Abstract
In the era of widespread antiretroviral therapy (ART), consequences of being HIV-exposed is unclear for children, especially in rural communities. A population sample of consecutive births (470/493) in the Eastern Cape of South Africa (SA) were recruited and reassessed at five points over the first 24 months. Maternal and child outcomes between mothers living with and without HIV were assessed using multiple linear and logistic regressions. At birth, 28% of the sample was mothers living with HIV and five additional mothers seroconverted. All mothers living with HIV reported taking ART. The rate of depressed mood and IPV was similar across serostatus. However, mothers living with HIV significantly decreased their alcohol use after learning about their pregnancy and were more likely to exclusively breastfeed when compared to mothers without HIV. Despite maternal HIV status, children had similar growth across the first 24 months of life. Future work is needed to assess if these developmental trajectories will persist.
Keywords: HIV Exposed children, HIV, Rural, South Africa, Exclusive breastfeeding
Introduction
South Africa (SA) has the largest HIV/AIDS epidemic globally (UNAIDS, 2016). Women are almost four times more likely to be infected in their early 20s as compared to men of the same age (Johnson et al., 2016). Women between the ages of 15–24 have the highest HIV incidence rate (2.01%) of any sex or age cohort in the country and represent 37% of new HIV infections in SA (SANAC, 2017). The prevention of vertical HIV transmission has been a global priority, as has been the management of HIV infection in pregnant women. In tandem with tuberculosis, HIV is still the greatest single underlying cause of maternal mortality in SA (National Committee on Confidential Enquiries into Maternal Deaths, 2018). Currently, about 250,000 South African mothers living with HIV have access to comprehensive vertical HIV transmission prevention services every year, which include lifelong antiretroviral therapy (ART), irrespective of their CD4 count (UNAIDS, 2016). As a result, perinatal transmission rates have fallen markedly from 30% in 2000 to less than 2% (Mayosi et al., 2012). In 2015, fewer than 6,000 infants contracted HIV, compared to 70,000 in 2004 (SANAC, 2017). As the number of HIV-infected infants has decreased, the focus has shifted towards HIV-exposed but uninfected infants who number about 3.2 million in SA (UNAIDS, 2017). Nearly 31% of all children born in SA in 2015 were HIV-exposed but uninfected (National Department of Health, 2017) and may face significant health deficits (Filteau, 2009; Goetghebuer, Rowland-Jones, & Kollmann, 2017a, 2017b; Evans, Jones, & Prendergast, 2016).
The majority of studies in the pre-ART era, most notably the ZVITAMBO study, a large cohort of 12,345 children (3135 HIV-exposed but uninfected), indicate that HIV-exposed children do worse than HIV unexposed children (Evans, Humphrey, Ntozini, & Prendergast, 2016). HIV-exposed children tend to have lower birth weights, impaired growth, and increased morbidity and mortality (Evans, Humphrey, et al., 2016; Evans, Chasekwa, Ntozini, Humphrey, & Prendergast, 2016; Omoni et al., 2017; Slogrove, Goetghebuer, Cotton, Singer, & Bettinger, 2016). However, a large South African study from 2001–2004 indicates that by 2 years, exclusive breastfeeding may minimize some of the negative impacts of HIV exposure (Patel et al., 2010). Since ART has become more widely available, there have been few large cohort studies comparing HIV-exposed and unexposed children and although there has been a greater heterogeneity of results, there are still major concerns about the sequelae of being born to mothers living with and without HIV (Goetghebuer et al., 2017b; Roux, Abrams, Nguyen, & Myer, 2016). Given these mixed findings, longitudinal studies examining the health of HIV-exposed children in the ART-era are key, especially from rural areas from where there is very little data.
The negative consequences of being born to a mother living with HIV are not entirely unexpected. The social and health impacts of HIV on mothers living with HIV and, by extension, their children, is lifelong. At its most extreme, this may involve a child being orphaned, but there may also be financial, psychological, and practical factors that cumulatively harm children of mothers living with HIV. In urban centers, mothers living with HIV have lower socio-economic status when compared to mothers without HIV. Specifically, they have lower household income and are more likely to experience food insecurity (Davis, Rotheram-Borus, Weichle, Rezai, & Tomlinson, 2017). At the same time, mothers living with HIV are at greater risk for multiple, interacting comorbidities: depression (Tomlinson et al., 2014), alcohol use (Le Roux et al., 2013), and intimate partner violence (IPV) (Jewkes, Dunkle, Nduna, & Shai, 2010). Mothers living with HIV also have more tasks to sustain their health and the health of their children over time than mothers without HIV and may have less physical capacity to do so (Gardner, McLees, Steiner, del Rio, & Burman, 2011). These social and health risks are often experienced concurrently and jeopardize maternal and child well-being (Jewkes et al., 2010).
Although the immunological impact of being born to a mothers living with HIV is not entirely understood, there is evidence that children exposed to HIV but uninfected have higher inflammatory markers, fewer T-Cells, and lower levels of maternal IgG transferred during pregnancy and breastfeeding (independent of maternal CD4) (Evans et al., 2016). This undermines their response to an infectious challenge – especially in the first year of life (Slogrove et al., 2016; von Mollendorf et al., 2015). In the era of ART for mothers living with HIV, there is also evidence that some of the ARV regimens used for vertical HIV transmission prevention may have an impact on birth weight and growth at 24 months, and therefore long-term well-being (Powis et al., 2016).
There is limited data on the the challenges facing rural mothers living with HIV and consequences of these challenges on their children’s developmental trajectories. Few studies in rural Africa examine the social, economic, and comorbid health challenges faced by rural mothers living with HIV and the impact of maternal HIV on the growth and development of their exposed children in the ART era (Filteau, 2009). This article examines child outcomes of children of mothers living with and without HIV in a deeply rural setting, at a time when the Option A* approach to vertical HIV transmission prevention was being implemented in South Africa.
Methods
This study was conducted with approval of the Health Research Ethics Committee of Stellenbosch University (N12/08/046) and the Institutional Review Board at the University of California, Los Angeles (IRB#16-001362).
