Abstract
Context
Across pregnancy, maternal serum cortisol levels increase up to 3-fold. It is not known whether maternal peripheral cortisol metabolism and clearance change across pregnancy or influence fetal cortisol exposure and development.
Objectives
The primary study objective was to compare maternal urinary glucocorticoid metabolites, as markers of cortisol metabolism and clearance, between the second and third trimester of pregnancy. Secondary objectives were to test associations of total maternal urinary glucocorticoid excretion, with maternal serum cortisol levels and offspring birth weight z score.
Design, Participants, and Setting
A total of 151 women with singleton pregnancies, recruited from prenatal clinic at the Pittsburgh site of the Measurement of Maternal Stress (MOMS) study, had 24-hour urine collections during both the second and third trimesters.
Results
Between the second and third trimester, total urinary glucocorticoid excretion increased (ratio of geometric means [RGM] 1.37, 95% CI 1.22-1.52, P < .001), and there was an increase in calculated 5β-reductase compared to 5α-reductase activity (RGM 3.41, 95% CI 3.04-3.83, P < .001). During the third trimester total urinary glucocorticoid excretion and serum cortisol were negatively correlated (r = –0.179, P = .029). Mean total urinary glucocorticoid excretion across both trimesters and offspring birth weight z score were positively associated (β = 0.314, P = .001).
Conclusions
The estimated activity of maternal enzymes responsible for cortisol metabolism change between the second and third trimester of pregnancy. Additionally, maternal peripheral metabolism and clearance of cortisol may serve as a novel mechanism affecting fetal cortisol exposure and growth.
Keywords: cortisol, glucocorticoid, metabolism, HPA, pregnancy, birth weight
Glucocorticoids play a critical role in fetal maturation. Although a surge in glucocorticoid exposure toward the end of pregnancy helps prime a fetus for life outside the womb (1), excess or inappropriately timed exposure can adversely program offspring development (2, 3). There is growing evidence that circulating levels of maternal cortisol influence both fetal cortisol exposure and development. Maternal blood cortisol levels correlate with cortisol levels measured in fetal blood (4) and amniotic fluid (5). Elevated cortisol levels measured in maternal blood or saliva are associated with offspring growth restriction and adverse neurodevelopment and metabolic health (6–8).
Maternal regulation of glucocorticoids changes profoundly across pregnancy, with circulating cortisol levels rising approximately 3-fold by delivery (9). Multiple factors contribute to maternal hypercortisolism, including rising cortisol-binding globulin (10), placental secretion of corticotropin-releasing hormone (CRH) (11), and reduced sensitivity of the hypothalamic–pituitary–adrenal (HPA) axis to glucocorticoid-mediated central negative feedback (12). Altered breakdown, clearance, and regeneration of cortisol within maternal peripheral tissues could also influence maternal serum levels and fetal glucocorticoid exposure.
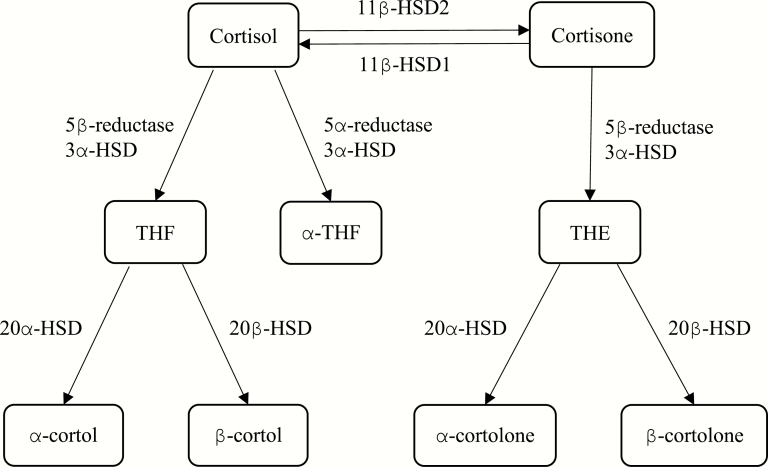
Relatively little intact cortisol is excreted from the body passively, with the majority instead being metabolized to compounds considered more inert before urinary excretion (13). Metabolism of cortisol to 5β-tetrahydrocortisol (THF), and its derivatives α-cortol and β-cortol, and 5α-tetrahydrocortisol (α-THF), are reliant on the activity of A-ring reductases, 5β-reductase, predominantly expressed in the liver, and 5α-reductase, expressed both in liver and fat. 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) acts in the kidney and placenta, converting cortisol to cortisone. In contrast, 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1) is most highly expressed in the liver, where it regenerates active cortisol from inert cortisone. These processes are outlined in Fig. 1. Peripheral glucocorticoid metabolism varies as a function of age, sex, and obesity and in many disease states (14–16).
Figure 1.
Peripheral cortisol metabolism enzymes and metabolites.
The sum of glucocorticoid metabolites measured in a 24-hour sample of urine represents total urinary glucocorticoid excretion. Because the majority of glucocorticoids are excreted in urine, this measurement has also been used as an estimate of glucocorticoid production by the adrenal gland (17). Additionally, comparison of the relative levels of metabolites offers insight into the activity of enzymes converting cortisol in peripheral tissues.
To date there has been limited investigation of maternal peripheral glucocorticoid metabolism and clearance in pregnancy. Longitudinal studies of maternal peripheral glucocorticoid metabolism in pregnancy have been limited by small sample size (18) or have relied on metabolites collected in spot urine or blood samples that are subject to diurnal variation (19, 20). There is growing evidence that maternal peripheral glucocorticoid metabolism and clearance are altered in preeclampsia (20–22). There are also preliminary data supporting a role for peripheral glucocorticoid metabolism influencing fetal development, with a higher plasma cortisone to cortisol ratio (representing more inert compared to active glucocorticoid) measured in mothers with psychiatric morbidity during the third trimester, being associated with higher offspring birth weight (23).
