Abstract
The study was designed to investigate whether sports-induced elevation of testosterone level impacts on the growth hormone/insulin-like growth factor-I (GH-IGF-I) axis and body composition, especially skeletal muscle mass. The study included 12 male wrestlers aged 21.1 ± 1.7 years and 10 male nonathletes aged 21.1 ± 1.2 years. Anthropometric and biochemical measurements in the group of nonathlete men were carried out once, while for wrestlers they were carried out twice, that is, on the 1st and 14th days of the training camp.
The levels of resting free testosterone (fT), cortisol (C), and human growth hormone (hGH) were significantly higher in the athletes than in nonathletes. A 2-week sports training induced a significant reduction in fT, IGF-I, and IGF binding protein-3 (IGFBP-3) levels and a rise in C level. Increased C level and reduced fT level in the athletes’ blood caused a rise in C/fT from the level of 39.95 ± 4.97 nmol/L to 59.73 ± 10.09 nmol/L (p < .05). A negative correlation was demonstrated between C/fT ratio and IGF-I level (r = −0.474, p < .05), which may indicate an inhibitory impact of high C level and low fT concentration on IGF-I release in response to sports training.
Sports activity induces significant changes in the C/fT ratio that can impact on the secretion of GH and IGF-I from the liver and finally on the fat-free body mass. The quantification of GH-IGF-I axis in relation to testosterone level could be a useful diagnostic tool in biochemical assessment of the regenerative ability of skeletal muscle or provide evidence of the early stages of muscle functional overload.
Keywords: muscle damage, satellite cells, body composition, sports training
The growth hormone/insulin-like growth factor-I (GH/IGF-I) axis, with binding proteins, plays a key role in the adaptation to exercise. Even moderate physical exertion can significantly affect the body by interacting with the GH/IGF-I axis. Attempting to precisely describe the impact of physical effort on the GH/IGF-I axis is a difficult task. The IGF-I response to exercise is particularly ambiguous (De Palo et al., 2008). The literature data on the relationship between circulating levels of IGF-I, in both total and free forms, demonstrate different results with increased or decreased circulatory levels in athletes (Antonelli et al., 2007). The response of GH to exercise has been more fully understood (De Palo et al., 2008). IGF-I is produced primarily in the liver, as a result of stimulation by GH, and participates in GH anabolic and myogenic activity. It affects the muscles directly by transmembrane receptor for IGF-I (IGF-IR) inducing phosphorylation of tyrosine residues and activation of tyrosine kinase (Harridge, 2007; Velloso, 2008).
IGF-I, which circulates in peripheral blood, is synthesized chiefly by hepatocytes and endothelial cells (Schoenfeld, 2010). Serum IGF-I concentration depends primarily on GH level (Velloso, 2008). IGF-I is also produced locally in muscle tissue where it stimulates proliferation and differentiation of satellite cells (SCs) by an auto- and paracrine signaling (Schoenfeld, 2010). The interaction between circulating IGF-I and its local expression can also play an important role in the regulation of muscle mass. Circulating IGF-I reduces the secretion of GH by a feedback loop, that is, high blood IGF-I level inhibits GH secretion and simultaneously impacts the local IGF-I synthesis (Velloso, 2008). Working muscles not only produce increased amounts of IGF-I but they also rely on the increased use of the circulating liver-derived IGF-I (Schoenfeld, 2010; Velloso, 2008).
The key role in SCs activation is played by IGF-I in relation to free testosterone (fT). As a hydrophobic molecule, testosterone diffuses through the membrane and, when binding with intracellular androgen receptor (AR), it enhances proliferation and differentiation of SCs mainly by inhibition of apoptosis (Kvorning et al., 2007; Morawin, 2014). Cellular response to testosterone–AR signal may involve inhibition to the expression of myostatin, which is a negative regulator of SCs proliferation and an inhibitor of muscle growth. Activated AR was also observed to induce expression of myogenic regulatory factor (MRF), such as MyoD, which occurs exclusively in SCs (Kvorning et al., 2007; Morawin, 2014; Schoenfeld, 2010). Testosterone binding with AR can also contribute to the increase in muscle protein synthesis by direct stimulation of gene expression for anabolic hormones such as hGH and IGF-I (Urban et al., 1995).
