Abstract
Background:
This study compares effectiveness of two commercially available signals, Pulsed Electromagnetic Field (PEMF) and Combined Magnetic Field (CMF) clinical signals, to stimulate bone healing in rabbit tibial osteotomies.
Methods:
One millimeter osteotomies in New Zealand White rabbits, stabilized with external fixators, were exposed daily to either signal for 30 minutes, three or six hours. Osteotomized sham controls received no signal exposure. Analyses of torsional strength, periosteal callus area and fracture healing stage demonstrated dose responses to increasing daily exposures to both signals.
Results:
By 14 days torsional strength increased over shams in the three and six hour-treated groups, significant only for the six hour groups (p<0.05). By 21 days both three and six hour-treated groups were significantly stronger than shams (p<0.05, p<0.005) and the PEMF 30 minute treated group also showed significance (p<0.05). PEMF versus CMF-treated groups were not different at any exposure time.
Conclusions:
Both CMF and PEMF signals were most effective in this model when used for six hours per day.
Clinical Relevance:
In this model we demonstrate that though both PEMF and CMF are “bioactive” and promote healing at shorter and longer exposure dosages, there exists an “optimal” threshold effect of 6 hours/day electromagnetic wave stimulation for bone healing.
Keywords: animal model, fracture healing, Combined Magnetic Field, Pulsed Electromagnetic Field
Introduction
Combined electromagnetic field and pulsed electromagnetic field technologies commercially available for use to promote osteogenesis are prescribed according to directives provided by manufacturer’s in keeping with indications as permitted by the FDA. Current treatment protocols are derived from submissions made commensurate with the clinical model testing provided. However, inadequate dosing literature exists to provide clinicians with guidance with respect to optimal application duration. Considerable variation in prescribed time of both PEMF and CMF signals is recommended by manufacturer’s.
Three different electromagnetic devices are currently available for use in the treatment of nonunion bone fractures.1-3 The recommended daily exposure times for these devices vary from 30 minutes to ten hours per day. All three signals are reported to stimulate healing of non-union fractures.4-8 A retrospective analysis of the PEMF clinical data indicated that the time it took to heal the nonunions showed a dose response related to the length of daily exposure.9 The median time to heal was reduced by 60% if the PEMF device was used for ten hours per day as compared to use for one hour per day. Garland et al.7 reported that a similar PEMF device when used fewer than three hours per day in a prospective clinical trial was less effective in healing non-union fractures (35.7%, 5/14) than when used three hours or more per day (80%, 108/135). No similar analysis of clinical dose response has been published for the CMF clinical data.
Therefore, this study was designed to quantitatively investigate whether the PEMF and CMF signals demonstrate different optimal exposure times in stimulating the repair of rabbit tibial osteotomies.
Methods
Skeletally mature male New Zealand White rabbits weighing 3-4 kg were obtained from Myrtle Rabbitry (Thompson Station, TN, USA). A one millimeter transverse osteotomy was created in the left tibia of each rabbit with a Gigli wire as previously described.10 The periosteum was preserved and the osteotomy stabilized with an external fixator (M100, Orthofix, Verona, Italy). All rabbits were permitted immediate unrestricted weight bearing in standard rabbit cages following surgery. Radiographs were taken post-surgery and weekly thereafter for the duration of the study.
Experimental Design
Each rabbit was randomly assigned to one of three treatment groups: unexposed sham controls, PEMF-exposed and CMF-exposed. All rabbits were placed in rabbit restrainers to undergo treatment. Exposures to the PEMF and CMF signals were for 30 minutes, three and six hours daily. Treatment was initiated 24 hours post-surgery and continued until sacrifice at 14 or 21 days at which times the treated tibiae underwent biomechanical testing. Sham controls at 14 days were handled identically with the rabbits placed in restrainers for 30 minutes, three and six hours daily but not exposed to either signal. In this group and in previous studies using this model, the length of time the sham rabbits were in restrainers had no effect on any biomechanical or radiographic healing parameters. For this reason the 21 day sham rabbits were all exposed for one hour per day to reduce the number of rabbits which would need to be euthanized.
