Abstract
Visceral hypersensitivity and pain result, at least in part, from increased excitability of primary afferents that innervate the colon. In addition to intrinsic changes in these neurons, emerging evidence indicates that changes in lining epithelial cells may also contribute to increased excitability. Here we review recent studies on how colon epithelial cells communicate directly with colon afferents. Specifically, anatomical studies revealed specialized synaptic connections between epithelial cells and nerve fibers, and studies using optogenetic activation of the epithelium showed initiation of pain-like responses. We review the possible mechanisms of epithelial-neuronal communication and provide an overview of the possible neurotransmitters and receptors involved. Understanding of the biology of this interface and how it changes in pathological conditions may provide new treatments for visceral pain conditions.
Keywords: visceral pain, sensory neurons, colon epithelium, gut-brain axis
Epithelial-neuronal signaling and visceral pain
Visceral hypersensitivity and pain are common debilitating symptoms of inflammatory bowel diseases (IBD) and irritable bowel syndrome (IBS). These disorders represent widespread health problems, estimated to affect up to 20% of the population [1]. Effective treatments to control pain and resolve hypersensitivity are lacking. Pathophysiological changes associated with IBS and IBD are thought to cause sensitization of spinal afferent neurons that innervate the colon, which transmit noxious stimuli to the spinal cord and central nervous system (CNS) [2, 3]. Pain research has largely focused on injury-evoked changes in spinal afferent and CNS neuron activity, but recent studies of skin, bladder, and colon have revealed new regulatory roles for epithelial cells in sensory transduction. In skin, epithelial cells (keratinocytes) have been shown to have direct communication with epidermal nerve fibers that is sufficient to drive action potential firing and nociceptive responses [4–6]. Augmentation of keratinocyte-neural signaling may also occur under inflammatory conditions [7–9]. The bladder epithelium (urothelium) not only serves a barrier function but also has an active role in mechanotransduction via release of neuroactive substances such as ATP, acetylcholine, and nitric oxide [10]. This release is increased in models of bladder inflammation and cystitis [11].
In the context of the colon, changes in epithelial structure and permeability are common in IBD (i.e., ulcerative colitis, Crohn’s disease). In active disease a compromised epithelium allows infiltration of bacteria, which provokes inflammatory immune cell responses [12, 13]. Activated immune cells (e.g., macrophages and mast cells) release cytokines and neuroactivators that can affect primary afferent function and result in pain [14, 15]. Biopsies of colon from IBD patients with pain often exhibit inflammation [14]. However, pain is reported by some patients with no evidence of inflammation or epithelial damage [16]. This finding and other preclinical studies suggest that epithelial regulation of sensory afferent activity may have a more significant role in pain signaling than previously thought and that subtle changes in epithelial function could lead to persistent pain. In support of this concept, several studies have shown that the intestinal epithelium produces and releases neurotransmitters such as ATP [17], glutamate [18], serotonin (5-HT) [19] and acetylcholine (ACh) [20], which act on colon nociceptive neurons. Additionally, channelrhodopsin-mediated selective activation of the colon epithelium is sufficient to evoke action potential firing in colon afferents and a pain-related visceromotor response [21]. Thus, the epithelium alone, in the absence of applied mechanical, chemical or thermal stimuli, can initiate sensory neuron activity and pain-like behavior. At the anatomical level, studies have indicated direct synaptic communication between specialized colon epithelial cells and surrounding neurons, providing a direct means of communication [22, 23]. Taken together, these observations indicate that with respect to structure and function, epithelial cells share many of the properties associated with neurons.
In this review, we summarize what is known about sensory afferents that innervate the colon and how they convey sensory information. We describe the different cell types that make up the colon epithelium, with a focus on enteroendocrine cells (EECs) and their role in sensory signaling. The chemical mechanisms of epithelial-neuronal communication in the colon are also discussed, with emphases on how these neurotransmitter systems are altered in response to colon inflammation.
Anatomical and functional organization of colon epithelial-neuronal communication
Sensory afferents that innervate the colon
Colon function is coordinated by intrinsic and extrinsic subpopulations of neurons. The colon receives extrinsic innervation from autonomic pathways (not discussed in the current article) and sensory pathways [24]. Extrinsic primary afferent neurons (hereafter referred to as “colon afferents”) convey sensory information from the colon to the central nervous system. They are the first in a chain of neurons that give rise to conscious sensations of pain, bloating, fullness and urgency [24]. Colon afferent somata are in dorsal root ganglia (DRG) at thoracolumbar and lumbosacral spinal levels. Thoracolumbar afferents project to the colon via the splanchnic nerve and lumbosacral afferents project via the pelvic nerve [25]. The vagus nerve is a third source of sensory innervation; vagal afferents that project to the colon have cell bodies in the nodose ganglion [25]. Single-unit electrophysiological recordings made in rodent ex vivo preparations have revealed five main classes of colon afferents, defined by their functional properties: muscular afferents, which respond to stretching of the colon, mucosal afferents, which respond to light distortion of the mucosa, muscular/mucosal afferents, which respond to stretch as well as light distortion of the mucosa, and vascular afferents, which respond to focal probing of the colon wall (serosal afferents) or mesentery (mesenteric afferents) [25, 26]. Muscular afferents include both low-threshold mechanosensors with a slowly adapting response to low-intensity stretch stimuli and high threshold fibers with response properties associated with dedicated nociceptors [26–28]. The low-threshold fibers are thought to respond to physiological distension induced by the passing of fecal matter. Mucosal afferents are also low-threshold responders and thought to participate in detecting luminal contents as part of defecatory reflexes [24]. In contrast, vascular afferents are high-threshold mechanosensors and display an adapting response to noxious stimuli, making them likely to transmit mechanically-induced pain stimuli [26, 29]. Mechanically insensitive afferents or “silent afferents” represent a fifth class of colon afferents that respond to chemical stimuli and, after activation by inflammatory mediators, to mechanical stimuli [30]. These five major classes of colon afferents have been demonstrated in both rodent and human [31].
The classification of colon afferents continues to be updated as more advanced techniques become available. A recent study using single-cell RNAseq analyses proposed distinct classes of extrinsic colon afferents based on molecular profiles [32]. These included peptidergic, non-peptidergic, and neurofilament-positive populations at both thoracolumbar and lumbosacral levels. This study also identified neurotransmitter receptors expressed across these populations. Ongoing molecular and functional studies aim to reveal the signaling mechanisms of these colon afferent classes and their role in visceral pain.
Colon epithelial cell types
Epithelial cells that line the small and large intestine form a simple columnar epithelium with two major functions: absorbing nutrients and water and forming a protective barrier (Figure 1). Epithelial morphology (i.e., number of villi vs. crypts) and secretory cell populations vary along the GI tract [33]. This review focuses on the colon epithelium, partly because most studies of IBD and IBS in humans examine colon biopsies. Additionally, measures and outcomes of most animal models of visceral pain target the colon epithelium and colon afferents. Most colon epithelial cells are absorptive enterocytes, with remaining types having secretory functions. Secretory cell types include goblet cells that secrete mucus for lubrication, tuft cells (also known as brush cells) that secrete opioids and immune mediators [34], and enteroendocrine cells (EECs) that secrete hormones and peptides [35]. There are over 10 types of EECs. Most prevalent in the human and mouse colon are L-cells, which express peptide YY (PYY) and glucagon-like peptides 1 and 2 (GLP-1 and GLP-2), and enterochromaffin (EC) cells, which release serotonin (5-HT) [36]. The mouse colon also contains a small number of I-cells that express cholecystokinin (CCK) [37] and the human colon contains a small proportion of somatostatin-expressing D-cells [38].