Setting
Zithulele Hospital is a rural hospital in the King Sabata Dalindyebo sub-district of the OR Tambo District of the Eastern Cape of SA. It is a 146-bed district hospital and has a catchment area with a population of approximately 130,000 people. It is situated in one of the poorest municipalities in SA (Business Tech, 2016), in the former Transkei homeland, a part of SA which was systematically neglected under apartheid. Zithulele Hospital, being a district hospital, cares for a lower risk maternity cohort than would be seen in a regional or tertiary hospital.
Between January and April 2013, a consecutive series of mothers giving birth at Zithulele Hospital and in the area covered by its 10 closest clinics were approached to participate in the study. This included mothers who delivered at home (10%) or on the way to a health facility (3%), as well as those who delivered at primary care clinics (9%). Mothers who travelled to the hospital from outside this catchment area to give birth at the hospital were excluded from the sample. Voluntary informed consent was obtained by 95% of mothers (470/493 live births; 5% refusal rate). When mothers were less than 18 years old, consent was also obtained from the parents/guardians of the adolescent mother. Nine of the 470 mothers gave birth to twins, and the second twin was excluded in the analysis. Mothers were approached while still in the hospital and typically interviewed in the first few days following birth. Those who delivered at home were recruited at their local clinic, which they typically visit as soon as possible after giving birth to secure a government Road to Health Card (RtHC). The RtHC is a type of health passport and, therefore, an important health record. It also serves as proof of birth and is used to apply for a birth certificate. We were notified about mothers who delivered at home by clinic sisters. These mother-infant pairs were typically visited within 2 weeks of delivery; mothers presenting to the hospital were interviewed within 1–3 days after giving birth.
Mothers were reassessed at five points over the course of the next two years. Twenty infants died in the first year and two infants died in the second year (9 of which were children of mothers living with HIV). The follow-up rates (with sample sizes adjusted for deaths) were: 85% at 3 months (n = 390/460), 92% at 6 months (n = 420/456), 88% at 9 months (n = 410/454), 91% at 12 months (n = 411/450), and 88% at 24 months post-birth (n = 396/450).
Assessments
Local, isiXhosa-speaking women were recruited and trained as field interviewers over a 6-week period. Training included interview techniques, administering the Edinburgh Postnatal Depression Scale (EPDS), the ethics of research, confidentiality and the use of mobile phones as a data collection tool. Field interviewers visited each home at 3, 6, 9, 12, and 24 months post-birth and collected data on mobile phones, which were pre-programmed with assessment questions by the Mobenzi mobile phone team (https://www.mobenzi.com/). The quality of the data entered into the Mobenzi Researcher Platform was checked routinely: on a daily basis for the first year by supervisors, and on a weekly basis for the second year.
Maternal measures
HIV status and testing and any positive TB test were self-reported by mothers at birth and at each assessment. Maternal HIV status was also verified on their maternity delivery record where this was available and the mother granted permission for the field worker to check the card. The HIV questions were skipped in interviews involving a child-minder. Tasks for preventing vertical HIV transmission. were self-reported by mothers living with HIV, including ART during pregnancy and post-birth, receiving nevirapine (NVP) for their child at birth, and HIV testing for their child. The child’s polymerase chain reaction (PCR) HIV test results were checked on the child’s RtHC at each visit post baseline, when permission was granted by the mother.
Demographic characteristics. Maternal age, years of education, marriage status, whether they were primipara, employed, or in school were self-reported by the mothers at birth.
Structural resources were identified as living with the father or in-laws, the number of people living in the household, monthly household income, having a water tank on the premises, and electricity. Food insecurity was assessed using one item (“How many days in the past week have you gone hungry?”) from The Household Food Insecurity Access Scale. This item has been found to be highly correlated with the nine-item scale used to distinguish food insecure from food secure households in South Africa (Tsai, Tomlinson, Comulada, & Rotheram-Borus, 2016).
The number of antenatal visits was self-reported, but typically verified by the interviewer or the mothers’ health cards.
Depression was assessed at each assessment using the Edinburgh Postnatal Depression Scale (EPDS) (Cox, Holden, & Sagovsky, 1987; Bruin, Swartz, Tomlinson, Cooper, & Molteno, 2004). The mean scale score is reported, as well as identifying mothers whose responses indicate probable depressive disorder (i.e., scores >13 to indicate depressed mood) ( Rochat et al., 2006). Alcohol use was self-reported as 0 (never drinking) or 1 (drinking in the month prior to recognizing pregnancy or during pregnancy). Intimate partner violence (IPV). Mothers reported whether they had been slapped, pushed or shoved, and/or threatened with a weapon by their current partner in the past 12 months at birth and at the 12-month assessment.
Child measures
A low birth weight was recorded for those infants whose weight was < 2500 grams (1) or not (0; ≥ 2500 grams). Weight and height were measured by trained and certified interviewers on measuring mats and on electronic scales that were recalibrated weekly. Children’s weight and height measures were then converted to Z-scores based on the World Health Organization’s (WHO) age-adjusted norms (http://www.who.int/childgrowth/standards/en/). Thus, growth at birth, 3, 6, 9,12, and 24 months is reflected in standardized scores (Z scores) for height-for-age (HAZ), weight-for-age (WAZ), and weight-for-length/height (WHZ). A Z-score below −2SD was considered a serious health deficit, as being stunted (< −2SD for HAZ), underweight-for-age (< −2 SD for WAZ) or wasted (< −2 SD for WHZ) (de Onis & Blössner, 2003).
Clinic and hospital visits. The mothers self-reported the number of clinic and hospital visits due to the child’s health and specially indicated episodes of diarrhea at 3 and 6 months post-birth.
Breastfeeding. Mothers self-reported on their feeding practices over the past 24 hours, 1 week and 3 months, and whether they were exclusively breastfeeding at 3- and 6-months post-birth.
Immunization status was recorded based on the national guidelines and reported on the child’s RtHC and coded as complete (1) or incomplete (0).
The child support grant (CSG) was recorded based on mother’s self-report as yes (1) or no (0).
