Abstract
Background: Proton pump inhibitors (PPIs) are often prescribed for elderly patients without appropriate indication, or for longer durations than recommended. Objective: To review appropriateness of PPI use prior to and in hospital, and deprescribing rates across different hospital units. Methods: Retrospective analysis of patients ≥65 years admitted to 5 acute care units: intensive care unit, acute care for elderly, orthopedics, surgery, and medicine. Patients who were “non-naive” (prehospital PPI use) or “naive” (new PPI initiated in hospital) users were included. For both groups, demographics, reason for admission, length of stay, comorbidities, name and number of home medications, PPI name, dose and indication, and PPI discharge instructions were collected. For naive patients, duration of in-hospital use and prescriber specialty was recorded. Results: Among non-naive patients (n = 377), for 37 patients (10%), the indication for a PPI was not appropriate, and for 92 patients (24%), the indication was unclear. Most patients had their home PPI continued while in hospital (87%) and at discharge (90%). Among naive (n = 93) patients, for 8 patients (9%), the indication for a PPI was not appropriate, and for 25 (27%) patients, the indication was unclear. PPI was prescribed to only 16 (18%) by the gastrointestinal consult service. Most patients had their new PPI continued at discharge (74%); only 7 (9%) were discharged with a plan to reassess PPI indication. Conclusion: PPIs are infrequently deprescribed during hospital admission, despite inappropriate or unclear indications for use. Thorough medication reconciliation, documentation of PPI indication and duration, and institutional focus on deprescribing are encouraged.
Keywords: proton pump inhibitor, medication therapy management, clinical pharmacy, geriatrics, aging
Introduction
The concurrent use of 5 or more medications per day is referred to as polypharmacy.1 It is most common in older adults, many of whom are living with multiple chronic conditions. Polypharmacy increases the risk of adverse drug events, drug interactions, nonadherence, hospitalization, and mortality, with a detrimental impact on patients, their caregivers, and health systems at large.2
Proton pump inhibitors (PPIs) are a class of acid-suppression medications that are often prescribed without an appropriate indication, or for longer durations than recommended.3-6 This contributes to polypharmacy, and use of these drugs is associated with adverse effects including Clostridium difficile infection, bone loss, fractures, and death.6,7 As such, the 2019 Beers criteria advise that PPIs should not be used for more than 8 weeks, with the exception of patients receiving long-term nonsteroidal anti-inflammatory drugs (NSAIDs), those with Barrett’s esophagus, hypersecretory conditions, or those with other demonstrated indications for ongoing PPI therapy.8 Furthermore, the Canadian Association of Gastroenterology,9 the American Gastroenterological Association,10 and Choosing Wisely Canada have recommended that PPI prescriptions be reevaluated at least annually for appropriateness, and that PPIs be deprescribed when no longer necessary.11-13
Hospitalization of older adults is an opportunity to assess the indication for ongoing PPIs and for deprescribing when appropriate. We hypothesize that PPIs are often prescribed for older adults without an appropriate indication, are continued longer than necessary when there is an appropriate indication, and that there are missed opportunities for deprescribing during acute care hospitalizations. The objective was to review the appropriateness of PPI use prior to and in hospital, and assess the deprescribing rates across different hospital units. These data will provide information on the extent of the problem, provide an avenue for mapping processes of care, and identify potential points for intervention.
Methods
This retrospective cohort study was conducted at Mount Sinai Hospital, a large academic teaching hospital affiliated with the University of Toronto. The study was approved by the Institutional Research Ethics Board, who waived the need for informed consent.
Participants
Included patients were 65 years of age or older and admitted to one of the following inpatient services between September 2017 and December 2017: the intensive care unit (ICU), acute care for the elderly (ACE) unit, orthopedic surgery, general surgery, and general internal medicine. The ACE unit is a ward designed to meet the needs of medically complex older adults. Patients were included if they were (1) already receiving a PPI at hospital admission (non-naive users) or (2) had a new PPI initiated during their hospital stay (naive users). Patients with documented palliative status, or for whom records were inaccessible through the electronic medical record system, were excluded.
