Notes
Editorial note
This review has been superseded by Cochrane Reviews: 'Antithrombotics after infra‐inguinal bypass grafting' (https://doi.org/10.1002/14651858.CD015141) and 'Antithrombotics after infra‐inguinal peripheral endovascular treatment' (https://doi.org/10.1002/14651858.CD015142).
Abstract
Background
Peripheral arterial disease (PAD) is frequently treated by either an infrainguinal autologous (using the patient's own veins) or synthetic graft bypass. The rate of occlusion of the graft after one year is between 12% and 60%. To prevent occlusion, patients are treated with an antiplatelet or antithrombotic drug, or a combination of both. Little is known about which drug is optimal to prevent infrainguinal graft occlusion. This is an update of a Cochrane review first published in 2003.
Objectives
To evaluate whether antithrombotic treatment improves graft patency, limb salvage and survival in patients with chronic PAD undergoing infrainguinal bypass surgery.
Search methods
The Cochrane Peripheral Vascular Diseases Group searched their Specialised Register (last searched August 2010) and the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 3).
Selection criteria
Randomised, controlled trials; two review authors independently assessed the methodological quality of each trial using a standardised checklist.
Data collection and analysis
Data collected included patient details, inclusion and exclusion criteria, type of graft, antithrombotic therapy, outcomes, and side effects.
Main results
A total of 14 trials were included in this review; 4970 patient results were analysed. Four trials evaluating vitamin K antagonists (VKA) versus no VKA suggested that oral anticoagulation may favour autologous venous, but not artificial, graft patency as well as limb salvage and survival. Two other studies comparing VKA with aspirin (ASA) or aspirin and dipyridamole provided evidence to support a positive effect of VKA on the patency of venous but not artificial grafts. Three trials comparing low molecular weight heparin (LMWH) to unfractionated heparin (UFH) failed to demonstrate a significant difference on patency. One trial comparing LMWH with placebo found no significant improvement in graft patency over the first postoperative year in a population receiving aspirin. One trial showed an advantage for LMWH versus aspirin and dipyridamol at one year for patients undergoing limb salvage procedures. Perioperative administration of ancrod showed no greater benefit when compared to unfractionated heparin. Dextran 70 showed similar graft patency rates to LMWH but a significantly higher proportion of patients developed heart failure with dextran.
Authors' conclusions
Patients undergoing infrainguinal venous graft are more likely to benefit from treatment with VKA than platelet inhibitors. Patients receiving an artificial graft benefit from platelet inhibitors (aspirin). However, the evidence is not conclusive. Randomised controlled trials with larger patient numbers are needed in the future to compare antithrombotic therapies with either placebo or antiplatelet therapies.
Plain language summary
Antithrombotic drugs to prevent further blood vessel blockage after bypass surgery using vein grafts obtained from the same person (autologous) or artificial grafts in the legs
Lower limb atherosclerosis can lead to blocked blood vessels causing pain on walking (intermittent claudication) or, if more severe, pain at rest, ulceration and gangrene (critical limb ischaemia).
Surgery to bypass the blockage uses either a piece of vein from another part of the person’s body or a synthetic graft. The bypass may help improve blood supply to the leg but the graft can also become blocked, even in the first year. To help prevent this, people are given aspirin (an antiplatelet drug) or a vitamin K antagonist (anti‐blood clotting or antithrombotic drug), or both, to try to stop loss of blood flow through the graft (patency). The review of trials found that patients undergoing venous grafts were more likely to benefit from treatment with vitamin K antagonists than platelet inhibitors. Patients receiving an artificial graft may benefit from platelet inhibitors (aspirin). However, the evidence is not conclusive. Although a total of 14 randomised, controlled trials involving 4970 patients were included in the review, trials with larger patient numbers are needed. This is because there was considerable variation between the included trials in whether patients received both types of drugs, anticoagulation levels and how they were measured, and the indications for surgery, intermittent claudication or critical limb ischaemia.
Background
Description of the condition
Lower limb atherosclerosis may manifest as pain on walking (intermittent claudication) or, if more severe, pain at rest, ulceration and gangrene (critical limb ischaemia). Intermittent claudication (IC) corresponds to Fontaine's classification (Fontaine 1954) stage II and critical limb ischaemia (CLI) refers to stages III and IV. In selected patients, treatment includes placement of a femoropopliteal or femorodistal bypass graft to divert blood past the occluded arterial segment, thereby improving blood perfusion of the limb, relieving the symptoms of claudication or rest pain, and avoiding amputation because of ulceration and gangrene (limb salvage). Several different materials may be used for bypass grafting. These include a section of the patient's own vein (autologous vein graft), an artificial graft material such as dacron or polytetrafluoroethylene (PTFE), treated human umbilical vein (taken from an umbilical cord), or a combination of these materials. Graft patency is dependent on many factors including the indication for surgery (IC or CLI), quality of arterial inflow and outflow, type of graft used (Cochrane 2010), operative technique, progression of atherosclerosis in the proximal or distal arteries, and graft stenosis due to remodelling and intimal hyperplasia (IH) (a narrowing of the graft due to excessive formation of cells in the inner lining).
Description of the intervention
There is evidence that patients with lower limb atherosclerosis frequently have a prothrombotic tendency (Swedenborg 1996; Woodburn 1996). The body's physiological stress response to surgery adds further to this. Additionally, platelets are implicated in the initial steps of IH development (Davies 1994). Therefore, a key question is whether postoperative treatment following infrainguinal bypass grafting should include long or short‐term antiplatelet drugs or anticoagulants in order to prevent graft occlusion (Kretschmer 1999) and, if so, whether the same treatment is optimal for all kinds of grafts and patients. Several reviews have been published (Cochrane 2010; Girolami 2000; Kraiss 1995; Lindblad 1995; Tangelder 1999; Watson 1999) trying to address these questions. A separate Cochrane review has been published on antiplatelet treatment in patients with symptomatic PAD undergoing infrainguinal bypass (Cochrane 2008); this present review will focus on the role of anticoagulants.
Why it is important to do this review
The overall one year incidence of graft failure (graft occlusion) for above‐knee femoropopliteal bypass grafts is 12% to 18% when a vein is used (Achermann 1998; Johnson 2000) and 20% to 25%% when PTFE is used (Abbott 1997; Johnson 2000). These figures rise to approximately 30% and 40%, respectively, for below‐knee popliteal grafts (Consensus 1991); 30% and 60% for femorotibial bypass grafts (Consensus 1991). Approximately 5% to 25% will fail within one month of the procedure (in the acute phase) depending on the type of graft material used; the highest failure rates are for prosthetic grafts to the tibial arteries (Parsons 1996). Almost 10% of early failures are due to the consequences of technical error (Alback 1998; Stept 1987) or to thrombogenic (clot forming) graft material (Hanson 1987). The remaining 5% to 10% are considered as unexpected early graft failures and might be caused by an increased prothrombotic state. Eighty per cent of all graft failures will occur within the first two postoperative years, due to development of graft stenosis (Bandyk 1987). Stenosis continues to develop at a rate of approximately 5% to 7% per year causing late vein graft failure (Schoder 2001). The Transatlantic Inter‐Society Consensus (TASC) for the management of peripheral arterial disease (Norgren 2007) states a five‐year patency rate of infrainguinal above‐knee bypass grafts of 74% to 76% for vein and 39% to 52% for PTFE grafts. If blood flow in the failed graft cannot be restored and further bypass surgery is not possible, then limb perfusion may, in some cases, be so poor that the limb cannot remain viable and amputation is required. Successful prevention of graft failure, and the need for surgical re‐intervention, is of major clinical and economic importance (Robinson 1997).
Objectives
To determine the efficacy of pharmacotherapy using anticoagulant drugs in patients with lower limb atherosclerosis (Intermittent claudication (IC) and critical limb ischaemia (CLI)) undergoing femoropopliteal and femorodistal bypass grafting. Outcomes include the overall success of therapy (graft patency and limb salvage rates) and complications of treatment. The null hypothesis tested is that antithrombotic therapy does not improve graft patency and limb salvage rates after femoropopliteal and femorodistal bypass surgery for lower limb atherosclerosis.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled clinical trials. Trials using alternation would be considered as quasi‐randomised controlled trials if included and a sensitivity analysis restricted to randomised controlled trials carried out to ensure the validity of any results.
Types of participants
All patients undergoing femoropopliteal or femorodistal bypass grafting for the treatment of IC and CLI. Patients undergoing bypass surgery for trauma were excluded. Patients undergoing bypass procedures using autologous vein grafts, synthetic (polytetrafluoroethylene (PTFE) or Dacron) grafts, treated human umbilical vein, or a combination of these materials were included.
Types of interventions
Trials in which participants were randomly allocated to receive either anticoagulant therapy versus placebo, one anticoagulant regimen versus another, or anticoagulant therapy versus an alternative treatment. The type of therapy, dosage, time of starting in relation to surgery (pre or postoperatively), and duration of therapy were recorded.
Types of outcome measures
Primary outcomes
Primary graft patency: patency rates after surgery with no further intervention, as determined by clinical examination, measurement of ankle‐brachial pressure index (ABPI), Doppler or duplex sonography, or angiography.
Assisted primary patency: patency rates after intervention to improve blood flow in a graft which has not occluded.
Secondary outcomes
Secondary graft patency: patency rates following secondary intervention to restore blood flow to the graft.
Objective assessment of lower limb blood flow: ankle‐brachial pressure index, exercise tolerance test.
Patient's quality of life.
Limb salvage rate: survival rates with limb intact.
Incidence of other cardiovascular events and mortality.
Side effects of treatment.
Search methods for identification of studies
Electronic searches
For this update the Cochrane Peripheral Vascular Diseases Group searched their Specialised Register (last searched August 2010) and the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 3), see Appendix 1 for details of the search strategy used to search CENTRAL. The Specialised Register is maintained by the Trials Search Co‐ordinator and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL, and AMED; and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used, are described in the Specialised Register section of the Cochrane Peripheral Vascular Diseases Group module in The Cochrane Library. Details of the search strategy used for the 2003 version of the review are shown in Appendix 2.
Searching other resources
Reference lists of papers and reports retrieved from the electronic searches were screened to identify potentially relevant additional studies.
Data collection and analysis
Selection of studies
Alistair Geraghty (AG) and Karen Welch (KW) independently selected which trials were suitable for the updated review. Disagreements were resolved by discussion. In the absence of consensus over the inclusion of a trial, a third opinion was sought.
Data extraction and management
For this update AG and KW independently extracted the following data using a standardised data extraction form.
Inclusion and exclusion criteria; patient details (age, gender, co‐morbidity).
Severity of arterial occlusive disease (as determined by the ankle‐brachial pressure index and the European Consensus definition of critical limb ischaemia).
Type of graft (autologous vein or synthetic); level of distal graft anastomosis (above‐knee popliteal, below‐knee popliteal, distal arteries). Type of anticoagulant therapy used (dose, commencement of therapy relative to surgery, duration of therapy, patient compliance).
Outcomes (as mentioned in Types of outcome measures). Treatment and control groups were compared for important prognostic factors and any differences described. Quality control measures to assess the bypass graft (such as Doppler and duplex sonography or angiography); the sites of the proximal and distal anastomosis; and the patients risk factors for graft occlusion were recorded.
Assessment of risk of bias in included studies
The authors used the criteria for assessing risk of bias as provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). We considered the six domains listed below with three possible responses. 'Yes' indicated a low risk of bias, 'no' indicated a high risk of bias, and if insufficient detail was reported the judgement was 'unclear'. We assessed the new studies included in the updated review and we re‐assessed the studies already included in the original review.
Sequence generation
Allocation concealment
Blinding
Incomplete data assessment
Selective outcome reporting
Other sources of bias
Assessment of heterogeneity
The I2 statistic was used to assess the appropriateness of pooling the data. This describes the percentage of the total variation in effect estimates that is due to heterogeneity rather than by chance. Values of I2 lie between 0% and 100% and can be categorized so that heterogeneity is low when I2 has a value of 25%, moderate if I2 has a value of 50%, and high when I2 has a value of 75%.
Data synthesis
Data were processed in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008) using RevMan 5 software, the Peto method, and a fixed‐effect model unless there was substantial heterogeneity, in which case we used a random‐effects model and the potential causes of heterogeneity were examined. For dichotomous data the numbers of events in the control and intervention groups of each study were calculated using the Peto odds ratio and 95% confidence interval.
Results
Description of studies
See the Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
For this update 40 potential publications were retrieved from a search of the Peripheral Vascular Diseases Groups Specialised Register and 2700 from searching CENTRAL. These were screened for relevance by reading the titles and abstracts (if available) and full text copies of 21 were obtained and subjected to further evaluation. Of these, 16 were additional publications to studies which had already been included in the previous version of the review.
Included studies
For this update there were14 eligible studies which investigated the efficacy of anticoagulant treatment in infrainguinal bypass surgery. Three additional studies were included in this update (Jivegard 2005; Logason 2001; Norgren 2004) and there were 11 studies which had already been included (Arfvidsson 1990; BOA 2000; Cole 1993; Edmondson 1994; Johnson 2002; Kretschmer 1992; Nydahl 1992; Samama 1994; Sarac 1998; Schneider 1979; Swedenborg 1996). A total of 4970 patients were included in the review analysis.
Vitamin K antagonist (VKA) versus no VKA
Four open randomised clinical trials (RCTs) were identified for a comparison of treatment with vitamin K antagonists (VKA) versus no VKA (Arfvidsson 1990; Johnson 2002; Kretschmer 1992; Sarac 1998).
Arfvidsson 1990 included 116 patients suffering from intermittent claudication (IC) or severe arterial insufficiency. Ninety‐two patients had critical or severe ischemia (peripheral arterial occlusive disease stages III and IV) and 24 patients suffered from claudication (stage IIb). Nine patients had femorodistal bypass, 25 had femoro‐above knee popliteal bypass and 48 had femoro‐below knee popliteal bypass. Forty‐nine patients had saphenous vein grafts and 33 had polytetrafluoroethylene (PTFE) grafts. Sixty‐one patients were assigned to treatment with dicoumarol and 55 patients to no anticoagulation. There were 34 patients who had thromboendarterectomy.
Johnson 2002 included 614 patients with ischaemic symptoms of disabling claudication, rest pain or tissue loss. Of these 207 patients had synthetic bypass grafts and 407 had vein bypass. Distal anastamosis site was above‐knee popliteal in 260 patients, below‐knee popliteal in 116 and tibial/peroneal in 238. Acetylsalicylic acid (ASA) (325 mg) was started one day preoperatively in both groups plus administration of warfarin (approximately 5 mg/day) in the treatment group on the first or second postoperative day.
Kretschmer 1992 involved a total of 130 patients: 62 with claudication (Fontaine stage II‐III) and 68 requiring limb salvage (stage III‐IV) underwent bypass procedures. All patients had femoropopliteal vein bypass grafts. Patients were assigned to treatment with coumarin derivatives (phenprocoumon) or no anticoagulation.
Sarac 1998 included 56 patients who underwent a total of 64 vein bypass grafts. There were 61 operations for rest pain or tissue loss, three were for short distance claudication. All these procedures were deemed high risk for occlusion with either a suboptimal venous conduit, poor arterial run‐off, or patients were undergoing a re‐do procedure. Patients were randomised to warfarin, starting on postoperative days two or three, with a target international normalised ratio (INR) of two to three and aspirin 325 mg/d or only aspirin 325 mg/d postoperatively.
In two of these trials patients received ASA (Johnson 2002; Sarac 1998) while in the other two studies patients did not receive any antiplatelet therapy (Arfvidsson 1990; Kretschmer 1992).
Vitamin K antagonist (VKA) versus acetylsalicylic acid (ASA) or dipyridamol (DIP)
Two RCTs were included comparing the administration of VKA versus ASA or DIP (BOA 2000; Schneider 1979).
BOA 2000 recruited 2690 patients of which 2650 completed the study; 51% of these patients had intermittent claudication and the remainder had pain at rest, ischaemic ulceration or gangrene. Participants underwent bypass operations from the femoral artery to the above‐knee popliteal (1222, 46%), below‐knee popliteal (897, 34%), crural (490,18%) or pedal (41, 2%) arteries. There were1546 patients (58%) who had vein bypass grafts and the remainder had synthetic grafts. Patients were assigned to coumarin derivatives (phenprocoumon or acenocoumarol) or to aspirin 80 mg daily, started within five days after surgery.
Schneider 1979 included 213 patients, 91 of whom had femoropopliteal bypass using a vein graft and could be included in the review. The remainder had thromboendarterectomy only. Of the included patients 37.3% had Fontaine stage III or IV disease and 61.7% had stage II disease. Patients were allocated to either coumarins or aspirin, or aspirin and dipyridamole.
Unfractionated heparin (UFH) versus low molecular weight heparin (LMWH)
Three RCTs were included which compared UFH to LMWH (Norgren 2004; Samama 1994; Swedenborg 1996).
Norgren 2004 examined 849 patients undergoing vascular surgery for: critical limb ischaemia (464), intermittent claudication (201), abdominal aortic aneurysm (118), other aneurysms (43) or other indications (35). This included 313 patients who underwent femoropopliteal bypass with either autologous or synthetic grafts, who could be included in this review.
Samama 1994 included 199 patients who underwent femorodistal bypass operations. There were135 patients who had autologous vein grafts, 47 who had PTFE grafts and 17 who had other types of graft.
Swedenborg 1996 included 18 patients who underwent infrainguinal bypass using vein grafts; three patients had intermittent claudication, six had rest pain and nine had ulcers or gangrene.
Low molecular weight heparin (LMWH) versus acetylsalicylic acid and dipyridamol (ASA/DIP)
Edmondson 1994 compared LMWH with ASA/DIP in 200 patients; 107 with IC and 93 patients with CLI. A total of 53 patients had vein grafts with the remainder having PTFE or dacron grafts.
Low molecular weight heparin (LMWH) versus no LMWH
One trial (Jivegard 2005) compared LMWH to placebo in a study population who all received aspirin. The trial included 284 patients who had either supra or infrainguinal bypass operations for lower limb ischaemia. The trialists were contacted and provided data for 229 patients who had infrainguinal bypass surgery.
Heparin versus ancrod
Cole 1993 compared ancrod versus UFH in 28 patients receiving 'high risk' infrainguinal bypass; the patients had distal anastamosis to either blind popliteal segment or a single outflow tibial vessel. Twenty‐five patients had vein grafts, two had PTFE grafts and one patient had a composite graft composed of both short saphenous veins.
Heparin versus antithrombin
In Nydahl 1992 12 patients undergoing infrainguinal bypass were randomised to perioperative treatment with UFH or antithrombin.
Heparin versus dextran
In Logason 2001 314 patients receiving infrainguinal bypass were randomised to receive either dextran 70 or LMWH in the perioperative period. Twenty‐eight patients were excluded after randomisation. The indication for surgery was IC in 47, 86 had rest pain, 147 had ulcers or gangrene and six had acute ischaemia. One hundred and forty‐nine patients had vein grafts, the remainder had synthetic or composite grafts.
Excluded studies
Thirteen studies were excluded for the reasons given in the Characteristics of excluded studies table.Two additional studies were excluded in this update (Kujath 2002; Waibel 1981).
Risk of bias in included studies
The risk of bias for the included trials is summarised in Figure 1 and Figure 2 and in the Characteristics of included studies table.
1.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
Vitamin K antagonists (VKA) versus no VKA
(Arfvidsson 1990; Johnson 2002; Kretschmer 1992; Sarac 1998) The number of events in these four studies were calculated from the survival curves when not otherwise reported.
Primary patency rate
Primary patency rates were analysed at three, six, 12, 24 months, and five years postoperatively comparing anticoagulant with no anticoagulant treatment for all graft types. There was a statistically non‐significant positive trend in favour of anticoagulant treatment on primary patency: odds ratio (OR) 0.65 (95% confidence interval (CI) 0.30 to 1.40) at three months (Analysis 1.1); OR 0.54 (95% CI 0.28 to 1.03) at six months (Analysis 1.2); OR 0.70 (95% CI 0.49 to 0.99) at 12 months (Analysis 1.3), OR 0.76 (95% CI 0.56 to 1.05) at 24 months (Analysis 1.4) and OR 0.80 (95% CI 0.61 to 1.07) at five years (Analysis 1.5).
1.1. Analysis.

