Imaging technologies in current clinical practice are not capable of accurately differentiating active bacterial infections from other causes of inflammation or oncologic processes. With antimicrobial resistance being one of the biggest threats to human health,1 there is an urgent need to improve diagnostic practices, especially for deep-seated infections which are not easily accessible, and optimize clinical management and antibiotic selection. Positron emission tomography (PET) offers the possibility to noninvasively and specifically detect bacteria in vivo, distinguish between different bacterial classes, identify antibiotic-resistant organisms, and monitor therapeutic responses.2 This potential is based on bacteria-class specific metabolism of small molecules (e.g., sugars) by pathogenic bacteria and has been in use for many decades to identify and differentiate bacteria in clinical microbiology laboratories.3 As the potential for clinical imaging of infections becomes evident, different approaches are emerging in the development of pathogen-specific imaging agents.4 In this issue of ACS Central Science, a team led by David Wilson reports the use of carbon-11 radiolabeled d-alanine to image clinically relevant bacterial infections using PET.5
The biochemical machinery that regulates microbial processes has historically been targeted for antibiotic discovery, and more recently for chemical sensors such as fluorescent probes and pathogen-specific imaging agents. The basic principle follows that if mammalian and bacterial cells have different biophysical structures and metabolic processes, chemical sensors can be designed and engineered to selectively target and accumulate in bacteria. Small molecule pathogen-specific radiotracers under development target differences in carbohydrate uptake and metabolism (e.g., 18F-fluorodeoxysorbitol and 18F-fluoromaltotriose), folate biosynthesis (e.g., 11C-para-aminobenzoic acid and 18F-fluoropropyl-trimethoprim), and bacterial cell wall biosynthesis (e.g., d-[11C]alanine, as reported in this issue), etc (Figure 1).
Figure 1.
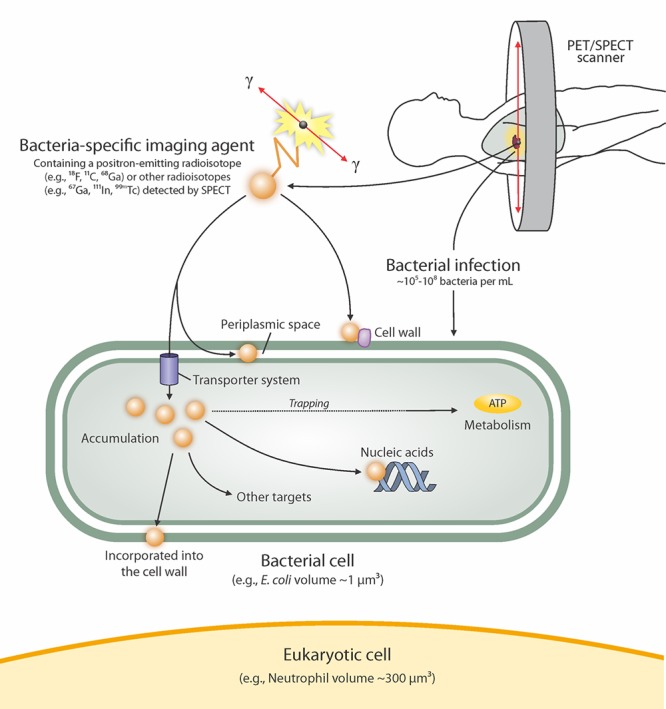
Bacteria-specific imaging. Mammalian and bacterial cells have different biophysical structures and metabolic processes. Thus, chemical sensors can be engineered to selectively target and accumulate in bacteria, and be developed for bacteria-specific imaging. Adapted from Ordonez et al.4 Reprinted with permission from ref (4). Copyright 2019 AAAS.
