurr1 binding sites were mutated) (Fig. 3d). Inhibition of Nurr1 significantly decreased promoter luciferase activity while overexpression of Nurr1 exhibited an opposite effect (Fig. 3e–g), supporting an indispensable role of direct regulation of CDK4 by Nurr1. ChIP analysis demonstrated that Nurr1 directly occupied the CDK4 promoter region (Fig. 3h). In brief, CDK4 was a direct target gene of Nurr1.
Fig. 3.
CDK4 is the direct target of Nurr1. (a) RT–PCR analysis of CDK4, E2F1, CCND2, P15, VEGF mRNA expression in BGC-823 cells transfected with Nurr1 siRNA. **P < 0.01, ***P < 0.001 by Student's t-test. (b) CDK4 mRNA expression in cells transfected with Nurr1 siRNA and in GES-1 cells transfected with Nurr1 overexpression plasmid. **P < 0.01, ***P < 0.001 by Student's t-test. (c) Western blot analysis of Nurr1, CDK4, Rb, p-Rb protein expression in cells transfected with Nurr1 siRNA or Nurr1-coding plasmid. (d) Nurr1 binding site in human CDK4 promoter and the corresponding base mutation. (e-g) Transcriptional activity of CDK4 assays using Luciferase reporter system. **P < 0.01, ***P < 0.001, by Student's t-test. (h) ChIP assay of Nurr1 directly bound to the promoter of CDK4. ***P < 0.001, by Student's t-test. (i-k) Primary tumor formation ability in nude mice xenograft model (i), tumor growth curve (j) and tumor weight (k). * P < 0.05, ****P < 0.0001 by Student's t-test.
We then explored whether CDK4 could promote GC cells proliferation. We found that CDK4 knockdown could impair GC cells proliferation by colony formation and CCK8 assays (Supplementary Fig. 3a and b). Next, we constructed BGC-823 cells that could stably express CDK4 shRNA (Supplementary Fig. 3c and d). Then these cells were subcutaneously injected into nude mice. Tumors formed in CDK4 shRNA group were smaller than that in control group, with regard to size and weight (Fig. 3i–k). The above results suggested that CDK4 promoted GC cells proliferation in vitro and in vivo.
3.4. H. pylori upregulates Nurr1 expression
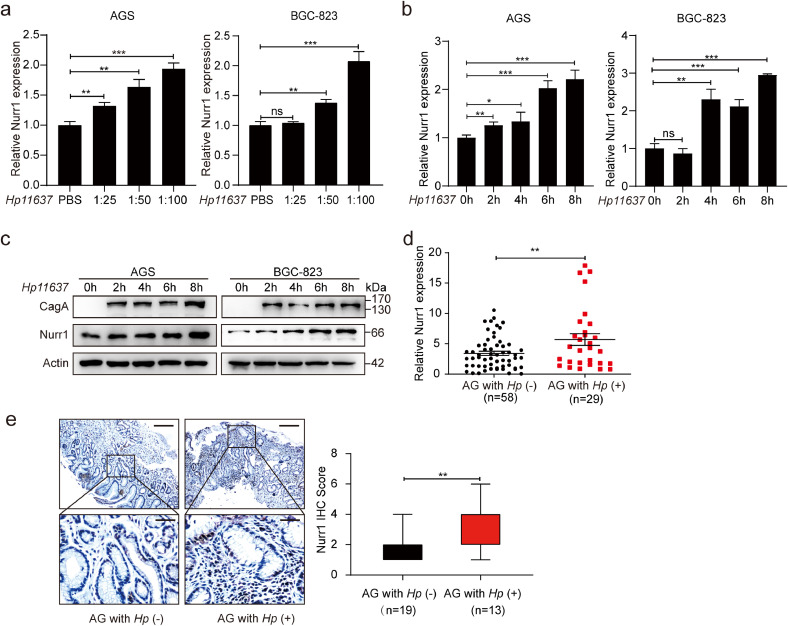
H. pylori infection is a primary factor for the occurrence of duodenal and gastric ulcers as well as GC [33,34]. Thus, we attempted to determine whether Nurr1 expression could be affected by H. pylori infection in GC cells. Nurr1 was induced both at mRNA (Fig. 4a and b, Supplementary Fig. 4a and b) and protein (Fig. 4c, Supplementary Fig. 4c) levels with infection of two H. pylori strains (Hp11637 and Hp26695). Induction of Nurr1 was found to be both dose (Fig. 4a, Supplementary Fig. 4a) and time (Fig. 4b and c, Supplementary Fig. 4b and c) dependent. Consistent with these results, Nurr1 mRNA and protein expression were also higher in H. pylori-positive AG samples than that in H. pylori-negative specimens (Fig. 4d, e). These results revealed that the Nurr1 expression could be induced by H. pylori infection.
Fig. 4.
Infection of H. pylori facilitates Nurr1 expression. (a-b) mRNA expression of Nurr1 in AGS and BGC-823 cells infected with H. pylori (Hp11637) at different MOI (a) and different time points (b). *P < 0.05, **P < 0.01, ***P < 0.001, by Student's t-test. (c) Western blot analysis of Nurr1 expression in AGS and BGC-823 cells infected with H. pylori (Hp11637) at different time points. (d) mRNA expression of Nurr1 in H. pylori-negative or H. pylori-positive AG human samples. **P < 0.01, by Student's t-test. (e) IHC staining for Nurr1 in H. pylori-negative or H. pylori-positive AG human samples. Scale bars: 200 μm (insets 50 μm). **P < 0.01, by Student's t-test.