Many studies have been conducted on the effects of sports training and/or single exercise on selected anabolic or catabolic factors that participate in skeletal muscle regeneration and their growth. However, no information has been found concerning the changes in the GH/IGF-I axis in relation to circulating testosterone. Therefore, the study was designed to investigate whether sports-induced elevation of testosterone level impacts on the GH-IGF-I axis and body composition, especially skeletal muscle mass.
Material and Methods
Subjects
Twelve male wrestlers, members of the national team, aged 21.1 ± 1.7 years (Table 1), were observed during the preparatory training period. They participated in a 14-day training camp at the National Olympic Sport Centre. Throughout the camp all athletes lived at the same accommodation and followed the same training schedule and diet. Daily energetic value of food offered on the menu did not exceed 5,200 kcal and the protein dose varied from 1.6 to 1.8 g/kg of body mass. During the camp, the wrestlers consumed either an isotonic sports drink Vitargo (osmolality 317 mOsm/kg H2O) or plain water. The training loads were demonstrated using the TRENING/TREOB4 program prepared by the Department of Sport Theory at the University School of Physical Education, Warsaw (Table 2). The training took place twice a day and lasted for an hour and a half. The first training was between 10:00 and 11:00 a.m. and the second training between 4:00 and 6:00 p.m. Wrestling training is characterized by endurance and strength effort and a large proportion of eccentric exercises. Ten healthy men of age 21.1 ± 1.2 years, who did not participate in professional sports activities within the previous 3 years, were considered the reference group (Table 1). The current health status and lifestyle of the subjects were estimated by a doctor of sports medicine. Anthropometric and biochemical measurements in the group of nonathlete men were carried out once, while for wrestlers, these were carried out twice, that is, on the 1st and 14th days of training.
Table 1.
Anthropometric and Body Composition Data of Nonathletes and Wrestlers (x ± SD).
| Wrestlers n = 12 |
Wrestlers vs. nonathletes |
|||
|---|---|---|---|---|
| Nonathletes n = 10 | 1st day of camp | 14th day of camp | 1st day of camp | |
| BM [kg] | 77.8 ± 7.4 | 72.9 ± 5.8 | 73.5 ± 5.7 | p > .05 |
| Height [cm] | 182.5 ± 7.1 | 170.9 ± 4.0 | 170.9 ± 4.0 | p < .01 |
| BMI [kg/m2] | 23.5 ± 1.1 | 25.0 ± 2.2 | 25.2 ± 2.2 | p > .05 |
| FM [kg] | 14.9 ± 2.6 | 6.3 ± 2.0 | 5.7 ± 1.8 | p < .001 |
| FM% | 19.7 ± 3.1 | 8.5 ± 2.2 | 7.7 ± 2.2* | p < .001 |
| FFM [kg] | 60.7 ± 4.4 | 66.5 ± 5.0 | 67.8 ± 5.1* | p < .05 |
| FFMI [kg/m2] | 18.3 ± 1.1 | 22.8 ± 1.9 | 23.2 ± 2.0* | p < .001 |
| FFM% | 80.3 ± 3.1 | 91.5 ± 2.2 | 92.3 ± 2.2* | p < .001 |
Note. BM = body mass; BMI = body mass index; FM = fat mass; FFM = fat-free mass; FFMI = fat-free mass index.
Statistically significant differences (p < .05) between the 1st and 14th days of the camp.
Table 2.
Exercise Intensity During Training Period and Creatine Kinase (CK) Activity.
| Training period | Type of training | Percentage of training load during the camp | CK initial level IU/L | CK peak level IU/L |
|---|---|---|---|---|
|
Preparatory
preseason phase December; n = 12 |
Endurance Directed Special/wrestling |
55 20 25 |
152 ± 131 | 409 ± 124 |
Note. Endurance training: team games, marches, and cross-country running, cross-country skiing, acrobatic exercises, climbing at ropes, pull-ups, exercises with partner.
Directed training: intervals, toss from knees, back suplex, reverse waist, turns.
Special/wrestling training: elevation from low position, keys, trolleys, throws with different amplitude of movement, gym.
All the subjects were informed of the study aim and their written consent for participation in the project was obtained. The protocol of the study was approved by the ethics committee at Medical University, Poznań (No 482/15), in accordance with the Helsinki Declaration.
