Abstract
Objectives: To assess the efficacy and safety of a methylphenidate hydrochloride extended-release capsule (MPH-MLR) formulation in treating attention-deficit/hyperactivity disorder (ADHD) in preschool children.
Methods: Children aged 4 to <6 years with qualifying ADHD Rating Scale Fourth Edition (ADHD-RS-IV) Preschool Version scores (≥90th percentile for age/gender) participated in four behavior management training (BMT) sessions or immediately entered (based on investigator assessment of symptom severity or previous participation) into a 6-week, open-label, flexible MPH-MLR dose optimization phase. After BMT, children with <30% improvement in ADHD-RS-IV score and ≥3 score on the Clinical Global Impression—Improvement (CGI-I) scale also entered the open-label period. All children began the open-label period with MPH-MLR 10 mg once daily; weekly adjustments permitted once-daily maximum of up to 40 mg. Children with ≥30% improvement in ADHD-RS-IV total score and a CGI-I score of 1–2 at open-label completion were randomized to their optimized dose of MPH-MLR or placebo for 2 weeks (double blind [DB]). Safety measures included adverse events (AEs), vital signs, and electrocardiograms.
Results: Open-label enrollment was 119 children. Mean (SD) ADHD-RS-IV total scores at open-label start and open-label end was 40.8 (10.4) and 19.5 (11.1), respectively. Ninety children were enrolled in the DB phase. Mean (SD) ADHD-RS-IV total scores for the MPH-MLR and placebo group were similar at DB beginning and was 25.8 (14.6) and 34.9 (14.1), respectively, at DB end. Mean change from baseline in ADHD-RS-IV total score during DB was significantly greater in children randomized to placebo compared with MPH-MLR; least squares mean change difference from baseline was −11.2, p = 0.002. During open-label dosing, the most common AEs (≥10%) were decreased appetite, decreased weight, insomnia, hypertension, emotional disorder, and affect lability.
Conclusion: Results demonstrate MPH-MLR efficacy in preschool children and a safety profile consistent with known AEs of methylphenidate when used for ADHD.
Keywords: ADHD, preschool, methylphenidate extended-release, MPH-MLR
Introduction
Evidence-based management of attention-deficit/hyperactivity disorder (ADHD) in children of preschool age is an emerging priority for clinicians and regulatory agencies (Center for Drug Evaluation and Research 2016). The reported prevalence of ADHD in preschool children has been estimated to range from 0.5% to 12.2% (Suvarna and Kamath 2009; Baker et al. 2010; Brault and Lacourse 2012; Wichstrom et al. 2012; Nomura et al. 2014; Ezpeleta and Granero 2015; Danielson et al. 2017). In children <6 years of age, ADHD is the most common psychiatric reason for referral to a specialized child and adolescent psychiatry clinic (Gadow et al. 2001; Wilens et al. 2002; Lumu et al. 2015), and is associated with substantial burden for children and their families. More than 40% of preschoolers who meet ADHD diagnostic criteria are suspended from school or daycare, compared with only 0.5% of children without ADHD; ∼16% are expelled (Egger and Angold 2006).
Well-investigated treatment options for preschoolers with ADHD are limited. Dextroamphetamine is the only medication approved by the U.S. Food and Drug Administration (FDA) to treat ADHD in children in this age group; however, randomized controlled trials supporting its efficacy and safety are lacking (Wolraich et al. 2011). In its 2011 guidance, the American Academy of Pediatrics (AAP) Clinical Practice Guidelines for the Diagnosis, Evaluation and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents recommended behavior therapy as first-line treatment in 4- to 5-year-olds, and treatment with methylphenidate (MPH) if ADHD symptoms do not improve significantly with behavior therapy (Wolraich et al. 2011). These recommendations were advanced despite the lack of an FDA-approved MPH product, based on placebo-controlled data demonstrating the efficacy and safety of immediate-release (IR) MPH in children aged 4 to 5 years in the National Institute of Mental Health-funded Preschool ADHD Treatment Study (PATS) (Greenhill et al. 2006; Kollins et al. 2006; Abikoff et al. 2007).
Many child psychiatrists do not follow the AAP guidelines. A survey that included 339 board-certified child and adolescent psychiatrists found that only 7.4% of practitioners first used behavioral therapy in preschool children (Chung et al. 2016). Physicians cited physical safety and educational concerns as the most important reasons to begin immediate pharmacological treatment. Therefore, it is important for clinicians to have data to support treatment choices.
Many children of preschool age are treated with extended duration stimulant formulations, despite the fact that these medications are not FDA approved for 4- and 5-year-olds. Prescription data from Oregon Medicaid in 2012, after release of the AAP guidelines for children <6 years of age with ADHD, showed that 91.4% of prescriptions for ADHD treatment in children <6 years of age were off-label (Panther et al. 2017). Of the MPH prescriptions in this population, 457 were for IR MPH and 459 were for extended-release (ER) MPH. Yet, class labeling for all MPH formulations begins at age 6. Consequently, randomized controlled trials for ER MPH in preschoolers are needed to provide essential efficacy and safety data for this vulnerable population, and are now required by FDA.
Methylphenidate hydrochloride ER capsules (Aptensio XR®; Rhodes Pharmaceuticals L.P., Coventry, RI), formulated as multiple-layer release (MLR) beads in hard gelatin capsules (methylphenidate hydrochloride extended-release capsules [MPH-MLR]), received FDA approval in April 2015 for treatment of ADHD in children ≥6 years of age to manage ADHD symptomatology (Aptensio XR® [methylphenidate extended release] 2017); approval was based on efficacy persisting from Hour 1 postdose to Hour 12 postdose (Wigal et al. 2014, 2015). After oral dosing, MPH-MLR exhibits a biphasic plasma profile by achieving a first peak concentration in a similar manner to IR MPH, and a second peak concentration at ∼6 to 8 hours (Adjei et al. 2014).
In response to the FDA mandate for additional studies of MPH in preschool-age children, and consistent with the AAP guidelines for treatment of ADHD in this population, this study was undertaken to evaluate the safety and efficacy of MPH-MLR in preschool children who did not benefit from ∼4 weeks of behavior management training (BMT; NCT02683265).
Methods
Study design
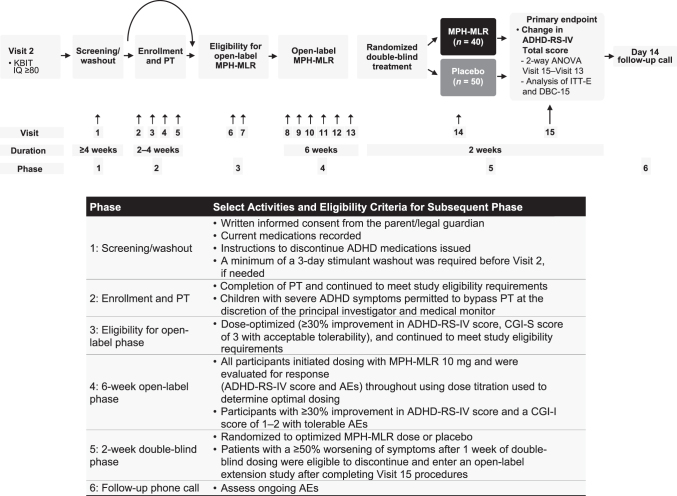
The study was a randomized, double-blind (DB), flexible-dose, placebo-controlled, parallel-group design conducted in six sequential phases (Fig. 1) at 11 U.S. sites. Phase 1 was screening/washout and lasted up to 28 days. A semistructured interview, the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children—Present and Lifetime version (K-SADS-PL) (Birmaher et al. 2009) was administered by trained clinicians to confirm the diagnosis of ADHD and identify comorbid psychiatric conditions. If the child had previously received stimulant medication, a minimum of 3-day washout period was required before the child entered Phase 2.