3.5. H. pylori upregulates Nurr1 expression by the PI3K/AKT pathway
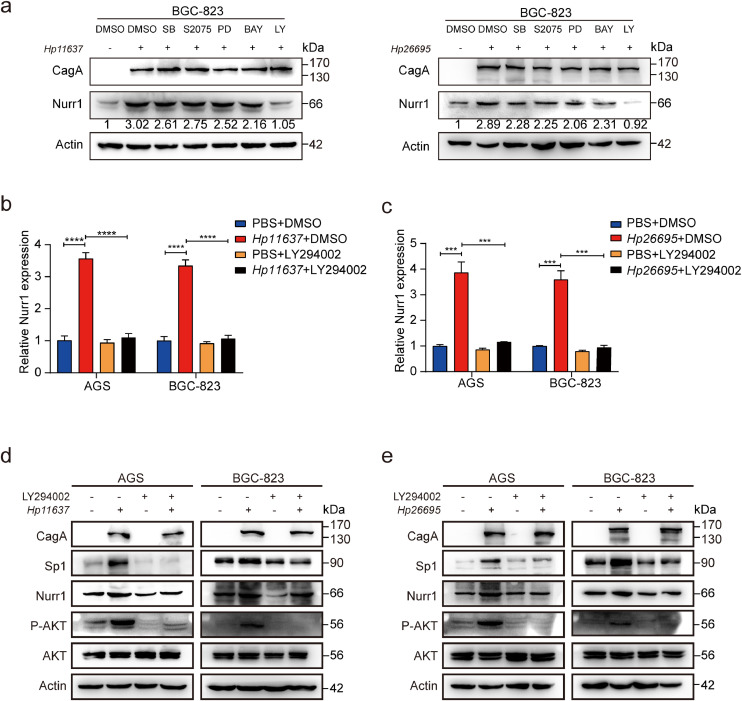
In order to determine how H. pylori infection upregulated Nurr1 expression, five key signaling pathways inhibitors were added before infection with H. pylori in BGC-823 cells. The upregulation of Nurr1 protein expression induced by H. pylori infection was blocked by PI3K/AKT inhibitor (LY294002) strongly (Fig. 5a). In addition, induction of Nurr1 mRNA expression by H. pylori was also blocked by LY294002 treatment (Fig 5b and c). These findings suggested that the PI3K/AKT pathway was mainly responsible for the induction of Nurr1 by H. pylori. Treatment alone with LY294002 attenuated the induction of Nurr1 by H. pylori in GC cells. We further confirmed the protein changes of AKT, p-AKT, Sp1 (discussed later) and Nurr1 by western blot (Fig. 5d and e). Our results indicated that infection of H. pylori increased Nurr1 expression through activation of the PI3K/AKT pathway.
Fig. 5.
H. pylori infection promotes Nurr1 expression depending on the PI3K/AKT pathway. (a) Western blot analysis of Nurr1 protein expression in BGC-823 cells with H. pylori infection and inhibitors treatment. SB (SB203580), PD (PD18435), LY(LY294002). (b and c) RT-PCR analysis of Nurr1 mRNA expression in AGS and BGC-823 cells with H. pylori infection and LY294002 (PI3K inhibitor) treatment. ***P < 0.001, ****P < 0.0001, by Student's t-test. (d–e) Western blot analysis of Nurr1, Sp1, P-AKT protein expression in AGS and BGC-823 cells with H. pylori infection and LY294002 treatment. Cells were harvested at 8 h after H. pylori (MOI=100) infection in (a-e).
3.6. Nurr1 is transcriptionally activated by Sp1
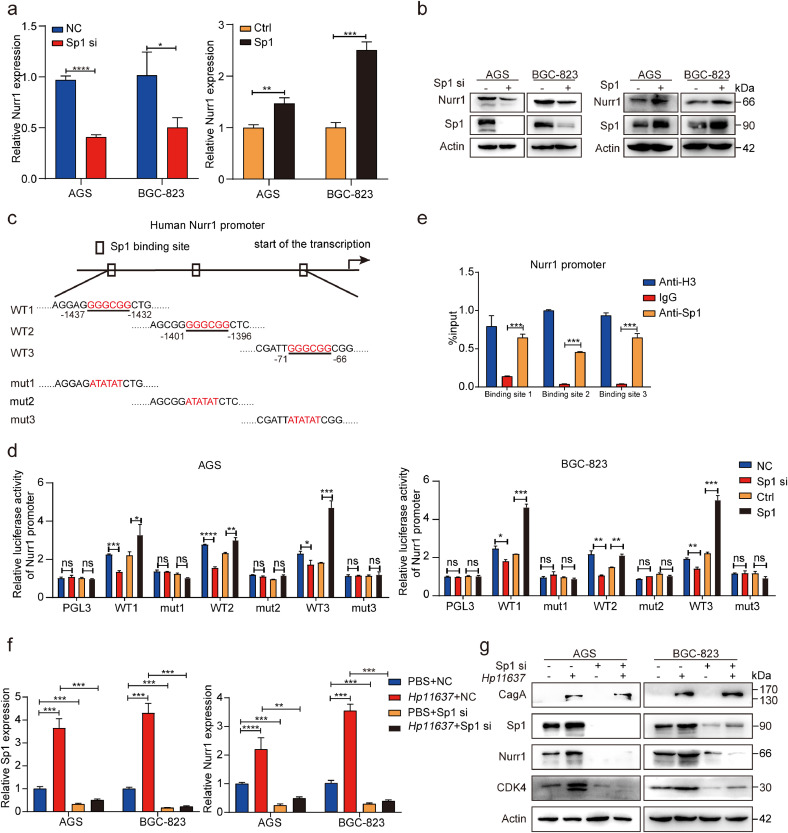
Previous studies have reported a strong association between Sp1 and PI3K/AKT pathway in various diseases and Sp1 served as a downstream protein of PI3K/AKT [35], [36], [37]. Thus, we attempted to determine whether the upregulation of Nurr1 induced by H. pylori depends on the PI3K/AKT-Sp1 pathway. We found that H. pylori infection increased the expression of Sp1 and Nurr1 simultaneously, and the LY294002 relieved the upregulation of these proteins induced by H. pylori at the same time (Fig. 5d and e). These results suggested that there was a potential regulatory relationship between Sp1 and Nurr1. Indeed, knockdown of Sp1 significantly decreased Nurr1 expression at both the mRNA and protein levels while overexpression