Developmental Milestones. The gross motor developmental milestones of the WHO for children at 6 (WHO1), 9 (WHO3), 12 (WHO1–5) and 24 months (WHO1–6) were administered (Lansdown et al., 1996). Tasks were coded as follows: if the milestones were completed (1), if the child refused or was unable to complete the task (0).
Analysis
Demographic and structural characteristics were compared between mothers living with and without HIV using logistic regression models for binary variables and linear regression models for continuous or discrete variables (i.e., age, education, number of household members, and child growth scores). Maternal HIV status was defined as a mother reporting a positive status at any time point. Childcare characteristics assessed at multiple time points were analyzed using longitudinal models with random intercepts for the mother to control for the longitudinal nature of the assessments. Child growth was assessed using longitudinal linear models with a random intercept for child and adjusted for maternal age, parity, and time to acquire CSG. One HIV-positive child was omitted due to death and another mother and child were omitted due to a coding error. Missing data are assumed missing at random; we used all observations that were possible to use, up to the point where mothers or children died or were lost to follow-up. Mothers and children could return to the study even if they missed an assessment. The regression models were carried out using IBM SPSS Statistics (Version 20, Armonk, NY: IBM Corp).
Results
Almost all mothers were tested for HIV during pregnancy or the pregnant mother already knew that she was HIV positive (464/468; 99%). Of those who tested, 92% were tested at their first antenatal visit. More than one quarter of mothers (28%) were known to be HIV positive when their children were born (132/468), four reported seroconverting in the next 12 months, and one between 12 and 24 months. Of the mothers without HIV at baseline, 60% (201/336) were re-tested for HIV by 12-months post-birth and 70% (236/336) had tested at least once by 24 months after birth.
Demographic, structural, health, and risk comparisons between mothers living with and without HIV
Table 1 summarizes the differences on demographic and structural characteristics between mothers living with and without HIV. At birth, compared to mothers without HIV, mothers living with HIV were older (B = 4.03, Std. Error = 0.71, 95% C.I. = [2.62, 5.43], p <.001) and were less likely to be primipara (OR = 0.34. 95% C.I. = [0.21, 0.53], p <.001), an adolescent (OR = 0.08. 95% C.I. = [0.03, 0.27], p <.001), or in school (OR = 0.16. 95% C.I. = [0.06, 0.39], p <.001). There were no significant differences between mothers living with and without HIV on maternal education, employment, or structural resources. Mothers living with and without HIV had about an eighth-grade education, few were employed (4%), about half had monthly household incomes above R2000 ($217 USD) (48%). Food insecurity was reported by 30% of mothers living with and without HIV. Nearly 48% of households used unsafe river water as their main source of drinking water, while only 16% of mothers living with and without HIV had a water tank on-site (for rainwater harvesting from roofs – safer than river water); similarly only 16% had electricity in their homes. About one in three were married or lived with their partner (33%) and mothers were part of households containing, on average, 8 members (including the new baby).
Table 1.
Differences in demographic and structural characteristics between mothers without HIV and mothers living with HIV
| Mothers without HIV n=336 %(n)/ M(SD) | Mothers living with HIV n=132 %(n)/ M(SD) | All mothers n=468 %(n)/ M(SD) | |
|---|---|---|---|
| Demographic, birth | |||
| Mean age (SD)*** | 23.8 (7.2) | 27.8 (6.1) | 24.9 (7.2) |
| Adolescent (<18 years old)*** | 22% (73) | 2% (3) | 16% (76) |
| Mean highest education level (SD) | 8.6 (2.3) | 8.7 (2.6) | 8.6 (2.4) |
| Primipara*** | 46% (155) | 23% (30) | 40% (185) |
| Employed | 3% (11) | 5% (7) | 4% (18) |
| In school*** | 20% (68) | 4% (5) | 16% (73) |
| Structural, birth | |||
| Married or lives with father of child | 32% (109) | 36% (47) | 33% (156) |
| Number of people in household (SD)a | 8.0 (2.9) | 7.7 (3.0) | 7.9 (2.9) |
| Monthly household income >R2000 | 50% (158/318) | 44% (57/129) | 48% (215/447) |
| Water tank on site | 17% (57) | 14% (19) | 16% (76) |
| Electricity | 15% (49) | 18% (24) | 16% (73) |
| Food Insecurity | 31% (104) | 26% (34) | 30% (138) |
Note.
p<.05
p <.01
p <.001
Number of people in the household including the mother and the new baby.
Vertical HIV transmission prevention tasks for mothers living with HIV: All of the mothers living with HIV reported taking either antiretroviral therapy (ART) (57%) or AZT (43%) during pregnancy as per the “Option A” vertical HIV transmission prevention protocol. At two years post-birth, 73% of mothers living with HIV were on ART – the rest either did not qualify for treatment or had defaulted. The majority of mothers living with HIV received NVP for their child at birth (93%), however only about half of HIV-exposed children who had their RtHC available (55%;48/87) had had an HIV test and the results written in to the card by 6 months post-birth. By 12 months, 89% of mothers living with HIV who were interviewed said their children had had a PCR test but only half of them had the results written in their RtHC (40/81;49%). By 24 months, 61% (88/145) of mothers living with HIV had children with an HIV PCR result written in their RtHC, while no result could be found in 21% (31/145).
About half of mothers living with and without HIV attended the recommended four antenatal clinic visits (47%). mothers living with HIV were more likely to have ever had TB (OR = 6.16, 95% C.I. = [3.20, 11.86], p <.001). While at baseline, only (54/132) 40.9% mothers living with HIV had disclosed their HIV status to the father of the child, (66/132) or 50.25% of mothers living with HIV reported that they knew that the child’s father had tested for HIV.
The rate of mothers reporting depressed moods (EPDS > 13) decreased from birth at 6 months regardless of HIV status (B = −1.31, Std. Error = 0.39, 95% C.I. = [−2.08, −0.53], p = .001). Compared to assessments at birth, depressed mood was significantly lower at each assessment: 9 months (B = −1.19, Std. Error = 0.39, 95% C.I. = [−1.94, −0.43], p = .002), 12 months (B = −1.14, Std. Error = 0.40, 95% C.I. = [−1.94, −0.35], p = .005), and 24 months (B = −2.69, Std. Error = 0.43, 95% C.I. = [−3.52, −1.85], p < .001), regardless of HIV status.
