Abstract
Background:
In ST-segment elevation myocardial infarction (STEMI) patients treated with percutaneous coronary intervention, direct transport from the scene to the catheterisation laboratory bypassing the emergency department has been shown to shorten times to reperfusion. The aim of this study was to investigate the effects of emergency department bypass on mortality in both haemodynamically stable and unstable STEMI patients.
Methods:
The analysis is based on a large cohort of STEMI patients prospectively included in the German multicentre Feedback Intervention and Treatment Times in ST-Elevation Myocardial Infarction (FITT-STEMI) trial.
Results:
Out of 13,219 STEMI patients who were brought directly from the scene by emergency medical service transportation and were treated with percutaneous coronary intervention, the majority were transported directly to the catheterisation laboratory bypassing the emergency department (n=6740, 51% with emergency department bypass). These patients had a significantly lower in-hospital mortality than their counterparts with no emergency department bypass (6.2% vs. 10.0%, P<0.0001). The reduced mortality related to emergency department bypass was observed in both stable (n=11,594, 2.8% vs. 3.8%, P=0.0024) and unstable patients presenting with cardiogenic shock (n=1625, 36.3% vs. 46.2%, P<0.0001). Regression models adjusted for the Thrombolysis In Myocardial Infarction (TIMI) risk score consistently confirmed a significant and independent predictive effect of emergency department bypass on survival in the total study population (odds ratio 0.64, 95% confidence interval 0.56–0.74, P<0.0001) and in the subgroup of shock patients (OR 0.69, 95% CI 0.54–0.88, P=0.0028).
Conclusion:
In STEMI patients, emergency department bypass is associated with a significant reduction in mortality, which is most pronounced in patients presenting with cardiogenic shock. Our data encourage treatment protocols for emergency department bypass to improve the survival of both haemodynamically stable patients and, in particular, unstable patients.
Clinical Trial Registration: NCT00794001
ClinicalTrials.gov: NCT00794001
Keywords: ST-segment elevation myocardial infarction (STEMI), percutaneous coronary intervention (PCI), cardiogenic shock, bypassing emergency department, treatment times, mortality, outcome
Introduction
Prompt treatment with primary percutaneous coronary intervention (PCI) increases the likelihood of survival for patients presenting with ST-segment elevation myocardial infarction (STEMI). Given the close relationship between ischaemia time and hypoxia-induced loss of contractile myocardium, recent advances in the therapy of STEMI patients have mainly focused on the time to reperfusion as a key performance indicator for better outcomes in terms of morbidity and mortality.1–10 Rapid and accurate interpretation of pre-hospital electrocardiograms in combination with bypassing the emergency department (ED) has been proposed as a feasible strategy to optimise and shorten re-perfusion times by preventing treatment delays from hospital arrival to balloon inflation and coronary stenting.11–21
As the successful restoration of antegrade coronary flow by early reperfusion therapy improves the prognosis of STEMI, the current guidelines of the European Society of Cardiology (ESC) for the treatment of STEMI patients underscore the significance of fast treatment pathways to primary PCI for emergency revascularisation by recommending fast tracking from the field directly to the catheterisation laboratory of a PCI-capable hospital.22 However, clinical evidence for this approach is rather limited and the American College of Cardiology (ACC)/American Heart Association (AHA) has not issued comparable guidance on this matter.23 The rather weak ESC recommendation is based mainly on the results from a large US registry which, however, reported similar adjusted mortality risks between patients bypassing and not bypassing the ED.18 A recently published systematic review on the impact of direct admission to the catheterisation laboratory as compared to transport to the ED confirmed the reduced delay to the start of revascularisation but did not produce a clear evidence-based benefit with respect to outcome.24 Thus, conflicting data exist as to whether a direct transfer without stopping at the ED will lower the incidence of adverse events during in-hospital treatment.10,11,12,25,26
Recently, we reported in data from the ongoing FITT–STEMI (Feedback Intervention and Treatment Times in ST-Elevation Myocardial Infarction) trial that shortened contact-to-balloon times were associated with improved survival, particularly in patients with cardiogenic shock.27,28 Using key driver analysis, we found that direct transmission to the catheterisation laboratory was a significant determinant of time to PCI treatment. However, neither from this study nor the existing literature is it clear whether ED bypass has any significant impact on mortality after adjustment to clinically relevant confounders. In the present paper, we addressed this important clinical issue in both haemodynamically stable patients and unstable patients presenting with cardiogenic shock.
Methods
Participating hospitals
The present paper reports outcome data from the prospective and ongoing multicentre FITT–STEMI study. The study protocol included systematic data analysis and standardised feedback interventions on treatment times and mortality in STEMI patients treated in different regional cardiac care networks.29,30 All consecutive PCI-treated STEMI patients presenting within 24 hours of symptom onset from 1 January 2006 to 31 December 2015, who were transported directly from the field by emergency medical transportation and treated with PCI within 360 minutes after first medical contact, were considered eligible for inclusion in this analysis. The existing study protocol encouraged immediate activation of the catheterisation laboratory by a single call, when the patient was triaged to primary PCI, combined with a direct transfer to the laboratory in order to prevent treatment delays in the time to reperfusion.27 The participating 48 PCI-capable hospitals, which are located all over Germany, endorsed the key strategies of the ACC initiative for the management of STEMI patients, including the establishment of multidisciplinary treatment teams for around the clock invasive treatment. For enrollment in the FITT-STEMI consortium, the candidate PCI centre had to fulfill the following requirements: a 24-hour PCI accessibility for at least one year before study participation, affiliation of at least two interventional cardiologists qualified to take the incoming calls of the emergency medical transportation team, a minimum of 250 PCI procedures per year and more than 50 annual PCI treatments in STEMI patients.
Among the total study participants with STEMI (n=20,964), the majority of patients (n=15,153) were directly transported from the scene to the PCI hospital by emergency medical services (EMS), and of those n=13,365 underwent prompt interventional reperfusion therapy with primary PCI (Figure 1). Not eligible for this outcome analysis were patients with: (a) interfacility transfer from a non-PCI-capable hospital to a receiving on-site study centre (n=3136); (b) self-admission to the PCI hospital (n=2174); and (c) myocardial infarction during hospital treatment at the PCI hospital (n=501). Complete records were available for n=13,219 patients (98.9%) transported by EMS transportation and were treated for reperfusion by primary PCI within 360 minutes after the first medical contact. Among them, n=6740 patients (51.0%) were directly transported from the pre-hospital setting to the catheterisation laboratory, while the other half (n=6479) had a stop at the ED on their way to PCI treatment. Cardiogenic shock was diagnosed in 1625 patients (12.3%), among them 684 patients (42.1%) were transported directly to the catheterisation laboratory.