of Sp1 had the opposite effects (Fig. 6a and b). These results supported that the expression of Nurr1 could be regulated positively by Sp1. Acting as a classical transcription factor, Sp1 occupied the promoter region of target genes through GGGCGG elements to enhance their transcription [38,39]. We found three Sp1 binding sites in Nurr1 promoter, and then constructed luciferase reporter plasmids containing Nurr1 promoters (WT1, WT2, and WT3: the binding sites were intact; mut1, mut2 and mut3: the binding sites were mutated accordingly) (Fig. 6c). Knockdown of Sp1 reduced the luciferase activity of Nurr1 promoters, whereas the overexpression of Sp1 increased the promoter activity (Fig. 6d), indicating that all the sites were indispensable for the transcriptional regulation of Nurr1 by Sp1. Next, ChIP assay was carried to verify the direct binding of Sp1 to Nurr1 promoter through the three binding sites (Fig. 6e). The results demonstrated that Nurr1 was a direct target gene of Sp1.
Fig. 6.
Sp1 targets Nurr1 promoter and mediates the regulation of Nurr1 by H. pylori. (a) RT-PCR analysis of Nurr1 mRNA expression in AGS and BGC-823 cells transfected with Sp1 siRNA or Sp1-coding plasmid. *P < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001, by Student's t-test. (b) Western blot analysis of Nurr1 and Sp1 protein expression in AGS and BGC-823 cells transfected with Sp1 siRNA or Sp1-coding plasmid. (c) Sp1 binding sites in human Nurr1 promoter and the corresponding base mutation. (d) The transcriptional activation of the Nurr1 promoter by Sp1 was detected by using a dual luciferase assay in AGS and BGC-823. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, by Student's t-test. (e) ChIP assay of Sp1 bound to the promoter of Nurr1 in BGC-823. ***P < 0.001, by Student's t-test. (f-g) Sp1 and Nurr1 mRNA (f) and protein (g) expression analysis in AGS and BGC-823 cells with Sp1 siRNA and H. pylori (Hp11637) treatment. **P < 0.01, ***P < 0.001, ****P < 0.0001, by Student's t-test.
It is also important to reveal whether Nurr1 was induced by H. pylori via Sp1. We suppressed Sp1 and then infected these cells with H. pylori. Both Nurr1 and its target gene CDK4 expression at mRNA (Fig 6f, Supplementary Fig. 4d) and protein (Fig. 6g, Supplementary Fig. 4e) levels induced by H. pylori were relieved by Sp1 suppression indicating an indispensable role of Sp1 in mediating transcriptional activation of Nurr1 with H. pylori infection.