The aims of this study were to assess how maternal urinary glucocorticoid excretion, measured in 24-hour urine, changes between the second and third trimester of pregnancy, and to test the associations of total urinary glucocorticoid excretion with maternal serum cortisol levels and offspring birth weight z score. We tested the hypothesis that total urinary glucocorticoid excretion, as a marker of maternal adrenal cortisol production, increases across pregnancy, and is negatively associated with offspring birth weight z score.
Materials and Methods
Participants and clinical protocol
The Measurement of Maternal Stress (MOMS) study was a multisite prospective cohort that recruited women with singleton pregnancies from antenatal clinics in Pittsburgh, Pennsylvania; Chicago, Illinois; Schuylkill County, Pennsylvania; and San Antonio, Texas between June 2013 and May 2014. Exclusion criteria were fetal congenital abnormality, chromosomal abnormalities, progesterone use before 14 weeks’ gestation, or regular maternal corticosteroid use. All participating women gave written informed consent, and the study protocol was approved by the institutional review board of each site. A description of the cohort has been presented previously (24).
This study reports data from a subset (151 of 200) of mother-baby dyads, recruited from the Pittsburgh site, who had 24-hour urine collected for measurement of total glucocorticoids and metabolites on 2 occasions during pregnancy, between 12.7 and 22.1 weeks’ gestation (second trimester), and between 31.9 and 36.4 weeks’ gestation (third trimester).
Participants also had blood collected for measurement of serum cortisol at study visits during the second and third trimester. Maternal demographic and medical information, including body mass index (BMI), age, ethnicity, diabetes mellitus, preeclampsia, gestational hypertension, and offspring outcomes including birth weight and birth gestation, were recorded either during study visits or on review of participants’ medical records. Offspring birth weight z scores were calculated according to International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st) standards (25).
Laboratory methods
Serum.
Serum was obtained by centrifuging whole blood at 1000 g at 4°C for 15 minutes, then aliquoting serum into 2-mL cryovials. Cortisol was assessed by radioimmunoassay at the Development, Health and Disease Research Program’s laboratory at the University of California, Irvine. Ten percent of samples were measured in duplicate, and interassay and intra-assay coefficients of variation were less than 10%.
Urinary glucocorticoids.
Urinary glucocorticoid metabolites were analyzed by gas chromatography–triple quadrupole mass spectrometry at the Edinburgh Clinical Research Facility Mass Spectrometry Core as previously described (26). The interassay and intra-assay coefficients of variation were less than 13%. Analytes included cortisol (F), cortisone (E), α-THF, THF, α-cortol, β-cortol, tetrahydrocortisone (THE), α-cortolone, and β-cortolone. The sum of these measured analytes is referred to as total urinary glucocorticoid excretion.
The following ratios of urinary metabolites were used as parameters to estimate peripheral glucocorticoid metabolism:
i) 11β-HSD2 activity = F/E
ii) 11β-HSD total activity = (THF + α-THF)/THE
iii) Relative 5β-reductase and 5α-reductase activity = THF/α-THF
iv) 5α-reductase activity = F/α-THF
v) 5β-reductase metabolism of F = F/(THF + α-cortol + β-cortol)
vi) 5β-reductase metabolism of E = E/(THE + α-cortolone + β-cortolone)
Statistical Analysis
All analyses were performed using IBM SPSS Statistics version 24. Data distributions were assessed for normality visually using histograms. Serum cortisol levels were normally distributed among the study population. Levels of all excreted urinary glucocorticoid metabolites were positively skewed, and log base 10–transformed before statistical analysis.
Demographic data are presented as mean ± SD. Change of urinary metabolite excretion between the second and third trimester was tested using paired t tests, and the degree of change is represented through the ratio of the geometric means (RGM), with 95% CIs. To assess whether peripheral metabolism has a maintained trait component across pregnancy, the rank stability— the similarity of where participants’ estimated enzymatic function fell within the study population’s distribution, at the second compared to the third trimester—was tested by a linear regression model adjusting for the gestation of urine sampling. The relationship between maternal total urinary glucocorticoid excretion and serum cortisol levels was tested using the Pearson coefficient within both the whole study population and in a subgroup of patients with blood sampled before 10 am. Finally, the association of maternal total urinary glucocorticoid excretion and offspring birth weight z score was tested by linear regression adjusting for confounding factors. These included the gestation at urine sampling and maternal ethnicity, smoking status, age, preeclampsia, gestational hypertension, diabetes mellitus (pregestational and gestational), BMI, and gravidity. Associations with birth weight z score were tested both for second and third trimester glucocorticoid excretion, and for mean glucocorticoid excretion across pregnancy. A P value less than .05 was considered statistically significant.
Results
Demographics
Table 1 shows the characteristics of study participants. Mothers were age 30.5 ± 5.0 years, with BMI 27.6 ± 7.1 kg/m2, and were predominantly white nonsmokers. Mean gestational age at birth was 39.4 ± 1.4 weeks, and mean birth weight was 3487 ± 489 g.
Table 1.