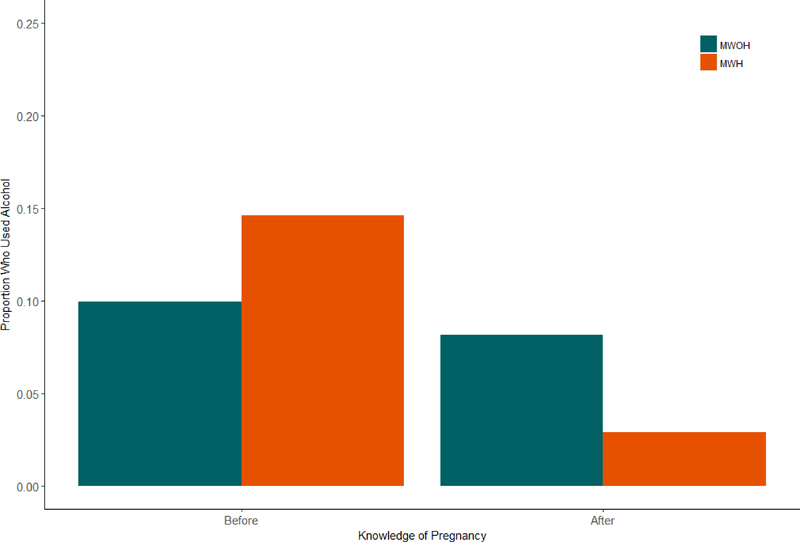
Figure 1 shows that mothers living with and without HIV had a similar rate of alcohol use before learning of their pregnancy (11%), but only mothers living with HIV significantly decreased their alcohol use after learning about their pregnancy when compared to mothers without HIV (8% vs. 3%) (B = 1.52, Std. Error = 0.16, 95% C.I. = [0.48, 2.56], p = .004). IPV is significantly higher during pregnancy (22%) when compared to prior to becoming pregnant (11%) and is similar across serostatus (B = −0.93, Std. Error = 0.16, 95% C.I. = [−1.24, −0.61], p < .00).
Figure 1.
Proportion of Mothers Who Used Alcohol Stratified by Mothers Living with and without HIV Before and After the Birth of their Child.
Child developmental comparisons between children of mothers living with and without HIV
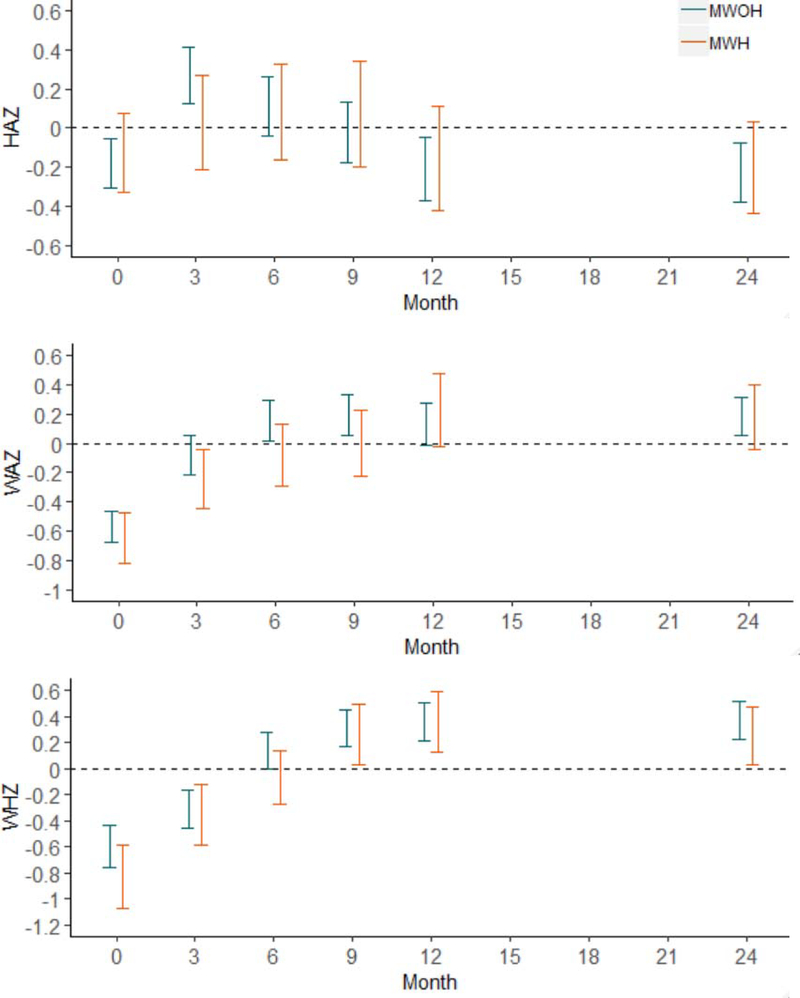
At birth, about 11% of children of mothers living with and without HIV were born with low birth weights; 7% had HAZ in the stunted range (HAZ < −2SD), 10% had WAZ in the malnourished range (WAZ < −2SD), and 17% had WHZ in the wasting range (WHZ < −2SD). Despite maternal HIV status, age, and parity, child growth (HAZ, WAZ, WHZ) was similar in the current cohort across the first 24 months post-birth (See Figure 2). However, delays in obtaining the CSG was associated with significantly lower HAZ (Estimate = −.01, Std. Error = .00, 95% C.I. = −.01, −.00, p = .042).
Figure 2.
95% Confidence Intervals (horizontal bars) for Child Growth (HAZ, WAZ, WHZ) stratified by mothers living with and without HIV over the first 24 months post-birth.