3.7. Validation of relationship between Nurr1 expression and pathogenic process
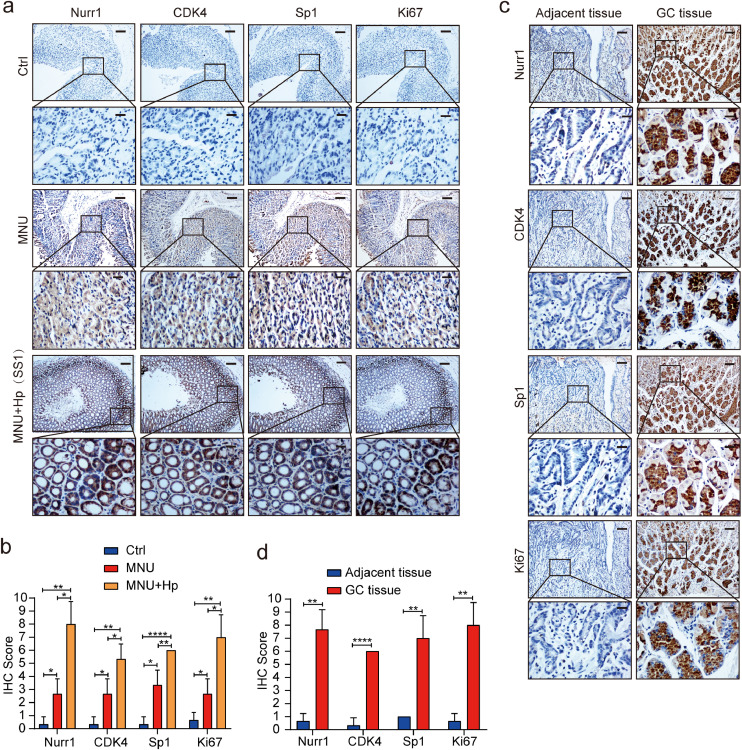
Next, we generated MNU (N-methyl-N-nitrosourea)-treated gastritis mouse model with H. pylori infection to detect the expression of Nurr1 and associated genes [40,41]. IHC staining showed that, compared with the control group, Nurr1 expression in MNU group was slightly upregulated, while the expression of Nurr1 in MNU plus H. pylori infection group was significantly upregulated (Fig. 7a and b). Importantly, we found the co-expression of Sp1, Nurr1, CDK4 as well as the cells proliferation marker Ki67 in gastritis tissues (Fig. 7a and b, Supplementary Fig. 5a), confirming the regulatory relationship between these genes. In the end, we detected the expression of Nurr1, Sp1, CDK4, and Ki67 in human GC samples and adjacent normal samples. As expected, the expression of these proteins was increased notably in GC samples with respect to adjacent normal samples (Fig. 7c and d). Similarly, we identified the co-expression of Sp1, Nurr1, CDK4 as well as Ki67 in GC tissues (Fig. 7c and d, Supplementary Fig. 5b), further confirming our hypothesis about the regulatory relationship among them.
Fig. 7.
Confirmation of correlation between the expression of Nurr1 and pathogenic process in mice tissues and clinical specimen. (a) IHC staining for Nurr1, CDK4, Sp1 and Ki67 in mucosal epithelial tissues of control, MNU and MNU-Hp treated mice. Scale bars: 200 μm (insets 50 μm). (b) IHC score. *P < 0.05, **P < 0.01, ****P < 0.0001, by Student's t-test. (c) IHC staining for Nurr1, CDK4, Sp1 and Ki67 in GC samples and corresponding normal samples. Scale bars: 200 μm (insets 50 μm). (d) IHC score. **P < 0.01, ****P < 0.0001, by Student's t-test.
4. Discussion
Nuclear receptors (NRs) serve as potential drug targets and exhibit vital roles in many diseases such as obesity and cancers [42], [43], [44]. There are 48 NRs superfamily including adopted receptors, endocrine receptors and multiple orphan nuclear receptors [45]. Nurr1 (NR4A2), Nur77(NR4A1) and NOR1(NR4A3) are the members of NR4A subfamily [46,47]. NR4A receptors regulate cell-intrinsic program of T cell hypo responsiveness. Tumor-bearing mice with CAR T cells lacking NR4A receptors present tumor regression and prolonged survival [48]. The effect of inhibiting the function of NR4A is similar to PD-1 blockade but it involves more regulatory elements. Therefore, NR4A is considered as a promising target for cancer immunotherapy. To investigate nuclear receptor genes differentially expressed between gastritis and GC, we performed gene expression profiling on three atrophic gastritis and GC samples. Results suggested that nine genes were upregulated while four genes were downregulated. Nurr1 expression was significantly upregulated in GC samples (Fig. 1a). Therefore, we wanted to explore the function and mechanism of Nurr1 in GC. In our study, we proved that the aberrant upregulation of Nurr1 accelerated GC cells proliferation. Furthermore, CDK4 was a direct downstream target of Nurr1. The Nurr1 DNA-binding domain could recognize AAAGGTCA motifs on the CDK4 promoter and could transcriptionally activate the CDK4 expression. We described an oncogenic role of Nurr1 in GC. However, whether Nurr1 also facilitates the malignant progression of GC requires further investigation.
It is well established that H. pylori infection is the strongest singular factor for malignancy within the stomach [21,49,50]. H. pylori infection induces the release of inflammatory factors facilitating the development of chronic gastritis, and in some cases GC [51]. Thus, we explored the correlation between Nurr1 and H. pylori in gastric carcinogenesis. In our research, we discovered that Nurr1 expression was induced by H. pylori and the PI3K/AKT-Sp1 pathway was responsible for the direct activation of Nurr1. In our study, we also proved that H. pylori infection promoted the expression of Nurr1 in the MNU treated gastritis mouse model. Following H. pylori infection, CagA (Cytotoxin-associated antigen A) [52] and PGN (Peptidoglycan) are injected into host cells by T4SS (Type IV secretion system) and this facilitates the transformation of the infected cells [53], [54], [55]. CagA is an important toxin and CagA positive strains are associated with high risk for GC [56]. CagA stimulates epithelial cells proliferation through mitotic signaling pathways such as the MEK-ERK pathway and decreases epithelial cells apoptosis by interfering with tumor suppressors such as P53 [20,57,58]. In our study, we did not explore if CagA from H. pylori can induce Nurr1 expression and it requires further study.
Transcription factors are important to promote the expression of key oncogenes in tumors. For instance, c-Jun directly binds to the GLS promoter and regulates metabolic reprogramming by increasing GLS expression in cancer cells [59]. In the current study, Nurr1 was positively regulated by both c-Jun and Sp1. However, only Sp1 could directly bind to the Nurr1 promoter and could enhance its transcription. Interestingly, Sp1 is involved in the development and progression of multiple cancers and is a downstream target gene of the AKT signaling pathway. Therefore, it was concluded that Nurr1 was induced by H. pylori through PI3K/AKT-Sp1 axis.