Amino acids are formed of amine and carboxyl functional groups, and a side chain that is linked to a chiral α-carbon, and thus exist as l- and d-enantiomers. While the more abundant l-amino acids are the building blocks of proteins in most cell types, their d-enantiomers have distinct functions. d-Amino acids are essential to the composition of bacteria, but are not utilized in the same way by most mammalian tissues. The bacterial cell wall, which is absent in mammalian cells, is composed of glycan strands cross-linked by peptides formed of d-amino acids. The most abundant d-amino acid in this structure is d-alanine, which is either biosynthesized from racemization of l-alanine or captured from exogenous sources.6 Parker et al. were able to radiolabel d-alanine with 11C, with >90% stereochemical purity. Since d-[11C]alanine is chemically identical to d-alanine, it is recognized by the same enzymes and incorporated into the bacterial cell wall, giving bacteria a measurable radioactive output in a wide range of pathogenic bacteria.
The progression of bacteria-specific imaging agents from concept to clinical translation requires a robust preclinical evaluation of radiotracer candidates in clinically relevant animal models.4 Pathogen-specific imaging agents should be able to distinguish between true infection and sterile inflammation, provide a signal that is proportional to the bacterial burden, and ideally have low background retention in nontarget organs. In rodent models of acute bacterial infection d-[11C]alanine outperformed more conventional PET agents 18F-fluorodeoxyglucose and 68Ga-citrate in distinguishing infection from sterile inflammation, and it also successfully monitored treatment response. However, the behavior of any given radiotracer in vivo is dependent not only on its target efficacy but also on other factors such as pharmacokinetics, intracellular enzyme kinetics, and bacterial and host metabolism. Indeed, d-amino acids are enantioselectively recognized by certain enzymes (e.g., d-amino acid oxidase) present in mammalian cells, which may explain the background signal observed in the animals that received d-[11C]alanine.
Bacteria residing at infected sites in situ could also have altered or reduced metabolism due to the local microenvironment or antibiotic treatments. Therefore, it would be critical to select highly active and conserved metabolic pathways in bacteria. Indeed, the incorporation of d-amino acids into the peptidoglycan in the bacterial cell is a highly conserved process, lending a robust strategy to label bacteria. However, data on whether the metabolic state of the bacteria could affect readouts and the sensitivity of this approach would need to be determined. Some bacteria-specific imaging agents currently under development, e.g., 11C-para-aminobenzoic acid, are not affected by the bacterial growth phase.7 A sensitivity threshold for bacteria-specific radiotracers of ∼105-6 bacteria/ml would be promising for the management of acute infections, which generally present with high bacterial burdens (108 bacteria/ml).4 As the efficacy spectrum of many antibiotics is dependent on the class of bacteria (Gram-positive or Gram-negative organisms), this distinction will allow narrowing of the antimicrobial regimen. Therefore, both general bacteria-specific as well as bacteria-class specific imaging agents are needed, and dual-imaging strategies utilizing radiotracers with short physical half-lives could allow sophisticated methods to both specifically detect bacteria in vivo as well as distinguish between different bacterial classes. Finally, while many recent studies have focused on the discovery and development of bacteria-specific radiotracers, this approach is broadly applicable to other classes of infectious pathogens (e.g., fungi).
From a discovery standpoint, the data presented by Parker et al. offer an opportunity for synthetic chemists to design second generation radiolabeled amino acids with optimized pharmacokinetics and reduced mammalian metabolism. Several fluorescent-labeled d-amino acids have been described and are shown to get incorporated into the bacterial cell wall.8 It would be interesting to explore the promiscuity of the peptidoglycan biosynthesis using functionalized non-natural radiolabeled d-amino acids. While fluorinated analogues of l- and d-alanine have shown defluorination in vivo, leading to high bone uptake, these were fluoroalkylated, and the use of alternative radiofluorinated side-chains remains largely unexplored.9 By employing diverse chemical approaches and different radioisotopes, the imaging protocols could also be modified. Since the rate of incorporation of d-amino acids into the bacterial cell wall is variable and depends on the organism and growth phase, longer lived radioisotopes could offer the opportunity of imaging at later time points, allowing more time for bacterial incorporation of the radiotracer. The findings by the Wilson team successfully demonstrate that radiolabeled d-amino acids can flag bacteria in vivo and have clinical translation potential. In addition, they highlight the opportunity for new and innovative chemical design to be applied to the development of radiotracers for bacterial imaging.