Data Collection and Analysis
Data were collected from electronic patient records. Two authors (NM and FMG) collected the data, and consistency was ensured through collecting data of the first patient from each unit together. For the entire cohort, the following information was collected: age, sex, reason for admission, length of stay, comorbidities, name and number of home medications, whether the PPI was initiated prior to admission or during the index hospitalization, name and dose of PPI patient was taking, occurrence of C difficile infections during the admission,14 and PPI discharge instructions. The name and number of home medications were obtained from medication history and reconciliation performed on admission, which involves speaking with patients and families, checking the provincial drug benefit database, checking pill bottles, and, if necessary, calling pharmacies and family physicians. If the patient developed a C difficile infection during this admission, the concurrent use of PPI was recorded, and whether the infection resulted in significant morbidity (megacolon, shock, ICU admission, surgery) or death. The C difficile infection was classified as “severe” if the white blood cell count was >15 000 cells/mm, or if serum creatinine was >132 mmol/L or greater than 1.5 times the patients’ baseline.15
For “non-naive” patients who were receiving PPI prior to hospital at time of the medication history and reconciliation, the indication, prehospital PPI name and dose, as well as whether the PPI was continued during the hospital admission and at discharge were recorded. The indication was evaluated based on the patient’s active or recent medical problems recorded in their medical records. For “naive” patients who had not been taking a PPI at home, and who had a new PPI initiated in hospital, the indication, medication name, dose and duration of in-hospital use, specialty of the prescribing clinician, and whether the new PPI was continued at discharge were recorded.
If patients were prescribed a new PPI in the ICU, the presence of the following variables potentially associated with a higher risk of gastrointestinal (GI) bleeding were recorded: invasive mechanical ventilation >48 hours; platelets <50, international normalized ratio >1.5, or partial thromboplastin time >2 times the control value; history of GI ulcers and/or GI bleeding within the past year; traumatic brain injury, traumatic spinal cord injury, or burn injury; or 2 or more of the following minor criteria: sepsis, ICU stay >7 days, occult GI bleeding for 6 or more days, or glucocorticoid therapy (more than 250 mg hydrocortisone daily or equivalent).16,17
Appropriateness of PPI indication was determined by identifying whether patients had one of the indications listed in the Choosing Wisely Canada PPI Toolkit documented as an active medical problem.11 The toolkit has been endorsed by the Canadian Association of Gastroenterology, College of Family Physicians of Canada, and Canadian Pharmacists Association, among other groups.11 Appropriate indications listed in the toolkit are as follows: esophagitis, gastroesophageal reflux disease, peptic ulcer disease, ICU stress ulcer prophylaxis, uncomplicated Helicobacter pylori, Barrett’s esophagus, chronic NSAID use, and documented history of bleeding GI ulcer.11 Indication for PPI use was categorized as either appropriate or inappropriate, or was marked unclear if we could not find the indication.
Deprescribing rates for non-naive and naive patient cohorts was calculated by dividing the number of patients discharged from hospital without a PPI on their medication list, or whose discharge notes contained instructions to stop the PPI, by the total number of PPI users in that hospital ward. This method was chosen as we are unable to establish whether patients were on PPI for an inappropriate duration of time, and how many of those patients with unclear indications did not have an appropriate indication.
Descriptive statistics were calculated for all variables. Mean and standard deviation were calculated for normally distributed variables; and median and interquartile range for nonnormally distributed variables.
Results
Demographics
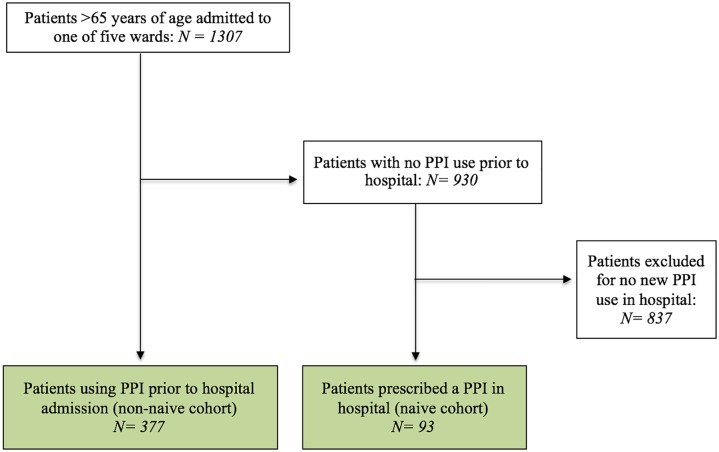
Over the 4-month period, there were 1307 patients admitted to the 5 medical units. Overall, 470 of these patients met the inclusion criteria of either using a PPI prior to hospitalization (n = 377), or being prescribed a new PPI in hospital (n = 93). Figure 1 shows the flow of patient screening. The majority of included patients were from the general medicine wards (n = 212). The mean age of patients was similar across the 5 wards (78.6 years). The sex distribution was equal in the entire cohort, although there was a slightly higher proportion of male patients in the ICU (67%) and a slightly lower proportion of male patients in orthopedic surgery (39%). The majority of patients were admitted from home (n = 374), followed by admission from other hospitals (n = 52), and from long-term care (n = 43). Table 1 shows patient demographics.