There are some studies concerning the function and mechanism of Nurr1 in GC and other tumors. For example, PGE2 increases Nurr1 expression via cAMP/PKA and NF-κB signaling pathways in colorectal cancer [60]. Different from the above reports, we primarily focused on the signaling pathways associated with H. pylori infection and found that H. pylori could induce Nurr1 expression via PI3K/AKT signaling pathway. What's more, it has also been reported that Nurr1 expression is upregulated by COX-2 and high expression of Nurr1 can block apoptosis in colorectal cancer [61]. Down-regulation of Nurr1 can reduce cells proliferation and elevate cells apoptosis in Hela cells [62]. The above studies indicate that Nurr1 plays a significant role of anti-apoptotic in cancer cells. In our study, we found that the apoptosis of GC cells didn't change significantly after silencing of Nurr1, while the proliferation of GC cells was significantly inhibited (Supplementary Fig. 6a-b). Therefore, we mainly investigated the effect of Nurr1 on proliferation of GC cells. Whereas our study found that the expression of Nurr1 was increased in GC samples when compared with adjacent samples, two previous work in GC found the opposite [56,63]. The different results may be due to the heterogeneity of samples and possible bias in data mining. Consistent with the previous reports, our results also showed that Nurr1 is an indicator of poor prognosis in three GC patient cohorts of GEO database. Further clinical study is required to confirm the preliminary findings.
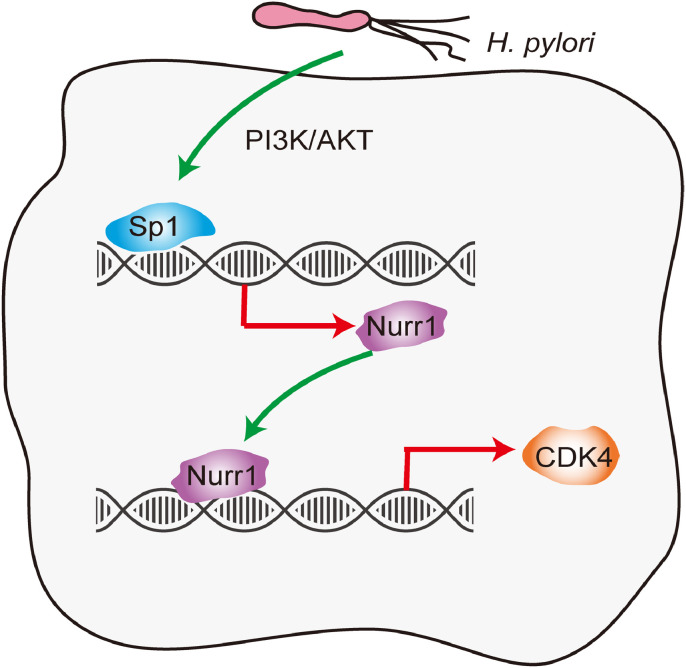
In summary, we present the first report that H. pylori could up-regulate Nurr1 expression through PI3K/AKT-Sp1 axis, and that Sp1 directly bound to Nurr1 promoter to activate its transcription. Nurr1 subsequently promoted CDK4 transcription by directly binding to its promoter, facilitating GC cells proliferation (Fig. 8). Therefore, Nurr1 may be a novel target for the diagnosis and treatment of GC.
Fig. 8.
Schematic model of the study. Infection of H. pylori enhanced Nurr1 expression by PI3K/AKT-Sp1 signaling pathway and Sp1 directly transcriptionally activated Nurr1 expression. Nurr1 bound to the promoter of CDK4 and increased its expression. In this study, we revealed the mechanism through which Nurr1 was upregulated and how Nurr1 promoted GC cells proliferation. Therefore, Nurr1 might be a new target for the diagnosis and treatment of GC.
Declaration of Competing Interest
The authors declare no competing interests.
Acknowledgments
Acknowledgements
Not applicable
Funding sources
This work was supported by the National Natural Science Foundation of China (Nos 81801983, 81871620, 81971901, 81772151 and 81571960), and the Department of Science and Technology of ShandongProvince (2018CXGC1208). The funders had no role in study design, data collection, data analysis, interpretation or writing of the report. The corresponding authors confirm that they have full access to all the data in the study and had final responsibility for the decision to submit for publication.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2020.102672.
Contributor Information
Lin Ma, Email: 2375@sdhospital.com.cn.
Jihui Jia, Email: jiajihui@sdu.edu.cn.
