Abstract
Background
Oral theophylline has, for many years, been used as a bronchodilator in patients with COPD. Despite the introduction of new drugs, and its narrow therapeutic index, theophylline is still recommended for COPD treatment.
Objectives
To determine the effectiveness of oral theophylline when compared to placebo in patients with stable COPD.
Search methods
We searched the Cochrane Airways Group trial register and Cochrane Central Register of Controlled Trials Cochrane Controlled Clinical Registers were searched.
Selection criteria
All studies were randomised controlled trials (RCTs).
Data collection and analysis
Two reviewers independently abstracted data and asessed the methodological quality.
Main results
Twenty RCTs met the inclusion criteria. Concomitant therapy varied from none to any other bronchodilator plus corticosteroid (oral and inhaled). The following outcomes were significantly different when compared to placebo.
Forced expiratory volume in one second (FEV1) improved with treatment: Weighted Mean Difference (WMD) 100 ml; 95% Confidence Interval (CI) 40 to 160 ml. Similarly for forced vital capacity (FVC): WMD 210 ml 95%CI 100 to 320. Two studies reported an improvement in maximum oxygen consumption (VO2 max); WMD 195 ml/min, 95%CI 113 to 278. At rest, arterial oxygen tension at rest (PaO2) and arterial carbon dioxide tension at rest (PaCO2) both improved with treatment (WMD 3.2 mm Hg; 95%CI 1.2 to 5.1, and WMD ‐2.4 mm Hg; 95%CI ‐3.5 to ‐1.2, respectively). Walking distance tests did not improve (four studies, Standardised Mean Difference 0.30, 95%CI ‐0.01 to 0.62), neither did Visual Analogue Score for breathlessness in two small studies (WMD 3.6, 95%CI ‐4.6 to 11.8). The Relative Risk (RR) of nausea was greater with theophylline (RR 7.7; 95%CI 1.5 to 39.9). However, patients' preference for theophylline was greater than that for placebo (RR 2.27; 95%CI 1.26 to 4.11). Very few participants withdrew from these studies for any reason.
Authors' conclusions
Theophylline has a modest effect on FEV1 and FVC and slightly improves arterial blood gas tensions in moderate to severe COPD. These benefits were seen in participants receiving a variety of different concomitant therapies. Improvement in exercise performance depended on the method of testing. There was a very low dropout rate in the studies that could be included in this review, which suggests that recruited participants may have been known by the investigators to be theophylline tolerant . This may limit the generalisability of these studies.
Keywords: Humans; Administration, Oral; Bronchodilator Agents; Bronchodilator Agents/administration & dosage; Pulmonary Disease, Chronic Obstructive; Pulmonary Disease, Chronic Obstructive/drug therapy; Randomized Controlled Trials as Topic; Theophylline; Theophylline/administration & dosage
Plain language summary
Oral theophylline compared to placebo for people with chronic obstructive pulmonary disease (COPD)
Theophylline treatment is commonly used in people with chronic obstructive pulmonary disease (COPD). This systematic review shows that orally administered theophylline improves lung function and levels of oxygen and carbon dioxide in the blood. However, there is limited data on its effect on symptoms, exercise capacity or quality of life. Despite being associated with increased side effects, particularly nausea, participants preferred theophylline over placebo.
Background
Chronic obstructive pulmonary disease (COPD), by definition, is characterised by limited reversibility with bronchodilator therapy (Folgering 1994; ATS 1995). Patients often have major limitations of physical activity, especially breathlessness during exercise. Oral theophylline is a bronchodilator that has been used for many years although sympathomimetic and inhaled anticholinergic agents are now used more often (Mulloy 1993). Despite this change in prescribing patterns, there is still a perception that theophylline confers additional benefit over that produced by the newer agents (Raguso 1996). Traditional methods of assessing clinical benefit with theophylline have been based upon physiological measurements of airways function, but some studies have shown improvement in exercise performance, breathlessness and health status (quality of life) with minimal change in lung function (Vereen 1986).
The major therapeutic benefit of theophylline has been attributed to its phosphodiesterase inhibitor activity, which inhibits the degradation of cyclic adenosine monophosphate (cAMP). Other mechanisms of action proposed for the action of theophylline include: inhibition of bronchoconstrictors (prostaglandins) as a result of the increased levels of cellular cAMP (Horrobin 1977); increased cellular calcium uptake and distribution involved in smooth muscle contraction (Aubier 1985); increases in endogenous secretion of cortisol; stimulation of release of endogenous catecholamines (Atuk 1967; Higbee 1982); positive inotropic effect on the heart (Matthay 1986) and theophylline is also known to be a mild diuretic (Johannesson 1985). Increased release of endogenous cortisol and catecholamines by theophylline may have a similar effect to that of the administration of corticosteroids (Mendella 1982).
Theophylline is known to have a central effect on respiration. In COPD, xanthine derivatives can bring about acute and long‐term enhancement of the hypoxemic ventilatory response (Aubier 1983) by increasing inspiratory muscle drive, resulting in tidal volume increases (Spinelli 1991).
The role of theophylline in the management of COPD has not been fully defined. Studies have not consistently shown theophylline to be beneficial in the management of stable COPD (Alexander 1980; Eaton 1980; Murciano 1989). The BTS 1997 guidelines on management of COPD recommends use of xanthine derivatives as a last resort and only after all other treatments have failed to show a response. The ATS guidelines on COPD (ATS 1995) makes stronger recommendations for the use of theophylline in both stable and acute management of COPD but due to its narrow therapeutic index (Woodcock 1983) it also recommends cautious use. Due to the increasing numbers of guidelines on COPD management and the lack of evidence‐based documentation, the US National Heart Lung and Blood Institute and the World Health Organisation have jointly developed evidence‐based guidelines for the management of COPD (Pauwels 2000; Gomez 2002). This project known as the Global Initiative for Chronic Obstructive Lung Disease (GOLD) aims to produce guidelines that are applicable globally and are based on well‐controlled clinical studies (where available) and not on consensus. However, the latter would be used where there was insufficient evidence. The current GOLD guideline recommends the use of theophylline as second line option since many studies have shown its bronchodilator effectiveness in COPD (web address: http://www.goldcopd.com).
A major disadvantage with this class of drugs is the incidence of adverse effects, particularly those involving the gastro‐intestinal tract, even when the plasma level is within the therapeutic range. The evaluation of efficacy and the assessment of adverse effects are important factors to consider when dose titrating with theophylline preparations (Persson 1986).
Most studies have been performed on small number of patients with short duration. To our knowledge, there is no systematic review regarding theophylline in stable COPD. Owing to the differing recommendations from various COPD guidelines and to the different conclusions from the many clinical studies this systematic review of the literature was performed to assess the effect of theophylline in patients with stable COPD.
Objectives
To determine the efficacy of oral theophylline compared to placebo in patients with stable COPD.
Methods
Criteria for considering studies for this review
Types of studies
All studies were randomised controlled trials which involved treatment with theophylline or placebo in patients with COPD. Studies of any duration were considered for inclusion but single dose studies were excluded.
Types of participants
Studies in patients with COPD were considered for inclusion. COPD was defined by internationally accepted criteria (e.g. ATS 1995; ERS 1995; BTS 1997) or defined objectively as a disorder characterised by reduced expiratory flow and slow forced emptying of the lungs and features which do not change markedly over several months (ERS 1995).
Types of interventions
The use of oral theophylline compared to placebo in a randomised fashion.
Types of outcome measures
PRIMARY OUTCOMES MEASURES (1) Exercise capacity: timed walking tests, endurance tests and incremental exercise tests on a treadmill or cycle ergometer.
(2) Lung function measurements (e.g. forced expiratory volume in one second (FEV1), forced vital capacity (FVC)).
(3) Health status (quality of life) scores: These include the Chronic Respiratory Disease Questionnaire (CRQ) or the St George's Respiratory Questionnaire (SGRQ).
SECONDARY OUTCOME MEASURES (1) Arterial blood gas tensions (partial pressure oxygen (PaO2), partial pressure carbon dioxide (PaCO2)) and oxygen saturation (SaO2) on exercise and rest.
(2) Dyspnoea: measured directly at rest or during exercise, or indirectly by self report on symptom diaries (e.g. visual analogue scale, Borg scale or Likert scale or any other validated measurement).
(3) Participant preference for treatment
(4) Adverse effects: frequency of gastric effects (nausea, diarrhoea, mild abdominal discomfort), insomnia, and arrhythmia's (atrial fibrillation, tachycardia, ventricular extrasystole).
(5) Acute exacerbations
(6) Mortality: proportion of deaths
(7) Dropout rate: number of participants dropping out of the study.
Search methods for identification of studies
We carried out a search of the Cochrane Airways Group trial register and the Cochrane Central Register of Controlled Trials using the following search strategy: obstructive OR bronchitis OR pulmonary emphysema OR bronchial hyperreactivity OR COPD OR COLD OR emphysema AND aminophylline OR theophylline OR theo* OR uniphyl OR nuelin. We also conducted separate and additional searches using MEDLINE (1966 to Feb 2002), EMBASE (1982 to Feb 2002), CINAHL (Feb 2002), and LILACS (1982 to Feb 2002) databases. We considered atudies of any duration or in any language. We identified other potential studies by writing to key authors, and examining the bibliographies of included studies and relevant review articles.
Data collection and analysis
LOCATING AND SELECTING STUDIES Two reviewers (FR, SC) independently assessed the titles and abstracts of all reports of trials identified by electronic searching. The full text copies of all potential trials were obtained. Disagreements between reviewers were resolved with discussion.
STUDY QUALITY Two reviewers independently assessed the methodological quality. We assessed all included studies using two study quality scales. The Cochrane assessment of allocation concealment was used, Grade A: Adequate concealment, Grade B: Uncertain about the method of allocation concealment, Grade C: Clearly inadequate allocation concealment & Grade D: Allocation concealment not used.
In addition, we also graded studies using the five point Jadad 1996 study quality score:
Was the study described as randomised? Yes = 1; No = 0 Was the study described as double blind? Yes = 1; No = 0 Was there a description of the withdrawals and dropouts? Yes = 1; No = 0 Was the method of randomisation well described and appropriate? Yes = 1; No = 0 Was the method of double blinding well described and appropriate? Yes = 1; No = 0 Deduct one point if methods for randomisation or blinding were inappropriate.
STATISTICAL ANALYSIS Where possible, we pooled trial outcome data. As planned in the protocol, for continuous variables, we calculated the results of individual studies as fixed effect weighted mean difference (WMD) or standardised mean difference (SMD) including the 95% confidence interval (CI) for each outcome. Where results were expressed as dichotomous variables, we calculated odds ratio (OR) or relative risks (RR) with 95% CI for individual outcomes.
The intention was to analyse separately trials employing a crossover design from those using a parallel‐group design. If first‐arm data from crossover trials was reported or if we had been able to obtain this data from the authors, then this would have been combined with data from parallel design trials. In the event, all trials were crossover in design, therefore aggregate means (from both study arms) were entered into RevMan and pooled.
SENSITIVITY ANALYSIS For pooled effects, we carried out a test for heterogeneity; p < 0.05 was considered statistically significant. If there was significant heterogeneity, we would have performed sensitivity tests using study quality, duration of study, dose and type of theophylline preparation and level of concomitant medication usage. When data were missing (in the case of SDs) these were calculated using the average of other trial SDs for that outcome. If range values were reported, SD was computed from the range using the following [SD = (UR ‐ LR) / (2 x 1.96), 1.96 is for 95% range but changes to 1.64 and 2.58 for 90% and 99% reference ranges]. If only p values were reported, a pooled estimate of SD was computed by converting the p value to a Student's t‐value; thereafter calculating SD using the following [SD = (square‐root {n1 x n2} / {n1 + n2}) x (difference in means / t‐value)]. Wherever these estimates of SDs were necessary, we conducted a sensitivity analysis of the overall result, excluding the calculated SDs, to test if the treatment effect differed significantly.
Results
Description of studies
Please refer to the table "Characteristics of included studies" for detailed descriptions of each included study. A brief summary is provided below.
SEARCH FOR STUDIES From 310 abstracts, we retrieved 86 full text papers for closer assessment. We selected twenty‐four studies for inclusion. Four studies were multiple publications of the same cohort of participants. Alexander 1980 had one follow‐up publication, Guyatt 1987 had two additional publications and the Iversen 1992 study had one duplicate publication. Therefore, there were 20 included studies (excluding four duplicate publications). The 62 excluded studies with their reasons for exclusion are listed in the table "Characteristics of excluded studies".
LOCATION OF STUDIES Six studies were conducted in USA (Schmidt 1979; Alexander 1980, Marvin 1983; Mahler 1985; Dullinger 1986; Kongragunta 1988), three in Canada (Guyatt 1987; Rivington 1988; Thomas 1992), two each in the UK (Anderson 1982; Chrystyn 1988), Israel (Fink 1994; Newman 1994), Ireland (Power 1992; Mulloy 1993) and Japan (Nishimura 1993; Nishimura 1995) and one each in Denmark (Iversen 1992), France (Murciano 1989) and Germany (Machraoui 1994).
TYPES OF PARTICIPANTS All studies included adult participants with COPD defined using objective criteria of less than 15% in FEV1 reversibility after inhaling a bronchodilator in six studies (Schmidt 1979; Mahler 1985; Chrystyn 1988; Murciano 1989; Power 1992; Mulloy 1993) or 25% in two studies (Dullinger 1986; Guyatt 1987). The MRC definition of COPD was used in two studies (Anderson 1982; Chrystyn 1988) and the ATS definition in one (Nishimura 1995). One study (Thomas 1992) did not include participants who had a greater than 20% change in either FEV1 or FVC over the previous two years. Most of the studies also used a pre‐defined criterion based on predicted FEV1 or FEV1/FVC ratio for including participants in their study, typical values for FEV1 were less than 60 to 70% and for FEV1/FVC ratio it was less than 0.6 to 0.7. One study (Nishimura 1995) included participants with a post‐bronchodilator FEV1/FVC ratio of less than 70%. All of the studies included participants who were either ex or current smokers and excluded participants who had asthma. Baseline mean FEV1 for the participants in the 20 studies ranged from 0.96 to 1.15 L. Mean age ranged from 58 to 69 years.
COMMITTANT MEDICATION Four of the studies did not allow use of bronchodilators during the study period (Alexander 1980; Guyatt 1987; Murciano 1989; Thomas 1992). Twelve studies permitted use of regular bronchodilators and inhaled corticosteroids for the duration of the study (Anderson 1982; Marvin 1983; Mahler 1985; Chrystyn 1988; Kongragunta 1988; Rivington 1988; Mulloy 1993; Nishimura 1993; Fink 1994; Machraoui 1994; Newman 1994; Nishimura 1995). Four studies did not describe concomitant medication use (Schmidt 1979; Dullinger 1986; Iversen 1992; Power 1992). TYPES OF INTERVENTION All 20 included studies were of crossover design and used dosing schedules to obtain plasma theophylline levels in the therapeutic range (10 to 20 mg/ml). Five studies used short acting or immediate release theophylline preparations (Schmidt 1979; Alexander 1980; Marvin 1983; Guyatt 1987; Machraoui 1994) while the remaining 15 studies used long acting or sustained release theophylline preparations. Where studies have reported both pre and post‐bronchodilator (e.g. salbutamol, terbutaline, ipratropium bromide) study measurements, only the pre‐bronchodilator measurements were used (for the meta‐analysis) as the purpose of this review was to observe the effects of oral theophylline administration and not to estimate the effects of immediate post‐bronchodilator therapy.
The duration of the studies ranged from 7 to 90 days. The duration of each study was entered into RevMan (as days) under user defined category, in order to observe any influence of study duration on effect size.
Nine of the studies reported adequate washout periods between their crossover arms ranging from three days to two weeks (Anderson 1982; Mahler 1985; Guyatt 1987; Kongragunta 1988; Murciano 1989; Mulloy 1993; Fink 1994; Newman 1994; Machraoui 1994). The remaining 11 studies did not report any washout period. This, however, may not mean that no washout period was employed by these 11 studies. To date we have not received any correspondence from the authors to verify this information.
DROPOUT AND WITHDRAWALS Only one study (Iversen 1992) failed to report dropouts. In the others, with the exception of Guyatt 1987 (eight withdrawals from 27 recruited) the dropout out rate was generally very low. Nine studies reported no dropouts (Marvin 1983; Mahler 1985; Dullinger 1986; Rivington 1988; Murciano 1989; Power 1992; Nishimura 1993; Fink 1994; Newman 1994).
Risk of bias in included studies
There was total agreement between two reviewers on the inclusion of studies and for the quality scores. Four studies were graded as Cochrane 'A' (adequate allocation concealment) (Schmidt 1979; Marvin 1983; Rivington 1988; Mulloy 1993), and the remaining 16 studies scored a 'B' grade (unclear allocation concealment). The mean (SD) score for Jadad study quality was 2.8 (0.81) and the range was 1 to 5. Most of the included studies (12) received a Jadad score of three, five studies received a score of two, and three studies each received a score of one, four and five. Overall the studies were of adequate quality as none of the studies scored 'C' with the Cochrane grading and the mean Jadad score was 2.8, which is considered of adequate‐to‐good quality.