Figure 1.
Flow diagram of the FITT–STEMI study cohort.
Outcome measures
In order to achieve continuous high-quality management, ongoing outcome monitoring with respect to treatment times was performed using an electronic case report form for data collection and a web-based data transfer system to the principal coordinating centre. For each consecutive STEMI patient, the participating PCI centres collected detailed information on pre- and in-hospital treatment times from initial contact with the medical system to balloon inflation. Key time points for out-of-hospital treatment included time of arrival at the field by EMS transportation usually staffed in Germany with a trained physician and experienced paramedics, treatment time at the scene, and transport time to the hospital. Assessment of in-hospital treatment times included information on whether there was a direct transfer to the catheterisation laboratory or, alternatively, an indirect transfer by the ED, as well as the time on arrival at the catheterisation laboratory, and the times of puncture and first balloon inflation at the culprit lesion. For each STEMI patient, the following interventional variables were documented as predefined key quality indicators by ambulance personnel or attending physicians: time of pre-hospital ECG recording within or longer than 10 minutes after arrival at the scene, telephone announcement in advance and telemetry-ECG transmission, as well as average and median components of times to treatment, including those from the first medical contact to balloon inflation. Information about in-hospital mortality after PCI was obtained for each patient. Additional data were obtained on medical history, cardiac risk factors, prior medication, medical comorbidity, Thrombolysis In Myocardial Infarction (TIMI) risk score as well as results from coronary angiography and the PCI procedure. Patients were classified as being in cardiogenic shock (Killip class IV) when the following clinical criteria were fulfilled and confirmed by experienced cardiologists: hypotension (systolic blood pressure of <90 mmHg or the need for supportive measures to maintain a systolic blood pressure of ⩾90 mmHg), signs of critical end-organ hypoperfusion, and a heart rate of 60 beats/minute or greater.31 The study protocol was approved by the ethics committee of the medical faculty at the University of Göttingen and the local ethics committees of all participating PCI centres.
Data assessment
Outcome data for each local study site were provided through a cooperative agreement which included regular and systematic data analysis and formalised feedback interventions in order to implement procedures of standardised quality management for timely reperfusion therapy. For this purpose, out-of-hospital emergency ambulance teams consisting of qualified physicians, trained in emergency medicine, and the medical staff working in the ED of the participating centres were regularly instructed by the local principal investigators to make precise diagnoses of the acute coronary syndrome and early PCI team activation in order to increase the frequency of direct transports to the catheterisation laboratory for STEMI patients. Feedback interventions were given on a quarterly basis during the first month of each quarter beginning in the third quarter after attending the FITT–STEMI consortium. At each local study site, the formalised feedback presentations were discussed in periodic and interactive sessions with members of the participating interdisciplinary STEMI treatment teams, including staff from the local EMS, physicians and nurses working in the ED and the emergency responding system, as well as staff from the catheterisation laboratory and interventional cardiologists. Particular attention was given to site-specific descriptive statistics with regard to improvements in treatment times elapsed from the first medical contact to direct hand-off in the catheterisation laboratory.
Data from two other infarct surveys were used to validate the completeness of our recruitment strategy, namely (a) insurance reimbursement data based on the International Statistical Classification of Diseases and Related Health Problems 10 (ICD-10) codes I21.0 to I21.3 for acute and subacute transmural infarction; and (b) data from the mandatory German hospital quality report on PCI procedures for the indication ‘ST-segment elevation myocardial infarction within 24 hours after ECG diagnosis’.27 The latter survey also included subacute myocardial infarctions, and the participation in the survey was compulsory for all certified PCI-capable catheterisation laboratories up to the year 2016. The stable percentages of annually included STEMI patients for the ICD-coded diagnosis of transmural infarction (69.7 ± 6.8 percentage points) as well as the considerable consilience with routine PCI procedures (95.6 ± 11.5 percentage points) underscore the overall integrity and completeness of the enrollment strategy used in the FITT–STEMI study.
Statistical analysis
Statistical analyses were performed using the SAS system (version 9.4). Raw data were used to calculate time intervals along the treatment pathway for each patient. Continuous data given as means and standard deviations were compared between the two groups of direct and non-direct transfer to the catheterisation laboratory using Student’s t tests. Categorical variables were analysed using chi-square tests. To assess whether bypassing the ED impacted on survival, a series of different logistic regression models were constructed with in-hospital mortality as the dependent variable and direct transfer as the independent variable adjusted for potential confounders known to be associated with outcomes. To search for potential factors that may account for the beneficial effect of direct transfer on survival, we optionally included door-to-balloon time as an interventional variable for in-hospital treatment time. For the models, a backward selection method was used to enter factors into the regression models. The results from these regression models are presented as odds ratios (ORs) with 95% confidence intervals (CIs). All analyses were two-tailed and a P value of 0.05 was considered statistically significant.
Results
Treatment times in patients with and without ED bypass
Although STEMI patients transported directly from the scene to the catheterisation laboratory differed with respect to age, sex and TIMI risk group from those not directly transported, there was a great overlap between the two groups (Supplementary Figure 1). In the total study cohort, angiographic results showed a high rate of successful revascularisation, as TIMI angiographic flow grade scoring 3 was achieved in more than nine out of 10 patients in the two groups (94.1% vs. 92.1%, P<0.0001). A detailed description of the total study population including the comparison between the groups with and without direct transfer is given in Table 1.
Table 1.