Comparison 1: Occlusion in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 1: Occlusion in all bypasses, 3 months
1.2. Analysis.

Comparison 1: Occlusion in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 2: Occlusion in all bypasses, 6 months
1.3. Analysis.

Comparison 1: Occlusion in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 3: Occlusion in all bypasses, 12 months
1.4. Analysis.

Comparison 1: Occlusion in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 4: Occlusion in all bypasses, 24 months
1.5. Analysis.

Comparison 1: Occlusion in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 5: Occlusion in all bypasses, 5 years
When venous grafts were analysed separately in patients treated with VKA versus control patients, a more positive but non‐significant effect for venous grafts was shown: OR 0.44 (95% CI 0.14 to 1.42) at three months (Analysis 4.1); OR 0.40 (95% CI 0.17 to 0.95) at six months (Analysis 4.2); OR 0.75 (95% CI 0.49 to 1.14) at 12 months (Analysis 4.3); OR 0.80 (95% CI 0.55 to 1.17) at 24 months (Analysis 4.4); and OR 1.00 (95% CI 0.71 to 1.40) at five years (Analysis 4.5). There was considerable heterogeneity between the trials as will be discussed later and the results were heavily weighted by the (Johnson 2002) study; but the effect measures did not vary significantly when a random‐effects method was used (OR 0.80; 95% CI 0.35 to 1.81). In the (Johnson 2002) study warfarin therapy was withdrawn in most patients with a vein bypass when the data monitoring board found that there was no benefit on patency for vein bypass patients but a probable increase in morbidity.
4.1. Analysis.

Comparison 4: Occlusion in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 1: Occlusion in venous bypasses, 3 months
4.2. Analysis.

Comparison 4: Occlusion in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 2: Occlusion in venous bypasses, 6 months
4.3. Analysis.

Comparison 4: Occlusion in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 3: Occlusion in venous bypasses, 12 months
4.4. Analysis.

Comparison 4: Occlusion in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 4: Occlusion in venous bypasses, 24 months
4.5. Analysis.

Comparison 4: Occlusion in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 5: Occlusion in venous bypasses, 5 years
In contrast, the effect in artificial conduits at the early time points was less pronounced: OR 0.89 (95% CI 0.13 to 5.95) at three months (Analysis 8.1); OR 0.87 (95% CI 0.20 to 3.82) at six months (Analysis 8.2). After 12 months and at later time points the antithrombotic effect seemed to be stronger in preventing bypass failure, with statistical significance after five years: OR 0.59 (95% CI 0.31 to 1.13; fixed) at 12 months; OR 0.72 (95% CI 0.40 to 1.29; fixed) at 24 months; and OR 0.43 (95% CI 0.26 to 0.73) at five years.
8.1. Analysis.

Comparison 8: Occlusion in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 1: Occlusion in artificial bypasses, 3 months
8.2. Analysis.

Comparison 8: Occlusion in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 2: Occlusion in artificial bypasses, 6 months
Limb salvage
The effect of VKAs on limb salvage was calculated for 139 patients in the treatment group and 129 controls. It should be noted that for seven patients with early occlusion in each group of the Arfvidsson 1990 study no follow‐up data were provided. There was a tendency for coumarins to reduce limb loss during the observation time: OR 0.27 (95% CI 0.1 to 0.72) at three months (Analysis 2.1); OR 0.29 (95% CI 0.11 to 0.76) at six months (Analysis 2.2); OR 0.48 (95% CI 0.21 to 1.11) at 12 months (Analysis 2.3); OR 0.50 (95% CI 0.26 to 0.96) at 24 months (Analysis 2.4); and OR 0.36 (95% CI 0.19 to 0.69) at five years (Analysis 2.5). There was considerable heterogeneity (81%) in Analysis 2.5, which may have been due to there being a higher proportion of patients with critical or severe ischaemia in the Arfvidsson 1990 trial.
2.1. Analysis.

Comparison 2: Limb loss in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 1: Limb loss in all bypasses, 3 months
2.2. Analysis.

Comparison 2: Limb loss in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 2: Limb loss in all bypasses, 6 months
2.3. Analysis.

Comparison 2: Limb loss in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 3: Limb loss in all bypasses, 12 months
2.4. Analysis.

Comparison 2: Limb loss in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 4: Limb loss in all bypasses, 24 months
2.5. Analysis.

Comparison 2: Limb loss in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 5: Limb loss in all bypasses, 5 years
Survival
For survival, a non‐significant trend was observed: OR 0.36 (95% CI 0.10 to 1.31) at six months (Analysis 3.2); OR 0.66 (95% CI 0.38 to 1.15) at 24 months (Analysis 3.4).
3.2. Analysis.

Comparison 3: Deaths in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 2: Deaths in all bypasses, 6 months
3.4. Analysis.

Comparison 3: Deaths in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 4: Deaths in all bypasses, 24 months
Analysis for assisted primary patency, secondary patency, site of distal anastomosis, and bleeding complications in the different patient groups could not be performed due to inaccessible raw data.
Overall, the effect of coumarins on patency, limb salvage, and survival was similar in the two trials (Arfvidsson 1990; Kretschmer 1992) in which patients did not receive aspirin. In the Sarac trial (Sarac 1998) all patients received aspirin and only venous grafts were used. In this cohort a similar effect was seen on primary patency as in the patients with a venous graft, who did not receive aspirin in the two other trials (Arfvidsson 1990; Kretschmer 1992). In addition, similar odds ratios for limb salvage and survival were observed.
Side effects
Bleeding complications requiring hospitalisation occurred in eight patients in the treatment group and in none in the control group in. One patient in the treatment group in Kretschmer 1992 had a lethal bleeding complication. In Sarac 1998 four (12.5%) patients in the treatment group required operative evacuation of wound haematoma compared with only one (4.2%) in the control group. Furthermore, in the VKA and ASA group one patient had gastrointestinal bleeding compared with three patients in the ASA group; one case in each group was reported with a bleeding event of the central nervous system, while three patients suffered from genitourinary bleeding in the VKA and ASA group compared with none in the control group.
Vitamin K antagonist (VKA) versus acetylsalicylic acid and dipyridamole (ASA/DIP)
Primary graft patency
Primary graft patency for all grafts, including 1356 patients in the VKA group and 1385 in the ASA group, at three, six, 12, and 24 months postoperatively showed almost no difference for VKA versus ASA (OR 0.89; 95% CI 0.69 to 1.15 (Analysis 11.1); OR 0.99; 95% CI 0.81 to 1.22 (Analysis 11.2); OR 0.92; 95% CI 0.77 to 1.11 (Analysis 11.3); OR 0.91; 95% CI 0.77 to 1.08 (Analysis 11.4) respectively).
11.1. Analysis.

Comparison 11: Occlusion in all bypasses, vitamin K antagonist (VKA) versus ASA/DIP, Outcome 1: Occlusion in all bypasses, 3 months
11.2. Analysis.

Comparison 11: Occlusion in all bypasses, vitamin K antagonist (VKA) versus ASA/DIP, Outcome 2: Occlusion in all bypasses, 6 months
11.3. Analysis.

Comparison 11: Occlusion in all bypasses, vitamin K antagonist (VKA) versus ASA/DIP, Outcome 3: Occlusion in all bypasses, 12 months
11.4. Analysis.

Comparison 11: Occlusion in all bypasses, vitamin K antagonist (VKA) versus ASA/DIP, Outcome 4: Occlusion in all bypasses, 24 months
Intention‐to‐treat analysis for venous grafts included 814 patients randomised to VKA treatment versus 823 to ASA. Vitamin K antagonists had a statistically significant favourable effect on patency rates compared with antiplatelet treatment either with ASA alone or with a combination of ASA and DIP (OR 0.66; 95% CI 0.46 to 0.93 at three months (Analysis 12.1); OR 0.71; 95% CI 0.53 to 0.95 at six months (Analysis 12.2); OR 0.65; 95% CI 0.49 to 0.85 at 12 months (Analysis 12.3); OR 0.59; 95% CI 0.46 to 0.76 at 24 months (Analysis 12.4)). For patients treated with an artificial conduit, a group that had been analysed only by the BOA trialists (BOA 2000) (542 in the VKA group versus 562 in the ASA group), no statistically significant positive effect was found for VKAs (OR 1.32; 95% CI 0.89 to 1.95 at three months (Analysis 13.1); OR 1.47; 95% CI 1.08 to 1.99 at six months (Analysis 13.2); OR 1.33; 95% CI 1.02 to 1.74 at 12 months (Analysis 13.3); OR 1.41; 95% CI 1.11 to 1.80 at 24 months (Analysis 13.4)).
12.1. Analysis.

Comparison 12: Occlusion in venous bypasses, vitamin K antagonist (VKA) versus ASA/DIP, Outcome 1: Occlusion in venous bypasses, 3 months
12.2. Analysis.

Comparison 12: Occlusion in venous bypasses, vitamin K antagonist (VKA) versus ASA/DIP, Outcome 2: Occlusion in venous bypasses, 6 months
12.3. Analysis.

Comparison 12: Occlusion in venous bypasses, vitamin K antagonist (VKA) versus ASA/DIP, Outcome 3: Occlusion in venous bypasses, 12 months
12.4. Analysis.

Comparison 12: Occlusion in venous bypasses, vitamin K antagonist (VKA) versus ASA/DIP, Outcome 4: Occlusion in venous bypasses, 24 months
13.1. Analysis.

Comparison 13: Occlusion in artificial bypasses, vitamin K antagonist (VKA) versus ASA/DIP, Outcome 1: Occlusion in non‐venous bypasses, 3 months
13.2. Analysis.

Comparison 13: Occlusion in artificial bypasses, vitamin K antagonist (VKA) versus ASA/DIP, Outcome 2: Occlusion in non‐venous bypasses, 6 months
13.3. Analysis.

Comparison 13: Occlusion in artificial bypasses, vitamin K antagonist (VKA) versus ASA/DIP, Outcome 3: Occlusion in non‐venous bypasses, 12 months
13.4. Analysis.

Comparison 13: Occlusion in artificial bypasses, vitamin K antagonist (VKA) versus ASA/DIP, Outcome 4: Occlusion in non‐venous bypasses, 24 months
No distinct data were reported for assisted primary patency or secondary patency rates.
Limb salvage and survival
Neither of the trials reported data on limb salvage or survival that were suitable for a formal meta‐analysis. However, in the BOA trial (BOA 2000) limb amputation had to be performed in 100 (7.5%) coumarin‐treated patients and 110 (8.3%) aspirin‐treated patients.
Side effects
In the BOA 2000 trial, haemorrhage requiring hospital admittance was reported for 119 (9%) patients in the VKA group and 59 (4.5%) patients in the ASA group. Sixteen (1.2%) patients died from fatal bleeding in the VKA group compared with 12 (0.9%) patients in the ASA group. In the Schneider 1979 trial, adverse effects were reported for two patients (0.6%), who stopped VKA treatment for bleeding complications, and 13 patients (21%) who stopped ASA for varying reasons. Three patients in each group died within two years.
Unfractionated heparin (UFH) versus low molecular weight heparin (LMWH)
Primary early graft patency
In the Norgren 2004 trial, analysis performed at 24 hours showed seven of 176 (3.9%) versus four of 137 (2.9%) occlusions in each group, which was a statistically insignificant difference (OR 1.38; 95% CI 0.39 to 4.8) (Analysis 14.1). This difference remained insignificant at 30 days with 18 of 174 (10.3%) versus 19 of 134 (14.2%) occlusions (OR 0.7; 95% CI 0.35 to 1.39) (Analysis 14.3).
14.1. Analysis.