Effects of interventions
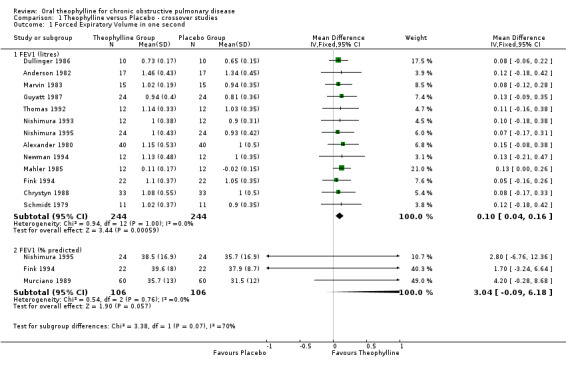
OUTCOMES REPORTED IN TWO OR MORE INCLUDED STUDIES: STATISTICALLY SIGNIFICANT EFFECT FEV1 ‐ L [Comparison 01:01:01] Thirteen studies with 244 patients contributed data towards this outcome which showed a significant improvement of 100 ml with treatment (WMD 100 ml; 95%CI 40 to 160). One of the thirteen studies did not report any SD for FEV1 (Dullinger 1986) so it was estimated from the values reported for range. A sensitivity analysis without the Dullinger 1986 study did not alter the mean overall result (WMD 110 ml; 95%CI 40 to 170) compared to when all 13 studies were included. There was no evidence of heterogeneity in this effect size across the studies.
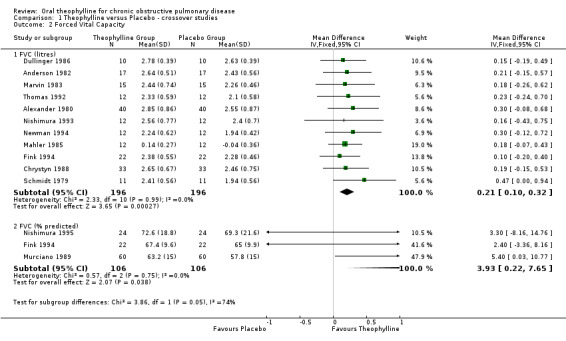
FVC ‐ L [Comparisons 01:02:01& 01:02:02] Eleven studies with 196 patients contributed data towards this outcome which showed significant improvement of 210 ml with treatment (WMD 210 ml; 95%CI 100 to 320). Two of the 11 studies did not report any SD's for FVC (Schmidt 1979; Dullinger 1986) so they were estimated from the reported range and p values, respectively. A sensitivity analysis without the Dullinger 1986 and Schmidt 1979 studies did not alter the mean overall result (WMD 200 ml; 95%CI 70 to 320) compared to when all 11 studies were included. FVC reported as percent predicted by three studies was also significant (WMD 3.93; 95%CI 0.22 to 7.65).
VO2 max ‐ ml/min [Comparison 01:09] Two studies with 32 patients reported this outcome (Fink 1994; Newman 1994) which showed significant improvement with treatment (WMD 195 ml/min; 95%CI 113 to 278).
PaO2 ‐ mm Hg at rest [Comparison 01:10] Six studies with 156 patients reported this outcome (Alexander 1980; Mahler 1985; Murciano 1989; Mulloy 1993; Fink 1994; Newman 1994) which showed significant improvement with treatment (WMD 3.18 mm Hg; 95%CI 1.23 to 5.13).
PaCO2 ‐ mm Hg at rest [Comparison 01:11] Six studies with 156 patients reported this outcome (Alexander 1980; Murciano 1989, Mahler 1985, Mulloy 1993; Fink 1994; Newman 1994) which showed significant decrease with treatment (WMD ‐2.36 mm Hg; 95%CI ‐3.52 to ‐1.21).
Patient preference for theophylline or placebo [Comparison 01:21] Two studies with a total of 50 (cross over) subjects (Alexander 1980; Mulloy 1993) reported that participants preferred theophylline to placebo (RR 2.27; 95%CI 1.26 to 4.11).
Nausea [Comparison 01:23] Three studies reported data on nausea (Alexander 1980; Mulloy 1993; Newman 1994). The risk of experiencing nausea when on treatment with theophylline was significantly increased (RR 7.67; 95%CI 1.47 to 39.94).
There did not appear to be any influence of study duration on outcome effect size (using user‐defined category in Forest plots).
OUTCOMES REPORTED IN TWO OR MORE STUDIES: NO STATISTICALLY SIGNIFICANT EFFECT
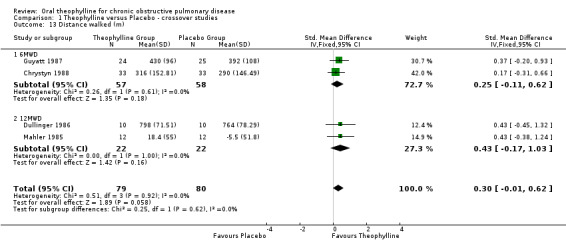
Distance walked (m) [Comparison 01:23] Two studies (58 patients) reported distance walked in six minutes (Guyatt 1987; Chrystyn 1988) and two (22 patients) the distance walked in 12 minutes (Mahler 1985; Dullinger 1986). In neither group of studies was the effect significant and when all four studies were combined using an SMD the effect was still not significant (SMD 0.30; 95%CI ‐0.01 to 0.62). The mean difference in the six‐minute walk studies was 33 m which is not at the threshold of clinical significance.
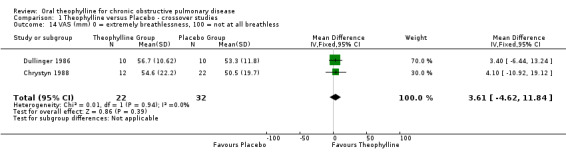
Visual Analogue Scale (VAS) for breathlessness [Comparison 01:14] Two studies (32 patients) reported this (Dullinger 1986; Chrystyn 1988), there was no effect.
Symptoms of wheeze and dyspnea [Comparison 01:18] Two studies (21 patients) reported these (Dullinger 1986; Chrystyn 1988), there was no effect.
Exacerbations [Comparison 01:20] Two studies (45 patients) reported these (Rivington 1988; Nishimura 1995), there was no effect.
Drop outs It was possible to compare drop out rates between treatment arms in only three studies totaling 74 patients (Anderson 1982; Fink 1994; Newman 1994). There was no difference. No data were available for health status and mortality.
Discussion
This review has shown that administering oral theophylline for at least seven days, to partients with moderate to severe COPD improves lung function, ventilatory capacity and arterial blood gas tensions in patients with stable COPD. Participants preference for theophylline was greater than that for placebo. However, the number of adverse effects (nausea) was greater with theophylline treatment.
The magnitudes of the lung function changes are relatively small, but similar to those reported in another Cochrane review of long‐acting beta2‐agonists (Appleton 2002). Meaningful symptomatic responses from bronchodilators in the presence of only trivial changes in FEV1 and FVC have been reported (Mahler 1985; Wolkove 1989; Hay 1992). Unfortunately, there were very small numbers of studies reporting symptoms in this review. The included studies that attempted to measure improvements in symptoms (e.g. dyspnoea, quality of life, wheeze) all showed improvements, but, owing to minimal reporting and the use of different methodologies the data could not be collated meaningfully. Individual studies did, however, report benefits. Alexander 1980 used a six point scale which measured dyspnoea, wheezing, cough, sputum, walking and feelings that showed improvements in all categories with the use of theophylline. The study by Guyatt 1987 reported significant improvements in dyspnoea and quality of life scores measures by CRQ. Chrystyn 1988 and Dullinger 1986 reported a modest improvement in dyspnoea with theophylline treatment. Iversen 1992 reported significant improvements in dyspnoea scores during theophylline treatment. Marvin 1983 and Thomas 1992 reported improvements in both wheezing and shortness of breath with theophylline.
Other mechanisms have been proposed to explain how theophylline could improve symptoms or reduce breathlessness in partients with COPD. The Chrystyn 1988 study measured the effects of theophylline on 33 participants with stable COPD. In their study a dose of theophylline that resulted in serum concentrations of 15‐20 mg/L, only led to an increase in FEV1 of 13% (130 mls) but there was a significant 64% decrease in trapped gas volume (1.84 L to 0.67 L ). Unfortunately, this was the only study to report data on trapped gas volume in this review.
Other workers have shown that inhaled beta 2‐agonists and ipratropium bromide reduce exertional breathlessness in subjects with stable COPD and this correlates strongly with decreases in thoracic gas entrapment (Chrystyn 1988; Webb 1990) and dynamic hyperinflation (Belman 1996; O'Donnell 1999). The improvements in lung function seen with theophylline in this review may be due to dilatation of the small airways with a consequent reduction in gas trapping. A fall in trapped gas volume (and thus FRC) is likely to improve the mechanical advantage of the diaphragm and chest wall muscles and may well explain many of the reported effects of theophylline on the respiratory muscles (Murciano 1984).
Theophylline has also been demonstrated to increase the pressure generated by respiratory muscles (Umut 1992) and increase diaphragmatic strength (Kongragunta 1988). Its effect has been shown to be greater in fatigued diaphragm (Murciano 1984) as has been shown in severe COPD. In one study, theophylline increased trans‐diaphragmatic pressure by 16% and this increase persisted even after 30 days of treatment with theophylline (Murciano 1984). In therapeutic doses theophylline is also known to increase respiratory drive independent of its effect on lung function (Ashutosh 1997). It is also known to increase respiratory muscle function in normal participants (Sherman 1996) and in COPD (Umut 1992) as measured by increases in maximal inspiratory and expiratory pressures. It has also been suggested that theophylline reduces breathlessness by improving diaphragmatic contractility. The Murciano 1989 trial demonstrated an improvement in respiratory muscle performance as indicated by a decline in the ratio of inspiratory pleural pressure during quiet breathing to the maximal pleural pressure (data not reported in this review as an outcome).
Another interpretation is that the improvement in respiratory muscle function is due not to an increase in diaphragmatic contractility but to an improvement in the length‐tension relationship of the diaphragm. This is because there is a reduction in gas trapping and a recent study by Hatipoglu 1999 supports this interpretation. It is possible and likely that these effects of theophylline had a role in slightly but significantly improving lung function as has been shown by this review.
There were small but statistically significant improvements in arterial blood gas tensions in patients treated with theophylline. In severe cases of COPD, respiratory rate is increased, and this may be combined with shallow breathing that is pronounced by carbon dioxide retention. It is known that theophylline improves minute ventilation in humans (Darnall‐Jr 1985) and animals (Javaheri 1989) and also alters the ventilatory response in COPD seen as improved ventilatory capacity measured as increased VO2 max. This ventilatory response results in an increase in tidal volume, which may be responsible for the improvement seen in blood gas tensions. Both these changes (increase in VO2 max and improved blood gas tensions) could be related to either a direct positive inotropic effect of theophylline on the respiratory muscles (Okubo 1987; Kongragunta 1988; Landsberg 1990; Marsh 1993) or due to its action via a central stimulatory pathway (Cooper 1987; Javaheri 1990) or both. It is known that theophylline is capable of stimulating the medullary respiratory center (Ritchie 1975).
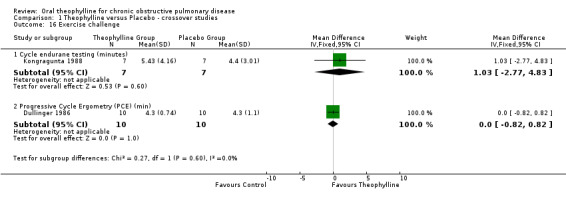
Although only two studies provided data for VO2 max, this is an important significant finding as greater exercise performance is implied by increases in VO2 max. Unfortunately there were not enough studies providing data on exercise performance (distance walked, cycle endurance or progressive cycle ergometry) to permit us to relate the increase in VO2 max to exercise performance. The effect size in the two studies that reported it was approximately 30 metres for a six minute walk which is not clinically significant.
Theophylline has a narrow therapeutic index and adverse effects are common even when the serum concentrations are in the 'therapeutic range' of 10 to 20 mg/L. In this review, nausea was reported by significantly more participants treated with theophylline compared with placebo. Another more serious adverse effect of theophylline in patients with COPD, is supraventricular arrhythmias (Levine 1985; Varriale 1993), however, this was not reported in this review. Nevertheless, the benefits of theophylline in stable COPD have to be weighed against the risk of adverse effects. All of the included studies in this review aimed for theophylline concentrations within the therapeutic range. In patients with asthma theophylline exerts beneficial effects at serum concentrations lower than the traditional therapeutic range of 10 to 20 mg/L (Mitenko 1973; Evans 1997). Lower concentrations of theophylline have the advantage that they are associated with fewer adverse effects. In future studies of theophylline for stable COPD it may be appropriate to have a lower target concentration of theophylline. An alternative approach would be to study specific inhibitors of type IV phosphodiesterases such as Airflo® that are reported to be effective in the treatment of asthma but which have fewer adverse effects compared to theophylline (Compton 2000; Barnette 2000; Giembycz 2000).
A possible pitfall of crossover studies, such as those included in this review, is the presence of carry‐over effects of the first treatment into the second treatment period, leading to an underestimation of the real difference among treatments (Cleophas 1993). Nine of the studies reported adequate washout periods between their crossover arms ranging from three days to two weeks. The remaining 11 studies did not either have a washout period or failed to report any washout period. To date we have not received any correspondence from the authors to verify this information.
A second possible pitfall associated with crossover designs, is that the software we used (RevMan) forces us to analyse crossover studies as if they were parallel studies. It is known (Cleophas 1996) that the two methods give identical results if the response to the two treatments, in the same individual, is completely unrelated. However, parallel analysis may lead to decreased statistical power when compared to paired analysis, if the response to the two treatments is positively correlated (i.e. if patients improving during bronchodilator are also more likely to improve somewhat during placebo). This possibility cannot be discounted in our review. The results of the statistical analysis from two‐period crossover trials make two main assumptions, no period effect and no treatment‐period interaction. But none of the authors reported these findings (correlation between the responses to the two treatments) from their studies and the presentation of the data did not permit these types of analysis. Therefore, we cannot exclude that our analysis underestimated the statistical significance of the observed differences, as compared to a paired analysis.
Authors' conclusions
Implications for practice.
Theophyllines produce a small improvement in FEV1 that is similar in size to that reported for long acting beta2‐agonists in COPD patients, with and without adjuvant bronchodilator therapy. There also appears to be a small mean improvement in arterial blood gas tensions. The evidence for symptomatic benefit or improved exercise performance is less consistent. There is an increased incidence of nausea, but on average, the patients in the studies reviewed here preferred theophylline. The tolerance of theophylline in the patients recruited to these studies (as reflected by the absence of an increased drop out rate) is a little surprising since clinical experience suggests that a significant number of patients cannot tolerate the gastro‐intestinal side‐effects. We conclude that, with close monitoring of individual patients and their serum theophylline levels, it appears that beneficial effects may be obtained in those who remain symptomatic from COPD despite first‐line bronchodilator therapy.
Implications for research.
Larger parallel randomised controlled trials with explicit clinical and diagnostic criteria, sufficient duration of follow‐up and description of all relevant clinical outcome measures appear warranted. Many previously conducted studies have relied heavily on the readily available physiological tests (e.g. FEV1, FVC, PEFR). As these are not particularly sensitive measures of change in this group of patients (Wolkove 1989; Celli 2000) we suggest that other relevant outcome measures should be used (e.g. symptoms, health status, adverse effects, exercise capacity & endurance, length of hospital stay, incidence of exacerbations, health care utilisation and cost effectiveness). Future studies should also endeavour to define which 'types' of patients are most likely to respond to treatment with theophylline. Studies also need to examine the role of theophylline in comparison, and in conjunction, with newer agents such as long acting bronchodilators. Further investigation of the effect of theophylline on ventilatory mechanics would be helpful to delineate the non‐bronchodilator effects of theophylline, which appear important. Because of a lower incidence of adverse effects, it will be interesting to observe the efficacy of specific inhibitors of type IV phosphodiesterases in people with COPD.
Feedback
inconsistent (?) reporting
Summary
Dear Madam or Sir,
I have encountered the difficulty interpreting the results of the review by Ram et al. "Oral theophylline for chronic obstructive pulmonary disease".
1. In Results section it states: "Two studies with a total of 100 subjects (Anderson 1982, Mulloy 1993) reported that subjects preferred theophylline..."
2. In the metaview graph we see 50 patients (cross‐over): 40 in Anderson study and 10 in Mulloy study.
3. In "Cachacteristics of the included studies" section, we read:
‐ Anderson 1982: Number of patients: 21 (I have confirmed that with Medline abstract)
‐ Mulloy: Number of patients: 10
My problem is the final number of patients that expressed their preference towards theophylline. Is it 100, as the authors say, 50 as appears from the metaview graph, or 31 (10 ‐ Mulloy and 21 ‐ Anderson) as one would expect from the total number of subjects in both trials?
I would appreciate your prompt response.
Sincerely,
Jan Brozek
I certify that I have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of my criticisms.
Reply
Dear Dr Brozek,
Thank you for your interest in our review. You are correct in pointing out that the patient numbers do not add‐up from the two studies for the outcome "preference".
The numbers in the results and meta‐view are correct as they stand but unfortunately an incorrect reference was entered for one of the studies. Anderson 1982 should read as Alexander 1980.
In addition, the total number of patients in the results section should read as 50 (cross‐over) not 100.
Once again thank you for your interest in our review and we apologise for the incorrect study reference. We will correct this error for the next issue of the Cochrane library.
Sincerely
Felix Ram
Contributors
Brozek J.
What's new
| Date | Event | Description |
|---|---|---|
| 5 June 2014 | Amended | PLS title amended |
History
Protocol first published: Issue 3, 1997 Review first published: Issue 4, 2002
| Date | Event | Description |
|---|---|---|
| 26 September 2008 | Amended | Converted to new review format. |
| 17 April 2002 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The author's would like to thank the members of the Cochrane Airways Group based at St George's Hospital Medical School, University of London and Kirsty Olsen who has copy edited this review.
