Abstract
Understanding the regulatory mechanisms that control intracellular stress has fundamental importance since its failure results in cell death. Evidence has emerged indicating that the intracellular signals that are induced in response to diverse stresses include the deoxyribonucleic acid damage response, the unfolded protein response, the mitochondrial and/or endoplasmic reticulum stress responses, and the autophagy signals to degrade dangerous protein aggregates. These signals bring changes to the stressed cells that may support systemic homeostasis or contribute to disease pathology. In normal thyroid cells, both reactive oxygen species (ROS) and antioxidant (AOD) activity is low. An increase in ROS balanced by AOD leads only to mild inflammation, but unopposed increases in ROS lead to a strong inflammatory response and may result in apoptosis. A balance between ROS and AOD is, therefore, needed to maintain thyrocyte homeostasis. This perspective describes how thyroid cells are subjected to multiple insults and how they try to protect themselves using these different cellular responses.
A recent paper in The Journal of Clinical Endocrinology & Metabolism by Diana et al (1) has emphasized the role of oxidative stress reflected in sera from patients with Graves disease, which is induced by thyroid-stimulating hormone receptor (TSHR) antibodies. They found that after 48 hours of exposure, both TSH and stimulating TSHR antibodies induced ROS in TSHR-transfected Chinese hamster ovary cells and confirmed the response with human thyrocytes. In contrast, a TSH-blocking monoclonal antibody was unable to induce ROS. However, the investigators did not document the end point of such ROS induction leaving the reader uncertain as to the significance of the observations. This short perspective attempts to place this information in the context of what is already known about thyroid cell stress and the complex response to thyroid antibody interactions.
Cellular stress is defined as processes within a cell that are triggered by an acute or chronic change from the usual cellular homeostasis with the intent of protecting the cell or organism. Cellular stress can be in the form of endoplasmic reticulum (ER) stress, mitochondrial stress, oxidative stress, or a combination of all 3 stresses. Thus cells, either within an organism or in vitro, respond to stress in various ways. Minor stress may be necessary for cellular homeostasis, while greater stress may lead to activation of cellular and metabolic pathways for cell survival and in extreme cases even cellular destruction by inducing signaling pathways, leading to programmed cell death or apoptosis and subsequent elimination of damaged cells. Hence, this is another biological example of a nonmonotonous dose response where minor stress and major stress produce distinctly different responses. A cell’s immediate response to a stressful stimulus is to help itself defend against, and recover from, the insult. If the danger signal is too large to be resolved, cells then autoactivate signaling pathways leading to cell death. There are many different types of cellular stress, and the responses that a cell mounts to deal with these conditions will, therefore, depend on the type of cells, the level of the insult, and the environment in which the cells reside. Thus, cellular stress like other biological phenomena is a highly coordinated and spatiotemporally regulated cellular process.
Protective responses such as the heat shock response or the unfolded protein response mediate an increase in chaperone protein activity within the ER, which enhances the protein-folding capacity of the cell (2). This counteracts the stress due to protein misfolding and promotes cell survival. Hence, the adaptive capacity of a cell ultimately determines its fate. Therefore, depending on the level and types of stress, different defense mechanisms and prosurvival strategies are mounted. Mechanisms underlying cell death include apoptosis, necrosis, pyroptosis, autophagy, and ferroptosis (see Box 1) and depend on the ability of the cell to cope or succumb to the conditions to which it is exposed. In addition, with extreme cellular stress leading to cell death, there follows the prompt and efficient clearance of the cellular debris to prevent secondary necrosis, and this is understood to be orchestrated by soluble “find-me” and “eat-me” signals exhibited by the dying cells (3).
Box 1: Types of cell death.
Apoptosis is a form of programmed cell death that occurs in multicellular organisms.
Necrosis is a form of cell death in which the cell membrane falls apart and cellular enzymes leak out and ultimately digest the cell.
Necroptosis is a programmed form of necrosis or inflammatory cell death.
Autophagic cell death: Cells that undergo an extreme amount of stress experience cell death either through apoptosis or necrosis.
Pyroptosis is a form of cell death that is associated with activation of a cytosolic danger-sensing protein complex called the inflammasome.