Figure 1.
Flowchart of hospitalized patients included in the retrospective cohort study.
Table 1.
Demographics of the Study Populationa.
| ICU | ACE | General Surgery | Orthopedic Surgery | General Medicine | Total | |
|---|---|---|---|---|---|---|
| Patients >65 years admitted to the ward, n | 138 | 161 | 113 | 289 | 606 | 1307 |
| Patients meeting study criteria, n (%) | ||||||
| PPI use prior to hospital (non-naive) | 42 (30.4%) | 47 (29.2%) | 28 (24.7%) | 77 (26.6%) | 183 (30.2%) | 377 (28.8%) |
| PPI newly started in hospital (naive) | 24 (17.4%) | 19 (11.8%) | 3 (2.7%) | 18 (6.2%) | 29 (4.8%) | 93 (7.1%) |
| Age in years, mean (SD) | 75.5 (7.1) | 81.5 (9.0) | 74.9 (7.7) | 77.0 (7.9) | 80.0 (9.7) | 78.6 (8.9) |
| Male, % | 67% | 52% | 45% | 39% | 50% | 50% |
| Location prior to hospitalization, n | ||||||
| Home | 42 | 51 | 30 | 81 | 170 | 374 |
| Other hospital | 21 | 5 | 1 | 11 | 14 | 52 |
| Long-term care | 2 | 10 | 0 | 3 | 28 | 43 |
| Unknown | 1 | 0 | 0 | 0 | 0 | 1 |
| Number of home medications, mean (range) | 10.9 (0-30) | 10.8 (1-26) | 9.8 (1-19) | 10.0 (2-27) | 11.7 (0-27) | 11.0 (0-30) |
| Length of hospital stay, days, mean (SD) | 24.7 (38.9) | 12.8 (14.1) | 3.8 (4.9) | 4.6 (3.7) | 6.5 (5.7) | 9.4 (21.3) |
Abbreviations: ICU, intensive care unit; ACE, acute care for the elderly unit; PPI, proton pump inhibitors.
Data are shown as n (%), or mean and standard deviation (SD), or mean and range.
Prior to hospital admission, patients were taking between 0 and 30 (mean = 11.0, SD = 5.2) medications overall, with the highest number in patients admitted to general medicine (mean = 11.7, SD = 5.1) and lowest in patients admitted to general surgery (mean = 9.8, SD = 4.8). The average length of hospital stay was 9.4 days (SD = 21.3) overall, with patients staying the longest in the ICU (24.7 days) and shortest in general surgery (3.8 days). The primary reasons for admission varied across different units, and only 40 (8.5%) patients were admitted for GI causes. Over half of patients were discharged directly home from hospital (n = 259, 55.1%).
Non-Naive Cohort
Overall, 377 patients were using a PPI on admission (non-naive cohort), which represents 29% of the total number of patients admitted during the study period. The indications for use varied, with the most common indications being gastroesophageal reflux disease (39%), NSAID use (16%), and previous GI bleed (5%). For 37 patients (10%), the indication was not appropriate according to the Choosing Wisely toolkit,11 and for 92 patients (24%), the indication for a PPI was unclear. The inappropriate indications were corticosteroid use, hiatal hernia, chemotherapy, anemia, and chronic cough. The majority of patients were using pantoprazole (n = 250, 66%). The initial PPI prescriber could not be determined from patients’ electronic health records. Most non-naive patients (n = 326, 87%) had their home PPI continued while in hospital. Most surviving non-naive patients had a PPI continued at hospital discharge (319/354, 90%). The majority (98%) had their home PPI continued, while 2% were switched to a different PPI. Of the 35 surviving non-naive patients who did not have a PPI continued at discharge, 18 had an inappropriate or unclear indication. Overall, 5 of 354 surviving patients (1%) were discharged with a specific plan to reevaluate the indication for PPI use. No discharge notes contained a plan to deescalate the PPI. Complete details of PPI use in non-naive patients are presented in Table 2, stratified by patient ward.