Baseline characteristics of PCI-treated STEMI patients transported by emergency medical services and stratified by direct transfer to the catheterization laboratory (ED bypass) versus non-direct transfer by the emergency department (non-ED bypass).
| Total study population (n=13,219) | ED bypass (n=6740; 51%) | Non-ED bypass (n=6479; 49%) | P value | |
|---|---|---|---|---|
| Demographic data | ||||
| Male gender | 9733 (74%) | 5080 (75%) | 4653 (72%) | <0.0001 |
| Age ± SD | 63.6 ± 12.9 | 63.0 ± 12.6 | 64.3 ± 13.2 | <0.0001 |
| Age >80 years | 1375 (10%) | 600 (9%) | 775 (12%) | <0.0001 |
| Body mass index (kg/m²) (mean, SD) | 27.5 ± 4.6 | 27.5 ± 4.5 | 27.4 ± 4.6 | 0.2161 |
| Clinical data | ||||
| Hypertension | 7845 (59%) | 3954 (59%) | 3891 (60%) | 0.1036 |
| Diabetes mellitus | 2281 (17%) | 1106 (16%) | 1175 (18%) | 0.0087 |
| Prior angina pectoris | 1709 (13%) | 895 (13%) | 814 (13%) | 0.2205 |
| Hyperlipoproteinaemia | 3849 (29%) | 1911 (28%) | 1938 (30%) | 0.0486 |
| Family history | 2511 (19%) | 1291 (19%) | 1220 (19%) | 0.6347 |
| Current smoker | 5577 (42%) | 2930 (43%) | 2647 (41%) | 0.0023 |
| Previous myocardial infarction | 1450 (11%) | 651 (10%) | 799 (12%) | <0.0001 |
| Previous stroke | 550 (4%) | 249 (4%) | 301 (5%) | 0.0062 |
| Previous angioplasty | 1,474 (11%) | 687 (10%) | 787 (12%) | 0.0004 |
| Previous CABG | 301 (2%) | 120 (2%) | 181 (3%) | 0.0001 |
| Renal failure | 619 (5%) | 248 (4%) | 371 (6%) | <0.0001 |
| Cardiopulmonary resuscitation | 1256 (10%) | 528 (8%) | 728 (11%) | <0.0001 |
| Cardiogenic shock | 1625 (12%) | 684 (10%) | 941 (15%) | <0.0001 |
| Intra-aortic balloon counterpulsation | 264 (2%) | 156 (2%) | 108 (2%) | 0.0078 |
| Off hours (nights/weekends) | 7607 (58%) | 3265 (48%) | 4342 (67%) | <0.0001 |
| Pre-hospital ECG | 12,202 (92%) | 6604 (98%) | 5598 (86%) | <0.0001 |
| Telemetry ECG | 3072 (23%) | 1924 (29%) | 1148 (18%) | <0.0001 |
| Pre-announcement by telephone | 10,877 (82%) | 6513 (97%) | 4364 (67%) | <0.0001 |
| TIMI risk score | ||||
| 0–2 | 4679 (35%) | 2592(38%) | 2087 (32%) | <0.0001 |
| 3–4 | 3787 (29%) | 1964 (29%) | 1823 (28%) | |
| 5–8 | 4102 (31%) | 1889 (28%) | 2213 (34%) | |
| >8 | 651 (5%) | 295 (4%) | 356 (5%) | |
| Treatment with glycoprotein IIb/IIIa receptor blockers (n=11,942) | 5143 (43%) | 2567 (42%) | 2576 (44%) | 0.0063 |
| Angiographic results | ||||
| No. of coronary arteries narrowed: | ||||
| 0 | 31 (0.2%) | 8 (0.1%) | 23 (0.4%) | <0.0001 |
| 1 | 5274 (40%) | 2748 (41%) | 2526 (39%) | |
| 2 | 4072 (31%) | 2143 (32%) | 1929 (30%) | |
| 3 | 3742 (28%) | 1796 (27%) | 1946 (30%) | |
| LMCA | 99 (0.8%) | 45 (0.7%) | 54 (0.8%) | |
| CTO in NIRA | 1494 (11%) | 691 (10%) | 803 (12%) | |
| Recanalisation vessel | ||||
| LAD | 5814 (44%) | 2912 (43%) | 2902 (45%) | <0.0001 |
| RCA | 5470 (41%) | 2939 (44%) | 2531 (39%) | |
| LCX | 1676 (13%) | 786 (12%) | 890 (14%) | |
| LMCA | 124 (1%) | 54 (0.8%) | 70 (1.1%) | |
| Graft | 134 (1%) | 49 (0.7%) | 85 (1.3%) | |
| ECG (STEMI site) | ||||
| Anterior | 5841 (44%) | 2927 (43%) | 2914 (45%) | <0.0001 |
| Inferior | 6577 (50%) | 3477 (52%) | 3100 (48%) | |
| Lateral | 672 (5%) | 298 (4%) | 374 (6%) | |
| LBBB | 129 (1%) | 38 (1%) | 91 (1%) | |
| TIMI angiographic flow grade before PCI | ||||
| Score 0–2 | 12,227 (92%) | 6281 (93%) | 5946 (92%) | 0.0022 |
| Score 3 | 991 (7%) | 459 (7%) | 532 (8%) | |
| TIMI angiographic flow grade after PCI | ||||
| Score 0–2 | 905 (7%) | 395 (6%) | 510 (8%) | <0.0001 |
| Score 3 | 12,313 (93%) | 6345 (94%) | 5968 (92%) | |
| Outcome | ||||
| In-hospital mortality rate | 1062 (8.0%) | 417 (6.2%) | 645 (10.0%) | <0.0001 |
Data are presented as percentages or means and standard deviations. P-values refer to the comparisons between the two groups.
STEMI: ST-segment elevation myocardial infarction; ED: emergency department; PCI: percutaneous coronary intervention; CABG: coronary artery bypass grafting; CTO: chronic total occlusion; LBBB: left bundle branch block; LCA: left coronary artery; LCX: left circumflex artery; LMCA: left main coronary artery; NIRA: non-infarct-related artery; RCA: right coronary artery; SD: standard deviation; TIMI: Thrombolysis In Myocardial Infarction.
Whereas scene-to-door time was longer in the group bypassing versus not bypassing the ED (18.3 ± 11.5 vs. 16.6 ± 22.8 minutes, P<0.0001), the mean door-to-catheterisation time was considerably shorter in the ED bypass group (5.4 ± 5.6 vs. 47.8 ± 38.8 minutes, P<0.0001) (Table 2). Likewise, the mean door-to-balloon time in the direct transfer group was 45 minutes shorter than in the non-bypassing group (P<0.0001; Figure 2). Overall, this resulted in a gain of treatment time from the first medical contact to balloon inflation of more than 44 minutes for the direct transfer group as compared to the group with a stop at the ED (76.5 ± 22.3 vs. 121.2 ± 47.3 minutes, P<0.0001). Notably, there was no difference in the cath-to-puncture time between directly and not directly transported patients (11.6 ± 6.6 vs. 11.8 ± 7.2, P=0.1937).