Children of mothers living with and without HIV had similar rates of clinic and hospital visits due to illness in the previous 3 months at 3 (42%; 12%) and 6 (38%; 9%) months post-birth. Children also had more episodes of diarrhea at 6 months when compared to 3 months (B = 0.74, Std. Error = 0.19, 95% C.I. = [0.38, 1.10], p < .001), despite maternal HIV status. Mothers reported taking their children an average of once in the past three months to the clinic for illness (3 months: M = 1.2, SD = 0.5, Range: 0–4; 6 months: M = 1.4, SD = 0.7, Range: 0–6). These patterns were similar across maternal serostatus.
Table 2 summarizes the differences in caretaking between mothers living with and without HIV. Mothers living with HIV were more likely to exclusively breastfeed their children for 3 months (OR = 1.95, 95% C.I. = [1.17, 3.25], p =.010) and 6 months (OR = 1.86, 95% C.I. = [1.01, 3.15], p = .021) than mothers without HIV. One in five mothers living with and without HIV exclusively breastfed for at least 6 months (20%). Only 73% of children had up-to-date immunizations at 12 months but reached 95% by 24 months and were similar across HIV exposure. Mothers living with HIV were more likely to obtain the child support grant by 3 months (OR = 1.70, 95% C.I. = [1.09, 2.66], p = .019) and by 6 months (OR = 1.76, 95% C.I. = [1.09, 2.83], p = .020) when compared to mothers without HIV. By 9 months post-birth, most mothers (75%) were receiving the child support grant and the rates were similar across serostatus.
Table 2.
Differences in childcare characteristics between children of mothers with and without HIV
| Mothers without HIV n=336 %(n)/ M(SD) | Mothers living with HIV n=132 %(n)/ M(SD) | All mothers n=468 %(n)/ M(SD) | |
|---|---|---|---|
| Exclusive breastfeeding 3mo* | |||
| 3 months* | 18% (48/219) | 29% (33/113) | 21% (81/388) |
| 6 months* | 17% (50/287) | 28% (29/103) | 20% (79/390) |
| Immunization up-to-date | |||
| 3 months | 49% (123/252) | 48% (51/106) | 49% (174/358) |
| 6 months | 73% (207/283) | 73% (85/116) | 73% (292/399) |
| 12 months | 74% (192/260) | 71% (75/106) | 73% (267/366) |
| Child support grant (CSG) secured | |||
| 3 months* | 34% (94/275) | 47% (53/113) | 38% (147/388) |
| 6 months* | 63% (187/299) | 75% (88/118) | 66% (275/417) |
| 9 months | 75% (217/291) | 78% (84/108) | 75% (301/399) |
| 12 months | 78% (229/295) | 78% (88/113) | 78% (317/408) |
| 24 months | 90% (256/285) | 91% (100/110) | 90% (356/395) |
Note.
p < .05
Developmental milestones were similar for children of mothers living with and without HIV at each assessment over 24 months. Despite maternal serostatus, the majority of all children at 6 (88%), 9 (94%), 12 (76%), and 24 (98%) months post-birth completed the WHO developmental milestones.
Discussion
This study examines how maternal HIV status as well as maternal resources, risk histories and caretaking influence child outcomes in deeply rural areas over the first two years of life. More than one quarter of mothers (28%) in this study are HIV positive at the birth of their children and the self-reported incidence rate of new HIV infections in this cohort of women is about 0.74% annually. It is encouraging that almost all mothers (99%) know their HIV status during pregnancy. In fact, nearly 92% of mothers without HIV are tested for HIV at their first antenatal visit. However, for just under one third of mothers without HIV (30%), HIV testing during pregnancy is the only HIV test received over the two years of the study. Pregnancy may increase the risk of acquiring HIV (Thompson et al, 2018; MUGO et al., 2011; Gray et al., 2005), and three mothers seroconverted during the first 6 months after birth. Although primary care clinics and mothers living with HIV may be doing well in some vertical HIV transmission prevention caretaking tasks, there are also significant gaps in care. Although we only have one confirmed child seroconversion in this study, only half of children have HIV results recorded in their health record as late as twelve months post-birth. Optimizing adherence to vertical HIV transmission prevention remains an important goal.
Contrary to most research in urban and peri-urban areas, mothers living with HIV are no more likely to experience depressed mood, to use alcohol, or to experience IPV than mothers without HIV (Haberland, n.d.). In this study, mothers living with HIV are 2.5 times more likely to stop drinking after learning out about their pregnancy. This finding is consistent with one other study that reports lower alcohol use among mothers living with HIV compared to mothers without HIV (Donald et al., 2017). Some researchers find that family and social connections are fostered by being geographically isolated. Rural areas may be protective to mothers of young children (Emmott, 2016; Tamsen Jean Rochat, Tomlinson, Bärnighausen, Newell, & Stein, 2011). A rural community’s perception of HIV may influence the health of mothers living with HIV more than individual stigma (Treves-Kagan et al., 2017). It is possible that mothers living with HIV have more exposure to friends and family members with HIV in a rural setting. HIV stigma may also be lower. We did not assess stigma. However, other studies in high-income countries report increased HIV stigma in rural areas (Kalichman, Katner, Banas, & Kalichman, 2017). The avoidance of alcohol is an important aspect of the counseling provided to all patients (men and women) who initiate ART in the Zithulele ARV program. This additional counseling and support may contribute to lower rates of alcohol use amongst mothers living with HIV.
Research suggests that exclusive breastfeeding decreases infant morbidity and mortality in LMIC and may improve child development outcomes as children grow (World Health Organization & UNICEF, 2016). In this study, mothers living with HIV are also more likely than mothers without HIV to exclusively breastfeed for three- and six-months post-birth. mothers living with HIV may be doing better than mothers without HIV in these domains, due to additional time spent counseling mothers living with HIV during antenatal care visits. Participation in vertical HIV transmission prevention has benefits outside of simply reducing HIV transition, such as reducing depression (Peltzer, Phaswana-Mafuya, & Treger, 2010). It is possible that the additional counseling encourages more mothers living with HIV to exclusively breastfeed; however, those messages are just as important for mothers without HIV.