Comparison 14: Occlusion in all bypasses, low molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 1: Occlusion in all bypasses, 24 hours
14.3. Analysis.

Comparison 14: Occlusion in all bypasses, low molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 3: Occlusion,in all bypasses, day 30
An intention‐to‐treat analysis performed by Samama 1994 yielded eight of 99 (7.9%) versus 22 of 100 (22%) occlusions on day 10 (OR 0.34; 95% CI 0.16 to 0.74) (Analysis 14.2) and 11 of 99 (10.9%) versus 24 of 100 (24%) on day 30 (OR 0.41; 95% CI 0.20 to 0.85; fixed) (Analysis 14.3) showing a statistically significant positive effect for LMWH. In addition, a separate intra‐protocol analysis was performed comparing 67 grafts in the LMWH group with 64 grafts in the UFH group. Early graft thrombosis within 10 days occurred in four and eight cases, for LMWH or UFH respectively; a difference that did not reach statistical significance.
14.2. Analysis.

Comparison 14: Occlusion in all bypasses, low molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 2: Occlusion in all bypasses, day 10
In the Swedenborg 1996 trial early graft occlusion occurred in two cases in each group. No time points were included and so the data could not be included in the meta‐analysis.
Combined analysis of the Norgren 2004 and Samama 1994 trials regarding primary graft patency showed an OR of 0.54 (95% CI 0.33 to 0.90; fixed) for day 30, marginally favouring LMWH versus UFH for early graft thrombosis.
Low molecular weight heparin (LMWH) versus acetylsalicylic acid and dipyridamol (ASA/DIP)
Primary patency
In the 94 patients randomised to LMWH and the 106 patients to ASA/DIP, there were 12 versus 30 occlusions at six months (OR 0.39; 95% CI 0.20 to 0.78) (Analysis 15.1) and 21 versus 38 occlusions at 12 months (OR 0.52; 95% CI 0.29 to 0.96) (Analysis 15.2), showing a significant positive effect for LMWH. Further subgroup analysis found that this positive effect was only significant in patients undergoing operations for limb salvage and not in patients having operations for claudication.
15.1. Analysis.

Comparison 15: Occlusion in all bypasses, low molecular weight heparin (LMWH) versus ASA/DIP, Outcome 1: Occlusion in all bypasses, 6 months
15.2. Analysis.

Comparison 15: Occlusion in all bypasses, low molecular weight heparin (LMWH) versus ASA/DIP, Outcome 2: Occlusion in all bypasses, 12 months
Nine patients in the LMWH group (four with patent grafts) and two in the aspirin group died during follow up. No major bleeding or adverse events occurred.
Low molecular weight heparin (LMWH) versus no LMWH
Of the 229 patients with infrainguinal bypasses 116 were randomised to receive LMWH, 113 received placebos, and all received aspirin. At one month there were eight versus 11 occlusions (OR 0.69; 95% CI 0.27 to 1.78) (Analysis 16.1); at three months there were 20 versus 25 occlusions (OR 0.75; 95% CI 0.39 to 1.44) (Analysis 16.2); and at 12 months 44 versus 46 occlusions (OR 0.97; 95% CI 0.56 to 1.69) (Analysis 16.3); and therefore no statistically significant difference between groups at each time point.
16.1. Analysis.

Comparison 16: Occlusion in all bypassess, low molecular weight heparin (LMWH) versus no LMWH, Outcome 1: Occlusion in all bypasses, 1 month
16.2. Analysis.

Comparison 16: Occlusion in all bypassess, low molecular weight heparin (LMWH) versus no LMWH, Outcome 2: Occlusion in all bypasses, 3 months
16.3. Analysis.

Comparison 16: Occlusion in all bypassess, low molecular weight heparin (LMWH) versus no LMWH, Outcome 3: Occlusion in all bypasses, 12 months
Heparin versus ancrod
(Cole 1993) Among the 14 patients randomised to either heparin or ancrod, one graft in each group occluded within 24 hours postoperatively. One patient suffered from postoperative bleeding and graft failure in the ancrod group. No further events occurred during one month follow up. Primary patency at one month was 12/13 and 13/14 with an OR of 1.08 (95% CI 0.06 to 18.18; fixed). Ancrod was equally efficient as UFH. No data on limb salvage, survival, or primary assisted patency were reported.
Heparin versus antithrombin (AT)
(Nydahl 1992) In five out of six patients in the AT group graft thrombosis occurred intraoperatively, whereas no occlusion occurred in the heparin group. The study was stopped before completion. One patient in the heparin group suffered from major bleeding; and one patient in the heparin group died from myocardial infarction on the second postoperative day.
Heparin versus dextran
In 314 patients randomised to receive either Dextran 70 or enoxaparin in the perioperative period for femorodistal bypass, there were no significant differences in graft patency rates at days one, five to seven, 30, and at three months. However, there was a significant increase in the number of patients developing heart failure in the dextran group (12.8% versus 0.7%, P < 0.001).
Discussion
Summary of main results
Differences in patency between venous and artificial grafts have been apparent for many years, with artificial grafts having a higher rate of occlusion than their venous counterparts (Abbott 1997; Achermann 1998; Parsons 1996). This review demonstrates that antithrombotic drug treatments also vary in their effects on patency depending on the type of graft used. The initial pooled analyses (Analysis 1.1 to Analysis 1.3) would suggest that all patients undergoing infrainguinal bypass surgery might benefit from VKA in terms of patency rates, limb salvage and survival at three, six, 12 and 24 months regardless of graft type (although the effect does not reach statistical significance for graft occlusion or survival). However, since graft type has a major influence on patency the effects of antithrombotic treatments on venous and artificial grafts must be considered separately.
For venous grafts, meta‐analysis suggests that VKA may have a positive effect on patency compared to no VKA (Analysis 4.1 to Analysis 4.5), however at no point did this result reach statistical significance. In fact of the four included trials (Arfvidsson 1990; Johnson 2002; Kretschmer 1992; Sarac 1998) only (Kretschmer 1992) found a statistically significant result in favour of VKA, at 12 and 24 months and at five years. The remaining trials failed to reach statistical significance at any point and the results were heavily weighted by the Johnson 2002 trial. The Johnson 2002 trial appeared to favour no VKA but this result again failed to reach statistical significance; the trialists concluded that there was no difference in the patency of in venous grafts between patients receiving VKA and no VKA. In this study warfarin therapy was withdrawn in most patients with a vein bypass when the data monitoring board found that there was no benefit on patency for vein bypass patients but a probable increase in morbidity. Similar trends were seen for limb loss, limb salvage and death in patients with venous bypasses receiving VKA. It is worth noting that there was considerable heterogeneity between the included trials. In Sarac 1998 and Johnson 2002 all patients received aspirin, but not in Arfvidsson 1990 or Kretschmer 1992. In addition anticoagulation levels were variable and measured differently, and indications for surgery (intermittent clauducation versus critical limb ischaemia) varied between trials.
A further meta‐analysis demonstrated that VKA has a positive effect on patency compared to ASA and DIP for up to 24 months in patients receiving venous grafts (OR 0.59; 95% CI 0.46 to 0.76) (Analysis 12.4).
For artificial grafts only two trials compared VKA to no VKA (Arfvidsson 1990; Johnson 2002) and the meta‐analysis was heavily weighted by the Johnson 2002 trial. The data showed no significant difference in patency at three, six,12 or 24 months. There was however an apparent improvement in patency with VKA at five years (OR 0.43; 95% CI 0.26 to 0.73) (Analysis 8.5). In the Johnson 2002 trial, patency rates varied between 6 mm and 8 mm bypass subgroups and there was a significant difference in patency in the 6 mm group, 71.4% in the warfarin plus aspirin group compared with 57.9% in the aspirin group. No significant differences in limb loss or death were identified.
8.5. Analysis.

