Abstract
Chronic stress and depressive-like behaviors in basic neuroscience research have been associated with impairments of neuroplasticity, such as neuronal atrophy and synaptic loss in the medial prefrontal cortex (mPFC) and hippocampus. The current review presents a novel integrative model of neuroplasticity as a multi-domain neurobiological, cognitive, and psychological construct relevant in depression and other related disorders of negative affect (e.g., anxiety). We delineate a working conceptual model in which synaptic plasticity deficits described in animal models are integrated and conceptually linked with human patient findings from cognitive science and clinical psychology. We review relevant reports including neuroimaging findings (e.g., decreased functional connectivity in prefrontal-limbic circuits), cognitive deficits (e.g., executive function and memory impairments), affective information processing patterns (e.g., rigid, negative biases in attention, memory, interpretations, and self-associations), and patient-reported symptoms (perseverative, inflexible thought patterns; inflexible and maladaptive behaviors). Finally, we incorporate discussion of integrative research methods capable of building additional direct empirical support, including using rapid-acting treatments (e.g., ketamine) as a means to test this integrative model by attempting to simultaneously reverse these deficits across levels of analysis.
Introduction
Depression is the leading cause of disability worldwide with a public disease burden of staggering proportions1. While efficacious treatments have been available for decades, remission rates are low, relapse rates are high, and disorder prevalence rates remain notably stagnant, with only 12.7% of patients receiving minimally adequate treatment2. At the molecular level, depression has been characterized as a failure of neuroplasticity, including neuronal atrophy and synaptic depression in the medial prefrontal cortex (mPFC) and hippocampus3–5. At the neurocognitive level, depression has been called a disorder of impaired cognitive flexibility and prefrontal inhibition6–8, leading to inflexible negative biases in cognition, such as rigidly held negative beliefs9.
Impaired neuroplasticity is theorized to underlie depression, but an empirical divide separates molecular models from cognitive/information processing models that motivate gold-standard behavioral treatments for depression. In this integrative review, we propose a model of neuroplasticity as a multi-level construct, conceptually linking relevant empirical findings across molecular/neuronal, neural network, cognitive, implicit information processing, and clinical levels of analysis. We highlight research approaches that help to bridge this divide. As an example, we discuss the potential for ketamine—which exhibits both rapid plasticity-enhancing effects in animal models4,10 and rapid clinical effects in human patients11,12—to provide a test of the predictions of this integrative model, including simultaneous and correlated reversals of multiple plasticity-related deficits across levels of analysis.
Neuroplasticity models of depression
Studies of the molecular and cellular mechanisms underlying depressive-like behaviors in rodent models and convergent brain imaging and postmortem studies of depressed patients have provided significant advances in our understanding of mood disorders. These findings reveal alterations at the levels of intracellular signaling, gene expression, neurotrophic factors, neurogenesis, neuroinflammation, excitatory and inhibitory neurotransmission, and synaptic number and function, and have been described in several brain regions implicated in depression13–22. The signaling pathways and types of molecular and cellular events vary depending on the brain regions studied. Studies have focused on PFC, hippocampus, amygdala, the ventral tegmental area-nucleus accumbens (VTA-NAc) dopamine system, and the HPA axis. These findings have resulted in complementary theories of depression and antidepressant response that have been linked, either directly or indirectly, to the molecular and cellular signaling mechanisms that mediate synaptic plasticity, and have therefore contributed to a broader neuroplasticity hypothesis of depression3–5. One of the leading theories highlights the roles of the PFC and hippocampus, including disruption of neurotrophic factors and synaptic connectivity that are related to neuroplasticity mechanisms4,5. According to this model of depression, chronic stress leads to sustained decreases in neuroprotective factors [e.g., brain-derived neurotrophic factor (BDNF) expression and signaling] that damage or hinder plasticity, fostering neuronal atrophy and decreased synaptic number and function, particularly in the mPFC and hippocampus3,4. This results in deficient adaptation to the environment, compromising learning and stress coping, and to downstream gain of activity in some ‘limbic network’ regions regulated by the PFC. One of the key efferent targets of mPFC is the amygdala, a region involved in control of fear