Data and analyses
Comparison 1. Theophylline versus Placebo ‐ crossover studies.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Forced Expiratory Volume in one second | 14 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 FEV1 (litres) | 13 | 488 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [0.04, 0.16] |
| 1.2 FEV1 (% predicted) | 3 | 212 | Mean Difference (IV, Fixed, 95% CI) | 3.04 [‐0.09, 6.18] |
| 2 Forced Vital Capacity | 13 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 FVC (litres) | 11 | 392 | Mean Difference (IV, Fixed, 95% CI) | 0.21 [0.10, 0.32] |
| 2.2 FVC (% predicted) | 3 | 212 | Mean Difference (IV, Fixed, 95% CI) | 3.93 [0.22, 7.65] |
| 3 Slow Vital Capacity (liters) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
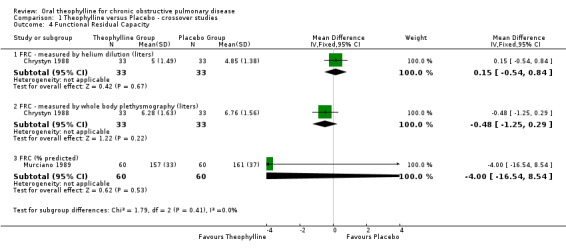
| 4 Functional Residual Capacity | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 FRC ‐ measured by helium dilution (liters) | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.54, 0.84] |
| 4.2 FRC ‐ measured by whole body plethysmography (liters) | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐1.25, 0.29] |
| 4.3 FRC (% predicted) | 1 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐16.54, 8.54] |
| 5 Peak Expiratory Flow Rate (liters/min) | 5 | 196 | Mean Difference (IV, Fixed, 95% CI) | 14.82 [‐9.39, 39.04] |
| 6 Total Lung Capacity | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 TLC ‐ measured by helium dilution (liters) | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | 0.52 [‐0.25, 1.29] |
| 6.2 TLC ‐ measured by whole body plethysmography (liters) | 1 | 66 | Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐1.03, 0.51] |
| 7 Residual Volume (litres) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8 Trapped gas volume (liters) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9 VO2max ‐maximum oxygen consumption (ml/min) | 2 | 64 | Mean Difference (IV, Fixed, 95% CI) | 195.27 [112.71, 277.83] |
| 10 PaO2 mmHg (arterial oxygen tension at rest) | 6 | 312 | Mean Difference (IV, Fixed, 95% CI) | 3.18 [1.23, 5.13] |
| 11 PaCO2 mmHg (arterial carbon dioxide tension at rest) | 6 | 312 | Mean Difference (IV, Fixed, 95% CI) | ‐2.36 [‐3.52, ‐1.21] |
| 12 SaO2 (oxygen saturation at rest %) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 13 Distance walked (m) | 4 | 159 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.01, 0.62] |
| 13.1 6MWD | 2 | 115 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.25 [‐0.11, 0.62] |
| 13.2 12MWD | 2 | 44 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.43 [‐0.17, 1.03] |
| 14 VAS (mm) 0 = extremely breathlessness, 100 = not at all breathless | 2 | 54 | Mean Difference (IV, Fixed, 95% CI) | 3.61 [‐4.62, 11.84] |
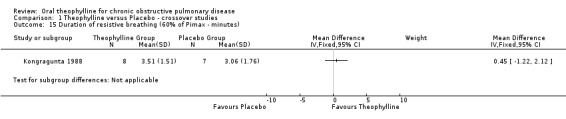
| 15 Duration of resistive breathing (60% of Pimax ‐ minutes) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 16 Exercise challenge | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 16.1 Cycle endurane testing (minutes) | 1 | 14 | Mean Difference (IV, Fixed, 95% CI) | 1.03 [‐2.77, 4.83] |
| 16.2 Progressive Cycle Ergometry (PCE) (min) | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.82, 0.82] |
| 17 Heart rate (bpm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18 Symptoms | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 18.1 Wheezing (simple scales) | 2 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.58, 0.19] |
| 18.2 Shortness of breath (simple scales) | 2 | 42 | Mean Difference (IV, Fixed, 95% CI) | ‐0.32 [‐0.89, 0.25] |
| 19 PC20 (mg/ml) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 20 Acute exacerbations | 2 | 90 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.10, 1.14] |
| 21 Patient preference for theophylline or placebo | 2 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.27 [1.26, 4.11] |
| 22 Drop‐outs | 3 | 148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.76] |
| 23 Adverse effects | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 23.1 Nausea | 3 | 78 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.67 [1.47, 39.94] |
| 23.2 Insomnia | 1 | 34 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.65] |
| 23.3 Dyspepsia | 1 | 66 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.38] |
| 23.4 Headaches | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 67.06] |
1.1. Analysis.
Comparison 1 Theophylline versus Placebo ‐ crossover studies, Outcome 1 Forced Expiratory Volume in one second.
1.2. Analysis.
Comparison 1 Theophylline versus Placebo ‐ crossover studies, Outcome 2 Forced Vital Capacity.
1.3. Analysis.
Comparison 1 Theophylline versus Placebo ‐ crossover studies, Outcome 3 Slow Vital Capacity (liters).
1.4. Analysis.
Comparison 1 Theophylline versus Placebo ‐ crossover studies, Outcome 4 Functional Residual Capacity.
1.5. Analysis.
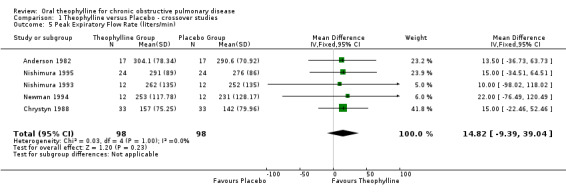
Comparison 1 Theophylline versus Placebo ‐ crossover studies, Outcome 5 Peak Expiratory Flow Rate (liters/min).
1.6. Analysis.
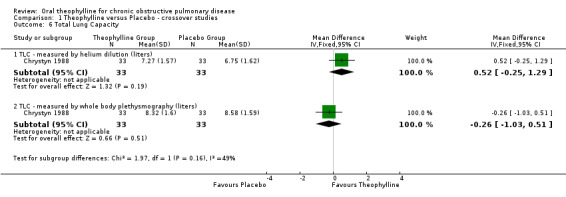
Comparison 1 Theophylline versus Placebo ‐ crossover studies, Outcome 6 Total Lung Capacity.
1.7. Analysis.
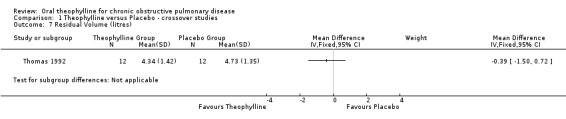
Comparison 1 Theophylline versus Placebo ‐ crossover studies, Outcome 7 Residual Volume (litres).
1.8. Analysis.
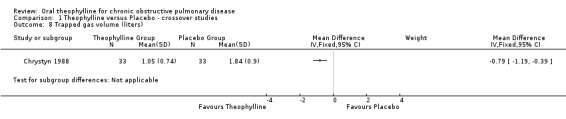
Comparison 1 Theophylline versus Placebo ‐ crossover studies, Outcome 8 Trapped gas volume (liters).
1.9. Analysis.
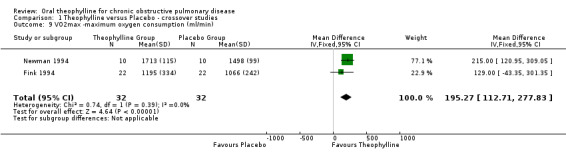
Comparison 1 Theophylline versus Placebo ‐ crossover studies, Outcome 9 VO2max ‐maximum oxygen consumption (ml/min).
1.10. Analysis.
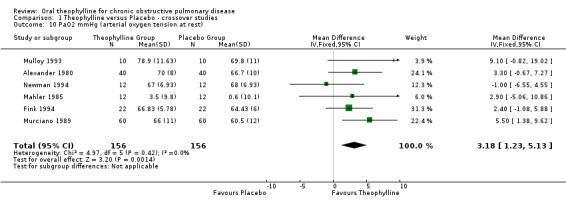
Comparison 1 Theophylline versus Placebo ‐ crossover studies, Outcome 10 PaO2 mmHg (arterial oxygen tension at rest).
1.11. Analysis.
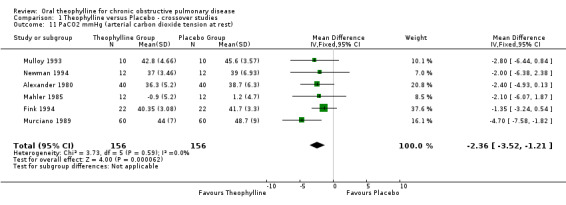
Comparison 1 Theophylline versus Placebo ‐ crossover studies, Outcome 11 PaCO2 mmHg (arterial carbon dioxide tension at rest).
1.12. Analysis.
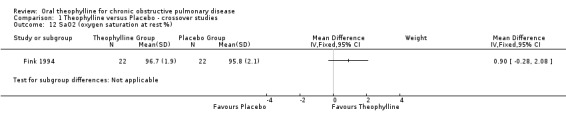
Comparison 1 Theophylline versus Placebo ‐ crossover studies, Outcome 12 SaO2 (oxygen saturation at rest %).
1.13. Analysis.
Comparison 1 Theophylline versus Placebo ‐ crossover studies, Outcome 13 Distance walked (m).
1.14. Analysis.
Comparison 1 Theophylline versus Placebo ‐ crossover studies, Outcome 14 VAS (mm) 0 = extremely breathlessness, 100 = not at all breathless.
1.15. Analysis.
Comparison 1 Theophylline versus Placebo ‐ crossover studies, Outcome 15 Duration of resistive breathing (60% of Pimax ‐ minutes).
1.16. Analysis.
Comparison 1 Theophylline versus Placebo ‐ crossover studies, Outcome 16 Exercise challenge.
1.17. Analysis.
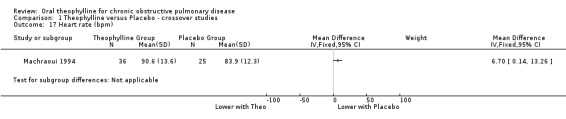
Comparison 1 Theophylline versus Placebo ‐ crossover studies, Outcome 17 Heart rate (bpm).
1.18. Analysis.
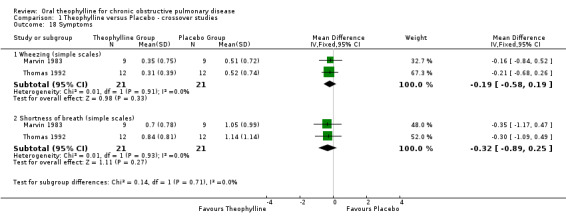
Comparison 1 Theophylline versus Placebo ‐ crossover studies, Outcome 18 Symptoms.
1.19. Analysis.
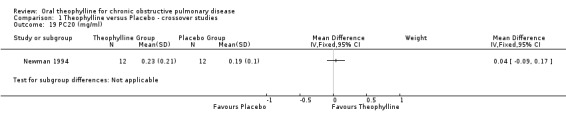
Comparison 1 Theophylline versus Placebo ‐ crossover studies, Outcome 19 PC20 (mg/ml).
1.20. Analysis.
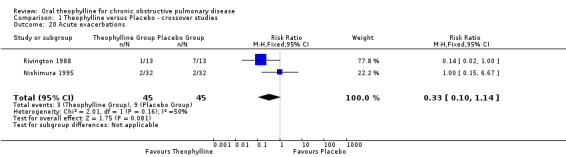
Comparison 1 Theophylline versus Placebo ‐ crossover studies, Outcome 20 Acute exacerbations.
1.21. Analysis.
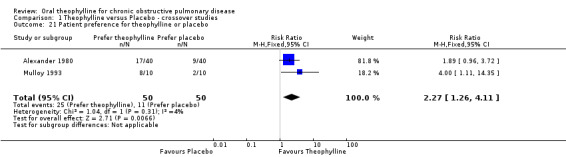
Comparison 1 Theophylline versus Placebo ‐ crossover studies, Outcome 21 Patient preference for theophylline or placebo.
1.22. Analysis.
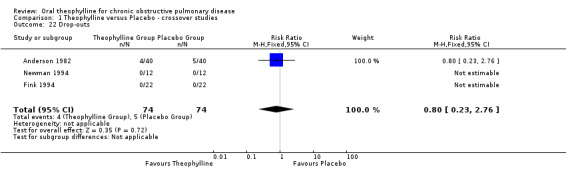
Comparison 1 Theophylline versus Placebo ‐ crossover studies, Outcome 22 Drop‐outs.
1.23. Analysis.
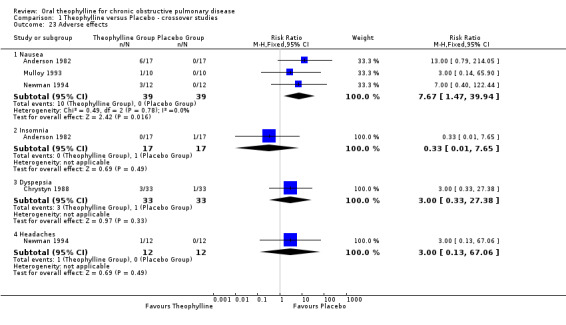
Comparison 1 Theophylline versus Placebo ‐ crossover studies, Outcome 23 Adverse effects.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alexander 1980.
| Methods | 1) Randomisation: not described. 2) Allocation concealment: not described. 3) Masking: double blind, identical looking placebo theophylline tablets were used. 4) Withdraws / drop‐outs: 13 drop‐outs. 5 placebo group and 4 theophylline group did not finish both study periods and are not included in the data analysis. 2 from each drug regimen dropped out as a result of acute respiratory distress that was attributed to pulmonary infection. 5) Duration of intervention: 4 weeks. 6) Design: crossover groups. 7) Jadad quality score: 3 8) Location: Iowa, USA | |
| Participants | 1) Inclusion criteria: Outpatients with moderate to severe COPD, stable, FEV1 less than 60 % of predicted, all had smoking histories of greater than 20 pack‐years. 2) Definition of COPD: FEV1 at least less than 60% of predicted and a chronic, steadily declinig FEV1 and forced expiratory flow during the middle half of the forced vital capacity (FEF 25% to 75%). 3)Type of exercise test: None. 4) Definition of stable COPD: not described. 5) Age: Mean = 59.3. 6) FEV1: not described. 7) Number of patients: 53 men. Drop‐out: 13. Final number of patients: 40. 8) Baseline therapy: Bronchodilators and corticosteroids were not allowed during the study, but other maintence medications were continued. 9) Exclusion criteria: Non‐compliant patients (identified by pill counts and by observing serum theophylline concentration during the dose titration period, history of asthma, sputum and peripheral blood eosinophilia or another known cause for pulmonary insufficiency, fluctuation results in pulmonary function tests during a period of several years. | |
| Interventions | THEOPHYLLINE GROUP (n = 40, completed the study)
1) Drug: Theophylline.
2) Short or long action: Short‐action
3) Dose: 100 mg four times daily
4) Washout period: not mentioned.
5) Theophylline blood level: during titration period to establish steady‐state (10 to 20 ug/ml) theophylline serum levels (1.5 to 2.0 hours after the second dose of the day) and on the day of entry into the trial, and on the final day of each treatment period. Mean serum theophylline level during active therapy was 15.1 (SD 4.22) ug/ml. PLACEBO GROUP ( n = 40, completed the study ). |
|
| Outcomes | 1) Pulmonary function tests (FVC (L), FVC (% pred), FEV1 (L), FEV1 (% Pred). 2) Resting arterial blood gases 3) Maximal voluntary ventilation. MVV (maximum voluntary ventilation (L), MVV (% pred). 4) Diary questionnaire, about pulmonary symptoms. | |
| Notes | 1) Intention‐to‐treat analyses: No 2) Sample size and statistical power: not reported. 3) Representativity: not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Anderson 1982.
| Methods | 1) Randomisation: not described. 2) Allocation concealment: not described. 3) Masking: Double blind, placebo was used and had the same appearance as theophylline tablets. 4) Withdraw / Drop‐out: 4 withdraws, (one because of a confusional state attributed to alcohol withdrawal and 3 because of side effects ‐ nightmares in one, headache in one and nausea and headache in one. (4/17 = 23%). 5) Duration of intervention: 3 periods of 8 days each, with one week washout. 6) Design: Cross‐over groups. 7) Jadad quality Score: 3 8) Location: Newport, UK | |
| Participants | 1) Inclusion criteria: patients that fulfilled the Medical Research Council criteria for chronic bronchitis, all patients were ex‐smokers. 2) Definition of COPD: The Medical Research Council criteria for chronic bronchitis (MRC, 1965). 3)Type of exercise test: none. 4) Definition of stable COPD: not described. 5) Age: Mean = 58, (range 34 to 70 years). 6) FEV1: Mean = 1.34, SD 0.122 (L). 7) Number of patients: 21 (17 men and 4 women). 8) Baseline therapy: corticosteroids were not allowed, but other maintence medications were permitted including inhaled bronchodilators. 9) Exclusion criteria: bronchial asthma. | |
| Interventions | THEOPHYLLINE GROUP ( n = 21, completed the study ).
1) Drug: Theophylline (Nuelin SA)
2) Short or long action: Long‐action (Nuelin SA)
3) Dose: 350 mg daily theophylline for 4 days followed by 700 mg daily for four days with matching placebo for 8 days with one week washout period between crossover.
4) Washout period: 1 week.
