Abstract
Androgen deprivation therapy (ADT) is a central part of prostate cancer (PCa) treatment. Pharmacologic androgen deprivation includes gonadotropin-releasing hormone (GnRH) agonism and antagonism, androgen receptor inhibition, and CYP17 inhibition. Studies in the past decade have raised concerns about the potential for ADT to increase the risk of adverse cardiovascular events such as myocardial infarction, stroke, and cardiovascular mortality, possibly by exacerbating cardiovascular risk factors. In this review, we summarize existing data on the cardiovascular effects of ADT. Among the therapies, abiraterone stands out for increasing risk of cardiac events in meta-analyses of both RCTs and observational studies. We find a divergence between observational studies, which show consistent positive associations between ADT use and cardiovascular disease, and randomized controlled trials (RCTs), which do not show these associations reproducibly.
Keywords: cardio-oncology, androgen deprivation therapy, prostate cancer, cardiotoxicity, gonadotropin releasing hormone agonists
Graphic Abstract
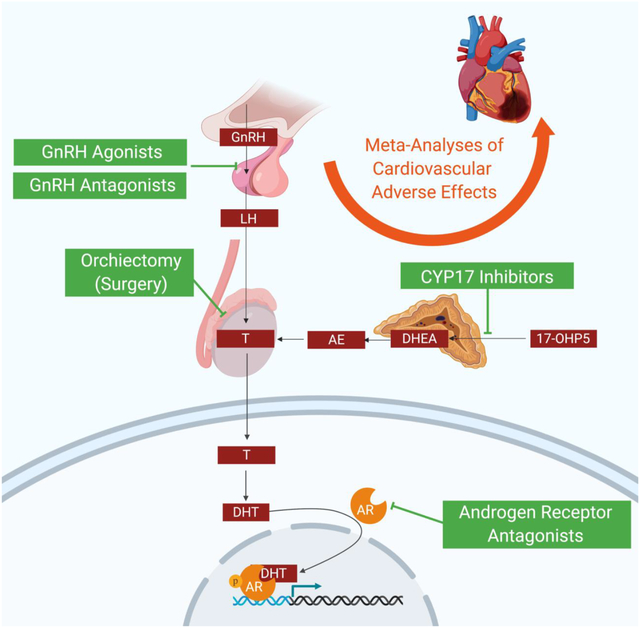
Introduction
Prostate cancer (PCa) is the second most common cancer in men, with an estimated incidence of 1,276,000 cases and 359,000 deaths globally in 20181. In the United States, 174,650 new cases and 31,620 deaths are projected to occur in 20192. The cornerstone of systemic treatment for PCa is pharmacologic or surgical androgen deprivation therapy (ADT). Pharmacologic ADT traditionally refers to treatment with a gonadotropin-releasing hormone (GnRH) agonist (e.g. leuprolide) or GnRH antagonist (e.g. degarelix). Suppression of androgen signaling can also be accomplished with androgen receptor (AR) inhibitors (e.g. enzalutamide) or CYP17 inhibitors (e.g. abiraterone). The inhibition of testosterone secretion by ADTs and AR-directed therapies results in a state of low plasma testosterone, a condition referred to as androgen deprivation. As advances in therapy improved the survival of PCa patients, growing reports have suggested a contribution of ADT to cardiovascular (CV) adverse sequelae. These reports led the American Heart Association, American Cancer Society, and American Urological Association to jointly issue a science advisory on the increased CV risks of ADT3. This review will summarize existing meta-analyses of the cardiovascular adverse effects of traditional ADTs as well as meta-analyses of AR-directed therapy. While the state of low testosterone may arise from a multitude of etiologies, in this review we will focus on androgen deprivation resulting from the drugs used in the treatment of prostate cancer for prostate cancer patients.
Physiology
PCa is an androgen-sensitive cancer that relies on signaling from the HPG axis. The HPG axis begins at the hypothalamus, which releases luteinizing hormone releasing hormone (LHRH) in a pulsatile manner (Figure 1). Binding of LHRH to the LHRH receptors on the anterior pituitary causes release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH). LH stimulates LH receptors on Leydig cells in the testes to produce testosterone.
Figure 1:
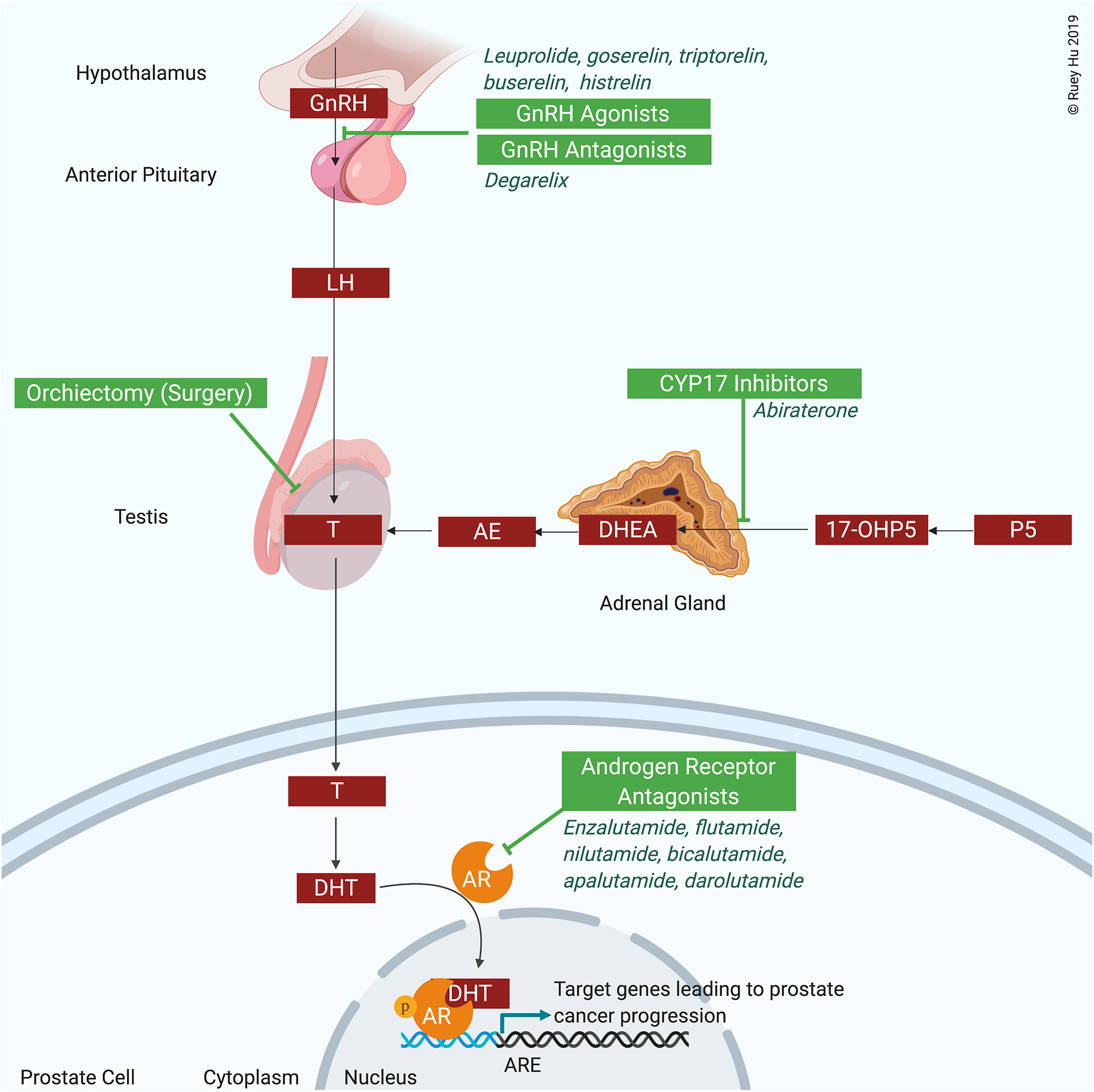
The hypothalamic-pituitary-gonadal axis and targets for androgen deprivation therapy in prostate cancer
Abbreviations: 17-OHP5: 17α-hydroxypregnenolone; AE: androstenedione; AR: androgen receptor; DHEA: dihydroepiandrosterone; DHT: dihydrotestosterone; GnRH: gonadotropin releasing hormone; LH: luteinizing hormone; P5: pregnenolone; T: testosterone
In the testes, and to a lesser degree in the adrenal glands, testosterone synthesis from cholesterol relies on a cascade of CYP17-dependent reactions involving the conversion pregnenolone to dehydroepiandrosterone (DHEA) and progesterone into androstenedione, both of which are converted to testosterone and subsequently dihydrotestosterone (Figure 1). Dihydrotestosterone binding to the androgen receptor (AR) in the ligand binding pocket causes translocation of AR from the cytoplasm to the nucleus, where it binds to DNA and promotes transcription of cancer growth-promoting genes. When testosterone levels are depleted during PCa treatment, PCa cells can continue to respond to androgens synthesized in the adrenal gland. This pathway was the rationale for developing CYP17 inhibitors, which block synthesis of androgens in adrenal gland.
Types of Androgen Deprivation Therapy
GnRH agonists
The most common type of ADT are the GnRH agonists. GnRH agonists bind to GnRH receptors on gonadotropin-producing cells in the anterior pituitary4. The resulting continuous (non-pulsatile) release of GnRH causes a transient surge in LH and FSH and increase in testosterone production from Leydig cells. Subsequently, the negative feedback downregulates GnRH receptors on gonadotropin-producing cells, decreased pituitary production of LH and FSH, and testosterone is reduced to castration levels. Leuprolide, goserelin, triptorelin, buserelin, and histrelin are examples of GnRH agonists. Their pharmacology has been described previously5 (Supplemental Table 1). All are available as intramuscular or subcutaneous formulations and are typically administered once every 1 to 6 months.