Ferroptosis is a type of programmed cell death that depends on iron and is characterized by lipid peroxidation. It is genetically and biochemically distinct from other forms of programmed cell death such as apoptosis. Ferroptosis is induced by the failure of the glutathione-dependent antioxidant defense.
Reactive oxygen species (ROS), also called oxygen free radicals, are one of the main causes of oxidative cell stress. ROS are highly reactive molecules induced by partially reduced forms of oxygen resulting from normal and altered cellular metabolism. It appears that early in evolution, nature selected ROS as a signal transduction mechanism to allow for adaptation to changes in environmental nutrients and an oxidative environment (4). ROS are mainly produced at sites on mitochondrial complexes I and III of the electron transport chain (5) and may include hydrogen peroxide, hydroxyl radicals, superoxide anions, and lipid peroxides (6), all of which have inherent chemical properties that confer reactivity to different biological targets. ROS associated with oxidative stress can induce pathology by damaging lipids, proteins, and deoxyribonucleic acid, leading to genotoxic responses (5). However, in the past 2 decades, it has also become apparent that ROS may serve as a signaling molecule to regulate cellular homeostasis (7). Thus, ROS may be good or bad depending on the context in which they are produced. Even in prokaryotes there are well-documented mechanisms whereby ROS may directly activate signaling molecules for adaptation to stress (8).
In eukaryotes, to counteract cellular stress, there exists a balance between oxidant and antioxidant systems. Thus, under abnormal pathophysiological conditions, balance is not always achieved, and oxidative damage is believed to contribute to a variety of diseases including cardiovascular, neurodegenerative, and neoplastic diseases and has also been implicated in Graves orbitopathy (6,9-11). Evaluation of human cellular defense systems (oxidant vs anti-oxidant) in thyroid tissue from patients with Graves disease (GD) undergoing thyroidectomy has revealed increased levels of free radicals and their scavengers compared to normal thyroid tissue, (11) and similar findings of increased oxidative parameters have been seen in the serum of hyperthyroid patients with GD (1,6). Although stimulating antibodies in patient serum are implicated in the production of ROS, increased oxidative parameters in the serum are hard to separate from other causes such as increased levels of thyroid hormone that are known to increase ROS (9) production, thus compounding the interpretation of such observations.
We first observed the induction of ROS in thyroid cells exposed to thyroid-stimulating hormone receptor (TSHR) antibodies of the “neutral” variety (12,13) (seeBox 2). We initially hypothesized that antibodies, by their large size, stressed the thyroid cells when bound to their cell membrane receptors because these endocytosed macrocomplexes are difficult cargoes for the cellular degradative process. However, under our culture conditions, this phenomenon was not seen with stimulating TSHR antibodies or with exposure to TSH. We now know this was because of their ability to induce prosurvival signals, especially adenosine 3',5'-cyclic monophosphate (cAMP) (13). However, it would appear that low levels of ROS can be induced by stimulating antibodies under certain conditions (1). In our early studies it was clear that the neutral antibodies, which we prefer to call hinge region antibodies or cleavage antibodies depending on their binding epitopes, were especially potent inducers of ROS. Since our first description of this response, we have shown that such induction of ROS is indeed inhibited by the cAMP/protein kinase A (PKA) pathway, explaining the low level or lack of ROS induction with stimulating TSHR antibodies and TSH itself (Fig. 1). Furthermore, these results are compatible with previous literature on cardiac myocytes (14) and islet β-cells, (15) which showed reciprocal inhibition between the cAMP/PKA pathway and ROS. The reduced insulin secretion from islet β-cells and the life-threatening arrhythmias associated with increased ROS production in myocytes were enhanced by lower cAMP levels (14,15). Furthermore, we have shown that a specific cAMP/PKA inhibitor such as H89 allowed ROS induction in the presence of stimulating TSHR antibodies or TSH. These findings reinforce the observation that the cAMP/PKA signaling loop is able to suppress ROS production in thyrocytes.
Box 2: Types of antibodies to TSH receptor.
Stimulating TSHR-Ab: Activates cAMP, which is necessary for thyrocyte survival.