Contrary to previous studies (Parsons, Young, Rochat, Kringelbach, & Stein, 2012) (Rahman, Malik, Sikander, Roberts, & Creed, 2008), children of mothers living with and without HIV had similar growth and developmental outcomes in the rural Eastern Cape of South Africa. Children of mothers living with HIV are typically found to experience reduced growth and slower achievement of developmental milestones (Omoni et al., 2017; Evans, Chasekwa, et al., 2016). However, it needs to be noted that on average, children of mothers living with and without HIV suffer notable deficits in our study. In our first assessment shortly after birth, 47% of children are either stunted, malnourished, wasted, or experience some combination of these growth deficits, regardless of the international standard. The mean HAZ, WAZ, and WHZ are also significantly lower at birth. In the current study, children gain weight faster than the standard growth curve, and by 24 months, children have mean WAZ and WHZ above the WHO standard. However, a better indicator of long-term under-nutrition is a child’s height and in this rural setting, it is lower than the WHO averages. Although weight and height improve significantly in the first few months of the infants’ life, they remain below average at 12- and 24-months post-birth, regardless of maternal serostatus. Children growing up in deeply rural areas of South Africa may face chronic under-nutrition due to limited access to nutritious foods. This may result in stunting and can have long-term consequences for cognitive development and school achievement (Black et al., 2013; Dewey & Begum, 2011). Yet, this issue is the same for children of mothers living with or without HIV.
It is somewhat surprising that, in a poor, rural area with inadequate water and sanitation and where almost a one-third of families experience food insecurity, children of mothers living with and without HIV appear to be equally vulnerable to the challenges to growing up. It may be that despite the challenges of rural living, mothers living with HIV in rural areas may be less vulnerable and more integrated into their communities. The mothers living with HIV in this study are older and more experienced mothers than mothers without HIV, and may also benefit from the ARV program and counseling provided by Zithulele Hospital and its surrounding clinics (Young & Gaunt, 2014). This appears to be reflected in their lower use of alcohol once mothers living with HIV realize they are pregnant and their higher rates of exclusive breastfeeding. The higher rates of exclusive breastfeeding by mothers living with HIV may also have an “equalizing effect” on their children, as seen in Patel et al. (Patel et al., 2010). Finally, many of the mothers living with HIV are on AZT instead of TDF/FTC/EFV, which has more recently been implicated with growth restriction in utero (Powis et al., 2016). This may be another factor why children of mothers living with HIV appear to be doing as well as children of mothers without HIV.
Conclusions
Rural mothers living with HIV do not appear to experience higher rates of alcohol use, depression, or IPV: comorbidities that potentially have negative impacts on the lives of both mothers and children. Mothers living with HIV are also more likely to abstain from alcohol during pregnancy and exclusively breastfeed their children for six months. In this study, children of mothers living with HIV in a deeply rural area are doing as well as children of mothers without HIV in the first two years of life. However, whether these developmental trajectories will persist remains unknown.
Limitations
This study is limited to gathering data largely through maternal self-report. No laboratory or diagnostic tests are available to confirm maternal HIV status or to test children’s immune status. However, clinic visits, immunizations, and HIV results for mothers and children were confirmed in the mother’s antenatal care record and the child’s RtHC, which serves as a medical record. Most families (>90%) have a RtHC, an important document allowing each child to apply for a child support grant. Although the interviewers are trained to ask questions in a non-stigmatizing manner, it is likely that some mothers withheld information on highly stigmatized behaviors, such as drinking alcohol. For those questions a rapid diagnostic test, such as a Peth test, or a more private means of answering, such as using a tablet or mobile phone, may be preferable.
Acknowledgments
Funding: This study was funded by the National Institute of Mental Health (NIMH; R01MH111391), the Center for HIV Identification, Prevention and Treatment Services (CHIPTS; P30MH058107), the National Institute on Alcohol Abuse and Alcoholism (NIAAA; R01AA017104 and R24AA022919), the Postdoctoral HIV Research Training Program for HIV Combination Prevention (T32MH109205), and the Elma Foundation. Mark Tomlinson is supported by the National Research Foundation, South Africa and is a Lead Investigator of the Centre of Excellence in Human Development, University Witwatersrand, South Africa.
Footnotes
Option A involved: for mothers with a CD4 of <350, full ART was offered for life. Mothers with a CD4 greater that 350 were given AZT twice daily until birth, with a dose of Truvada®, given at them time of going into labour. Infants were given NVP syrup until the cessation of breastfeeding
References
- Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, Onis M. de, … Uauy R (2013). Maternal and child undernutrition and overweight in low-income and middle-income countries. The Lancet, 382(9890), 427–451. 10.1016/S0140-6736(13)60937-X [DOI] [PubMed] [Google Scholar]
- Bruin GPD, Swartz L, Tomlinson M, Cooper PJ, & Molteno C (2004). The factor structure of the Edinburgh Postnatal Depression scale in a South African peri-urban settlement. South African Journal of Psychology, 34(1), 113–121. Retrieved from https://journals.co.za/content/sapsyc/34/1/EJC98258 [Google Scholar]
- Business Tech. (2016). The richest and poorest municipalities in South Africa. Retrieved February 10, 2019, from https://businesstech.co.za/news/wealth/127213/the-richest-and-poorest-municipalities-in-south-africa/
