Abstract
Vogt–Koyanagi–Harada disease (VKHD) is a rare systemic granulomatous autoimmune disease that affects melanocyte‐rich organs such as eye, inner ear, meninges, skin, and hair. VKHD leads to chronic uveal inflammation accompanied by a decline in visual acuity in some patients when appropriate corticosteroid treatment was not initiated in an early phase. Immune checkpoint inhibitors (ICIs) are widely used in the treatment of several kinds of cancers and chemoimmunotherapy has become the standard of care in the first‐line treatment of non‐small cell lung cancer (NSCLC). While ICIs induce immune‐related adverse events, drug‐induced VKHD is quite rare with only four reports in the ICI monotherapy; three patients with melanoma and one patient with NSCLC. We describe the first case of VKHD during chemoimmunotherapy including pembrolizumab in a patient with NSCLC, which was successfully treated with corticosteroid without any sequela.
Keywords: Chemoimmunotherapy, immune‐related adverse event, non‐small cell lung cancer, pembrolizumab, Vogt–Koyanagi‐Harada disease
Vogt–Koyanagi–Harada disease (VKHD) is very rare and drug‐induced VKHD is quite rare. This would be the first report on VKHD during chemoimmunotherapy.
Introduction
The introduction of immune checkpoint inhibitors (ICIs) has revolutionized the treatment strategy of non‐small cell lung cancer (NSCLC), especially in patients with NSCLC without oncogenic driver mutation or translocation. The addition of pembrolizumab to standard chemotherapy with carboplatin and either paclitaxel or nanoparticle albumin‐bound (nab)‐paclitaxel, as compared with chemotherapy alone, brought more prolonged overall survival and median progression‐free survival in patients with squamous NSCLC 1.
Immune‐related adverse events (irAEs) occur with a certain frequency and Vogt–Koyanagi–Harada disease (VKHD) is a quite rare irAE with only one report in the treatment of NSLC with pembrolizumab monotherapy 2. In this report, VKHD during chemoimmunotherapy in a patient with NSCLC was successfully treated with corticosteroid.
Case Report
A 71‐year‐old male patient with NSCLC (not otherwise specified) without EGFR‐sensitizing mutation or ALK translocation (cT3N2M0, stage IIIA) was treated with a combination of carboplatin, nab‐paclitaxel, and pembrolizumab for four cycles, followed by pembrolizumab maintenance therapy for two cycles. This led to a partial response with remarkable tumour shrinkage.
However, he complained of headache on day 6 of second cycle of pembrolizumab maintenance therapy, which was followed by low vision and auditory disturbance after nine days.
He referred to the Department of Ophthalmology and the following findings were obtained in both eyes: anterior granulomatous uveitis with mutton‐fat keratic precipitates on the corneal endothelium via anterior segment photography (Fig. 1A, B), posterior synechia after atropine instillation, retinal detachment via fundus photography (Fig. 1C, D), and optical coherence tomography (OCT; Fig. 1E, F), as well as disseminated spotted choroidal hyperfluorescence via fluorescein fundus angiography. The cerebrospinal fluid (CSF) contained 215 white blood cells per mm3 with increased lymphocyte up to 98%. CSF protein was elevated at 196 mg/dL and CSF glucose was as low as 38 mg/dL when plasma glucose was 123 mg/dL. He showed alopecia as a cutaneous finding. Audiograms showed auditory disturbance (Fig. 2I).
Figure 1.
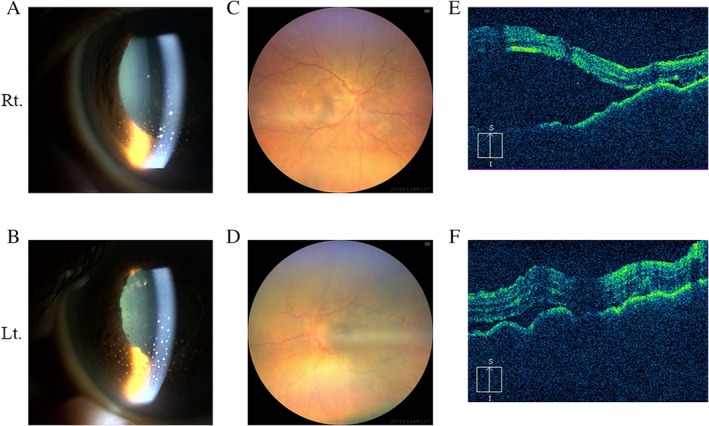
Anterior segment photography showed anterior granulomatous uveitis with mutton‐fat keratic precipitates on the corneal endothelium of bilateral eyeball (A, B). Fundus photography exhibited disc oedema with multifocal serous detachment at the posterior pole (C, D). Optical coherence tomography images also showed bilateral marked serous retinal detachment (E, F).