GnRH antagonists
GnRH antagonists bind to GnRH receptors on gonadotropin-producing cells in the anterior pituitary to inhibit release of LH or FSH without an initial increase in testosterone release6. Degarelix is an example of a GnRH antagonist. Degarelix is administered as a monthly subcutaneous injection. Degarelix causes a rapid fall in testosterone levels within 2–3 days, which is significantly more rapid than GnRH agonists.7,8 Degarelix causes greater and more rapid PSA reduction than GnRH agonists, and has a lower rate of PSA failure9. Degarelix also causes greater and more rapid LH and FSH reduction than GnRH agonists9. Degarelix is more likely to cause injection site reactions than GnRH agonists, while flushing is equally common in both groups10. Interestingly, degarelix causes improved health-related quality of life compared to GnRH agonists11. GnRH antagonists are less susceptible to the resistance that GnRH agonists may experience due to decreased sensitivity of the GnRH receptor from continuous exposure to GnRH agonists8.
Androgen receptor antagonists
Androgen receptor antagonists, also known as antiandrogens, competitively inhibit dihydrotestosterone binding to the androgen receptor (AR) at the androgen binding site12. These agents inhibit nuclear translocation of the AR and interaction of the AR with the promoter at the AR response element. The inhibition of AR-dependent transcription impairs cell proliferation and triggers apoptosis. Nonsteroidal androgen receptor antagonists, discussed below, spare the patient from anti-mineralocorticoid, anti-gonadotropic, and progestogenic effects. Flutamide, nilutamide, and bicalutamide are first-generation androgen receptor antagonists. Enzalutamide, apalutamide, and darolutamide are next-generation (more potent) androgen receptor antagonists5. They are administered orally. A new compound that is a hybridization of abiratarone and enzalutamide has shown promising results for treating enzalutamide-resistant PCa13. AR antagonists are commonly used with GnRH agonists to alleviate the effects of the testosterone surge that occurs with a GnRH agonist. Extended AR antagonists may be used with GnRH agonists or antagonists to achieve combined androgen blockade (CAB).
CYP17 inhibitors
CYP17, an enzyme found in the testes, adrenal glands, and prostate tumor tissue, possesses both 17α-hydroxylase and C17,20-lyase activity, which generate testosterone from testosterone precursors14. CYP17 inhibitors block these reactions. Additionally, CYP17 inhibition reduces cortisol synthesis and may induce increased ACTH release promoting synthesis of mineralocorticoid precursors, leading to hypertension, edema, and hypokalemia. Corticosteroids are thus co-administered to prevent unwanted ACTH release. Ketoconazole and abiraterone are CYP17 inhibitors. Although androgen receptor antagonists and CYP17 inhibitors are androgen axis directed therapies rather than strictly androgen deprivation therapies, they have been grouped under the umbrella of ADTs in many studies, and will be treated as such in the data synthesis below.
Cardiovascular Adverse Effects of ADT
Keating and colleagues first identified an increased risk of incident diabetes, coronary heart disease, myocardial infarction (MI), and sudden cardiac death in association with GnRH agonists in a Surveillance, Epidemiology, and End Results (SEER)-Medicare database15. This finding has spurred numerous observational studies and retrospective studies from randomized controlled trials (RCTs).
Cardiovascular Adverse Effects of ADTs as a Pooled Group
Among the three available meta-analyses of observational trials, ADTs had positive associations (although not always significant) with CV events, CV death, and MI (Table 1)16–18. The comparator (non-ADT) group in these studies could include radical prostatectomy, radiotherapy, or watchful waiting. When the comparator group was watchful waiting, ADT was significantly associated with any non-fatal CV disease and stroke17,18. The strengthened effect size when the comparator group was restricted to watchful waiting suggests that there is CV risk associated with some non-ADT therapies, which may be minimizing the true CV effect difference between ADT and non-ADT. Among the three available meta-analyses of RCTs, there were no significant associations with CV outcomes except for a positive association with non-fatal CV disease compared in one analysis15. Therefore, in patients with low cardiovascular risk enrolled in RCTs, there is a suggestion but no conclusive increase in risk of cardiovascular adverse effects from ADT.