- Cox JL, Holden JM, & Sagovsky R (1987). Detection of Postnatal Depression: Development of the 10-item Edinburgh Postnatal Depression Scale. The British Journal of Psychiatry, 150(6), 782–786. 10.1192/bjp.150.6.782 [DOI] [PubMed] [Google Scholar]
- Davis EC, Rotheram-Borus MJ, Weichle TW, Rezai R, & Tomlinson M (2017). Patterns of Alcohol Abuse, Depression, and Intimate Partner Violence Among Township Mothers in South Africa Over 5 Years. AIDS and Behavior, 21(2), 174–182. 10.1007/s10461-017-1927-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Onis M, & Blössner M (2003). The World Health Organization Global Database on Child Growth and Malnutrition: methodology and applications. International Journal of Epidemiology, 32(4), 518–526. 10.1093/ije/dyg099 [DOI] [PubMed] [Google Scholar]
- Dewey KG, & Begum K (2011). Long-term consequences of stunting in early life. Maternal & Child Nutrition, 7(s3), 5–18. 10.1111/j.1740-8709.2011.00349.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald KAM, Fernandez A, Claborn K, Kuo C, Koen N, Zar H, & Stein DJ (2017). The developmental effects of HIV and alcohol: a comparison of gestational outcomes among babies from South African communities with high prevalence of HIV and alcohol use. AIDS Research and Therapy, 14(1), 28 10.1186/s12981-017-0153-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmott E (2016). Access to Alloparents In Weekes-Shackelford V, Shackelford TK, & Weekes-Shackelford VA (Eds.), Encyclopedia of Evolutionary Psychological Science (pp. 1–4). Cham: Springer International Publishing; 10.1007/978-3-319-16999-6_112-1 [DOI] [Google Scholar]
- Evans C, Chasekwa B, Ntozini R, Humphrey JH, & Prendergast AJ (2016). Head circumferences of children born to HIV-infected and HIV-uninfected mothers in Zimbabwe during the preantiretroviral therapy era. AIDS (London, England), 30(15), 2323–2328. 10.1097/QAD.0000000000001196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C, Humphrey JH, Ntozini R, & Prendergast AJ (2016). HIV-Exposed Uninfected Infants in Zimbabwe: Insights into Health Outcomes in the Pre-Antiretroviral Therapy Era. Frontiers in Immunology, 7 10.3389/fimmu.2016.00190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C, Jones CE, & Prendergast AJ (2016). HIV-exposed, uninfected infants: new global challenges in the era of paediatric HIV elimination. The Lancet Infectious Diseases, 16(6), e92–e107. 10.1016/S1473-3099(16)00055-4 [DOI] [PubMed] [Google Scholar]
- Filteau S (2009). The HIV-exposed, uninfected African child. Tropical Medicine & International Health, 14(3), 276–287. 10.1111/j.1365-3156.2009.02220.x [DOI] [PubMed] [Google Scholar]
- Gardner EM, McLees MP, Steiner JF, del Rio C, & Burman WJ (2011). The Spectrum of Engagement in HIV Care and its Relevance to Test-and-Treat Strategies for Prevention of HIV Infection. Clinical Infectious Diseases, 52(6), 793–800. 10.1093/cid/ciq243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetghebuer T, Rowland-Jones SL, & Kollmann TR (2017). Editorial: Immune Mechanisms Underlying the Increased Morbidity and Mortality of HIV-Exposed Uninfected (HEU) Children. Frontiers in Immunology, 8 10.3389/fimmu.2017.01060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray RH, Li X, Kigozi G, Serwadda D, Brahmbhatt H, Wabwire-Mangen F, … Wawer MJ (2005). Increased risk of incident HIV during pregnancy in Rakai, Uganda: a prospective study, 366, 7. [DOI] [PubMed] [Google Scholar]
- Haberland N (n.d.). Addressing intimate partner violence and power in relationships in HIV testing services: Results of an intervention piloted in Nairobi, Kenya, 50. [DOI] [PMC free article] [PubMed]
- Jewkes RK, Dunkle K, Nduna M, & Shai N (2010). Intimate partner violence, relationship power inequity, and incidence of HIV infection in young women in South Africa: a cohort study. The Lancet, 376(9734), 41–48. 10.1016/S0140-6736(10)60548-X [DOI] [PubMed] [Google Scholar]
- Johnson LF, Chiu C, Myer L, Davies M-A, Dorrington RE, Bekker L-G, … Meyer-Rath G (2016). Prospects for HIV control in South Africa: a model-based analysis. Global Health Action, 9 10.3402/gha.v9.30314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalichman S, Katner H, Banas E, & Kalichman M (2017). Population Density and AIDS-Related Stigma in Large-Urban, Small-Urban and Rural Communities of the Southeastern United States. Prevention Science : The Official Journal of the Society for Prevention Research, 18(5), 517–525. 10.1007/s11121-017-0761-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansdown RG, Goldstein H, Shah PM, Orley JH, Di G, Kaul KK, … Reddy V (1996). Culturally appropriate measures for monitoring child development at family and community level: a WHO collaborative study. Bulletin of the World Health Organization, 74(3), 283–290. Retrieved from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2486921/ [PMC free article] [PubMed] [Google Scholar]
- LE ROUX IM, TOMLINSON M, HARWOOD JM, O’CONNOR MJ, WORTHMAN CM, MBEWU N, … ROTHERAM-BORUS MJ (2013). Outcomes of Home Visits for Pregnant Mothers and their Infants: a Cluster Randomised Controlled Trial. AIDS (London, England), 27(9), 1461–1471. 10.1097/QAD.0b013e3283601b53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayosi BM, Lawn JE, Niekerk A van, Bradshaw D, Karim SSA, & Coovadia HM (2012). Health in South Africa: changes and challenges since 2009. The Lancet, 380(9858), 2029–2043. 10.1016/S0140-6736(12)61814-5 [DOI] [PubMed] [Google Scholar]
- MUGO NR, HEFFRON R, DONNELL D, WALD A, WERE EO, REES H, … BAETEN JM (2011). Increased Risk of HIV-1 Transmission in Pregnancy: A Prospective Study among African HIV-1 Serodiscordant Couples. AIDS (London, England), 25(15), 1887–1895. 10.1097/QAD.0b013e32834a9338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Committee on Confidential Enquiries into Maternal Deaths. (2018). Saving Mothers 2014–2016: Seventh triennial report on confidential enquiries into maternal deaths in South Africa: Short report. Pretoria, South Africa: National Department of Health; Retrieved from https://www.sasog.co.za/Content/Docs/Saving_Mothers.pdf [Google Scholar]