Table 2.
Patients Who Were Using PPI Prior to Hospitalization (Non-Naive Users).
| ICU | ACE | General Surgery | Orthopedic Surgery | General Medicine | Total | |
|---|---|---|---|---|---|---|
| Total number (% of total patients admitted) | 42 (30.4%) | 47 (29.2%) | 28 (24.7%) | 77 (26.6%) | 183 (30.2%) | 377 (28.8%) |
| Indication for use, n | ||||||
| GERD | 10 | 17 | 14 | 41 | 71 | 153 |
| NSAID use | 6 | 0 | 2 | 10 | 41 | 59 |
| Peptic ulcer disease | 1 | 1 | 1 | 2 | 7 | 12 |
| Previous GI bleed | 4 | 6 | 1 | 4 | 4 | 19 |
| Barrett’s esophagus | 1 | 0 | 1 | 0 | 3 | 5 |
| Other (not indicated) | 8 | 1 | 5 | 9 | 14 | 37 |
| Unclear indication | 12 | 22 | 4 | 11 | 43 | 92 |
| PPI used, n | ||||||
| Pantoprazole | 31 | 28 | 19 | 51 | 121 | 250 |
| Omeprazole | 2 | 6 | 3 | 8 | 36 | 55 |
| Lansoprazole | 5 | 6 | 3 | 8 | 12 | 34 |
| Rabeprazole | 3 | 5 | 1 | 6 | 10 | 25 |
| Dexlansoprazole | 0 | 0 | 1 | 2 | 1 | 4 |
| Esomeprazole | 1 | 2 | 1 | 2 | 3 | 9 |
| Clostridium difficile infection | 2 | 4 | 0 | 1 | 4 | 11 |
| Using PPI | 2 | 2 | 0 | 1 | 4 | 9 |
| Severea | 0 | 1 | 0 | 1 | 2 | 4 |
| PPI continued in hospital, n (% of users)b | 35 (83%) | 36 (77%) | 22 (79%) | 66 (86%) | 167 (91%) | 326 (87%) |
| PPI continued at discharge (% of survivors) | 27/31 (87%) | 31/44 (72%) | 27/28 (96%) | 70/75 (93%) | 164/176 (93%) | 319/354 (90%) |
| Plan to reassess PPI indication (% of survivors) | 1/31 (3%) | 1/44 (2%) | 0/28 (0%) | 1/75 (1%) | 2/176 (1%) | 5/354 (1%) |
Abbreviations: ICU, intensive care unit; ACE, acute care for the elderly unit; GERD, gastroesophageal reflux disease; NSAID, nonsteroidal anti-inflammatory drug; GI, gastrointestinal; PPI, proton pump inhibitor.
Severe Clostridium difficile was defined as blood cell count >15 000 cells/mm3, or serum creatinine >132 mmol/L or greater than 1.5 times the patients’ baseline.13
Same or different PPI drug.
Naive Cohort
Overall, 93 patients (7% of patients admitted during the study period) were newly prescribed a PPI during their hospital stay (naive cohort); 16 (18%) of these prescriptions were by the GI consult service. The indications for use were primarily GI bleeding in hospital (30%), NSAID use (22%), and gastroesophageal reflux disease (11%). For 8 patients (9%) the indication was not appropriate according to the Choosing Wisely toolkit,11 and for 25 (27%) patients, the indication for PPI use was unclear. The inappropriate indications were corticosteroid use, anemia, gastrostomy tube, and mild dyspepsia. Once again, the most commonly prescribed PPI was pantoprazole (n = 74, 80%). Most surviving patients were continued on their new PPI at hospital discharge (57/77, 74%). For 5 (7%) patients, it was unknown whether the PPI was continued at discharge. Of the 20 surviving naive patients who did not have a PPI continued at discharge, 11 had an inappropriate or unclear indication. Overall, 7 of 77 surviving patients (9%) were discharged with a specific plan to reevaluate the indication for PPI use. No discharge notes contained a plan to de-escalate the PPI. Results from the naive patient cohort are presented in Table 3, stratified by patient ward.