Figure 2.
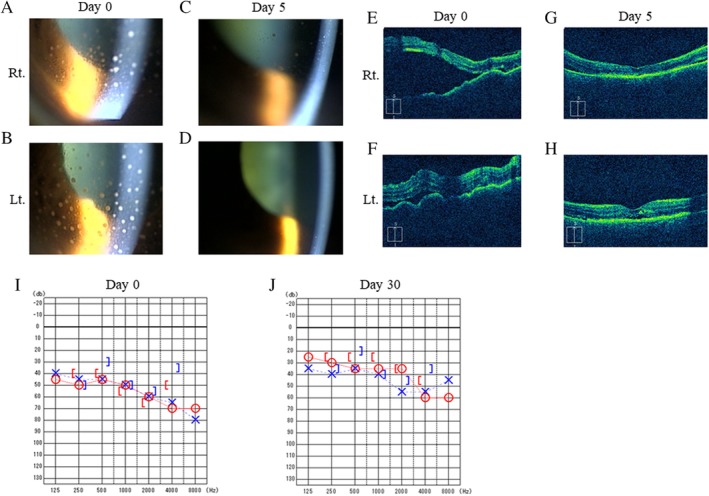
Anterior segment photography before pulse corticosteroid therapy (A, B) and after five days (C, D). Mutton‐fat keratic precipitates on the corneal endothelium were improved after steroid therapy. Optical coherence tomography images before pulse corticosteroid therapy (E, F) and after five days (G, H). Bilateral retinal detachment showed significant improvement after steroid therapy. Audiograms obtained before treatment (I) and 30 days after steroid therapy (J). Bilateral auditory acuity showed improvement after steroid therapy.
Thus, he was diagnosed as VKHD and pulse corticosteroid therapy with 500 mg of methylprednisolone for three days was initiated, which was followed by 30 mg of oral prednisolone therapy which was tapered off according to the ocular status and was discontinued after 12 weeks. The visual and auditory acuity improved and headache was relieved after treatment, which was accompanied by the improvement in findings five days after steroid initiation in anterior segment photography (Fig. 2C, D) and OCT (Fig. 2G, H). Auditory disturbance also improved through steroid treatment after 30 days (Fig. 2J). The therapy was quite effective and the patient remained symptom free for three months after the discontinuation of oral corticosteroid therapy.
Discussion
Immune‐related ocular toxicities are uncommon and the incidence and risk have not been substantially assessed 3. However, immune‐related ocular toxicities are serious adverse events as they could lead to the loss of eyesight. Uveitis and dry eyes are relatively common among them, the incidence ranging from 0.3% to 6% and 1.2% to 24.2%, respectively 3. The differences in the incidence of ocular toxicities between the types of ICIs have not elucidated because of the low incidence.
VKHD is a rare systemic anti‐melanocyte granulomatous autoimmune disease that affects eye, inner ear, meninges, skin, and hair 4. Several studies demonstrated that HLA (human leucocyte antigen)‐DR4 is strongly associated with VKHD. The clinical features of VKHD are the following: bilateral chronic iridocyclitis, posterior uveitis, neurological signs compatible with meningitis, and cutaneous findings of alopecia, poliosis, or vitiligo 4. VKHD is important cause of noninfectious uveitis especially in the individuals of pigmented skin including Asians, and its incidence among all cases of uveitis is estimated to be approximately 7% in Japan 4.