Blocking TSHR-Ab: Blocks TSH binding to the TSHR and is unable to activate cAMP.
Partial blocking-Ab: Weak agonist able to activate cAMP at a low level and inhibit TSH action.
Neutral/hinge or cleavage TSHR-Ab: Unable to activate cAMP and induce cell death or apoptosis when unopposed by cAMP.
Abbreviations: Ab, antibody; cAMP, adenosine 3',5'-cyclic monophosphate; TSH, thyroid-stimulating hormone; TSHR, thyroid-stimulating hormone receptor.
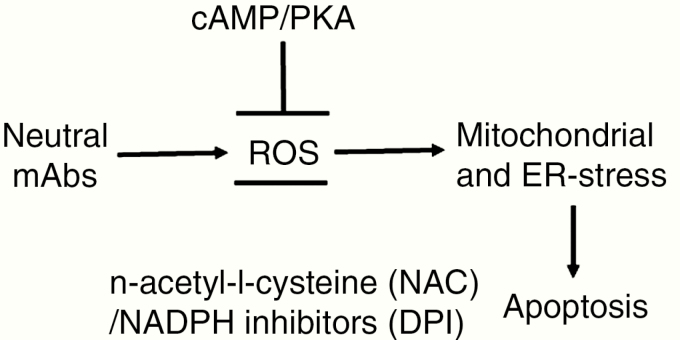
Figure 1.
Model explaining how cAMP/PKA reduces mROS: TSHR antibodies that do not induce cAMP/PKA are able to induce ROS generation. Neutral mAbs activate ROS, and ROS then induces mitochondrial and ER stress leading to apoptosis of thyrocytes. In contrast, induction of cAMP/PKA (by TSH or stimulating TSHR-Ab) suppresses ROS and prevents apoptosis. (NAC and DPI are inhibitors of ROS and also prevent apoptosis).
Abbreviations: cAMP, adenosine 3',5'-cyclic monophosphate; DPI, diphenylneiodonium; ER, endoplasmic reticulum; mAb, monoclonal antibody; NAC, n-acetyl-l-cysteine; PKA, protein kinase A; mROS, mitochondrial reactive oxygen species; TSH, thyroid-stimulating hormone; TSHR, thyroid-stimulating hormone receptor.
Our studies have also demonstrated that the end point of ROS induction is often thyroid cell apoptosis via induction of both mitochondrial and ER stress when unopposed by the cAMP/PKA signal (13). We also explored the mechanism of such cell death by studying the sorting and vesicular trafficking of TSHR and TSHR–monoclonal antibody (mAb) complexes in thyrocytes (16). This revealed that neutral mAbs were in fact less able to induce TSHR sorting and vesicular trafficking. The neutral antibodies failed to induce endosomes and lysosomes due to the lack of induction of Ras/Rab signaling proteins (16). Hence, the cAMP/PKA signaling pathway is vital for thyrocyte survival and may be one reason for thyroid cells to have constitutively active TSHRs with elevated basal cAMP levels (17). This ligand-independent constitutive activity of the TSHR (17), although only well demonstrated in vitro, may be an important in vivo asset in receptor homeostasis and thyroid cell survival.
In conclusion, in the autoimmune thyroid diseases such as GD and Hashimoto thyroiditis, the thyroid cell is under constant stress by the binding of antibodies to their cell membrane receptors and not merely by immune attack from infiltrating T cells, macrophages, and other immune cells. Most of this antibody-induced cellular stress is well handled by thyrocytes, and any accumulating cellular debris is disposed of appropriately via effective endosome and lysosome mechanisms when controlled by an active cAMP/PKA signaling pathway. Failure of such active signaling leads to the inability of the cell to handle the endocytosed antibodies resulting in high levels of ROS and, ultimately, thyroid cell death. Our recent demonstration of intrathyroidal oxidative stress in vivo by the administration of a cleavage region mAb to normal mice (18) is proof of the concept that antibody-induced cell damage is likely to be an active mechanism in thyroidal and extrathyroidal pathology associated with GD. Knowledge gained by studying thyroid cell stress may lead to the development of improved therapeutic interventions for the modulation of thyroid disease and thyroid cancer.