and anxiety and widely implicated in human depression23; other output regions include the dorsal raphe, which has been linked to helplessness behavioral deficits (e.g., loss of control); the lateral habenula, associated with anhedonic and aversive responses; and the bed nucleus of the stria terminalis, another region linked with anxiety and negative emotion22. Conversely, when neuroplasticity is enhanced (e.g., by treatment), synaptic contacts increase, enhancing adaptability by allowing activity-dependent competition to stabilize the neural structures that best represent internal and external conditions24–26. These basic neuroscience findings are linked directly to shifts in depression-like behaviors in animal models, such as performance on the forced swim test, a probe of “despair,” the novelty suppressed feed test, a probe of “anxiety”, and the sucrose preference test, a probe of “anhedonia.”27
Molecular and cellular studies have examined the intracellular signaling pathways underlying the regulation of synaptic function by stress and antidepressant treatments. Repeated stress decreases the expression of BDNF in limbic and cortical brain regions, notably the hippocampus and PFC4,5,22. In addition, repeated stress exposure decreases mTORC1 signaling, which is required for synapse formation and neuroplasticity28, and inhibition of mTORC1 decreases synapse formation in the PFC and is sufficient to cause depression-like behaviors in rodents in the absence of stress exposure, demonstrating a causal relationship between synapses and behavior. Recent evidence demonstrates that chronic stress also leads to activation of microglia, the brain’s resident immune cells, which engulf synapses on nearby pyramidal neurons and thereby contribute to neuronal atrophy29. It is also notable that in some brain circuits, stress and depression may lead to enhancement of neuroplasticity mechanisms. For example, studies demonstrate that social defeat stress increases BDNF in the VTA-NAc pathway, leading to enhanced function that is thought to contribute to disruption of reward and motivation behaviors in depression20,21. There is also evidence that repeated stress causes hypertrophy of pyramidal neurons in the basolateral nucleus of the amygdala that could contribute to altered anxiety and emotion30,31. These findings demonstrate the diversity of the disruptions of plasticity in depression that vary according to brain circuitry and the underlying function regulated by different brain regions.
Clinical evidence provides some further support for the relevance of neuroplasticity mechanisms in depressed patients, though not all findings are consistent. Ketamine, a glutamatergic agent used routinely for induction and maintenance of anesthesia, exhibits well-replicated, rapid, potent antidepressant effects in randomized controlled trials (i.e., metaanalytic Cohen’s d=1.4, a large effect)12, even in difficult-to-treat conditions such as treatment-resistant depression32 and bipolar depression33. In addition, the FDA has recently approved a nasal application of (S)-ketamine, referred to as esketamine (Spravato), for treatment resistant depression. The rapidity and magnitude of ketamine’s effects have been attributed to its ability to rapidly reverse neuroplasticity deficits in animal models3,4,10,34. A single dose of ketamine increases BDNF release and stimulates mTORC1 signaling, which leads to increased levels of synaptic proteins (i.e., GluR1, PSD95, and synapsin 1) and increased number and function of synapses in the PFC4,22,35. An elegant recent study provided further evidence that new synapse formation is causally related to the antidepressant actions of ketamine10. Using in vivo two photon imaging, ketamine reversed the loss of synapses caused by stress, while selective deletion of these new synapses blocked the sustained antidepressant-like behavioral actions of ketamine.
Of note, however, rapastinel, another drug that reverses synaptic plasticity deficits and exhibits antidepressant-like effects in rodent models36,37, has been studied in 3 clinical trials to date with relatively weak evidence for its efficacy38. Given that clinical studies in depression are subject to well-known confounds including strong placebo responsivity and heterogeneous clinical presentations, further trials may be warranted to clarify rapastinel’s antidepressant efficacy. Regarding the specific role of mTORC1, a recent preliminary study39 found that rapamycin, an agent capable of blocking mTORC1, when given concurrent to ketamine infusion, did not block ketamine’s antidepressant efficacy (as was expected), but rather extended the window of ketamine’s antidepressant effect. However, the authors speculated that the dose of rapamycin (6 mg oral) may in fact have been insufficient to block mTORC1 in the brain, and that rapamycin’s potent, peripheral anti-inflammatory actions most likely account for the observed paradoxical effect. Both basic and clinical research have vital and complementary roles to play in the ongoing developing, testing, and refining of neuroplasticity theories of depression.