The PEMF signal consists of a pulsed magnetic field comprised of 4.5 ms duration bursts of 20 pulses repeated at 15 Hz. During each pulse the magnetic field rises from 0 to 1.6 mT in 200 ms and then decays back to zero in 25 ms.2
The CMF signal consists of the collinear sum of two magnetic fields; a 76.6 Hz sine wave of 20 mT amplitude (40 mT peak to peak) superimposed with a net static magnetic field of 20 mT.3 To more closely mimic the clinical use of these signals, no attempt was made to offset the Earth’s magnetic field other than in the case of the CMF signal where the net static field parallel to the 76.6 Hz sine wave is normally held at 20 mT as part of the device. For both signals, paired Helmholtz coils were attached to the rabbit restrainers in a vertical position and centered over the location of the osteotomy site.
Investigators were blinded to the knowledge of which treatment each specimen received.
Radiographical Studies
Anteroposterior and lateral radiographs were taken at weekly intervals to evaluate callus formation and assess adequacy of fixation. The area of periosteal callus and diaphyseal bone at the osteotomy site was measured using digitized images of X-rays. Metrics software by Orthographics (Salt Lake City, UT, USA) was used for the analysis. Periosteal callus and diaphyseal bone were outlined along the bone between the two inner-most fixator screws in both the lateral and anteroposterior films. Area was calculated from each view and was expressed as a ratio of periosteal callus to diaphyseal bone.
Biomechanical Testing
The osteotomized tibiae were harvested, cleaned of soft tissues, and immediately prepared for biomechanical testing as previously described.10 Mechanical tests were performed using a custom-made torsion apparatus on an MTS 810 uniaxial testing machine (MTS, Minneapolis, MN, USA). The proximal end of the specimen was firmly anchored on the apparatus. Torque was applied to the distal end of the specimen at a rate of 1.76 degrees per second until failure occurred, and the torque versus angular deformation data were recorded. Torque was calculated as the product of the tensile force (measured in the cable with a load cell) and the length of the moment arm. Torsional strength (Nm) and torsional stiffness (Nm per degree) were determined for each specimen by the methods of White et al.11 Torsional strength was defined as the maximum torque before the first abrupt drop in the torque versus angular deformation curve. Stiffness, which is the slope of the rising portion of the curve, was estimated as the ratio of the torsional strength and the angle of failure. A statistical power analysis was performed based on previous experience with rabbit tibia torsion tests. Assuming a variation of 13.25% for torsional strength and a target of 80% confidence, a sample size of six was found to be sufficient to identify a 20% difference between treatment groups. The difference between groups was analyzed by one-way analysis of variance and a Mann-Whitney test.
Biomechanical Stages of Fracture Healing
Post torsional testing radiographs of the specimens were taken to characterize the location of the failure site. The location of the failure fracture was combined with biomechanical stiffness data to assign one of four biomechanical stages of fracture repair to each tibia, as described by White et al.11 The four stages were defined as follows: Stage I - failure through the original fracture site with low stiffness; Stage II - failure through the original fracture site with high stiffness; Stage III - failure partially through the original fracture site and partially through intact bone, with high stiffness; and Stage IV - failure entirely through intact bone, with high stiffness.
Results
Of 111 rabbits used in this study, 97 were available for assessment. Five rabbits died of unknown etiology (one sham, three PEMF and one CMF). Nine suffered secondary fractures through the proximal pins prior to study completion (one sham, six PEMF and two CMF).
Biomechanical Testing
Torque versus angular deformation curves had a very consistent shape for all specimens, including a highly linear portion that rose to a peak value and then dropped abruptly at the point of failure. Biomechanical results are presented in Tables 1 and 2 and Figures 1 and 2. At 14 days, osteotomies exposed for 30 minutes per day to either signal showed no increase in mean torsional strength over sham controls. Mean torsional strength values of both groups treated with PEMF or CMF for three hours per day were increased over sham controls, but this difference was not statistically significant. A significant increase in mean torsional strength was found in both signal-exposed groups treated for six hours per day when compared to sham controls. Comparison of torsional strengths between the PEMF and CMF treated groups showed no significant differences at any exposure time. The sham controls also showed no significant differences in mean torsional strength whether restrained for 30 minutes, three or six hours per day.
Table 1.