Appendix. Supplementary materials
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Correa P. Human gastric carcinogenesis: a multistep and multifactorial process–First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52(24):6735–6740. [PubMed] [Google Scholar]
- 3.Villanueva M.T. Therapeutics: gastric cancer gets a red carpet treatment. Nat Rev Cancer. 2014;14(10) doi: 10.1038/nrc3825. [DOI] [PubMed] [Google Scholar]
- 4.Leung W.K., Wu M.S., Kakugawa Y. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol. 2008;9(3):279–287. doi: 10.1016/S1470-2045(08)70072-X. [DOI] [PubMed] [Google Scholar]
- 5.Van Cutsem E., Sagaert X., Topal B., Haustermans K., Prenen H. Gastric cancer. Lancet. 2016;388(10060):2654–2664. doi: 10.1016/S0140-6736(16)30354-3. [DOI] [PubMed] [Google Scholar]
- 6.Lin S.C., Kao C.Y., Lee H.J. Dysregulation of miRNAs-COUP-TFII-FOXM1-CENPF axis contributes to the metastasis of prostate cancer. Nat Commun. 2016;7:11418. doi: 10.1038/ncomms11418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baek S.H., Kim K.I. Emerging roles of orphan nuclear receptors in cancer. Annu Rev Physiol. 2014;76:177–195. doi: 10.1146/annurev-physiol-030212-183758. [DOI] [PubMed] [Google Scholar]
- 8.Wagner M., Zollner G., Trauner M. Nuclear receptors in liver disease. Hepatology. 2011;53(3):1023–1034. doi: 10.1002/hep.24148. [DOI] [PubMed] [Google Scholar]
- 9.Decressac M., Volakakis N., Bjorklund A., Perlmann T. NURR1 in Parkinson disease–from pathogenesis to therapeutic potential. Nat Rev Neurol. 2013;9(11):629–636. doi: 10.1038/nrneurol.2013.209. [DOI] [PubMed] [Google Scholar]
- 10.Bensinger S.J., Tontonoz P. A Nurr1 pathway for neuroprotection. Cell. 2009;137(1):26–28. doi: 10.1016/j.cell.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 11.Saijo K., Winner B., Carson C.T. A Nurr1/CoREST pathway in microglia and astrocytes protects dopaminergic neurons from inflammation-induced death. Cell. 2009;137(1):47–59. doi: 10.1016/j.cell.2009.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Z., Benoit G., Liu J. Structure and function of Nurr1 identifies a class of ligand-independent nuclear receptors. Nature. 2003;423(6939):555–560. doi: 10.1038/nature01645. [DOI] [PubMed] [Google Scholar]
- 13.Wallen-Mackenzie A., Mata de Urquiza A., Petersson S. Nurr1-RXR heterodimers mediate RXR ligand-induced signaling in neuronal cells. Genes Dev. 2003;17(24):3036–3047. doi: 10.1101/gad.276003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perlmann T., Jansson L. A novel pathway for vitamin a signaling mediated by RXR heterodimerization with NGFI-B and NURR1. Genes Dev. 1995;9(7):769–782. doi: 10.1101/gad.9.7.769. [DOI] [PubMed] [Google Scholar]
- 15.Loppi S., Kolosowska N., Karkkainen O. HX600, a synthetic agonist for RXR-Nurr1 heterodimer complex, prevents ischemia-induced neuronal damage. Brain Behav Immun. 2018;73:670–681. doi: 10.1016/j.bbi.2018.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le W.D., Xu P., Jankovic J. Mutations in NR4A2 associated with familial Parkinson disease. Nat Genet. 2003;33(1):85–89. doi: 10.1038/ng1066. [DOI] [PubMed] [Google Scholar]
- 17.Sacchetti P., Carpentier R., Segard P., Olive-Cren C., Lefebvre P. Multiple signaling pathways regulate the transcriptional activity of the orphan nuclear receptor NURR1. Nucleic Acids Res. 2006;34(19):5515–5527. doi: 10.1093/nar/gkl712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z., Wu D., Ng C.F. Nuclear receptor profiling in prostatospheroids and castration-resistant prostate cancer. Endocr Relat Cancer. 2018;25(1):35–50. doi: 10.1530/ERC-17-0280. [DOI] [PubMed] [Google Scholar]
- 19.Li X., Tai H.H. Activation of thromboxane A(2) receptors induces orphan nuclear receptor Nurr1 expression and stimulates cell proliferation in human lung cancer cells. Carcinogenesis. 2009;30(9):1606–1613. doi: 10.1093/carcin/bgp161. [DOI] [PubMed] [Google Scholar]
- 20.Amieva M., Peek R.M., Jr. Pathobiology of Helicobacter pylori-Induced gastric cancer. Gastroenterology. 2016;150(1):64–78. doi: 10.1053/j.gastro.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peek R.M., Jr., Fiske C., Wilson K.T. Role of innate immunity in Helicobacter pylori-induced gastric malignancy. Physiol Rev. 2010;90(3):831–858. doi: 10.1152/physrev.00039.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox J.G., Wang T.C. Inflammation, atrophy, and gastric cancer. J Clin Invest. 2007;117(1):60–69. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malfertheiner P. Helicobacter pylori treatment for gastric cancer prevention. N Engl J Med. 2018;378(12):1154–1156. doi: 10.1056/NEJMe1800147. [DOI] [PubMed] [Google Scholar]