FIG. 1.
Study design. ADHD, attention-deficit/hyperactivity disorder; ADHD-RS-IV, ADHD Rating Scale Fourth Edition; AE, adverse event; ANOVA, analysis of variance; CGI-I, Clinical Global Impression—Improvement; CGI-S, Clinical Global Impression—Severity; DBC-15, children in the double-blind compliant population who had 15 ± 3 days of participation in the double-blind phase of the study; ITT-E, intention-to-treat—efficacy evaluable; KBIT, Kaufman Brief Intelligence Test; MPH-MLR, methylphenidate hydrochloride extended-release capsules; PT, parental behavior training.
BMT was required (four visits ≥90-minute duration) during a 2- to 4-week period; the primary caregivers were the primary attendees for these sessions. The content for this training was adapted from empirically validated interventions (Cunningham et al. 1995; Barkley 1997; Wells et al. 2000). Waivers for BMT were available if the child and primary caregiver had, in the previous 12 months, participated in a documented course of nonpharmacological treatment with minimal benefit, or the child exhibited ADHD symptoms and impairment that warranted immediate pharmacological treatment, as assessed by the investigator. Evidence for a waiver was reviewed and approved by the study's principal investigator and medical monitor.
Phase 3 began with two baseline visits after completion of BMT. During the first baseline visit, study eligibility was confirmed and the ADHD Rating Scale Fourth Edition (ADHD-RS-IV) Preschool Version (McGoey et al. 2007), the Clinical Global Impression—Severity (CGI-S), and Clinical Global Impression—Improvement (CGI-I) (Guy 1976) assessments were administered. Continuation to the open-label Phase 4 required that children had a <30% improvement from screening in the ADHD-RS-IV Preschool Version total score (Birmaher et al. 2009) and a CGI-I score of ≥3 (minimally improved or less) after BMT. If continued eligibility was confirmed, medical evaluation, including vital signs and collection of blood for laboratory assessment, was completed and study drug was dispensed.
During the 6-week, open-label Phase 4, treatment was initiated for each child with a once-daily dose of MPH-MLR 10 mg administered in the morning. At weekly visits, a child's current dose was maintained, increased, or decreased based on clinician assessment of response and reported adverse events (AEs), until an optimized dose or the protocol-specified maximum dose of 40 mg was reached. When compared with scores recorded just before Phase 4 entry, the optimal dose was defined as the dose associated with a reduction of ADHD symptoms ≥30%, a CGI-I score of “much improved” or “very much improved,” and tolerable AEs.
Children who achieved an optimized dose during Phase 4 were eligible to participate in the 2-week, parallel-arm, DB Phase 5. Children were randomized in a 1:1 ratio through a computer-generated randomization schedule to either continue their optimized dose or receive matching placebo. Children who after 1 week of DB dosing had a ≥50% worsening of symptoms from the start of Phase 5 (based on their ADHD-RS-IV total score) and a CGI-I of “much worse” or “very much worse” compared with the start of Phase 5 were eligible to discontinue the DB phase and enter the open-label extension study.
At the end of the 2-week, DB period, ADHD-RS-IV Preschool Version, CGI-S, CGI-I, physical examination, and electrocardiogram were repeated and AEs were recorded. Children for whom these procedures were not completed were ineligible to continue with the open-label extension study. Children who completed all of Phase 5 and continued to meet all inclusion/exclusion criteria were eligible to enter the 12-month, open-label extension safety study. A follow-up call (Phase 6), during which ongoing AEs and concomitant medications were recorded, occurred ∼2 weeks after discontinuation of DB treatment, unless the child was eligible and enrolled in the open-label extension study.
This study was conducted in accordance with the ethical principles that originate from the Declaration of Helsinki and consistent with the International Conference on Harmonisation of Technical Requirements for Registration for Pharmaceuticals for Human Use guidelines on good clinical practice and regulatory requirements, as applicable.
Participants
Male and female children aged 4 years and 0 months to 5 years and 8 months were enrolled if they met the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, criteria for ADHD with symptoms present for ≥6 months. Diagnosis was confirmed with the K-SADS-PL. Children must have also had age- and sex-adjusted ratings of ≥90th percentile total score on the ADHD-RS-IV Preschool Version (rated over the previous 6 months), a Child Global Assessment Scale (Shaffer et al. 1983) score of <65, a CGI-S score ≥4, and an IQ ≥80, as assessed using the Kaufman Brief Intelligence Test, Second Edition (Kaufman and Kaufman 2004). Blood pressure (BP; systolic and diastolic) was required to be <95th percentile for age and gender.
Children were excluded from participation if they showed a lack of response to a trial of adequate dose and duration of MPH and intolerance to previous MPH treatment; were receiving psychotropic medication other than clonidine, guanfacine, atomoxetine, and/or stimulants; had taken an investigational drug in the 30 days before screening; or had used monoamine oxidase inhibitors within 14 days before screening.
Treatment
Doses of MPH-MLR permitted by protocol in Phases 4 and 5 of this study were 10, 15, 20, 30, and 40 mg. Nonsedating antihistamines, acetaminophen, ibuprofen, antibiotics for treatment of a minor illness, and vitamins were permitted during the study. Children participating in psychotherapy before study initiation were permitted to continue; however, initiation of psychotherapy was not permitted during the study.
Endpoints
The primary efficacy endpoint was change in ADHD-RS-IV total score during the DB phase. Secondary endpoints were change in ADHD-RS-IV hyperactivity/impulsivity and inattention subscales, change in CGI-S and CGI-I ratings, and change in the Conners Early Childhood Behavior—Parent Short Response scale (Conners 2009).
Safety evaluations included the profile and frequency of AEs, clinical laboratory evaluations (hematology, serum chemistry, and urinalysis), physical examinations (including height and body weight), vital signs (temperature, BP, and heart rate), electrocardiogram, the Columbia Suicide Severity Rating Scale, and the Children's Sleep Habits Questionnaire (CSHQ) (Owens et al. 2000). Specific guidance was provided for the measurement of BP: (1) BP had to be recorded after 5 minutes of rest in a sitting position; (2) manual or automated BP systems were permitted; (3) interpretation of on-study BP used the Department of Health and Human Services pocket guide (National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents 2007); and (4) individual instances of BP measurement that met the criteria for hypertension were to be recorded as an AE.