Body Composition
Body mass (BM) and body composition (fat-free mass [FFM] and fat mass [FM]) were estimated using bioelectrical impedance (BIA) by using Tanita Body Composition Analyzer MC-980 (Japan) calibrated prior to each test session in accordance with the manufacturer’s guidelines. Duplicate measurements were taken from the participant in a standing position; the average value was used for the final analysis. The repeatability of measurement amounted to 98%. The measurements were taken between 7:00 and 8:00 a.m. before blood sampling.
Blood Sampling
Blood samples were taken from the median cubital vein between 7:00 and 8:00 a.m. after 15 min of rest (and an overnight sleep). Within 20 min, the samples were centrifuged at 3,000 g and +4oC for 10 min. Aliquots of serum were stored at −80°C.
Skeletal Muscle Damage
Serum total creatine kinase (CK) activity was used as a marker of sarcolemma disruption and evaluated using commercially available reagents and Dr. Lange analyzer (Germany) at a temperature of 20–25oC. The CK activity was measured immediately after serum collection for the consecutive days of the training camp.
Hormones and Growth Factors
Serum fT and C concentrations were determined using DRG kits (Poland); fT and C detection limits were estimated at 0.35 nmol/mL and 10 nmol/mL, respectively. The intra-assay coefficient of variation for the fT and C kits was <7%. Serum IGF-I, IGF binding protein-3 (IGFBP-3), and GH levels were determined by enzyme immunoassay methods using commercial kits from R&D Systems (USA). The detection limits for IGF-I, IGFBP-3, and GH were estimated at 0.026 ng/mL, 0.02 ng/mL, and 0.64 pg/mL, respectively. The average intra-assay coefficient of variation for the IGF-I, IGFBP-3, and GH kits was <5%.
Statistical Analysis
Statistical analyses were performed by means of statistical software Statistica 13.1 (StatSoft Inc., Tulsa, OK, USA). All the data were tested for distribution normality by the one-way analysis of variance ANOVA and Tukey’s post hoc test. Comparisons of repeated measurements among wrestlers were assessed by the Wilcoxon signed-ranks test. ANOVA was used for evaluation of significant differences between the resting values of wrestlers and nonathletes and the sports training–induced increases in wrestlers. Associations between measured parameters were analyzed using Pearson’s linear regression (coefficient, r). Statistical significance was set at p < .05. The results are expressed as mean and standard deviation (x ± SD).
Results
Body Composition
In the study, significantly higher FFM percentage (FFM%) and significantly lower FM percentage (FM%) were recorded in the athletes in comparison with the nonathletes (Table 1). The wrestlers exhibited high FFM and FFM% and low FM and FM%. The 2-week wrestling training camp in the preparatory period led to the reduction in FM and FM% and an increase in FFM and FFM%. Negative correlation was observed between FFM and IGF-I (r = −0.620, p < .01).
CK/Skeletal Muscle Damage
Blood CK activity in the nonathletes (110 ± 22 IU/L) did not differ significantly when compared with the wrestlers (1st day of the training camp: 152 ± 131 IU/L). The 2-week wrestling training resulted in a statistically significant increase of CK activity in the study wrestlers (14th day of the training camp: 409 ± 124 IU/L; Table 2).
Catabolic/Anabolic Balance
C and fT levels in the study athletes were twice as high as in the nonathletes (Table 3). As a result of the 2-week training, C level in the wrestlers rose statistically significantly and exceeded the upper limit of the normal range, 690 nmol/L, whereas fT level was lowered by approximately 30%. Changes in blood C levels in the athletes were positively correlated with CK (r = 0.533, p < .001), while the changes in fT were found to be negatively correlated with the activity of CK (r = −0.721, p < .01) and C (r = −0.460, p < .05) but these correlated positively with IGF-I (r = 0.570, p < .01).
Table 3.
Concentrations of Factors Regulating Skeletal Muscle Growth and Regeneration in Nonathletes and Wrestlers (x ± SD).
| Wrestlers n = 12 |
Wrestlers vs. nonathletes |
|||
|---|---|---|---|---|
| Nonathletes n = 10 | 1st day of camp | 14th day of camp | 1st day of camp | |
| C [nmol/L] | 168 ± 52 | 691 ± 70 | 784 ± 86* | p < .001 |
| fT [nmol/L] | 7.16 ± 1.10 | 19.92 ± 1.72 | 13.32 ± 1.68** | p < .001 |
| hGH [pg/ml] | 60 ± 11 | 151 ± 30 | 170 ± 32 | p < .001 |
| IGF-I [ng/mL] | 85 ± 13 | 91 ± 14 | 75 ± 11** | p > .05 |
| IGFBP-3 [ng/mL] | 2645 ± 153 | 2820 ± 197 | 2532 ± 203# | p < .05 |
Note. C = cortisol; fT = free testosterone; hGH = human growth hormone; IGF-I = insulin-like growth factor I; IGFBP-3 = insulin-like growth factor binding protein 3.