Maternal, infant, and sampling demographics
| Maternal Demographics | Number (%), Mean ± SD |
|---|---|
| Maternal age, y | 30.5 ± 5.0 |
| Maternal BMI, kg/m2 | 27.6 ± 7.1 |
| Gravidity | |
| 1 | 50 (33.1%) |
| 2 | 41 (27.2%) |
| ≥ 3 | 60 (39.7%) |
| Ethnicity | |
| Hispanic white | 1 (0.7%) |
| White | 118 (78.1%) |
| Black | 27 (17.9%) |
| Other | 5 (3.3%) |
| Current smoker | |
| Yes | 10 (6.6%) |
| No | 141 (93.4%) |
| Preeclampsia | |
| Yes | 4 (2.8%) |
| No | 139 (97.2%) |
| Hypertension | |
| Yes | 15 (10.5%) |
| No | 128 (89.5%) |
| Diabetes | |
| Yes | 9 (6.3%) |
| No | 134 (93.7%) |
| Infant demographics | |
| Infant sex | |
| Female | 61 (42.7%) |
| Male | 82 (57.3%) |
| Birth weight, g | 3487 ± 489 |
| Birth gestation, wks | 39.4 ± 1.4 |
| Birth weight z score | 0.56 ± 0.99 |
| Sampling demographics | |
| Second trimester urine sample gestation, wks | 17.3 ± 2.4 |
| Third trimester urine sample gestation, wks | 33.9 ± 1.2 |
| Second trimester blood sample gestation, wks | 16.7 ± 2.4 |
| Third trimester blood sample gestation, wks | 33.3 ± 1.1 |
| Second trimester blood sample time, h after midnight | 11.0 ± 2.2 |
| Third trimester blood sample time, h after midnight | 10.6 ± 2.5 |
Of the 151 participants included in the study, the following data were missing: maternal BMI n = 2, infant demographics and maternal health during pregnancy n = 8, second trimester serum cortisol n = 1, third trimester serum cortisol n = 2.
Abbreviation: BMI, body mass index.
Changing glucocorticoid levels across pregnancy
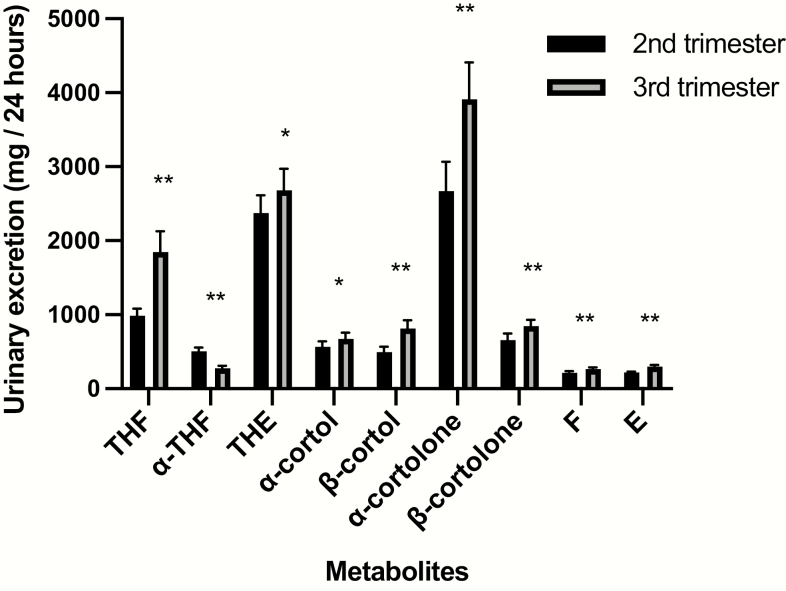
Fig. 2 and Table 2 depict urinary glucocorticoid metabolite excretion for collections during the second and third trimester. Across pregnancy total urinary glucocorticoid excretion increased (RGM 1.37, P < .001). Excretion of all individual metabolites increased except for α-THF, which decreased between the second and third trimester (RGM 0.55, P < .001). Assessing individual metabolic pathways, the ratio of F/E (RGM 0.90, P < .001) decreased, likely representing increased estimated 11β-HSD2 (inactivation of cortisol to cortisone) activity across pregnancy. Total body 11β-HSD activity represented by (THF + α-THF)/THE (RGM 1.27, P < .001) shifted in favor of excretion of cortisol metabolites relative to cortisone metabolites. The activity of A-ring reductases shifted toward 5β-reductase metabolism compared to 5α-reductase metabolism, with increased THF/α-THF ratio (RGM 3.41, P < .001). Between the second and third trimester serum cortisol also increased (ratio of means 1.63, 95% CI 1.40-1.85, P < .001).
Figure 2.
Geometric mean and 95% CIs of glucocorticoid metabolites from 24-hour urine collections during the second and third trimester. *P < .01, **P < .001.
Table 2.
Changes in urinary metabolites excretion and ratios across pregnancy
| Second Trimester: Median (Lower Quartile-Upper Quartile) | Third Trimester: Median (Lower Quartile-Upper Quartile) | Change Across Gestations: RGM (95% CI) | |
|---|---|---|---|
| Urinary metabolites, mg/24 h | |||
| THF | 1043 (691-1397) | 1768 (1066-3269) | 1.88 (1.65-2.15)b |
| α-THF | 494 (331-781) | 291 (177-436) | 0.55 (0.50-0.61)b |
| THE | 2500 (1588-3579) | 2799 (1805-4222) | 1.13 (1.04-1.23)a |
| α-cortol | 586 (368-917) | 641 (455-1140) | 1.19 (1.05-1.34)a |
| β-cortol | 545 (259-947) | 849 (540-1410) | 1.65 (1.45-1.88)b |
| α-cortolone | 2420 (1589-4473) | 3685 (2371-6241) | 1.46 (1.25-1.71)b |
| β-cortolone | 632 (424-979) | 796 (574-1189) | 1.29 (1.13-1.47)b |
| F | 231 (160-315) | 272 (215-361) | 1.23 (1.13-1.35)b |
| E | 228 (171-292) | 316 (227-410) | 1.36 (1.26-1.48)b |
| Total urinary glucocorticoids | 9691 (6157-12 805) | 13 523 (8955-18 269) | 1.37 (1.22-1.52)b |
| Ratios of metabolites | |||
| 11β-HSD2 activity = F/E | 0.99 (0.78-1.28) | 0.88 (0.73-1.16) | 0.90 (0.86-0.95)b |
| 11β-HSD total activity = (THF + α-THF)/THE | 0.61 (0.52-0.85) | 0.76 (0.48-1.23) | 1.27 (1.14-1.42)b |
| Relative 5β -reductase and 5α -reductase activity = THF/α-THF | 1.78 (1.33-2.83) | 7.19 (3.64-11.74) | 3.41 (3.04-3.83)b |
| 5α -reductase activity = F/α-THF | 0.45 (0.27-0.60) | 0.98 (0.61-1.51) | 2.24 (2.00-2.50)b |
| 5β-reductase metabolism of F = F/(THF + α- cortol + β-cortol) | 0.10 (0.07-0.14) | 0.07 (0.05-0.11) | 0.72 (0.65-0.81)b |
| 5β-reductase metabolism of E = E/(THE + +α-cortolone+ β-cortolone) | 0.04 (0.02-0.06) | 0.04 (0.03-0.06) | 1.05 (0.96-1.15) |
Paired t test (2-tailed) of log-transformed urine values.