Integrative hypothesis
In spite of these findings suggesting crucial links between neuroplasticity mechanisms and behavioral tests in animal models, a fundamental translational question remains with respect to the alleviation of complex, multifaceted human conditions: how, precisely, might neuroplasticity mechanisms profoundly alter human experience? Our focus in the current review is to specify potential downstream results of molecular plasticity impairment observable at more ‘macro’ levels of analysis. In humans, depression and chronic negative affect are associated not only with decreases in convergent molecular and cellular neuroplasticity markers (e.g., BDNF40; prefrontal synapses measured post-mortem41), but also with altered functional integration across PFC and limbic (e.g., hippocampus, amygdala, striatum) circuits8,42–44. Such neural network alterations are posited to contribute to impaired regulatory control of stimulus-driven affective processing7,45, producing rigid negative biases evident across a wide range of implicit information processing domains (e.g., negative appraisals of self, the environment, and the future46; preferential attention and memory for negative stimuli45,47). These neural and implicit cognitive patterns may in turn contribute to impaired overall cognitive and behavioral flexibility6,48 and help to maintain and reinforce a state of high negative affect by fostering overestimation of the personal shortcomings, dangers, and misfortunes inherent to the individual’s life9. Although psychological and neurocognitive accounts of depression have not typically been united with neuroplasticity findings into a single integrative theory, strong parallels are suggested by overlap in the implicated brain regions (e.g., mPFC, hippocampus, amygdala) and behavioral sequelae (rigid responses to the environment). On this basis, we propose an integrative model of neuroplasticity and mood that bridges these levels of analysis (Table 1; Figure 1).
Table 1.
Neuroplasticity Markers Across Levels of Analysis
| Level of analysis | Dysfunctional State | Treatment Goal State |
|---|---|---|
| Molecular/cellular | ↓synaptic number and function, neuronal atrophy | ↑synaptogenesis, ↑neurotrophic factors |
| Neural network | ↓PFC-limbic circuit connectivity | ↑PFC-limbic connectivity and ↑PFC regulatory control over limbic regions |
| Cognitive Function | ↓flexibility, ↓cognitive control, ↓goal-directed inhibition/excitation of lower-order functions | ↑flexibility and cognitive control, ↑goal-directed inhibition/excitation capacity |
| Affective Information Processing | rigid negative biases in implicit information processing (e.g., attention, memory, interpretations, self-representations) | unbiased and flexible information processing |
| Clinical/self-report | Perseverative negative thoughts, repetitive maladaptive behaviors, depression | novel positive thoughts/perceptions, diversified behavioral repertoire, euthymic mood, improved function/engagement |
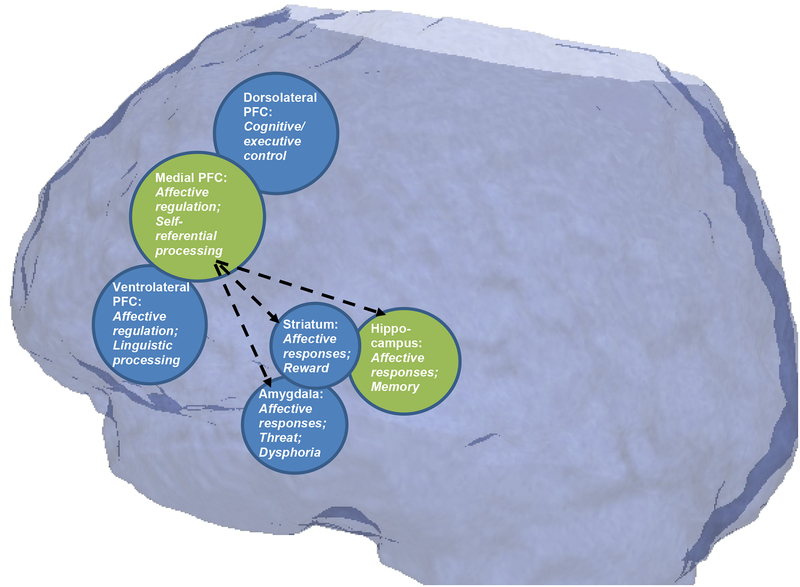
Figure 1:
Regions with prominent neuroplasticity deficits in animal models of depression4,5 (in green) and functionally interconnected regions within a cortico-mesolimbic circuit relevant to mood regulation (blue). Some proposed functions of these regions with relevance in our integrative model are highlighted. “Medial prefrontal cortex (PFC),” as implicated in animal models, includes a number of subdivisions implicated in human depression including subgenual anterior cingulate cortex (ACC) and ventro- and dorso-medial PFC areas. Dashed lines represent primary hypothesized impairments in prefrontal cortex connectivity and top-down regulation of limbic regions, resulting in impairments in behavioral and cognitive flexibility across levels of analysis.