Table 2.
Relevant interventional time intervals in the two groups with direct and non-direct transportation to the catheterisation laboratory (ED bypass versus non-ED bypass).
| Total study population (n=13,219) |
ED bypass (n=6740) |
Non-ED bypass (n=6479) |
P value | |
|---|---|---|---|---|
| Symptom-to-contact (min) | 153.7 ± 221.4 | 152.6 ± 217.6 | 154.8 ± 225.3 | 0.5568 |
| Time at scene (min) | 22.9 ± 22.1 | 22.0 ± 11.4 | 23.8 ± 29.4 | <0.0001 |
| Transport time (min) | 17.4 ± 17.9 | 18.3 ± 11.5 | 16.6 ± 22.8 | <0.0001 |
| Door-to-cath time (min) | 26.2 ± 34.7 | 5.4 ± 5.6 | 47.8 ± 38.8 | <0.0001 |
| Cath-to-puncture time (min) | 11.7 ± 6.9 | 11.6 ± 6.6 | 11.8 ± 7.2 | 0.1937 |
| Puncture-to-balloon time (min) | 20.6 ± 12.9 | 19.2 ± 12.0 | 22.0 ± 13.6 | <0.0001 |
| Door-to-balloon time (min) | 58.5 ± 39.4 | 36.2 ± 14.8 | 81.6 ± 43.4 | <0.0001 |
| Contact-to-balloon time (min) | 98.4 ± 43.0 | 76.5 ± 22.3 | 121.2 ± 47.3 | <0.0001 |
Data are given as means and standard deviations.
ED: emergency department.
Figure 2.

Frequencies of door-to-balloon time intervals as demonstrated by histograms separately for percutaneous coronary intervention (PCI)-treated ST-segment elevation myocardial infarction (STEMI) patients with direct transfer to the catheterisation laboratory bypassing the emergency department (ED) (a) and with indirect transfer to the catheterisation laboratory due to a transient stop in the ED (b).
In patients presenting with cardiogenic shock, symptom-to-contact (P=0.1654) and contact-to-door times (P=0.0692) did not significantly differ with regard to ED bypass (Supplementary Table 1). However, the mean contact-to-balloon time was 49 minutes shorter in haemodynamically unstable patients with ED bypass as compared to their not directly transported counterparts (P<0.0001). For stable patients with no clinical signs of cardiogenic shock, this gain in time to PCI treatment was in the same range (43 minutes).
Reasons for non-direct transfer to the catheterisation laboratory
Despite a universal strategy among all study sites for pre-arrival notification of the catheterisation laboratory by the emergency medical transportation team and an endorsement for direct transport to the catheterisation laboratory, approximately half of the total study cohort was not directly transferred (49%). Using a standardised item on the case report form allowing multiple answers, we assessed the reasons for failed direct transfer. Direct transport was failed due to late arrival of the catheterisation laboratory staff (27.8%), no announcement or failure of timely announcement by EMS (20.0%), ambiguous pre-hospital STEMI diagnosis (17.1%), occupancy of the catheterisation laboratory by another patient at the time of arrival (16.6%), time-consuming primary care in the ED (10.2%), elaborate diagnostic procedures to exclude significant comorbidity including computed tomography (0.7%) and miscellaneous causes (14.9%). In summary, the reasons for failed direct transmission were often multifactorial, most probably due to problems in pre-hospital diagnosis and delays in catheterisation laboratory team readiness.
Overall beneficial impact of ED bypass on survival
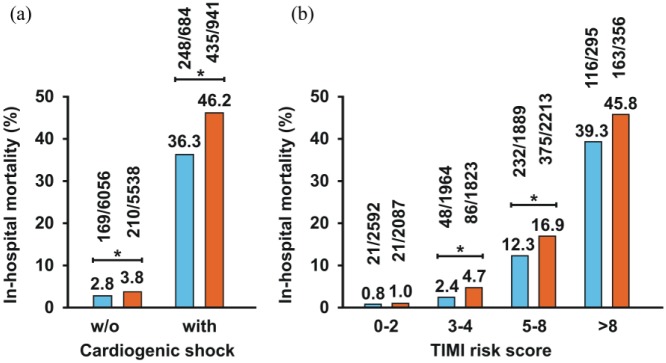
Among the study participants who went through the ED, there were 645 deaths (10.0%), whereas mortality was significantly lower in the patient group brought directly to the catheterisation laboratory (417 deaths, 6.2%, P<0.0001). The improved survival related to ED bypass was observed for both haemodynamically stable (n =11,594, 2.8% vs. 3.8%, P=0.0024) and unstable patients with cardiogenic shock (n=1625, 36.3% vs. 46.2%, P<0.0001) (Figure 3(a)). Corroborating these findings, in all four predefined TIMI risk score subgroups, mortality was lower in patients bypassing the ED (Figure 3(b)). Using a logistic regression model with in-hospital mortality as the dependent variable adjusted to the TIMI risk score, we confirmed that direct transport to the catheterisation laboratory was a statistically significant and independent predictor of better survival (OR 0.64, 95% CI 0.56–0.74, P<0.0001; Table 3).
Figure 3.
Mortality rates in percutaneous coronary intervention (PCI)-treated ST-segment elevation myocardial infarction (STEMI) patients with (blue columns) and without (red columns) emergency department bypass by cardiogenic shock (a) and predefined Thrombolysis In Myocardial Infarction (TIMI) risk score intervals (b). Significant group differences are marked with asterisks.
Table 3.
Logistic regression model with in-hospital mortality as dependent variable and contact-to-door time as independent variable adjusted for TIMI risk score.
| Variable | Odds ratio | 95% CI | P value |
|---|---|---|---|
| ED bypass | 0.639 | 0.555–0.736 | <0.0001 |
| Contact-to-door time | 1.030 | 1.026–1.034 | <0.0001 |
| TIMI risk score | |||
| 3–4 vs. ⩽2 | 3.817 | 2.687–5.424 | <0.0001 |
| 5–8 vs. ⩽2 | 17.164 | 12.492–23.582 | |
| >8 vs. ⩽2 | 71.341 | 50.518–100.748 | |
ED: emergency department; TIMI: Thrombolysis In Myocardial Infarction; CI: confidence interval.