Abbreviations: E, cortisone; F, cortisol; HSD, hydroxysteroid dehydrogenase; HSD2, hydroxysteroid dehydrogenase type 2; RGM, ratio of geometric means; THE, tetrahydrocortisone; THF, 5β-tetrahydrocortisol.
a P less than .01. bP less than .001.
Individual stability in peripheral glucocorticoid metabolism
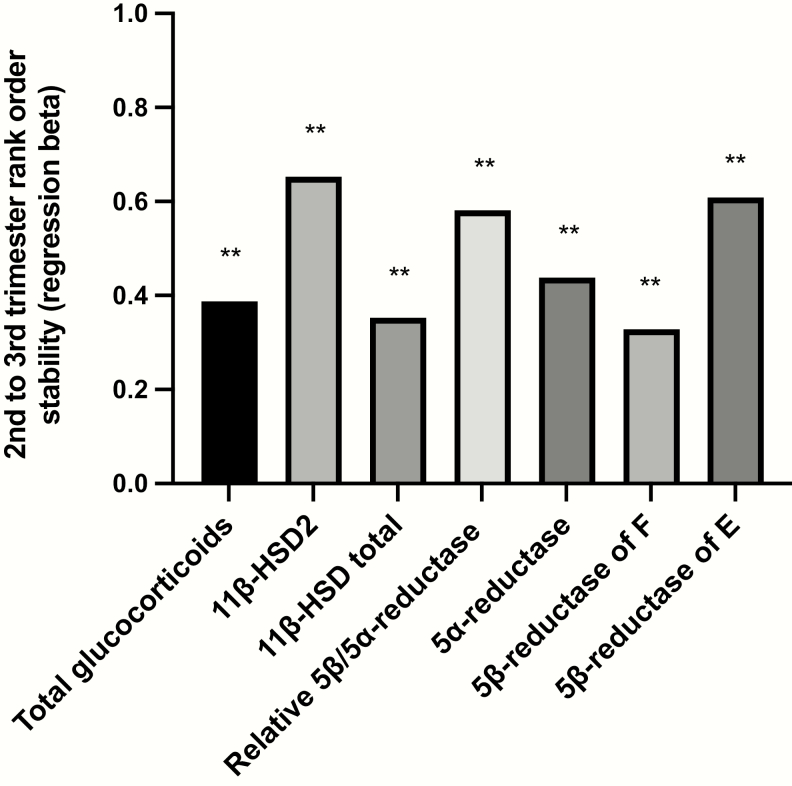
Table 3 and Fig. 3 represent rank-order stability of total urinary glucocorticoid excretion and estimates of peripheral metabolism of glucocorticoids for participants across the second and third trimester. Despite the whole-group changes in peripheral glucocorticoid metabolism across pregnancy, the relative enzymatic activity of individual participants compared to the whole group was well maintained across both time points, with women with higher estimated activity for peripheral glucocorticoid metabolism during the second trimester tending to have higher estimated enzyme activity measured in the third trimester.
Table 3.
Rank correlation across the second and third trimesters of participant total urinary glucocorticoid excretion or estimated enzymatic function
| Standardized Coefficient, β | |
|---|---|
| Total urinary glucocorticoids | .387a |
| 11β-HSD2 activity = F/E | .652a |
| 11β-HSD total activity = (THF + α-THF)/THE | .352a |
| Relative 5β -reductase and 5α -reductase activity = THF/α-THF | .581a |
| 5α -reductase activity = F/α-THF | .438a |
| 5β-reductase metabolism of F = F/(THF + α-cortol + β-cortol) | .328a |
| 5β-reductase metabolism of E = E/(THE + +α-cortolone + β-cortolone) | .608a |
Adjusted according to the gestation of urine collection.
Abbreviations: E, cortisone; F, cortisol; HSD, hydroxysteroid dehydrogenase; HSD2, hydroxysteroid dehydrogenase type 2; THE, tetrahydrocortisone; THF, 5β-tetrahydrocortisol.
a P less than .001.
Figure 3.
Rank correlation across the second and third trimesters of participant total urinary glucocorticoid excretion or estimated enzymatic function. **P < .001.
Associations between total urinary glucocorticoid excretion and serum cortisol levels
During the second trimester serum cortisol was not associated with total urinary glucocorticoid excretion (r = 0.076, P = .358). During the third trimester, total urinary glucocorticoid excretion was negatively associated with serum cortisol within the whole group (r = –0.179, P = .029). This association between third trimester serum cortisol and total urinary glucocorticoid excretion was largely driven by the subgroup of participants with third trimester blood samples taken before 10 am (n = 66, r = –0.354, P = .004). In contrast, participants’ results with third trimester blood taken after 10 am were n = 83, r = –0.096, and P = .390.