VKHD as an irAE was first reported in a 43‐year‐old female patient with malignant melanoma (MM) 5 who developed bilateral serious retinal detachments two weeks after first‐line ipilimumab therapy. The second report was of a 54‐year‐old female patient with MM 6 and VKHD was observed 13 months after second‐line ipilimumab therapy, in which initial symptoms were headache, poliosis, and tinitis, and HLA‐DR4 was not detected. The third report was of a 59‐year‐old male patient with MM 7 and VKHD was observed 16 months after fifth‐line ipilimumab therapy, in which initial symptoms were poliosis and vitiligo, and HLA‐DR4 was detected. The fourth report was of a 61‐year‐old male patient with NSCLC 2 and VKHD was observed two months after first‐line pembrolizumab therapy, in which initial symptoms were ocular pain and auditory change, and HLA‐DR4 was not assessed.
In the current case, VKHD was observed four months after first‐line chemoimmunotherapy for NSCLC, in which initial symptom was headache followed by low vision and auditory disturbance, and HLA‐DR4 was not detected.
The mechanism of VKHD is considered as an autoimmune aggression against antigens associated with melanocytes after herpes family viral infection in genetically susceptible individuals 4. The immune response is targeting melanocyte‐specific proteins in VKHD, leading to several systemic symptoms previously mentioned. In the pathogenesis of VKHD, CD4+ and CD8+ T lymphocytes are considered to play important roles and there is no report on VKHD as a complication of cytotoxic chemotherapy. Thus, pembrolizumab might have activated these T lymphocytes in some of the susceptible patients during chemoimmunotherapy, leading to the development of VKHD.
Although VKHD as an irAE is rare, more cases with VKHD could be expected as the number of cases treated with ICIs is increasing worldwide. The multidisciplinary approaches to diagnose and treat the patients developing rare irAEs is important.
Disclosure Statement
Appropriate written informed consent was obtained for publication of this case report and accompanying images.
Kurono, Y , Takeda, T , Kunimatsu, Y , Tani, N , Hashimoto, I , Hirose, K . (2020) Vogt–Koyanagi–Harada disease during chemoimmunotherapy for non‐small cell lung cancer. Respirology Case Reports, 8(3), e00545 10.1002/rcr2.545
Associate Editor: Kazuhisa Takahashi
References
- 1. Paz‐Ares L, Luft A, Vicente D, et al. 2018. Pembrolizumab plus chemotherapy for squamous non‐small‐cell lung cancer. N. Engl. J. Med. 379:2040–2051. [DOI] [PubMed] [Google Scholar]
- 2. Tamura T, Akimoto E, Matsumoto C, et al. 2018. Vogt‐Koyanagi‐Harada syndrome induced by pembrolizumab in a patient with non‐small cell lung cancer. J. Thorac. Oncol. 13:1606–1607. [DOI] [PubMed] [Google Scholar]
- 3. Abdel‐Rahman O, Oweira H, Petrausch U, et al. 2017. Immune‐related ocular toxicities in solid tumor patients treated with immune checkpoint inhibitors: a systematic review. Expert Rev. Anticancer Ther. 17:387–394. [DOI] [PubMed] [Google Scholar]
- 4. Lavezzo MM, Sakata VM, Morita C, et al. 2016. Vogt‐Koyanagi‐Harada disease: review of a rare autoimmune disease targeting antigens of melanocytes. Orphanet J. Rare Dis. 11:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wong RK, Lee JK, and Huang JJ. 2012. Bilateral drug (ipilimumab)‐induced vitritis, choroiditis, and serous retinal detachments suggestive of Vogt‐Koyanagi‐Harada syndrome. Retin. Cases Brief Rep. 6:423–426. [DOI] [PubMed] [Google Scholar]
- 6. Crosson JN, Laird PW, Debiec M, et al. 2015. Vogt‐Koyanagi‐Harada‐like syndrome after CTLA‐4 inhibition with ipilimumab for metastatic melanoma. J. Immunother. 38:80–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bricout M, Petre A, Amini‐Adle M, et al. 2017. Vogt‐Koyanagi‐Harada‐like syndrome complicating pembrolizumab treatment for metastatic melanoma. J. Immunother. 40:77–82. [DOI] [PubMed] [Google Scholar]