5) Theophylline blood level: measured on the last day of each study period. The mean daily dose of theophylline for the whole group was 9.2 mg/Kg range 6‐13.2 mg/Kg. PLACEBO GROUP: n = 21 completed the study. |
|
| Outcomes | 1) Pulmonary function tests PEFR, (FVC (L), FVC (% pred), FEV1 (L), FEV1 (% Pred). 2) Symptom scores 3) Side effects: 6 patients had nausea or headache in theophylline group. One patient had insomnia during the placebo week. | |
| Notes | 1) Intention‐to‐treat analyses: No. 2) Sample size and statistical power: not reported. 3) Representativity: not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Chrystyn 1988.
| Methods | 1) Randomisation: patients were randomly allocated to the order of treatment by four identical Latin squares. 2) Allocation concealment: Not described. 3) Masking: The dose changes were effected in a single blind manner with matching placebo tablets. 4) Withdraw/Drop‐out: 5 drop‐out /38 (13,16%). 5) Duration of intervention: 2 months. 4 consecutive two month treatment periods (total = 8 months). Four periods: placebo, low, medium and high theophylline serum concentrations. 6) Design: Crossover groups. 7) Jadad uality score: 2 8) Location: West Yorkshire, UK | |
| Participants | 1) Inclusion criteria:COPD, moderate to severe obstruction of airflow. 2) Definition of COPD: Medical Research Council definition. 3)Type of exercise test: Six min waking test 4) Definition of stable COPD: not defined. But no patients were recruited who had an acute exacerbation in the previous 2 weeks. 5) Age: Mean = 61.2, SD 5.71 years (range 53 to 73) 6) FEV1: Mean = 29.1, SD 12.4 (% predc) 7) Initial total number of patients: 38. Drop‐out: 5. Final patients number: 33 ( 30 men and 3 women). 8) Baseline therapy: Inhalation and oral corticosteroids and other bronchodilators were continued. 9) Exclusion criteria:Asthma or allergy, > = 15% improvement in FEV1 20 min after inhaling 500 ug terbutaline sulphate, known sensitivity to methylxanthine, severe cardiac disease or other disease that might interfere with exercise testing. | |
| Interventions | THEOPHYLLINE 3 GROUP‐ low dose ( n = 19), medium dose (n = 12), high dose (n = 10), completed the study.
1) Drug: Theophylline
2) Short or long action: Long‐action
3) Dose: 3 doses used; low (5 to 10 mg/l), medium (10 to 15 mg/l) and high (15 to 20 mg/l). Used the medium dose only for the review as it is clinically the most relevant dose resulting in therapeutic plasma levels.
4) Washout period: not mentioned.
5) Theophylline blood level: measured at the end of each two month period. PLACEBO GROUP ( n = 22 ) |
|
| Outcomes | 1) Pulmonary function tests (FEV1), (FVC), (SVC) (L), TLC measured by helium dilution (L), FRC measured by helium dilution (L), TLC measured by whole body plesthymography (L), FRC measured by whole body plesthymography (L) and trapped gas volume (L). 2) Exercise testing : six minute walking test 3) Visual analogue scale for dyspnoea (10 cm) 4) Peak expiratory flow (L/min.) 5) Side effects: nausea, insomnia, dyspepsia, headache, cramp, tremor (theophylline group) | |
| Notes | 1) Intention‐to‐treat analyses: Yes 2) Sample size and statistical power: not reported. 3) Representativity: not specified | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Dullinger 1986.
| Methods | 1) Randomisation: not described (block design). 2) Allocation concealment: not described. 3) Masking: Double blind, placebo tables were identical in appearance to theophylline tablets. 4) Withdraw/Drop‐out: none. 5) Duration of intervention: 1 x 4 week as 4 different combinations of treatments were tested. 6) Design: Cross‐over group 7) Jadad quality score: 3 8) Location: Minneapolis, USA | |
| Participants | 1) Inclusion criteria: COPD, outpatients. 2) Definition of COPD: FEV1 less than 1.5 L and FEV1/FVC of less than 60%. 3)Type of exercise test: 12 minute walk test 4) Definition of stable COPD: not described. 5) Age: Mean = 61 years ( range 53 to 72 years). 6) FEV1: Mean = 2.63 L (range 1.69 to 3.74) 7) Number of patients: 10 men. withdraw: 0 8) Baseline therapy: Not described. 9) Exclusion criteria: presence of significant chest abnormalities on x‐ray, asthma, history of atopy, sputum or blood eosinophilia, absence of long‐term smoking, frequent episodic attacks of wheeziness, regular use of corticosteroids, FEV1 response to an inhaled beta‐agonist greater than 25% of baseline or co‐existing disease which might interfere with exercise testing. | |
| Interventions | THEOPHYLLINE GROUP VERSUS PLACEBO ( n = 10, completed the study) 1) Drug: Theophylline (Theo‐Dur, Key Pharmaceuticals) 2) Short or long action: Long‐action 3) Dose: twice daily in amounts to provide average plasma concentrations of 10 to 15 ug/ml. 4) Washout period: not mentioned. 5) Theophylline blood level: measured on the first day of the initial baseline study and at day 6 of each period. | |
| Outcomes | 1) Pulmonary function tests: FVC (L), FEV1 (L). 2) Exercise testing (12 minute walking test and Incremental cycle ergometry test) 3) Dyspnea (oxygen cost diagram ‐ OCD and breathlessness rating ‐ BR) 4) Side effects: No reports of adverse effects from any of the treatments. | |
| Notes | 1) Intention‐to‐treat analyses: Yes 2) Sample size and statistical power: not reported. 3) Representativity: not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Fink 1994.
| Methods | 1) Randomisation: not described. 2) Allocation concealment: not described. 3) Masking: Double blind, using identical placebo tablets to active treatment. 4) Withdraw/Drop‐out: none. 5) Duration of intervention: 1 month. 6) Design: Cross‐over groups 7) Jadad quality Score: 2 8) Location: Tel Aviv, Israel | |
| Participants | 1) Inclusion criteria:COPD, severe, stable, smokers or former smokers. 2) Definition of COPD: FEV1 less than 50% of predicted. 3)Type of exercise test: Incremental cycle‐ergometer. 4) Definition of stable COPD: not described. 5) Age: Mean = 68.5, SD 3.3 years 6) FEV1: Mean = 38.3, SD 8.6 (% pred) and Mean = 1.06, SD 2.8 (L) 7) Number of patients: 22 ( 17 men and 5 women), withdraw: 0 8) Baseline therapy: Inhalation corticosteroids and other bronchodilators. 9) Exclusion criteria: known cardiac disease or cardiac disorders shown on the baseline incremental exercise test. | |
| Interventions | THEOPHYLLINE GROUP (n = 22, completed the study)
1) Drug: Theophylline (Theotrim, Trima Lab., Israel)
2) Short or long action: Long‐action
3) Dose: 300 mg theophylline (twice daily). Patients weighing less than 60 Kg took 200 mg twice per day.
4) Washout period: 2 weeks.
5) Theophylline blood level: measured on the day of the initial baseline study and after the first week of treatment. The dose was adjusted if the level was below 55.5umol/l. This adjustment was repeated until all patients had a blood level above 55.5umol/l. PLACEBO GROUP ( n = 22, completed the study ) |
|
| Outcomes | 1) Pulmonary function tests FVC (L), FVC (% pred), FEV1 (L), FEV1 (% pred). 2) Exercise testing (incremental exercise cycle ergometer test ‐ WR, VO2max, HR, respiratory rate and ventilation, VO2max/HR (maximum oxygen pulse), VEmax/MVV (dyspnoea index), anaerobic threshold (VE/VO2slope). 3) Resting arterial blood gases 4) Maximal voluntary ventilation (MVV) (L), MVV (% pred). 5) Side effects: not described. | |
| Notes | 1) Intention‐to‐treat analyses: Yes 2) Sample size and statistical power: not reported. 3) Representativity: not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Guyatt 1987.
| Methods | 1) Randomisation: not described. 2) Allocation concealment not described. 3) Masking: Double blind, placebo tablets were identical in appearance to active treatment. 4) Withdraw/Drop‐out: 8 drop‐outs. 5) Duration of follow up: 4 treatment periods, each of 2 week duration, during which they received the follwing combination: placebo‐placebo, placebo‐salbutamol (inhaler), placebo‐theophylline, and salbutamol (Inhaler)‐theophylline. 6) Design: Crossover group 7) Jadad quality Score: 3 8) Location: Ontario, Canada | |
| Participants | 1) Inclusion criteria: COPD. 2) Definition of COPD: FEV1 less than 70% and FEV1/FVC of less than 0.7. 3) Type of exercise test: 6 minute walk test 4) Definition of stable COPD: change in respiratory medication in the month prior to entry or hospitalisation in the previous 2 months. 5) Age: Mean = 65.3 years, SD 7.4. 6) FEV1: Mean = 1.02 L, SD 0.38. 7) Number of patients: 27 men. Dropout: 8 (1 angina, 1 for surgery to remove a lipoma, 1 found the study too inconvenient, and 1 dropped out after a respiratory tract infection (during salbutamol period). Final number of patients: 19. 8) Baseline therapy: Patients were instructed not to use their own medication under any circumstances and to contact a physician, who was available full‐time. If patients deteriorated, they were seen immediately, all outcome measures were obtained, and the patient was started on the next period's medication without the code being broken. 9) Exclusion criteria: a) inability to tolarate a theophylline level of greater than 12 ug/ml, b) a documented improvement in FEV1 of 25% or more in response to a trial of orally administered steroids, c) a documented improvement in FEV1 of 25% or more after inhaling 200 umg of salbutamol, d) asthma, e) clinical instability, f) use of orally administered or inhaled anticholonergic preparations. | |
| Interventions | THEOPHYLLINE GROUP (n = 19, completed the study)
1) Drug: Theophylline
2) Short or long action: Short‐action
3) Dose: Patients were given a previously titrated dose of theophylline to achieve a level of at least 65, and, if possible, close to 100 mmol/L.
4) Washout period: There was no washout period, but data from the first 3 days of each period was excluded.
5) Theophylline blood level: measured before the start of the study. In each treatment periods (the mean theophylline level during periods of active drug was 12.3 +‐ 2.9 ug/ml. PLACEBO GROUP (n = 19, completed the study) |
|
| Outcomes | 1) Pulmonary function tests (FEV1 and FVC and Peak flow) 2) Exercise testing (6 minute walking test) 3) Dyspnoea (visual analogue scale) 4) Quality of life questionaire 4) Side effects: 3 shortness of breath, 1 gastrointestinal upset (periods had to be terminated before). | |
| Notes | 1) Intention‐to‐treat analyses: Yes 2) Sample size and statistical power: not reported. 3) Representativity: Yes. Patients were recruited of more than 1,000 patients with CAL including all such patients seen in 10 secondary care respirology practices in a metropolitan area of approximately 500,000 people in the previous 2 years. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Iversen 1992.
| Methods | 1) Randomisation: not described. 2) Allocation concealment: not described. 3) Masking: double blind, identical looking placebo theophylline tablets were used. 4) Withdraws / drop‐outs: not mentioned. 5) Duration of intervention:4 weeks. 6) Design: crossover groups. 7) Jadad quality score: 2 8) Location: Hillerod, Denmark | |
| Participants | 1) Inclusion criteria: severe COPD. 2) Definition of COPD: FEV1 was mean 0.99 SD 0.45 L, FVC mean 2.2 SD 0.68 L. 3)Type of exercise test: None. 4) Definition of stable COPD: not described. 5) Age: not reported as abstract only published. 6) FEV1: not described. 7) Number of patients:48 patients. Drop‐out: not reported. 8) Baseline therapy: not reported in abstract. 9) Exclusion criteria: not reported in abstract. | |
| Interventions | THEOPHYLLINE GROUP (n = 48)
1) Drug: Theophylline.
2) Short or long action: Long action, sustained release Theo‐Dur
3) Dose: 300 mg twice daily
4) Washout period: not mentioned.
5) Theophylline blood level: mena 7.1 SD 3.6 mg/l. PLACEBO GROUP ( n = 48) |
|
| Outcomes | 1) Pulmonary function tests. 2) Arterial blood gases 3) Dyspnoea scores 4) Patients 'sense of well‐being' 5) Daily beta‐agonists usage | |
| Notes | As this study was reported as an abstract it was devoid of many details. 1) Intention‐to‐treat analyses: No 2) Sample size and statistical power: not reported. 3) Representativity: not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Kongragunta 1988.
| Methods | 1) Randomisation: not described. 2) Allocation concealment: not described. 3) Masking: Double blind, identical placebo used. 4) Withdraw/Drop‐out: 0 withdraw and 2 drop‐outs (1 in cycle exercise runs and 1 in duration of resistive breathing). 5) Duration of intervention: 3 days of treatment with theophylline (or placebo) to reach therapeutic plasma level than study measurements done. 6) Design: Cross‐over 7) Jadad quality Score: 3 8) Location: Chicago, USA | |
| Participants | 1) Inclusion criteria:moderately severe COPD (mean FEV1 1.08), none hypercapnic. 2) Definition of COPD: not described. 3)Type of exercise test: Incremental cycle‐ergometer exercise testing. 4) Definition of stable COPD: not described. 5) Age: not reported. 6) FEV1: Mean = 1.10 (L), SD (0.30) and Mean = 36.1 (% pred), SD (12.9) 7) Number of patients: 8. withdraw: 0 8) Baseline therapy: All medications were given in their regular dose, no patients were on corticosteroids at the time of the study. 9) Exclusion criteria: not described. | |
| Interventions | THEOPHYLLINE GROUP ( n = 8, completed the study )
1) Drug: Theophylline.
2) Short or long action: Long‐action
3) Dose: not reported but therapeutic levels reach after third dose, mean 12.8 SD 4.4 ug/ml.
4) Washout period: 3 days.
5) Theophylline blood level: mean 12.8 SD 4.4 ug/ml. PLACEBO GROUP ( n=8, completed the study ) |
|
| Outcomes | 1) Maximal transdiaphragmatic pressures(Pdimax) 2) Exercise testing (Endurance) 3) Electromyograms of the quadriceps femoris 4) Inspiratory resistive breathing 5) Duration of resistive breathing runs 6) Changes in respiratory rate and duty cycle 7) Side effects: not described. | |
| Notes | 1) Intention‐to‐treat analyses: yes. 2) Sample size and statistical power: not reported. 3) Representativity: not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Machraoui 1994.
| Methods | 1) Randomisation: not described. 2) Allocation concealment: not described. 3) Masking: double blind, placebo tablets were used. 4) Withdraws / drop‐outs: not mentioned. 5) Duration of intervention:4 days. 6) Design: crossover groups. 7) Jadad quality score: 3 8) Location: Germany | |
| Participants | 1) Inclusion criteria: COPD 2) Definition of COPD: 3)Type of exercise test: None. 4) Definition of stable COPD: not described. 5) Age: 6) FEV1: 7) Number of patients: 25 (18M). Drop‐out: not reported. Final number of patients: not reported. 8) Baseline therapy: all concomitant therapy continued for the duration of the study. 9) Exclusion criteria: Left ventricular disease excluded with history, clinical diagnosis and chest x‐ray. No electrolyte imbalances, dysproteinaemia or liver and kidney dysfunction or acidosis allowed. | |
| Interventions | THEOPHYLLINE GROUP (n = 25)
1) Drug: Theophylline.
2) Short or long action: Short‐action
3) Dose: 400 mg bd for 4 days.
4) Washout period: 3 days
5) Theophylline blood level: done on the 4th and 11 days of the study. PLACEBO GROUP ( n = 25) |
|
| Outcomes | 1) Arterial blood gases 2) ECG 3) Heart rate | |
| Notes | 1) Intention‐to‐treat analyses: No 2) Sample size and statistical power: not reported. 3) Representativity: not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Mahler 1985.
| Methods | 1) Randomisation: not described. 2) Allocation concealment: not described. 3) Masking: Double blind, with placebo identical in appearance to theophylline tablets. 4) Withdraw/Drop‐out: none. 5) Duration of intervention: 2 x 4 weeks 6) Design: Cross‐over groups 7) Jadad quality score: 3 8) Location: West Haven, Connecticut, USA | |
| Participants | 1) Inclusion criteria:COPD, stable, outpatients, at least moderate air‐flow obstruction, less than 65 % of predicted, ability to exercise on an upright bicycle ergometer, and willingness to discontinue all medications for the period of the study. 2) Definition of COPD: FEV1 at least less than 65% of predict, with nonreversible airway obstruction, defined as less than 15% improvement in FEV1 after an inhaled bronchodilator. 3)Type of exercise test: 12 min walking test and submaximal steady state as well as progressive, incremental exercise on the bicycle ergometer. 4) Definition of stable COPD: not described. 5) Age: Mean = 60, SD 7 years 6) FEV1: Mean = 40 (% pred) and Mean= 1.36, SD 0.67 (L) 7) Number of patients: 12 men. withdraw: 0 8) Baseline therapy: Patients were instructed to use only an inhaled bronchodilator for respiratory symptoms. 9) Exclusion criteria: History of asthma or electrocardiographic evidence of coronary artery disease, valvular heart disease, hypertension, or primary myocardial disease. | |
| Interventions | THEOPHYLLINE GROUP (n = 12, completed the study)
1) Drug: Theophylline (Theo‐Dur, Key Pharmaceutical, Inc., Miami, FL)
2) Short or long action: Long‐action
3) Dose: Initial 13 mg/Kg/day in 2 divided doses based on lean body weight.