Statistically significant differences (p < .05) between the 1st and 14th days of the camp.
Statistically significant differences (p < .01) between the 1st and 14th days of the camp.
#Statistically significant differences (p < .001) between the 1st and 14th days of the camp.
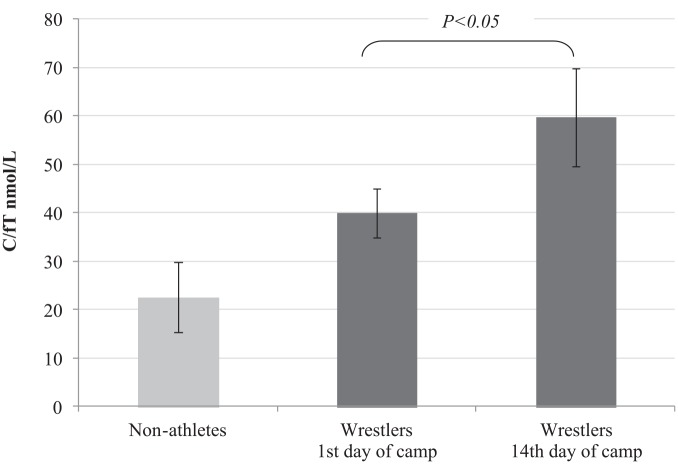
The blood C/fT ratio in the wrestlers was significantly higher (p < .05) than in the nonathletes mainly due to high C level (Figure 1). A twofold increase in C/fT was observed in the wrestlers’ blood on completion of the 2-week training. The 2-week training caused C/fT in the wrestlers to increase by 50%, mainly owing to significantly increased C level and the reduction of fT level. The change in C/fT was negatively correlated with IGF-I level (r = −0.474, p < .05), which may potentially indicate a transient inhibitory impact of C increase and fT decrease on the release of IGF-I during an intensive training period.
Figure 1.
The Cortisol (C)-to-Free Testosterone (fT) Ratio in Nonathletes (n = 10) and Wrestlers (n = 12).
GH-IGF-I Axis
Significantly higher resting values of hGH and IGFBP-3 were recorded in the wrestlers than in the analyzed nonathletes (Table 3). No statistically significant differences were observed in the level of IGF-I and bioavailable IGFI-I (IGF-I/IGFBP3) in the analyzed nonathletes. On completion of the 2-week training, no significant alterations in hGH level were observed in the wrestlers; however, IGF-I and IGFBP-3 level was found to be significantly decreased. The changes in IGF-I level were negatively correlated with FFM value in the study wrestlers (r = −0.620, p < .01). No significant relationships between training-induced changes to hGH level and the other catabolic/anabolic factors were identified in the athletes.
Discussion
There are many factors that can affect hormone levels, including body composition, sleep rhythm, nutritional status, and energy balance. One of the important factors affecting changes in hormone levels is physical activity. The response of the internal secretion system depends on the duration and intensity of the effort as well as the level of training and the type of exercise performed (De Palo et al., 2008). Physical effort causes damage to muscle fibers, which begins a number of processes leading to the reconstruction of muscle fibers, among others, by stimulating an increase in the concentration of anabolic hormones such as testosterone, IGF-I whether GH. Testosterone and IGF-I stimulate the activation, proliferation, and differentiation of SCs. Skeletal muscle damage triggers signaling paths, which lead to skeletal muscle hypertrophy. Damage to muscle fiber and proteolysis are significant factors that stimulate muscle mass increase (Paulsen et al., 2012; Schoenfeld, 2010).