Table 1:
Cardiovascular mortality and cardiovascular disease associated with androgen deprivation therapy (ADT) as a pooled group compared to non-ADT, according to results of meta-analyses from 2010 to 2019.
| Type | Treatment Agent (no. of patients) | Comparator Agent (no. of patients) | CV mortality | Any non-fatal CVD | Myocardial Infarction | Stroke | |
|---|---|---|---|---|---|---|---|
| Nguyen 2011 | RCT | ADT (n=2200) | Non-immediate ADT (n=1941) | RR 0.93 (CI 0.79–1.10, p=0.41, I2=0%, N=8) | |||
| Bourke 2013 | RCT | ADT (n=1065) | Non-immediate ADT (n=814) | RR 1.06 (CI 0.80–1.40, p=0.69, I2=0%, N=4) | |||
| Zhao 2014 | Obs. | ADT (n=129,802)^ | Non-ADT (n=165,605)^ | HR 1.17 (CI 1.04–1.32, p = 0.01, I2=57%, N=6) | HR 1.10 (CI 1.00–1.21, p = 0.06, I2=72%, N=6) | HR 1.10 (CI 0.97–1.26, p=0.14, I2=68%, N=6) | |
| Zhao 2014 | Obs. | ADT (n=39,465)^ | Watchful waiting (n=43,648)^ | HR 1.30 (CI 1.13–1.50, p=0.0003, I2=0%, N=4) | HR 1.19 (CI 1.08–1.30, p=0.0004, I2=0%, N=3) | ||
| Carneiro 2015 | Obs. | ADT (n= 52,308) | Non-ADT (n=74,590) | OR 1.92 (CI 0.79–4.68, p 0.15, I2=97%, N=3) | OR 1.06 (CI 0.70–1.61, p<0.78, I2=100%, N=2) | OR 2.05 (CI 1.93–2.17, p<0.00001, I2=100%, N=2) | OR 1.07 (CI 0.66–1.72, p=0.79, I2=99%, N=2) |
| Carneiro 2015 | RCT | ADT (n=8,388) | Non-ADT (n=8,411) | OR 0.97 (CI 0.81–1.18, p 0.79, I2 0%, N=6) | OR 1.55 (CI 1.09–2.20, p=0.01, I2 0%, N=3) | OR 1.23 (CI 0.92–1.64, p=0.16, I2: 0%, N=2) |
OR 1.02 (CI 0.71–1.46, p=0.93, I2=0%, N=2) |
| Meng 2016 | Obs. | ADT (n=74,538) | Non-ADT (n= 85,947) | HR 1.12 (CI 0.95–1.32, p=0.16, I2=85%, N=6) | |||
| Meng 2016 | Obs. | ADT (n=39,029) | Watchful waiting (n=42,073) | HR 1.16 (CI 1.03–1.31, p = 0.01, I2=0%, N=2) |
Abbreviations: RR: relative risk; OR: odds ratio; HR: hazard ratio; CI: confidence interval; ADT: androgen deprivation therapy; CV: cardiovascular: CVD: cardiovascular disease: MI: myocardial infarction; RCT: meta-analysis of randomized controlled trials; Obs.: meta-analysis of observational studies
Small n: total number of patients examined in the meta-analysis
Large N: number of studies or trials available for that outcome in the meta-analysis
The exact participant count in Zhao (2014) varies by outcome.
Cardiovascular Adverse Effects of GnRH Agonists
Among the ADTs, the strongest CV adverse event signal comes from observational studies of the GnRH agonists. In the three meta-analyses of GnRH agonists compared to non-ADT, positive associations were found between GnRH agonists and CV death, non-fatal CV disease, MI, and stroke (Table 2)16,17,20. There are currently no meta-analyses of RCTs of GnRH agonists and CV adverse events.
Table 2:
Cardiovascular mortality and cardiovascular disease associated with GnRH agonists compared to non-ADT, according to results of meta-analyses from 2010 to 2019.
| Type | Treatment Agent (no. of patients) | Comparator Agent (no. of patients) | CV death | Any non-fatal CVD | Myocardial Infarction | Stroke | |
|---|---|---|---|---|---|---|---|
| Zhao 2014 | Obs. | GnRH agonist (n=89865)^ | Non-ADT (n=126219)^ | HR 1.36 (CI 1.10, 1.68, p=0.004, I2=91%, N=4) | HR 1.19 (CI 1.04, 1.36, p = 0.01, I2=86%, N = 3) | HR 1.20 (CI 1.05–1.38, p = 0.008, I2=82%, N = 4) | |
| Bosco 2015 | Obs. | GnRH agonist | Non-ADT | RR 1.38 (CI 1.29–1.48, p <0.001, I2=85%, N=16) | RR 1.57 (CI 1.26–1.94, p <0.001, I2=92%, N=6) | RR 1.51 (CI 1.24–1.84, p<0.001, I2=90%, N=5) | |
| Meng 2016 | Obs. | GnRH agonist (n = 49292) | Non-ADT (n= 47309) | HR 1.20 (CI 1.12–1.28, P<0.001, I2 = 0%, N=3) |
Abbreviations: RR: relative risk; OR: odds ratio; HR: hazard ratio; CI: confidence interval; ADT: androgen deprivation therapy; CV: cardiovascular: CVD: cardiovascular disease: MI: myocardial infarction; RCT: meta-analysis of randomized controlled trials; Obs.: meta-analysis of observational studies
The exact participant count in Zhao (2014) varies by outcome.
Small n: total number of patients examined in the meta-analysis
Large N: number of studies or trials available for that outcome in the meta-analysis
Cardiovascular Adverse Effects of Androgen Receptor Antagonists
The CV adverse effect signal from androgen receptor antagonists was mixed. In the three meta-analyses of observational studies of androgen receptor antagonists compared to non-ADT, there were mixed associations between ADT and non-fatal CV disease and MI17,20, and no associations between ADT and CV death and stroke16,17 (Supplemental Table 2). There are currently no meta-analyses of RCTs of androgen receptor antagonists and CV adverse events.
Cardiovascular Adverse Effects of Combined Androgen Blockade
Combined androgen blockade, which refers to the use of a GnRH agonist together with an androgen receptor antagonist, showed increased risk for CV adverse effects. In two meta-analyses of observational studies which examined combined androgen blockade compared to non-ADT, there was a positive association with CV death, non-fatal CV disease, and stroke16,17 (Supplemental Table 3). The association with MI was not statistically significant17.