The reduced mortality in ED bypass patients is achieved through shorter treatment times
We then considered whether the positive impact of ED bypass on outcome resulted from a gain in time to reperfusion therapy. To this end, we computed a second regression model again using the TIMI risk score and contact-to-door time as confounders and, in addition to ED bypass, entered door-to-balloon time as an independent and competing variable. In contrast to the first model, direct transfer completely lost its significance in predicting outcome (OR 0.88, 95% CI 0.74–1.04, P=0.1313), whereas in this model shorter door-to-balloon time was a highly significant predictor of better survival (P<0.0001; Table 4).
Table 4.
Similar model to that demonstrated in Table 3 except that door-to-balloon time was additionally entered as a clinically relevant confounder.
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| ED bypass | 0.877 | 0.740–1.040 | 0.1313 |
| Contact-to-door time | 1.030 | 1.026–1.033 | <0.0001 |
| Door-to-balloon time | 1.006 | 1.004–1.008 | <0.0001 |
| TIMI risk score | |||
| 3–4 vs. ⩽2 | 3.700 | 2.603–5.258 | <0.0001 |
| 5–8 vs. ⩽2 | 16.365 | 11.908–22.491 | |
| >8 vs. ⩽2 | 66.964 | 47.392–94.620 | |
ED: emergency department; TIMI: Thrombolysis In Myocardial Infarction; OR: odds ratio; CI: confidence interval.
Similar results were obtained in two independent models when a set of clinically relevant confounders was substituted for the TIMI risk score. Again, ED bypass was a highly significant predictor of better survival (OR 0.77, 95% CI 0.65–0.91, P=0.0027, Supplementary Table 2), but completely lost its predictive role (P=0.6897) when door-to-balloon time was additionally entered as a significant variable (P<0.0001; Supplementary Table 3). These observations suggest that the beneficial effect of ED bypass resulted simply from the prevention of a treatment delay during the time from hospital arrival to restoring coronary blood flow.
Similar findings as for the total study population were also revealed in adjusted models including only patients with cardiogenic shock. Again, ED bypass was a significant predictor of better survival in shock patients (OR 0.69, 95% CI 0.54–0.88, P=0.0028), but lost its significance for predicting death (P=0.8756) when competing with door-to-balloon time entered as an additional and highly significant confounder (P<0.0001).
Discussion
The present paper demonstrates outcome data of the FITT–STEMI trial, a prospective and multicentre study designed to evaluate the association between treatment delays from first medical contact to PCI and in-hospital mortality in a large, unselected cohort of consecutive STEMI patients. Results showed that mortality was significantly lower in the group of STEMI patients with direct transmission to the catheterisation laboratory compared with their non-directly transmitted counterparts. Notably, the gain in survival resulting from ED bypass was observed for both haemodynamically stable and unstable patients presenting with cardiogenic shock. In particular, the latter group benefitted most from direct transport to the catheterisation laboratory, as determined by the low number needed to be treated to save one additional life.
Although the decision for direct transport was generally favoured in younger STEMI patients with less medical comorbidity and a lower TIMI risk score, the highly significant relationship between survival and direct transport remained stable also in models adjusted for these clinically relevant confounders. Another important finding of our study is that, when door-to-balloon time was entered in these models as an independent variable to compete with direct transport in its relevance for in-hospital survival, the bypassing of the ED completely lost its predictive value, whereas shorter treatment time from hospital arrival to balloon inflation became a highly associated predictor of better survival. The prognosis with regard to in-hospital mortality was much better in the ED bypass group, because time-consuming delays associated with the transient stop at the ED were prevented. This underscores the assumption that the beneficial effect of bypassing the ED on survival simply resulted from a gain in time to revascularisation.
By collecting detailed information on treatment times along the entire pre- and intra-hospital treatment pathways, we found that nearly all STEMI patients bypassing the ED with direct transport to the catheterisation laboratory had received a pre-hospital ECG recording and were pre-announced by the EMS. We observed that this did not prolong treatment times at the scene, whereas transport times were minimally longer in this group. This finding could be related to the fact that longer transport times increased the prospects of direct transport to the catheterisation laboratory due to timely activation of the catheterisation laboratory team. In total, in STEMI patients bypassing the ED, we measured no delay in pre-hospital treatment times (contact-to-door), whereas in-hospital times (door-to-balloon) were markedly reduced. This gain in time to reperfusion was exclusively related to the reduction in the time from arrival at the hospital to admission to the catheterisation laboratory.
Our data are in line with recently published studies demonstrating that field triage and the bypassing of the ED in STEMI patients transported by EMS resulted in reduced in-hospital treatment times. Although several studies have supported the idea that a direct-access catheterisation laboratory pathway for timely PCI treatment shortens the time interval to reperfusion therapy and ameliorates hypoxia-induced left ventricular dysfunction, the impact of this approach on survival is still controversial.26 Most studies failed to show a significant effect on mortality in adjusted models or did not exclude transfer patients and patients who did not receive PCI treatment.12,13,15,17 In a single-centre study, Farshid and colleagues observed an improved prognosis in those 190 PCI-treated STEMI patients in whom catheterisation laboratory activation was initiated prior to hospital arrival (24% of all study participants) compared with those with in-hospital catheterisation laboratory activation.21 In a total of 1859 study participants, Estévez-Loureiro et al. demonstrated a long-term prognostic benefit of field triage and direct transfer to the catheterisation laboratory when compared with patients transmitted to the ED.19 However, the proportion of patients transferred by the two treatment pathways was imbalanced as only 425 patients (23%) of the total study cohort were transported directly to PCI treatment and, in addition, the use of the glycoprotein IIb/IIIa receptor antagonist abciximab was higher in the direct transfer group.19 In a report from the AHA Mission Lifeline Program with data from 12,581 STEMI patients with a pre-hospital ECG diagnosis, ED bypass occurred only in 1316 patients (10.5%) and was associated numerically with a lower mortality. The mortality in multivariate analysis, however, was similar in the two groups with and without ED bypass.18
Both the number of study participants with STEMI diagnosis and the ED bypass rate were considerably higher in our large multicentre study compared with previous reports. Our findings confirmed that the algorithm to shorten intervention times from the first medical contact in the field to in-hospital mechanical reperfusion by regular feedback-driven quality controls is feasible and successful in local networks of STEMI care.27 As shown in our previous feasibility studies, formalised interactive data feedback led to an increased number of STEMI patients bypassing the ED in both single-centre and multicentre settings.29,30 However, only half of our total STEMI population was transported directly to the catheterisation laboratory, despite the general endorsement of preventing delays by activating the cardiac catheterisation call team already in the pre-hospital setting.