Figure 1. The mechanisms of communication between colon epithelial cells and colon afferents.
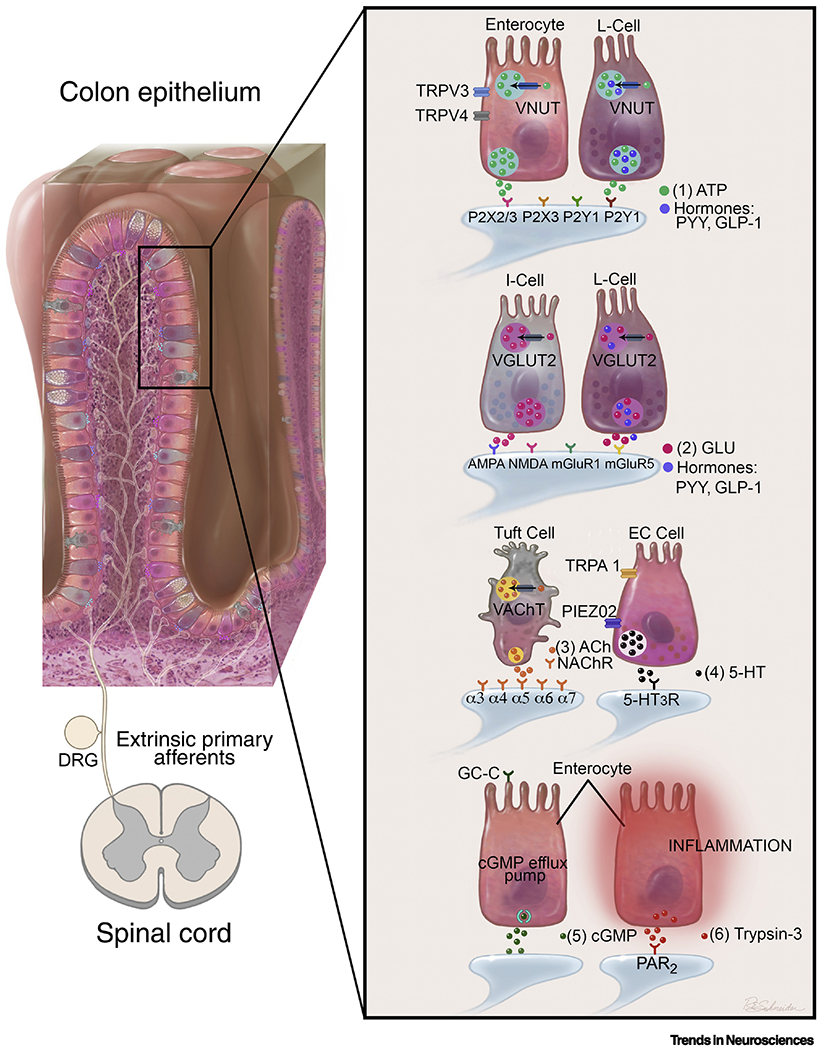
This graphic summarizes some of the key mechanisms of epithelial-neuronal communication, as discussed in this review. Neuroactive substances released by specific epithelial cell types are illustrated, along with the corresponding receptors that are found on colon afferent terminals. In some cells, mechanisms of release are also illustrated, including the ion channels and vesicular transporters involved. 1) ATP: Both enterocytes and EECs release ATP (along with other cell types) and VNUT expression is ubiquitous in the epithelium. ATP is also co-released with hormones from L-cells [49–51, 56]. 2) Glu: VGLUT2 is expressed in I-cells and L-cells, and glutamate is co-released with hormones from L-cells [18, 47, 77]. 3) ACh: VAChT is specifically expressed in tuft cells [85]. 4) 5-HT: EC cells are a primary source of 5-HT and express the sensory receptors TRPA1 and Piezo2. Evidence shows that 5-HT may be released upon activation of these receptors [22, 41, 45, 69]. 5) cGMP: Activation of the GC-C receptor on enterocytes causes release of cGMP [100, 101]. 6) Trypsin-3: Epithelial cells express trypsin-3 and release it in the presence of inflammation [96]. (Illustration credit: Roy Schneider)
Although EECs comprise about 1% of the epithelium, they are the most likely cell type to participate in epithelial-neuronal communication in the colon. EECs function as endocrine regulators; they sense luminal content and release hormones that regulate secretion, motility and satiety signals [39]. EECs express many receptors typically found on afferent neurons, e.g., taste receptors that sense glucose, amino acids and fatty acids [40]. EECs also express receptors implicated in mechanotransduction and nociceptive signaling, such as Piezo2 and transient receptor potential ankyrin 1 (TRPA1) [41–45]. These epithelial cells are electrically excitable and can form synaptic structures with neurons [22, 23]. Using EEC-specific fluorescent reporter lines and transcriptional profiling, axon-like basal processes (a.k.a. neuropods) and presynaptic vesicle proteins were identified [46]. Other studies targeted rabies virus to EECs in the colon and demonstrated synaptic connectivity between EECs (specifically, PYY-expressing cells) and surrounding neurons [23], which include extrinsic primary afferents with cell bodies in lumbar DRG [47]. This EEC-colon afferent connection provides at least one potential cellular substrate for visceral pain transmission.
Transmitters involved in epithelial-primary afferent communication in the colon
Diverse cell types within the colon express numerous signaling molecules and receptors that are likely to facilitate epithelial-neuronal communication. Some of these are highlighted in Figure 1.
Adenosine triphosphate (ATP)
One mechanism of colon mechanosensory transduction was proposed by Geoffrey Burnstock, a pioneer of the purinergic signaling field, who posited that colon distension leads to ATP release from epithelial cells, which then acts on purinergic receptors expressed on primary afferents [17]. This hypothesis was supported by studies showing that mechanical stimulation of the mucosa resulted in ATP release and that afferent responses to mechanical stimulation were attenuated in the presence of purinergic receptor antagonists [48]. Distension-evoked release of ATP may be mediated, at least in part, by activation of transient receptor potential channel V4 (TRPV4), which is present in human and mouse epithelial cells [49–51]. TRPV3 channel activation is also linked to ATP release; one study showed that addition of the TRPV3 agonist carvacrol to cultured colon epithelial cells increased ATP in culture supernatants [52].