Changes in Strength and Stiffness as a Function of Daily Exposure Time
| Hours of Exposure/Day | 0.5 | 3 | 6 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | (SD) | n | Mean | (SD) | n | Mean | (SD) | ||
| Torque (Nm) 14 Days | ||||||||||
| Sham | 6 | 1.18 | (0.35) | 6 | 0.98 | (0.51) | 4 | 0.93 | (0.17) | |
| CMF | 8 | 1.13 | (0.66) | 8 | 1.34 | (0.43) | 8 | 1.58 | (0.33) | |
| PEMF | 6 | 0.89 | (0.28) | 7 | 1.41 | (0.29) | 6 | 1.77 | (0.23) | |
| Torque (Nm) 21 Days | ||||||||||
| Sham | 6 | 2.10 | (0.27) | 6 | 2.1 | (0.27) | 6 | 2.10 | (0.27) | |
| CMF | 5 | 2.28 | (0.23) | 7 | 2.59 | (0.41) | 7 | 2.96 | (0.40) | |
| PEMF | 4 | 2.50 | (0.26) | 7 | 2.59 | (0.39) | 7 | 3.14 | (0.38) | |
| Stiffness (Nm/Deg) 14 Days | ||||||||||
| Sham | 6 | 0.12 | (0.05) | 6 | .12 | (0.03) | 4 | 0.10 | (0.08) | |
| CMF | 8 | 0.11 | (0.05) | 8 | 0.14 | (0.09) | 8 | 0.15 | (0.04) | |
| PEMF | 6 | 0.09 | (0.06) | 7 | 0.13 | (0.09) | 6 | 0.16 | (0.07) | |
| Stiffness (Nm/Deg) 21 Days | ||||||||||
| Sham | 6 | 0.18 | (0.04) | 6 | 0.18 | (0.04) | 6 | 0.18 | (0.04) | |
| CMF | 6 | 0.18 | (0.05) | 7 | 0.18 | (0.03) | 7 | 0.20 | (0.06) | |
| PEMF | 4 | 0.18 | (0.04) | 7 | 0.18 | (0.06) | 7 | 0.24 | (0.09) | |
Table 2.
Statistical Analysis of Differences in Torsional Strength (One Way Anova)
| 14 DAYS | vs. CMF | vs. Sham | |
|---|---|---|---|
| 0.5 Hr./Day | PEMF | p=0.436 | p=0.153 |
| CMF | p=0.870 | ||
| 3 Hrs./Day | PEMF | p=0.749 | p=0.083 |
| CMF | p=0.169 | ||
| 6 Hrs./Day | PEMF | p=0.449 | p<0.001* |
| CMF | p<0.004* | ||
| 21 DAYS | |||
| 0.5 Hr./Day | PEMF | p=0.216 | p=0.047* |
| CMF | p=0.267 | ||
| 3 Hrs./Day | PEMF | p=0.795 | p=0.027* |
| CMF | p=0.052* | ||
| 6 Hrs./Day | PEMF | p=0.312 | p<0.001* |
| CMF | p<0.001* | ||
Statistically significant
Figure 1.
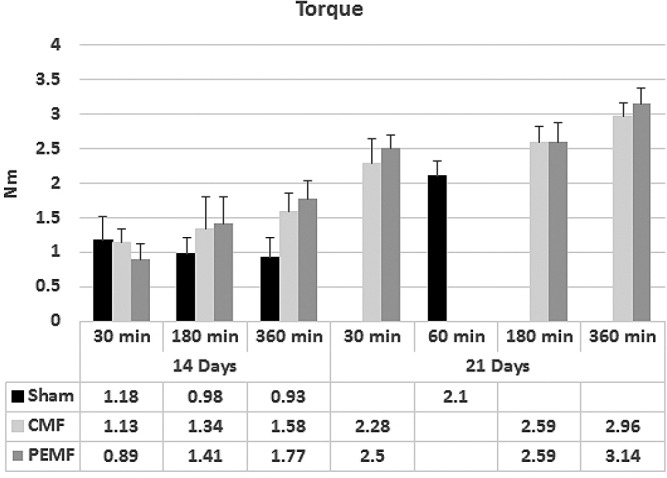
Biomechanical testing of osteotomy healing showing torsional strength values as a function of treatment with PEMF or CMF signals at three different daily exposure times after 14 days (a) and 21 days (b) post-osteotomy.
Figure 2.