The bypassing of the ED is a complex process and requires strong collaboration between different systems and treatment groups, which includes correct ECG-based STEMI diagnosis in the field as well as the bypassing of non-PCI hospitals and pre-activation of catheterisation laboratory teams. If the patient arrives at the PCI hospital during off hours before the cardiac intervention team, there is a need for the availability of in-hospital urgent care teams to receive the patient at the catheterisation laboratory. For the first time, our study protocol systematically determined both system- and patient-related reasons for the non-bypassing of the ED. Failed pre-announcement by EMS and late arrival of the catheterisation laboratory staff during off hours were identified as relevant factors impeding direct transfer, which raises the potential for further improvements.
Although the percentage of direct transfers achieved in unstable STEMI patients was lower compared with stable patients (42.1% vs. 52.2%), they benefitted considerably from ED bypass with respect to outcome. In the group of shock patients, ED bypass was significantly associated with better survival, as revealed in univariate comparison and multivariate regression models. One additional life out of 10 PCI-treated patients with cardiogenic shock can be saved when these patients are directly transported to the catheterisation laboratory. Given this low number needing to be treated, more effort should be made to increase the number of direct transports particularly in this high-risk STEMI group.
Limitations
The findings from this prospective study need to be interpreted carefully in the light of several limitations, which mainly result from the observational design as a randomised controlled trial not being feasible due to ethical considerations. In the absence of a randomisation procedure, causality between direct transport to the catheterisation laboratory and mortality cannot formally be concluded. Although we noted that indirect transfer by way of the ED was associated with a considerable delay from hospital arrival to PCI treatment, unmeasured confounding variables and selection bias may impact on decision-making for direct/indirect transfer. To address this important limitation, we used a standardised questionnaire to assess reasons for system delays in those patients not directly transported to the catheterisation laboratory. In addition, the prognostic value of ED bypass may be less pronounced in STEMI management care systems in other countries, in which physicians experienced in emergency medicine do not participate in EMS as they do in Germany.28 However, the beneficial effect of ED bypass can most likely be extrapolated to other health services with similar sophisticated EMS systems and high numbers of PCI centres as in most other developed countries, regardless of whether the EMS are physician-manned or exclusively paramedic-staffed. Finally, only in-hospital mortality data were available in our study, and future studies are needed to examine the association between in-hospital delays before PCI treatment and long-term outcomes.
In summary, outcome data from the prospective FITT–STEMI trial indicate the prognostic significance of ED bypass to improve the process of care in STEMI patients treated by PCI. Our adjusted models support the conclusion that the improved prognosis observed for those patients directly transferred to the catheterisation laboratory simply results from their reduced time to PCI treatment. Based on this finding, we encourage existing PCI-capable networks to promote their healthcare system readiness and response to STEMI by pre-hospital announcement of STEMI diagnosis and direct access to the catheterisation laboratory. Protocols favouring the transport of patients from the field directly to the catheterisation laboratory may improve the survival of both haemodynamically stable and unstable patients in established local STEMI treatment networks. In our view, these recommendations do not only apply to the healthcare system in Germany where the data were collected, but can be extrapolated to other settings as faster treatment is very likely to result in improved patient outcomes.
Supplemental Material
Supplemental material, Scholz_et_al_Supplemental_data for Prognostic significance of emergency department bypass in stable and unstable patients with ST-segment elevation myocardial infarction by Karl Heinrich Scholz, Tim Friede, Thomas Meyer, Claudius Jacobshagen, Björn Lengenfelder, Jens Jung, Claus Fleischmann, Hiller Moehlis, Hans G Olbrich, Rainer Ott, Albrecht Elsässer, Stephen Schröder, Christian Thilo, Werner Raut, Andreas Franke, Lars S Maier and Sebastian KG Maier in European Heart Journal: Acute Cardiovascular Care
Footnotes
Conflict of interest: Tim Friede reports personal fees for consultancies (including data monitoring committees) from Novartis, Bayer, Biogen, AstraZeneca, Janssen, Grünenthal, Pharmalog, SGS and Roche, all outside the submitted work. Furthermore, he has received research funding by the European Commission for statistical analyses on the EUTrigTreat (NCT01209494) and EU-CERT-ICD (NCT02064192) clinical studies. All relationships declared are modest. All other authors declare no conflict of interest.
Funding: This study was supported by a grant from the German Heart Foundation and the Arbeitsgemeinschaft Leitender Kardiologischer Krankenhausärzte to KHS.