S.K.J. is funded by the National Institutes of Health [Director’s Transformative Research Award R01-EB020539, R01-HL131829, R01-EB025985, R56-AI145435, and the Department of Defense’s Congressionally Directed Medical Research Programs PR-171338P1.
The authors declare the following competing financial interest(s): S.K.J. is Co-PI with Dr. Wilson on R01-EB025985 and PR-171338P1. S.K.J. is an inventor on pending patent US20150250906A1 on bacteria-specific labeled substrates as imaging biomarkers, filed by Johns Hopkins University.
References
- World Health Organization . Ten Threats to Global Health in 2019; WHO: Geneva, 2019. [Google Scholar]
- Weinstein E. A.; Ordonez A. A.; DeMarco V. P.; Murawski A. M.; Pokkali S.; MacDonald E. M.; Klunk M.; Mease R. C.; Pomper M. G.; Jain S. K. Imaging Enterobacteriaceae infection in vivo with 18F-fluorodeoxysorbitol positron emission tomography. Sci. Transl. Med. 2014, 6 (259), 259ra146. 10.1126/scitranslmed.3009815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Faddin J. F.Biochemical Tests for Identification of Medical Bacteria; Williams & Wilkins: Baltimore, 1976. [Google Scholar]
- Ordonez A. A.; Sellmyer M. A.; Gowrishankar G.; Ruiz-Bedoya C. A.; Tucker E. W.; Palestro C. J.; Hammoud D. A.; Jain S. K. Molecular imaging of bacterial infections: Overcoming the barriers to clinical translation. Sci. Transl. Med. 2019, 11 (508), eaax8251 10.1126/scitranslmed.aax8251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M. F. L.; Luu J. M.; Schulte B.; Huynh T. L.; Stewart M. N.; Sriram R.; Yu M.; Jivan S.; Turnbaugh P. J.; Flavell R. R.; Rosenberg O. S.; Ohlinger M. A.; Wilson D. M.. Sensing living bacteria in vivo using d-alanine derived 11C radiotracers. ACS Central Science 2020, 10.1021/acscentsci.0c00743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radkov A. D.; Moe L. A. Bacterial synthesis of d-amino acids. Appl. Microbiol. Biotechnol. 2014, 98 (12), 5363–74. 10.1007/s00253-014-5726-3. [DOI] [PubMed] [Google Scholar]
- Ordonez A. A.; Weinstein E. A.; Bambarger L. E.; Saini V.; Chang Y. S.; DeMarco V. P.; Klunk M. H.; Urbanowski M. E.; Moulton K. L.; Murawski A. M.; Pokkali S.; Kalinda A. S.; Jain S. K. A Systematic Approach for Developing Bacteria-Specific Imaging Tracers. J. Nucl. Med. 2017, 58 (1), 144–150. 10.2967/jnumed.116.181792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y. P.; Rittichier J.; Kuru E.; Yablonowski J.; Pasciak E.; Tekkam S.; Hall E.; Murphy B.; Lee T. K.; Garner E. C.; Huang K. C.; Brun Y. V.; VanNieuwenhze M. S. Full color palette of fluorescent d-amino acids for in situ labeling of bacterial cell walls. Chem. Sci. 2017, 8 (9), 6313–6321. 10.1039/C7SC01800B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L. M.; Zha Z. H.; Qu W. C.; Qiao H. W.; Lieberman B. P.; Plossl K.; Kung H. E. Synthesis and evaluation of F-18 labeled alanine derivatives as potential tumor imaging agents. Nucl. Med. Biol. 2012, 39 (7), 933–943. 10.1016/j.nucmedbio.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]