Analysis
Several populations were defined for purposes of data analysis: (1) safety population, including all children receiving at least one dose or partial dose of study treatment; (2) intention-to-treat (ITT) population, including all children randomized to treatment in the DB Phase 5; and (3) ITT—efficacy evaluable (ITT-E) population, including all children in the ITT population who completed ADHD-RS-IV assessments at the end of the open-label Phase 4 (DB phase baseline) and had at least one postbaseline ADHD-RS-IV assessment.
Post hoc analyses examined data in the population of DB compliant (DBC) subjects—that is, children in the ITT-E population with nonmissing ADHD-RS-IV total scores for Visits 13, 14, and 15. The DBC-15 population included those subjects in the DBC with 15 ± 3 days of participation in Phase 5. These analyses were intended to explore the possible effects of protocol-specified permission for children to withdraw from the DB phase before completing 2 weeks on DB treatment.
Sample size determination was based on a two-sample t-test, assuming a mean increase from baseline in the ADHD-RS-IV total score of 1.5 in the MPH-MLR arm and 10 in the placebo arm, and assuming a standard deviation of 11 for the mean change in either group. Enrollment of 74 children (37 in each treatment group) would result in 90% power to detect a treatment difference of −8.5 points on the ADHD-RS-IV total score at a two-sided significance level of 0.05.
Analysis of the primary efficacy endpoint was through a two-way analysis of variance (ANOVA), using each child's change in ADHD-RS-IV total score during the DB phase. Statistical tests were two-sided and values of p ≤ 0.05 were considered statistically significant. The ANOVA model included two fixed factors, treatment group and investigative site; the model also included a term for treatment by site interaction. The dependent variable in the ANOVA model was the change in ADHD-RS-IV total score from baseline to the last postbaseline ADHD-RS-IV total score available for each subject. Sites with fewer than 10 subjects in the analysis population were combined into a single site for purposes of data analysis. There was no imputation of missing values of ADHD-RS-IV total scores in the primary analysis.
Analyses of the ADHD-RS-IV subscale scores and the Conners Early Childhood Behavior—Parent Short Response score were conducted the same way as for the ADHD-RS-IV total score. Analysis of the change in CGI-S from the end of the open-label phase to the end of the DB phase was using the Cochran–Mantel–Haenszel chi-square test.
Our effect size (ES) calculation was based on a study that demonstrated that a change of 10 to 15 points in ADHD-RS-IV total scores corresponded to a change of one level in CGI-I rating (Goodman et al. 2010), and on calculating the difference between treatment groups in change from baseline during Phase 5. The ES calculation was supported by the additional calculation of dividing the mean difference measurement by the square root of the measurement variance.
Results
Participants
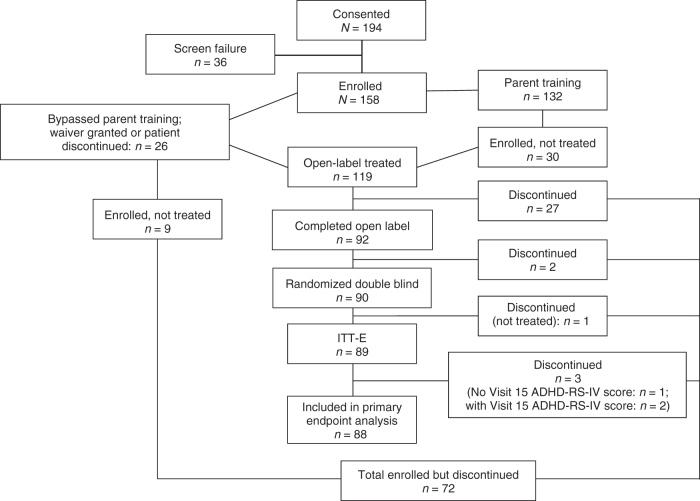
Of the 194 children screened, 132 entered the enrollment and parent training phase, 128 entered the eligibility for open-label treatment phase, 119 entered open-label treatment, and 90 were randomized to DB treatment (40 to MPH-MLR, 50 to placebo; Fig. 2). Twenty children bypassed the BMT. Baseline demographics of enrolled children are given in Table 1. In the safety population (N = 119), the most common ADHD diagnosis subtype was combined with 88.0% and 89.9% reported for the placebo and MPH-MLR treatment groups, respectively. The percentages of children with hyperactive/impulsive subtype were 12.0% and 8.4% in the placebo and MPH-MLR treatment groups, respectively. A small percentage of children (1.7%) in the MPH-MLR treatment group were diagnosed with the inattentive subtype. Mean (SD) ADHD-RS-IV total scores at open-label start and open-label end were 40.8 (10.4) and 19.5 (11.1), respectively. The median optimized dose during the open-label phase was 30 mg (mean, 27.5 mg).
FIG. 2.
Disposition. ADHD-RS-IV, Attention-Deficit/Hyperactivity Disorder Rating Scale Fourth Edition; ITT-E, intention-to-treat—efficacy evaluable.
Table 1.
Baseline Demographics and Characteristics
| Parameter | ITT population |
Enrolled population |
||
|---|---|---|---|---|
| MPH-MLR (n = 40) | Placebo (n = 50) | Overall (N = 90) | Overall (N = 158) | |
| Age, months | ||||
| Mean (SD) | 58.5 (5.6) | 59.2 (6.4) | 58.9 (6.1) | 58.8 (6.2) |
| Median (Q1, Q2) | 57.0 (55.0, 63.5) | 59.0 (54.0, 64.0) | 59.0 (55.0, 64.0) | 58.5 (53.0, 64.0) |
| Minimum, maximum | 49, 69 | 48, 70 | 48, 70 | 48, 70 |
| Sex, n (%) | ||||
| Male | 29 (72.5) | 39 (78.0) | 68 (75.6) | 121 (76.6) |
| Female | 11 (27.5) | 11 (22.0) | 22 (24.4) | 37 (23.4) |
| Race, n (%) | ||||
| White | 24 (60.0) | 30 (60.0) | 54 (60.0) | 82 (51.9) |
| Black or African American | 15 (37.5) | 18 (36.0) | 33 (36.7) | 70 (44.3) |
| Asian | 0 | 1 (2.0) | 1 (1.1) | 1 (0.6) |
| Other | 1 (2.5) | 1 (2.0) | 2 (2.2) | 4 (2.5) |
| Hispanic or Latino | 5 (12.5) | 6 (12.0) | 11 (12.2) | 17 (10.8) |
| Characteristic | ||||
| Mean (SD) height, cm | 110.4 (4.9) | 110.8 (5.8) | 110.6 (5.4) | 110.9 (5.4)a |
| Mean (SD) weight, kg | 20.2 (3.1) | 20.5 (3.8) | 20.3 (3.4) | 20.4 (3.4)a |
| Mean (SD) body mass index, kg/m2 | 16.5 (1.7) | 16.6 (2.4) | 16.6 (2.2) | 16.5 (2.0)a |
n = 157.
ITT, intention-to-treat; MPH-MLR, methylphenidate hydrochloride extended-release capsules.