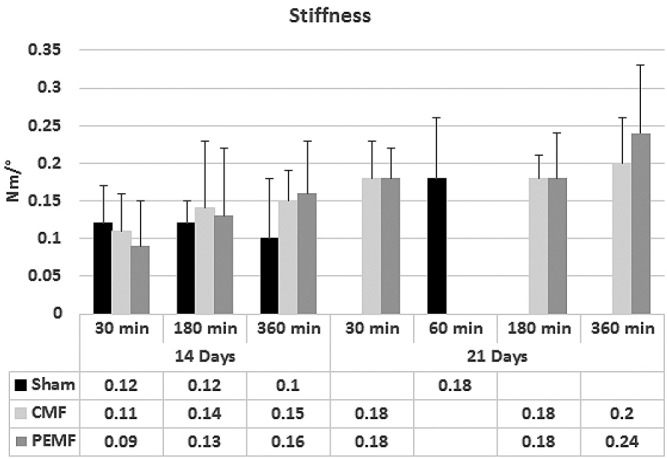
Biomechanical testing of osteotomy healing showing stiffness as a function of treatment with PEMF or CMF signals at three different daily exposure times after 14 days (a) and 21 days (b) post-osteotomy.
At 21 days postoperatively, tibial osteotomies treated with PEMF showed a mean torsional strength value significantly higher than sham controls for all three exposure times. Mean torsional strength in the CMF group was significantly higher than sham controls in the three and six hour per day exposure groups but not in the 30 minutes group. No significant differences were found between PEMF and CMF treatment groups at any exposure. Osteotomies treated for six hours per day with either PEMF or CMF were significantly stronger than those treated for ½ hour per day (p=0.016 PEMF, p=0.007 CMF). The PEMF group treated for six hours per day was also stronger than the three hour-treated group (p=0.02).
Increased stiffness over shams was seen in the three and six hour-treated groups at 14 days and in the six hour-treated groups at 21 days, although none reached significance of p<0.05 (Figure 2). Both PEMF and CMF six hour-treated groups at 21 days achieved torsional stiffness not significantly different from normal intact bone whereas shams had not. The stiffness and strength values in the 6 hour-treated groups are rising toward normal intact values regardless of treatment modality.
Radiological Studies
Both CMF and PEMF signals stimulated increases in mean periosteal callus area over non-stimulated shams at 14 days when used for 3 and 6 hours per day, but not for 30 minutes per day. Six hour daily exposures produced the largest callus areas. At 21 days, mean callus areas were increased over shams at all three daily exposure times to both signals with maximal differences seen in the groups exposed for three hours per day (Figure 3). There were no significant differences in the mean periosteal callus areas between CMF and PEMF-treated osteotomies under any of the experimental conditions.
Figure 3.

Radiographic analysis of the relative area of periosteal callus to diaphyseal bone as a function of daily exposure time to the PEMF or CMF signal 14 days (a) and 21 days (b) post-osteotomy.
Biomechanical Stages of Fracture Healing
Results presented in Table 3 show that exposure to either the CMF or the PEMF signals for three or six hours per day stimulated earlier appearance of the more mature fracture stages (III and IV) as compared to the sham controls. At 14 days sham controls and groups exposed to PEMF or CMF for 30 minutes per day fractured only through the osteotomy site (Stages I and II). At three hour daily exposures to either signal, increasing numbers of tibias reached Stage II, and one CMF and two PEMF fractured through adjacent bone (Stage III). With six hour daily treatment, 50% of the CMF and 67% of the PEMF group reached Stage III. No sham controls reached Stage III by 14 days. At 21 days no improvement over shams was observed for either group treated for 30 minutes per day. At three hours per day, 57% of both CMF and PEMF-treated osteotomies had reached Stage III as compared to 33% of the shams. With six hour daily exposures, 57% of the CMF and the PEMF groups reached Stage III compared to 33% of the shams. 14% of CMF and 29% of PEMF-treated groups fractured entirely through bone adjacent to the osteotomy (Stage IV). No sham controls reached Stage IV by 21 days.
Table 3.