Associations between total urinary glucocorticoid excretion and infant birth weight z score
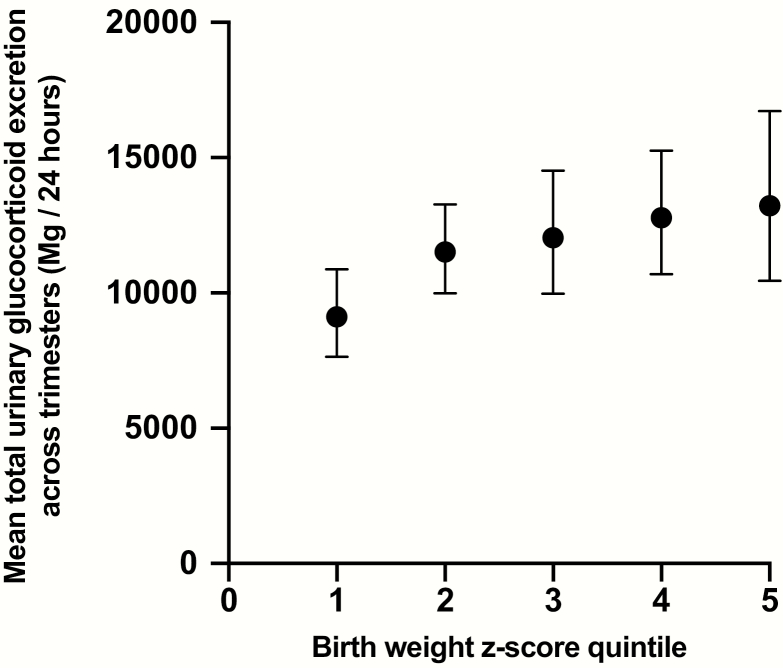
In the adjusted models, there were positive associations between total urinary glucocorticoid excretion during the second trimester and offspring birth weight z score (β = 0.198, r-square change 0.028, P = .033), total urinary glucocorticoid excretion during the third trimester and offspring birth weight z score (β = 0.202, r-square change 0.032, P = .023), and mean total glucocorticoid excretion across both trimesters with offspring birth weight z score (β = 0.314, r-square change 0.066, P = .001). In contrast, there was no association between mean serum cortisol levels and offspring birth weight z score. A visual representation of maternal glucocorticoid excretion across trimesters according to infant birth weight z score quintile is shown in Fig. 4.
Figure 4.
Geometric means and 95% CIs of mothers’ mean total urinary glucocorticoid excretion across trimesters according to offspring birth weight z score quintile.
Associations between glucocorticoid metabolite ratios, with serum cortisol and infant birth weight z score
Having demonstrated that total urinary glucocorticoid excretion was negatively associated with serum cortisol during the third trimester and positively associated with birth weight z score, further exploratory analysis was undertaken to investigate whether these effects were being driven by the action of individual metabolic pathways. In this exploratory analysis, higher third trimester serum cortisol was associated with estimates of reduced 5α-reductase activity (F/α-THF; whole group r = 0.168, P = .041; venipuncture < 10 am subgroup r = 0.318, P = .009), and reduced 5β-reductase activity (F/(THF + α-cortol + β-cortol); whole group r = 0.206, P = .012; venipuncture < 10 am subgroup r = 0.281, P = .022) and (E/(THE + α-cortolone + β-cortolone); whole group r = 0.252, P = .002; venipuncture < 10 am subgroup r = 0.251, P = .042). No associations were seen between third trimester serum cortisol and estimated 11β-HSD1 or 11β-HSD2 activity. Additionally, no associations were seen between infant birth weight z score and urine metabolite ratios.
Discussion
In this study of pregnant women with detailed measurements of glucocorticoid metabolism, we have demonstrated that glucocorticoid metabolism changes across pregnancy, and that total urinary glucocorticoid excretion is positively associated with offspring birth weight z score.
Within the cohort total maternal glucocorticoid excretion increased between the second and third trimester. This builds on previous observations of increased urinary free cortisol excretion across pregnancy (9) and likely represents an increase in adrenal cortisol release across pregnancy. There were also differences in the ratios of urinary metabolites between the second and third trimester. This provides evidence that the global actions of enzymes working to metabolize cortisol in peripheral tissues change across pregnancy. A reduced F/E ratio represents increased 11β-HSD2 activity. An increase in (THF + α-THF)/THE ratio, in the context of estimated increased 11β-HSD2 likely represents an increase in 11β-HSD1 activity across pregnancy. The ratio of A-ring reductase metabolism shifted profoundly toward 5β-reductase metabolism compared to 5α-reductase metabolism with increased THF/α-THF ratio (27). A reduction of 5α-reductase cortisol metabolism is in keeping with results from a study in which α-THF excretion measured in maternal urine increased across the first year postpartum (28). The action of 5α-reductase in pregnancy has received attention because of its important role in converting testosterone to dihydrotestosterone, with 5α-reductase genetic mutation or pharmacological inhibition causing in utero undervirilization of male offspring (29). 5α-reductase metabolism of progesterone has also been investigated in the context of parturition, with 5α-reductase type 1–deficient mice failing to undergo cervical ripening at term (30). However, to our knowledge the physiological importance of 5α-reductase metabolism of cortisol in pregnancy has not previously been considered.
Changes in glucocorticoid metabolism may offer specific advantages to the mother and fetus. In addition to controlling systemic cortisol inactivation and clearance, peripherally located enzymes play an important role in regulating glucocorticoid exposure to specific tissues. This is most commonly discussed in relation to the kidney, where local 11β-HSD2 acts to prevent excessive activation of mineralocorticoid receptors by cortisol (13). 5α-reductase influences cortisol clearance and action within the liver, and its activity has been shown to be modifiable either by early life stress (31) or by variation in nutritional demands (32, 33). Within pregnancy, marked reduction in 5α-reductase activity during the third trimester may act to enhance cortisol activity in the liver, allowing mobilization of fuels at a time of increased metabolic requirements.