Cardiovascular Adverse Effects of Orchiectomy
Individuals undergoing orchiectomy may have increased risk of CV events. In three meta-analyses of observational data examining orchiectomy compared to non-ADT, there was a positive association between orchiectomy and non-fatal CV disease16,17,20 (Supplemental Table 4). Individual associations between orchiectomy and CV death, MI, and stroke were positive but did not achieve statistical significance. There are currently no meta-analyses of RCTs of orchiectomy CV adverse events.
Cardiovascular Adverse Effect Differences Between ADT Types
The mechanism of specific ADTs may differently affect CV event risk. In one meta-analysis, GnRH antagonists were associated with lower CV events than GnRH agonists (HR 0.44, CI 0.26–0.74, p=0.002, I2=42%, N=3)21. In a broader meta-analysis comparing all types of ADT with each other (Supplemental Table 5)22, orchiectomy had the most unfavorable CV risk profile. Orchiectomy had a near-doubling of MI risk compared to combined androgen blockade (CAB), which appears to have the least harmful CV risk profile. Differences were modest among the other ADT types. GnRH antagonists were associated with a 58% decreased risk of MI compared to GnRH agonists21,22. Between continuous ADT and intermittent ADT, there was no difference in the development of CV events or thromboembolic events, but there was a marginally significant increase in CV death from continuous ADT23.
Cardiovascular Adverse Effects of Abiraterone and Enzalutamide
Two agents, enzalutamide (an androgen receptor antagonist) and abiraterone (a CYP17 inhibitor), have drawn specific attention for their association with CV risk. In a meta-analysis of observational studies and a meta-analysis of RCTs, enzalutamide did not increase risk of cardiac events, but increased the risk of hypertension (Supplemental Table 6)24,25. Abiraterone was associated with increased risk of cardiac events and the risk of hypertension in both meta-analyses (Table 3). The strength of abiraterone’s association with any cardiac events and hypertension suggests that further scrutiny should be given to the CYP17 inhibitors in future clinical trials. Furthermore, pharmacovigilance studies show that abiraterone have increased risk of atrial tachyarrhythmias and heart failure compared to other ADTs26, an area that should be studied in future meta-analyses.
Table 3:
Cardiovascular events associated with abiraterone (a CYP17 inhibitor) compared to non-ADT, according to results of meta-analyses from 2010 to 2019.
| Type | Treatment Agent (no. of patients) | Comparator Agent (no. of patients) | Any cardiac events | CTCAE Grade ≥3 cardiac events | Any hypertension | CTCAE Grade ≥3 hypertension | |
|---|---|---|---|---|---|---|---|
| Moreira 2017 | RCT | Abiraterone and prednisone (n=1,343) | Prednisone (n=940) | RR 1.28 (CI 1.06–1.55, P = 0.01, I2=0%, N=2) | RR 1.76 (CI1.12–2.75, P=0.01, I2=0%, N=2) | ||
| Iacovelli 2018 | RCT | Abiraterone and prednisone (n=2,878) | Prednisone (n=2,496) | RR 1.41 (CI 1.21–1.64, P<0.001, I2=0%, N=4) | RR 2.22 (CI 1.60–3.27, P<0.001, I2=0%, N=4) | RR 1.79 (CI 1.45–2.21, P<0.001, I2=68%, N=4) | RR 2.19 (CI 1.73–2.78, P<0.001, I2=34%, N=4) |
Abbreviations: RR: relative risk; CI: confidence interval; ADT: androgen deprivation therapy; CTCAE: Common terminology criteria for adverse events; RCT: meta-analysis of randomized controlled trials; Obs.: meta-analysis of observational studies
Small n: total patients examined in the meta-analysis
Large N: number of studies or trials available for that outcome in the meta-analysis
The Impact of Study Population on Cardiovascular Risk
Several factors related to trial design could partially explain these divergent results between meta-analyses of RCTs and meta-analyses of observational studies. RCTs may underestimate CV risk because the primary endpoints in RCTs are measures of PCa disease control, not CV events. Firstly, CV events in RCTs for PCa therapies are not defined or adjudicated in a standardized way as done in large prospective CV outcomes trials. Secondly, these RCTs are not sufficiently powered to look for unexpected CV events. Thirdly, the duration of follow-up is rarely as long as in observational studies. Fourthly, patients in the control arm in some trials did end up receiving ADT as well, just in a deferred time frame, thus attenuating any positive effect of ADT on CV risk. Fifthly, PCa RCTs suffer from selection bias as they exclude patients with high CV risk from enrolment.
On the other hand have greater susceptibility to confounding, less control over adherence to treatment, and may have outcome reporting bias, potentially leading to an overestimation of CV risk. Population-based databases, such as SEER, do not exclude elderly patients or those with concurrent CV disease or CV risk factors, thus more closely resembling the population of patients who get PCa27. The critical role that baseline CV risk factors and comorbidities play in overall survival was demonstrated in a study on a long-term follow-up of a prostate cancer RCT28.
Finally, the heterogeneity varies widely between the studies, ranging from 0–100%, so results should be interpreted with caution. Studies varied greatly in follow-up time and regions included (Supplemental Table 7). On balance, the data support a cardiovascular risk association that needs to be further characterized. Pragmatic trials may overcome these limitations and offer the methodological innovation needed to address this research question.
Mechanisms
The increased risk of adverse CV outcomes from ADT is thought to be related to its role in promoting atherosclerosis, dyslipidemia, adiposity, and insulin resistance.
ADT and atherosclerosis
Multiple murine models of androgen deprivation have supported the hypothesis that androgen deprivation worsens atherosclerosis lesion formation. Firstly, orchiectomized LDL-receptor knockout (Ldlr−/−) mice consuming a high fat diet developed larger atherosclerotic lesions as compared to sham-treated mice29. Testosterone supplementation in the orchiectomy model significantly reduced atherosclerotic lesion size compared to placebo, but this reduction did not occur if testosterone was administered in the presence of an aromatase inhibitor, which blocks conversion of testosterone to 17β-estradiol. This suggests that testosterone may inhibit atherosclerosis indirectly through its conversion to 17β-estradiol. Indeed, 17β-estradiol supplementation reduced atherosclerotic lesion size to the same degree as testosterone treatment29.
Secondly, androgen receptor knockout (ARKO) in the context of an apolipoprotein E deficiency model led to larger atherosclerotic lesions in the aortic root compared to animals with an intact androgen receptor30. As in the Ldlr−/− model, testosterone supplementation reduced lesion size in both ARKO and wild-type mice, although the effect was blunted in ARKO mice. This suggests disruption of testosterone signaling is atherogenic via both AR-dependent and AR-independent mechanisms.
Thirdly, in vitro, testosterone dose-dependently augmented cholesterol efflux from human monocyte-derived macrophages via upregulation of scavenger receptor B1, thereby providing a possible mechanism for how testosterone can reduce the cholesterol content of atherosclerotic lesions31. Collectively, these preclinical models support the hypothesis that androgen deprivation drives atherosclerosis.