4) Washout: 2 weeks
5) Theophylline blood level: measured on the fourth day of the study, theophylline blood level measured using HPLC in patients receiving theophylline as well as placebo. If the level was less than 10 um/ml, then the dosage was increased by 200 to 400 mg/day; in those receiving placebo, the dosage was randomly changed on the fourth day in some patients. In all patients who had a change in initial dosage of medication, theophylline level was remeasured on the seventh day. All patients receiving theophylline had a therapeutic blood level (10 to 20 ug/ml). PLACEBO GROUP ( n = 12, completed the study ). |
|
| Outcomes | 1) Pulmonary function tests (FVC (L), TLC (by body plethysmography), DLCO (ml/min/mmHg), FEV1 (L), FEV1 (% Pred). 2) Exercise testing: 12 min walking test (incremental and endurance exercise cycle ergometer test ‐ WR, VO2max, HR, respiratory rate and ventilation, VO2max/HR, VEmax/MVV, anaerobic threshold (VE/VO2slope). 3) Resting arterial blood gases 4) Dyspnea index 5) Side effects: none occured. All patients tolerated the theophylline and placebo medications without adverse effects. | |
| Notes | 1) Intention‐to‐treat analyses: Yes 2) Sample size and statistical power: not reported. 3) Representativity: yes. 20 patients with COPD were selected from outpatient clinics. After specific tests 12 patients were selected for the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Marvin 1983.
| Methods | 1) Randomisation: not described 2) Allocation concealment: coded in the pharmacy. 3) Masking: Double blind, placebo controlled. The code was not broken under any circumstances until completion of the entire study. 4) Withdraw/Drop‐out: none. 5) Duration of intervention: 10 days (four arms tothe study therefore 10 days x 4). 6) Design: Cross‐over 7) Jadad quality score: 3 8) Location: Little Rock, USA | |
| Participants | 1) Inclusion criteria:COPD severe outpatients, clinically stable, smoking history of greater than 20 pack‐years. 2) Definition of COPD: FEV1 less than 50% of predicted. 3) Type of exercise test: Incremental cycleergometer and steady‐state exercise testing 4) Definition of stable COPD: not refered. 5) Age: Mean = (?) range 50 to 69 years 6) FEV1: Mean = 1.03, SD 0.34 (L) 7) Number of patients: 15 men. withdraw: 0 8) Baseline therapy: All medications with cardiopulmonary effect were discontinued for 72 hours. 9) Exclusion criteria: Asthma, presence of greater than 5% eosinophils on peripheral blood smear, primary cardiovascular disease (angina, systemic hypertension, ventricular arrhythmia), evidence of left ventricular decompensation, other complicating systemic illness. | |
| Interventions | THEOPHYLLINE GROUP (n = 15, completed the study)
1) Drug: Theophylline (Elixophylline)
2) Short or long action: Short‐action
3) Dose: 200 mg theophylline (four times daily) for 10 days.
4) Washout: not reported.
5) Theophylline blood level: measured before and after 10 days of theophylline treatment. The dose was not adjusted. PLACEBO GROUP ( n = 15, completed the study ) |
|
| Outcomes | 1) Compliance with medication 2) Exercise testing (steady‐state exercise (60% of the maximal work load during 6 minutes) 3) Shortness of breath: using a simple graded scale (absent = 0, mild = 1, moderate = 2, severe = 3). 4) Wheezing: same simple scale as shortness of breath (above). 5) Side effects: 'intolerance' in theophylline group (only one patient) 6) Lung function | |
| Notes | 1) Intention‐to‐treat analyses: Yes 2) Sample size and statistical power: not reported. 3) Representativity: not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Mulloy 1993.
| Methods | 1) Randomisation: not specified. 2) Allocation concealment: The treatment packs containing medications were sealed in advance. 3) Masking: Double blind, placebo controlled. 4) Withdraw/Drop‐out: 2/12 (17%). 5) Duration of intervention: 3 weeks x 2 (first week dose adjustments followed by 2 weeks of treatment). 6) Design: Crossover group. 7) Jadad quality score: 3 8) Location: Dublin, Ireland | |
| Participants | 1) Inclusion criteria:COPD, clinically stable. 2) Definition of COPD: FEV1 less than 50% of predicted, with less than 15% improvement in FEV1 2O min. after 400 ug of salbutamol via inhaler. 3)Type of exercise test: Incremental treadmill exercise testing 4) Definition of stable COPD: Yes, (none had exacerbation of the COPD for at least 6 wk before entry into the study. 5) Age: (range 51 to 84). Mean = 4.2, SD 5.39 6) FEV1: 0.74 to 1.39 (range 20 to 47%). Mean = 0.91, SD 0.21 7) Number of patients: 12 ( 9 men and 1 woman). 2 drop‐out. Final number of patients: 10. 8) Baseline therapy: Predinisone, duovent inhaler, beclomethasone inhaler, ipratropium inhaler, salbutamol inhaler, theophylline. 9) Exclusion criteria: history of cardiac (other than cor pulmonale) or hepatic disease. Taking hypnotics, sedatives, or any medication known to interfere with the metabolism or absorption of theophylline, smokers, asthma, blood eosinophilia. | |
| Interventions | THEOPHYLLINE GROUP (n=10, completed the study)
1) Drug: Theophylline (Napp Lab, Cambridge)
2) Short or long action: Long‐action
3) Dose: 400 mg theophylline bd (10:00 AM and 10 PM). Patients weighing less than 70 kg took 300 mg bd.
4) Washout: 1 week, when dose adjustments were made for both the active and placebo arms.
5) Theophylline blood level: measured during the first week in baseline period and during the subsequent week in both active and placebo groups. The dose was adjusted in a blinded fashion in those on active treatment. Adjustments were made until all patients had a blood level between 10 and 20 mg/l. PLACEBO GROUP (n = 10, completed the study). |
|
| Outcomes | 1) Pulmonary function tests 2) Incremental exercise testing 3) Resting arterial blood gases and oxygen saturation. 4) Sleep alterations 5) Side effects: Nausea, only one patient (this patient had not taken theophylline before as the other 9 had). | |
| Notes | 1) Intention‐to‐treat analyses: No. 2) Sample size and statistical power: not reported. 3) Representativity: Yes. Randomised outpatients. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Murciano 1989.
| Methods | 1) Randomisation: not described. 2) Allocation concealment: not described. 3) Masking: Double blind, placebo identical to theophylline in appearance. 4) Withdraw/Drop‐out: none. 5) Duration of follow up: 2 months. (two months of placebo and two months of treatment + 8‐day washout period between periods). 6) Design: Cross‐over group design. 7) Jadad quality score: 3 8) Location: Clichy, France | |
| Participants | 1) Inclusion criteria:COPD, clinically stable. Smokers (15/ 60 ‐ 25%), former smokers (43/ 60 ‐ 72%) and nonsmokers (2/60 ‐ 3%). 2) Definition of COPD: a change in FEV1 of less 15% after the administration of 400ug albuterol. 3)Type of exercise test: none. 4) Definition of stable COPD: not described. 5) Age: Mean = 61, SD 8 years 6) FEV1: Mean = 31.6, SD 12.5 (% pred) 7) Number of patients: 60. 8) Baseline therapy: No inhalation corticosteroids or other bronchodilators. Antibiotics only when infection was evidenced by an increased production of purulent sputum. Oxygen therapy (13 patients) was maintained at an identical flow and duration throughout the study. 9) Exclusion criteria: not reported. | |
| Interventions | THEOPHYLLINE GROUP (n = 60, completed the study)
1) Drug: Theophylline (Theostat, Theoplus, Sinbio Laboratories, Paris).
2) Short or long action: Long‐action
3) Dose: 10 mg per Kg of body weight per day of theophylline (twice daily).
4) Washout: 8 days.
5) Theophylline blood level: measured after one week in each treatment period with use of HPLC, and the results were reviewed by an independent observer. If necessary, the dose of theophylline was adjusted to obtain a plasma level of 10‐20 mg/l. PLACEBO GROUP (n = 60, completed the study) |
|
| Outcomes | 1) Pulmonary function tests (% of predicted), FEV1(% of predicted), FRC (% of predicted), Tidal volume (L). 2) Minute ventilation (L/min) 3) Airway resistence (cm of H2O/L/sec), 2) Resting arterial blood gases 3) Respiratory‐Muscle Performance ( Ppl, Ppl max, Ppl/Ppl max) 4) Dyspnea (visual ‐ analogue scale) 5) Side effects: not reported. 6) Respiratory Rate (breaths/min). | |
| Notes | 1) Intention‐to‐treat analyses: Yes 2) Sample size and statistical power: not reported. 3) Representativity: not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Newman 1994.
| Methods | 1) Randomisation: not described. 2) Allocation concealment: not described. 3) Masking: Double blind, placebo controlled. 4) Withdraw/Drop‐out: none. 5) Duration of intervention: 2 x 4 weeks. 6) Design: Cross‐over groups 7) Jadad quality score: 2 8) Location: Jerusalem, Israel | |
| Participants | 1) Inclusion criteria: COPD moderate to severe. 2) Definition of COPD: FEV1 25‐60% of predict, PaO2 >= 55mmHg and PCO2 < = 50mmHg. 3) Type of exercise test: Incremental cycle‐ergometer exercise testing 4) Definition of stable COPD: not described. 5) Age: Mean = 62.4, SD 5.6 years 6) FEV1: Mean = 43.4, SD 10.7 (% pred) and Mean = 1.15, SD 0.3 (L) 7) Number of patients: 12 (11 men and 1 women). withdraw: 0. 15 met the inclusion criteria but only 12 wanted to participate in the study. 8) Baseline therapy: Inhaled bronchodilators were allowed only to relieve symptoms, and the doses consumed was recorded. 9) Exclusion criteria: Asthma, blood eosinophils of > 500 cell/mm3, more severe COPD or symptomatic coronary artery or cerebrovascular disease. | |
| Interventions | THEOPHYLLINE GROUP (n = 12, completed the study)
1) Drug: Theophylline (Theotrim, Trima Lab., Israel)
2) Short or long action: Long‐action
3) Dose: was titrated to achieve blood levels of 10 to 20 mg/l.
4) Washout: not reported.
5) Theophylline blood level: measured 2 to 3 h after the morning dose of 10 to 20 mg/L at 2 to 3 h after morning dose. PLACEBO GROUP (n = 12, completed the study) |
|
| Outcomes | 1) Pulmonary function tests (FEV1 (L), FVC (L), flow volume loop). 2) Peak flow (L/min.) 3) Exercise testing: Incremental cycle ergometer test (VO2max (ml/min.), AT=anaerobic threshold (ml VO2/min., HR max (beats/min), VO2/HR max (ml/beat), VE max (L/min), exercise BR (L/min), R max) 4) Resting arterial blood gases 5) Maximal voluntary ventilation 6) PC20 (provocative concentration of inhalent needed to induce 20% fall in FEV1) 7) Neuropsychological evaluation tests (memory, attention, concentration) 8) Side effects: nausea, headache, loss of recent memory. | |
| Notes | 1) Intention‐to‐treat analyses: Yes. 2) Sample size and statistical power: not reported. 3) Representativity: not specified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Nishimura 1993.
| Methods | 1) Randomisation: not described. 2) Allocation concealment: not described. 3) Masking: Double blind, placebo used which was identical to active treatment. 4) Withdraw/Drop‐out: none. 5) Duration of intervention: 2 x 4 weeks. 6) Design: Cross‐over groups 7) Jadad quality score: 3 8) Location: Kyoto, Japan | |
| Participants | 1) Inclusion criteria: COPD moderate to severe. 2) Definition of COPD: FEV1 < 60% predicted, FEV1/FVC < 0.7, aged between 50 to 75 yrs. 3)Type of exercise test: none 4) Definition of stable COPD: no exacerbation in the preceeding 3 months, no treatment with inhaled or systematic steroids in the preceeding 2 weeks. 5) Age: Mean = 64.3, SD 5.9 yrs 6) FEV1: Mean = 0.92, SD 0.38 (L) and Mean % predicted = 35.3, SD 14.0. 7) Number of patients: 12 (11 men and 1 women). withdraw: 1. 8) Baseline therapy: both Inhaled salbutamol (400ug) and ipratropium bromide (40ug) were allowed qid. 9) Exclusion criteria: asthma, heart disease or any other illness. | |
| Interventions | THEOPHYLLINE GROUP (n = 12, completed the study)
1) Drug: Theophylline (Slow‐bid, RPR, Japan)
2) Short or long action: Long‐action
3) Dose: 400mg for 2 weeks, followed by 600mg for another 2 weeks.
4) Washout: not reported.
5) Theophylline blood level: ?? PLACEBO GROUP (n=12, completed the study) |
|
| Outcomes | 1) Pulmonary function tests (FEV1 (L), FVC (L). 2) Peak flow (L/min.) 3) Daily symptoms of cough, sputum, wheeze, and dyspnoea based on a smple scale of 1 to 4 (1 best, 4 worst) 4) Side effects: nausea, gastrointestinal effects. | |
| Notes | 1) Intention‐to‐treat analyses: No. 2) Sample size and statistical power: not reported. 3) Representativity: not specified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Nishimura 1995.
| Methods | 1) Randomistion: not described. 2) Allocation concealment: not described. 3) Masking: Double blind with matching placebo. 4) Withdraw/Drop‐out: 7 drop‐outs. One patient because he complained of urinary tract infection. Four patients because of exacerbation due to respiratory tract infection: Two during the theophylline period and two during the placebo period. Two in theophylline group because of gastrointestinal side effects. One patient in the theophylline group did not have theophylline level detected, he was excluded from the data analysis. Withdraw: 1. 5) Duration intervention: 4 weeks. 6) Design: cross‐over groups 7) Jadad quality score: 2 8) Location: Kyoto, Japan | |
| Participants | 1) Inclusion criteria:COPD, stable, age older 55 years, history of cigarette smoking of more than 20 pack‐years, chest radiograph showing hyperinflation with or without a vascular deficiency pattern suggestive of pulmonary emphysema, smokers, former smokers, a best postbronchodilator ratio of the FEV1 to FVC of less than 70%. 2) Definition of COPD: FEV1 of less than 80% of the predicted value, according to the American Thoracic Society. 3)Type of exercise test: none. 4) Definition of stable COPD: no acute exacerbation of airflow obstruction within the preceding 3 months. 5) Age: Mean = 63.3, SD 4.7 years, range 55 to 73. 6) FEV1: Mean = 36.8, SD 17 (% pred) and Mean = 0.96, SD 0.43 (L) 7) Number of patients: 32 men. Drop‐out: 7. Withdraw: 1. Final number of patients: 24. 8) Baseline therapy: salbutamol 400 ug and ipratropium bromide 80 ug qid. 9) Exclusion criteria: any history suggestive of asthma, heart disease, or any other illness. Patients treated with inhaled or systemic steroids in the preceding 3 weeks were also excluded. | |
| Interventions | THEOPHYLLINE GROUP (n = 24, completed the study)
1) Drug: Theophylline
2) Short or long action: Long‐action
3) Dose: the daily doses of theophylline were predetermined to provide average serum concentration of more than 10 ug/ml.
4) Washout: not reported.
5) Theophylline blood level: was measured by fluorescence polarization immunoassay. The moment of the measured during the study and adjusted dose criteria was not described. PLACEBO GROUP (n = 24, completed the study ) |
|
| Outcomes | 1) Pulmonary function tests by body plethysmography (FVC (L), FVC (% pred), FEV1 (L), FEV1 (% Pred), 2) Peak expiratory flow rate. 3) Symptoms of cough, sputum, wheezing and shortness of breath, rated on a simple scale of one to four. 4) Side effects: 2 patents had gastrointestinal side effects in the theophylline group. | |
| Notes | 1) Intention‐to‐treat analyses: No 2) Sample size and statistical power: not reported. 3) Representativity: not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Power 1992.
| Methods | ABSTRACT ONLY 1) Randomisation: not described. 2) Allocation concealment: not described. 3) Masking: Single blind, placebo controlled. 4) Withdraw/Drop‐out: not reported. 5) Duration of intervention: 2 x 4 weeks. 6) Design: Cross‐over groups 7) Jadad quality score: 1 8) Location: Dublin, Ireland | |
| Participants | 1) Inclusion criteria: COPD patients who did not demonstrate a bronchodilator response 'irreversible'. 2) Definition of COPD: not reported. 3) Type of exercise test: none 4) Definition of stable COPD: not described. 5) Age: Mean = 67.4, SD 6.6 years 6) FEV1: Mean = 1.06, SD 0.53 (L), FVC 2.44, SD 0.88 (L). 7) Number of patients: 37 8) Baseline therapy: not reported. 9) Exclusion criteria: not reported. | |
| Interventions | THEOPHYLLINE GROUP (n = 37)
1) Drug: Theophylline
2) Short or long action: Long‐action
3) Dose: not reported but dosing was bd.
4) Washout: not reported.
5) Theophylline blood level: not reported. PLACEBO GROUP (n = 37) |
|
| Outcomes | 1) Spirometry 2) Peak flow (L/min.) 3) Symptom scores 4) 6 MW distance 5) Quality of life: CRDQ & Nottingham Health Profile Index). | |
| Notes | 1) Intention‐to‐treat analyses: not reported. 2) Sample size and statistical power: not reported. 3) Representativity: not specified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
Rivington 1988.
| Methods | 1) Randomisation: not described. 2) Allocation concealment: randomly generated sequence. 3) Masking: Double blind, placebo controlled, 3 way crossover. 4) Withdraw/Drop‐out: None but seven of the 12 who completed the active phase of the study were removed from the placebo phase because of increasing symptom severity. 5) Duration of intervention: 2 weeks x 3. Three arms in study but we will only use one active arm and the placebo arm as to avoid double counting the placebo group. 6) Design: Crossover groups 7) Jadad quality score: 3 8) Location: Ontario, Canada | |
| Participants | 1) Inclusion criteria: Stable COPD, not have asthma, aged 50 yrs and over and require chronic bronchodilator therapy. 2) Definition of COPD: FEV1:FVC ratio of < 60% predicted and documented chronic airflow limitation. 3) Definition of stable COPD: not stated. 4) Type of exercise test: none 5) FEV1 % predicted Mean 37.4 SE 4.0, PaO2 Mean 73.3 SE 2.2 mmHg, PaCO2 Mean 40.9 SE 1.0 mmHg, Age Mean 64.7 SE 2.1. 6) Number of patients: 12 (8 men and 4 women). Withdraw: 1 due to exacerbation in active active phase. Initial number of patients: 13 patients. 7) Baseline therapy: During the trial patients were encouraged not to use bronchodilators other than their usual salbutamol inhaler and their usual inhaled or oral steroids. 8) Exclusion criteria: patients with clinically significant cardiac, renal, hepatic or metabolic disease, patients known to have taken drugs known to interfer with theophylline metabolism, and patients needing supplemental oxygen or were experiencing chronic respiratory failure = PaCO2 > 55mmHg). | |
| Interventions | THEOPHYLLINE GROUP (n = 12, completed the study)
1) Drug: Uniphyl or Theo‐Dur (we usedthe results from the Uniphyl vs placebo comparison)
2) Short or long action: Long‐action.