Alternatively, changing glucocorticoid metabolism across pregnancy may be a bystander influenced by other physiological changes in the mother across pregnancy. Maternal glucocorticoid metabolism could be influenced by a changing inflammatory milieu. For example, it has both been demonstrated that tumor necrosis factor alpha (TNF-α) increases across pregnancy (27), and that inhibiting TNF-α in patients with inflammatory arthritis increases 5α-reductase activity (34). Changing biliary physiology may also influence maternal glucocorticoid metabolism, with bile acids holding the potential to inhibit A-ring reductases and 11β-HSDs (35). Increases in insulin resistance across pregnancy may also influence glucocorticoid metabolism. However, insulin-sensitizing therapies and weight loss have both previously been associated with decreases in 5α-reductase activity (36, 37), making it unlikely that changes in insulin sensitivity are driving the reductions in 5α-reductase activity seen during the third trimester. There is also likely to be a placental contribution to maternal whole-body glucocorticoid metabolism estimated through urinary glucocorticoids. In an ex vivo placental perfusion model, the majority of cortisone converted from cortisol at term gestation was transferred back into the maternal circulation rather than fetal circulation (38).
During the second trimester there was no association between maternal urinary glucocorticoid excretion and serum cortisol, whereas during the third trimester higher serum cortisol correlated with lower total urinary glucocorticoid excretion. Additionally, in exploratory analysis, higher serum cortisol in the third trimester was associated with lower estimated activity of 5β-reductase and 5α-reductase. Individual differences in peripheral glucocorticoid metabolism and clearance may influence serum cortisol levels in the later stages of pregnancy. In healthy, nonpregnant populations differences in peripheral glucocorticoid metabolism are generally not associated with serum cortisol levels, likely because of compensatory glucocorticoid release by the HPA axis in response to changing negative feedback (39, 40). However, in critically ill patients reduced peripheral metabolism and clearance of cortisol contributes to increased serum cortisol levels (16). Throughout pregnancy regulation of the maternal HPA axis changes, becoming progressively less sensitive to negative feedback by glucocorticoids (12). It therefore seems physiologically plausible that by the third trimester individual differences in glucocorticoid metabolism and clearance influence serum cortisol levels.
An unexpected finding was the modest positive association between total urinary glucocorticoid excretion and offspring birth weight z score, with maternal total urinary glucocorticoid excretion measured in the second and third trimesters of pregnancy explaining 6.6% of variance in offspring birth weight z score. Previous studies have typically reported a negative association between synthetic glucocorticoid exposure (2), or maternal cortisol levels measured in saliva (7) or blood (41), with infant birth weight. A negative association has also previously been reported between urinary free cortisol measured in the morning between 18 and 20 weeks’ gestation and fetal growth (42). The relationship between total urinary glucocorticoid excretion and infant birth weight z score has not previously been tested. Increased maternal peripheral metabolism and clearance of glucocorticoids may serve as a mechanism reducing cortisol exposure to the fetus. This theory is strengthened by the negative association found between serum cortisol and total urinary glucocorticoids observed in the third trimester. In the exploratory analyses no associations were found between birth weight z score and any of the urinary metabolite ratios used to estimate peripheral enzymatic function, so it cannot be concluded that this relationship is driven through the effects of a single enzyme’s function. Alternatively, the relationship between maternal total urinary glucocorticoid excretion and infant birth weight z score could be mediated by other maternal factors. For example, increased urinary glucocorticoid excretion has previously been associated with insulin resistance (36), and increased maternal insulin resistance during pregnancy may also act to increase offspring birth weight (43).
Despite whole-group changes in peripheral metabolism across pregnancy, individuals’ rank within the cohort remained relatively stable with those who had higher calculated enzymatic activity during the second trimester also tending to have higher activity during the third trimester. This implies that individual’s peripheral metabolism shows a consistent trait across pregnancy, increasing the likelihood that peripheral glucocorticoid metabolism could influence fetal exposure to cortisol and play a role in fetal development.
Strengths of this study include the use of a modern technique for accurate quantification of urinary glucocorticoid metabolites (26), the large sample size, and longitudinal study design allowing comparison of urinary metabolites across pregnancy. Limitations include the fact that there was variation in the time of day blood samples were collected, that participants did not fast before venipuncture, and the lack of measurement of other serum glucocorticoid metabolites in addition to cortisol.
Conclusions
Between the second and third trimester the ratios of urinary glucocorticoids, acting as markers of peripheral metabolism, changed suggesting a relative decrease in 5α-reductase metabolism and a relative increase in 5β-reductase metabolism of cortisol. However, interindividual differences among study participants were relatively well preserved between the 2 testing periods. The negative association between total urinary glucocorticoids and third trimester serum cortisol, along with the positive association between total urinary glucocorticoids and birth weight z score, provides preliminary data that peripheral glucocorticoid metabolism may influence fetal glucocorticoid exposure and fetal growth.
Acknowledgments
We are grateful for the support of the MOMS Study Collaboration including research staff and participants. We also appreciate the important contribution of the MOM-led pilot study collaboration. We acknowledge the support of the University of Edinburgh Mass Spectrometry Core.
Financial Support: This work was supported by The National Children’s Study, Vanguard Study, Task Order 5 (HHSN275201200007I-HHSN27500005), and Auxiliary Research Scholar and Research Career Development Awards from NorthShore University Health System (to A.E.B.B.), the British Heart Foundation, and a fellowship from Theirworld (to D.Q.S.). This work was undertaken in the Medical Research Council Centre for Reproductive Health at the University of Edinburgh, which is funded by Medical Research Council Centre Grant (MRC G1002033). We acknowledge the support of the Wellcome Trust (202794/Z/16/Z).
Glossary
Abbreviations
- 11β-HSD2
11β-hydroxysteroid dehydrogenase type 2
- BMI
body mass index
- CRH
corticotropin-releasing hormone
- HPA
hypothalamic–pituitary–adrenal
- MOMS
Measurement of Maternal Stress
- RGM
ratio of geometric means
- THE
tetrahydrocortisone
- THF
5β-tetrahydrocortisol
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: The data set generated during the present study is not publicly available but is available from the corresponding author on reasonable request.