- 24.Wang F., Meng W., Wang B., Qiao L. Helicobacter pylori-induced gastric inflammation and gastric cancer. Cancer Lett. 2014;345(2):196–202. doi: 10.1016/j.canlet.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 25.Suzuki H., Moayyedi P. Helicobacter pylori infection in functional dyspepsia. Nat Rev Gastroenterol Hepatol. 2013;10(3):168–174. doi: 10.1038/nrgastro.2013.9. [DOI] [PubMed] [Google Scholar]
- 26.Kim Y.H., Shin S.W. Helicobacter pylori and prevention of gastric cancer. N Engl J Med. 2018;378(23):2244. doi: 10.1056/NEJMc1805129. [DOI] [PubMed] [Google Scholar]
- 27.Monteleone G., Del Vecchio Blanco G., Palmieri G. Induction and regulation of Smad7 in the gastric mucosa of patients with Helicobacter pylori infection. Gastroenterology. 2004;126(3):674–682. doi: 10.1053/j.gastro.2003.11.048. [DOI] [PubMed] [Google Scholar]
- 28.Zhu S., Soutto M., Chen Z. Helicobacter pylori-induced cell death is counteracted by NF-kappaB-mediated transcription of DARPP-32. Gut. 2017;66(5):761–762. doi: 10.1136/gutjnl-2016-312141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuo S.H., Yeh P.Y., Chen L.T. Overexpression of B cell-activating factor of TNF family (BAFF) is associated with Helicobacter pylori-independent growth of gastric diffuse large B-cell lymphoma with histologic evidence of MALT lymphoma. Blood. 2008;112(7):2927–2934. doi: 10.1182/blood-2008-02-137513. [DOI] [PubMed] [Google Scholar]
- 30.Teng Y.S., Liu Y.G., Chen X.H. Decreased IR-17RB expression impairs CD11b(+)CD11c(-) myeloid cell accumulation in gastric mucosa and host defense during the early-phase of Helicobacter pylori infection. Cell Death Dis. 2019;10(2):79. doi: 10.1038/s41419-019-1312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wagner J., Akerud P., Castro D.S. Induction of a midbrain dopaminergic phenotype in Nurr1-overexpressing neural stem cells by type 1 astrocytes. Nat Biotechnol. 1999;17(7):653–659. doi: 10.1038/10862. [DOI] [PubMed] [Google Scholar]
- 32.Sherr C.J., Beach D., Shapiro G.I. Targeting CDK4 and CDK6: from discovery to therapy. Cancer Discov. 2016;6(4):353–367. doi: 10.1158/2159-8290.CD-15-0894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hathroubi S., Servetas S.L., Windham I., Merrell D.S., Ottemann K.M. Helicobacter pylori biofilm formation and its potential role in pathogenesis. Microbiol Mol Biol Rev. 2018;82(2) doi: 10.1128/MMBR.00001-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uemura N., Okamoto S., Yamamoto S. Helicobacter pylori infection and the development of gastric cancer. N Engl J Med. 2001;345(11):784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 35.Sze K.M., Wong K.L., Chu G.K., Lee J.M., Yau T.O., Ng I.O. Loss of phosphatase and tensin homolog enhances cell invasion and migration through AKT/Sp-1 transcription factor/matrix metalloproteinase 2 activation in hepatocellular carcinoma and has clinicopathologic significance. Hepatology. 2011;53(5):1558–1569. doi: 10.1002/hep.24232. [DOI] [PubMed] [Google Scholar]
- 36.Ye Y., Jin L., Wilmott J.S. PI(4,5)P2 5-phosphatase A regulates PI3K/Akt signalling and has a tumour suppressive role in human melanoma. Nat Commun. 2013;4:1508. doi: 10.1038/ncomms2489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Choi J.A., Jung Y.S., Kim J.Y., Kim H.M., Lim I.K. Inhibition of breast cancer invasion by TIS21/BTG2/Pc3-Akt1-Sp1-Nox4 pathway targeting actin nucleators, mDia genes. Oncogene. 2016;35(1):83–93. doi: 10.1038/onc.2015.64. [DOI] [PubMed] [Google Scholar]
- 38.Zheng L., Liang X., Li S. CHAF1A interacts with TCF4 to promote gastric carcinogenesis via upregulation of c-MYC and CCND1 expression. EBioMedicine. 2018;38:69–78. doi: 10.1016/j.ebiom.2018.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fang Q., Tian M., Wang F. Amlodipine induces vasodilation via Akt2/Sp1-activated miR-21 in smooth muscle cells. Br J Pharmacol. 2019;176(13):2306–2320. doi: 10.1111/bph.14679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li S., Liang X., Ma L. MiR-22 sustains NLRP3 expression and attenuates H. pylori-induced gastric carcinogenesis. Oncogene. 2018;37(7):884–896. doi: 10.1038/onc.2017.381. [DOI] [PubMed] [Google Scholar]
- 41.Nam K.T., Hahm K.B., Oh S.Y. The selective cyclooxygenase-2 inhibitor nimesulide prevents Helicobacter pylori-associated gastric cancer development in a mouse model. Clin Cancer Res. 2004;10(23):8105–8113. doi: 10.1158/1078-0432.CCR-04-0896. [DOI] [PubMed] [Google Scholar]
- 42.Giguere V. Orphan nuclear receptors: from gene to function. Endocr Rev. 1999;20(5):689–725. doi: 10.1210/edrv.20.5.0378. [DOI] [PubMed] [Google Scholar]