ADT and adiposity
ADT increases visceral and subcutaneous fat32 while decreasing lean body mass33. This may occur via loss of androgen-mediated inhibition of stem cell differentiation into adipocytes34, as well as loss of androgen-mediated stimulation of lipolysis and androgen-mediated reduction of lipid accumulation35. Of note, 90% of the gain in adiposity is subcutaneous rather than visceral36.
ADT and insulin resistance
ADT leads to insulin resistance. Among men without diabetes, ADT has been associated with worsening fasting insulin, fasting glucose, leptin, and HOMAIR (homeostasis model of insulin resistance)37,38. More importantly, ADT has been associated with increased risk of developing diabetes39. Among men with diabetes, ADT has been associated with worsening A1c control40. This is plausible as testosterone has dose- and time-dependent effects on increasing cellular expression of insulin receptor substrate-1 and glucose transporter 441.
ADT and metabolic syndrome
In a meta-analysis of 9 studies of men treated with ADT for prostate cancer, ADT was associated with an increased risk of developing metabolic syndrome (relative risk: 1.75; CI: 1.27–2.41; I2: 0%)42. However, ADT raises both LDL and HDL levels, instead of decreasing HDL levels, as in metabolic syndrome43–45. The fat accumulation in ADT is primarily subcutaneous, rather than the visceral accumulation of metabolic syndrome36. Moreover, there is no significant increase in waist-to-hip-ratio. These data suggest that ADT acts via pathways other than the traditional insulin resistance-mediated development of metabolic syndrome.
ADT and hypertension
ADT was hypothesized to lead to hypertension since androgen-deprived states were shown to increase arterial stiffness46,47. However, only abiraterone and enzalutamide have consistently demonstrated associations with hypertension24. Increased mineralocorticoid production from an increase in ACTH resulting from suppression of cortisol has been suggested as a mechanism for abiraterone’s hypertensive effect48.
ADT and endothelial cell function
At the cellular level, ADT leads to endothelial cell (EC) dysfunction. In ECs from patients with diabetes, androgen signaling was negatively enriched49. However, despite this previously identified biology, GnRH agonists improved conduit artery flow-mediated vasodilation (FMD) in men with PCa at 3 months50. Discontinuation of GNRH agonist resulted in return of FMD to baseline after 6 months. The improvement in FMD occurred despite worsening insulin resistance and dyslipidemia. Other cross sectional studies have described similar effects of ADT on EC function,51 suggesting that EC dysfunction may not be a major determinant of atherosclerosis from ADT.
ADT and Arrhythmia
ADT, especially enzalutamide, may be associated with increased QT interval and acquired long QT syndrome (LQT)52. Testosterone shortens while estradiol lengthens QT prolongation (thus explaining, in part, the long standing observation that men have shorter QT than women)53. Similarly, an association between hypogonadism in men and LQT and risk of torsade de pointes (TdP) has been observed54,55. This association appears to be causal, as correction of hypogonadism by testosterone replacement therapy reduces QT prolongation56. These results suggest electrocardiographic monitoring may have a role in the surveillance of men treated with ADT.
GnRH Receptors Outside the Pituitary
Pituitary cells and cardiac myocytes have increased mRNA expression of GnRH receptor, LH receptor, and FSH receptor compared to other human cells57. In mice, GnRH has been shown to increase cardiac contractility via the protein kinase A (PKA) pathway58. However, further studies remain to be done to characterize the link between GnRH agonist use and GnRH receptor stimulation on cardiac myocytes. There is no evidence yet about whether this may be related to the QT interval prolongation reported from GnRH agonist use59. Intriguingly, FSH levels were positively associated with QT duration in two observational studies54,60.
Synopsis of Mechanisms
The aforementioned atherosclerosis, visceral adiposity, lipolysis inhibition, insulin resistance, and endothelial dysfunction result in an unfavorable cardiovascular risk profile predisposing to MI, stroke, and hypertension61,62. In addition to these structural changes, conduction abnormalities arise as androgen deprivation prolongs the QT interval. Plaque destabilization and insulin resistance are further worsened by the increased state of inflammation caused by elevated pro-inflammatory cytokines and adiponectin from androgen receptor-dependent and -independent mechanisms63.
Management
The CV adverse effects of ADT, such as atherosclerotic plaque growth, are insidious. The cornerstone of management relies on prevention. Prior to initiating an ADT, ideal management involves a multidisciplinary discussion with the patient about the risks and benefits of ADT. Physicians initiating ADT in patients with multiple CV risk factors or history of CV events should consider referral to cardiology or cardio-oncology. The components of cardiac prevention in prostate cancer survivors can be remembered by the ABCDE mnemonic: A for awareness and aspirin; B for blood pressure control; C for cholesterol and cigarettes; D for diabetes and diet; and E for exercise64. These principles do not differ from the principles of cardiac prevention in the general population.
Conclusion
In conclusion, meta-analyses demonstrate a recurring pattern whereby GnRH agonists, GnRH antagonists, androgen receptor antagonists (combined androgen blockade), and orchiectomy for prostate cancer show positive associations with CV events and CV death in observational studies. These effects are not consistently reproducible in RCTs. Notably, the CYP17 inhibitor abiraterone increases risk of CV events in both observational studies and RCTs. Animal and human studies suggest that the mechanisms by which ADT increases CV risk include increased atherosclerosis, dyslipidemia, metabolic syndrome, and insulin resistance. Our current work can provide the basis for a living network meta-analysis. Further pragmatic trials and meta-analyses are necessary to definitively characterize the impact of ADT and AR directed therapies on CV events.
Supplementary Material
Highlights.
As a pooled group, androgen deprivation therapy (ADT) had positive associations (although not always significant) with cardiovascular (CV) events, CV death, and myocardial infarction (MI) among the three meta-analyses of observational studies, but among none of the three meta-analyses of randomized controlled trials (RCTs).
GnRH agonists had strong positive associations with CV death, CV disease, MI, and stroke, among the three meta-analyses of observational trials.
GnRH antagonists had mixed associations with CV disease and MI, and no associations with CV death and stroke, among the three meta-analyses of observational studies.
Combined androgen blockade had positive associations with CV death, CV disease, and stroke, among two meta-analyses of observational studies
CYP17 inhibitors had positive associations with CV events and hypertension, among two meta-analyses of RCTs
Sources of Funding
This work was supported by NIH grants R56HL141466 and R01HL141466. Dr. Meijers was supported by funding from the Niels Stensen Fellowship and the Netherlands Heart Institute.