There have been numerous studies that indicate that regular physical activity causes fT level to rise, thereby increasing the skeletal muscle regeneration potential. Exercise intensifies the synthesis of testosterone and an increase in its concentration in the blood, but the changes depend on the intensity and duration of exercise. High-intensity efforts lasting less than 1 min do not change the concentration of fT, while efforts lasting an hour cause a significant increase in fT concentration. However, with efforts lasting more than an hour, there is an increase and then a decrease in fT concentration over the next 3 hr to resting values. Larger fT changes are observed in the blood of nontraining people (Chaudhary and Shenoy 2015; Mackey et al., 2007; Morawin, 2014; Mougios, 2006; Nemet et al., 2012). Several weeks’ preparatory training was reported to have increased testosterone level by 5% to 14% in tennis players, canoeists, and runners (Gomes et al., 2013; Hejazi & Hosseini, 2012; Sutkowy et al., 2014). In this study significantly higher fT level was observed in the wrestlers than in the analyzed nonathletes. A positive correlation was found between fT and IGF-I as well as between fT and FFM%, which confirms testosterone anabolic effect on skeletal muscle mass enhancement. High values of fT and FFM% were identified in the wrestlers, whereas in the untrained men these values were found to be low. A 2-week training at the camp resulted in 30% decrease of blood testosterone level with a simultaneous increase in CK activity in the wrestlers. The applied wrestling training at high intensity caused significant muscle damages, which weaken and delay skeletal muscle regeneration as a result. This is proven by a reduction of anabolic molecules such as fT and IGF-I, which may be caused by a local acute inflammation, a high and persistent level of pain and inflammation mediators, and disturbance to pro- and anti-inflammatory balance (Peake et al., 2005). A similar reduction of testosterone level after high-intensity exercise was reported by Kupchak et al. (2014) and Peltonen et al. (2018) after ultramarathon and 20-week training of maximal and explosive strength.
C is a catabolic hormone released from the adrenal cortex in response to emotional and/or physical stress; it enhances protein breakdown and inhibits its synthesis (Radak et al. 2008). In this study, after the wrestling training, C level was found to have increased statistically significantly. Similarly, exercise-induced C increase was reported by Protzner et al. (2015) in the study, which included football and handball players as well as athletes of cyclic sports, such as canoeing and triathlon, who underwent maximal exercise Bruce protocol treadmill test, whereas Barbas et al. (2011) demonstrated blood C level increase in wrestlers after each consecutive wrestling match during 1-day tournaments. The authors explained that C level was affected not only by physical stress but also by emotional stress prior to or during competition.
The measurement of blood C/fT ratio in athletes is used to assess catabolic/anabolic balance and the risk of nonfunctional overreaching and overtraining syndrome (Mougios, 2006). The assessment of C and testosterone in athletes is an early marker of the reduction in training load tolerance (Tian et al., 2015). This study demonstrated a twofold increase in C/fT ratio in the athletes in comparison with the study nonathletes. In the wrestlers’ blood, a twofold increase of C/fT after the 2-week training was recorded. The rise in C/fT levels has been observed in athletes of various sports, both prior to competition and during multiweek training.
Tissue growth and regeneration is dependent primarily on hGH/IGF-I axis, whose mechanism of response to exercise is yet to be fully understood. The level of hGH is known to increase within 10–20 min after exercise commencement to reach its maximal concentration directly after exercise completion, irrespective of the exercise type and duration. The level is maintained up to 2 hr after physical exercise. The hGH secretion was found to be positively correlated with the duration of exercise of constant intensity (Gibney et al., 2007). In the study, hGH concentration was significantly higher in the wrestlers than in nonathletes, which indicate an enhancement of anabolic processes by regular physical activity. No significant changes to hGH after the 2-week wrestling training level were observed.
Numerous studies suggest that every type of physical exercise induces an increase in hGH level. Ehrnborg et al. (2003) observed high blood hGH concentration after maximal test exercise in athletes practicing such sports as cycling, athletics, rowing, swimming, triathlon, tennis, weight lifting, football, and alpine and cross-country skiing. De Palo at al. (2008) reported high blood hGH levels in cyclists both after short-term exercise of maximal intensity and after prolonged exercise of the intensity of 70%–80% maximal oxygen uptake (VO2max), whereas Smilios et al. (2013) and Manini et al. (2012) recorded elevated hGH concentration induced by strength exercise.