List of contributors: (in order of number of patients included up to 31 December 2015) Universitätsklinikum Göttingen (Claudius Jacobshagen, Kristina Schröder, Swetlana Hartmann, Lars S. Maier); Universitätsklinikum Würzburg (Björn Lengenfelder, Verena Reinhart, Sebastian K.G. Maier); St. Bernward-Krankenhaus Hildesheim (Karl H. Scholz, Dorothee Ahlersmann); Helios Klinikum Krefeld (Rainer Ott, Heinrich G. Klues, Alexander Bufe); Klinikum Oldenburg (Albrecht Elsässer, Susanne Grafmüller, Annette Schütz); Klinikum Wolfsburg (Claus Fleischmann, Rolf Engberding, Rüdiger Becker); Klinikum Darmstadt (Gerald S. Werner, Hiller Moehlis); Asklepios Klinik Langen (Hans G. Olbrich, Kerstin Eck); Städtisches Klinikum München Neuperlach (Harald Mudra, Martin Hug, Anamaria Stote); Klinikum Ingolstadt (Harald Franck, Monika-Krista Zackl, Karlheinz Seidl); Klinik am Eichert Göppingen (Stephen Schröder, Marion Steindl, Josef Steindl, Sophia Atseles); Klinikum Worms (Jens Jung, Birgit Nicklas); Klinikum Augsburg (Christian Thilo, Georg Waidhauser, Wolfgang v. Scheidt); Universitätsklinikum Jena (Attila Yilmaz, Hans R. Figulla, Daniel Kretzschmar, Corinna Schneider, Christian Schulze); Klinikum Lüneburg (Christian Weiß, Claus H. Müller); Krankenhaus Buchholz (Werner Raut, Klaus Hertting); Krankenhaus Landshut-Achdorf (Bernhard Zrenner, Josef Haimerl, Ute Zrenner); KRH Klinikum Hannover-Siloah (Andreas Franke, Jan Fürste); Klinikum Lippe-Detmold (Dirk Härtel, Melanie Kriete, Ulrich Tebbe, Stephan Gielen); Klinikum Leer (Christian Vahlhaus, Ralf-G. Pretzsch); Klinikum Ludwigsburg (Ralph Berroth, Joachim Geiger, Friederike Wunsch, Christian Wolpert); Robert-Bosch-Krankenhaus Stuttgart (Stephan Hill, Andrea Bullinger, Udo Sechtem); Klinikum Deggendorf (Edmond Skenderaj, Ulrich Valta-Seufzer, Martin Giesler); Kreiskrankenhaus Eschwege (Marco Lubitz, Peter Schott); Regio Klinikum Pinneberg (Konrad Gorski, Thomas Hofmann); Klinikverbund Kempten-Oberallgäu (Carsten Bauer, Wulf Ito); Klinikum Viersen (Nicolas v. Beckerath); Medizinische Hochschule Hannover (Jörn Tongers, Benedikta Ritter, Karin Hohenleitner); Sana Kliniken Lübeck (Hans Martin Grusnick, Joachim Weil); Klinikum St. Elisabeth Straubing (Sebastian K.G. Maier, Elke Grassl); Klinikum Regiomed-Kliniken Coburg (Caroline Kleinecke, Andrea Linss, Kerstin Truthan, Hans-Joachim Goller, Johannes Brachmann); Asklepios Harzklinik Goslar (Gaby Lehnert, Stefan Lange, Tobias Steffen, Arnd B. Buchwald, Christoph Engelhardt); Kliniken Ostallgäu-Kaufbeuren, Füssen (Simon Delladio, Martin Hinterseer, Myriam Parvanov); Hermann-Josef-Krankenhaus Erkelenz (Klaus Dieter Winter, Christina Ziesen); Kliniken Maria Hilf Mönchengladbach (Jürgen vom Dahl, Dierk Rulands); SLK Kliniken Heilbronn (Marcus Hennersdorf, Jens Martin Maier, Eva Schropp); Krankenhaus Rothenburg ob der Tauber (Christian Wacker); Kreiskrankenhaus Dormagen (Benjamin Orth, Georg Haltern); Marienkrankenhaus Soest (Roland Bürger, Markus Flesch); SLK Kliniken Am Plattenwald Bad Friedrichshall (Thomas Dengler); Universitätsklinikum Regensburg (Christina Strack, Dierk Endemann, Lars S. Maier); Klinikum Landkreis Erding (Lorenz Bott-Flügel); Klinikum Neumarkt (Veronika Lingg); Krankenhaus Bethanien Moers (Alexander Donath, Stefan Möhlenkamp); Vinzenzkrankenhaus Hannover (Beate Bugdoll, Petra Wucherpfennig, Jan-Bernd Schüttert, Christian Zellerhoff); Krankenhaus Henriettenstift Hannover (Thomas Weiss, Thorsten Grundmann); Evangelisches Krankenhaus Bethesda Mönchengladbach (Thomas Lickfeld); DRK-Krankenhaus Clementinenhaus Hannover (Heinz-Peter Remmlinger).
References
- 1. Bradley EH, Herrin J, Wang Y, et al. Strategies for reducing the door-to-balloon time in acute myocardial infarction. N Engl J Med 2006; 355: 2308–2320. [DOI] [PubMed] [Google Scholar]
- 2. Francone M, Bucciarelli-Ducci C, Carbone I, et al. Impact of primary coronary angioplasty delay on myocardial salvage, infarct size, and microvascular damage in patients with ST-segment elevation myocardial infarction: insight from cardiovascular magnetic resonance. J Am Coll Cardiol 2009; 54: 2145–2153. [DOI] [PubMed] [Google Scholar]
- 3. Leurent G, Fougerou C, Pennec PY, et al. Door-to-balloon delays before primary angioplasty in the Regional Acute Myocardial Infarction Registry of Brittany. An analysis of the Observatoire Régional Breton sur l’Infarctus du myocarde (ORBI). Arch Cardiovasc Dis 2009; 102: 777–784. [DOI] [PubMed] [Google Scholar]
- 4. Baran KW, Kamrowski KA, Westwater JJ, et al. Very rapid treatment of ST-segment elevation myocardial infarction: utilizing prehospital electrocardiograms to bypass the emergency department. Circ Cardiovasc Qual Outcomes 2010; 3: 431–437. [DOI] [PubMed] [Google Scholar]
- 5. Grosgurin O, Plojoux J, Keller PF, et al. Prehospital emergency physician activation of interventional cardiology team reduces door-to-balloon time in ST-elevation myocardial infarction. Swiss Med Wkly 2010; 140: 228–232. [DOI] [PubMed] [Google Scholar]
- 6. Studnek JR, Garvey L, Blackwell T, et al. Association between prehospital time intervals and ST-elevation myocardial infarction system performance. Circulation 2010; 122: 1464–1469. [DOI] [PubMed] [Google Scholar]
- 7. Terkelsen CJ, Sørensen JT, Maeng M, et al. System delay and mortality among patients with STEMI treated with primary percutaneous coronary intervention. JAMA 2010; 304: 763–771. [DOI] [PubMed] [Google Scholar]
- 8. Müller UM, Eitel I, Eckrich K, et al. Impact of minimising door-to-balloon times in ST-elevation myocardial infarction to less than 30 min on outcome: an analysis over an 8-year period in a tertiary care centre. Clin Res Cardiol 2011; 100: 297–309. [DOI] [PubMed] [Google Scholar]
- 9. Sanchez-Ross M, Oghlakian G, Maher J, et al. The STAT–MI (ST-Segment Analysis Using Wireless Technology in Acute Myocardial Infarction) trial improves outcomes. JACC Cardiovasc Interv 2011; 4: 222–227. [DOI] [PubMed] [Google Scholar]
- 10. Cockburn J, Karimi K, Hoo S, et al. Outcomes by day and night for patients bypassing the emergency department presenting with ST-segment elevation myocardial infarction identified with a pre-hospital electrocardiogram. J Interv Cardiol 2015; 28: 24–31. [DOI] [PubMed] [Google Scholar]