Plasticity impairments in neural networks
Consistent with the predictions of animal models, hippocampal and PFC volumes are robustly decreased in depressed patients, according to in vivo structural imaging5. Recently compiled large imaging corpuses, which include >1000 unipolar depressed patients and many thousands of healthy control participants, have documented particularly robust decreases in hippocampal volume49, which were driven by individuals with recurrent depression and early age of onset (≤age 21), suggesting the impact of the depressed state on hippocampal volume may be cumulative across time and/or episodes. Amygdalar volumes were likewise decreased, but only in patients with early-onset depression, and this finding was not statistically robust after correcting for multiple comparisons. In PFC subregions, robust decreases in cortical thickness were observed in medial and orbital areas of the PFC and anterior cingulate cortex (ACC)50, which constitute subdivisions of the rodent “mPFC” homologue (Figure 1). Convergent meta-analytic findings suggest disrupted white matter microarchitecture in depressed patients in key white matter tracts that facilitate inter- and intra-hemispheric integration across PFC and limbic regions, including the corpus collosum, front-occipital fasciculus, and PFC projection fibers51.
Given that brain structure constrains functional networks52, these volumetric changes are hypothesized to underlie abnormalities observed in functional neuroimaging measures within the same circuits. Brain processes may best be characterized as the coordinated activity of disparate brain regions over time53. Functional connectivity measures (i.e., the temporal correlation between spatially remote neurophysiological events) may be a particularly relevant marker of plasticity deficits, as these indices directly quantify the degree to which regions of the brain act in a coherent fashion and/or influence one another over a second-to-second timeframe. Thus, connectivity indices, often measured with neuroimaging methods such as fMRI, may provide a crucial intermediary, helping to bridge structural neuroplasticity markers (e.g., synaptic dysconnectivity) to downstream alterations in the brain’s ‘output,’ i.e., mental and cognitive processes. When using meta-analytic techniques to identify the most robust patterns across individual studies, depression has been associated with decreased ‘intrinsic’ functional connectivity (i.e., connectivity observed during a resting state) within and between PFC and limbic networks, including decreased connectivity between mPFC and limbic/affective regions including the hippocampus and amygdala54. Depression has likewise been linked to decreased ‘global’ intrinsic connectivity between the medial and lateral PFC and all other regions of the brain55, and to decreased inter- and intra-hemispheric integration within and across dorsolateral PFC, medial PFC/ACC, hippocampus, and parietal regions56,57. Notably, these connectivity decreases are evident in spite of simultaneously increased intrinsic connectivity in other, spatially overlapping networks (e.g., within the midline “default mode network”54,58), suggesting connectivity aberrations in depression are circuit-specific.
Similarly, during task states requiring processing of affective stimuli (e.g., evaluating the personal relevance of negative/positive attributes; viewing negative pictures), depressed patients have shown decreased functional connectivity between lateral PFC and amygdala44 and/or hippocampus59 and across medial PFC/ACC and limbic regions60,61. During ‘cognitive reappraisal’ tasks, in which volitional attempts are made to down-regulate subjective negative responses to negative stimuli62, depressed patients have shown decreased activation in lateral and medial PFC coupled with increases in amygdalar response63,64, and altered mPFC-amygdalar connectivity65, suggesting impaired volitional PFC regulation of the amygdala. Collectively, these patterns observed in human patients are consistent with the premise that neuronal and molecular plasticity deficits may feed forward to impairments in the coordinated function of prefrontal and limbic regions66, resulting in impaired PFC regulation and unchecked stimulus-driven responses to salient negative stimuli.