- 43.Puigserver P., Wu Z., Park C.W., Graves R., Wright M., Spiegelman B.M. A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell. 1998;92(6):829–839. doi: 10.1016/s0092-8674(00)81410-5. [DOI] [PubMed] [Google Scholar]
- 44.Bensinger S.J., Tontonoz P. Integration of metabolism and inflammation by lipid-activated nuclear receptors. Nature. 2008;454(7203):470–477. doi: 10.1038/nature07202. [DOI] [PubMed] [Google Scholar]
- 45.Germain P., Staels B., Dacquet C., Spedding M., Laudet V. Overview of nomenclature of nuclear receptors. Pharmacol Rev. 2006;58(4):685–704. doi: 10.1124/pr.58.4.2. [DOI] [PubMed] [Google Scholar]
- 46.Zhao Y., Bruemmer D. NR4A orphan nuclear receptors: transcriptional regulators of gene expression in metabolism and vascular biology. Arterioscler Thromb Vasc Biol. 2010;30(8):1535–1541. doi: 10.1161/ATVBAHA.109.191163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woronicz J.D., Calnan B., Ngo V., Winoto A. Requirement for the orphan steroid-receptor Nur77 in apoptosis of T-Cell hybridomas. Nature. 1994;367(6460):277–281. doi: 10.1038/367277a0. [DOI] [PubMed] [Google Scholar]
- 48.Won H.Y., Hwang E.S. Transcriptional modulation of regulatory T cell development by novel regulators NR4As. Arch Pharm Res. 2016;39(11):1530–1536. doi: 10.1007/s12272-016-0803-z. [DOI] [PubMed] [Google Scholar]
- 49.Polk D.B., Peek R.M., Jr. Helicobacter pylori: gastric cancer and beyond. Nat Rev Cancer. 2010;10(6):403–414. doi: 10.1038/nrc2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Harjes U. Gastric cancer: risk analysis. Nat Rev Cancer. 2018;18(2):66. doi: 10.1038/nrc.2018.9. [DOI] [PubMed] [Google Scholar]
- 51.Hocker M., Hohenberger P. Helicobacter pylori virulence factors–one part of a big picture. Lancet. 2003;362(9391):1231–1233. doi: 10.1016/S0140-6736(03)14547-3. [DOI] [PubMed] [Google Scholar]
- 52.Wei J., Noto J.M., Zaika E. Bacterial CagA protein induces degradation of p53 protein in a p14ARF-dependent manner. Gut. 2015;64(7):1040–1048. doi: 10.1136/gutjnl-2014-307295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Saadat I., Higashi H., Obuse C. Helicobacter pylori CagA targets PAR1/MARK kinase to disrupt epithelial cell polarity. Nature. 2007;447(7142):330–333. doi: 10.1038/nature05765. [DOI] [PubMed] [Google Scholar]
- 54.Viala J., Chaput C., Boneca I.G. Nod1 responds to peptidoglycan delivered by the Helicobacter pylori cag pathogenicity island. Nat Immunol. 2004;5(11):1166–1174. doi: 10.1038/ni1131. [DOI] [PubMed] [Google Scholar]
- 55.Greenberg E.R., Chey W.D. Defining the role of sequential therapy for H. pylori infection. Lancet. 2013;381(9862):180–182. doi: 10.1016/S0140-6736(12)61849-2. [DOI] [PubMed] [Google Scholar]
- 56.Han Y., Cai H., Ma L. Expression of orphan nuclear receptor NR4A2 in gastric cancer cells confers chemoresistance and predicts an unfavorable postoperative survival of gastric cancer patients with chemotherapy. Cancer. 2013;119(19):3436–3445. doi: 10.1002/cncr.28228. [DOI] [PubMed] [Google Scholar]
- 57.Mimuro H., Suzuki T., Tanaka J., Asahi M., Haas R., Sasakawa C. Grb2 is a key mediator of helicobacter pylori CagA protein activities. Mol Cell. 2002;10(4):745–755. doi: 10.1016/s1097-2765(02)00681-0. [DOI] [PubMed] [Google Scholar]
- 58.Keates S., Keates A.C., Warny M., Peek R.M., Jr., Murray P.G., Kelly C.P. Differential activation of mitogen-activated protein kinases in AGS gastric epithelial cells by cag+ and cag- Helicobacter pylori. J Immunol. 1999;163(10):5552–5559. [PubMed] [Google Scholar]
- 59.Lukey M.J., Greene K.S., Erickson J.W., Wilson K.F., Cerione R.A. The oncogenic transcription factor c-Jun regulates glutaminase expression and sensitizes cells to glutaminase-targeted therapy. Nat Commun. 2016;7:11321. doi: 10.1038/ncomms11321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Han Y.F., Cao G.W. Role of nuclear receptor NR4A2 in gastrointestinal inflammation and cancers. World J Gastroenterol. 2012;18(47):6865–6873. doi: 10.3748/wjg.v18.i47.6865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holla V.R., Mann J.R., Shi Q., DuBois R.N. Prostaglandin E2 regulates the nuclear receptor NR4A2 in colorectal cancer. J Biol Chem. 2006;281(5):2676–2682. doi: 10.1074/jbc.M507752200. [DOI] [PubMed] [Google Scholar]
- 62.Ke N., Claassen G., Yu D.H. Nuclear hormone receptor NR4A2 is involved in cell transformation and apoptosis. Cancer Res. 2004;64(22):8208–8212. doi: 10.1158/0008-5472.CAN-04-2134. [DOI] [PubMed] [Google Scholar]
- 63.Chang W., Ma L., Lin L. Identification of novel hub genes associated with liver metastasis of gastric cancer. Int J Cancer. 2009;125(12):2844–2853. doi: 10.1002/ijc.24699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.