Table 3.
Patients Who Were Newly Started on PPI in Hospital (Naive Users).
| ICU | ACE | General Surgery | Orthopedic Surgery | General Medicine | Total | |
|---|---|---|---|---|---|---|
| Total number (% of total patients admitted) | 24 (17.4%) | 19 (11.8%) | 3 (2.7%) | 18 (6.2%) | 29 (4.8%) | 93 (7.1%) |
| Indication for use | ||||||
| GI bleed in hospital | 15 | 4 | 0 | 0 | 9 | 28 |
| GERD | 2 | 2 | 0 | 2 | 4 | 10 |
| NSAID use | 0 | 0 | 0 | 14 | 6 | 20 |
| Stress ulcer prophylaxis | 2 | 0 | 0 | 0 | 0 | 2 |
| Other (not indicated) | 0 | 3 | 1 | 0 | 4 | 8 |
| Unclear | 5 | 10 | 2 | 2 | 6 | 25 |
| PPI useda | ||||||
| Pantoprazole | 17 | 15 | 1 | 18 | 23 | 74 |
| Lansoprazole | 10 | 2 | 2 | 0 | 3 | 17 |
| Omeprazole | 1 | 2 | 0 | 0 | 0 | 3 |
| Rabeprazole | 0 | 0 | 0 | 0 | 3 | 3 |
| Prescriber | ||||||
| GI service | 5 | 10 | 0 | 0 | 1 | 16 |
| Other | 19 | 9 | 3 | 18 | 28 | 77 |
| Clostridium difficile Infection, n (%) | 1 | 0 | 0 | 0 | 0 | 1 |
| Using PPI | 1 | 0 | 0 | 0 | 0 | 1 |
| Severeb | 1 | 0 | 0 | 0 | 0 | 1 |
| Average duration of in-hospital PPI use (days) | 19.7 | 7.4 | 7.0 | 3.9 | 4.9 | 9.1 |
| PPI continued at discharge (% of survivors with known discharge medication) | 9/11 (82%) | 11/16 (69%) | 1/2 (50%) | 16/16 (100%) | 20/28 (71%) | 57/77 (74%) |
| Unknownc | 4 | 0 | 1 | 0 | 0 | 5 |
| Plan to reassess PPI indication (% of survivors) | 2/15 (13%) | 1/16 (6%) | 0/2 (0%) | 0/16 (0%) | 4/28 (14%) | 7/77 (9%) |
Abbreviations: ICU, intensive care unit; ACE, acute care for the elderly unit; GI, gastrointestinal; GERD, gastroesophageal reflux disease; NSAID, nonsteroidal anti-inflammatory drug; PPI, proton pump inhibitor.
Four patients were given more than one PPI drug, and so were included multiple times.
Severe Clostridium difficile was defined as blood cell count >15 000 cells/mm3, or serum creatinine >132 mmol/L or greater than 1.5 times the patients’ baseline.13
Discharge medications not found.
Of the 377 non-naive users, 11 (2.9%) developed C difficile infection; 9 were receiving PPI at the time of diagnosis. Four of these patients had severe C difficile infection. Of the 93 naive users, 1 (1%) developed C difficile infection, was receiving PPI at the time of diagnosis, and this case was severe. None of the 12 patients required ICU admission or surgery, and none of them died. The rate of hospital-acquired C difficile infection at Mount Sinai Hospital overall was 19 per 1000 adult admissions during study period—a rate of 0.2%.
Discussion
In this retrospective review of hospitalized elderly patients, the average number of home medications was 11 indicating a high level of polypharmacy.1 Approximately one third of these elderly inpatients were receiving PPIs on hospital admission, and 7% had PPIs newly started in hospital. There was often unclear documentation of the indication and the planned duration of PPI use, both for patients using PPI at home and those newly started in hospital. Rates of deprescribing were low across all hospital units, despite the high proportion of patients with unclear indication for PPI. Additionally, plans to reevaluate or de-escalate PPIs were seldom found in discharge documentation.