Acknowledgments
We thank Dr. Rauf Latif for review of the manuscript and Dr. Risheng Ma for technical advice.
Financial Support: This work was supported in part by DK069713 from the National Institutes of Health, the Segal Family Endowment, and the veterans administration (VA) Merit Review Program (to T.F.D.).
Glossary
Abbreviations
- AOD
antioxidant
- cAMP
adenosine 3',5'-cyclic monophosphate
- ER
endoplasmic reticulum
- GD
Graves disease
- mAb
monoclonal antibody
- PKA
protein kinase A
- ROS
reactive oxygen species
- TSH
thyroid-stimulating hormone
- TSHR
thyroid-stimulating hormone receptor
Additional Information
Disclosure statement: The authors have nothing to disclose.
Data availability: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. Diana T, Daiber A, Oelze M, et al. Stimulatory TSH-receptor antibodies and oxidative stress in graves disease. J Clin Endocrinol Metab. 2018;103(10):3668–3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13(2):89–102. [DOI] [PubMed] [Google Scholar]
- 3. Ravichandran KS. Find-me and eat-me signals in apoptotic cell clearance: progress and conundrums. J Exp Med. 2010;207(9):1807–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Finkel T. Signal transduction by mitochondrial oxidants. J Biol Chem. 2012;287(7):4434–4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Marchi S, Giorgi C, Suski JM, et al. Mitochondria-ros crosstalk in the control of cell death and aging. J Signal Transduct. 2012;2012:329635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mano T, Shinohara R, Iwase K, et al. Changes in free radical scavengers and lipid peroxide in thyroid glands of various thyroid disorders. Horm Metab Res. 1997;29(7):351–354. [DOI] [PubMed] [Google Scholar]
- 7. Cross CE, Halliwell B, Borish ET, et al. Oxygen radicals and human disease. Ann Intern Med. 1987;107(4):526–545. [DOI] [PubMed] [Google Scholar]
- 8. Wood ZA, Poole LB, Karplus PA. Peroxiredoxin evolution and the regulation of hydrogen peroxide signaling. Science. 2003;300(5619):650–653. [DOI] [PubMed] [Google Scholar]
- 9. Venditti P, Di Meo S. Thyroid hormone-induced oxidative stress. Cell Mol Life Sci. 2006;63(4):414–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Milenkovic M, De Deken X, Jin L, et al. Duox expression and related H2O2 measurement in mouse thyroid: onset in embryonic development and regulation by TSH in adult. J Endocrinol. 2007;192(3):615–626. [DOI] [PubMed] [Google Scholar]
- 11. Zarković M. The role of oxidative stress on the pathogenesis of Graves’ disease. J Thyroid Res. 2012;2012:302537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morshed SA, Ando T, Latif R, Davies TF. Neutral antibodies to the TSH receptor are present in Graves’ disease and regulate selective signaling cascades. Endocrinology. 2010;151(11):5537–5549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morshed SA, Ma R, Latif R, Davies TF. How one TSH receptor antibody induces thyrocyte proliferation while another induces apoptosis. J Autoimmun. 2013;47:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang Z, Kirton HM, Al-Owais M, et al. Epac2-Rap1 signaling regulates reactive oxygen species production and susceptibility to cardiac arrhythmias. Antioxid Redox Signal. 2017;27(3):117–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li N, Li B, Brun T, et al. NADPH oxidase NOX2 defines a new antagonistic role for reactive oxygen species and cAMP/PKA in the regulation of insulin secretion. Diabetes. 2012;61(11):2842–2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Morshed SA, Ma R, Latif R, Davies TF. Biased signaling by thyroid-stimulating hormone receptor-specific antibodies determines thyrocyte survival in autoimmunity. Sci Signal. 2018;11(514):eaah4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kleinau G, Biebermann H. Constitutive activities in the thyrotropin receptor: regulation and significance. Adv Pharmacol. 2014;70:81–119. [DOI] [PubMed] [Google Scholar]
- 18. Morshed SA, Ma R, Latif R, Davies TF. Cleavage region thyrotropin receptor antibodies influence thyroid cell survival in vivo. Thyroid. 2019;29(7):993–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]