Primary endpoint
Mean (SD) ADHD-RS-IV total scores at the beginning of the DB period (Visit 13) were similar in children randomized to MPH-MLR (19.5 [11.1]) and placebo (17.8 [9.9]; p = 0.309). Mean (SD) ADHD-RS-IV total scores for the MPH-MLR and placebo group were 25.8 (14.6) and 34.9 (14.1), respectively, at DB end.
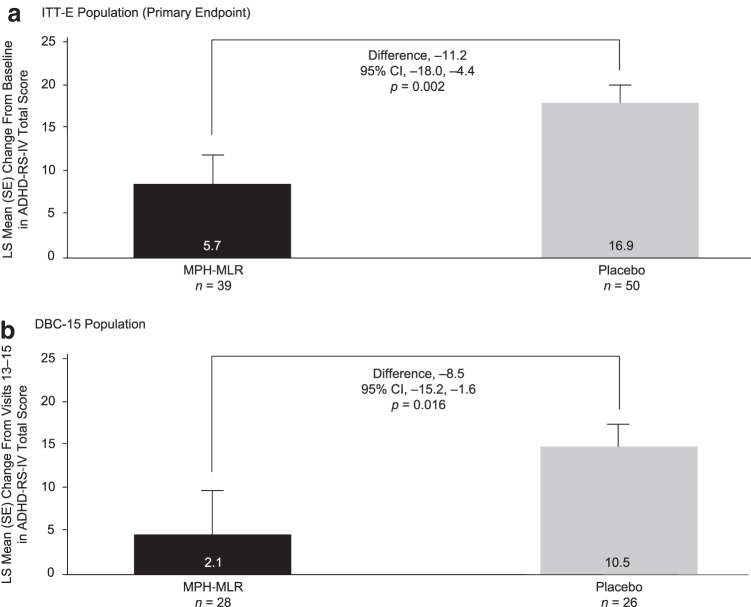
For the ITT-E population, the mean change from baseline in ADHD-RS-IV total score during the DB Phase 5 was significantly greater in children randomized to placebo compared with MPH-MLR. The difference in the least squares mean change from baseline (MPH-MLR group least squares mean change from baseline minus placebo group least squares mean change from baseline) was −11.2 (95% confidence interval [CI] = −18.0 to −4.4; p = 0.002; Fig. 3a), meaning that the loss of pre-established treatment effect after open-label treatment with MPH-MLR was significantly greater in children randomized to placebo than those randomized to continue on MPH-MLR. The ES was −1.1, slightly more than a minimally clinically significant difference in ADHD-RS-IV total score in favor of the MPH-MLR group. When this analysis was repeated in the DBC-15 population, the difference in least squares mean change from baseline in ADHD-RS-IV total scores during Phase 5 was also significantly greater in children randomized to placebo compared with MPH-MLR (Fig. 3b).
FIG. 3.
Primary endpoint: change in ADHD-RS-IV total score from baseline (Visit 13) to Visit 15 (end of double-blind Phase 5) in (a) the ITT-E population and (b) the DBC-15 population. The smaller change in the MPH-MLR group suggests that symptom control was better maintained in the MPH-MLR group versus the placebo group. ADHD-RS-IV, Attention-Deficit/Hyperactivity Disorder Rating Scale, Fourth Edition; CI, confidence interval; DBC-15, children in the double-blind compliant population who had 15 ± 3 days of participation in the double-blind phase of the study; ITT-E, intention-to-treat—efficacy evaluable; LS, least squares; MPH-MLR, methylphenidate hydrochloride extended-release capsules.
Secondary efficacy endpoints
In the ITT-E population, mean change from baseline in ADHD-RS-IV hyperactivity/impulsivity and inattention scores during the DB Phase 5 were significantly greater in children randomized to placebo compared with MPH-MLR; the difference in the least squares mean change from baseline (MPH-MLR group least squares mean change from baseline minus placebo group least squares mean change from baseline) was hyperactivity/impulsivity −6.2 (95% CI = −9.8 to −2.6; p = 0.001) and inattention −5.0 (95% CI = −8.4 to −1.6; p = 0.005).
CGI-S was evaluated at Visit 13, baseline for the DB Phase 5, and again at the end of Phase 5 (Visit 15; Table 2). Analysis of each treatment group at the end, relative to the beginning of Phase 5, indicated that children in both treatment groups had significantly higher severity ratings at Visit 15; the higher severity rating in the MPH-MLR group is likely an artifact of the study design, which permitted children to opt out of the DB phase before completing 2 weeks of treatment and enter open-label treatment if they had significant worsening of ADHD symptoms. In the DBC-15 population, between-group analysis indicated no significant difference between treatment groups at Visit 13; at Visit 15, the MPH-MLR group had significantly lower CGI-S rating than placebo. At Visit 15, the placebo group had a significantly higher CGI-S rating than at Visit 13.
Table 2.
Summary of Clinical Global Impression—Severity Ratings at Beginning and End of the Double-Blind Phase 5
| Number (%) with the rating | ITT-E |
DBC-15 |
||
|---|---|---|---|---|
| MPH-MLR (n = 39) | Placebo (n = 50) | MPH-MLR (n = 28) | Placebo (n = 26) | |
| CGI-S (Visit 13) | ||||
| 1 = Normal, not at all ill | 7 (17.9) | 13 (26.0) | 5 (17.9) | 3 (11.5) |
| 2 = Borderline mentally ill | 15 (38.5) | 14 (28.0) | 10 (35.7) | 6 (23.1) |
| 3 = Mildly ill | 8 (20.5) | 11 (22.0) | 6 (21.4) | 6 (23.1) |
| 4 = Moderately ill | 7 (17.9) | 11 (22.0) | 6 (21.4) | 10 (38.5) |
| 5 = Markedly ill | 1 (2.6) | 1 (2.0) | 1 (3.6) | 1 (3.8) |
| 6 = Severely ill | 1 (2.6) | 0 | 0 | 0 |
| 7 = Among the most extremely ill subjects | 0 | 0 | 0 | 0 |
| CGI-S (Visit 15) | ||||
| 1 = Normal, not at all ill | 4 (10.3) | 3 (6.0) | 4 (14.3) | 2 (7.7) |
| 2 = Borderline mentally ill | 12 (30.8) | 5 (10.0) | 11 (39.3) | 4 (15.4) |
| 3 = Mildly ill | 5 (12.8) | 4 (8.0) | 5 (17.9) | 4 (15.4) |
| 4 = Moderately ill | 6 (15.4) | 10 (20.0) | 5 (17.9) | 6 (23.1) |
| 5 = Markedly ill | 10 (25.6) | 19 (38.0) | 2 (7.1) | 8 (30.8) |
| 6 = Severely ill | 2 (5.1) | 7 (14.0) | 1 (3.6) | 2 (7.7) |
| 7 = Among the most extremely ill subjects | 0 | 2 (4.0) | 0 | 0 |
| Within-group Cochran–Mantel–Haenszel statistic: row mean scores difference p-valuea | 0.021 | <0.0001 | 0.586 | 0.039 |
Cochran–Mantel–Haenszel p-value for the comparison within the indicated treatment group of Visit 15 to Visit 13 distribution of CGI-S ratings across the seven possible responses.