Effect of PEMF and CMF Exposure on the Four Biomechanical Stages of Fracture Healing
| 14 Days | 21 Days | |||||||
|---|---|---|---|---|---|---|---|---|
| Fracture Stage | I | II | III | IV | I | II | III | IV |
| 0.5 Hour/Day | ||||||||
| Sham | 2 | 3 | - | - | - | 4 | 2 | - |
| CMF | 4 | 4 | - | - | - | 4 | 2 | - |
| PEMF | 4 | 2 | - | - | - | 3 | 1 | - |
| 3 Hours/Day | ||||||||
| Sham | 4 | 2 | - | - | - | 4 | 2 | - |
| CMF | 2 | 5 | 1 | - | - | 3 | 4 | - |
| PEMF | 1 | 4 | 2 | - | - | 3 | 4 | - |
| 6 Hours/Day | ||||||||
| Sham | 3 | 1 | - | - | - | 4 | 2 | - |
| CMF | 1 | 3 | 4 | - | - | 2 | 4 | 1 |
| PEMF | 1 | 1 | 4 | - | - | 1 | 4 | 2 |
Discussion
This study has demonstrated a clear dose response correlating daily exposure time with the rate of healing of rabbit tibial osteotomies. Both the CMF and PEMF signals are “bioactive”, stimulating greater biomechanical strength when used for six hours per day than when used for three hours or 30 minutes daily exposures. There were no significant differences in mean biomechanical strength between osteotomies treated with PEMF or CMF when exposed for similar time periods (30 minutes, three or six hours per day). Both signals stimulated increases in torsional stiffness but not to the same extent as torsional strength. This may indicate that the signals are changing the composition of the healing bone resulting in an increased capacity to absorb energy prior to fracture (i.e. accelerated remodeling).
Increases in callus area and fracture healing maturation stage also showed a dose dependence on daily exposure time to both of the signals. As fractures advance from Stage III to IV, the torsion test changes from a measure of fracture gap tissue properties to a measure of the adjacent intact bone properties. This explains why the six hour-treated groups at 21 days achieved stiffness values not different from intact bone. The fact that torsional strengths in these groups still fall short of intact bone might be due to changes in bone density associated with disuse.
Other studies using the PEMF or CMF signals have also shown a dose relationship to different daily exposure times. Factors such as signal intensity and pulse number can also have an influence on healing. Using the PEMF signal to enhance bone formation in a model of fracture callus formation in the rat, Aaron and Ciombor12 have shown increased stimulation of cartilage and bone in animals treated for eight hours per day compared to those treated for four or one hour per day. Fitton-Jackson and Bassett13 showed that 14Ca uptake and synthesis of collagen by chick embryonic limb rudiments in vitro was also differentially stimulated by an increase in exposure time to the PEMF signal. By manipulating dosage, intensity, and duration of PEMF therapy, Girolamo et al.14 found that specific conditions were most effective at enhancing cell viability and up-regulating the production of various growth factors in human tendon cells. Using PEMF therapy Li et al.15 discovered that, at 400 kV/m, the proliferation of osteoblasts increased from 0 to 400 pulses, but decreased once pulses reached 2800.
The CMF signal used to stimulate bone healing in a rabbit fibular osteotomy model16 showed that the greatest biomechanical stiffness resulted from 24 hour exposures with three and ½ hour daily exposures showing smaller increases over controls. Smith et al.17 treated eight day chick femoral rudiments in vitro for 30 minutes , one, four or 24 hour daily exposures to the CMF signal. Femur width and diaphyseal collar length was greatest in rudiments treated for four hours per day, and diaphyseal collar was maximally thickened at one hour per day. The authors state that the results, “. . . clearly show that exposures of one or four hours per day seem to produce more marked results for most of the parameters than either 24 hours or 30 minutes per day.” There was a clear dose effect with femora treated with minimal exposure time, 30 minutes, demonstrating little improvement over controls. In a castration induced rat model of osteoporosis, Magee et al.18 reported that exposure to the CMF signal for two hours per day (30 minutes four times per day) was more effective in inhibiting bone loss than treatment for 30 minutes per day.
The CMF studies reported by Ryaby et al.19 found that rat fibular fractures exposed to the CMF signal for 30 minutes per day showed no significant differences in torsional strength or stiffness in comparison to untreated controls after either 21 or 30 days. This corroborates findings in the present study which also demonstrated limited stimulation at 30 minutes per day exposure to CMF.
A dose response relationship has also been reported for stimulation of proteoglycan synthesis20 and for osteotomy repair21 using electromagnetic signals other than the PEMF and CMF signals described in this study.