References
- 1. Fowden AL, Li J, Forhead AJ. Glucocorticoids and the preparation for life after birth: are there long-term consequences of the life insurance? Proc Nutr Soc. 1998;57(1):113–122. [DOI] [PubMed] [Google Scholar]
- 2. Reynolds RM. Glucocorticoid excess and the developmental origins of disease: two decades of testing the hypothesis—2012 Curt Richter Award Winner. Psychoneuroendocrinology. 2013;38(1):1–11. [DOI] [PubMed] [Google Scholar]
- 3. Moisiadis VG, Matthews SG. Glucocorticoids and fetal programming part 1: Outcomes. Nat Rev Endocrinol. 2014;10(7):391–402. [DOI] [PubMed] [Google Scholar]
- 4. Gitau R, Cameron A, Fisk NM, Glover V. Fetal exposure to maternal cortisol. Lancet. 1998;352(9129):707–708. [DOI] [PubMed] [Google Scholar]
- 5. Glover V, Bergman K, Sarkar P, O’Connor TG. Association between maternal and amniotic fluid cortisol is moderated by maternal anxiety. Psychoneuroendocrinology. 2009;34(3):430–435. [DOI] [PubMed] [Google Scholar]
- 6. Zijlmans MA, Riksen-Walraven JM, de Weerth C. Associations between maternal prenatal cortisol concentrations and child outcomes: a systematic review. Neurosci Biobehav Rev. 2015;53:1–24. [DOI] [PubMed] [Google Scholar]
- 7. Cherak SJ, Giesbrecht GF, Metcalfe A, Ronksley PE, Malebranche ME. The effect of gestational period on the association between maternal prenatal salivary cortisol and birth weight: a systematic review and meta-analysis. Psychoneuroendocrinology. 2018;94:49–62. [DOI] [PubMed] [Google Scholar]
- 8. Stinson LJ, Stroud LR, Buka SL, et al. Prospective evaluation of associations between prenatal cortisol and adulthood coronary heart disease risk: the New England family study. Psychosom Med. 2015;77(3):237–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jung C, Ho JT, Torpy DJ, et al. A longitudinal study of plasma and urinary cortisol in pregnancy and postpartum. J Clin Endocrinol Metab. 2011;96(5):1533–1540. [DOI] [PubMed] [Google Scholar]
- 10. Qureshi AC, Bahri A, Breen LA, et al. The influence of the route of oestrogen administration on serum levels of cortisol-binding globulin and total cortisol. Clin Endocrinol. 2007;66(5):632–635. [DOI] [PubMed] [Google Scholar]
- 11. Sasaki A, Shinkawa O, Yoshinaga K. Placental corticotropin-releasing hormone may be a stimulator of maternal pituitary adrenocorticotropic hormone secretion in humans. J Clin Invest. 1989;84(6):1997–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Odagiri E, Ishiwatari N, Abe Y, et al. Hypercortisolism and the resistance to dexamethasone suppression during gestation. Endocrinol Jpn. 1988;35(5):685–690. [DOI] [PubMed] [Google Scholar]
- 13. Walker BR, Seckl JR. Cortisol metabolism. In: Bjorntorp P, ed. International Textbook of Obesity. Chichester, UK: John Wiley & Sons Inc; 2001:241–268. [Google Scholar]
- 14. Nixon M, Upreti R, Andrew R. 5α-reduced glucocorticoids: a story of natural selection. J Endocrinol. 2012;212(2):111–127. [DOI] [PubMed] [Google Scholar]
- 15. Andrew R, Phillips DI, Walker BR. Obesity and gender influence cortisol secretion and metabolism in man. J Clin Endocrinol Metab. 1998;83(5):1806–1809. [DOI] [PubMed] [Google Scholar]
- 16. Boonen E, Vervenne H, Meersseman P, et al. Reduced cortisol metabolism during critical illness. N Engl J Med. 2013;368(16):1477–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Remer T, Maser-Gluth C, Wudy SA. Glucocorticoid measurements in health and disease—metabolic implications and the potential of 24-h urine analyses. Mini Rev Med Chem. 2008;8(2):153–170. [DOI] [PubMed] [Google Scholar]
- 18. Stirrat LI, O’Reilly JR, Riley SC, et al. Altered maternal hypothalamic-pituitary-adrenal axis activity in obese pregnancy is associated with macrosomia and prolonged pregnancy. Pregnancy Hypertens. 2014;4(3):238. [DOI] [PubMed] [Google Scholar]
- 19. Mistry HD, Eisele N, Escher G, et al. Gestation-specific reference intervals for comprehensive spot urinary steroid hormone metabolite analysis in normal singleton pregnancy and 6 weeks postpartum. Reprod Biol Endocrinol. 2015;13:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vasku M, Kleine-Eggebrecht N, Rath W, Mohaupt MG, Escher G, Pecks U. Apparent systemic 11ß-dehydroxysteroid dehydrogenase 2 activity is increased in preeclampsia but not in intrauterine growth restriction. Pregnancy Hypertens. 2018;11:7–11. [DOI] [PubMed] [Google Scholar]
- 21. Jayasuriya NA, Hughes AE, Sovio U, Cook E, Charnock-Jones DS, Smith GCS. A Lower maternal cortisol-to-cortisone ratio precedes clinical diagnosis of preterm and term preeclampsia by many weeks. J Clin Endocrinol Metab. 2019;104(6):2355–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kosicka K, Siemiątkowska A, Szpera-Goździewicz A, Krzyścin M, Bręborowicz GH, Główka FK. Increased cortisol metabolism in women with pregnancy-related hypertension. Endocrine. 2018;61(1):125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hellgren C, Edvinsson Å, Olivier JD, et al. Tandem mass spectrometry determined maternal cortisone to cortisol ratio and psychiatric morbidity during pregnancy—interaction with birth weight. Psychoneuroendocrinology. 2016;69:142–149. [DOI] [PubMed] [Google Scholar]