- 11. van de Loo A, Saurbier B, Kalbhenn J, et al. Primary percutaneous coronary intervention in acute myocardial infarction: direct transportation to catheterization laboratory by emergency teams reduces door-to-balloon time. Clin Cardiol 2006; 29: 112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Steg PG, Cambou JP, Goldstein P, et al. Bypassing the emergency room reduces delays and mortality in ST elevation myocardial infarction: the USIC 2000 registry. Heart 2006; 92: 1378–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Carstensen S, Nelson GC, Hansen PS, et al. Field triage to primary angioplasty combined with emergency department bypass reduces treatment delays and is associated with improved outcome. Eur Heart J 2007; 28: 2313–2319. [DOI] [PubMed] [Google Scholar]
- 14. Dorsch MF, Greenwood JP, Priestley C, et al. Direct ambulance admission to the cardiac catheterization laboratory significantly reduces door-to-balloon times in primary percutaneous coronary intervention. Am Heart J 2008; 155: 1054–1058. [DOI] [PubMed] [Google Scholar]
- 15. Majumder B, Mavroudis C, Smith C, et al. Superior outcome with direct catheter laboratory access vs ED-activated primary percutaneous coronary intervention. Am J Emerg Med 2012; 30: 1118–1124. [DOI] [PubMed] [Google Scholar]
- 16. Antman EM. Bypassing the emergency department to improve the process of care for ST-elevation myocardial infarction: necessary but not sufficient. Circulation 2013; 128: 322–324. [DOI] [PubMed] [Google Scholar]
- 17. Bagai A, Al-Khalidi HR, Muñoz D, et al. Bypassing the emergency department and time to reperfusion in patients with prehospital ST-segment elevation: findings from the reperfusion in acute myocardial infarction in Carolina Emergency Departments project. Circ Cardiovasc Interv 2013; 6: 399–406. [DOI] [PubMed] [Google Scholar]
- 18. Bagai A, Jollis JG, Dauerman HL, et al. Emergency department bypass for ST-segment elevation myocardial infarction patients identified with a prehospital electrocardiogram: a report from the American Heart Association Mission: Lifeline program. Circulation 2013; 128: 352–359. [DOI] [PubMed] [Google Scholar]
- 19. Estévez-Loureiro R, Calviño-Santos R, López-Sainz A, et al. Long-term prognostic benefit of field triage and direct transfer of patients with ST-segment elevation myocardial infarction treated by primary percutaneous coronary intervention. Am J Cardiol 2013; 111: 1721–1726. [DOI] [PubMed] [Google Scholar]
- 20. Anderson LL, French WJ, Peng SA, et al. Direct transfer from the referring hospitals to the catheterization laboratory to minimize reperfusion delays for primary percutaneous coronary intervention: insights from the National Cardiovascular Data Registry. Circ Cardiovasc Interv 2015; 8: e002477. [DOI] [PubMed] [Google Scholar]
- 21. Farshid A, Allada C, Chandrasekhar J, et al. Shorter ischaemic time and improved survival with pre-hospital STEMI diagnosis and direct transfer for primary PCI. Heart Lung Circ 2015; 24: 234–240. [DOI] [PubMed] [Google Scholar]
- 22. Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018; 39: 119–177. [DOI] [PubMed] [Google Scholar]
- 23. O’Gara PT, Kushner FG, Ascheim DD, et al. 2013 ACCF/AHA Guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation 2013; 127: e362–e425. Erratum in: Circulation 2013; 128: e481. [DOI] [PubMed] [Google Scholar]
- 24. Hagiwara MA, Bremer A, Claesson A, et al. The impact of direct admission to a catheterisation lab/CCU in patients with ST-elevation myocardial infarction on the delay to reperfusion and early risk of death: results of a systematic review including meta-analysis. Scand J Trauma Resusc Emerg Med 2014; 22: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sandouk A, Ducassé JL, Grolleau S, et al. Compliance with guidelines in patients with ST-segment elevation myocardial infarction after implementation of specific guidelines for emergency care: results of RESCA+31 registry. Arch Cardiovasc Dis 2012; 105: 262–270. [DOI] [PubMed] [Google Scholar]
- 26. Silvain J, Vignalou JB, Beygui F, et al. Impact of transfer time on mortality in acute coronary syndrome with ST-segment elevation treated by angioplasty. Arch Cardiovasc Dis 2012; 105: 639–648. [DOI] [PubMed] [Google Scholar]
- 27. Scholz KH, Maier SKG, Maier LS, et al. Impact of treatment delay on mortality in ST-segment elevation myocardial infarction (STEMI) patients presenting with and without haemodynamic instability: results from the German prospective, multicentre FITT–STEMI trial. Eur Heart J 2018, 39: 1065–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wijns W, Naber CK. Reperfusion delay in patients with high-risk ST-segment elevation myocardial infarction: every minute counts, much more than suspected. Eur Heart J 2018; 39: 1075–1077. [DOI] [PubMed] [Google Scholar]
- 29. Scholz KH, Hilgers R, Ahlersmann D, et al. Contact-to-balloon time and door-to-balloon time after initiation of a formalized data feedback in patients with acute ST-elevation myocardial infarction. Am J Cardiol 2008; 101: 46–52. [DOI] [PubMed] [Google Scholar]
- 30. Scholz KH, Maier SK, Jung J, et al. Reduction in treatment times through formalized data feedback: results from a prospective multicenter study of ST-segment elevation myocardial infarction. JACC Cardiovasc Interv 2012; 5: 848–857. [DOI] [PubMed] [Google Scholar]
- 31. Hochman JS, Sleeper LA, Webb JG, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. N Engl J Med 1999; 341: 625–634. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Scholz_et_al_Supplemental_data for Prognostic significance of emergency department bypass in stable and unstable patients with ST-segment elevation myocardial infarction by Karl Heinrich Scholz, Tim Friede, Thomas Meyer, Claudius Jacobshagen, Björn Lengenfelder, Jens Jung, Claus Fleischmann, Hiller Moehlis, Hans G Olbrich, Rainer Ott, Albrecht Elsässer, Stephen Schröder, Christian Thilo, Werner Raut, Andreas Franke, Lars S Maier and Sebastian KG Maier in European Heart Journal: Acute Cardiovascular Care