The mechanisms that underlie ATP release from epithelial cells are only partially known. As shown for neurons and adrenal chromaffin cells [53, 54]. ATP may be released in combination with other neurotransmitters from enterochromaffin cells or other EECs [55]. ATP can also be co-released with hormones from EECs, e.g., L-cells release ATP along with GLP-1 and PYY [56]. ATP is also likely released on its own from diverse epithelial cell types. Ubiquitous expression of vesicular nucleotide transporter (VNUT) in the mouse colon epithelium and in human intestinal epithelium cell lines suggest all epithelial types are capable of ATP release. In addition, VNUT-mediated exocytosis of ATP-containing vesicles may be dependent on TRPV4 activation [51]. Other mechanisms of ATP release from colon epithelial cells, including ATP-permeable channels (e.g., pannexins), have not been ruled out [57].
Targets of ATP release on colon afferent endings include ionotropic P2X3 and P2X2/3 receptors [48, 58] as well as metabotropic P2Y1 and P2Y2 receptors [59] expressed by muscular and vascular colon afferents [58, 59]. Recent findings from our group support these targets, as epithelial-neuronal signaling was blocked using a cocktail of P2X3, P2X2/3, and P2Y2 antagonists [21]. Additionally, ATP released during distension has been shown to activate and sensitize TRPV1 channels on colon afferents [60].
Models of visceral hypersensitivity suggest inflammation-induced changes occur in purinergic signaling. In the trinitro benzene sulfonic acid (TNBS) model of IBD, increased distension-evoked ATP release augmented colon afferent firing in response to ATP application and this was correlated with upregulation of P2X3 receptors in lumbosacral dorsal root ganglia [61]. ATP signaling is also implicated in colon hypersensitivity resulting from intracolonic infusion of zymosan (a model of post-infectious IBS), based in part on the absence of hypersensitivity in P2X3−/− mice [58]. In addition, P2X3 protein level is higher in colon biopsies from IBD subjects (which is often accompanied by persistent pain) compared with control tissue [62]. The authors hypothesized that both submucosal enteric neurons and primary afferent nerve endings contributed to the increase in ATP. Together, these studies suggest that inflammation-induced changes in ATP release (possibly from epithelial cells) and ATP receptor activity (on colon sensory neurons) contribute to hypersensitivity.
Serotonin (5-hydroxytryptophan)
Serotonin (5-HT) in the gut regulates peristalsis, secretion and nociceptive signaling. Enterochromaffin (EC) cells make up less than 1% of the epithelium, yet they synthesize and release over 95% of the body’s 5-HT [19]. The 5-HT release machinery, located at the basal surface of EC cells, is in close apposition to nerve fibers [22, 63]. Secretory granules at the apical surface may facilitate release of 5-HT into the gut lumen [64]. EC cells are the major source of 5-HT whereas virtually all epithelial cells in the gut express the serotonin-selective reuptake transporter (SERT) and thus take part in controlling 5-HT levels [65].
The excitatory ionotropic 5-HT3 receptor (5-HT3R) is widely expressed on colon afferents [32] and has been implicated in visceral pain signaling. In the mouse model of dextran sulfate sodium (DSS)-induced colitis, an increase in 5-HT3R-positive nerve fibers in the colon mucosa was measured [66]. In vivo studies of colon sensitivity, measured by responses to colorectal distension (CRD), have shown that i.v. administration of alosetron, a 5-HT3R antagonist, diminishes these responses. Alosetron administration also decreased c-Fos positive neurons in the spinal cord dorsal horn in response to CRD [67]. Importantly, alosetron has been used to treat IBS-D (diarrhea-predominant) and randomized controlled trials showed pain relief. However, due to side effects of constipation and colitis, alosetron was withdrawn from the market [68].
A study examining both intestinal cryosections and organoids derived from small intestine tissue showed that EC cells form synapse-like contacts with 5-HT3R-expressing nerve endings [22]. In the same study, functional connectivity between EC cells and nerve fibers was revealed using a colon-nerve ex vivo preparation in which selective pharmacological activation of EC cells caused action potential firing in 5-HT3 positive colon mucosal afferents, presumably via the release of 5-HT [22]. Communication between nerves and EC cells could be initiated via EC expressed molecules that transduce sensory stimuli. For example, studies show that some EC cells express the mechanosensitive Piezo2 receptor, localized adjacent to 5-HT vesicles [45, 69]. Stretching of the colon tissue can activate Piezo2, providing a link between sensations associated with colon distension and 5-HT signaling. However, it should be noted that colon mechanotransduction is likely to be a shared responsibility of EC cells and colon afferents; colon afferents also express Piezo2, most frequently in neurofilament-positive populations [32]. TRPA1 is another potential stimulus-transducing molecule expressed by EC cells. In the context of gustatory sensation, this ligand-gated receptor is responsible for the oral cavity’s ability to detect molecules like allyl isothiocyanate (found in mustard oil, wasabi, and horseradish) and cinnamaldehyde (found in cinnamon). The presence of TRPA1 in colon EC cells supports the concept that these cells can act as “taste buds” of the colon [70] and as sensors of chemical irritants. Like Piezo2, TRPA1 is expressed in colon afferents and its deletion degrades, but does not eliminates mechanosensory function [71], indicating mechanistic redundancy for this sensory modality that could include a role for the epithelium.
Several studies have examined the role of 5-HT in visceral pain disorders. One study reported that inflamed colon tissue samples from IBD patients have decreased 5-HT compared to controls, decreased SERT levels and fewer 5-HT-immunoreactive EC cells per crypt in the mucosa [72]. In contrast, animal models of colitis (TNBS and DSS) display increases in EC cell count and mucosal 5-HT content [73, 74]. The discrepancy in EC cell count and 5-HT levels could be due to limitations of the models, where acute colitis does not directly compare to the chronic inflammation in human IBD [63].
Studies of 5-HT signaling in IBS have also shown conflicting results. One study compared patients with IBS-D (diarrhea-predominant) and IBS-C (constipation-predominant). Both groups had decreased mucosal SERT expression and no change in EC density or in tissue agitation-evoked 5-HT release [72]. Another study showed that colon samples from post-infectious (PI) IBS patients had significantly more 5-HT-immunoreactive EC cells per imaging field compared to healthy controls, whereas non-PI-IBS samples showed no difference in EC cell count [75]. In an analysis of samples from IBS patients of all phenotypes, no difference in EC cell count or 5-HT content was found between patients with and without hypersensitivity [76]. Thus, 5-HT signaling may be altered in IBS, but its role in colon hypersensitivity and pain remain unclear.
Glutamate
Glutamate is another epithelial-derived transmitter involved in sensory signaling. PYY- and CCK-expressing EECs have been shown to release glutamate [47] and the vesicular glutamate transporter 2 (VGLUT2) colocalizes with PYY and GLP-1 throughout the intestines [77]. Additional culture studies using a neuroendocrine cell line (GLUTag) showed that glutamate is co-released with GLP-1 in response to KCl or glucose [18].