3) Dose: once daily.
4) Washout: one day.
5) Theophylline blood level: measured to give a morning trough of at least 7 ug/ml. PLACEBO GROUP (n = 12) |
|
| Outcomes | 1) Peak flow rate 2) Heart rate (mean and max). 3) ECG 4) Side effects: headache, nausea, vomiting recorded on a 0 to 6 scale. 5) Symptoms: cough, dyspnoea, wheeze, chest tightness recorded on a 0 to 6 scale. 6) Use of daily inhaled beta‐agonists. | |
| Notes | 1) Intention‐to‐treat analyses: No. 2) Sample size and statistical power: not reported. 3) Representativity: not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Schmidt 1979.
| Methods | 1) Randomisation: not reported. 2) Allocation concealment: the numbers 1 to 12 were randomly assigned to packages of six bottles containing 180 capsules of 200 mg of either theophylline or placebo. Six of these packages contained three bottles labeled months 4,5, and 6. Six packages contained drug and placebo in reverse manner. 3) Masking: Double blind. Placebo tablets had the same appearance as theophylline. 4) Withdraw/Drop‐out: one dropped out after month 4 treatment. 3 patients had to be crossed over because of the treament efficacy, one after several days, one after 2 weeks, and the third after months. 5) Duration intervention: 3 months x 2 6) Design: Cross‐over groups. 7) Jadad quality score: 5 8) Location: Pennsylvania, USA | |
| Participants | 1) Inclusion outpatients criteria:COPD, without improve 15% response to inhaled isoproterenol. 2) Definition of COPD: FEV1 less than 70% of predicted. 3)Type of exercise test: none. 4) Definition of stable COPD: not described. 5) Age: not described (range 30 to 70) 6) FEV1: Mean = 1.05, SD not described. 7) Number of patients:12. Drop‐out: 1 (11 patients completed the study). 8) Baseline therapy: not reported. 9) Exclusion criteria: not reported. | |
| Interventions | THEOPHYLLINE GROUP (n = 11, completed the study)
1) Drug: Elixophyllin 200mg capsules.
2) Short or long action: Short‐action
3) Dose: 800 mg theophylline per day total (dosing was 200mg qid).
4) Washout: not reported
5) Theophylline blood level: not measured. PLACEBO GROUP (n = 11, completed the study) |
|
| Outcomes | 1) Pulmonary function tests (FVC (L), FVC (% pred), FEV1 (L), FEV1 (% pred), MMFER (L/min). Maximal mid flow expiratory rate. 2) Patient questionnaire pulmonary status 3) Symptom: subjective 4) Heart rate | |
| Notes | 1) Intention‐to‐treat analyses: no. 2) Sample size and statistical power: not reported. 3) Representativity: not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Thomas 1992.
| Methods | 1) Randomisation: not described. 2) Allocation concealment: not described. 2) Masking: Double blind. Placebo was indistinguishable from the active medication. 3) Withdraw/Drop‐out: Two drop‐out (one during the theophylline titration period for reasons unrelated to the study and the other during the second treatment phase due to severe dyspnoea ‐ at which time he was receiving theophylline. 4) Duration of intervention: 2 weeks x 4. Four arms in study but we need only use 2 (3 versus 4). 6) Design: Crossover groups 7) Jadad quality score: 4 8) Location: Toronto, Canada | |
| Participants | 1) Inclusion criteria: Stable COPD, outpatients. 2) Definition of COPD: FEV1 less than 60 % of predicted normal and FEV1/FVC ratio less than 0.7. 3) Definition of stable COPD: not requiring additional therapeutic intervention. 5) Age: Mean= 63.1 years ( SD 4.6 years). 6) FEV1: Mean= 1.09 L (SD 0.35 and range 0.6 to 1.6 L) 7) Number of patients: 12 (6 men and 6 women). Withdraw: 2 .Initial number of patients: 14 patients. 8) Baseline therapy: No bronchodilators, other than the test medications or steroids were allowed during the trial. Medications patients were taking at time of study entry were continued at stable dosages throughout the trial. 9) Exclusion criteria: FEV1 or FVC values differed by more than 20 % in any spirometric tests performed during the preceding two years. Evidence of severe cardiac, hepatic, or renal disease; and if taking allopurinol, beta‐blockers, oral corticosteroids, phenytoin, or cimetidine. | |
| Interventions | THEOPHYLLINE GROUP (n = 12, completed the study)
1) Drug: Phyllocontin, Purdue Frederick.
2) Short or long action: Long‐action theophylline.
3) Dose: twice daily, at a previously determined dosage.
4) Washout: not reported.
5) Theophylline blood level: measured on the open‐label theophylline dose‐titration period during which their dosage of oral was adjusted to produce serum theophylline concentration of at least 10 mg/L, and as close as possible to 16.5 mg/L ‐ between 4 and 6 h after dose. PLACEBO GROUP (n=12) |
|
| Outcomes | 1) Pulmonary function tests: FVC (L), FEV1 (L), FVC, IC, FRC, Raw, TLC, RV. 2) Patient diary data. 3) Dyspnoea, cough, wheezing and sputum production: using a five‐point simple scale 0 = none; 1 = mild; 2 = moderate; 3 = severe; 4 = intolerable. 4) Side effects: using a simple five‐point scale 0 = none; 1= mild; 2 = moderate; 3 = severe; 4 = intolerable. | |
| Notes | 1) Intention‐to‐treat analyses: No. 2) Sample size and statistical power: not reported. 3) Representativity: not described. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | B ‐ Unclear |
6MW: Six minute walk distance AT: Anaerobic threshold (mlVO2/min) DLCO: Diffusing capacity of the lung for carbon monoxide (ml/min/mmHg) FEV1: Forced expiratory volume in one second (L) FVC: Forced Vital Capacity (L) FRC: Functional Residual Capacity (L) HR: Heart Rate MMFER: Maximal mid flow expiratory rate MVV: Maximum Voluntary Ventilation (L) PaCO2: Arterial carbon dioxide tension (mmHg or KPa) PaO2: Arterial oxygen tension (mmHg or KPa) PC20: concentration of inhalent necessary to produce a 20% fall in FEV1 PEFR: Peak expiratory flow rate RV: Residual volume (L) SVC: Slow vital capacity (L) TGV: Trapped gas volume (L) TLC: Total Lung Capacity (L) VEmax/MVV: dyspnoea index VE/VO2slope: anaerobic threshold VO2max/HR : maximum oxygen pulse VO2 max: Maximum oxygen consumption WR: Work Rate
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adelroth 1998 | Review article. |
| Arnold 1990 | No placebo arm. Study compared enprofylline versus theophylline. |
| Ashutosh 1997 | The objective of this study was to observe the effect theophylline on respiratory drive. The study was also only of single doses of theophylline. |
| Bachmann 1995 | All patients were taking theophylline as study was seeing the effects of cimetidine and famotidine on theophylline. |
| Berry 1991 | Because of the objective of this study is investigate the effect to theophylline on sleep‐disordered breathing in patients with COPD. |
| Beulcke 1995 | No placebo arm. Study compared two derivatives of xanthine. |
| Bleecker 1991 | Single dose study lasting 2 hours and probably not an RCT. |
| Blom 1985 | Study did not involve the use of theophylline. |
| Boos 1989 | No placebo arm in study. |
| Carvalho‐Pinto 1991 | No placebo arm in study. |
| Chapman 1990 | The population studied had reversible obstructive airway disease (asthma) |
| Conradson 1987 | The population studied have bronchial asthma (23) and/or chronic bronchitis (1). |
| Crimi 1995 | No placebo arm in study. Comparison was between nedocromil sodium and theophylline. |
| Cusack 1986 | No placebo arm in study. |
| Di Lorenzo 1998 | There is no control group, only salmeterol and theophylline intervention groups. |
| Dini 2001 | Not an RCT. |
| Dorow 1978 | Allocation of treatment not randomised, although double‐blinding was used. |
| Dull 1981 | This study presents data for post isoproterenol nebulisation response on the final day of 4‐wk placebo and theophylline period. |
| Evans 1984 | Single dose study. Patients were given theophylline at 2200hrs on the eve of each study day and all patients were studies 12 hours later. Varying single doses were used (200, 400, 600 and 800 mg) on separate days. |
| Fitzpatrick 1992 | The subjects included in this study are healthy volunteeers |
| Greening 1981 | The population is not COPD. They have asthma. |
| Harkaway 1985 | No placebo arm, study comparing 2 preparations of theophylline. |
| Horiguchi 1999 | Not COPD but study done in patients with asthma. |
| Hudson 1973 | No placebo arm in study. |
| Javaheri 1996 | The population studied in this paper have heart failure not COPD |
| Karpel 1994 | There are four grops in this study : ipratropium + theophylline + albuterol vs theophylline + albuterol vs ipratropium vs placebo. But there are no theophylline only group. |
| Lamont 1982 | Study included patients with greater than 20% reversibility in FEV1 after beta2 agonists from pMDI, therefore COPD patients only not included in study. |
| Lefcoe 1982 | Because of the distribution of the grous: P = placebos (2 oral, plus one aerosol), F + T = fenoterol + teophylline, I = Ipratropium bromide + placebo + theophylline, F+ T + I = fenoterol+ theophylline + ipratropium bromide. So, there is not a isolated theophylline study group. |
| Leitch 1981 | Single dose study lasting 3 hours. |
| Leuenberger 1997 | Not an RCT but a review paper. |
| Lunell 1983 | No placebo arm in study. |
| Mahon 1999 | This was not a conventional RCT but an N of 1 trial, therefore it was excluded as only conventional RCT's were included. Patients in this study switched (crossover) to the "other" blinded treatment arm if the first treatment did not appear to work. |
| Man 1996 | No placebo arm in study. |
| Marlin 1978 | Not COPD patients. |
| Martin 1992 | No placebo group in study. |
| McHardy 1981 | Study specifically looked at chronic bronchitis patients only but also included some patients who had COPD. But the data for COPD patients is not presented separately but mentioned in text. |
| Melani 1994 | There is no placebo group in this study. |
| Melillo 1989 | There is no control group. |
| Morandini 1989 | No placebo arm in study. |
| Oren 1997 | This study is not randomised. |
| Poukkula 1989 | Not patients with COPD. |
| Pulido 1989 | Bamyphylline is a theophylline derivate. Our protocol includes only aminophylline and theophylline not their derivates. |
| Sacco 1995 | In this study there was no placebo group. It was doxofylline versus theophylline groups. |
| Shivaram 1997 | Single dose study lasting 120 minutes. |
| Siemon 1991 | Study comparing two different theophylline preparations. |
| Steen 1980 | No placebo arm in study. Tedral versus theophylline only. |
| Sutton 1981 | Not COPD patients. |
| Svedmyr 1982 | Single dose study in patients with asthma. Oral theophylline used in combination with terbutaline. |
| Taccola 1999 | No placebo arm. |
| Tanzj 1974 | Two different theophylline preparations compared with no placebo arm in study. |
| Tatsis 1988 | This study does not a placebo group and includes bronchial asthmatics patients |
| Taylor 1985 | Not COPD patients, mean bronchodilator reversibility in FEV1 of > 26%. |
| Tsukino 1998 | Single dose study. |
| Umut 1992 | Not an RCT. |
| Van Andel 1999 | Not a RCT. |
| Vereen 1986 | Use of iv aminophylline preparation. |
| Villani 1997 | Because doxophylline is a theophylline derivate. Our protocol includes only aminophylline and theophylline. |
| Vyse 1989 | No placebo group. |
| Wiessmann 1975 | Allocation of treatment not randomised, although double‐blinding was used. |
| Winter 1984 | No placebo arm. Study only included a comparison between theophylline versus salbutamol. |
| Yang 2001 | Not COPD patients but asthma. |
| ZuWallack 2001 | Study was primarily comparing theophylline plus salmeterol as a combination treatment. Although the study had theophylline only arm it did not have a placebo only arm so the study cannot be included in the review. |
Contributions of authors
List includes: Ram FSF (FR), Jones PW (PJ), Castro AA (AAC), de Brito Jardim JR (JRJ), Atallah AN (ANA), Lacasse Y (YL), Mazzini R (RM), Goldstein R (RG), Cendon S (SC).
Proposing the idea of the review: PJ Designing the review: SC, AAC Coordinating the review: FR, SC Data collection for the review: FR, SC, AAC, RM Developing search strategy: FR, AAC Undertaking searches: FR, AAC Screening search results: AAC, FR Organising retrieval of papers: FR, SC Screening retrieved papers against inclusion criteria: FR, SC, AAC Appraising quality of papers: FR, SC, AAC Abstracting data from papers: FR, SC, AAC, RM Writing to authors of papers for additional information: FR Obtaining and screening data on unpublished studies: FR Data management for the review: FR, SC, AAC Entering data into RevMan: FR, SC, AAC, RM Analysis of data: FR, SC Interpretation of data: FR, SC Providing a methodological perspective: FR, SC, AAC Providing a clinical perspective: FR, SC, AAC Providing a policy perspective: FR, SC, AAC Providing a consumer perspective: FR, SC, AAC Writing the review: FR Responsible for future updates of the review: FR
Sources of support
Internal sources
Pneumology Division, Universidade Federal de São Paulo, Brazil.
Clinical Trial Meta‐analysis Unit, Universidade Federal de São Paulo, Brazil.
External sources
FAPESP ‐ São Paulo Foundation of Support for Research. Proc.: 96/12506‐9, Brazil.
Felix Ram received funding from The Netherlands Asthma Foundation, Netherlands.