Interestingly, the osteogenic response in this osteotomy model appears to be similar whether the rabbits were exposed to the PEMF or CMF signal for each of the doses, 30 minutes, three or six hours per day. The peak magnetic fields of the two signals differ from each other quite radically; the CMF signal being about 40X smaller than the PEMF signal. In addition, the CMF signal contains a 76.6 Hz sine wave, whereas the PEMF signal is a pulse burst signal consisting of frequency components from 15 Hz to greater than 10 kHz. Although the frequency content of the two signals are very different, Fourier analysis of the PEMF signal reveals frequency components of less than 100 Hz (including one at 75 Hz) with amplitudes similar to that of the CMF signal. Since there is evidence suggesting that it is the low frequency components of the pulsed electromagnetic fields which are responsible for the osteogenic response,22 this may be the reason why both CMF and PEMF have similar effects on osteogenesis in the rabbit osteotomy model.
Both PEMF and CMF signals have been shown clinically to affect healing nonunion and delayed union fractures.4, 5, 8, 23-26 Recommended daily use for the PEMF signal is ten hours per day, which has been reported to stimulate the fastest time to heal.20 While treating tibial delayed unions and non-union fractures, it was noted that increased union probability coincided with increased PEMF exposure; however, it was not statistically significant due to study limitations.27 Healing will be enhanced with shorter daily exposures to the PEMF signal, but the time to heal is longer. To our knowledge, there are no published clinical studies using the CMF signal for more than one-half hour per day, so clinical efficacy with longer daily usage remains unknown.
The PEMF and CMF signals do not stimulate maximal osteogenic responses when used for ½ hour per day in this rabbit tibial osteotomy model or in the other animal models discussed above. Although none of these animal studies are nonunion models, these results suggest that longer exposures to either the PEMF or CMF signal may provide the optimal treatment regimen for obtaining fusion of nonunions.
Acknowledgement
Funding was provided to the University of Iowa by a grant from EBI Medical Systems, Inc., Parsippany, New Jersey.
References
- 1.American Medical Electronics Physio-Stimä. PMA No. 850007, February 21, 1986.
- 2.Bi-Osteogenâ System 204. PMA No. 790002, November 6, 1979.
- 3.OrthoLogicâ 1000 Bone Growth Stimulator. PMA No.910066, March 1994.
- 4.Bassett CA, Mitchell SN, Gaston SR. Pulsing electromagnetic field treatment in ununited fractures and failed arthrodeses. JAMA. 1982;247(5):623–628. [PubMed] [Google Scholar]
- 5.Bassett CA, Mitchell SN, Gaston SR. Treatment of ununited tibial diaphyseal fractures with pulsing electromagnetic fields. J Bone Joint Surg Am. 1981;63(4):511–523. [PubMed] [Google Scholar]
- 6.Frykman GK, Taleisnik J, Peters G, Kaufman R, Helal B, Wood VE, Unsell RS. Treatment of nonunited scaphoid fractures by pulsed electromagnetic field and cast. J Hand Surg Am. 1986;11(3):344–349. doi: 10.1016/s0363-5023(86)80140-x. [DOI] [PubMed] [Google Scholar]
- 7.Garland DE, Moses B, Salyer W. Long-term follow-up of fracture nonunions treated with PEMFs. Contemporary orthopaedics JID - 8219527. [PubMed]
- 8.Perry CR, Zoltan JD, Gershuni DH, et al. Treatment of problem fractures using resonant magnetic fields. Trans comb orthop mtg 63. 1992.
- 9.Pethica BA, Brownell J. Transactions of the eighth annual meeting of the bioelectrical repair and growth society; October 9-12. Washington, D.C.: 1988. The dose-response relationship in PEMF therapy of ununited fractures. [Google Scholar]
- 10.Fredericks DC, Nepola JV, Baker JT, Abbott J, Simon B. Effects of pulsed electromagnetic fields on bone healing in a rabbit tibial osteotomy model. Journal of orthopaedic trauma JID - 8807705. [DOI] [PubMed]
- 11.White AA, Panjabi MM, Southwick WO. The four biomechanical stages of fracture repair. J Bone Joint Surg Am. 1977;59(2):188–192. [PubMed] [Google Scholar]
- 12.Aaron RK, McCiombor DK. Acceleration of experimental endochondral ossification by biophysical stimulation of the progenitor cell pool. J Orthop Res. 1996;14(4):582–589. doi: 10.1002/jor.1100140412. [DOI] [PubMed] [Google Scholar]
- 13.Fitton-Jackson S. The response of skeletal tissues to pulsed magnetic fields. Tissue Culture in Medical Research. 1980;2:21–28. [Google Scholar]
- 14.ViganÃ2M de Girolamo L, Galliera E, Stanco D, Setti S, Marazzi MG, Thiebat G, Corsi Romanelli MM, Sansone V. In vitro functional response of human tendon cells to different dosages of low-frequency pulsed electromagnetic field. Knee Surgery, Sports Traumatology, Arthroscopy. 2015;23(11):3443–3453. doi: 10.1007/s00167-014-3143-x. [DOI] [PubMed] [Google Scholar]
- 15.Li K, Ma S, Li Y, Ding G, Teng Z, Liu J, Ren D, Guo Y, Ma L, Guo G. Effects of PEMF exposure at different pulses on osteogenesis of MC3T3-E1 cells. Arch Oral Biol. 2014;59(9):921–927. doi: 10.1016/j.archoralbio.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 16.Weinstein AM, McLeod BR, Smith SD, Liboff AR. Ion resonance turned electromagnetic fields increase healing rate in osteotomized rabbitts. Trans orthop res soc 36th annual meeting. 1990.