Glutamate receptors are expressed by colon afferents and are implicated in visceral hypersensitivity [78]. Single cell RNA sequencing indicates several types of glutamate receptors are expressed across subtypes of colon afferents, including the ionotropic AMPA and NMDA receptors, and the metabotropic receptors mGluR1-5 [32]. Studies in rat showed mGluR5 antagonists diminish visceromotor responses to CRD and, in an ex vivo colon-nerve preparation, decrease muscular and serosal colon afferent responses to mechanical stimulation [79]. Changes in NMDA receptors (NMDAR) are also associated with IBS. Specifically, IBS patients were shown to have upregulation of NMDAR, which correlated with abdominal pain scores, whereas mice showed increased sensitivity to colorectal distension in response to intracolonic administration of NMDA [80]. These findings complemented previous reports showing that NMDAR antagonists prevented hypersensitivity in a mouse model of TNBS-induced colitis [81].
A recent study employed rabies virus retrograde tracing in mice to show that PYY- and CCK-positive EECs synapse with DRG afferent fibers [47]. Many EECs were shown to co-express VGLUT2 and CCK. Optogenetic activation in vitro confirmed that CCK-positive EECs release glutamate, causing activation of vagal neurons. These studies support the idea that EECs use glutamate to signal to colon afferents.
Acetylcholine (ACh)
The ACh synthesizing enzyme choline acetyltransferase (ChAT) is one of the commonly used indicators of ACh production. Expression of ChAT in the intestinal epithelium has been recognized for decades [82]. ACh may be an important transmitter between tuft cells, which express ChAT, and surrounding neurons [83]. The vesicular acetylcholine transporter (VAChT), another indicator of ACh production, has been detected via immunohistochemistry in both human [84] and mouse [85] intestinal epithelium. In mice, VAChT is expressed in only about 20% of ChAT-expressing tuft cells in the proximal colon and not detected in distal colon or small intestine [85]. In VAChT+ tuft cells, ACh may be released through synaptic vesicles, but in VAChT− cells, ACh may be released via organic cation transporters [86], or gap junctions [87].
Nicotinic ACh receptors (nAChR) are expressed on colon afferents, but their role in visceral pain is unclear. Single cell RNAseq analysis has shown high levels of nAChRα subunits 3-7 in colon afferents [32]. Another study showed nAChRα3 expression in about 50% of peptidergic afferents that innervate visceral organs. This study also showed that in skin, mechanically insensitive afferents (MIAs), also known as “silent” nociceptors, can be defined by expression of nAChRα3 [88], raising the possibility that MIAs that innervate the colon also express nAChRα3.
Although tuft cells represent only 0.4% of intestinal epithelial cells (in the mouse), they have an important role in mediating immune responses [34]. They are a primary source of interleukin-25, which recruits helper T cells and innate lymphoid cells during infection [89]. They also express cyclooxygenase enzymes (COX1 and COX2), which produce inflammatory prostaglandins [90]. Recent evidence suggests tuft cells also initiate a type 2 immune circuit in response to succinate, a metabolite secreted by parasitic helminths [91]. Visceral hypersensitivity, which often occurs with infection, is thought to result from an inflammatory response to the parasite. As suggested by this study, this response may be initiated by succinate activation of tuft cells and subsequent activation of sensory signaling pathways. The role of tuft cells in prolonged inflammatory conditions such as IBD is unclear, but alteration in the gut’s non-neuronal cholinergic system is a possible link. Evidence consistent with this idea comes from immunohistochemical and mRNA analyses of human colon tissue, which show that patients with ulcerative colitis expressed significantly lower levels of ChAT and VAChT in the colon epithelium [84].
Proteases
Proteases released by colon epithelial cells are likely regulators of colon afferent hypersensitivity. Supernatants obtained from IBS colon biopsies increase the excitability of sensory neurons in culture, an effect that is blocked by protease inhibitors [92]. These supernatants also induce visceral hypersensitivity in mice, but not in the presence of a protease-activated receptor-2 (PAR2) antagonist or in PAR2-deficient mice [93]. In addition, application of a PAR2 agonist (SLIGRL-NH2) into the colon lumen results in hypersensitivity to colorectal distension and increased Fos expression in the spinal cord dorsal horn [94]. Electrophysiology studies have shown PAR2 sensitizes serosal afferents via a TRPV4-dependent mechanism [95]. Colon epithelial cells, enterocytes in particular, are a source of proteases; addition of lipopolysaccharide (LPS) to Caco-2 cells in culture caused release of the protease trypsin-3 [96]. Trypsin-3 has been shown to induce colorectal hypersensitivity in a PAR2-dependent manner and is increased in epithelium of colon biopsies of human IBS patients and rat IBS models [96].
Efforts to identify proteases active in IBS and IBD patients are ongoing. Functional proteomic assays of colon tissue and supernatants from IBD patients (both ulcerative colitis and Crohn’s disease), showed increased trypsin-like activity as well as overactive cathepsin G and thrombin activity in IBD patients [97]. Flow human colon afferents respond to proteases is also unclear; although PAR2 is expressed in 40% of human sensory afferent neurons, neuronal responses to supernatants from IBS patients are mediated by PAR1 [98].
Cyclic guanosine-3’,5’-monophosphate (cGMP)
Signaling molecules released from colon epithelial cells may also inhibit neural activity. One such molecule is cGMP, which is released upon activation of the epithelial guanylate cyclase C (GC-C) receptor [99]. Linaclotide, a drug prescribed for IBS-C for relief of pain and constipation, is a peptide agonist of GC-C, a transmembrane receptor expressed on the luminal aspect of the intestinal epithelium that also binds bacterial enterotoxins (responsible for traveler’s diarrhea) and peptide hormones (e.g., guanylin) [100]. In randomized controlled trials, linaclotide reduced abdominal pain in over 60% of patients [101]. Linaclotide’s binding to GC-C stimulates the synthesis and release of cGMP from epithelial cells, which has effects that are twofold: stimulation of fluid production in the intestinal lumen and inhibition of colon afferent activity [102]. The increase in epithelial cGMP initiates a protein kinase-dependent pathway which activates the cystic fibrosis transmembrane regulator (CFTR), increasing secretion of bicarbonate and chloride into the lumen. These secretions inhibit the sodium/hydrogen exchanger, leading to fluid secretion into the lumen [103]. The increased production of cGMP in the epithelial cells leads to more extracellular cGMP, which has been shown to inhibit colon afferent firing via a membrane receptor target, though little is known about the identity of this target [104]. Linaclotide itself does not reduce afferent excitability; only cGMP released from the epithelium has this effect.
In rodent ex vivo preparations, cGMP applied to the colon lumen decreased colon afferent firing rates [101, 105]. This effect was more robust in a mouse model of TNBS-induced colon inflammation [104]. Patch clamp analysis of cultured human DRG neurons also showed greater effectiveness of cGMP in the presence of inflammatory mediators [104]. Although the mechanisms involved in cGMP-induced inhibition remain unclear, further investigation of cGMP targets is warranted as this approach has significant therapeutic potential.
Concluding remarks and future perspectives
Colon epithelial cells, EECs in particular, are likely to have significant influence on the activity of colon afferents and visceral pain signaling. The neuroactivators released by epithelial cells that are most likely to be involved in this epithelial-neuronal communication are summarized in Table 1. This table shows each neurotransmitter and its epithelial cell sources, as well as the model systems and techniques that have been used to identify the sources of each transmitter.
Table 1.