Changes in blood IGF-I level are related to exercise frequency (single bout vs. repeated), exercise type (eccentric vs. concentric), and exercise duration (short vs. long term). In physically active people IGF-I activity may be connected with inflammatory response intensity, mainly with changes to the level of interleukin-1β (IL-1β) and IL-6, which reduce the concentration of IGFBP-3 and modulate bioavailability of IGF-I (Dall et al., 2001; Grandys et al., 2017). The majority of literature reports an increased IGF-I level induced by maximal exercise test in the blood of athletes engaged in both endurance and strength/speed sports such as rowing, cycling, weight lifting, cross-country skiing, tennis, football, swimming, and decathlon (Dall et al., 2001; De Palo et al., 2008; Ehrnborg et al., 2003). Single-bout, short-term exercise, such as Wingate test or 30-s run, causes a rise in IGF-I level (Stokes et al., 2005). Long-term exercise, in turn, such as 1.5-h football training and wrestling training, reduces blood IGF-I concentration while simultaneously increasing proinflammatory cytokine level (Nemet et al., 2002; Stokes et al., 2005). Grandys et al. (2017) and Nemet et al. (2002) suggested that IGF-I reduction was caused by proinflammatory response to speed-strength exercise.
In the research conducted in this study, 2-week wrestling training was observed to lead to decreased levels of IGF-I and IGFBP-3. Changes to pro- and/or anti-inflammatory cytokines were not investigated in this study. Earlier analyses that included wrestlers indicated high IL-1β level in athletes attending a training camp in the preparatory period (Zembron-Lacny et al., 2017).
The source of blood IGF-I is located not only in the liver and endothelium but also in skeletal muscles. Via its auto- and paracrine activity, IGF-I synthesized in the muscles stimulates proliferation and differentiation of SCs. Damage to muscle fiber can trigger IGF-I release to the circuit (Berg & Bang, 2004; Grandys et al., 2017). In this study, the severity of muscle damage after 2-week training, determined by total CK activity was not correlated with IGF-I. This indicates that there was no relation between changes to IGF-I level and muscle damage.
A negative correlation was found between IGF-I level and C/fT ratio (r = −0.465, p < .01), which may indicate a transient inhibitory influence of high C level on IGF-I release during a high-intensity training period.
The above-mentioned studies imply that IGF-I response to physical exercise is not unequivocal, which may indicate that IGF-I synthesis and release from the liver and endothelium or from the muscles can be affected by a range of metabolic and physical factors. Increased levels of IGF-I have been demonstrated after both short- and long-term exercise of moderate intensity, whereas its reduction has been observed after prolonged exercise of high-intensity resistance or interval training (Nemet et al., 2009).
Conclusions
Sports activity induces significant changes in C/fT ratio that can impact on the secretion of GH and IGF-I from liver and finally on the fat-free body mass. The quantification of GH-IGF-I axis in relation to testosterone level could be a useful diagnostic tool in biochemical assessment of the regenerative ability of skeletal muscle or provide evidence of the early stages of muscle functional overload.
Acknowledgments
We are grateful for cooperation and financial support from the Polish Wrestling Federation.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by statutory funds from the University School of Physical Education, Poznań, Poland.
ORCID iD: Morawin Barbara  https://orcid.org/0000-0002-5874-1886
https://orcid.org/0000-0002-5874-1886
References
- Antonelli G., Cappellin E., Gatti R., Chiappin S., Spinella P., De Palo E. F. (2007). Measurement of free IGF-I saliva levels: Perspectives in the detection of GH/IGF axis in athletes. Clinical Biochemistry, 40(8), 545–550. [DOI] [PubMed] [Google Scholar]
- Barbas I., Fatouros I. G., Douroudos I. I., Chatzinikolaou A., Michailidis Y., Draganidis D., Jamurtas A. Z., Nikolaidis M. G., Parotsidis C., Theodorou A. A., Katrabasas I., Margonis K., Papassotiriou I., Taxildaris K. (2011). Physiological and performance adaptations of Greco-Roman wrestlers during a one-day tournament. European Journal of Applied Physiology, 111(7), 1421–1436. [DOI] [PubMed] [Google Scholar]