Abbreviations
- ACTH
adrenocorticotropic hormone
- ADT
androgen deprivation therapy
- AR
androgen receptor
- ARKO
androgen receptor knockout
- CAB
combined androgen blockade
- CI
confidence interval
- CV
cardiovascular
- DHEA
dehydroepiandrosterone
- EC
endothelial cell
- FMD
flow-mediated vasodilation
- FSH
follicle stimulating hormone
- GnRH
gonadotropin-releasing hormone
- HOMA
homeostasis model of insulin resistance
- HPG
hypothalamic-pituitary-gonadal
- HR
hazard ratio
- LH
luteinizing hormone
- LHRH
luteinizing hormone releasing hormone
- LQT
long QT syndrome
- MI
myocardial infarction
- OR
odds ratio
- PCa
prostate cancer
- PKA
protein kinase A
- RCT
randomized, controlled trial
- RR
relative risk
- SEER
Surveillance, Epidemiology, and End Results
- TdP
torsade de pointes
Footnotes
Disclosures
Dr. Moslehi reports receiving consulting fees from Pfizer, Takeda/Millennium, Ariad, Bristol-Myers Squibb, Acceleron, Vertex, Incyte, and Verastem. Dr. Beckman reports consulting fees from Amgen, Astra Zeneca, Antidote Pharmaceuticals, Bristol Myers Squib, Merck, and Sanofi. He reports Data Safety Monitoring Board fees from Bayer and Novartis.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA: A Cancer Journal for Clinicians. 2019;69(1):7–34. doi: 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- 3.Levine GN, D’Amico AV, Albertsen P, et al. Androgen-Deprivation Therapy in Prostate Cancer and Cardiovascular Risk. Circulation. 2010;121(6):833–840. doi: 10.1161/CIRCULATIONAHA.109.192695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beyer DA, Amari F, Thill M, et al. Emerging gonadotropin-releasing hormone agonists. Expert Opinion on Emerging Drugs. 2011;16(2):323–340. doi: 10.1517/14728214.2010.547472 [DOI] [PubMed] [Google Scholar]
- 5.Barber M, Nguyen LS, Wassermann J, Spano J-P, Funck-Brentano C, Salem J-E. Cardiac arrhythmia considerations of hormone cancer therapies. Cardiovasc Res. 2019;115(5):878–894. doi: 10.1093/cvr/cvz020 [DOI] [PubMed] [Google Scholar]
- 6.Shore ND, Abrahamsson P-A, Anderson J, Crawford ED, Lange P. New considerations for ADT in advanced prostate cancer and the emerging role of GnRH antagonists. Prostate Cancer and Prostatic Diseases. 2013;16(1):7–15. doi: 10.1038/pcan.2012.25 [DOI] [PubMed] [Google Scholar]
- 7.Van Poppel H Evaluation of degarelix in the management of prostate cancer. Cancer Manag Res. 2010;2:39–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tombal B, Berges R. Optimal Control of Testosterone: A Clinical Case-Based Approach of Modern Androgen-Deprivation Therapy. European Urology Supplements. 2008;7(1):15–21. doi: 10.1016/j.eursup.2007.11.001 [DOI] [Google Scholar]
- 9.Klotz L, Boccon‐Gibod L, Shore ND, et al. The efficacy and safety of degarelix: a 12-month, comparative, randomized, open-label, parallel-group phase III study in patients with prostate cancer. BJU International. 2008;102(11):1531–1538. doi: 10.1111/j.1464-410X.2008.08183.x [DOI] [PubMed] [Google Scholar]
- 10.Shore ND. Experience with degarelix in the treatment of prostate cancer. Therapeutic Advances in Urology. 2013;5(1):11–24. doi: 10.1177/1756287212461048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gittelman M, Pommerville PJ, Persson B-E, Jensen J-K, Olesen TK, Degarelix Study Group. A 1-year, open label, randomized phase II dose finding study of degarelix for the treatment of prostate cancer in North America. J Urol. 2008;180(5):1986–1992. doi: 10.1016/j.juro.2008.07.033 [DOI] [PubMed] [Google Scholar]
- 12.Rathkopf D, Scher HI. Androgen Receptor Antagonists in Castration-Resistant Prostate Cancer. The Cancer Journal. 2013;19(1):43. doi: 10.1097/PPO.0b013e318282635a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu J, Zhang L, Yan G, et al. Discovery and biological evaluation of novel androgen receptor antagonist for castration-resistant prostate cancer. European Journal of Medicinal Chemistry. 2019;171:265–281. doi: 10.1016/j.ejmech.2019.03.041 [DOI] [PubMed] [Google Scholar]
- 14.Vasaitis TS, Bruno RD, Njar VCO. CYP17 inhibitors for prostate cancer therapy. J Steroid Biochem Mol Biol. 2011;125(1–2):23–31. doi: 10.1016/j.jsbmb.2010.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keating NL, O’Malley AJ, Smith MR. Diabetes and Cardiovascular Disease During Androgen Deprivation Therapy for Prostate Cancer. JCO. 2006;24(27):4448–4456. doi: 10.1200/JCO.2006.06.2497 [DOI] [PubMed] [Google Scholar]
- 16.Carneiro A, Sasse AD, Wagner AA, et al. Cardiovascular events associated with androgen deprivation therapy in patients with prostate cancer: a systematic review and meta-analysis. World J Urol. 2015;33(9):1281–1289. doi: 10.1007/s00345-014-1439-6 [DOI] [PubMed] [Google Scholar]
- 17.Meng F, Zhu S, Zhao J, et al. Stroke related to androgen deprivation therapy for prostate cancer: a meta-analysis and systematic review. BMC Cancer. 2016;16(1):180. doi: 10.1186/s12885-016-2221-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao J, Zhu S, Sun L, et al. Androgen Deprivation Therapy for Prostate Cancer Is Associated with Cardiovascular Morbidity and Mortality: A Meta-Analysis of Population-Based Observational Studies. PLOS ONE. 2014;9(9):e107516. doi: 10.1371/journal.pone.0107516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourke L, Kirkbride P, Hooper R, Rosario AJ, Chico TJA, Rosario DJ. Endocrine therapy in prostate cancer: time for reappraisal of risks, benefits and cost-effectiveness? British Journal of Cancer. 2013;108(1):9–13. doi: 10.1038/bjc.2012.523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nguyen PL, Je Y, Schutz FAB, et al. Association of Androgen Deprivation Therapy With Cardiovascular Death in Patients With Prostate Cancer: A Meta-analysis of Randomized Trials. JAMA. 2011;306(21):2359–2366. doi: 10.1001/jama.2011.1745 [DOI] [PubMed] [Google Scholar]
- 21.Bosco C, Bosnyak Z, Malmberg A, Adolfsson J, Keating NL, Van Hemelrijck M. Quantifying Observational Evidence for Risk of Fatal and Nonfatal Cardiovascular Disease Following Androgen Deprivation Therapy for Prostate Cancer: A Meta-analysis. European Urology. 2015;68(3):386–396. doi: 10.1016/j.eururo.2014.11.039 [DOI] [PubMed] [Google Scholar]