While there is a large body of literature demonstrating that PPIs are overused,3-6 there are few studies evaluating deprescribing. This study adds to this literature by showing that PPIs are infrequently deprescribed during hospital admission, despite cases where the indication for PPI is unknown or unclear. There were consistently low rates of deprescribing across all hospital units, suggesting room for enhanced medication monitoring and deprescribing. The highest rates of deprescribing were present in the ACE unit (31% of surviving patients). The ACE unit at Mount Sinai Hospital takes a multidisciplinary team-based approach to providing holistic care, and nurses and allied health staff in the unit have advanced training in geriatrics. As such, the relatively high rates of deprescribing observed may be due to a greater focus on medication safety on this ward designed to manage acutely ill older adults.
Several studies offer strategies for deprescribing PPIs in older hospitalized adults, led by either physicians or pharmacists.18 There are a number of opportunities for deprescribing PPI during a hospital stay. Medication reconciliation at admission presents an opportunity to inquire about the indication for PPI and duration of treatment through communication with family physicians, the prescribing clinician, or the dispensing pharmacy. For PPIs prescribed in hospital, complete and clear documentation of the indication and recommended duration of PPI use would facilitate appropriate deprescribing, particularly at transitions of care. Patient and caregivers should be clearly informed about the indication and expected duration of PPI therapy. While identifying PPI indication for non-naive patients may be more time-consuming and difficult, deprescribing for naive patients should be more straightforward as the reasons for initiating PPI therapy should be easier to establish. At an institutional level, staff can be familiarized with PPI deprescribing algorithms, and a culture of appropriate prescribing/deprescribing should be fostered. Measures such as computerized physician order entry alerts (notifications of potentially inappropriate prescriptions based on patient’s clinical indications) to encourage appropriate prescribing and electronic decision support tools to augment deprescribing can be implemented.19
This study has limitations. First, the retrospective design restricts the data source to the electronic medical record, and as such, only information that was adequately documented could be obtained. For example, it is possible that some patients were deprescribed their PPI at discharge or on admission, but this information was not clearly documented in the patient record. Patients may also have had an appropriate indication for PPI that was not indicated in the record for this admission. Additionally, rates of deprescribing were considered equivalent to the number of patients who did not have PPI continued on discharge. There is potential for inaccuracies in these numbers, for example, if the PPI was accidentally left off the discharge note. Furthermore, there was no follow-up on whether PPI deprescribing efforts were successful in those patients who were deprescribed or began to be tapered off the medication. Next, historical information on when patients had initiated medications was unavailable. As such, the assessment of appropriateness of the duration of therapy for a given indication was not performed. Additional limitations are the short study duration, the small number of patients, and that all hospital wards were not sampled.
Conclusion and Relevance
In summary, hospitalized elderly patients have a high degree of polypharmacy, and there are missed opportunities for deprescribing PPIs during acute hospitalizations. Minimizing PPI use and polypharmacy is important to optimize the health of elderly patients. We encourage hospitals to craft local strategies that include medication reconciliation, documentation of PPI indication and duration, and deprescribing.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded jointly by Canadian Frailty Network (Technology Evaluation in the Elderly Network), which is supported by the Government of Canada through the Networks of Centers of Excellence (NCE) program, and the Savlov/Schmidt Scholars Program at Mount Sinai Hospital.