- National Department of Health. (2017). The 2015 National Antenatal Sentinel HIV & Syphilis Survey, South Africa, National Department of Health; Pretoria, South Africa: Retrieved from https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=13&ved=2ahUKEwievcHa6KngAhWUqHEKHQZfAXkQFjAMegQIABAC&url=http%3A%2F%2Fwww.health.gov.za%2Findex.php%2Fshortcodes%2F2015-03-29-10-42-47%2F2015-04-30-08-18-10%2F2015-04-30-08-21-56%3Fdownload%3D2584%3A2015-national-antenatal-hiv-prevalence-survey-final-23oct17&usg=AOvVaw2vK04-A9rKxY7S28PZk7GQ [Google Scholar]
- Omoni AO, Ntozini R, Evans C, Prendergast AJ, Moulton LH, Christian PS, & Humphrey JH (2017). Child Growth According to Maternal and Child HIV Status in Zimbabwe. The Pediatric Infectious Disease Journal, 36(9), 869–876. 10.1097/INF.0000000000001574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons CE, Young KS, Rochat TJ, Kringelbach ML, & Stein A (2012). Postnatal depression and its effects on child development: a review of evidence from low- and middle-income countries. British Medical Bulletin, 101(1), 57–79. 10.1093/bmb/ldr047 [DOI] [PubMed] [Google Scholar]
- Patel D, Bland R, Coovadia H, Rollins N, Coutsoudis A, & Newell M-L (2010). Breastfeeding, HIV status and weights in South African children: a comparison of HIV-exposed and unexposed children. AIDS, 24(3), 437 10.1097/QAD.0b013e3283345f91 [DOI] [PubMed] [Google Scholar]
- Peltzer K, Phaswana-Mafuya N, & Treger L (2010). Use of traditional and complementary health practices in prenatal, delivery and postnatal care in the context of hiv transmission from mother to child (PMTCT) in the Eastern Cape, South Africa. African Journal of Traditional, Complementary and Alternative Medicines, 6(2). 10.4314/ajtcam.v6i2.57087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powis KM, Smeaton L, Hughes MD, Tumbare EA, Souda S, Jao J, … Shapiro R (2016). In-utero triple antiretroviral exposure associated with decreased growth among HIV-exposed uninfected infants in Botswana. AIDS (London, England), 30(2), 211–220. 10.1097/QAD.0000000000000895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Malik A, Sikander S, Roberts C, & Creed F (2008). Cognitive behaviour therapy-based intervention by community health workers for mothers with depression and their infants in rural Pakistan: a cluster-randomised controlled trial. The Lancet, 372(9642), 902–909. 10.1016/S0140-6736(08)61400-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochat Tamsen J., Richter LM, Doll HA, Buthelezi NP, Tomkins A, & Stein A (2006). Depression Among Pregnant Rural South African Women Undergoing HIV Testing. JAMA, 295(12), 1373–1378. 10.1001/jama.295.12.1376 [DOI] [PubMed] [Google Scholar]
- Rochat Tamsen Jean, Tomlinson M, Bärnighausen T, Newell M-L, & Stein A (2011). The prevalence and clinical presentation of antenatal depression in rural South Africa. Journal of Affective Disorders, 135(1–3), 362–373. 10.1016/j.jad.2011.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux S. M. le, Abrams EJ, Nguyen K, & Myer L (2016). Clinical outcomes of HIV-exposed, HIV-uninfected children in sub-Saharan Africa. Tropical Medicine & International Health, 21(7), 829–845. 10.1111/tmi.12716 [DOI] [PubMed] [Google Scholar]
- SANAC. (2017). The National Strategic Plan. Retrieved from http://sanac.org.za/the-national-strategic-plan/
- Slogrove AL, Goetghebuer T, Cotton MF, Singer J, & Bettinger JA (2016). Pattern of Infectious Morbidity in HIV-Exposed Uninfected Infants and Children. Frontiers in Immunology, 7 10.3389/fimmu.2016.00164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson KA, Hughes J, Baeten JM, John-Stewart G, Celum C, Cohen CR, … & Heffron R (2018). Increased risk of female HIV-1 acquisition throughout pregnancy and postpartum: a prospective per-coital act analysis among women with HIV-1 infected partners. The Journal of Infectious Diseases, 218(1), 16–25. doi: 10.1093/infdis/jiy113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomlinson M, O’Connor MJ, le Roux IM, Stewart J, Mbewu N, Harwood J, & Rotheram-Borus MJ (2014). Multiple Risk Factors During Pregnancy in South Africa: The Need for a Horizontal Approach to Perinatal Care. Prevention Science, 15(3), 277–282. 10.1007/s11121-013-0376-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treves-Kagan S, El Ayadi AM, Pettifor A, MacPhail C, Twine R, Maman S, … Lippman SA (2017). Gender, HIV testing and stigma: The association of HIV testing behaviors and community-level and individual-level stigma in rural South Africa differ for men and women. AIDS and Behavior, 21(9), 2579–2588. 10.1007/s10461-016-1671-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai AC, Tomlinson M, Comulada WS, & Rotheram-Borus MJ (2016). Food Insufficiency, Depression, and the Modifying Role of Social Support: Evidence from a Population-Based, Prospective Cohort of Pregnant Women in Peri-Urban South Africa. Social Science & Medicine (1982), 151, 69–77. 10.1016/j.socscimed.2015.12.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNAIDS. (2017). UNAIDS DATA 2017. Retrieved from http://www.unaids.org/en/resources/documents/2017/2017_data_book
- UNAIDS. Country Reports: South Africa. (2016). South Africa. Retrieved February 6, 2019, from http://www.unaids.org/en/regionscountries/countries/southafrica
- von Mollendorf C, von Gottberg A, Tempia S, Meiring S, de Gouveia L, Quan V, … Cohen C (2015). Increased Risk for and Mortality From Invasive Pneumococcal Disease in HIV-Exposed but Uninfected Infants Aged <1 Year in South Africa, 2009–2013. Clinical Infectious Diseases, 60(9), 1346–1356. 10.1093/cid/civ059 [DOI] [PubMed] [Google Scholar]
- World Health Organization, & UNICEF. (2016). Guideline. the duration of breastfeeding, and support from health services to improve feeding practices among mothers living with HIV. Retrieved from http://www.ncbi.nlm.nih.gov/books/NBK379872/ [PubMed]
- Young C, & Gaunt B (2014). Providing high-quality HIV care in a deeply rural setting – the Zithulele experience. Southern African Journal of HIV Medicine, 15(1), 28–29. 10.4102/sajhivmed.v15i1.40 [DOI] [Google Scholar]