Comparison 8: Occlusion in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 5: Occlusion in artificial bypasses, 5 years
Comparison of VKA to ASA and DIP in artificial grafts showed a significant beneficial effect on patency with ASA and DIP at six, 12 and 24 months (Analysis 13.1 to Analysis 13.4).
Comparing rates of bleeding complications associated with anticoagulation was generally difficult due to differences in reporting. However, VKA was seen to be associated with a higher rate of bleeding complications compared to both no VKA and aspirin (Arfvidsson 1990; BOA 2000; Kretschmer 1992; Sarac 1998). Both Arfvidsson 1990 and BOA 2000 estimated a major bleeding complication rate of approximately 4% to 5%; this compares to a rate of 2.5% with aspirin in BOA 2000. The rate of wound haematoma also appears significantly increased with VKA compared with aspirin (Sarac 1998). Inter‐study differences in the therapeutic ranges of anticoagulation clearly have implications on the rate of haemorrhagic complications. Generally, it should be noted that comparison between the different studies was hampered by the fact that the target international normalised ratio (INR) differed between trials; the (BOA 2000) trialists aimed at 3.0 to 4.5 whereas the investigators of the Johnson 2002 trial aimed at a value of 1.4 to 2.8. In other studies, a Quick value was used instead of the INR, making direct comparison almost impossible.
The effect of LMWH and UFH on early patency was evaluated in three trials that included a total of 509 patients. Results were largely inconclusive with only one trial (Samama 1994) showing a positive effect for LMWH above UFH when comparing graft occlusion at day 10 and day 30. Neither Norgren 2004 nor Swedenborg 1996 showed any significant difference. Additionally a per protocol analysis of the Samama 1994 data failed to demonstrate significance. Pooled intention‐to‐treat data at day 30 did show a marginally positive effect for LMWH over UFH but a much larger cohort of patients receiving venous and artificial bypasses would have to be evaluated for reliable comparison in the future.
One trial comparing LMWH versus ASA and DIP (Edmondson 1994) found improved graft patency for the LMWH group at six and 12 months but this advantage was only in patients undergoing surgery for limb salvage and not for claudication. Of note, only a quarter of the patients in the study had vein grafts. A larger study is required both to confirm this result and to allow for subgroup analysis comparing graft type.
Overall completeness and applicability of evidence
The evidence for VKA in venous bypasses is weak and at times inconsistent. VKA does appear to be more favourable than antiplatelet therapy in terms of graft patency but there may be an increase in bleeding complications. The evidence for antiplatelet drugs in artificial bypasses is much more consistent and reliable. In order to support these findings and to rule out any divergencies, further large trials with homogeneous, precisely reported patient characteristics of the study participants and study designs are needed. It should be mentioned that extracting data from the included trials was difficult as most publications did not provide raw data, which is ideally needed for an appropriate analysis. Contacting the authors was usually not very successful, either because raw data were no longer available or authors did not reply. Therefore, the numbers of patients and events had to be calculated from the survival curves.
Agreements and disagreements with other studies or reviews
Despite the heterogeneity of the included studies, our findings show a tendency towards agreement between the different trials, which is also biologically plausible. Venous and prosthetic grafts appear to have varying mechanisms of failure. Venous grafts, once they have been incorporated into the high pressure system in the human leg, lose their endothelial layer within days (Sasaki 2000). Exposure of the subendothelial layers to the blood stream triggers the expression and release of tissue factor (Channon 1997; Muluk 1998), one of the activators of the coagulation cascade. This process results in locally increased thrombin generation with subsequent thrombus formation that is enhanced by a simultaneous inflammatory process through the release of interleukins (interleukin‐1b, interleukin‐6, tumour necrosis factor), which attract and activate granulocytes and monocytes. The latter are able to express additional tissue factor, thus enhancing the local thrombogenic process. Vein grafts undergo an active remodelling process over several months following arterialisation, with increases in wall thickness and stiffness and changes to the luminal diameter (Jacot 2004). Venous grafts fail after developing stenosis either at or around anastomotic sites or at points along their length. It is postulated that it is the combination of venous remodeling and intimal hyperplasia (IH) that leads to luminal narrowing and subsequent failure in venous grafts (Owens 2010). Although activated platelets play an important role in thrombus formation and IH, it seems that the activated coagulation system at the graft site of endothelial injury also has a role and thrombin inhibition with VKA may therefore have some benefit. On the other hand, inhibition of platelet deposition on prosthetic grafts might be the more efficient therapy for patients with PTFE and dacron bypasses. IH at the distal anastamosis appears to be the principal cause of prosthetic graft failure. Compliance mismatch between the synthetic graft and the native artery results in altered wall stress and endothelial damage around the anastamosis. Areas of IH develop, particularly at the heel and toe of the anastamosis and on the floor of the recipient artery. Introduction of a vein cuff at the anastamosis reduces the IH response (Kissin 2000) and it is postulated this may be due to a reduction in compliance mismatch (Sarkar 2006). A key step in the initiation of IH is the adherance and aggregation of platelets to the injured endothelium; it may be that this is the main therapeutic target in preventing prosthetic bypass graft failure, where IH has a dominant role. The recently reported CASPAR trial (CASPAR 2010) combined antiplatelet therapy with clopidogrel and aspirin versus aspirin alone and showed significant improvement in graft patency in prosthetic grafts (hazard ratio (HR) 0.65; 95% CI 0.45 to 0.95) but not venous grafts (HR1.25; 95% CI 0.94 to 1.67), which would seem to support this argument.
Authors' conclusions
Implications for practice.
The evidence suggests that patients subjected to infrainguinal venous graft surgery are more likely to benefit from VKA than platelet inhibitors. Patients receiving an artificial graft usually benefit from platelet inhibitors (aspirin).
Implications for research.
Evidence‐based results suggest VKA may be of more benefit in venous bypasses. The evidence for antiplatelets in prosthetic grafts is stronger and more consistent. Large randomised controlled trials are required to further evaluate the effects of VKA on venous bypasses and to determine the optimal dose (INR value aimed at) and length of treatment. Additionally, the effect of LMWH compared with UFH in perioperative treatment should be evaluated in larger RCTs that include a subgroup analysis of venous and artificial grafts. In view of the new available antithrombotic agents, such as direct thrombin inhibitors (for example dabigatran) or the factor Xa inhibitors (rivaroxaban and apixaban), many new trials should investigate the efficacy and safety of these agents for bypass patency, limb salvage, and survival. At present graft patency is variably assessed between trials (including clinically, the ankle‐brachial pressure index, duplex scanning, angiography) and it would be useful to reach a consensus as to how this should be defined, including the degree of stenosis that may indicate a failing graft that requires intervention. This would allow improved comparison between trials. Furthermore, presentation of data should be much more detailed, and not only show survival curves for overall patency. Tables showing the raw data would improve the transparency of the trial performance and allow comparison of endpoints at consecutive time points of follow up. Thus, the reader would be enabled to identify the number of occlusions or other endpoints at different time points in each comparison group, as well as in subgroups defined by bypass material, above‐knee and below‐knee anastomosis, and inflow and outflow conditions.
Feedback
Anticoagulant feedback, 14 February 2011
Summary
Feedback received on this review, and other reviews and protocols on anticoagulants, is available on the Cochrane Editorial Unit website at http://www.editorial-unit.cochrane.org/anticoagulants-feedback.
CAPPA feedback, 10 April 2014
Summary
First of all we like to compliment authors with their extensive work1 on the topic of antithrombotics and infrainguinal arterial bypass surgery. Periprocedural prophylactic antithrombotics (PPAT) involves every day practice around the world during arterial interventions in the vascular patient, but is subject to much discussion and evidence based consensus is lacking.
To increase insight in periprocedural prophylactic anticoagulation and to develop evidence based guidelines on this topic for vascular surgery and interventional radiology, a study group was instituted in the Netherlands: CAPPA: Consensus on Arterial PeriProcedural Anticoagulation. This group consists of Dutch vascular surgeons and interventional radiologists and is supported by the Dutch Boards of Vascular Surgery and Interventional Radiology. In our effort to elucidate PPAT we have found two misinterpretations of study data in your review. Correction of this data alters the interpretation and outcome of part of the meta‐analysis in your review.
After 2 extensive surveys on daily practice of antithrombotics amongst vascular surgeons2 and interventional radiologists3, we performed a systematic review4 on the subject of "prophylactic periprocedural antithrombotics in open and endovascular abdominal aortic (AAA) repair". Another systematic review was performed on the subject of "prophylactic intraoperative antithrombotics in open infrainguinal bypass surgery (IABS)". This review has recently been accepted for publication by the Journal of Cardiovascular Surgery (Torino).
While performing this systematic review, we thoroughly studied the manuscripts of the Cochrane review on antithrombotics administered during surgical procedure, especially the sub‐heading "Unfractionated heparin (UFH) versus low molecular weight heparin (LMWH)", on page 6, 10 and 12. For accurate interpretation of the data we have contacted authors of included studies. The work by Norgren et al5 is one of the key studies of the review and the exact definition of distal reconstructions depicted in his study was not completely clear for us. Dr Norgren clarified to us by mail:
"Distal reconstructions imply a distal anastomosis below the BK popliteal artery (mainly crural arteries or ADP). Proximal reconstructions mean aorto‐iliac, aorto‐femoral and iliaco‐femoral". This means that the numbers depicted in the meta‐analysis in the Cochrane review should be corrected for the patency and mortality at day 30: included patients for infrainguinal reconstructions should be: 174 +77 for the LMWH group and 221 for the UFH group. This alteration of data affects the outcome of the meta‐analysis, since now no significant difference between the two groups can be found.
Patency at day 30, see Figure 76
3.
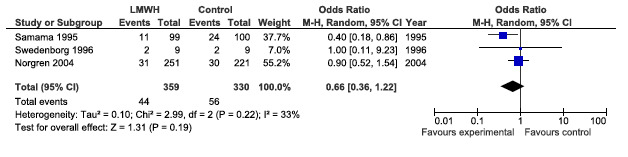
CAPPA feedback April 2014: Patency at day 30
Mortality at day 30, see Figure 77
4.
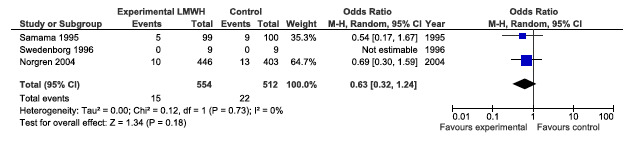
CAPPA feedback April 2014: Mortality at day 30
On the study of Swedenborg et al7, it was stated on page 10 of the Cochrane review, that "No time points were included and so the data could not be included in the meta‐analysis". After additionally contacting prof. Swedenborg by mail, it was established that the follow up in his study7 was 30 days. Therefore we included the data from that study in our meta‐analysis.5‐7
Stated by the authors of the Cochrane in the discussion on page 12 is that "Pooled intention‐to‐treat data at day 30 did show a marginally positive effect for LMWH over UFH but a much larger cohort of patients receiving venous and artificial bypasses would have to be evaluated for reliable comparison in the future". From our forest plot it can be deducted that not even this marginal effect is present.
We realize that our remarks are of only very minor importance and we agree with the conclusion of authors that more RCTs should be executed on this topic before any reliable conclusion can be drawn on the topic of LMWH versus UFH as periprocedural prophylactic antithrombotic. We would be obliged if our, small, additions to the Cochrane Review will be published in your Journal.
References
1 Geraghty AJ, Welch K. Antithrombotic agents for preventing thrombosis after infrainguinal arterial bypass surgery. Cochrane Database of Systematic Reviews 2011, Issue 6. Art. No.: CD000536. DOI: 10.1002/14651858.CD000536.pub2.
2 Wiersema AM, Bruijninckx CMA, Reijnen MMPJ, Vos JA, van Delden OM, Vahl A, Zeebregts CJ and Moll FL; The CAPPA study group (Consensus on Arterial Periprocedural Anticoagulation). Perioperative prophylactic antithrombotic strategies in vascular surgery: current practice in the Netherlands. Journal of Cardiovascular Surgery 2013; Jan 22. [Epub ahead of print].
3 Wiersema AM, Vos JA, Bruijninckx CM, van Delden OM, Reijnen MM, Vahl A, Zeebregts CJ and Moll FL. Periprocedural prohylactic antithrombotic strategies in interventional radiology: current practice in The Netherlands and comparison with The United Kingdom. Cardiovascular and Interventional Radiology. 2013;36(6):1477‐92.
4 Wiersema AM, Jongkind V, Bruijninckx CMA, Reijnen MMPJ, Vos JA, van Delden OM, Zeebregts CJ and Moll FL; The CAPPA study group (Consensus on Arterial Periprocedural Anticoagulation). Prophylactic rerioperative anti‐thrombotics in open and endovascular abdominal aortic aneurysm (AAA) surgery: A systematic review. European Journal of Vasccular and Endovascular Surgery 2012;44(4):359‐67.
5 Norgren L. Can low molecular weight heparin replace unfractionated heparin during peripheral arterial reconstruction? An open label prospective randomized controlled trial. Journal of Vascular Surgery 2004;39(5):977‐84
6 Samama CM, Gigou F, Ill P. Low‐molecular‐weight heparin vs. unfractionated heparin in femorodistal reconstructive surgery: a multicenter open randomized study. Enoxart Study Group. Annals of Vascular Surgery 1995;9 Suppl:S45‐53.
7 Swedenborg J, Nydahl S, Egberg N. Low molecular mass heparin instead of unfractionated heparin during infrainguinal bypass surgery. European Journal of Vascular and Endovascular Surgery 1996;11(1):59‐64.
Reply
This review has been taken on by new authors who are currently updating the review. The review authors will respond to this feedback as part of the updated review.
Contributors
Feedback: On behalf of the CAPPA study group
Arno M. Wiersema, MD Department of Surgery, Westfriesgasthuis, Hoorn Department of Surgery, Division of Vascular Surgery, University Medical Center Utrecht, University of Utrecht
Vincent Jongkind, MD, PhD Department of Surgery, University Medical Center Vrije Universiteit, Vrije Universiteit, Amsterdam
Cornelis M.A. Bruijninckx, MD, PhD Department of Surgery, Equipe Zorgbedrijven, Rotterdam
Reply: Marlene Stewart, Managing Editor Cochrane PVD Group.
What's new
| Date | Event | Description |
|---|---|---|
| 23 January 2023 | Amended | This review has been superseded by Cochrane Reviews: 'Antithrombotics after infra‐inguinal bypass grafting' (doi.org/10.1002/14651858.CD015141) and 'Antithrombotics after infra‐inguinal peripheral endovascular treatment' (doi.org/10.1002/14651858.CD015142) and will no longer be updated. |
History
Protocol first published: Issue 4, 1997 Review first published: Issue 4, 2003
| Date | Event | Description |
|---|---|---|
| 21 April 2014 | Feedback has been incorporated | Feedback submitted and added to review. |
| 23 March 2011 | New search has been performed | Searches were re‐run and the review updated. Three additional studies were included and two additional studies were excluded. |
| 23 March 2011 | New citation required but conclusions have not changed | The review was updated by new authors. |
| 14 February 2011 | Amended | Link to anticoagulant feedback added |
Notes
Acknowledgements
The authors would like to acknowledge the contribution of the authors of the original review, Janine Dörffler‐Melly, Harry R Büller, Marianne M Koopman and Martin H Prins. We would also like to thank Janet Wale who wrote the Plain Language Summary.
Appendices
Appendix 1. 2010 CENTRAL search strategy
| #1 | MeSH descriptor Anticoagulants explode all trees | 7204 |
| #2 | anticoagul* or warfarin or (vitamin near3 antagonist*) or VKA or Nicoumalone or phenindione or acenocoumarol* or Sinthrome or dicoumarol* or nicoumalone or phenprocoumon or Marcoumar or Marcumar or Falithrom or AVK or bishydroxycoumarin* or coumarin* or phenprocoumon* or Ximelagatran or Exanta or Exarta or H 376/95 or dabigatran or rivaroxaban or BAY59‐7939 or TTP889 or odiparcil or LY517717 or YM150 or DU‐176b or apixaban or betrixaban | 6313 |
| #3 | MeSH descriptor Platelet Aggregation Inhibitors explode all trees | 7255 |
| #4 | antiplatelet* or anti‐platelet* or antiaggreg* or anti‐aggreg* or (platelet* adj5 inhibit*) or (thrombocyt* adj5 inhibit*) | 1825 |
| #5 | aspirin* or ASA or dipyridamol* or epoprostenol* or iloprost* or ketanserin* or milrinone* or mopidamol* or pentoxifyllin* or ticlopidine* or trapidil or "eicosapentaenoic Acid" or tirofiban or Aggrastat or eptifibatide or Integrilin or SCH 530348 or "acetyl salicylic acid*" or "acetylsalicylic acid" or "acetyl‐salicylic acid" or clopidogrel* or picotamide* or suloctidil* or defibrotide* or cilostazol or Pletal or satigrel or sarpolgrelate or kbt3022 or kbt‐3022 or isbogrel or cv4151 or cv‐4151 or triflusal or Dispril or Albyl* or Ticlid* or Persantin* or Plavix or Aggrenox or Plasugrel or Ticagrelor or Cangrelor or Portola | 18149 |
| #6 | (((glycoprotein iib* or gp iib*) near3 (antagonist* or inhibitor*)) or GR144053 or GR‐144053 or abciximab or tirofiban* or eftifibatid or eptifibatide or ReoPro or Integrilin* or Aggrastat of indobufen) | 1039 |
| #7 | antithrombotic* | 1132 |
| #8 | UFH or bivalirudin* | 508 |
| #9 | (LMWH OR UH or heparin or nadroparin* OR fraxiparin* OR enoxaparin OR Clexane OR klexane OR lovenox OR dalteparin OR Fragmin OR ardeparin OR normiflo OR tinzaparin OR logiparin OR Innohep OR certoparin OR sandoparin OR reviparin OR clivarin* OR danaproid OR danaparoid or antixarin OR ardeparin* OR bemiparin* OR Zibor OR cy 222 OR embolex OR monoembolex OR parnaparin* OR "rd 11885" Or tedelparin OR Kabi‐2165 OR Kabi 2165 or emt‐966 or emt‐967 or "pk‐10 169" or pk‐10169 or pk10169 or cy‐216 or cy216 or seleparin* or tedegliparin or seleparin* or tedegliparin* or wy90493 or "wy 90493" or "kb 101" or kb101 or lomoparan or orgaran or parnaparin or fluxum or lohepa or lowhepa or "op 2123" or parvoparin or AVE5026) | 7691 |
| #10 | (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9) | 30182 |
| #11 | MeSH descriptor Femoral Artery explode all trees | 660 |
| #12 | MeSH descriptor Popliteal Artery explode all trees | 228 |
| #13 | MeSH descriptor Saphenous Vein explode all trees | 455 |
| #14 | MeSH descriptor Peripheral Vascular Diseases explode all trees | 2055 |
| #15 | MeSH descriptor Arterial Occlusive Diseases, this term only | 727 |
| #16 | MeSH descriptor Arteriolosclerosis, this term only | 0 |
| #17 | MeSH descriptor Arteriosclerosis Obliterans explode all trees | 67 |
| #18 | MeSH descriptor Atherosclerosis explode all trees | 265 |
| #19 | MeSH descriptor Intermittent Claudication explode all trees | 658 |
| #20 | MeSH descriptor Ischemia, this term only | 678 |
| #21 | (peripheral near (arter* or vasc*)) or atherosclerosis or arteriosclerosis or PVD or PAOD or PAD | 16435 |
| #22 | claudic* or (peripher* or leg or (lower next extremit*)) | 25982 |
| #23 | isch* or CLI | 14891 |
| #24 | infrainguinal or (infra near inguin*) | 149 |
| #25 | femoropopliteal or (fem* near pop*) | 1782 |
| #26 | femorodistal or (femoro* near distal) | 53 |
| #27 | infrapopliteal or (infra near poplite*) | 54 |
| #28 | (#11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27) | 51978 |
| #29 | bypass or surgery or construct* or reconstruct* or graft* | 97587 |
| #30 | occlu* or reocclu* or re‐occlu* | 8491 |
| #31 | MeSH descriptor Blood Vessel Prosthesis explode all trees | 407 |
| #32 | MeSH descriptor Blood Vessel Prosthesis Implantation explode all trees | 412 |
| #33 | (#29 OR #30 OR #31 OR #32) | 103185 |
| #34 | (#28 AND #33) | 14362 |
| #35 | (#34 AND #10) | 2700 |
Appendix 2. 2003 CENTRAL search strategy
#1 ARTERIAL OCCLUSIVE DISEASES single term (MeSH) #2 (peripheral next arterial next occlusive next dis*) #3 (arterial next occlusive next dis*) #4 (#1 or #2 or #3) #5 INTERMITTENT CLAUDICATION single term (MeSH) #6 (intermittent next claudication) #7 (#5 or #6) #8 (critical next limb next isch*) #9 (#4 or #7 or #8) #10 (femoropopliteal next bypass) #11 (bypass next surgery) #12 (peripheral next arterial next bypass*) #13 femorodistal* #14 (femoro next distal*) #15 (#10 or #11 or #12 or #13 or #14) #16 ANTICOAGULANTS explode all trees (MeSH) #17 anticoagulant* #18 antithrombotic* #19 (#16 or #17 or #18) #20 (#9 and #15 and #19)
Data and analyses
Comparison 1. Occlusion in all bypasses, vitamin K antagonist (VKA) versus no VKA .
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Occlusion in all bypasses, 3 months | 3 | 276 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.30, 1.40] |
| 1.2 Occlusion in all bypasses, 6 months | 3 | 276 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.28, 1.03] |
| 1.3 Occlusion in all bypasses, 12 months | 4 | 890 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.70 [0.49, 0.99] |
| 1.4 Occlusion in all bypasses, 24 months | 4 | 890 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.56, 1.05] |
| 1.5 Occlusion in all bypasses, 5 years | 3 | 833 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.61, 1.07] |
Comparison 2. Limb loss in all bypasses, vitamin K antagonist (VKA) versus no VKA .
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Limb loss in all bypasses, 3 months | 3 | 276 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.27 [0.10, 0.72] |
| 2.2 Limb loss in all bypasses, 6 months | 3 | 276 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.29 [0.11, 0.76] |
| 2.3 Limb loss in all bypasses, 12 months | 3 | 276 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.48 [0.21, 1.11] |
| 2.4 Limb loss in all bypasses, 24 months | 3 | 276 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.50 [0.26, 0.96] |
| 2.5 Limb loss in all bypasses, 5 years | 2 | 212 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.36 [0.19, 0.69] |
Comparison 3. Deaths in all bypasses, vitamin K antagonist (VKA) versus no VKA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Deaths in all bypasses, 3 months | 3 | 268 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.20 [0.04, 1.04] |
| 3.2 Deaths in all bypasses, 6 months | 3 | 268 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.36 [0.10, 1.31] |
| 3.3 Deaths in all bypasses, 12 months | 3 | 268 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.45 [0.21, 0.97] |
| 3.4 Deaths in all bypasses, 24 months | 3 | 268 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.66 [0.38, 1.15] |
| 3.5 Deaths in all bypasses, 5 years | 2 | 212 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.60 [0.35, 1.05] |
3.1. Analysis.