- 24. Miller GE, Culhane J, Grobman W, et al. Mothers’ childhood hardship forecasts adverse pregnancy outcomes: role of inflammatory, lifestyle, and psychosocial pathways. Brain Behav Immun. 2017;65:11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Villar J, Cheikh Ismail L, Victora CG, et al. ; International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH-21st) International standards for newborn weight, length, and head circumference by gestational age and sex: the Newborn Cross-Sectional Study of the INTERGROWTH-21st Project. Lancet. 2014;384(9946):857–868. [DOI] [PubMed] [Google Scholar]
- 26. Homer N, Kothiya S, Rutter A, Walker BR, Andrew R. Gas chromatography tandem mass spectrometry offers advantages for urinary steroids analysis. Anal Biochem. 2017;538:34–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Beckmann I, Visser W, Struijk PC, van Dooren M, Glavimans J, Wallenburg HC. Circulating bioactive tumor necrosis factor-alpha, tumor necrosis factor-alpha receptors, fibronectin, and tumor necrosis factor-alpha inducible cell adhesion molecule VCAM-1 in uncomplicated pregnancy. Am J Obstet Gynecol. 1997;177(5):1247–1252. [DOI] [PubMed] [Google Scholar]
- 28. Rogers SL, Hughes BA, Jones CA, et al. Diminished 11β-hydroxysteroid dehydrogenase type 2 activity is associated with decreased weight and weight gain across the first year of life. J Clin Endocrinol Metab. 2014;99(5):E821–E831. [DOI] [PubMed] [Google Scholar]
- 29. Imperato-McGinley J, Guerrero L, Gautier T, Peterson RE. Steroid 5alpha-reductase deficiency in man: an inherited form of male pseudohermaphroditism. Science. 1974;186(4170):1213–1215. [DOI] [PubMed] [Google Scholar]
- 30. Mahendroo MS, Porter A, Russell DW, Word RA. The parturition defect in steroid 5alpha-reductase type 1 knockout mice is due to impaired cervical ripening. Mol Endocrinol. 1999;13(6):981–992. [DOI] [PubMed] [Google Scholar]
- 31. Yehuda R, Bierer LM, Andrew R, Schmeidler J, Seckl JR. Enduring effects of severe developmental adversity, including nutritional deprivation, on cortisol metabolism in aging Holocaust survivors. J Psychiatr Res. 2009;43(9):877–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tomlinson JW, Finney J, Gay C, Hughes BA, Hughes SV, Stewart PM. Impaired glucose tolerance and insulin resistance are associated with increased adipose 11beta-hydroxysteroid dehydrogenase type 1 expression and elevated hepatic 5alpha-reductase activity. Diabetes. 2008;57(10):2652–2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stimson RH, Johnstone AM, Homer NZ, et al. Dietary macronutrient content alters cortisol metabolism independently of body weight changes in obese men. J Clin Endocrinol Metab. 2007;92(11):4480–4484. [DOI] [PubMed] [Google Scholar]
- 34. Nanus DE, Filer AD, Hughes B, et al. TNFα regulates cortisol metabolism in vivo in patients with inflammatory arthritis. Ann Rheum Dis. 2015;74(2):464–469. [DOI] [PubMed] [Google Scholar]
- 35. McNeilly AD, Macfarlane DP, O’Flaherty E, et al. Bile acids modulate glucocorticoid metabolism and the hypothalamic-pituitary-adrenal axis in obstructive jaundice. J Hepatol. 2010;52(5):705–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tomlinson JW, Finney J, Hughes BA, Hughes SV, Stewart PM. Reduced glucocorticoid production rate, decreased 5alpha-reductase activity, and adipose tissue insulin sensitization after weight loss. Diabetes. 2008;57(6):1536–1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Glintborg D, Hermann AP, Hagen C, et al. A randomized placebo-controlled study on the effects of pioglitazone on cortisol metabolism in polycystic ovary syndrome. Fertil Steril. 2009;91(3):842–850. [DOI] [PubMed] [Google Scholar]
- 38. Stirrat LI, Sengers BG, Norman JE, et al. Transfer and metabolism of cortisol by the isolated perfused human placenta. J Clin Endocrinol Metab. 2018;103(2):640–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Purnell JQ, Brandon DD, Isabelle LM, Loriaux DL, Samuels MH. Association of 24-hour cortisol production rates, cortisol-binding globulin, and plasma-free cortisol levels with body composition, leptin levels, and aging in adult men and women. J Clin Endocrinol Metab. 2004;89(1):281–287. [DOI] [PubMed] [Google Scholar]
- 40. White PC, Mune T, Agarwal AK. 11 beta-hydroxysteroid dehydrogenase and the syndrome of apparent mineralocorticoid excess. Endocr Rev. 1997;18(1):135–156. [DOI] [PubMed] [Google Scholar]
- 41. Goedhart G, Vrijkotte TG, Roseboom TJ, van der Wal MF, Cuijpers P, Bonsel GJ. Maternal cortisol and offspring birthweight: results from a large prospective cohort study. Psychoneuroendocrinology. 2010;35(5):644–652. [DOI] [PubMed] [Google Scholar]
- 42. Diego MA, Field T, Hernandez-Reif M, Schanberg S, Kuhn C, Gonzalez-Quintero VH. Prenatal depression restricts fetal growth. Early Hum Dev. 2009;85(1):65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yamashita H, Yasuhi I, Fukuda M, et al. The association between maternal insulin resistance in mid-pregnancy and neonatal birthweight in uncomplicated pregnancies. Endocr J. 2014;61(10):1019–1024. [DOI] [PubMed] [Google Scholar]