Major regulators of epithelial-neuronal communication in the small intestine and colon.
| Transmitter | Epithelial Cell Source | Model System | Techniques Used to Identify Source of Transmitter | Ref |
|---|---|---|---|---|
| Acetylcholine (ACh) | Tuft cells | Mouse: small intestine and colon tissue | Immunohistochemistry (IHC), in situ hybridization, transgenic fluorescent reporter | [82] |
| Adenosine triphosphate (ATP) | All | Rat: colon tissue | Luciferin-luciferase ATP release assay | [48] |
| All | Human: CCD 841 cell line | Luciferin-luciferase ATP release assay | [51] | |
| All | Mouse: colon epithelium primary culture | Luciferin-luciferase ATP release assay | [52] | |
| L-cells | GLUTag cell line Mouse: cultures of small intestine epithelium Human: culture of colon epithelium |
Epithelial-neuronal co-cultures, luciferin-luciferase ATP release assay, sniffer patch IHC |
[56] | |
| All | CCD 841 cell line Mouse: colon tissue |
qRT-PCR, IHC, luciferin-luciferase assay qRT-PCR, IHC |
[51] | |
| Glutamate (Glu) | L-cells | Rat: small intestine tissue | IHC | [77] |
| L-cells | GLUTag cell line | In vitro chemically evoked release | [18] | |
| I-cells | Mouse: small intestine tissue | IHC, in vitro optogenetics | [47] | |
| Cyclic guanosine-3’,5’-monophosphate (cGMP) | All | Human: T84 cell line | Enzyme immunoassay | [102] |
| Serotonin (5-HT) | EC cells | Rat: small intestine tissue | Immunoelectron microscopy | [64] |
| EC cells | Mouse: small intestine, cultured organoids, ex vivo colon-nerve preparation | IHC, in vitro electrophysiology, 5-HT biosensors (sniffer patch) | [22] | |
| EC cells | Mouse: small intestine and colon, cultured organoids | IHC, in vitro calcium imaging, 5-HT biosensors (sniffer patch) | [69] | |
| Trypsin-3 | All | Rat: colon tissue Human: colon tissue, Caco-2 cell line |
IHC, Western blot | [96] |
As summarized in Figure 1, colon epithelial cells express some of the same sensory receptors that are found in colon afferents, indicating that they have a role in monitoring the environment in the gut lumen. This is supported by studies showing that colon afferent responses to mechanical stimuli are diminished when epithelium-released neurotransmitters are blocked [21, 48]. In many cases, transmission from epithelial cells may be diffuse and have slow or indirect actions on colon afferent terminals. However, communication from some EECs to colon afferents may be achieved through direct synaptic transmission. Enterochromaffin cells (ECs) are one type of EEC that show evidence of these synapses and they contain the receptors TRPA1 and Piezo2, which are critical in mechanosensation and pain signaling [22, 69]. This receptor expression profile and connectivity with surrounding colon afferents may indicate that ECs have a more salient role in sensory signaling than other cell types.
Data discussed in the previous sections support the hypothesis that the combination of all epithelial cell types is necessary for normal sensation in the colon, but further studies are needed for confirmation (see Outstanding Questions). To determine the contribution of each cell type to sensory signaling, it would be imperative to develop cell type-specific manipulation paradigms applicable to the colon physiology, for instance via optogenetics and chemogenetics. More comprehensive studies are also needed to identify all sensory receptors expressed in each colon epithelial cell type, similar to the single-cell RNAseq analysis of small intestine epithelium [106]. Molecular techniques should also be employed to determine whether other epithelial-released molecules, such as neuropeptides, play a role in modulation of colon afferent activity. Functional analysis of specific colon afferent receptors is also needed to determine which receptors are important in epithelial-neural signaling.
Outstanding Questions.
Can visceral pain be triggered by a single epithelial cell type, or does this signaling depend on a combination of cell types?
Several sensory receptors have been reported as expressed by specific colon epithelial cell types. Are there other such sensory receptors in addition to those already identified?
What other epithelial-released neuroactive molecules influence colon afferent activity?
How is the release of neurotransmitters from epithelial cells affected in inflammatory conditions such as IBD and IBS?
Can epithelial cells be a therapeutic target for colon associated pain?
Acute and chronic inflammation likely affect epithelial-neuronal signaling in the colon, and more studies are required to examine how alterations at this interface affect disease processes and symptoms. One possibility is that, like neurons, epithelial cells become hypersensitive with inflammation, thus amplifying the sensory signaling pathways. This could occur via inflammation-induced changes in voltage-sensitive channels that regulate electrically excitable epithelial cell types (such as ECs) and/or via changes in proteins that regulate exocytosis/release of neuroactive substances. In addition, appropriate animal models that elicit the hallmarks of colon diseases with different etiology should be employed (e.g., DSS or TNBS (IBD), zymosan or repeated stress (IBS), and parasite infection models) to assess the full range of epithelial responses to different disease challenges. A thorough analysis of epithelial changes will require multimodal strategies including calcium imaging and electrophysiology techniques, high resolution anatomical analysis (e.g., 3D electron microscopic reconstruction) as well as neurotransmitter release assays. This comprehensive approach is required to fully understand the complexity of epithelial-nerve interactions.
Highlights:
Colon epithelial cells express some of the same mechano- and chemosensors expressed on extrinsic colon afferents, and they release neurotransmitters that can act on these neurons.
Specific activation of colon epithelial cells can initiate action potential firing in colon afferents and pain-like responses, suggesting a significant role for these cells in sensory transduction and pain signaling.
Mediators of colon epithelial cell-colon afferent communication include ATP, serotonin, glutamate, acetylcholine, proteases, and cGMP.
Alterations in the colon epithelium are common in inflammatory bowel disease and irritable bowel syndrome disorders. It is likely, therefore, that changes in the colon epithelium contribute to the pain associated with these disorders.