- 22.Albertsen PC, Klotz L, Tombal B, Grady J, Olesen TK, Nilsson J. Cardiovascular Morbidity Associated with Gonadotropin Releasing Hormone Agonists and an Antagonist. European Urology. 2014;65(3):565–573. doi: 10.1016/j.eururo.2013.10.032 [DOI] [PubMed] [Google Scholar]
- 23.Scailteux L-M, Naudet F, Alimi Q, Vincendeau S, Oger E. Mortality, cardiovascular risk, and androgen deprivation therapy for prostate cancer: A systematic review with direct and network meta-analyses of randomized controlled trials and observational studies. Medicine. 2016;95(24):e3873. doi: 10.1097/MD.0000000000003873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin C, Fan Y, Meng Y, et al. A meta-analysis of cardiovascular events in intermittent androgen-deprivation therapy versus continuous androgen-deprivation therapy for prostate cancer patients. Prostate Cancer and Prostatic Diseases. 2016;19(4):333–339. doi: 10.1038/pcan.2016.35 [DOI] [PubMed] [Google Scholar]
- 25.Iacovelli R, Ciccarese C, Bria E, et al. The Cardiovascular Toxicity of Abiraterone and Enzalutamide in Prostate Cancer. Clinical Genitourinary Cancer. 2018;16(3):e645–e653. doi: 10.1016/j.clgc.2017.12.007 [DOI] [PubMed] [Google Scholar]
- 26.Moreira RB, Debiasi M, Francini E, et al. Differential side effects profile in patients with mCRPC treated with abiraterone or enzalutamide: a meta-analysis of randomized controlled trials. Oncotarget. 2017;8(48):84572–84578. doi: 10.18632/oncotarget.20028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bretagne M, Lebrun-Vignes B, Pariente A, et al. Heart failure and atrial tachyarrhythmia on abiraterone: a pharmacovigilance study. Archives of Cardiovascular Diseases. 2019;In Press. [DOI] [PubMed] [Google Scholar]
- 28.Moslehi JJ. Cardiovascular Toxic Effects of Targeted Cancer Therapies. New England Journal of Medicine. 2016;375(15):1457–1467. doi: 10.1056/NEJMra1100265 [DOI] [PubMed] [Google Scholar]
- 29.D’Amico AV, Chen M-H, Renshaw A, Loffredo M, Kantoff PW. Long-term Follow-up of a Randomized Trial of Radiation With or Without Androgen Deprivation Therapy for Localized Prostate Cancer. JAMA. 2015;314(12):1291–1293. doi: 10.1001/jama.2015.8577 [DOI] [PubMed] [Google Scholar]
- 30.Nathan L, Shi W, Dinh H, et al. Testosterone inhibits early atherogenesis by conversion to estradiol: Critical role of aromatase. Proc Natl Acad Sci U S A. 2001;98(6):3589–3593. doi: 10.1073/pnas.051003698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bourghardt J, Wilhelmson ASK, Alexanderson C, et al. Androgen Receptor-Dependent and Independent Atheroprotection by Testosterone in Male Mice. Endocrinology. 2010;151(11):5428–5437. doi: 10.1210/en.2010-0663 [DOI] [PubMed] [Google Scholar]
- 32.Langer C, Gansz B, Goepfert C, et al. Testosterone up-regulates scavenger receptor BI and stimulates cholesterol efflux from macrophages. Biochemical and Biophysical Research Communications. 2002;296(5):1051–1057. doi: 10.1016/S0006-291X(02)02038-7 [DOI] [PubMed] [Google Scholar]
- 33.Hamilton EJ, Gianatti E, Strauss BJ, et al. Increase in visceral and subcutaneous abdominal fat in men with prostate cancer treated with androgen deprivation therapy. Clinical Endocrinology. 2011;74(3):377–383. doi: 10.1111/j.1365-2265.2010.03942.x [DOI] [PubMed] [Google Scholar]
- 34.Hara N, Ishizaki F, Saito T, Nishiyama T, Kawasaki T, Takahashi K. Decrease in Lean Body Mass in Men With Prostate Cancer Receiving Androgen Deprivation Therapy: Mechanism and Biomarkers. Urology. 2013;81(2):376–380. doi: 10.1016/j.urology.2012.10.050 [DOI] [PubMed] [Google Scholar]
- 35.Chazenbalk G, Singh P, Irge D, Shah A, Abbott DH, Dumesic DA. Androgens inhibit adipogenesis during human adipose stem cell commitment to predipocyte formation. Steroids. 2013;78(9):920–926. doi: 10.1016/j.steroids.2013.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta V, Bhasin S, Guo W, et al. Effects of dihydrotestosterone on differentiation and proliferation of human mesenchymal stem cells and preadipocytes. Mol Cell Endocrinol. 2008;296(1–2):32–40. doi: 10.1016/j.mce.2008.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in Body Composition during Androgen Deprivation Therapy for Prostate Cancer. J Clin Endocrinol Metab. 2002;87(2):599–603. doi: 10.1210/jcem.87.2.8299 [DOI] [PubMed] [Google Scholar]
- 38.Basaria S, Muller DC, Carducci MA, Egan J, Dobs AS. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer. 2006;106(3):581–588. doi: 10.1002/cncr.21642 [DOI] [PubMed] [Google Scholar]
- 39.Rubinow KB, Snyder CN, Amory JK, Hoofnagle AN, Page ST. Acute testosterone deprivation reduces insulin sensitivity in men. Clinical Endocrinology. 2012;76(2):281–288. doi: 10.1111/j.1365-2265.2011.04189.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tsai H-T, Keating NL, Van Den Eeden SK, et al. Risk of Diabetes among Patients Receiving Primary Androgen Deprivation Therapy for Clinically Localized Prostate Cancer. J Urol. 2015;193(6):1956–1962. doi: 10.1016/j.juro.2014.12.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keating NL, Liu P-H, O’Malley AJ, Freedland SJ, Smith MR. Androgen Deprivation Therapy and Diabetes Control among Diabetic Men with Prostate Cancer. Eur Urol. 2014;65(4):816–824. doi: 10.1016/j.eururo.2013.02.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen X, Li X, Huang H, Li X, Lin J. [Effects of testosterone on insulin receptor substrate-1 and glucose transporter 4 expression in cells sensitive to insulin]. Zhonghua Yi Xue Za Zhi. 2006;86(21):1474–1477. [PubMed] [Google Scholar]
- 43.Bosco C, Crawley D, Adolfsson J, Rudman S, Van Hemelrijck M. Quantifying the Evidence for the Risk of Metabolic Syndrome and Its Components following Androgen Deprivation Therapy for Prostate Cancer: A Meta-Analysis. PLoS One. 2015;10(3). doi: 10.1371/journal.pone.0117344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Braga-Basaria M, Muller DC, Carducci MA, Dobs AS, Basaria S. Lipoprotein profile in men with prostate cancer undergoing androgen deprivation therapy. International Journal of Impotence Research. 2006;18(5):494. doi: 10.1038/sj.ijir.3901471 [DOI] [PubMed] [Google Scholar]