- Berg U., Bang P. (2004). Exercise and circulating insulin-like growth factor I. Hormone Research, 62(1), 50–58. [DOI] [PubMed] [Google Scholar]
- Chaudhary S., Shenoy S. (2015). Analysis of hormonal responses to aerobic and anaerobic zone training. Journal of Medical Science and Clinical Research, 3(3), 4677–4683. [Google Scholar]
- Dall R., Lange K. H., Kjaer M., Jørgensen J. O., Christiansen J. S., Orskov H., Flyvbjerg A. (2001). No evidence of insulin-like growth factor-binding protein 3 proteolysis during a maximal exercise test in elite athletes. The Journal of Clinical Endocrinology and Metabolism, 86(2), 669–674. [DOI] [PubMed] [Google Scholar]
- De Palo E. F., Antonelli G., Gatti R., Chiappin S., Spinela P., Cappellin E. (2008). Effects two different types of exercise on GH/IGF-I axis in athletes Is the free/Total IGF-I ratio a new investigate approach? Clinica Chimica Acta, 387(1–2), 71–74. [DOI] [PubMed] [Google Scholar]
- Ehrnborg C., Lange K. H., Dall R., Christiansen J. S., Lundberg P. A., Baxter R. C., Boroujerdi M. A., Bengtsson B. A., Healey M. L., Pentecost C., Longobardi S., Napoli R., Rosén T. & GH-2000 Study Group. (2003). The growth hormone/insulin-like growth factor-I axis hormones and bone markers in elite athletes in response to a maximum exercise test. The Journal of Clinical Endocrinology and Metabolism, 88(1), 394–401. [DOI] [PubMed] [Google Scholar]
- Gibney J., Healy M. L., Sönksen P. H. (2007). The growth hormone/insulin-like growth factor-I axis in exercise and sport. Endocrine Reviews, 28(6), 603–624. [DOI] [PubMed] [Google Scholar]
- Gomes R. V., Moreira A., Lodo L., Nosaka K., Coutts A. J., Aoki M. S. (2013). Monitoring training loads, stress, immune-endocrine responses and performance in tennis players. Biology of Sport, 30(3), 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandys M., Majerczak J., Kuczek P., Sztefko K., Duda K., Zoladz J. A. (2017). Endurance training-induced changes in the GH-IGF-I axis influence maximal muscle strength in previously untrained men. Growth Hormone & IGF Research, 32(2017), 41–48. [DOI] [PubMed] [Google Scholar]
- Harridge S. D. R. (2007). Plasticity of human skeletal muscle: Gene expression to in vivo function. Experimental Physiology, 92(5), 783–797. [DOI] [PubMed] [Google Scholar]
- Hejazi K., Hosseini S. R. A. (2012). Influence of selected exercise on serum immunoglobulin, testosterone and cortisol in semi-endurance elite runners. Asian Journal of Sports Medicine, 3(3), 185–192. [PMC free article] [PubMed] [Google Scholar]
- Kupchak B. R., Kraemer W. J., Hoffman M. D., Phinney S. D., Volek J. S. (2014). The impact of an ultramarathon on hormonal and biochemical parameters in men. Wilderness & Environmental Medicine, 25(3), 278–288. [DOI] [PubMed] [Google Scholar]
- Kvorning T., Andersen M., Brixen K., Schjerling P., Suetta C., Madsen K. (2007). Suppression of testosterone does not blunt mRNA expression of myoD, myogenin, IGF-I, myostatin or androgen receptor post strength training in humans. The Journal of Physiology, 578(Pt2), 579–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackey A., Kjaer M., Dandanell S., Mikkelsen K. H., Holm L., Døssing S., … Langberg H. (2007). The influence of anti-inflammatory medication on exercise-induced myogenic precursor cell response in humans. Journal of Applied Physiology, 103(2), 425–431. [DOI] [PubMed] [Google Scholar]
- Manini T. M., Yarrow J. F., Buford T. W., Clark B. C., Conover C. F., Borst S. E. (2012). Growth hormone response to acute resistance exercise with vascular restriction in young and old men. Growth Hormone & IGF Research, 22(5), 167–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morawin B. (2014). Rola testosteronu w regeneracji mięśni szkieletowych po wysiłku fizycznym. Rocznik Lubuski, 40(2), 95–105. [Google Scholar]
- Mougios V. (2006). Enzymes and hormones. In Mougios V. (Ed.), Exercise biochemistry (pp. 295–303). Human Kinetics. [Google Scholar]