Acknowledgements
We thank Mr. Roy E. Schneider (University of Toledo, OH) for the graphic design of the illustration in Figure 1 and Dr. Michael S. Gold for helpful suggestions in the preparation of this manuscript. Grant support from the National Institutes of Health (T32 NS073548, T32DK063922, 10T2 OD023859 and R01AR069951) is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abdul Rani R, Raja Ali RA, and Lee YY, Irritable bowel syndrome and Inflammatory bowel disease overlap syndrome: pieces of the puzzle are falling into place. Intest Res, 2016. 14(4): p. 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gold MS and Gebhart GF, Nociceptor sensitization in pain pathogenesis. Nat Med, 2010. 16(11): p. 1248–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azpiroz F, et al. , Mechanisms of hypersensitivity in IBS and functional disorders. Neurogastroenterol Motil, 2007. 19(1 Suppl): p. 62–88. [DOI] [PubMed] [Google Scholar]
- 4.Baumbauer KM, et al. , Keratinocytes can modulate and directly initiate nociceptive responses. Elife, 2015. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moore C, et al. , UVB radiation generates sunburn pain and affects skin by activating epidermal TRPV4 ion channels and triggering endothelin-1 signaling. Proc Natl Acad Sci U S A, 2013. 110(34): p. E3225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pang Z, et al. , Selective keratinocyte stimulation is sufficient to evoke nociception in mice. Pain, 2015. 156(4): p. 656–65. [DOI] [PubMed] [Google Scholar]
- 7.Kohda F, et al. , Histamine-induced IL-6 and IL-8 production are differentially modulated by IFN-gamma and IL-4 in human keratinocytes. J Dermatol Sci, 2002. 28(1): p. 34–41. [DOI] [PubMed] [Google Scholar]
- 8.Shi X, et al. , Neuropeptides contribute to peripheral nociceptive sensitization by regulating interleukin-1beta production in keratinocytes. Anesth Analg, 2011. 113(1): p. 175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Song IS, et al. , Substance P induction of murine keratinocyte PAM 212 interleukin 1 production is mediated by the neurokinin 2 receptor (NK-2R). Exp Dermatol, 2000. 9(1): p. 42–52. [DOI] [PubMed] [Google Scholar]
- 10.Winder M, et al. , Signalling molecules in the urothelium. Biomed Res Int, 2014. 2014: p. 297295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sun Y, et al. , Augmented stretch activated adenosine triphosphate release from bladder uroepithelial cells in patients with interstitial cystitis. J Urol, 2001. 166(5): p. 1951–6. [PubMed] [Google Scholar]
- 12.Peterson LW and Artis D, Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol, 2014. 14(3): p. 141–53. [DOI] [PubMed] [Google Scholar]
- 13.Roda G, et al. , Intestinal epithelial cells in inflammatory bowel diseases. World J Gastroenterol, 2010. 16(34): p. 4264–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akbar A, Walters JR, and Ghosh S, Review article: visceral hypersensitivity in irritable bowel syndrome: molecular mechanisms and therapeutic agents. Aliment Pharmacol Ther, 2009. 30(5): p. 423–35. [DOI] [PubMed] [Google Scholar]
- 15.Sharkey KA and Kroese AB, Consequences of intestinal inflammation on the enteric nervous system: neuronal activation induced by inflammatory mediators. Anat Rec, 2001262(1): p. 79–90. [DOI] [PubMed] [Google Scholar]
- 16.Bielefeldt K, Davis B, and Binion DG, Pain and inflammatory bowel disease. Inflamm Bowel Dis, 2009. 15(5): p. 778–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burnstock G, Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci, 2001. 22(4): p. 182–8. [DOI] [PubMed] [Google Scholar]
- 18.Uehara S, et al. , Vesicular storage and secretion of L-glutamate from glucagon-like peptide 1-secreting clonal intestinal L cells. J Neurochem, 2006. 96(2): p. 550–60. [DOI] [PubMed] [Google Scholar]
- 19.Gershon MD, 5-Hydroxytryptamine (serotonin) in the gastrointestinal tract. Curr Opin Endocrinol Diabetes Obes, 2013. 20(1): p. 14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Klapproth H, et al. , Non-neuronal acetylcholine, a signalling molecule synthezised by surface cells of rat and man. Naunyn Schmiedebergs Arch Pharmacol, 1997. 355(4): p. 515–23. [DOI] [PubMed] [Google Scholar]
- 21.Makadia PA, et al. , Optogenetic Activation of Colon Epithelium of the Mouse Produces High-Frequency Bursting in Extrinsic Colon Afferents and Engages Visceromotor Responses. J Neurosci, 2018. 38(25): p. 5788–5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bellono NW, et al. , Enterochromaffin Cells Are Gut Chemosensors that Couple to Sensory Neural Pathways. Cell, 2017. 170(1): p. 185–198 e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bohorquez DV, et al. , Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J Clin Invest, 2015. 125(2): p. 782–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brierley SM, Hibberd TJ, and Spencer NJ, Spinal Afferent Innervation of the Colon and Rectum. Front Cell Neurosci, 2018. l2: p. 467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brookes SJ, et al. , Extrinsic primary afferent signalling in the gut. Nat Rev Gastroenterol Hepatol, 2013. 10(5): p. 286–96. [DOI] [PubMed] [Google Scholar]
- 26.Brierley SM, et al. , Splanchnic and pelvic mechanosensory afferents signal different qualities of colonic stimuli in mice. Gastroenterology, 2004. 127(1): p. 166–78. [DOI] [PubMed] [Google Scholar]
- 27.Hughes PA, et al. , Post-inflammatory colonic afferent sensitisation: different subtypes, different pathways and different time courses. Gut, 2009. 58(10): p. 1333–41. [DOI] [PubMed] [Google Scholar]
- 28.Malin SA, et al. , TPRV1 expression defines functionally distinct pelvic colon afferents. J Neurosci, 2009. 29(3): p. 743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brierley SM, et al. , Selective role for TRPV4 ion channels in visceral sensory pathways. Gastroenterology, 2008. 134(7): p. 2059–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng B and Gebhart GF, Characterization of silent afferents in the pelvic and splanchnic innervations of the mouse colorectum. Am J Physiol Gastrointest Liver Physiol, 2011. 300(1): p. G170–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hockley JRF, Smith ESJ, and Bulmer DC, Human visceral nociception: findings from translational studies in human tissue. Am J Physiol Gastrointest Liver Physiol, 2018. 315(4): p. G464–G472. [DOI] [PubMed] [Google Scholar]
- 32.Hockley JRF, et al. , Single-cell RNAseq reveals seven classes of colonic sensory neuron. Gut, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wong AC, Vanhove AS, and Watnick PI, The interplay between intestinal bacteria and host metabolism in health and disease: lessons from Drosophila melanogaster. Dis Model Mech, 2016. 9(3): p. 271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gerbe F and Jay P, Intestinal tuft cells: epithelial sentinels linking luminal cues to the immune system. Mucosal Immunol, 2016. 9(6): p. 1353–1359. [DOI] [PubMed] [Google Scholar]
- 35.Leushacke M and Barker N, Ex vivo culture of the intestinal epithelium: strategies and applications. Gut, 2014. 63(8): p. 1345–54. [DOI] [PubMed] [Google Scholar]