Comparison 3: Deaths in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 1: Deaths in all bypasses, 3 months
3.3. Analysis.

Comparison 3: Deaths in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 3: Deaths in all bypasses, 12 months
3.5. Analysis.

Comparison 3: Deaths in all bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 5: Deaths in all bypasses, 5 years
Comparison 4. Occlusion in venous bypasses, vitamin K antagonist (VKA) versus no VKA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Occlusion in venous bypasses, 3 months | 3 | 243 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.44 [0.14, 1.42] |
| 4.2 Occlusion in venous bypasses, 6 months | 3 | 243 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.40 [0.17, 0.95] |
| 4.3 Occlusion in venous bypasses, 12 months | 4 | 650 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.49, 1.14] |
| 4.4 Occlusion in venous bypasses, 24 months | 4 | 650 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.80 [0.55, 1.17] |
| 4.5 Occlusion in venous bypasses, 5 years | 3 | 586 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.00 [0.71, 1.40] |
Comparison 5. Limb loss in venous bypasses, vitamin K antagonist (VKA) versus no VKA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Limb loss in venous bypasses, 3 months | 2 | 179 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.08, 1.62] |
| 5.2 Limb loss in venous bypasses, 6 months | 2 | 179 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.35 [0.08, 1.62] |
| 5.3 Limb loss in venous bypasses, 12 months | 2 | 179 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.43 [0.11, 1.65] |
| 5.4 Limb loss in venous bypasses, 24 months | 2 | 179 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.44 [0.18, 1.07] |
| 5.5 Limb loss in venous bypasses, 5 years | 2 | 179 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.29 [0.14, 0.60] |
5.1. Analysis.

Comparison 5: Limb loss in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 1: Limb loss in venous bypasses, 3 months
5.2. Analysis.

Comparison 5: Limb loss in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 2: Limb loss in venous bypasses, 6 months
5.3. Analysis.

Comparison 5: Limb loss in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 3: Limb loss in venous bypasses, 12 months
5.4. Analysis.

Comparison 5: Limb loss in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 4: Limb loss in venous bypasses, 24 months
5.5. Analysis.

Comparison 5: Limb loss in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 5: Limb loss in venous bypasses, 5 years
Comparison 6. Limb salvage venous grafts, vitamin K antagonist (VKA) versus no VKA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Limb salvage 6 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
6.1. Analysis.

Comparison 6: Limb salvage venous grafts, vitamin K antagonist (VKA) versus no VKA, Outcome 1: Limb salvage 6 months
Comparison 7. Deaths in venous bypasses, vitamin K antagonist (VKA) versus no VKA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Deaths in venous bypasses, 3 months | 2 | 179 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.11 [0.00, 5.55] |
| 7.2 Deaths in venous bypasses, 6 months | 2 | 179 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.40 [0.04, 4.08] |
| 7.3 Deaths in venous bypasses, 12 months | 2 | 179 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.48 [0.18, 1.31] |
| 7.4 Deaths in venous bypasses, 24 months | 2 | 179 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.32, 1.23] |
| 7.5 Deaths in venous bypasses, 5 years | 2 | 179 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.30, 1.00] |
7.1. Analysis.

Comparison 7: Deaths in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 1: Deaths in venous bypasses, 3 months
7.2. Analysis.

Comparison 7: Deaths in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 2: Deaths in venous bypasses, 6 months
7.3. Analysis.

Comparison 7: Deaths in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 3: Deaths in venous bypasses, 12 months
7.4. Analysis.

Comparison 7: Deaths in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 4: Deaths in venous bypasses, 24 months
7.5. Analysis.

Comparison 7: Deaths in venous bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 5: Deaths in venous bypasses, 5 years
Comparison 8. Occlusion in artificial bypasses, vitamin K antagonist (VKA) versus no VKA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Occlusion in artificial bypasses, 3 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 8.2 Occlusion in artificial bypasses, 6 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 8.3 Occlusion in artificial bypasses, 12 months | 2 | 240 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.31, 1.13] |
| 8.4 Occlusion in artificial bypasses, 24 months | 2 | 240 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.72 [0.40, 1.29] |
| 8.5 Occlusion in artificial bypasses, 5 years | 2 | 240 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.43 [0.26, 0.73] |
8.3. Analysis.

Comparison 8: Occlusion in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 3: Occlusion in artificial bypasses, 12 months
8.4. Analysis.

Comparison 8: Occlusion in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 4: Occlusion in artificial bypasses, 24 months
Comparison 9. Limb loss in artificial bypasses, vitamin K antagonist (VKA) versus no VKA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Limb loss in artificial bypasses, 3 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 9.2 Limb loss in artificial bypasses, 6 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 9.3 Limb loss in artificial bypasses, 12 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 9.4 Limb loss in artificial bypasses, 24 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 9.5 Limb loss in artificial bypasses, 5 years | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
9.1. Analysis.

Comparison 9: Limb loss in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 1: Limb loss in artificial bypasses, 3 months
9.2. Analysis.

Comparison 9: Limb loss in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 2: Limb loss in artificial bypasses, 6 months
9.3. Analysis.

Comparison 9: Limb loss in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 3: Limb loss in artificial bypasses, 12 months
9.4. Analysis.

Comparison 9: Limb loss in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 4: Limb loss in artificial bypasses, 24 months
9.5. Analysis.

Comparison 9: Limb loss in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 5: Limb loss in artificial bypasses, 5 years
Comparison 10. Deaths in artificial bypasses, vitamin K antagonist (VKA) versus no VKA.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Deaths in artificial bypasses, 3 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 10.2 Deaths in artificial bypasses, 6 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 10.3 Deaths in artificial bypasses, 12 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 10.4 Deaths in artificial bypasses, 24 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 10.5 Deaths in artificial bypasses, 5 years | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
10.1. Analysis.

Comparison 10: Deaths in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 1: Deaths in artificial bypasses, 3 months
10.2. Analysis.

Comparison 10: Deaths in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 2: Deaths in artificial bypasses, 6 months
10.3. Analysis.

Comparison 10: Deaths in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 3: Deaths in artificial bypasses, 12 months
10.4. Analysis.

Comparison 10: Deaths in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 4: Deaths in artificial bypasses, 24 months
10.5. Analysis.

Comparison 10: Deaths in artificial bypasses, vitamin K antagonist (VKA) versus no VKA, Outcome 5: Deaths in artificial bypasses, 5 years
Comparison 11. Occlusion in all bypasses, vitamin K antagonist (VKA) versus ASA/DIP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Occlusion in all bypasses, 3 months | 2 | 2741 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.89 [0.69, 1.15] |
| 11.2 Occlusion in all bypasses, 6 months | 2 | 2741 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.81, 1.22] |
| 11.3 Occlusion in all bypasses, 12 months | 2 | 2741 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.92 [0.77, 1.11] |
| 11.4 Occlusion in all bypasses, 24 months | 2 | 2741 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.77, 1.08] |
Comparison 12. Occlusion in venous bypasses, vitamin K antagonist (VKA) versus ASA/DIP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Occlusion in venous bypasses, 3 months | 2 | 1637 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.46, 0.92] |
| 12.2 Occlusion in venous bypasses, 6 months | 2 | 1637 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.53, 0.95] |
| 12.3 Occlusion in venous bypasses, 12 months | 2 | 1637 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.49, 0.85] |
| 12.4 Occlusion in venous bypasses, 24 months | 2 | 1637 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.59 [0.46, 0.76] |
Comparison 13. Occlusion in artificial bypasses, vitamin K antagonist (VKA) versus ASA/DIP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Occlusion in non‐venous bypasses, 3 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 13.2 Occlusion in non‐venous bypasses, 6 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 13.3 Occlusion in non‐venous bypasses, 12 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 13.4 Occlusion in non‐venous bypasses, 24 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
Comparison 14. Occlusion in all bypasses, low molecular weight heparin (LMWH) versus unfractionated heparin (UFH).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Occlusion in all bypasses, 24 hours | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 14.2 Occlusion in all bypasses, day 10 | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 14.3 Occlusion,in all bypasses, day 30 | 2 | 507 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.33, 0.90] |
| 14.4 Per protocol | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
14.4. Analysis.

Comparison 14: Occlusion in all bypasses, low molecular weight heparin (LMWH) versus unfractionated heparin (UFH), Outcome 4: Per protocol
Comparison 15. Occlusion in all bypasses, low molecular weight heparin (LMWH) versus ASA/DIP.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 15.1 Occlusion in all bypasses, 6 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 15.2 Occlusion in all bypasses, 12 months | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
Comparison 16. Occlusion in all bypassess, low molecular weight heparin (LMWH) versus no LMWH.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 16.1 Occlusion in all bypasses, 1 month | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 16.2 Occlusion in all bypasses, 3 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 16.3 Occlusion in all bypasses, 12 months | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 17. Survival venous grafts, Sarac study.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 17.1 Survival 6 months, intention to treat | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected | |
| 17.2 Survival 2 years, intention to treat | 2 | 147 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.70 [0.22, 2.21] |
17.1. Analysis.

Comparison 17: Survival venous grafts, Sarac study, Outcome 1: Survival 6 months, intention to treat
17.2. Analysis.

Comparison 17: Survival venous grafts, Sarac study, Outcome 2: Survival 2 years, intention to treat
Comparison 18. Early graft thrombosis, ancrod versus heparin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 18.1 Early graft thrombosis, 24 h and 1 month | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Totals not selected |
18.1. Analysis.

Comparison 18: Early graft thrombosis, ancrod versus heparin, Outcome 1: Early graft thrombosis, 24 h and 1 month
Comparison 19. Early graft thrombosis, unfractionated heparin (UFH) versus antithrombin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 19.1 Antithrombin versus UFH, intraoperative graft thrombosis | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 19.2 Antithrombin versus UFH, 1 month occlusion (following thrombendarterectomy) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
19.1. Analysis.

Comparison 19: Early graft thrombosis, unfractionated heparin (UFH) versus antithrombin, Outcome 1: Antithrombin versus UFH, intraoperative graft thrombosis
19.2. Analysis.

Comparison 19: Early graft thrombosis, unfractionated heparin (UFH) versus antithrombin, Outcome 2: Antithrombin versus UFH, 1 month occlusion (following thrombendarterectomy)
Comparison 20. Early graft occlusion, low molecular weight heparin (LMWH) versus dextran.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 20.1 Early graft occlusion, 30 days | 1 | 277 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.16, 1.02] |
20.1. Analysis.