- 45.Goldberg RB, Rabin D, Alexander AN, Doelle GC, Getz GS. Suppression of Plasma Testosterone Leads to an Increase in Serum Total and High Density Lipoprotein Cholesterol and Apoproteins A-I and B. J Clin Endocrinol Metab. 1985;60(1):203–207. doi: 10.1210/jcem-60-1-203 [DOI] [PubMed] [Google Scholar]
- 46.Moorjani S, Dupont A, Labrie F, et al. Increase in plasma high-density lipoprotein concentration following complete androgen blockage in men with prostatic carcinoma. Metabolism. 1987;36(3):244–250. doi: 10.1016/0026-0495(87)90183-1 [DOI] [PubMed] [Google Scholar]
- 47.Dockery F, Bulpitt CJ, Agarwal S, Donaldson M, Rajkumar C. Testosterone suppression in men with prostate cancer leads to an increase in arterial stiffness and hyperinsulinaemia. Clinical Science. 2003;104(2):195–201. doi: 10.1042/cs1040195 [DOI] [PubMed] [Google Scholar]
- 48.Smith JC, Bennett S, Evans LM, et al. The Effects of Induced Hypogonadism on Arterial Stiffness, Body Composition, and Metabolic Parameters in Males with Prostate Cancer. J Clin Endocrinol Metab. 2001;86(9):4261–4267. doi: 10.1210/jcem.86.9.7851 [DOI] [PubMed] [Google Scholar]
- 49.Attard G, Reid AHM, Auchus RJ, et al. Clinical and Biochemical Consequences of CYP17A1 Inhibition with Abiraterone Given with and without Exogenous Glucocorticoids in Castrate Men with Advanced Prostate Cancer. J Clin Endocrinol Metab. 2012;97(2):507–516. doi: 10.1210/jc.2011-2189 [DOI] [PubMed] [Google Scholar]
- 50.Beckman JA, Doherty SP, Feldman ZB, et al. Comparative Transcriptomics of Ex Vivo, Patient-Derived Endothelial Cells Reveals Novel Pathways Associated With Type 2 Diabetes Mellitus. J Am Coll Cardiol Basic Trans Science. 2019;4(5):567–574. doi: 10.1016/j.jacbts.2019.05.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nguyen PL, Jarolim P, Basaria S, et al. Androgen Deprivation Therapy Reversibly Increases Endothelium‐Dependent Vasodilation in Men With Prostate Cancer. Journal of the American Heart Association. 2015;4(4):e001914. doi: 10.1161/JAHA.115.001914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herman SM, Robinson JT, McCredie RJ, Adams MR, Boyer MJ, Celermajer DS. Androgen deprivation is associated with enhanced endothelium-dependent dilatation in adult men. Arterioscler Thromb Vasc Biol. 1997;17(10):2004–2009. [DOI] [PubMed] [Google Scholar]
- 53.Salem J-E, Yang T, Moslehi JJ, et al. Androgenic Effects on Ventricular Repolarization A Translational Study From the International Pharmacovigilance Database to iPSC-Cardiomyocytes. Circulation. 2019;(140). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Makkar RR, Fromm BS, Steinman RT, Meissner MD, Lehmann MH. Female Gender as a Risk Factor for Torsades de Pointes Associated With Cardiovascular Drugs. JAMA. 1993;270(21):2590–2597. doi: 10.1001/jama.1993.03510210076031 [DOI] [PubMed] [Google Scholar]
- 55.Abehsira G, Bachelot A, Badilini F, et al. Complex Influence of Gonadotropins and Sex Steroid Hormones on QT Interval Duration. J Clin Endocrinol Metab. 2016;101(7):2776–2784. doi: 10.1210/jc.2016-1877 [DOI] [PubMed] [Google Scholar]
- 56.Salem Joe-Elie, Waintraub Xavier, Carine Courtillot, et al. Hypogonadism as a Reversible Cause of Torsades de Pointes in Men. Circulation. 2018;138(1):110–113. doi: 10.1161/CIRCULATIONAHA.118.034282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gagliano-Jucá T, İçli TB, Pencina KM, et al. Effects of Testosterone Replacement on Electrocardiographic Parameters in Men: Findings From Two Randomized Trials. J Clin Endocrinol Metab. 2017;102(5):1478–1485. doi: 10.1210/jc.2016-3669 [DOI] [PubMed] [Google Scholar]
- 58.Kakar SS, Jennes L. Expression of gonadotropin-releasing hormone and gonadotropin-releasing hormone receptor mRNAs in various non-reproductive human tissues. Cancer Lett. 1995;98(1):57–62. [PubMed] [Google Scholar]
- 59.Dong F, Skinner DC, Wu TJ, Ren J. The Heart: A Novel Gonadotrophin-Releasing Hormone Target. Journal of Neuroendocrinology. 2011;23(5):456–463. doi: 10.1111/j.1365-2826.2011.02119.x [DOI] [PubMed] [Google Scholar]
- 60.Garnick MB, Pratt CM, Campion M, Shipley J. The effect of hormonal therapy for prostate cancer on the electrocardiographic QT interval: phase 3 results following treatment with leuprolide and goserelin, alone or with bicalutamide, and the GnRH antagonist abarelix. JCO. 2004;22(14_suppl):4578–4578. doi: 10.1200/jco.2004.22.90140.4578 [DOI] [Google Scholar]
- 61.Canpolat U, Tokgözoğlu L, Yorgun H, et al. The association of premature ovarian failure with ventricular repolarization dynamics evaluated by QT dynamicity. Europace. 2013;15(11):1657–1663. doi: 10.1093/europace/eut093 [DOI] [PubMed] [Google Scholar]
- 62.Libby P The vascular biology of atherosclerosis In: Bonow R, Mann D, Zipes D, Libby P, eds. Braunwald’s Heart Disease: Textbook of Cardiovascular Medicine. 9th ed Philadelphia, PA: Elsevier Saunders; 2012. [Google Scholar]
- 63.Hajer GR, van Haeften TW, Visseren FLJ. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur Heart J. 2008;29(24):2959–2971. doi: 10.1093/eurheartj/ehn387 [DOI] [PubMed] [Google Scholar]
- 64.Tzortzis V, Samarinas M, Zachos I, Oeconomou A, Pisters LL, Bargiota A. Adverse effects of androgen deprivation therapy in patients with prostate cancer: focus on metabolic complications. Hormones. 2017;16(2):115–123. doi: 10.14310/horm.2002.1727 [DOI] [PubMed] [Google Scholar]
- 65.Bhatia N, Santos M, Jones LW, et al. Cardiovascular Effects of Androgen Deprivation Therapy for the Treatment of Prostate Cancer: ABCDE Steps to Reduce Cardiovascular Disease in Patients With Prostate Cancer. Circulation. 2016;133(5):537–541. doi: 10.1161/CIRCULATIONAHA.115.012519 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