- 36.Gunawardene AR, Corfe BM, and Staton CA, Classification and functions of enteroendocrine cells of the lower gastrointestinal tract. Int J Exp Pathol, 2011. 92(4): p. 219–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egerod KL, et al. , A major lineage of enteroendocrine cells coexpress CCK, secretin, GIP, GLP-1, PYY, and neurotensin but not somatostatin. Endocrinology, 2012. 153(12): p. 5782–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sjolund K, et al. , Endocrine cells in human intestine: an immunocytochemical study. Gastroenterology, 1983. 85(5): p. 1120–30. [PubMed] [Google Scholar]
- 39.Janssen S and Depoortere I, Nutrient sensing in the gut: new roads to therapeutics? Trends Endocrinol Metab, 2013. 24(2): p. 92–100. [DOI] [PubMed] [Google Scholar]
- 40.Latorre R, et al. , Enteroendocrine cells: a review of their role in brain-gut communication. Neurogastroenterol Motil, 2016. 28(5): p. 620–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Doihara H, et al. , QGP-1 cells release 5-HT via TRPA1 activation; a model of human enterochromaffin cells. Mol Cell Biochem, 2009. 331(1-2): p. 239–45. [DOI] [PubMed] [Google Scholar]
- 42.Murthy SE, et al. , The mechanosensitive ion channel Piezo2 mediates sensitivity to mechanical pain in mice. Sci Transl Med, 2018. 10(462). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nozawa K, et al. , TRPA1 regulates gastrointestinal motility through serotonin release from enterochromaffin cells. Proc Natl Acad Sci U S A, 2009. 106(9): p. 3408–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Szczot M, et al. , PIEZO2 mediates injury-induced tactile pain in mice and humans. Sci Transl Med, 2018. 10(462). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang F, et al. , Mechanosensitive ion channel Piezo2 is important for enterochromaffin cell response to mechanical forces. J Physiol, 2017. 595(1): p. 79–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bohorquez DV and Liddle RA, Axon-like basal processes in enteroendocrine cells: characteristics and potential targets. Clin Transl Sci, 20114(5): p. 387–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaelberer MM, et al. , A gut-brain neural circuit for nutrient sensory transduction. Science, 2018. 361(6408). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wynn G, et al. , Purinergic mechanisms contribute to mechanosensory transduction in the rat colorectum. Gastroenterology, 2003. 125(5): p. 1398–409. [DOI] [PubMed] [Google Scholar]
- 49.Cenac N, et al. , Transient receptor potential vanilloid-4 has a major role in visceral hypersensitivity symptoms. Gastroenterology, 2008. 135(3): p. 937–46, 946, e1–2. [DOI] [PubMed] [Google Scholar]
- 50.D’Aldebert E, et al. , Transient receptor potential vanilloid 4 activated inflammatory signals by intestinal epithelial cells and colitis in mice. Gastroenterology, 2011. 140(1): p. 275–85. [DOI] [PubMed] [Google Scholar]
- 51.Mihara H, et al. , Involvement of VNUT-exocytosis in transient receptor potential vanilloid 4-dependent ATP release from gastrointestinal epithelium. PLoS One, 2018. 13(10): p. e0206276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ueda T, et al. , TRPV3, a thermosensitive channel is expressed in mouse distal colon epithelium. Biochem Biophys Res Commun, 2009. 383(1): p. 130–4. [DOI] [PubMed] [Google Scholar]
- 53.Burnstock G, Purinergic cotransmission. Brain Res Bull, 1999. 50(5-6): p. 355–7. [DOI] [PubMed] [Google Scholar]
- 54.Zhang Q, et al. , Differential Co-release of Two Neurotransmitters from a Vesicle Fusion Pore in Mammalian Adrenal Chromaffin Cells. Neuron, 2019. 102(1): p. 173–183 e4. [DOI] [PubMed] [Google Scholar]
- 55.Winkler H and Westhead E, The molecular organization of adrenal chromaffin granules. Neuroscience, 1980. 5(11): p. 1803–23. [DOI] [PubMed] [Google Scholar]
- 56.Lu VB, et al. , Adenosine triphosphate is co-secreted with glucagon-like peptide-1 to modulate intestinal enterocytes and afferent neurons. Nat Commun, 2019. 10(1): p. 1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwiebert EM and Zsembery A, Extracellular ATP as a signaling molecule for epithelial cells. Biochim Biophys Acta, 2003. 1615(1-2): p. 7–32. [DOI] [PubMed] [Google Scholar]
- 58.Shinoda M, Feng B, and Gebhart GF, Peripheral and central P2X receptor contributions to colon mechanosensitivity and hypersensitivity in the mouse. Gastroenterology, 2009. 137(6): p. 2096–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hockley JR, et al. , P2Y Receptors Sensitize Mouse and Human Colonic Nociceptors. J Neurosci, 2016. 36(8): p. 2364–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lakshmi S and Joshi PG, Co-activation of P2Y2 receptor and TRPV channel by ATP: implications for ATP induced pain. Cell Mol Neurobiol, 2005. 25(5): p. 819–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wynn G, et al. , Purinergic component of mechanosensory transduction is increased in a rat model of colitis. Am J Physiol Gastrointest Liver Physiol, 2004. 287(3): p. G647–57. [DOI] [PubMed] [Google Scholar]
- 62.Yiangou Y, et al. , ATP-gated ion channel P2X(3) is increased in human inflammatory bowel disease. Neurogastroenterol Motil, 2001. 13(4): p. 365–9. [DOI] [PubMed] [Google Scholar]
- 63.Mawe GM and Hoffman JM, Serotonin signalling in the gut--functions, dysfunctions and therapeutic targets. Nat Rev Gastroenterol Hepatol, 2013. 10(8): p. 473–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fujimiya M, Okumiya K, and Kuwahara A, Immunoelectron microscopic study of the luminal release of serotonin from rat enterochromaffin cells induced by high intraluminal pressure. Histochem Cell Biol, 1997. 108(2): p. 105–13. [DOI] [PubMed] [Google Scholar]
- 65.Wade PR, et al. , Localization and function of a 5-HTtransporter in crypt epithelia of the gastrointestinal tract. J Neurosci, 1996. 16(7): p. 2352–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matsumoto K, et al. , Experimental colitis alters expression of 5-HT receptors and transient receptor potential vanilloid 1 leading to visceral hypersensitivity in mice. Lab Invest, 2012. 92(5): p. 769–82. [DOI] [PubMed] [Google Scholar]
- 67.Kozlowski CM, et al. , The 5-HT(3) receptor antagonist alosetron inhibits the colorectal distention induced depressor response and spinal c-fos expression in the anaesthetised rat. Gut, 2000. 46(4): p. 474–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fayyaz M and Lackner JM, Serotonin receptor modulators in the treatment of irritable bowel syndrome. Ther Clin Risk Manag, 2008. 4(1): p. 41–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alcaino C, et al. , A population of gut epithelial enterochromaffin cells is mechanosensitive and requires Piezo2 to convert force into serotonin release. Proc Natl Acad Sci U S A, 2018. 115(32): p. E7632–E7641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bertrand PP, The cornucopia of intestinal chemosensory transduction. Front Neurosci, 2009. 3: p. 48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Brierley SM, et al. , The ion channel TRPA1 is required for normal mechanosensation and is modulated by algesic stimuli. Gastroenterology, 2009. 137(6): p. 2084–2095 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Coates MD, et al. , Molecular defects in mucosal serotonin content and decreased serotonin reuptake transporter in ulcerative colitis and irritable bowel syndrome. Gastroenterology, 2004. 126(7): p. 1657–64. [DOI] [PubMed] [Google Scholar]