- Nemet D., Oh Y., Kim H. S., Hill M., Cooper D. M. (2002). Effect of intense exercise on inflammatory cytokines and growth mediators in adolescent boys. Pediatrics, 110(4), 681–689. [DOI] [PubMed] [Google Scholar]
- Nemet D., Meckel Y., Bar-Sela S., Zaldivar F., Cooper D. M., Eliakim A. (2009). Effect of local cold-pack application on systemic anabolic and inflammatory response to sprint-interval training: A prospective comparative trial. European Journal of Applied Physiology, 107(4), 411–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemet D., Portal S., Zadik Z., Pilz-Burstein R., Adler-Portal D., Meckel Y., Eliakim A. (2012). Training increases anabolic response and reduces inflammatory response to a single practice in elite male adolescent volleyball players. Journal of Pediatric Endocrinology & Metabolism, 25(9–10), 875–880. [DOI] [PubMed] [Google Scholar]
- Paulsen G., Mikkelsen U. R., Raastad T., Peake J. M. (2012). Leucocytes, cytokines and satellite cells: What role do they play in muscle, damage and regeneration following eccentric exercise? Exercise Immunology Review, 18, 42–97. [PubMed] [Google Scholar]
- Peake J. M., Suzuki K., Wilson G., Hordern M., Nosaka K., Mackinnon L., Coombes J. S. (2005). Exercise-induced muscle damage, plasma cytokines, and markers of neutrophil activation. Medicine and Science in Sports and Exercise, 37(5), 737–745. [DOI] [PubMed] [Google Scholar]
- Peltonen H., Walker S., Hackney A. C., Avela J., Häkkinen K. (2018). Increased rate of force development during periodized maximum strength and power training is highly individual. European Journal of Applied Physiology, 118(5), 1033–1042. [DOI] [PubMed] [Google Scholar]
- Protzner A., Szmodis M., Udvardy A., Bosnyák E., Trájer E., Komka Z., Györe I., Tóth M. (2015). Hormonal neuroendocrine and vasoconstrictor peptide responses of ball game and cyclic sport elite athletes by treadmill test. PLoS One, 10(12), e0144691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radak Z., Chung H. Y., Goto S. (2008). Systemic adaptation to oxidative challenge induced by regular exercise. Free Radical Biology & Medicine, 44(2), 153–159. [DOI] [PubMed] [Google Scholar]
- Schoenfeld B. J. (2010). The mechanisms of muscle hypertrophy and their application to resistance training. Journal of Strength and Conditioning Research, 24(10), 2857–2872. [DOI] [PubMed] [Google Scholar]
- Smilios I., Tsoukos P., Zafeirdis A., Spassis A., Tokmakidis S. P. (2013). Hormonal responses after resistance exercise performed with maximum and submaximum movement velocitie. Applied Physiology, Nutrition, and Metabolism, 39(3), 351–357. [DOI] [PubMed] [Google Scholar]
- Stokes K., Nevill M., Frystyk J., Lakomy H., Hall G. (2005). Human growth hormone responses to repeated bouts of sprint exercise with different recovery periods between bouts. Journal of Applied Physiology, 99(4), 1254–1261. [DOI] [PubMed] [Google Scholar]
- Sutkowy P. B., Augustyńska B., Wożniak A., Rakowski A. (2014). Physical exercise combined with whole-body cryotherapy in evaluating the level of lipid peroxidation products and other oxidant stress indicators in kayakers. Oxidative Medicine and Cellular Longevity. DOI: 10.1155/2014/402631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., He Z., Zhao J., Tao D., Xu K., Midgley A., McNaughton L. (2015). An 8-year longitudinal study of overreaching in 114 elite female Chinese wrestlers. Journal of Athletic Training, 50(2), 217–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban T. J., Bodenburg Y. H., Gilkison C., Foxworth J., Coggan A. R., Wolfe R. R., Ferrando A. (1995). Testosterone administration to elderly men increases skeletal muscle strength and protein synthesis. The American Journal of Physiology, 269(5Pt11), E820–E826. [DOI] [PubMed] [Google Scholar]
- Velloso C. P. (2008). Regulation of muscle mass by growth hormone and IGF-I. British Journal of Pharmacology, 154(3), 557–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zembron-Lacny A., Ziemann E., Zurek P., Hübner-Wozniak E. (2017). Heat shock protein 27 response to wrestling training in relation to the muscle damage and inflammation. Journal of Strength and Conditioning Research, 31(5): 1221–1228. [DOI] [PubMed] [Google Scholar]