CGI-S, Clinical Global Impression—Severity scale; DBC-15, children in the double-blind compliant population who had 15 ± 3 days of participation in the double-blind phase of the study; ITT-E, intention-to-treat—efficacy evaluable; MPH-MLR, methylphenidate hydrochloride extended-release capsules.
Between-group analysis of the MPH-MLR and placebo groups at Visit 15 indicated that the CGI-I rating was significantly worse in the placebo group relative to the MPH-MLR group in the ITT-E population (Table 3). This difference in CGI-I rating was not mirrored in the DBC-15 populations, which may be related to the reduced sample size relative to the ITT-E population. Analysis of Conners Early Childhood Behavior—Parent Short Response T-Scores from baseline to Visit 15 did not show any significant differences between MPH-MLR and placebo in either the ITT-E or DBC-15 populations (data not shown).
Table 3.
Summary of Clinical Global Impression-Improvement Ratings at Beginning and End of the Double-Blind Phase 5
| Number (%) with the rating | ITT-E |
DBC-15 |
||
|---|---|---|---|---|
| MPH-MLR (n = 39) | Placebo (n = 50) | MPH-MLR (n = 28) | Placebo (n = 26) | |
| CGI-I (Visit 15) | ||||
| 1 = Very much improved | 6 (15.4) | 5 (10.0) | 5 (17.9) | 3 (11.5) |
| 2 = Much improved | 8 (20.5) | 6 (12.0) | 8 (28.6) | 6 (23.1) |
| 3 = Minimally improved | 3 (7.7) | 4 (8.0) | 3 (10.7) | 2 (7.7) |
| 4 = No change | 11 (28.2) | 13 (26.0) | 10 (35.7) | 10 (38.5) |
| 5 = Minimally worse | 4 (10.3) | 1 (2.0) | 2 (7.1) | 1 (3.8) |
| 6 = Much worse | 6 (15.4) | 16 (32.0) | 0 | 4 (15.4) |
| 7 = Very much worse | 1 (2.6) | 5 (10.0) | 0 | 0 |
| Between-group Cochran–Mantel–Haenszel statistic: row mean scores difference p-valuea | 0.045a | 0.128b | ||
Cochran–Mantel–Haenszel p-value for the comparison of the MPH-MLR treatment group to the placebo group distribution of CGI-S ratings across the seven possible responses at the given time point.
Cochran–Mantel–Haenszel p-value for the comparison of the MPH-MLR treatment group to the placebo group distribution of CGI-I ratings across the 7 possible responses at the given time point.
CGI-I, Clinical Global Impression—Improvement scale; DBC-15, children in the double-blind compliant population who had 15 ± 3 days of participation in the double-blind phase of the study; ITT-E, intention-to-treat—efficacy evaluable; MPH-MLR, methylphenidate hydrochloride extended-release capsules.
Safety
The profile of AEs was typical of that previously reported in children with ADHD treated with MPH. During the 6-week, open-label Phase 4, 95 (79.8%) children had at least one treatment-emergent adverse event (TEAE), most of which related to study treatment, which were mild or moderate in severity. The most common TEAEs considered to be either possibly or probably related to treatment were decreased appetite (18.5%), insomnia (15.1%), decreased weight (14.2%), and irritability (13.4%), all of which were considered by the investigator to be mild or moderate in severity except for one instance of severe insomnia. One child experienced a serious AE of Campylobacter infection during the open-label phase that was considered unrelated to study treatment. No deaths were reported during open-label treatment. No clinically relevant instances of abnormal laboratory values were observed.
During the 2-week, DB Phase 5, TEAEs were experienced by 10 (25.6%) children treated with MPH-MLR and 6 (12.0%) children treated with placebo (Table 4). The most commonly reported AEs considered to be either possibly or probably related to MPH-MLR were hypertension (7.7%), and emotional poverty, negativism, pollakiuria, onychophagia, decreased appetite, and tachycardia (2.6% each). All the TEAEs in the DB phase were deemed to be either mild or moderate in severity, except for a TEAE of severe negativism in one subject. No subjects experienced a serious AE during the DB phase and no deaths occurred.
Table 4.
Summary of the Most Common Treatment-Emergent Adverse Events (Incidence ≥2%) Encountered During the 2-Week Double-Blind Phase 5 (Safety Population)
| System organ class preferred term | MPH-MLR (n = 39) |
Placebo (n = 50) |
||
|---|---|---|---|---|
| n (%) | No. of events | n (%) | No. of events | |
| Any TEAE | 10 (25.6) | 14 | 6 (12.0) | 9 |
| Psychiatric disorders | ||||
| Emotional disorder | 0 | 0 | 1 (2.0) | 1 |
| Onychophagia | 1 (2.6) | 1 | 0 | 0 |
| Emotional poverty | 1 (2.6) | 1 | 0 | 0 |
| Negativism | 1 (2.6) | 1 | 0 | 0 |
| Infections and infestations | ||||
| Upper respiratory tract infection | 2 (5.1) | 2 | 0 | 0 |
| Cellulitis | 0 | 0 | 1 (2.0) | 1 |
| Sinusitis | 0 | 0 | 1 (2.0) | 1 |
| Metabolism and nutrition disorders | ||||
| Decreased appetite | 1 (2.6) | 1 | 0 | 0 |
| Gastrointestinal disorders | ||||
| Vomiting | 0 | 0 | 1 (2.0) | 1 |
| Gastritis | 1 (2.6) | 1 | 0 | 0 |
| Vascular disorders | ||||
| Hypertension | 3 (7.7) | 3 | 0 | 0 |
| General disorders and administration site conditions | ||||
| Pyrexia | 0 | 0 | 1 (2.0) | 1 |
| Adverse event | 1 (2.6) | 1 | 0 | 0 |
| Musculoskeletal and connective tissue disorders | ||||
| Pain in extremity | 0 | 0 | 1 (2.0) | 2 |
| Renal and urinary disorders | ||||
| Pollakiuria | 1 (2.6) | 1 | 0 | 0 |
| Urinary incontinence | 1 (2.6) | 1 | 0 | 0 |
| Injury, poisoning, and procedural complications | ||||
| Injury corneal | 0 | 0 | 1 (2.0) | 1 |
| Skin and subcutaneous tissue disorders | ||||
| Rash | 0 | 0 | 1 (2.0) | 1 |
| Cardiac disorders | ||||
| Tachycardia | 1 (2.6) | 1 | 0 | 0 |
MPH-MLR, methylphenidate hydrochloride extended-release capsules; TEAE, treatment-emergent adverse event.
The withdrawal of 13 subjects was attributed to TEAEs; 12 subjects withdrew from Phase 4 and one from Phase 5. At the time of withdrawal, five subjects were receiving MPH-MLR 15 mg, five subjects 20 mg, and three subjects 30 mg. One child had an emotional disorder (severe emotional dysregulation), which was considered severe and possibly treatment related by the investigator. The remaining TEAEs leading to withdrawal were considered mild or moderate in severity.