Comparison 20: Early graft occlusion, low molecular weight heparin (LMWH) versus dextran, Outcome 1: Early graft occlusion, 30 days
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Arfvidsson 1990.
| Study characteristics | ||
| Methods | An open, randomised clinical trial with patients undergoing infrainguinal revascularisation. | |
| Participants | 116 patients suffering from IC or severe arterial insufficiency were included in the study. 92 patients (79%) had critical or severe ischemia (PAOD stages III and IV) and 24 patients suffered from (21%) claudication (stage IIb). There was no significant difference between the treatment and the control group concerning age (71 ± 1 vs 73 ± 1 years), sex (37 m/24 f vs 36 m/19 f), duration of symptoms (26 ± 4 vs 25 ± 4 months), diabetes mellitus (15 vs 15 patients), stage of the disease (46 vs 46 patients with CLI), smoking (35 vs 29 patients), or type of reconstruction (27 vs 22 saphenous vein and 14 vs 19 PTFE grafts). Patients undergoing thrombendarterectomy were excluded from the analysis (20 vs 14). | |
| Interventions | 61 patients were assigned to treatment with dicoumarol. 55 patients no anticoagulation. All patients received 10 to 20,000 IU/24 hours iv heparin pre and postoperatively for three to five days. Dicoumarol was started within two days postoperatively; aimed for simplastin levels of 10% to 20%. |
|
| Outcomes | Graft occlusion, limb loss and death were primary endpoints at one, two, three, four, five, six, nine, and 12 months, thereafter every 12 months. Assessment of graft patency was performed by Doppler flow measurement and arteriography when in doubt. Bleeding complications requiring hospitalisation were recorded. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not discussed. |
| Allocation concealment (selection bias) | Low risk | Patients were randomly allocated to the two different treatment groups by a person not involved in the decision about the admission or non‐admission of the patients into the study. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No information given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Seven patients with occlusions within 2 weeks were excluded in each group, no patients lost to follow up. |
| Selective reporting (reporting bias) | Unclear risk | No explicit statement on primary/secondary outcomes of study, however there are no obvious omissions. |
| Other bias | Low risk | Appears to be free of other sources of bias |
BOA 2000.
| Study characteristics | ||
| Methods | An open, randomised, multicentre clinical trial of patients undergoing infrainguinal bypass surgery. | |
| Participants | 2690 patients undergoing infrainguinal venous or artificial bypass surgery were included. Baseline characteristics did not differ significantly between comparison groups regarding age (69 ± 10 vs 69 ± 10 years), sex (65 vs 63% male), stage of the disease (50% vs 52% claudicants), site of the distal anastomosis (45% vs 48% popliteal above knee, 36% vs 32% popliteal below knee, 18% vs 19% crural, 2% vs 1% pedal), graft material (59% vs 58% vein), and preoperative antithrombotic medication (63% vs 65%). There were 54% smokers, 39% with hypertension, 26% with diabetes mellitus, and 16% with hyperlipidemia in both groups. | |
| Interventions | 1339 were assigned to coumarin derivatives (phenprocoumon or acenocoumarol). 1351 to aspirin 80 mg daily, started within five days after surgery. The intended INR range was 3.0 to 4.5, which was achieved in 50% of the observed patient years. |
|
| Outcomes | Primary endpoint was graft patency assessed clinically by Doppler flow measurement or duplex sonography or by arteriography if indicated. Secondary endpoints were vascular death, myocardial infarction, stroke, amputation, vascular intervention, and major haemorrhage. Follow up was at three, six, 12, 18, and 24 months, with six monthly visits thereafter. Mean observation time was 21 months. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated blocked sequences of numbers based on different block lengths, stratified by centre. |
| Allocation concealment (selection bias) | Low risk | Randomisation by telephone at remote location. |
| Blinding (performance bias and detection bias) All outcomes | High risk | 'To avoid the ethical and practical difficulties of sham monitering of blood coagulation in the aspirin group, treatment assignment was open.' However, those adjudicating/classifying adverse events were 'unaware of treatment status.' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Small numbers of withdrawals, unlikely to influence result. '13 patients assigned oral anticoagulants were excluded after randomisation because they refused participation (6), did not undergo bypass surgery (3), or were already randomised (4). 27 patients assigned aspirin were withdrawn because they refused participation (7), did not have bypass surgery (17), or were already randomised (3)'. |
| Selective reporting (reporting bias) | Low risk | Planned outcomes discussed, adverse events discussed. |
| Other bias | Low risk | Appears to be free of other potential sources of bias. |
Cole 1993.
| Study characteristics | ||
| Methods | A randomised, open, single centre clinical trial of patients undergoing infrainguinal bypass surgery. | |
| Participants | 28 patients considered as 'high risk' for infrainguinal bypass grafting to either the blind popliteal segment or to a single outflow tibial vessel, were assigned to heparin treatment (n = 14; 13 venous bypasses and one PTFE graft) or to treatment with ancrod (n = 14; 12 venous, one PTFE, one composite graft). Patient characteristics were not further specified regarding age, sex, diabetes mellitus, arterial hypertension, hyperlipidemia, or smoking. | |
| Interventions | Heparin was administered intravenously during operation at a dose of 100 IU/kg. Ancrod was administered before operation for 12 hours at a dose of 70 IU, until fibrinogen levels were stabilised between 0.2 to 0.5 mg/L. Ancrod was continued postoperatively for 48 hours. |
|
| Outcomes | Primary outcomes were fibrinogen depletion and early graft patency. Graft patency was determined clinically by ABI and Doppler flow measurement before hospital discharge and one month postoperatively. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation ‐ generated by Rannor Call routine on Statistical Analysis System (SAS Institute Inc, Cary, NC). |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed but seems unlikely given nature of intervention. Unclear if those assessing patency were blind to treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 'One patient in the ancrod group withdrew from the study when surgery had to be delayed because the fibrinogen was found to be less than 0.2gm/L; the prothrombin time indicated that fibrinogen was completely absent and that surgery should be postponed until it was restored to the desired level. This patient was therefore removed from the study and from further analysis.' This is a potentially important side effect of ancrod treatment in a small sample size; exclusion may introduce bias. |
| Selective reporting (reporting bias) | High risk | No explicit statement on primary or secondary outcomes, no detailed analysis of adverse events. |
| Other bias | Low risk | Appears to be free of other potential sources of bias. |
Edmondson 1994.
| Study characteristics | ||
| Methods | An open, randomised, multicentre clinical trial in patients undergoing femoropopliteal bypass grafting. | |
| Participants | Patients with angiographically proven peripheral vascular disease undergoing venous or prosthetic femoropopliteal bypass surgery were included. Comparison groups were separated for patients with IC (n = 53 receiving LMWH, n = 54 ASA/DIP) and patients suffering from CLI (n = 41 and n = 52, respectively). Basic characteristics did not differ significantly between comparison groups such as age (66.8 vs 68.4 years), sex (65% vs 70% male), smoking (29% vs 31%), diabetes mellitus (15% vs 20%), and hyperlipidemia (5% vs 7%). There were 51% vs 56% claudicants in both groups, respectively. Approximately 20% of the patients had one patent run‐off vessel in both groups, 25% ‐ 30% had two patent vessels, and 50 % had three patent run‐off vessels. The distal anastomosis was located above knee in 68% vs 69% and below knee in 28% vs 27% of the patients. There were 29% vs 25% vein grafts in the two comparison groups. | |
| Interventions | 94 patients were randomised to receive 2500 IU LMWH (Fragmin) sc once daily. 106 patients to receive 300 mg aspirin and 100 mg dipyridamole, both three times per day for three months. |
|
| Outcomes | On the seventh postoperative day patients were assessed for primary graft occlusion, wound infection, thrombectomy, bleeding, and death. Total follow up was one year with visits at one, three, six, and 12 months. Graft patency was evaluated clinically and by ABI, and if indicated, supplemented by arteriography or duplex sonography. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Computer generated random numbers.' |
| Allocation concealment (selection bias) | Low risk | 'Central telephone randomisation.' |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | '3 on low molecular weight heparin and 2 on aspirin & dipiridymole were lost to follow up.' Similar numbers between 2 groups, unlikely to introduce bias. |
| Selective reporting (reporting bias) | Unclear risk | No explicit statement on primary/secondary outcomes, however there are no obvious admissions. |
| Other bias | Low risk | Appears to be free of other sources of bias. |
Jivegard 2005.
| Study characteristics | ||
| Methods | Multicentre, placebo controlled, randomised, double blind study of patients with lower limb ischaemia undergoing bypass surgery. Randomisation took place 1‐5 days postsurgery. | |
| Participants | 284 patients with lower limb ischaemia secondary to peripheral arterial disease with symptoms lasting more than 2 weeks who underwent a bypass procedure were randomised. Charecteristics between each group were similar; including re‐operation before randomisation (7% vs 6%), female sex (45% in each), present smoker (31% vs 30%), malignancy (2% vs 1%), diabetes (37% vs 36%) and age (73 vs 74 years). | |
| Interventions | 141 patients (116 of whom had infrainguinal bypasses) were randomised to receive 5000 iu dalteparin by a subcutaneous injection of 0.2mls once daily for 3 months. 140 patients (113 of whom had infrainguinal bypasses) received a 0.2ml placebo injection for 3 months. All patients received aspirin. |
|
| Outcomes | Primary objective was primary graft patency at three and 12 months postoperatively. Graft was considered patent if fulfilled one of eight specific criteria which defined standards for combinations of pulse palpation, doppler assessment, ankle‐brachial pressure index and intraoperative or autopsy findings. Secondary outcomes were healing of ischaemic ulcers and degree of rest pain in operated leg, acceptability of self injections after bypass grafting for lower limb ischaemia, incidence of other vascular events and death. | |
| Notes | Published data includes patients receiving both supra and infrainguinal bypass procedures analysed together. Communication with authors provided patency data for infrainguinal bypasses separately. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised using a computer algorithm method, taking into account 17 different parameters believed to be potential relevance for the patency of peripheral arterial bypass grafts. |
| Allocation concealment (selection bias) | Low risk | Randomisation at remote location via fax. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All patients, investigators, and study teams blind to intervention until all statistical analysis performed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Small number of withdrawals, unlikely to introduce bias. '2 patients withdrawn early after randomisation. One further patient with an occluded bypass by the time of randomisation also excluded.' |
| Selective reporting (reporting bias) | Low risk | Planned outcomes discussed, adverse events discussed. |
| Other bias | Low risk | Appears to be free of other potential sources of bias. |
Johnson 2002.
| Study characteristics | ||
| Methods | An open, randomised multicentre clinical trial in patients with PAD scheduled for elective axillofemoral, femoral‐femoral, femoropopliteal or femorodistal bypass surgery. Randomisation was between 1 October 1991 and 30 September 1995. | |
| Participants | A total of 614 patients with either IC or CLI (rest pain and/or tissue loss) undergoing either femoropopliteal above or below knee, or femorocrural prosthetic or venous bypass surgery were included. Those receiving axillofemoral, femoro‐femoral or femoropedal bypasses were excluded from comparison in this analysis. Patient characteristics included diabetes (85% to 91%), cholesterol levels (187 to 203 mg/dL), hypertension (85% to 91%), prior stroke (16.6% to 17,8%), prior myocardial infarction (18.6% to 30.0%). | |
| Interventions | 325 mg ASA was started one day preoperatively in both groups plus administration of warfarin approximately 5 mg/day in the treatment group on the first or second postoperative day. The low dose target INR was 1.4 to 2.8. | |
| Outcomes | Assisted primary graft occlusion, above‐ or below‐knee amputation, and death were clinical endpoints. Monthly monitoring of the INR was performed in the warfarin group. Follow‐up visits were every three months to assess bypass patency by ABI measurement and duplexsonography scanning if necessary. | |
| Notes | Randomisation was on the first postoperative day following stratification to prosthetic or venous graft material. Completion of randomisation was performed by a telephone call to the study coordinating center. Patency rates reported in this study were assisted primary patency rates, because bypass revision was permitted for bypasses identified as patient but stenotic. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not discussed. |
| Allocation concealment (selection bias) | Low risk | Allocated via telephone at remote location. |
| Blinding (performance bias and detection bias) All outcomes | High risk | 'Nonmasked clinical trial'. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 14 patients were lost to follow up ‐ 9 patients on warfarin + aspirin, 5 patients on aspirin alone. Comparable between two groups, unlikely to introduce bias given large sample size. |
| Selective reporting (reporting bias) | Low risk | Planned outcomes discussed, adverse events discussed. |
| Other bias | Low risk | Appears to be free of other sources of bias. |
Kretschmer 1992.
| Study characteristics | ||
| Methods | An open, randomised, single centre clinical trial of patients receiving femoropopliteal reversed vein grafts. | |
| Participants | 130 patients receiving an elective femoropopliteal venous bypass were assigned to treatment with coumarins or to no anticoagulant nor antiplatelet treatment. Patient characteristics such as sex (79% vs 78% male), age (62.5 vs 62.3 years), diabetes mellitus (35% vs 36%), arterial hypertension (27% vs 27%), stage of the disease (49% vs 53% claudicants), distal anastomotic site (42% vs 43% above knee), and poor run‐off with 0 to 1 patent vessel (80% vs 77%) did not differ significantly in the two comparison groups. | |
| Interventions | Patients were assigned to treatment with coumarin‐derivatives (phenprocoumon) or to no anticoagulation. Phenprocoumon was started in the second postoperative week until death or graft occlusion. Anticoagulant treatment was aimed at 15% to 25% for Quick values, 10% to 20% for Hepatoquick, or 5% to 12% for Thrombotest. | |
| Outcomes | Primary endpoints were graft occlusion, limb loss, and death. Graft patency assessment was performed by evaluation of pulsatile Doppler flow and ABI, which prompted an angiography at an ABI‐fall of 30%. Successfully treated stenoses were considered as patent bypasses. Follow‐up visits were at three‐monthly intervals during the first year and six‐monthly thereafter for 10 years. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were assigned to the two treatment groups by adaptive randomisation allowing optimum balancing of prognostic factors between groups according to Pocock (see Additional references). |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Reviewers were not blinded to treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No patients lost to follow up. |
| Selective reporting (reporting bias) | Unclear risk | Stated primary outcomes discussed, adverse events discussed in limited detail. No obvious admissions. |
| Other bias | Low risk | Appears to be free of other sources of bias. |
Logason 2001.
| Study characteristics | ||
| Methods | An open, randomised, multicentre clinical trial of patients undergoing femorodistal bypass. | |
| Participants | 314 patients from 16 centres undergoing femoro‐distal bypass were randomised to receive either dextran or LMWH in the perioperative period. Patient characteristics such as age (74 vs 73 years), diabetes (32% vs 37%), hypertension (46% vs 34%), cardiac problems (42% vs 46%), pulmonary problems (10% vs 8%) smoking (49% vs 46%), indication for surgery (acute ischaemia 1% vs 3%, intermittent claudication 15% vs 18%, rest pain 34% vs 26%, ulcer or gangrene 50% vs 53%) and type of graft (54% vs 50% vein) were similar between the two groups. 28 patients were excluded postrandomisation. | |
| Interventions | Patients were assigned treatment with dextran or LMWH in the perioperative period. The dextran group received a total dose of 2500mls Dextran 70; 500mls at anaesthetic induction and again immediately post‐operatively and a further 500mls on the morning of post‐operative days one, two and three. These patients received 5000 iu heparin at cross‐clamping. The LMWH group received 40mg enoxaparin sc the day before surgery and every evening for seven days postoperatively. At cross clamping this group received 20mg enoxaparin iv. |
|