ORCID iD: Nishila Mehta  https://orcid.org/0000-0002-6804-5075
https://orcid.org/0000-0002-6804-5075
References
- 1. Mansoon N, Shakib S, Kalisch-Ellet L, Caughey GE. What is polypharmacy? A systematic review of definitions. BMC Geriatr. 2017;17:230. doi: 10.1186/s12877-017-0621-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shah BM, Hajjar ER. Polypharmacy, adverse drug reactions and geriatric syndromes. Clin Geriatr Med. 2012;28:173-186. doi: 10.1016/j.cger.2012.01.002 [DOI] [PubMed] [Google Scholar]
- 3. Kelly OB, Dillane C, Patchett SE, Harewood GC, Murray FE. The inappropriate prescription of oral proton pump inhibitors in the hospital setting: a prospective cross-sectional study. Dig Dis Sci. 2015;60:2280-2286. doi: 10.1007/s10620-015-3642-8 [DOI] [PubMed] [Google Scholar]
- 4. Heidelbaugh JJ, Kim AH, Change R, Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol. 2012;5:219-232. doi: 10.1177/1756283X12437358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nauton M, Peterson GM, Deeks LS, Young H, Kosari S. We have had a gutful: the need for deprescribing proton pump inhibitors. J Clin Pharm Ther. 2018;43:65-72. doi: 10.1111/jcpt.12613 [DOI] [PubMed] [Google Scholar]
- 6. Heidelbaugh JJ, Goldberg KL, Inadomi JM. Overutilization of proton pump inhibitors: a review of cost-effectiveness and risk [corrected]. Am J Gastroenterol. 2009;104(suppl 2):S27-S32. doi: 10.1038/ajg.2009.49 [DOI] [PubMed] [Google Scholar]
- 7. Maes ML, Fixen DR, Linnebur SA. Adverse effects of proton-pump inhibitor use in older adults: a review of the evidence. Ther Adv Drug Saf. 2017;8:273-297. doi: 10.1177/2042098617715381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. American Geriatrics Society 2019 Updated AGE Beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc. 2019;67:674-694. doi: 10.1111/jgs.15767 [DOI] [PubMed] [Google Scholar]
- 9. Canadian Association of Gastroenterology. Five things physicians and patients should question. https://choosingwiselycanada.org/gastroenterology/. Accessed April 20, 2019.
- 10. Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton-pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152:706-715. doi: 10.1053/j.gastro.2017.01.031 [DOI] [PubMed] [Google Scholar]
- 11. Cahir C, Fahey T, Telijeur C, Bennett K. Proton pump inhibitors: potential cost reductions by applying prescribing guidelines. BMC Health Serv Res. 2012;12:408. doi: 10.1186/1472-6963-12-408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Choosing Wisely Canada. Toolkit: Bye-bye, PPI. https://choosingwiselycanada.org/perspective/ppi-toolkit/. Published May 8, 2017. Accessed May 30, 2019.
- 13. Farrell B, Pottie K, Thompson W, et al. Deprescribing proton pump inhibitors: evidence-based clinical practice guideline. Can Fam Physician. 2019;63:354-364. [PMC free article] [PubMed] [Google Scholar]
- 14. Moayeddi P, Eikelboom JW, Bosch J, et al. Safety of proton pump inhibitors based on a large, multi-year randomized control trial of patients receiving rivaroxaban or aspirin. Gastroenterology. 2019;157:682-691.e2. doi: 10.1053/j.gastro.2019.05.056 [DOI] [PubMed] [Google Scholar]
- 15. Cohen SH, Gerding DN, Johnson S, et al. Clinical practice guidelines for Clostridium difficile infection in adults: 2010 update by the Society for Healthcare Epidemiology of America (SHEA) and the Infectious Diseases Society of America (IDSA). Infect Control Hosp Epidemiol. 2010;31:431-455. doi: 10.1086/651706 [DOI] [PubMed] [Google Scholar]
- 16. Siddiqui AH, Siddiqui F. Curling Ulcer (Stress-Induced Gastric). Treasure Island, FL: StatPearls Publishing; 2019. https://www.statpearls.com/as/gastrointestinal/20173/. Accessed November 29, 2019. [Google Scholar]
- 17. Mohebbi L, Hesch K. Stress ulcer prophylaxis in the intensive care unit. Proc (Bayl Univ Med Cent). 2009;22:373-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wildson TD, Hendrix I, Thynne TR, Mangoni AA. Effectiveness of interventions to deprescribe inappropriate proton pump inhibitors in older adults. Drugs Aging. 2017;34:265-287. doi: 10.1007/s40266-017-0442-1 [DOI] [PubMed] [Google Scholar]
- 19. McDonald EG, Wu PE, Rashidi B, et al. The MedSafer study: a controlled trial of an electronic decision support tool for deprescribing in acute care. J Am Geriatr Soc. 2019;67:1843-1850. doi: 10.1111/jgs.16040 [DOI] [PubMed] [Google Scholar]