The CSHQ was used to assess the effect of MPH-MLR on sleep quality. During the 2-week, DB phase, the mean CSHQ total score increased by 0.2 for placebo and did not change for the MPH-MLR group, with no significant differences between the treatment groups (p = 0.858). The changes in subscale scores during the DB phase were minimal for both treatment groups, and no significant differences between groups were found.
Discussion
In this first randomized, placebo-controlled trial of an ER MPH formulation in preschool children aged 4 to <6 years, MPH-MLR dosages up to 40 mg were efficacious and well tolerated. During the open-label, dose-optimization phase, children whose MPH-MLR dose was optimized exhibited, on average, a 21.3-point reduction in their ADHD-RS-IV total score versus baseline, consistent with a robust improvement in ADHD symptoms (ES −1.1 for the DB Phase 5 and −2.7 for the dose-optimized, open-label Phase 4). This reduction in ADHD-RS-IV total score was maintained during the DB phase in children randomized to MPH-MLR but not placebo. ADHD-RS-IV total scores at baseline were not significantly different between treatment groups, indicating that the mean change in ADHD-RS-IV total score difference was not likely related to baseline severity. The safety profile of MPH-MLR in this study was consistent with what is already known about MPH use for ADHD, and it extends this information to the preschool population.
It is interesting to contrast the current findings with those in the National Institute of Mental Health-funded PATS. In PATS, 165 preschool children were treated with IR MPH in doses up to 22.5 mg, achieving a mean optimal dose of 14.2 ± 8.1 mg/day (0.7 ± 0.4 mg/kg/day) (Greenhill et al. 2006). Seven (4%) preschoolers were classified as nonresponders and 14 (8%) were classified as placebo responders. The ESs for MPH were 0.2–0.7 during the titration phase and 0.2–0.9 during the parallel phase. These ESs were smaller than those seen in school-aged children treated with MPH in the Multimodal Treatment Study of ADHD (Greenhill et al. 2001), and smaller than those observed in our study.
In PATS, AEs that were statistically more frequent on MPH than placebo included decreased appetite, insomnia, and weight loss during dose titration (Wigal et al. 2006). During the open-label phase of our study, we had a similar AE profile, which, with the exception of one report of severe insomnia, included AEs of mild or moderate severity. Of interest, in this study, the mean optimized dose was almost twice the mean optimal dose achieved during PATS (Greenhill et al. 2006). The IR component of MPH-MLR comprises ∼40% of the total dose (Aptensio XR® [methylphenidate extended release] 2017). In the PATS the 14 mg averaged optimized dose was divided into three daily doses. Thus, the initial IR MPH-MLR dose of 12 mg in this trial was ∼2.5 times the average PATS morning dose (Greenhill et al. 2006). Preschool children in our study tolerated the higher doses with a robust response comparable with what is seen when older children are administered MPH-MLR (Wigal et al. 2015). The ER component of MPH-MLR may have improved tolerability throughout the day.
We observed a relative increase in ADHD-RS-IV total score change from baseline in children randomized to MPH-MLR in the DB phase, not only those randomized to placebo. This slight worsening of symptoms is consistent with the negative effects of expectancy when children are transitioned from optimized active medication to randomized treatment with either active medication or placebo (Coghill et al. 2014).
In our study, preschool children had to fail BMT, considered the first-line treatment in the United States (Wolraich et al. 2011), before moving on to active dosing during the optimization phase. This suggests that children treated with MPH-MLR had impairments not adequately addressed with BMT.
In this study, MPH-MLR exerted minimal negative effects on sleep quality compared with placebo. It is possible that this finding is related to the fact that children with sleep problems either do not enroll in these studies or discontinued during the open-label titration phase. In two studies in older children and adolescents, optimizing the dose of MPH-MLR actually improved most CSHQ (or the adolescent version) measures (Owens et al. 2016).
Study limitations
This study was relatively brief, and sustained effects of treatment on longer term ADHD functioning cannot be inferred; this is an important direction for future research, given the chronic nature of ADHD. No standardized scale for assessment of TEAEs was used; rather, the presence of TEAEs was spontaneously reported. Although solicited reports of AEs have been shown to result in higher reporting rates, they have not led to improved detection of differences between treatment and placebo (Wernicke et al. 2005). In addition, although the CGI was used to assess global improvement, and found to be different between the drug and placebo groups, the primary focus on assessment of symptoms (ADHD-RS-IV), rather than other aspects of functioning, may have obscured other facets of functional impairment and their relationship to treatment. Of note, the relationship between symptom reduction and global improvement has been shown to be somewhat divergent in other clinical trials (Coghill et al. 2017; Kollins 2018; Weiss et al. 2018). Further investigation of the functional aspects of treatment response in the preschool population is therefore required.
Conclusions
MPH-MLR at doses up to 40 mg was efficacious and well tolerated in preschool children 4 to <6 years of age with ADHD. The safety profile of MPH-MLR in preschool children was consistent with the known safety profile of MPH in older children. These data are the first from a randomized, placebo-controlled trial to support the use of an ER MPH formulation, MPH-MLR, as an option for the clinical management of ADHD in preschool children when behavior therapy has not been successful.
Clinical Significance
These findings provide empirical data to support the use of MPH-MLR in young children with a verified diagnosis of ADHD. Of importance, to mirror recommended treatment guidelines for the preschool population, children in the trial participated in nonpharmacological treatment before enrolling (or were otherwise deemed eligible to initiate pharmacotherapy), and showed robust benefit from treatment, as measured by improvements in ADHD symptoms.
Acknowledgments
The authors acknowledge the contributions of all study participants, their parents/guardians, and the investigators. This study was funded by Rhodes Pharmaceuticals L.P. Medical writing support was provided by Linda Wagner, PharmD, Excel Scientific Solutions, and funded by Rhodes Pharmaceuticals L.P.
Authors' Contributions
Analyses were generally conducted by MedSource, Houston, TX; post hoc analyses were conducted by H.C.F.; data interpretation was provided by all authors. A.L.A. wrote the first draft and all authors provided input into revising the article and approved the final version for publication.