| Outcomes | Graft patency was assessed at day one, days five to seven, day 30 and three months. Patency was determined using pulse palpation, ABPI and clinical judgement. Duplex scanning and angiography were used for cases of uncertainty. Patency was assessed blindly. Groups were also compared for blood loss, transfusion rates, and complications such as allergy, haemorrhage, myocardial infarction, heart failure and death. Haemoglobin, platelet count, serum creatinine and APTT were compared at days five to seven. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random list. |
| Allocation concealment (selection bias) | Low risk | Randomisation occurred separately at each participating centre with sequentially numbered sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | 'For practical reasons the study was not blind, but the surgeon assessing patency was blinded to the group.' |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Total of 28 patients excluded after randomisation; five from each group had anticoagulation before or after surgery, five had improper randomisation (two from dextran group, three from enoxaparin group), five did not have the planned operation performed (three from the dextran group, two from the enoxaparin group), five had the incorrect treatment (one from the dextran group, four from the enoxaparin treatment) and three had a creatinine of >200μmol/l (one from the dextran group, two from the enoxaparin group). Exclusions are generally comparable between each group. Missing data for eight patients when comparing graft patency at three months. |
| Selective reporting (reporting bias) | Low risk | All planned outcomes discussed, no omissions apparent. |
| Other bias | Low risk | Appears to be free of other potential sources of bias. |
Norgren 2004.
| Study characteristics | ||
| Methods | An open, multicentre (20 centres), randomised trial of 849 patients undergoing vascular surgery between November 2000 and December 2002. | |
| Participants | Inclusion criteria: patients undergoing surgery for crtical limb ischemia (464), intermittent claudication (201), abdominal aortic aneurysm (118), other anurysm (43), other indication (35). This included 313 patients who underwent femoropopliteal bypass with either autologous or synthetic grafts who could be included in this Cochrane review. Exclusion criteria: carotid surgery Age: 40‐96 years (median, 74 years) Sex: 507 men, 342 women. |
|
| Interventions | 40mg of enoxaparin iv immediately before arterial clamping with a corresponding heparin solution used to irrigate the vessels locally. 5000 IU UFH iv intraoperatively immediately before arterial clamping with a corresponding heparin solution used to irrigate the vessels locally. Preoperative use of other antithrombotics aspirin, LMWH, UFH, clopidogrel, dextran was similar in both groups. Postoperative treatment was given at the discretion of the individual hospital, mostly aspirin 75 mg/d or 160 mg/d but dextran or LMWH was allowed. 313 patients underwent femoropopliteal reconstructions 176 received LMWH and 137 received UFH. |
|
| Outcomes | Patency of arterial reconstruction at days 1 and 30 was recorded as was perioperative blood loss and protamine use to stop bleeding. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocks of 20 patients per hospital. |
| Allocation concealment (selection bias) | Low risk | 'Sealed envelope principle.' |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open trial. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 23 patients died and that 9 were not followed up 'for other reasons' but no information regarding which groups they belonged to or reasons for dropouts. |
| Selective reporting (reporting bias) | Low risk | Stated primary outcomes discussed, adverse events discussed, no obvious admissions. |
| Other bias | Unclear risk | Pharmaceutical company supported trial. |
Nydahl 1992.
| Study characteristics | ||
| Methods | An open, randomised clinical trial in patients undergoing infrainguinal bypass surgery. | |
| Participants | 13 patients admitted for infrainguinal bypass surgery were assigned to the two treatment groups. Mean age was 62 vs 74 years in the heparin and antithrombin group, respectively, while 71% vs 83% suffered from claudication. All patients but one had below‐knee popliteal distal anastomosis, 57% vs 50% had diabetes mellitus. The number of smokers was higher in the antithrombin group (14% vs 50%). | |
| Interventions | Seven patients were randomised to perioperative treatment with a single intravenous dose of 5000 IU UFH. Six patients to treatment with 1500 IU antithrombin into the femoral artery. |
|
| Outcomes | Patency assessment of the graft was performed by ABI evaluation and Doppler flow measurement one month after surgery. | |
| Notes | The study was planned to include 20 patients, but was stopped after inclusion of 13 patients, because all patients receiving antithrombin presented intraoperative graft thrombosis. Randomisation procedure was not specified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions or withdrawals postrandomisation apparent. |
| Selective reporting (reporting bias) | Unclear risk | Stated outcomes and adverse events discussed. |
| Other bias | High risk | Small sample size ‐ the study was planned to include 20 patients, but was stopped after inclusion of 13 patients because all patients receiving antithrombin developed intraoperative graft thrombosis. |
Samama 1994.
| Study characteristics | ||
| Methods | An open, randomised multicentre study conducted between 1990 and 1992 in patients undergoing elective femorodistal reconstructive surgery. | |
| Participants | All patients scheduled for femorodistal reconstructive surgery under general anesthesia were eligible for the study. Patient characteristics such as age (72.4 vs 71.4 years), sex (2.3 vs. 2.0 m/f ratio), BMI (24.7 vs 24.1), diabetes mellitus (32.3% vs 33 %), dyslipidemia (28.3% vs. 23%), arterial hypertension (61.6% vs 63%), smoking (47.5% vs 49%), graft material (67.7% vs 68% autologous vein), and site of the distal anastomosis (8.1% vs 11% above‐knee; 39.4% vs 31% below‐knee femoropopliteal) did not significantly differ between comparison groups. | |
| Interventions | 100 patients were randomly assigned to treatment with UFH. 99 patients were randomly assigned to treatment with LMWH (Enoxaparin). Treatment was started intraoperatively with an intravenous bolus, followed by flushing the saphenous vein or prosthetic graft and subcutaneous injections twice daily for 10 days. |
|
| Outcomes | Primary endpoint was graft thrombosis at 10 days postoperatively, which was assessed by angiography on day 10 ± 2 or before if indicated and clinically on day 30. Bleeding complications were recorded, defining major hemorrhage as a loss of > 2 g/dl of hemoglobin or transfusion of more than 2 packed red cell units. | |
| Notes | Data was analysed with intention to treat for drug efficacy. In an additional intra‐protocol analysis 67 angiographically assessed grafts (LWMH‐group) were compared to 64 grafts (UFH‐group). Eligible patients were randomly allocated to either of the two treatment groups. Randomisation procedure was not specified, nor was any clarification given by the authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomization was balanced by blocks of four patients, which were separate for each centre.' |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis carried out. 'Two patients (allocated to the enoxaparin group) never received any treatment and were excluded from analysis.' |
| Selective reporting (reporting bias) | Unclear risk | Stated outcomes discussed, adverse events discussed, no omissions apparent. |
| Other bias | Low risk | Appears to be free of other potential sources of bias. |
Sarac 1998.
| Study characteristics | ||
| Methods | An open, randomised, two centre clinical trial with patients scheduled for venous infrainguinal bypass surgery. | |
| Participants | Country: USA. Sex: 44 male, 12 female. 56 patients were scheduled for venous infrainguinal bypass surgery and were defined as high risk patients for graft occlusion, meaning that there was only a marginal venous conduit and/or poor arterial run‐off, prior graft failure. Patient characteristics differed in a higher percentage of diabetes mellitus in the aspirin group (53% vs 79%), but not for mean age (69.4 vs 66.2 years), sex (81% vs 75%), arterial hypertension (84% vs 100%), smoking (97% vs 75%). |
|
| Interventions | 32 patients (37 grafts) were randomised to warfarin and aspirin 325 mg/d. Warfarin therapy was started on postoperative days two or three with a target INR of two to three. 24 patients (27 grafts) to only aspirin 325 mg/d postoperatively |
|
| Outcomes | Primary endpoints were graft patency and limb loss. Graft patency was assessed by evaluation of ABI and duplexsonography. A decrease of 0.15 in ABI and graft velocity of more than 150 cm/sec or less than 30 cm /sec were defined as a failing graft requiring arteriography. Follow up was at two and four weeks, three and six months and every six months thereafter for three years. Occurrence of death, bleeding complications, and evacuation of wound haematoma were recorded. | |
| Notes | Postoperative morbidity rates were similar between groups but a higher incidence of wound haematomas in the warfarin group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐assisted random number generator. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open trial. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Large numbers of patients withdrawn over course of study in both groups in tables assessing patency; no explanation whether this reflects censorship due to shorter follow‐up period only or withdrawals for other reasons. |
| Selective reporting (reporting bias) | Unclear risk | Stated outcomes discussed, adverse events discussed, no omissions apparent. |
| Other bias | Low risk | Appears to be free of other potential sources of bias. |
Schneider 1979.
| Study characteristics | ||
| Methods | An open, randomised, single centre clinical trial performed between 1974 and 1978. | |
| Participants | Patients underwent either thrombendarterectomy or femoropopliteal venous bypass surgery. Among 213 eligible patients 91 were randomised to receive a femoropopliteal venous bypass. Patient characteristics did not differ significantly regarding age, sex, diabetes mellitus, arterial hypertension, smoking, and hyperlipidemia. | |
| Interventions | All patients were treated with therapeutic doses of heparin and coumarins in the first one to two postoperative weeks and were then randomly allocated to either coumarins (Quick, aim 25% to 30%) or aspirin, or aspirin/dipyridamole. Coumarin‐derivate versus ASA 1g or ASA 1g and dipyridamole 225 mg in infrainguinal venous bypasses and TEA. | |
| Outcomes | Graft patency was assessed angiographically before dismissal, further by evaluation of segmental oscillography and a fall of the systolic pressure of at least 20 mmHg. Signs of occlusions were measured angiographically. Follow‐up period was two years with three‐monthly visits during the first year and six‐monthly contacts in the second year. Drug assessment was performed by measurement of prothrombin time for coumarins and ASA levels in urine. Death and bleeding complications were recorded. Side effects like gastrointestinal bleeding or ulceration or pain or other bleeding complications could not be disclosed, as results were only presented for all patients, including those undergoing TEA. | |
| Notes | After contacting the authors, analysis of two additional publications of the same data was enabled. Allocation concealment remained unclear. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Coumarins vs antiplatelet was treated as open trial. Aspirin vs Aspirin + dipyridamole was double blind. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 13 patients in the antiplatelet groups discontinued therapy, 2 patients in the coumarin group discontinued therapy ‐ no clear explanation for these dropouts given but postulates that may in part be related to side effects. |
| Selective reporting (reporting bias) | High risk | Stated outcomes discussed, limited discussion on adverse events. |
| Other bias | Low risk | Appears to be free of other potential sources of bias. |
Swedenborg 1996.
| Study characteristics | ||
| Methods | An open, randomised clinical trial of patients undergoing elective infrainguinal bypass surgery. | |
| Participants | 18 patients were investigated receiving 18 autologous saphenous vein grafts. Patient characteristics regarding age, sex, preoperative ABI, stage of the disease (claudication), smoking, site of the distal anastomosis did not differ significantly in the two comparison groups. | |
| Interventions | LMWH (Fragmin) or UFH in a dose of 70 anti‐Xa activity U/kg of UFH or LMWH (Fragmin) was administered intravenously once during surgery. | |
| Outcomes | The main focus of this study was to evaluate hypercoagulability as a risk factor, whereas early graft occlusion was only of secondary interest. Follow‐up duration was not specified, but the term 'postoperatively' implied occlusion shortly after operation but no longer than 24 hours. No further information was accessible for follow‐up evaluations of graft patency, limb salvage or survival. | |
| Notes | Patients scheduled for saphenous vein infrainguinal bypass were randomised to either treatment strategy. Randomisation procedure was not specified and attempts to contact the authors were unsuccessful. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions or dropouts apparent. |
| Selective reporting (reporting bias) | High risk | Stated outcomes discussed, no discussion of adverse events. |
| Other bias | High risk | Small sample size. |
ABI: ankle‐brachial pressure index ASA: acetylsalicylic acid (aspirin) BMI: body mass index CLI: critical limb ischaemia DIP: dipyridamole IC: intermittent claudication INR: international normalised ratio iv: intravenous
LMWH: low molecular weight heparin PAD: peripheral arterial disease
PTA: peripheral percutaneous angioplasty PTFE: polytetrafluoroethylene Simplastin: a reagent used to measure thrombin time TEA: thrombendarterectomy
UFH: unfractionated heparin vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alja‐Kulju 1990 | Retrospective study. |
| Benedetti 1988 | Not an RCT. No control group investigated. Heterogeneous cohort of different operations (aortofemoral, femoropopliteal, femorotibial, extraanatomical) and different graft materials such as Dacron, PTFE, composite, saphenous vein. Insufficient presentation of data. |
| Evans 1966 | No control group was used. |
| Fietze‐Fischer 1987 | Prostaglandin treatment |
| Gruss 1991 | Prostaglandin treatment |
| Kretschmer 1987 | Retrospective study. |
| Kretschmer 1988 | Retrospective study. |
| Kujath 2002 | Pilot study to identify optimal dosing regimen of LMWH, no control group, heterogeneic group of operations. |
| McMillan 1997 | Patients were not randomised. |
| Ohtake 1997 | Retrospective study of a platelet inhibitor compared to warfarin as antithrombotic therapy after femoro‐popliteal bypass surgery in PTFE grafts. |
| Ray 1997 | No control group was used. |
| Rosenthal 1987 | Retrospective study. |
| Waibel 1981 | Hetergeneic group of operations containing only small number of femoral bypasses. Insufficient information. |
PTFE: polytetrafluoroethylene RCT: randomised controlled trial
Contributions of authors
For this update Alistair Geraghty (AG) and Karen Welch (KW) independently selected trials, extracted data and carried out data analyses. AG updated the text of the review.
Sources of support
Internal sources
No sources of support provided
External sources
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
Arfvidsson 1990 {published data only}
- Arfvidsson B, Lundgren F, Drott C, Schersten T, Lundholm K. Influence of coumarin treatment on patency and limb salvage after peripheral arterial reconstructive surgery. American Journal of Surgery 1990;159(6):556-60. [DOI] [PubMed] [Google Scholar]
BOA 2000 {published data only}
- Algra A. A randomised trial of oral anticoagulants versus aspirin after infrainguinal bypass surgery. Tijdschrift voor Sociale Gezondheidszorg 1999;77(4):11.
- Ariesen MJ, Tangelder MJ, Lawson JA, Eikelboom BC, Grobbee DE, Algra A, et al. Risk of major haemorrhage in patients after infrainguinal venous bypass surgery: therapeutic consequences? The Dutch BOA (Bypass Oral Anticoagulants or Aspirin) study. European Journal of Vascular and Endovascular Surgery 2005;30(2):154-9. [DOI] [PubMed] [Google Scholar]
- Dutch Bypass Oral anticoagulants of Aspirin (BOA) Study Group. Efficacy of oral anticoagulants compared with aspirin after infrainguinal bypass surgery (The Dutch Bypass Oral anticoagulants or Aspirin study): a randomised trial. Lancet 2000;355(9201):346-51. [PubMed] [Google Scholar]
- Eikelboom BC. Prevention of occlusions after infrainguinal bypass surgery with oral anticoagulants or acetylsalicylic acid: A randomised trial. Nederlands Tijdschrift voor Geneeskunde 2001;145(22):1060-7. [PubMed] [Google Scholar]
- Gorter JW, Oostenbrink JB, Tangelder MJ, Stroke Prevention in Reversible Ischemia Trial (SPIRIT) Study Group, Dutch Bypass Oral, et al. Costs of outpatient anticoagulant treatment in patients with cerebral and peripheral arterial occlusive disease. Thrombosis and Haemostasis 2001;85(1):52-6. [PubMed] [Google Scholar]
- Oostenbrink JB, Tangelder MJ, Busschbach JJ, Van Hout BA, Buskens E, Algra A, et al. Cost-effectiveness of oral anticoagulants versus aspirin in patients after infrainguinal bypass grafting surgery. Journal of Vascular Surgery 2001;34(2):254-62. [DOI] [PubMed] [Google Scholar]
- Smeets L, Ho GH, Tangelder MJ, Algra A, Lawson JA, Eikelboom BC, et al. Outcome after occlusion of infrainguinal bypasses in the Dutch BOA Study: comparison of amputation rate in venous and prosthetic grafts. European Journal of Vascular and Endovascular Surgery 2005;30(6):604-9. [DOI] [PubMed] [Google Scholar]