- 73.Bertrand PP, et al. , Analysis of real-time serotonin (5-HT) availability during experimental colitis in mouse. Am J Physiol Gastrointest Liver Physiol, 2010. 298(3): p. G446–55. [DOI] [PubMed] [Google Scholar]
- 74.Linden DR, et al. , Serotonin transporter function and expression are reduced in mice with TNBS-induced colitis. Neurogastroenterol Motil, 2005. 17(4): p. 565–74. [DOI] [PubMed] [Google Scholar]
- 75.Lee KJ, et al. , The alteration of enterochromaffin cell, mast cell, and lamina propria T lymphocyte numbers in irritable bowel syndrome and its relationship with psychological factors. J Gastroenterol Hepatol, 2008. 23(11): p. 1689–94. [DOI] [PubMed] [Google Scholar]
- 76.Kerckhoffs AP, et al. , SERT and TPH-1 mRNA expression are reduced in irritable bowel syndrome patients regardless of visceral sensitivity state in large intestine. Am J Physiol Gastrointest Liver Physiol, 2012. 302(9): p. G1053–60. [DOI] [PubMed] [Google Scholar]
- 77.Hayashi M, et al. , Expression and localization of vesicular glutamate transporters in pancreatic islets, upper gastrointestinal tract, and testis. J Histochem Cytochem, 2003. 51(10): p. 1375–90. [DOI] [PubMed] [Google Scholar]
- 78.Blackshaw LA, Page AJ, and Young RL, Metabotropic glutamate receptors as novel therapeutic targets on visceral sensory pathways. Front Neurosci, 2011. 5: p. 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lindstrom E, et al. , Involvement of metabotropic glutamate 5 receptor in visceral pain. Pain, 2008. 137(2): p. 295–305. [DOI] [PubMed] [Google Scholar]
- 80.Qi Q, et al. , Colonic N-methyl-d-aspartate receptor contributes to visceral hypersensitivity in irritable bowel syndrome. J Gastroenterol Hepatol, 2017. 32(4): p. 828–836. [DOI] [PubMed] [Google Scholar]
- 81.Qi QQ, et al. , Colonic mucosal N-methyl-D-aspartate receptor mediated visceral hypersensitivity in a mouse model of irritable bowel syndrome. J Dig Dis, 2016. 17(7): p. 448–57. [DOI] [PubMed] [Google Scholar]
- 82.Porter AJ, et al. , Choline acetyltransferase immunoreactivity in the human small and large intestine. Gastroenterology, 1996. 111(2): p. 401–8. [DOI] [PubMed] [Google Scholar]
- 83.Gautron L, et al. , Neuronal and nonneuronal cholinergic structures in the mouse gastrointestinal tract and spleen. J Comp Neurol, 2013. 521(16): p. 3741–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jonsson M, Norrgard O, and Forsgren S, Presence of a marked nonneuronal cholinergic system in human colon: study of normal colon and colon in ulcerative colitis. Inflamm Bowel Dis, 2007. 13(11): p. 1347–56. [DOI] [PubMed] [Google Scholar]
- 85.Schutz B, et al. , Chemical coding and chemosensory properties of cholinergic brush cells in the mouse gastrointestinal and biliary tract. Front Physiol, 2015. 6: p. 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wessler I, et al. , Release of non-neuronal acetylcholine from the isolated human placenta is mediated by organic cation transporters. Br J Pharmacol, 2001. 134(5): p. 951–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang YA and Roper SD, Intracellular Ca(2+) and TRPM5-mediated membrane depolarization produce ATP secretion from taste receptor cells. J Physiol, 2010. 588(Pt 13): p. 2343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Prato V, et al. , Functional and Molecular Characterization of Mechanoinsensitive “Silent” Nociceptors. Cell Rep, 2017. 21(11): p. 3102–3115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fallon PG, et al. , Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J Exp Med, 2006. 203(4): p. 1105–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bezencon C, et al. , Murine intestinal cells expressing Trpm5 are mostly brush cells and express markers of neuronal and inflammatory cells. J Comp Neurol, 2008. 509(5): p. 514–25. [DOI] [PubMed] [Google Scholar]
- 91.Nadjsombati MS, et al. , Detection of Succinate by Intestinal Tuft Cells Triggers a Type 2 Innate Immune Circuit. Immunity, 2018. 49(1): p. 33–41 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Valdez-Morales EE, et al. , Sensitization of peripheral sensory nerves by mediators from colonic biopsies of diarrhea-predominant irritable bowel syndrome patients: a role for PAR2. Am J Gastroenterol, 2013. 108(10): p. 1634–43. [DOI] [PubMed] [Google Scholar]
- 93.Cenac N, et al. , Role for protease activity in visceral pain in irritable bowel syndrome. J Clin Invest, 2007. 117(3): p. 636–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Coelho AM, et al. , Proteinases and proteinase-activated receptor 2: a possible role to promote visceral hyperalgesia in rats. Gastroenterology, 2002. 122(4): p. 1035–47. [DOI] [PubMed] [Google Scholar]
- 95.Sipe WE, et al. , Transient receptor potential vanilloid 4 mediates protease activated receptor 2-induced sensitization of colonic afferent nerves and visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol, 2008. 294(5): p. G1288–98. [DOI] [PubMed] [Google Scholar]
- 96.Rolland-Fourcade C, et al. , Epithelial expression and function of trypsin-3 in irritable bowel syndrome. Gut, 2017. 66(10): p. 1767–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Denadai-Souza A, et al. , Functional Proteomic Profiling of Secreted Serine Proteases in Health and Inflammatory Bowel Disease. Sci Rep, 2018. 8(1): p. 7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Desormeaux C, et al. , Protease-activated receptor 1 is implicated in irritable bowel syndrome mediators-induced signaling to thoracic human sensory neurons. Pain, 2018. 159(7): p. 1257–1267. [DOI] [PubMed] [Google Scholar]
- 99.Huott PA, et al. , Mechanism of action of Escherichia coli heat stable enterotoxin in a human colonic cell line. J Clin Invest, 1988. 82(2): p. 514–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hannig G, et al. , Guanylate cyclase-C/cGMP: an emerging pathway in the regulation of visceral pain. Front Mol Neurosci, 2014. 7: p. 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Castro J, et al. , Linaclotide inhibits colonic nociceptors and relieves abdominal pain via guanylate cyclase-C and extracellular cyclic guanosine 3′,5′-monophosphate. Gastroenterology, 2013. 145(6): p. 1334–46 e1–11. [DOI] [PubMed] [Google Scholar]
- 102.Busby RW, et al. , Linaclotide, through activation of guanylate cyclase C, acts locally in the gastrointestinal tract to elicit enhanced intestinal secretion and transit. Eur J Pharmacol, 2010. 649(1-3): p. 328–35. [DOI] [PubMed] [Google Scholar]
- 103.Lacy BE, Levenick JM, and Crowell MD, Linaclotide: a novel therapy for chronic constipation and constipation-predominant irritable bowel syndrome. Gastroenterol Hepatol (N Y), 2012. 8(10): p. 653–60. [PMC free article] [PubMed] [Google Scholar]
- 104.Grundy L, et al. , Chronic linaclotide treatment reduces colitis-induced neuroplasticity and reverses persistent bladder dysfunction. JCI Insight, 2018. 3(19). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Feng B, et al. , Activation of guanylate cyclase-C attenuates stretch responses and sensitization of mouse colorectal afferents. J Neurosci, 2013. 33(23): p. 9831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Haber AL, et al. , A single-cell survey of the small intestinal epithelium. Nature, 2017. 551(7680): p. 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]