Disclosures
A.C.C.: research support from AEVI, Akili, Alcobra, Arbor, Forest Research Institute, Ironshore, KemPharm, Lilly USA, Lundbeck, Neos, Neurovance, Noven, Otsuka, Pearson, Pfizer, Purdue, Rhodes Pharmaceuticals L.P., Shire, Sunovion, Supernus, and Tris; consultant and/or advisory board member for and honoraria from AEVI, Akili, Arbor, Ironshore, KemPharm, Neos, Neurovance, NLS, Noven, Pfizer, Purdue, Rhodes Pharmaceuticals L.P., Shire, Sunovion, Supernus, and Tris; payment for lectures from Arbor, Neos, Pfizer, Shire, and Tris; and writing assistance on projects from Arbor, Ironshore, Neos, Pfizer, Purdue, Rhodes Pharmaceuticals L.P., Shire, Sunovion, and Tris. S.H.K.: research support and/or consulting fees from the following: Akili, Alcobra, Bose, Ironshore, Jazz, KemPharm, Medgenics, NLS, Purdue Canada, Rhodes Pharmaceuticals L.P., Shire, SK Life Science, and Sunovion. H.C.F.: consultant for Rhodes Pharmaceuticals L.P. J.H.N.: consultant/advisory board for Akili, Alcobra, Arbor, Cingulate, Enzymotec, Ironshore, KenPharm, Lundbeck, Medici, NLS, Pfizer, Rhodes, Shire, Sunovion, and Supernus; research support from Enzymotec, Otsuka, Shire, Supernus; honoraria for disease state lectures from Shire. G.M.: researcher for Akili, Alcobra, Alkermes, Allergan, Axsome, Boehringer, Forum, Genentech, Janssen, Lundbeck, Medgenics, NLS Pharma, Otsuka, Reckitt Benckiser, Roche, Sage, Shire, Sunovion, Supernus, Takeda, Taisho, and Teva; consultant for Alkermes, Allergan, Ironshore, Janssen, Lundbeck, Merck, Otsuka, Neos, NLS Pharma, Purdue, Rhodes Pharmaceuticals L.P., Shire, Sunovion, Takeda, Teva, and Vanda; speaker for Alkermes, Allergan, Janssen, Lundbeck, Merck, Otsuka, Neos, Shire, Sunovion, Takeda, and Teva. A.L.A. and R.J.K.: employees of Rhodes Pharmaceuticals L.P.
References
- Abikoff HB, Vitiello B, Riddle MA, Cunningham C, Greenhill LL, Swanson JM, Chuang SZ, Davies M, Kastelic E, Wigal SB, Evans L, Ghuman JK, Kollins SH, McCracken JT, McGough JJ, Murray DW, Posner K, Skrobala AM, Wigal T: Methylphenidate effects on functional outcomes in the Preschoolers with Attention-Deficit/Hyperactivity Disorder Treatment Study (PATS). J Child Adolesc Psychopharmacol 17:581–592, 2007 [DOI] [PubMed] [Google Scholar]
- Adjei A, Kupper RJ, Teuscher NS, Wigal S, Sallee FR, Childress A, Kollins S, Greenhill L: Steady-state bioavailability of methyphenidate extended-release (MPH-MLR) capsule versus methylphenidate immediate-release tablets (Ritalin®) in healthy adult volunteers. Clin Drug Investig 34:795–805, 2014 [DOI] [PubMed] [Google Scholar]
- Aptensio XR® [methylphenidate extended release]: Rhodes Pharmaceuticals L.P. January 2017. http://www.aptensioxr.com/resources/full-prescribing-information.pdf Accessed December20, 2018
- Baker BL, Neece CL, Fenning RM, Crnic KA, Blacher J: Mental disorders in five-year-old children with or without developmental delay: Focus on ADHD. J Clin Child Adolesc Psychol 39:492–505, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkley RA: Defiant Children: A Clinician's Manual for Assessment and Parent Training. New York, Guilford Press, 1997 [Google Scholar]
- Birmaher B, Ehmann M, Axelson DA, Goldstein BI, Monk K, Kalas C, Kupfer D, Gill MK, Leibenluft E, Bridge J, Guyer A, Egger HL, Brent DA: Schedule for affective disorders and schizophrenia for school-age children (K-SADS-PL) for the assessment of preschool children—A preliminary psychometric study. J Psychiatr Res 43:680–686, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault MC, Lacourse É: Prevalence of prescribed attention-deficit hyperactivity disorder medications and diagnosis among Canadian preschoolers and school-age children: 1994–2007. Can J Psychiatry 57:93–101, 2012 [DOI] [PubMed] [Google Scholar]
- Center for Drug Evaluation and Research: Letter regarding NDA 205831. May 11, 2016. https://www.fda.gov/downloads/Drugs/DevelopmentApprovalProcess/DevelopmentResources/UCM634218.pdf Accessed April1, 2019
- Chung J, Tchaconas A, Meryash D, Adesman A: Treatment of attention-deficit/hyperactivity disorder in preschool-age children: Child and adolescent psychiatrists' adherence to clinical practice guidelines. J Child Adolesc Psychopharmacol 26:335–343, 2016 [DOI] [PubMed] [Google Scholar]
- Coghill DR, Banaschewski T, Lecendreux M, Johnson M, Zuddas A, Anderson CS, Civil R, Dauphin M, Higgins N, Lyne A, Gasior M, Squires LA: Maintenance of efficacy of lisdexamfetamine dimesylate in children and adolescents with attention-deficit/hyperactivity disorder: Randomized-withdrawal study design. J Am Acad Child Adolesc Psychiatry 53:647–657.e641, 2014 [DOI] [PubMed] [Google Scholar]
- Coghill DR, Joseph A, Sikirica V, Kosinski M, Bliss C, Huss M: Correlations between clinical trial outcomes based on symptoms, functional impairments, and quality of life in children and adolescents with ADHD. J Atten Disord 2017. [Epub ahead of print]; DOI: 10.1177/1087054717723984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conners C: Conners Early Childhood. North Tonawanda, NY, Multi-Health Systems, Inc., 2009 [Google Scholar]
- Cunningham CE, Bremner R, Boyle M: Large group community-based parenting programs for families of preschoolers at risk for disruptive behaviour disorders: Utilization, cost effectiveness, and outcome. J Child Psychol Psychiatry 36:1141–1159, 1995 [DOI] [PubMed] [Google Scholar]
- Danielson ML, Visser SN, Gleason MM, Peacock G, Claussen AH, Blumberg SJ: A national profile of attention-deficit hyperactivity disorder diagnosis and treatment among US children aged 2 to 5 years. J Dev Behav Pediatr 38:455–464, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egger HL, Angold A: Common emotional and behavioral disorders in preschool children: Presentation, nosology, and epidemiology. J Child Psychol Psychiatry 47:313–337, 2006 [DOI] [PubMed] [Google Scholar]
- Ezpeleta L, Granero R: Executive functions in preschoolers with ADHD, ODD, and comorbid ADHD-ODD: Evidence from ecological and performance-based measures. J Neuropsychol 9:258–270, 2015 [DOI] [PubMed] [Google Scholar]
- Gadow KD, Sprafkin J, Nolan EE: DSM-IV symptoms in community and clinic preschool children. J Am Acad Child Adolesc Psychiatry 40:1383–1392, 2001 [DOI] [PubMed] [Google Scholar]
- Goodman D, Faraone S, Adler L, Dirks B, Hamdani M, Weisler R: Interpreting ADHD rating scale scores: Linking ADHD rating scale scores and CGI Levels in two randomized controlled trials of lisdexamfetamine dimesylate in ADHD. Prim Psychiatry 17:44–52, 2010 [Google Scholar]