Declarations of interest
There are no known conflicts of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Alexander 1980 {published data only}
- Alexander M, Dull W, Kasik J. Treatment of chronic obstructive pulmonary disease with orally administered theophylline. The Journal of the American Medical Association 1980;244:2286‐90. [PubMed] [Google Scholar]
- Dull WL, Alexander MR, Sadoul P, Woolson RF. The efficacy of isoproterenol inhalation for predicting the response to orally administered theophylline in chronic obstructive pulmonary disease. American Review of Respiratory Disease 1982;126(4):656‐9. [DOI] [PubMed] [Google Scholar]
Anderson 1982 {published data only}
- Anderson G, Peel ET, Pardoe T, Jones R. Sustained‐release theophylline in chronic bronchitis. British Journal of Diseases of the Chest 1982;76(3):261‐5. [PubMed] [Google Scholar]
Chrystyn 1988 {published data only}
- Chrystyn H, Mulley BA, Peake MD. Dose response relation to oral theophylline in severe chronic obstructive airways disease. British Medical Journal 1988;297:1506‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dullinger 1986 {published data only}
- Dullinger D, Kronenberg R, Niewoehner DE. Efficacy of inhaled metaproterenol and orally‐administered theophylline in patients with chronic airflow obstruction. Chest 1986;89(2):171‐3. [DOI] [PubMed] [Google Scholar]
Fink 1994 {published data only}
- Fink G, Kaye C, Sulkes J, Gabbay U, Spitzer SA. Effect of theophylline on exercise performance in patients with severe chronic obstructive pulmonary disease. Thorax 1994;49:332‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 1987 {published data only}
- Guyatt GH, Townsend M, Keller JL, Singer J. Should study subjects see their previous responses: data from a randomized control trial. Journal of Clinical Epidemiology 1989;42:913‐20. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Townsend M, Nogradi S, Pugsley SO, Keller JL, Newhouse MT. Acute response to bronchodilator. An imperfect guide for bronchodilator therapy in chronic airflow limitation. Archives of Internal Medicine 1988;148:1949‐52. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Townsend M, Pugsley SO, Keller JL, Short HD, Taylor DW, et al. Bronchodilators in chronic air‐flow limitation: effects on airway function, exercise capacity, and quality of life. American Review of Respiratory Disease 1987;135:1069‐74. [DOI] [PubMed] [Google Scholar]
Iversen 1992 {published data only}
- Iversen E, Maltbaek N, Toennesen, Eiken P, Nielsen L, Laursen LC, et al. Sustained‐release theophylline treatment of patients with irreversible chronic obstructive pulmonary disease. European Respiratory Journal 1992;5 Suppl (15):137. [Google Scholar]
- Iversen ET, Eiken PA, Nielsen LM, Laursen LC, Maltbaek N, Tonnesen FV, et al. Theophylline treatment of irreversible, chronic obstructive pulmonary disease [Article in Danish]. Ugeskr Laeger 1994;156(39):5696‐9. [PMID: 7985256] [PubMed] [Google Scholar]
Kongragunta 1988 {published data only}
- Kongragunta VR, Druz WS, Sharp JT. Dyspnea and diaphragmatic fatigue in patients with chronic obstructive pulmonary disease. American Review of Respiratory Disease 1988;137:662‐7. [DOI] [PubMed] [Google Scholar]
Machraoui 1994 {published data only}
- Machraoui A, Lawo T, Schmidt EW, Hoffarth HP, Barmeyer J, Ulmer WT. Ventricular arrythmias in chronic obstructive lung disease (COLD): effect of theophylline in therapeutic doses [Theophyllin bei ventrikulären rhythmusstörungen]. Atemwegs und Lungenkrankenheiten 1994;20(9):510‐4. [Google Scholar]
Mahler 1985 {published data only}
- Mahler DA, Matthay RA, Snyder PE, Wells CK, Loke J. Sustained‐release theophylline reduces dyspnea in nonreversible obstructive airway disease. American Review of Respiratory Disease 1985;131:22‐5. [DOI] [PubMed] [Google Scholar]
Marvin 1983 {published data only}
- Marvin PM, Baker BJ, Dutt AK, Murphy ML, Bone RC. Physiologic effects of oral bronchodilators during rest and exercise in chronic obstructive pulmonary disease. Chest 1983;84:684‐9. [DOI] [PubMed] [Google Scholar]
Mulloy 1993 {published data only}
- Mulloy E, McNicholas WT. Theophylline improves gas exchange during rest, exercise, and sleep in severe chronic obstructive pulmonary disease. American Review of Respiratory Disease 1993;148:1030‐6. [DOI] [PubMed] [Google Scholar]
Murciano 1989 {published data only}
- Murciano D, Auclair M, Pariente R, Aubier M. A randomized, controlled trial of theophylline in patients with severe chronic obstructive pulmonary disease. New England Journal of Medicine 1989;320(23):1521‐5. [DOI] [PubMed] [Google Scholar]
Newman 1994 {published data only}
- Newman D, Tamir J, Speed L, Newman J, Ben‐Dov I. Physiological and neuropsychological effects of theophylline in chronic obstructive pulmonary disease. Israel Journal of Medical Sciences 1994;30:811‐6. [PubMed] [Google Scholar]
Nishimura 1993 {published data only}
- Nishimura K, Koyama H, Ikeda A, Izumi T. Is oral theophylline effective in combination with both inhaled anticholinergic agent and inhaled beta 2‐agonist in the treatment of stable COPD. Chest 1993;104(1):179‐84. [PMID: 8325065] [DOI] [PubMed] [Google Scholar]
Nishimura 1995 {published data only}
- Nishimura K, Koyama H, Ikeda A, Sugiura N, Kawakatsu K, Izumi T. The additive effect of theophylline on a high‐dose combination of inhaled salbutamol and ipratropium bromide in stable COPD. Chest 1995;107(3):718‐23. [DOI] [PubMed] [Google Scholar]
Power 1992 {published data only}
- Power CK, Morris AM, Sreenan SK, Burke CM. An assessment of oral theophylline in patients with "reversible" chronic obstructive pulmonary disease. Irish Journal of Medical Science 1992;161(5):509. [Google Scholar]
Rivington 1988 {published data only}
- Rivington RN, Calcutt L, Hodder RV, Stewart JH, Aitken TTL. Safety and efficacy of once‐daily Uniphyl tablets compared with twice‐daily Theo‐Dur tablets in elderly patients with chronic airflow obstruction. American Journal of Medicine 1988;85(1B):48‐53. [DOI] [PubMed] [Google Scholar]
Schmidt 1979 {published data only}
- Schmidt R, Altschuler S. The usefulness of theophylline in nonasthmatic airway obstruction. Angiology 1979;30(11):744‐9. [DOI] [PubMed] [Google Scholar]
Thomas 1992 {published data only}
- Thomas P, Pugsley JA, Stewart JH. Theophylline and salbutamol improve pulmonary function in patients with irreversible chronic obstructive pulmonary disease. Chest 1992;101(1):160‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adelroth 1998 {published data only}
- Adelroth E, Barnes NC, Chung KF, Davies RJ, Poulter LW. Drug trials. European Respiratory Journal 1998;26 Suppl:36‐8. [PMID: 9585879] [PubMed] [Google Scholar]
Arnold 1990 {published data only}
- Arnold JD, Courtenay‐Evans RJ, Whitfield RR, O'Reilly JF, Petrie GR, Higgins AJ, et al. Comparative assessment of enprofylline and theophylline for chronic obstructive airways disease in the elderly. Respiratory Medicine 1990;84(3):211‐5. [DOI] [PubMed] [Google Scholar]
Ashutosh 1997 {published data only}
- Ashutosh K, Mohammad S, Fragale‐Jackson J. Effects of theophylline on respiratory drive in patients with chronic obstructive pulmonary disease. Journal of Clinical Pharmacology 1997;37:1100‐7. [DOI] [PubMed] [Google Scholar]
Bachmann 1995 {published data only}
- Bachmann K, Sullivan TJ, Reese JH, Jauregui L, Miller K, Scott M, et al. Controlled study of the putative interaction between famotidine and theophylline in patients with chronic obstructive pulmonary disease. Journal of Clinical Pharmacology 1995;35(5):529‐35. [DOI] [PubMed] [Google Scholar]
Berry 1991 {published data only}
- Berry RB, Desa MM, Branum JP, Light RW. Effect of theophylline on sleep and sleep‐disordered breathing in patients with chronic obstructive pulmonary disease. American Review of Respiratory Disease 1991;143(2):245‐50. [DOI] [PubMed] [Google Scholar]
Beulcke 1995 {published data only}
- Beulcke G. Acebrophylline and theophylline in the treatment of bronchial asthma. An open comparative, randomized study. Acta Therapeutica 1995;21(2):101‐11. [Google Scholar]
Bleecker 1991 {published data only}
- Bleecker E, Britt EJ. Acute bronchodilating effects of ipratropium bromide and theophylline in chronic obstructive pulmonary disease. American Journal of Medicine 1991;91Suppl:24‐7. [DOI] [PubMed] [Google Scholar]
Blom 1985 {published data only}
- Blom BB, Boe J, Bulow K, Hagelqvist I. A comparison of oral betasub 2‐agonist clenbuterol and salbutamol in obstructive lung disease. Currant Therapeutic Research, Clinical and Experimental 1985;37(1):51‐7. [Google Scholar]
Boos 1989 {published data only}
- Boos R, Kampen C, Weede W, Beck W. Nedocromil sodium therapy in asthma patients. Therapeutic effect in addition to treatment with oral theophylline and inhaled bronchodilator agents [In German]. Fortschritte Der Medizin 1989;107(33):712‐6. [PubMed] [Google Scholar]
Carvalho‐Pinto 1991 {published data only}
- Carvalho‐Pinto RM, Terra Filho M, Cukier A. Influence of the combination of theophylline and beta 2 agonists by oral route on pulmonary funtion of patients with chronic obstructive lung disease. Revista Do Hospital Das Clinicas; Faculdade De Medicina Da Universidade De Sao Paulo 1991;46(4):166‐9. [PubMed] [Google Scholar]
Chapman 1990 {published data only}
- Chapman KR, Boucher S, Hyland RH, Day A, Kreisman H, Rivington RR, et al. A comparison of enprofylline and theophylline in the maintenance therapy of chronic reversible obstructive airway disease. Journal of Allergy & Clinical Immunology 1990;85(2):514‐21. [DOI] [PubMed] [Google Scholar]
Conradson 1987 {published data only}
- Conradson TB, Eklundh G, Olofsson B, Pahlm O, Persson G. Arrhythmogenicity from combined bronchodilator therapy in patients with obstructive lung disease and concomitant ischemic heart disease. Chest 1987;91:5‐9. [DOI] [PubMed] [Google Scholar]
Crimi 1995 {published data only}
- Crimi E, Orefice U, Benedetto F, Grassi V, Brusasco V. Nedocromil sodium versus theophylline in the treatment of reversible obstructive airway disease. Annals of Allergy, Asthma & Immunology 1995;74(6):501‐8. [PubMed] [Google Scholar]
Cusack 1986 {published data only}
- Cusack BJ, Crowley JJ, Mercer GD, Charan NB, Vestal RE. Theophylline clearance in patients with severe chronic obstructive pulmonary disease receiving supplemental oxygen and the effect of acute hypoxemia. American Review of Respiratory Diseases 1986;133(6):1110‐4. [DOI] [PubMed] [Google Scholar]
Di Lorenzo 1998 {published data only}
- Lorenzo G, Morici G, Drago A, Pellitteri M E, Mansueto P, Melluso M, et al. Efficacy, tolerability, and effects on quality of life of inhaled salmeterol and oral theophylline in patients with mild‐to‐moderate chronic obstructive pulmonary disease. Clinical Therapeutics 1998;20(6):1130‐48. [DOI] [PubMed] [Google Scholar]
Dini 2001 {published data only}
- Dini FL, Cogo R. Doxofylline: a new generation xanthine bronchodilator devoid of major cardiovascular adverse effects. Current Medical Research & Opinion 2001;16(4):258‐68. [DOI] [PubMed] [Google Scholar]
Dorow 1978 {published data only}
- Dorow V P. The effect of an oral aminophylline compound with protracted activity on respiratory resistance and blood gases in obstructive respiratory diseases [Die Wirkung eines oralen Aminophyllin‐Praparates mit Retardwirkung auf die Atemwegswiderstande und arteriellen Blutgase bei obstruktiven Atemwegserkrankungen]. Arzneim Forsch Drug Res 1978;28(1):853‐7. [PubMed] [Google Scholar]
Dull 1981 {published data only}
- Dull WL, Alexander MR, Kasik JE. Isoproterenol challenge during placebo and oral theophylline therapy in chronic obstructive pulmonary disease. American Review of Respiratory Disease 1981;123:340‐2. [DOI] [PubMed] [Google Scholar]
Evans 1984 {published data only}
- Evans W V. Plasma theophylline concentrations, six minute walking distances, and breathlessness in patients with chronic airflow obstruction. British Medical Journal 1984;289:1649‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fitzpatrick 1992 {published data only}
- Fitzpatrick MF, Engleman HM, Boellert F, Mchardy R, Shapiro CM, Deary IJ, et al. Effect of therapeutic theophylline levels on the sleep quality and daytime cognitive performance of normal subjects. American Review of Respiratory Disease 1992;145:1355‐8. [DOI] [PubMed] [Google Scholar]
Greening 1981 {published data only}
- Greening A P, Baillie E, Gribbin H R, Pride N B. Sustained release oral aminophylline in patients with airflow obstruction. Thorax 1981;36(4):303‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harkaway 1985 {published data only}
- Harkaway PS, Conlon PF, Hirsh JD, Allen WT. Comparison of two sustained‐release theophylline preparations in adult paitents with obstructive airways disease. Journal of Clinical Pharmacology 1985;25(6):444‐7. [DOI] [PubMed] [Google Scholar]
Horiguchi 1999 {published data only}
- Horiguchi T, Tachikawa S, Kasahara J, Doi M, Shiga M, Miyazaki J, et al. Suppression of airway inflammation by theophylline in adult bronchial asthma. Respiration 1999;66(2):124‐7. [DOI] [PubMed] [Google Scholar]
Hudson 1973 {published data only}
- Hudson LD, Tyler ML, Petty TTL. Oral aminophylline and dikydroxypropyl theophylline in reversible obstructive airway disease: a single‐dose, double blind, crossover comparison. Current Therapeutic Research, Clinical & Experimental 1973;15(7):367‐72. [PubMed] [Google Scholar]
Javaheri 1996 {published data only}
- Javaheri S, Parker TJ, Wexler L, Liming JD, Lindower P, Roselle GA. Effect of theophylline on sleep‐disordered breathing in heart failure. New England Journal of Medicine 1996;335:562‐7. [DOI] [PubMed] [Google Scholar]
Karpel 1994 {published data only}
- Karpel JP, Kotch A, Zinny M, Pesin J, Alleyne W. A comparison of inhaled ipratropium, oral theophylline plus inhaled B‐agonist, and the combination of all three in patients with COPD. Chest 1994;105(4):1089‐94. [DOI] [PubMed] [Google Scholar]
Lamont 1982 {published data only}
- Lamont H, Straeten M, Pauwels R, Moerman E. The combined effect of theophylline and terbutaline in patients with chronic obstructive airway disease. European Journal of Respiratory Diseases 1982;63(1):13‐22. [PubMed] [Google Scholar]
Lefcoe 1982 {published data only}
- Lefcoe NM, Toogood JH, Blennerhassett G, Baskerville J, Paterson NAM. The addition of an aerosol anticholinergic to an oral beta agonist plus theophylline in asthma and bronchitis. Chest 1982;82:300‐5. [DOI] [PubMed] [Google Scholar]
Leitch 1981 {published data only}
- Leitch AG, Morgan A, Ellis DA, Bell G, Haslett C, McHardy GJR. Effect of oral salbutamol and slow‐release aminophylline on exercise tolerance in chronic bronchitis. Thorax 1981;36:787‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leuenberger 1997 {published data only}
- Leuenberger P, Anderhub HP, Brandli O, Keller R, Knoblauch A, Kuhn M, et al. Management 1997 of chronic obstructive pulmonary disease. Schweiz Med Wochenschr 1997;127:766‐82. [PubMed] [Google Scholar]
Lunell 1983 {published data only}
- Lunell E, Svedmyr N, Andersson KE, Persson CG. A novel bronchodilator xanthine apparently without adenosine receptor antagonism and tremorogenic effect. European Journal of Respiratory Diseases 1983;64(5):333‐9. [PubMed] [Google Scholar]
Mahon 1999 {published data only}
- Mahon JL, Laupacis A, Hodder R, McKim D, Paterson NAM, Wood TE, et al. Theophylline for irreversible chronic airflow limitation. Chest 1999;115:38‐48. [DOI] [PubMed] [Google Scholar]
Man 1996 {published data only}
- Man GC, Chapman KR, Habib AS, Darke AC. Sleep quality and nocturnal respiratory function with once‐daily theophylline (Uniphyl) and inhaled salbutamol in patients with COPD. Chest 1996;110(3):648‐53. [DOI] [PubMed] [Google Scholar]
Marlin 1978 {published data only}
- Marlin GE, Hartnett BJ, Berend N, Hacket NB. Assessment of combined oral theophylline and inhaled beta adrenoceptor agonist bronchodilator therapy. British Journal of Clinical Pharmacology 1978;5(1):45‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Martin 1992 {published data only}
- Martin RJ, Pak J. Overnight theophylline concentrations and effects on sleep and lung function in chronic obstructive pulmonary disease. American Review of Respiratory Disease 1992;145(3):540‐4. [DOI] [PubMed] [Google Scholar]
McHardy 1981 {published data only}
- McHardy GJ, Morgan A, Ellis DA, Haslett C, Leitch AG. Physical effort capacity after administration of salbutamol or aminophylline [Physische belastbarkeit nach gabe von salbutamol‐ und phyllocontin‐ tabletten]. Therapiewoche 1981;31:5153‐6. [Google Scholar]
Melani 1994 {published data only}
- Melani AS, Gregorio A. Long‐term therapy with inhaled salmeterol versus oral slow release theophylline. Effects on oxygen saturation during the walking test in patients with severe COPD [Terapia di lunga durata con salmeterol per via inalatoria Vs teofillina orale a lento rilascio: effetti sulla saturazione ossiemoglobinica durante il test del cammino in pazienti con BPCO severo. Lotta contro la TBC e malattie polmonari soc]. Lotta Contro La Tuberculosi e Le Malattie Polmonari Sociali 1994;64(4):331‐6. [Google Scholar]
Melillo 1989 {published data only}
- Melillo G, Balzano G, Jodice F, Felice A, Campisi V, Capone M, et al. Treatment of reversible chronic airways orbstruction with doxofylline compared with slow‐release theophylline: a double‐blind, randomized, multicentre trial. International Journal of Clinical Pharmacology Research 1989;9(6):397‐405. [PubMed] [Google Scholar]
Morandini 1989 {published data only}
- Morandini GC, Finiguerra M, Conti P, Bernocchi D, Manini G. Treatment of chronic bronchitis [Combined therapy with sustained‐release theophylline (Teonova R) and a mucoactive drug sobrerol (Sobrepin R)]. Clin Trials 1989;26(3):163‐74. [Google Scholar]
Oren 1997 {published data only}