- 17.Smith SD, McLeod BR, Liboff AR. Effects of resonant magnetic fields on chick femoral development in vitro. J Bioelectr. 1991;10(1-2):81–99. [Google Scholar]
- 18.Magee FP, Weinstein AM, Fitzsimmons RJ, et al. Electromagnetics in Medicine and Biology. San Francisco: San Francisco Press; 1991. The use of low-energy combined AC and DC magnetic fields in the prevention of osteopenia; pp. 171–176. In: Brighton CT, Pollack SR, editors. [Google Scholar]
- 19.Ryaby JT, Huene D, Magee FP, Naser PR. Effects of combined AC/DC magnetic fields on healing in a closed femoral fracture model. Trans orthop res soc 39th annual meeting. 1993.
- 20.Bee JA, Liu HX, Clarke N, Abbott J. Biomechanics and Cells. Society for Experimental Biology Seminar Series 54 ed. Cambridge: Cambridge University Press; 1994. Modulation of cartilage extracellular matrix turnover by pulsed electromagnetic fields (PEMF) pp. 224–269. In: Lyall F, El Haj AJ, editors. [Google Scholar]
- 21.Baker JT, Fredericks DC, Abbott J, Aper R, Brown T, Nepola JV. The effects of pulsed electromagnetic field (PEMF) stimulation on fresh fracture healing in the rabbit tibial osteotomy model. Trans orthop res soc 40th annual meeting. 1994.
- 22.McLeod KJ, Rubin CT. The effect of low-frequency electrical fields on osteogenesis. The Journal of bone and joint surgery. American volume JID - 0014030. [PubMed]
- 23.Sharrard WJ, Sutcliffe ML, Robson MJ, Maceachern AG. The treatment of fibrous non-union of fractures by pulsing electromagnetic stimulation. J Bone Joint Surg Br. 1982;64(2):189–193. doi: 10.1302/0301-620X.64B2.6978339. [DOI] [PubMed] [Google Scholar]
- 24.Sharrard WJ. A double-blind trial of pulsed electromagnetic fields for delayed union of tibial fractures. J Bone Joint Surg Br. 1990;72(3):347–355. doi: 10.1302/0301-620X.72B3.2187877. [DOI] [PubMed] [Google Scholar]
- 25.Streit A, Watson BC, Granata JD, Philbin TM, Lin HN, O’Connor JP, Lin S. Effect on clinical outcome and growth factor synthesis with adjunctive use of pulsed electromagnetic fields for fifth metatarsal nonunion fracture: A double-blind randomized study. Foot Ankle Int. 2016;37(9):919–923. doi: 10.1177/1071100716652621. [DOI] [PubMed] [Google Scholar]
- 26.Boopalan PRJVC, Chittaranjan SB, Balamurugan R, Nandakumar NS, Sabareeswaran A, Mohanty M. Pulsed electromagnetic field (PEMF) treatment for fracture healing. Ovid. 2009;20(4):423–428. [Google Scholar]
- 27.Assiotis A, Sachinis NP, Chalidis BE. Pulsed electromagnetic fields for the treatment of tibial delayed unions and nonunions. A prospective clinical study and review of the literature. Journal of Orthopaedic Surgery and Research. 2012;7(1):24. doi: 10.1186/1749-799X-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]