- Tangelder MJ, Algra A, Lawson JA, Hennekes S, Eikelboom BC. Optimal oral anticoagulant intensity to prevent secondary ischemic and hemorrhagic events in patients after infrainguinal bypass graft surgery. Dutch BOA Study Group. Journal of Vascular Surgery 2001;33(3):522-7. [DOI] [PubMed] [Google Scholar]
Cole 1993 {published data only}
- Cole CW, Bormanis J, Luna GK, Hajjar G, Barber GG, Harris KA et al. Ancrod versus heparin for anticoagulation during vascular surgical procedures. Journal of Vascular Surgery 1993;17(2):288-93. [DOI] [PubMed] [Google Scholar]
Edmondson 1994 {published data only}
- Edmondson RA, Cohen AT, Das SK, Wagner MB, Kakkar VV. Low-molecular weight heparin versus aspirin and dipyridamole after femoropopliteal bypass grafting. Lancet 1994;344:914-8. [DOI] [PubMed] [Google Scholar]
- Kakkar VV, Edmondson RA, Cohen AJ, Phillips MJ. A prospective, randomized trial of low molecular weight heparin (LMWH) versus persantin in femoropopliteal bypass grafts. Thrombosis and Haemostasis 1993;69(6):647-Abstract No 375.
Jivegard 2005 {published and unpublished data}
- Jivegard L, Drott C, Gelin J, Groth O, Hensater M, Jensen N, et al. Effects of three months of low molecular weight heparin (dalteparin) treatment after bypass surgery for lower limb ischemia - A randomised placebo-controlled double blind multicentre trial. European Journal of Vascular and Endovascular Surgery 2005;29(2):190-8. [DOI] [PubMed] [Google Scholar]
Johnson 2002 {published data only}
- Jackson MR, Johnson WC, Williford WO, Valentine RJ, Clagett GP. The effect of anticoagulation therapy and graft selection on the ischemic consequences of femoropopliteal bypass graft occlusion: results from a multicenter randomized clinical trial. Journal of Vascular Surgery 2002;35(2):292-8. [DOI] [PubMed] [Google Scholar]
- Johnson WC, Williford WO, Corson JD, Padberg FT Jr. Hemorrhagic complications during long-term postoperative warfarin administration in patients undergoing lower extremity arterial bypass surgery. Vascular 2004;12(6):362-8. [DOI] [PubMed] [Google Scholar]
- Johnson WC, Williford WO and members of the Department of Veterans Affairs Cooperative Study. Benefits, morbidity, and mortality associated with long-term administration of oral anticoagulant therapy to patients with peripheral arterial bypass procedures: A prospective randomized study. Journal of Vascular Surgery 2002;35(3):413-21. [DOI] [PubMed] [Google Scholar]
- Kibbe MR, Hassett AL, McSherry F, Conner P, Bontempo FA, Williford W, et al. Can screening for genetic markers improve peripheral artery bypass patency? Journal of Vascular Surgery 2002;36(6):1198-206. [DOI] [PubMed] [Google Scholar]
Kretschmer 1992 {published data only}
- Kretschmer G, Herbst F, Prager M, Sautner T, Wenzl E, Berlakovich GA, et al. A decade of oral anticoagulants treatment to maintain autologous vein grafts for femoropopliteal atherosclerosis. Archives of Surgery 1992;127:1112-5. [DOI] [PubMed] [Google Scholar]
- Kretschmer G, Wenzl E, Wagner O, Polterauer P, Piza F, Schemper M, et al. Can anticoagulants prevent graft reocclusion? In: Maurer PC, Becker HM, Heidrich H, Hoffmann G, Kriessmann A, Muller-Wiefel H, Pratorius C, editors(s). What is new in Angiology?. Zuckschwerdt W, 1986:200-1. [Google Scholar]
Logason 2001 {published data only}
- Logason K, Bergqvist D, Study Group on AntIthrombotic Prophylaxis of Femorodistal Bypass Surgery. Low molecular weight heparin (enoxaparin) versus dextran in the prevention of early occlusion following arterial bypass surgery distal to the groin. European Journal of Vascular and Endovascular Surgery 2001;21(3):261-5. [DOI] [PubMed] [Google Scholar]
- Logason K. Low-molecular weight heparin versus dextran in the prevention of early occlusion following arterial bypass surgery distal to the groin. European Journal of Surgery, Supplement 1998;(581):40-1. [DOI] [PubMed] [Google Scholar]
Norgren 2004 {published data only}
- Norgren L, Swedish EnoxaVasc Study Group. Can low molecular weight heparin replace unfractionated heparin during peripheral arterial reconstruction? An open label prospective randomized controlled trial. Journal of Vascular Surgery 2004;39(5):977-84. [DOI] [PubMed] [Google Scholar]
Nydahl 1992 {published data only}
- Nydahl S, Swedenborg J, Egberg N. Peroperative anticoagulation with antithrombin or heparin in infrainguinal bypass surgery. European Journal of Vascular Surgery 1992;6:610-5. [DOI] [PubMed] [Google Scholar]
Samama 1994 {published data only}
- Samama CM, Gigou F, Ill P. Low-molecular weight heparin vs. unfractionated heparin in femorodistal reconstructive surgery: a multicenter open randomized study. Annals of Vascular Surgery 1995;9 Suppl:45-53. [DOI] [PubMed] [Google Scholar]
- Samama CM. Low molecular weight heparin (enoxaparin) versus unfractionated heparin during and after arterial reconstructive surgery; A multicenter randomized study. Thrombosis and Haemostasis 1993;69(6):546-Abstract No 24.
Sarac 1998 {published data only}
- Sarac TP, Huber TS, Back MR, Ozaki CK, Carlton LM, Flynn TC, et al. Warfarin improves the outcome of infrainguinal vein bypass grafting at high risk for failure. Journal of Vascular Surgery 1998;28(3):446-517. [DOI] [PubMed] [Google Scholar]
Schneider 1979 {published data only}
- Bollinger A, Schneider E, Pouliadis G, Torres C, Brunner U. Antiplatelet drugs and anticoagulants after reconstructive surgery of the femoro-popliteal arteries. Results of a prospective study. In: Prophylaxis of Venous, Peripheral, Cardiac and Cerebral Vascular Diseases with Acetylsalicylic Acid. Stuttgart, New York: F.K. Schattauer Verlag, 1980:73-8.
- Schneider E, Brunner U, Bollinger A. Medical treatment for preventing recurrence after femoro-popliteal arterial reconstruction [Medikamentose Rezidivprophylaxe nach femoro-poplitealer Arterienrekonstruktion]. Angio 1979;2(1):73-7. [Google Scholar]
Swedenborg 1996 {published data only}
- Swedenborg J, Nydahl S, Egberg N. Low molecular mass heparin can be used as peri-operative anticoagulant during infrainguinal bypass surgery. Thrombosis and Haemostasis 1995;73(6):979-Abstract No 308.
- Swedenborg J, Nydahl S, Egberg N. Low molecular mass heparin instead of unfractionated heparin during infrainguinal bypass surgery. European Journal of Vascular and Endovascular Surgery 1996;11(1):59-64. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alja‐Kulju 1990 {published data only}
- Alja-Kulju K, Ketonen P, Salo J, Sipponen J, Verkkala, K, Harjola P-T. Effect of antiplatelet and anticoagulant therapy on patency of femorotibial bypass grafts. Journal of Cardiovascular Surgery 1990;31(5):651-5. [PubMed] [Google Scholar]
Benedetti 1988 {published data only}
- Benedetti-Valentini F, Irace L, Gattuso R, Ciocca F, Aracu A, Intrieri F, et al. Arterial repair of the lower limbs: prevention of prosthetic grafts occlusion by LMW-heparin. International Angiology 1988;7 Suppl 3:29-32. [PubMed] [Google Scholar]
Evans 1966 {published data only}
- Evans G, Irvine WT. Long-term arterial-graft patency in relation to platelet adhesiveness, biochemical factors, and anticoagulant therapy. Lancet 1966;2(7459):353-5. [DOI] [PubMed] [Google Scholar]
Fietze‐Fischer 1987 {published data only}
- Fietze-Fischer B, Gruss JD, Bartels D, et al. Prostaglandin E1 as an adjuvant therapy in the event of femoropopliteal and crural great saphenous vein in situ bypass surgery. Vasa 1987;16 Suppl 17:23-5. [PubMed] [Google Scholar]
- Fietze-Fischer B, Gruss JD, Bartels D, Vargas-Montano H, Stritter W. Prostaglandin E1 as an adjuvant therapy in the event of femoropopliteal and crural great saphenous vein in situ bypass surgery. Vasa 1987;16 Suppl 17:23-5. [PubMed] [Google Scholar]
Gruss 1991 {published data only}
- Gruss JD, Hiemer W, Kroiss A, Geissler C. Experience with adjuvant prostaglandin therapy in vascular surgery. Vasa 1991;20 Suppl 33:353-4. [PubMed] [Google Scholar]
Kretschmer 1987 {published data only}
- Kretschmer G, Wenzl E, Piza F, Polterauer P, Ehringer H, Minar E, et al. The influence of anticoagulant treatment on the probability of function in femoropopliteal vein bypass surgery: Analysis of a clinical series (1970 to 1985) and interim evaluation of a controlled clinical trial. Surgery 1987;102(3):453-9. [PubMed] [Google Scholar]
Kretschmer 1988 {published data only}
- Kretschmer G, Wenzl E, Schemper M, Huk I, Konecny U, Marosi L et al. Vein bypass surgery for femoro-popliteal ateriosclerosis: influence of different risk factors on patient survival and the importance of anticoagulant treatment. European Journal of Vascular Surgery 1988;2(2):77-81. [DOI] [PubMed] [Google Scholar]
Kujath 2002 {published data only}
- Kujath P, Eckmann C, Misselwitz F. Low-molecular-weight heparin in arterial reconstructive surgery: a double-blind, randomized dose-finding trial. Clinical and Applied Thrombosis/Hemostasis 2002;8(4):337-45. [DOI] [PubMed] [Google Scholar]
McMillan 1997 {published data only}
- McMillan WD, McCarthy WJ, Lin SJ, Matsumura JS, Pearce WH, Yao JST. Perioperative low molecular weight heparin for infrageniculate bypass. Journal of Vascular Surgery 1997;25(5):796-801. [DOI] [PubMed] [Google Scholar]
Ohtake 1997 {published data only}
- Ohtake H, Urayama H, Kimura, K, Yokoi K, Tsunezuka Y, Kawakami T, et al. Comparison of cilostazol with warfarin as antithrombotic therapy after femoro-popliteal bypass surgery using an e-PTFE graft. Minerva Cardioangiologica 1997;45(11):527-30. [PubMed] [Google Scholar]
Ray 1997 {published data only}
- Ray SA, Rowley MR, Bevan DH, Taylor RS, Dormandy JA. Hypercoagulable abnormalities and postoperative failure of arterial reconstruction. European Journal of Endovascular Surgery 1997;13(4):363-70. [DOI] [PubMed] [Google Scholar]
Rosenthal 1987 {published data only}
- Rosenthal D, Mittenthal MH, Ruben DM, Jones DH, Estes JW, Stanton PE, et al. The effects of aspirin, dipyridamole and warfarin in femorodistal reconstructions: long-term results. American Surgeon 1987;53(9):477-81. [PubMed] [Google Scholar]
Waibel 1981 {published data only}
- Waibel P, Geering P. Late results of anticoagulation in arterial reconstructions. Vasa 1981;10:308-9. [PubMed]
Additional references
Abbott 1997
- Abbott WM, Green RM, Matsumoto T, Wheeler JR, Miller N, Veith FJ, et al. Prosthetic above-knee femoropopliteal bypass grafting: results of a multicenter randomized prospective trial. Above-Knee Femoropopliteal Study Group. Journal of Vascular Surgery 1997;25(1):19-28. [DOI] [PubMed] [Google Scholar]
Achermann 1998
- Achermann A, Gurke L, Stirnemann P. Supragenicular bypass: venous in comparison with synthetic prosthesis (PTFE). Swiss Surgeon 1998;4(3):129-32. [PubMed] [Google Scholar]
Alback 1998
- Alback A, Lepantalo M. Immediate occlusion of in situ saphenous vein bypass grafts: a survey of 329 reconstructions. European Journal of Surgery 1998;164(10):745-50. [DOI] [PubMed] [Google Scholar]
Bandyk 1987
- Bandyk DF, Kaebnick HW, Stewart GW, Towne JB. Durability of the in situ saphenous vein arterial bypass: a comparison of primary and secondary patency. Journal of Vascular Surgery 1987;5(2):256-68. [DOI] [PubMed] [Google Scholar]
CASPAR 2010
- Belch JJ, Dormandy J, Biasi BM, Cairols M, Diehm C, Eikelboom B, et al. Results of the randomized, placebo-controlled clopidogrel and acetylsalicylic acid in bypass surgery for peripheral arterial disease (CASPAR) trial. Journal of Vascular Surgery 2010;52:825-33, 833. [DOI] [PubMed] [Google Scholar]
Channon 1997
- Channon KM, Fulton GJ, Davies MG, Peters KG, Ezekowitz MD, Hagen PO, et al. Modulation of tissue factor protein expression in experimental venous bypass grafts. Arteriosclerosis, Thrombosis, and Vascular Biology 1997;17(7):1313-9. [DOI] [PubMed] [Google Scholar]
Cochrane 2008
- Brown J, Lethaby A, Maxwell H, Wawrzyniak AJ, Prins MH. Antiplatelet agents for preventing thrombosis after peripheral arterial bypass surgery. Cochrane Database of Systematic Reviews 2008, Issue 4. Art. No: CD000535. [DOI: 10.1002/14651858.CD000535.pub2] [DOI] [PubMed] [Google Scholar]
Cochrane 2010
- Twine CP, McLain AD. Graft type for femoro-popliteal bypass surgery. Cochrane Database of Systematic Reviews 2010, Issue 5. Art. No: CD001487. [DOI: 10.1002/14651858.CD001487.pub2] [DOI] [PubMed] [Google Scholar]
Consensus 1991
- Anonymous. Second European Consensus Document on chronic critical leg ischaemia. Circulation 1991;84 Suppl 4:1-26. [PubMed] [Google Scholar]
Davies 1994
- Davies MG, Hagen PO. Pathobiology of intimal hyperplasia. The British Journal of Surgery 1994;81(9):1254-69. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fontaine 1954
- Fontaine VR, Kim M, Kieny R. Surgical treatment for peripheral vascular disease [Die chirurgische Behandlung der peripheren Durchblutungsstorungen]. Helvetica Chirurgica Acta 1954;5/6:499-533. [PubMed] [Google Scholar]
Girolami 2000
- Girolami B, Bernardi E, Prins MH, ten Cate JW, Prandoni P, Simioni P, et al. Antiplatelet therapy and other interventions after revascularisation procedures in patients with peripheral arterial disease: a meta-analysis. European Journal of Vascular and Endovascular Surgery 2000;19(4):370-80. [DOI] [PubMed] [Google Scholar]
Hanson 1987
- Hanson SR, Harker LA. Vascular graft thrombus formation. Annals of the New York Acadamy of Science 1987;516:653-61. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.2. [updated September 2009]. The Cochrane Collaboration, 2009. Available from www.cochrane-handbook.org.
Jacot 2004
- Jacot JG, Abdullah I, Belkin M, Gerhard-Herman M, Gaccione P, Polak JF, et al. Early adaptation of human lower extremity vein grafts: wall stiffness changes accompany geometric remodeling. Journal of Vascular Surgery 2004;39:547-55. [DOI] [PubMed] [Google Scholar]
Johnson 2000
- Johnson WC, Lee KK. A comparative evaluation of polytetrafluoroethylene, umbilical vein, and saphenous vein bypass grafts for femoral-popliteal above-knee revascularization: a prospective randomized Department of Veterans Affairs cooperative study. Journal of Vascular Surgery 2000;32(2):268-77. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kissin 2000
- Kissin M, Kansal N, Pappas PJ, DeFouw DO, Duran WN, Hobson RW. Vein interposition cuffs decrease the intimal hyperplastic response of polytetrafluoroethylene bypass grafts. Journal of Vascular Surgery 2000;31:69-83. [DOI] [PubMed] [Google Scholar]
Kraiss 1995
- Kraiss LW, Johansen K. Pharmacologic intervention to prevent graft failure. Surgical Clinics of North America 1995;75(4):761-72. [DOI] [PubMed] [Google Scholar]
Kretschmer 1999
- Kretschmer G, Holzenbein TH. Oral anticoagulation in peripheral vascular surgery: how intense, for how long, or at all? Journal of Internal Medicine 1999;245(4):389-97. [DOI] [PubMed] [Google Scholar]
Lindblad 1995
- Lindblad B, Wakefield TW, Stanley TJ, Bergqvist D, Nichol BJ, Greenfield LJ, et al. Pharmacological prophylaxis against postoperative graft occlusion after peripheral vascular surgery: a world-wide survey. European Journal of Vascular and Endovascular Surgery 1995;9(3):267-71. [DOI] [PubMed] [Google Scholar]
Muluk 1998
- Muluk SC, Vorp DA, Severyn DA, Gleixner S, Johnson PC, Webster MW. Enhancement of tissue factor expression by vein segments exposed to coronary arterial hemodynamics. Journal of Vascular Surgery 1998;27(3):521-7. [DOI] [PubMed] [Google Scholar]
Norgren 2007
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). Journal of Vascular Surgery 2007;45 Suppl S:5-67. [PMID: ] [DOI] [PubMed] [Google Scholar]
Owens 2010
- Owens CD. Adaptive changes in autogenous vein grafts for arterial reconstruction: clinical implications. Journal of Vascular Surgery 2010;51(3):736-46. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Parsons 1996
- Parsons RE, Suggs WD, Veith FJ, Sanchez LA, Lyon RT, Marin ML, et al. Polytetrafluoroethylene bypasses to infrapopliteal arteries without cuffs or patches: a better option than amputation in patients without autologous vein. Journal of Vascular Surgery 1996;23(2):347-54. [DOI] [PubMed] [Google Scholar]
Robinson 1997
- Robinson KD, Sato DT, Gregory RT, Gayle RG, DeMasi RJ, Parent FN 3rd. Long-term outcome after early infrainguinal graft failure. Journal of Vascular Surgery 1997;26(3):425-37. [DOI] [PubMed] [Google Scholar]
Sarkar 2006
- Sarkar S, Salacinski HJ, Hamilton G, Seifalian AM. The mechanical properties of infrainguinal vascular bypass grafts: their role in influencing patency. European Journal of Vascular and Endovascular Surgery 2006;31:627-36. [DOI] [PubMed] [Google Scholar]
Sasaki 2000
- Sasaki Y, Suehiro S, Becker AE, Kinoshita H, Ueda M. Role of endothelial cell denudation and smooth muscle cell dedifferentiation in neointimal formation of human vein grafts after coronary artery bypass grafting: therapeutic implications. Heart 2000;83(1):69-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schoder 2001
- Schoder M, Cejna M, Lammer J. Interventions in infrainguinal bypass grafts. ROFO-Fortschritte auf dem Gebiet der Rontgenstrahlen und der Bildgebenden Verfahren 2001;173(12):1059-68. [DOI] [PubMed] [Google Scholar]
Stept 1987
- Stept LL, Flinn WR, McCarthy WJ III, Bartlett ST, Bergan JJ, Yao JS. Technical defects as a cause of early graft failure after femorodistal bypass. Archives of Surgery 1987;122(5):599-604. [DOI] [PubMed] [Google Scholar]
Tangelder 1999
- Tangelder MJD, Lawson JA, Algra A, Eikelboom BC. Systematic review of randomized controlled trials of aspirin and oral anticoagulants in the prevention of graft occlusion and ischemic events after infrainguinal bypass surgery. Journal of Vascular Surgery 1999;30(4):701-9. [DOI] [PubMed] [Google Scholar]
Watson 1999
- Watson HR, Belcher G, Horrocks M. Adjuvant medical therapy in peripheral bypass surgery. The British Journal of Surgery 1999;86(8):981-91. [DOI] [PubMed] [Google Scholar]
Woodburn 1996
- Woodburn KR, Rumley A, Lowe GD, Love JG, Murray GD, Pollock JG. Clinical, biochemical, and rheological factors affecting the outcome of infrainguinal bypass grafting. Journal of Vascular Surgery 1996;24(4):639-46. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Cochrane 2003
- Dörffler-Melly J, Büller HR, Koopman MM, Prins MH. Antithrombotic agents for preventing thrombosis after infrainguinal arterial bypass surgery. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858] [DOI] [PubMed] [Google Scholar]