- Oren R, Beeri M, Hubert A, Kramer M R, Matzner Y. Effect of theophylline on erythrocytosis in chronic obstructive pulmonary disease. Archives of Internal Medicine 1997;157(13):1474‐8. [PubMed] [Google Scholar]
Poukkula 1989 {published data only}
- Poukkula A, Korhonen UR, Huikuri H, Linnaluoto M. Theophylline and salbutamol in combination in patients with obstructive pulmonary disease and concurrent heart disease: effect on cardiac arrhythmias. Journal of Internal Medicine 1989;226:229‐34. [DOI] [PubMed] [Google Scholar]
Pulido 1989 {published data only}
- Pulido E, Pupita F, Battistoni C. Treatment of patients with chronic airways obstruction: a controlled study with bamyphylline. Pharmatherapeutica 1989;5:416‐22. [PubMed] [Google Scholar]
Sacco 1995 {published data only}
- Sacco C, Braghiroli A, Grossi E, Donner CF. The effects of doxofylline versus theophylline on sleep architecture in COPD patients. Monaldi Archives for Chest Disease 1995;50:98‐103. [PubMed] [Google Scholar]
Shivaram 1997 {published data only}
- Shivaram U, Cash ME, Mateo F, Shim C. Effects of high‐dose ipratropium bromide and oral aminophylline on spirometry and exercise tolerance in COPD. Respiratory Medicine 1997;91:327‐34. [DOI] [PubMed] [Google Scholar]
Siemon 1991 {published data only}
- Siemon G, Huber W, Maier‐Stocker P. Euphylong in standard dosage: comparison with an international retard preparation for twice‐daily administration in recommended dosage [In German]. Pneumologie 1991;45:853‐7. [PMID: 1812479] [PubMed] [Google Scholar]
Steen 1980 {published data only}
- Steen SN, Smith R, Thomas JS. Clinical efficacy of theophylline combination therapy for chronic obstructive airways disease. Curr Ther Res Clin Exp 1980;27(4):608‐19. [Google Scholar]
Sutton 1981 {published data only}
- Sutton PP, Pavia D, Bateman JR, Clarke SW. The effect of oral aminophylline on lung mucociliary clerance in man. Chest 1981;80(6):889‐92. [PubMed] [Google Scholar]
Svedmyr 1982 {published data only}
- Svedmyr K. Effects of oral theophylline combined with oral and inhaled beta‐2 adrenostimulants in asthmatics. Allergy 1982;37(2):119‐27. [DOI] [PubMed] [Google Scholar]
Taccola 1999 {published data only}
- Taccola M, Bancalari L, Ghignoni G, Paggiaro PL. Salmeterol versus slow‐release theophylline in patients with reversible obstructive pulmonary disease. Monaldi Archives for Chest Disease 1999;54(4):302‐6. [PubMed] [Google Scholar]
Tanzj 1974 {published data only}
- Tanzj L, Andreini E, Romeo A. Use of orally administered hydro‐alcohol solution of theophylline in chronic obstructive bronchopathy: preliminary study [L'impiego di una soluzione idroalcoolica di teofillina per os nella broncopatia cronica obstruttiva: studio preliminare]. Cardiologia Pratica 1974;25(4):349‐54. [PubMed] [Google Scholar]
Tatsis 1988 {published data only}
- Tatsis G, Danos J, Gaga M, Pantelakis D, Veslemes M, Jordanoglou J. A single‐blind crossover study of two different slow release theophylline preparations. Journal of International Medical Research 1988;16(6):452‐8. [DOI] [PubMed] [Google Scholar]
Taylor 1985 {published data only}
- Taylor D R, Buick B, Kinney C, Lowry RC, Mcdevitt DG. The efficacy of orally administered theophylline, inhaled salbutamol, and a combination of the two as chronic therapy in the management of chronic bronchitis with reversible air‐flow obstruction. American Review of Respiratory Disease 1985;131:747‐51. [DOI] [PubMed] [Google Scholar]
Tsukino 1998 {published data only}
- Tsukino M, Nishimura K, Ikeda A, Hajiro T, Koyama H, Izumi T. Effects of theophylline and ipratropium bromide on exercise performance in patients with stable chronic obstructive pulmonary disease. Thorax 1998;53:269‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Umut 1992 {published data only}
- Umut S, Gemicioglu B, Yildirim N, Barlas, Ozuner Z. Effect of theophylline in chronic obstructive lung disease. International Journal of Clinicial Pharmacology and Therapeutic Toxicology 1992;30:149‐52. [PubMed] [Google Scholar]
Van Andel 1999 {published data only}
- Andel AE, Reisner C, Menjoge SS, Witek TJ. Analysis of inhaled corticosteroids and oral theophylline uas among patients with stable COPD from 1987 to 1995. Chest 1999;115(3):703‐7. [DOI] [PubMed] [Google Scholar]
Vereen 1986 {published data only}
- Vereen LE, Kinasewitz GT, George RB. Effect of aminophylline on exercise performance in patients with irreversible airway obstruction. Archives of Internal Medicine 1986;146(7):1349‐51. [PubMed] [Google Scholar]
Villani 1997 {published data only}
- Villani F, Maria P, Ronchi E, Galimberti M. Oral doxophylline in patients with chronic obstructive pulmonary disease. International Journal of Clinical Pharmacology and Therapeutics 1997;35:107‐11. [PubMed] [Google Scholar]
Vyse 1989 {published data only}
- Vyse T, Cochrane GM. Controlled release salbutamol tablets versus sustained release theophylline tablets in the control of reversible obstructive airways disease. Journal of International Medical Research 1989;17(1):93‐8. [DOI] [PubMed] [Google Scholar]
Wiessmann 1975 {published data only}
- Wiessmann KJ. Effect of a slow‐release aminophylline on pulmonary function in obstructive respiratory disease [Der einfluss eines oralen Aminophyllin‐Praparates mit Retardwirkung auf di Lungenfunktionsparameter bei obstruktiven Atemwegserkrankungen]. Deutsch Medizinische Wochenschrift 1975;36(5):1781‐90. [DOI] [PubMed] [Google Scholar]
Winter 1984 {published data only}
- Winter RJ, Langford JA, George RJ, Deacock SJ, Rudd RM. The effects of theophylline and salbutamol on right and left ventricular function in chronic bronchitis and emphysema. British Journal of Diseases of the Chest 1984;78(4):358‐62. [PubMed] [Google Scholar]
Yang 2001 {published data only}
- Yang J, Tu H‐Y, Li Q‐Q. Effect of theophylline on airway inflammation in asthma. Acta Pharmacol Sin 2001;22(5):475‐80. [PubMed] [Google Scholar]
ZuWallack 2001 {published data only}
- ZuWallack R, Mahler D, Reilly D, Chruch N, emmett A, Rickard K, Knobil K. Salmeterol plus theophylline combination therapy in the treatment of COPD. Chest 2001;119:1661‐70. [DOI] [PubMed] [Google Scholar]
Additional references
Appleton 2002
- Appleton S, Poole P, Smith B, Veale A, Bara A. Long acting beta2‐agonists for COPD patients with poorly reversible airflow limitation. The Cochrane Library 2002, Issue 2. [DOI] [PubMed] [Google Scholar]
ATS 1995
- American Thoracic Society. American Thoracic Society Standards for the diagnosis and care of patients with COPD. American Journal of Respiratory & Critical Care Medicine 1995;152 Suppl:77‐120. [PubMed] [Google Scholar]
Atuk 1967
- Atuk NO, Blaydes MC, Westervelt FB Jr, Wood JE Jr. Effect of aminophylline on urinary excretion of epinephrine and norepinephrine in man. Circulation 1967;35(4):745‐53. [DOI] [PubMed] [Google Scholar]
Aubier 1983
- Aubier M, Murciano D, Viires N, Lecocguic Y, Palacios S, Pariente R. Increased ventilation caused by improved diaphragmatic efficiency during aminophylline infusion. American Review of Respiratory Disease 1983;127(2):148‐54. [DOI] [PubMed] [Google Scholar]
Aubier 1985
- Aubier M, Roussos C. Effect of theophylline on respiratory muscle function. Chest 1985 1985;88 Suppl:91‐7. [DOI] [PubMed] [Google Scholar]
Barnette 2000
- Barnette MS, Underwood DC. New phosphodiesterase inhibitors as therapeutics for the treatment of chronic lung disease. Current Opinion in Pulmonary Medicine 2000;6:164‐9. [DOI] [PubMed] [Google Scholar]
Belman 1996
- Belman MJ, Botnick WC, Shin JW. Inhaled bronchodilators reduce dynamic hyperinflation during exercise in patients with chronic obstructive pulmonary disease. American Journal of Respiratory & Critical Care Medicine 1996;153:967‐75. [DOI] [PubMed] [Google Scholar]
Bergstrand 1980
- Bergstrand H. Phosphodiesterase inhibition and theophylline. European Journal of Respiratory Diseases 1980;109 Suppl:37‐44. [PubMed] [Google Scholar]
BTS 1997
- British Thoracic Society. British Thoracic Society Guidelines for the Management of Chronic Obstructive Pulmonary Disease. Thorax 1997;52 Suppl (5):1‐28. [Google Scholar]
Celli 2000
- Celli BR. The Importance of Spirometry in COPD and Asthma ‐ Effect on Approach to Management. Chest 2000;117:15‐19. [DOI] [PubMed] [Google Scholar]
Cleophas 1993
- Cleophas TJ. Carry‐over biases in clinical pharmacology. European Journal of Clinical Chemistry & Clinical Biochemistry 1993;31(12):803‐9. [DOI] [PubMed] [Google Scholar]
Cleophas 1996
- Cleophas TJ. Crossover trials are only useful when there is a positive correlation between the response to different treatment modalities. British Journal of Clinical Pharmacology 1996;41(3):235‐9. [DOI] [PubMed] [Google Scholar]
Compton 2000
- Compton C, Duggan M, Cedar E, Nieman RB, Amit O, Tabona MV, et al. Ariflo® efficacy in a 12 month study of patients with asthma. American Journal of Respiratory & Critical Care Medicine 2000;161:A505. [Google Scholar]
Cooper 1987
- Cooper CB, Davidson AC, Cameron IR. Aminophylline, respiratory muscle strength and exercise tolerance in chronic obstructive airway disease. Bulletin Europeen de Physiopathologie Respiratoire 1987;23(1):15‐22. [PubMed] [Google Scholar]
Darnall‐Jr 1985
- Darnall‐Jr. RA. Aminophylline reduces hypoxic ventilatory depression: possible role of adenosine. Pediatric Research 1995;19(7):706‐10. [DOI] [PubMed] [Google Scholar]
Eaton 1980
- Eaton ML, Green BA, Church TR, McGowan T, Niewoehner DE. Efficacy of theophylline in "irreversible" airflow obstruction. Annals of Internal Medicine 1980;92(6):758‐61. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. British Medical Journal 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
ERS 1995
- European Respiratory Society Consensus Statement. Optimal assessement and management of chronic obstructive pulmonary disease (COPD). European Respiratory Journal 1995;8:1398‐420. [DOI] [PubMed] [Google Scholar]
Evans 1997
- Evans DJ, Taylor DA, Zetterstrom U, Chung KF, O'Connor BJ, Barnes PJ. A comparison of low dose inhaled budesonide plus theophylline and high dose inhaled budesonide for moderate asthma. New England Journal of Medicine 1997;337:1412‐8. [DOI] [PubMed] [Google Scholar]
Folgering 1994
- Folgering H, Herwaarden CV. Exercise limitations in patients with pulmonary diseases. International Journal of Sports Medicine 1994;15:107‐11. [DOI] [PubMed] [Google Scholar]
Giembycz 2000
- Giembycz MA. Phosphodiesterase 4 inhibitors and the treatment of asthma: where are we now and where do we go from here?. Drugs 2000;59(2):193‐212. [DOI] [PubMed] [Google Scholar]
Gomez 2002
- Gomez FP, Rodriguez‐Roisin R. Global initiative for chronic obstructive lung disease (GOLD) guidelines for chronic obstructive pulmoary disease. Current Opinion in Pulmonary Medicine 2002;8(2):81‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Guyatt 1988
- Guyatt GH, Townsend M, Nogradi S, Pugsley SO, Keller JL, Newhouse MT. Acute response to bronchodilator: an imperfect guide for bronchodilator therapy in chronic airflow limitation. Archives of Internal Medicine 1988;148:1949‐52. [DOI] [PubMed] [Google Scholar]
Hatipoglu 1999
- Hatipoglu US, F Laghi F, Tobin MJ. Does inhaled albuterol improve diaphragmatic contractility in patients with Chronic Obstructive Pulmonary Disease. American Journal of Respiratory & Critical Care Medicine 1999;160:1916‐21. [DOI] [PubMed] [Google Scholar]
Hay 1992
- Hay JG, Stone P, Carter J, Church S, Eyre‐Brook A, Pearson MG, et al. Bronchodilator reversibility, exercise performance and breathlessness in stable chronic obstructive pulmonary disease. European Respiratory Journal 1992;5(6):659‐64. [PubMed] [Google Scholar]
Higbee 1982
- Higbee MD, Kumar M, Galant SP. Stimulation of endogenous catecholamine release by theophylline: a proposed additional mechanism of action for theophylline effects. Journal of Allergy & Clinical Immunology 1982;70(5):377‐82. [DOI] [PubMed] [Google Scholar]
Horrobin 1977
- Horrobin DF, Manku MS, Franks DJ, Hamet P. Methyl xanthine phosphodiesterase inhibitors behave as prostaglandin antagonists in a perfused rat mesenteric artery preparation. Prostaglandins 1977;13(1):33‐40. [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore A, Carroll D, Jenkinson C, Reynolds JM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: Is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Javaheri 1989
- Javaheri S, Evers JA, Teppema LJ. Increase in ventilation caused by aminophylline in the absence of changes in ventral medullary extracellular fluid pH and carbon dioxide tension. Thorax 1989;44(2):121‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Javaheri 1990
- Javaheri S, Guerra L. Lung function, hypoxic and hypercapnic ventilatory responses, and respiratory muscle strength in normal subjects taking oral theophylline. Thorax 1990;45(10):743‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Johannesson 1985
- Johannesson N, Andersson K, Joelsson B, Persson C. Relaxation of lower esophageal sphincter and stimulation of gastric secretion and diuresis by antiasthmatic xanthines. American Review of Respiratory Disease 1985;131:26‐31. [DOI] [PubMed] [Google Scholar]
Kerstjens 1999
- Kerstjens HAM. Stable chronic obstructive pulmonary disease. Clinical evidence. Issue 1. London: BMJ Publishing Group, 1999. [Google Scholar]
Landsberg 1990
- Landsberg KF, Vaughan LM, Heffner JE. The effect of theophylline on respiratory muscle contractility and fatigue. Pharmacotherapy 1990;10(4):271‐9. [PubMed] [Google Scholar]
Levine 1985
- Levine JH, Michael JR, Guarnieri T. Multifocal atrial tachycardia: a toxic effect of theophylline. Lancet 1985;i:12‐4. [DOI] [PubMed] [Google Scholar]
Marsh 1993
- Marsh GD, McFadden RG, Nicholson RL, Leasa DJ, Thompson RT. Theophylline delays skeletal muscle fatigue during progressive exercise. American Review of Respiratory Disease 1993;147(4):876‐9. [DOI] [PubMed] [Google Scholar]
Matthay 1986
- Matthay RA, Mahler DA. Theophylline improves global cardiac function and reduces dyspnea in chronic obstructive lung disease. Journal of Allergy & Clinical Immunology 1986;78(Pt 2):793‐9. [DOI] [PubMed] [Google Scholar]
Mendella 1982
- Mendella LA, Manfreda J, Warren CP, Anthonisen NR. Steroid response in stable chronic obstructive pulmonary disease. Annals of Internal Medicine 1982;96(1):17‐21. [DOI] [PubMed] [Google Scholar]
Mitenko 1973
- Mitenko PA, Ogilvie RI. Rational intravenous doses of theophylline. New England Journal of Medicine 1973;289:600‐3. [DOI] [PubMed] [Google Scholar]
Mulrow 1997
- Mulrow CD, Oxman AD. Cochrane Collaboration Handbook (updated 1 march 1997). The Cochrane Library 1997, Issue 1. [Google Scholar]
Murciano 1984
- Murciano D, Aubier M, Lecocguic Y, Pariente R. Effects of theophylline on diaphragmatic strength and fatigue in patients with chronic obstructive pulmonary disease. New England Journal of Medicine 1984;311(6):349‐53. [DOI] [PubMed] [Google Scholar]
O'Donnell 1999
- O'Donnell DE, Lam M, Webb KA. Spirometric correlates of improvement in exercise performance after anticholinergic therapy in chronic obstructive pulmonary disease. American Journal of Respiratory & Critical Care Medicine 1999;160:542‐9. [DOI] [PubMed] [Google Scholar]
Okubo 1987
- Okubo S, Konno K, Ishizaki T, Kubo M, Suganuma T, Takizawa T. Effect of theophylline on respiratory neuromuscular drive. European Journal of Clinical Pharmacology 1987;33(1):85‐8. [DOI] [PubMed] [Google Scholar]
Pauwels 2000
- Pauwels RA. National and International Guidelines for COPD ‐ The need for evidence. Chest 2000;117 Suppl:20‐2. [DOI] [PubMed] [Google Scholar]
Persson 1986
- Persson CGA. Overview of effects of theophylline. Journal of Allergy & Clinical Immunology 1986;78:780‐7. [DOI] [PubMed] [Google Scholar]
Raguso 1996
- Raguso CA, Coggan AR, Labros SS, Gastaldelli A, Wolfe R. Effect of theophylline on substrate metabolism during exercise. Metabolism 1996;45:1153‐60. [DOI] [PubMed] [Google Scholar]
Ritchie 1975
- Ritchie JM. Central nervous stimulants: the xanthines. In: Goodman LS, Gilman A editor(s). The Pharmacological Basis of Therapeutics. New York: Macmillan Publishing Co Inc, 1975:367‐8. [Google Scholar]
Sherman 1996
- Sherman MS, Lang DM, Matityahu A, Campbell D. Theophylline improves measurements of respiratory muscle efficiencyTheophylline improves measurements of respiratory muscle efficiencyTheophylline improves measurements of respiratory muscle efficiency. Chest 1996;110(6):1437‐42. [DOI] [PubMed] [Google Scholar]
Spinelli 1991
- Spinelli A, Fanelli A, Gorini M, Sanna A, Francois C, Scano G. Control of breathing in patients with chronic pulmonary obstructive disease: response to bamiphylline. Respiration 1991;58(5‐6):241‐8. [DOI] [PubMed] [Google Scholar]
Varriale 1993
- Varriale P, Ramaprasad S. Aminophylline induced atrial fibrillation. Pacing and Clinical Electrophysiology 1993;16:1953‐5. [DOI] [PubMed] [Google Scholar]
Verren 1986
- Verren LE, Kinasewitz GT, George RB. Effect of aminophylline on exercise performance in patients with irreversible airway obstruction. Archives of Internal Medicine 1986;146:1349‐51. [PubMed] [Google Scholar]
Webb 1990
- Webb K, Fox L, Muir C, O'Donnell DE. Ipratropium bromide reduces exertional dyspnea in patoents with severe chronic airflow limitation. Chest 1990;98:1135. [Google Scholar]
Wolkove 1989
- Wolkove N, Dajczman E, Colacone A, Kreisman H. The relationship between pulmonary function and dyspnea in obstructive lung disease. Chest 1989;96:1247‐51. [DOI] [PubMed] [Google Scholar]
Woodcock 1983
- Woodcock AA, Johnson MA, Geddes DM. Theophylline prescribing, serum concentrations, and toxicity. Lancet 1983;1:610‐3. [DOI] [PubMed] [Google Scholar]
Wrenn 1991
- Wrenn K, Slovis CM, Murphy F, Greenberg RS. Aminophylline therapy for acute bronchospastic disease in the emergency room. Annals of Internal Medicine 1991;115(4):241‐7. [DOI] [PubMed] [Google Scholar]
Yung 1998
- Yung M, South M. Randomised controlled trial of aminophylline for severe acute asthma. Archives of Disease in Childhood 1998;79(5):405‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]