- Greenhill L, Kollins S, Abikoff H, McCracken J, Riddle M, Swanson J, McGough J, Wigal S, Wigal T, Vitiello B, Skrobala A, Posner K, Ghuman J, Cunningham C, Davies M, Chuang S, Cooper T: Efficacy and safety of immediate-release methylphenidate treatment for preschoolers with ADHD. J Am Acad Child Adolesc Psychiatry 45:1284–1293, 2006 [DOI] [PubMed] [Google Scholar]
- Greenhill LL, Swanson JM, Vitiello B, Davies M, Clevenger W, Wu M, Arnold LE, Abikoff HB, Bukstein OG, Conners CK, Elliott GR, Hechtman L, Hinshaw SP, Hoza B, Jensen PS, Kraemer HC, March JS, Newcorn JH, Severe JB, Wells K, Wigal T: Impairment and deportment responses to different methylphenidate doses in children with ADHD: The MTA titration trial. J Am Acad Child Adolesc Psychiatry 40:180–187, 2001 [DOI] [PubMed] [Google Scholar]
- Guy W: ECDEU Assessment Manual for Psychopharmacology—Revised (DHEW Publ No ADM 76–338). Rockville, MD, U.S. Department of Health, Education, and Welfare, Public Health Services, Alcohol, Drug Abuse, and Mental Health Administration, NIMH Psychopharmacology Research Branch, Division of Extramural Research Programs, 1976
- Kaufman A, Kaufman N: Kaufman Brief Intelligence Test, Second Edition (KBIT-2). San Antonio, TX, Pearson, 2004 [Google Scholar]
- Kollins S, Greenhill L, Swanson J, Wigal S, Abikoff H, McCracken J, Riddle M, McGough J, Vitiello B, Wigal T, Skrobala A, Posner K, Ghuman J, Davies M, Cunningham C, Bauzo A: Rationale, design, and methods of the Preschool ADHD Treatment Study (PATS). J Am Acad Child Adolesc Psychiatry 45:1275–1283, 2006 [DOI] [PubMed] [Google Scholar]
- Kollins SH: Moving beyond symptom remission to optimize long-term treatment of attention-deficit/hyperactivity disorder. JAMA Pediatr 172:901–902, 2018 [DOI] [PubMed] [Google Scholar]
- Lumu L, Albertyn L, Szabo CP: Psychiatric services for preschoolers: An emerging need. J Child Adolesc Ment Health 27:113–124, 2015 [DOI] [PubMed] [Google Scholar]
- McGoey KE, DuPaul GJ, Haley E, Shelton T: Parent and teacher ratings of attention-deficit/hyperactivity disorder in preschool: The ADHD rating scale-IV preschool version. J Psychopathol Behav Assess 29:269–276, 2007 [Google Scholar]
- National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents: A Pocket Guide to Blood Pressure Measurement in Children. U.S. Department of Health and Human Services, May 2007. https://www.nhlbi.nih.gov/files/docs/bp_child_pocket Accessed January30, 2019
- Nomura K, Okada K, Noujima Y, Kojima S, Mori Y, Amano M, Ogura M, Hatagaki C, Shibata Y, Fukumoto R: A clinical study of attention-deficit/hyperactivity disorder in preschool children—Prevalence and differential diagnoses. Brain Dev 36:778–785, 2014 [DOI] [PubMed] [Google Scholar]
- Owens J, Weiss M, Nordbrock E, Mattingly G, Wigal S, Greenhill LL, Chang WW, Childress A, Kupper RJ, Adjei A: Effect of Aptensio XR (methylphenidate HCl extended-release) capsules on sleep in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 26:873–881, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens JA, Spirito A, McGuinn M: The Children's Sleep Habits Questionnaire (CSHQ): Psychometric properties of a survey instrument for school-aged children. Sleep 23:1043–1051, 2000 [PubMed] [Google Scholar]
- Panther SG, Knotts AM, Odom-Maryon T, Daratha K, Woo T, Klein TA: Off-label prescribing trends for ADHD medications in very young children. J Pediatr Pharmacol Ther 22:423–429, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaffer D, Gould MS, Brasic J, Ambrosini P, Fisher P, Bird H, Aluwahlia S: A children's global assessment scale (CGAS). Arch Gen Psychiatry 40:1228–1231, 1983 [DOI] [PubMed] [Google Scholar]
- Suvarna BS, Kamath A: Prevalence of attention deficit disorder among preschool age children. Nepal Med Coll J 11:1–4, 2009 [PubMed] [Google Scholar]
- Weiss M, Childress A, Mattingly G, Nordbrock E, Kupper RJ, Adjei AL: Relationship between symptomatic and functional improvement and remission in a treatment response to stimulant trial. J Child Adolesc Psychopharmacol 28:521–529, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells KC, Epstein JN, Hinshaw SP, Conners CK, Klaric J, Abikoff HB, Abramowitz A, Arnold LE, Elliott G, Greenhill LL, Hechtman L, Hoza B, Jensen PS, March JS, Pelham W, Pfiffner L, Severe J, Swanson JM, Vitiello B, Wigal T: Parenting and family stress treatment outcomes in attention deficit hyperactivity disorder (ADHD): An empirical analysis in the MTA study. J Abnorm Child Psychol 28:543–553, 2000 [DOI] [PubMed] [Google Scholar]
- Wernicke JF, Faries D, Milton D, Weyrauch K: Detecting treatment emergent adverse events in clinical trials: A comparison of spontaneously reported and solicited collection methods. Drug Saf 28:1057–1063, 2005 [DOI] [PubMed] [Google Scholar]
- Wichstrom L, Berg-Nielsen TS, Angold A, Egger HL, Solheim E, Sveen TH: Prevalence of psychiatric disorders in preschoolers. J Child Psychol Psychiatry 53:695–705, 2012 [DOI] [PubMed] [Google Scholar]
- Wigal SB, Greenhill LL, Nordbrock E, Connor DF, Kollins SH, Adjei A, Childress A, Stehli A, Kupper RJ: A randomized placebo-controlled double-blind study evaluating the time course of response to methylphenidate hydrochloride extended-release capsules in children with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 24:562–569, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigal SB, Nordbrock E, Adjei AL, Childress A, Kupper RJ, Greenhill L: Efficacy of methylphenidate hydrochloride extended-release capsules (Aptensio XR) in children and adolescents with attention-deficit/hyperactivity disorder: A Phase III, randomized, double-blind study. CNS Drugs 29:331–340, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigal T, Greenhill L, Chuang S, McGough J, Vitiello B, Skrobala A, Swanson J, Wigal S, Abikoff H, Kollins S, McCracken J, Riddle M, Posner K, Ghuman J, Davies M, Thorp B, Stehli A: Safety and tolerability of methylphenidate in preschool children with ADHD. J Am Acad Child Adolesc Psychiatry 45:1294–1303, 2006 [DOI] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Brown S, Tanguay S, Monuteaux MC, Blake C, Spencer TJ: Psychiatric comorbidity and functioning in clinically referred preschool children and school-age youths with ADHD. J Am Acad Child Adolesc Psychiatry 41:262–268, 2002 [DOI] [PubMed] [Google Scholar]
- Wolraich M, Brown L, Brown RT, DuPaul G, Earls M, Feldman HM, Ganiats TG, Kaplanek B, Meyer B, Perrin J, Pierce K, Reiff M, Stein MT, Visser S: ADHD: Clinical practice guideline for the diagnosis, evaluation, and treatment of attention-deficit/hyperactivity disorder in children and adolescents. Pediatrics 128:1007–1022, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]