Plasticity impairments in cognition
Structural and neural circuit alterations described above may contribute to performance deficits observed in depressed patients during cognitive and neuropsychological tasks. These deficits are consistent with an impaired capacity to engage flexibly with external stimuli and efficiently exert goal-directed cognition in support of task demands, thereby reducing flexible, adaptive behavior in complex environments67. Meta-analysis of executive functions in depressed patients suggest pronounced deficits (Cohen’s d ≥1.0) in cognitive flexibility, inhibition capacity, and verbal fluency, as well as moderate impairments in strategic planning and organization68,69. Attention and concentration are also broadly impaired70,71. These wide-ranging executive deficits suggest a broad impairment in the ability to control and regulate other lower-order functions and behaviors, including the ability to initiate and stop actions, to monitor and change behavior to match shifting demands of the environment, and to plan optimal behaviors in the face of novelty69. Such abilities rely on the integrity and coordinated function of both lateral and medial PFC and ACC subregions72,73, paralleling the neuronal and neural network levels (as described above), and are believed to contribute fundamentally to depressed patients’ substantial functional impairments74. Correspondingly, computerized cognitive training interventions, designed to rehabilitate core prefrontal cognitive functions (e.g., working memory) through repeated task practice, show preliminary meta-analytic evidence of acute efficacy in the treatment of depression, including moderate effects on symptom severity and daily functioning75,76.
Memory deficits are also apparent in depressed patients. These include impairments in both prospective memory for new information—across a variety of domains and task conditions77—and retrospective autobiographical memory retrieval, wherein a lack of specificity in retrieved memories (“overgeneral memory”) has been described78. Overgeneral memory is correlated with reduced problem-solving performance79 and imageability of future events80, suggesting the lack of specificity in recalling past life events constrains the ‘cognitive set’ of viable possible actions that are readily available to conscious awareness. Memory deficits in depressed patients have long been hypothesized to relate directly to neuroplasticity deficits within the hippocampus81, given its key role in memory formation and retrieval. However, circuit-level dysfunctions impacting connectivity within and across multiple PFC and limbic regions may best explain the breadth of cognitive impairments observed in depression, collectively reducing the individual’s capacity to flexibly adjust in response to a continually changing environment.
Plasticity impairments in affective information processing patterns
In addition to cognitive deficits on neuropsychological tests (utilizing standardized, ostensibly neutral stimuli), depression is characterized by rigid, valence-specific biases in the processing of affective information. These biases create preferential processing of negative information (i.e., up-regulation of the ‘negative valence system’), and decreased engagement with positive information (down-regulation of the ‘positive valence system’), across a range of information processing domains, including attention, memory, interpretation, implicit associations (e.g., negative representations of one’s self), and learning and decision-making8,82.
Attention, which acts as an initial filter on the information entering conscious awareness, shows valence-specific alterations in depressed patients including attentional preference for dysphoric stimuli and biases away from positive stimuli45,47,83. In addition, depressed patients show impaired inhibition of negative information7. As these factors result in a greater share of negative (relative to neutral and positive) information being passed forward for further processing, they likely contribute to additional biases documented at later, more ‘elaborative’ stages of cognition, including preferential recall of negative over positive information84,85, and biases towards negative interpretations of ambiguous words and word fragments, images (e.g., facial expressions), and scenarios86–88. Finally, depression has been associated with stronger implicit associations between the mental concept of oneself and negative characteristics, e.g., lower implicit self-esteem, as measured by performance-based indices89–91. Stronger implicit associations between self and suicide-related constructs (e.g., death)92, as well as stronger attentional bias towards suicide-related words93, have further been linked to prospective risk of suicide attempts, suggesting a link between affective processing biases and the most severe consequences of depression.
Within the positive valence system, depression involves prominent alterations in the processing of reward cues, such as decreased hedonic pleasure or ‘liking’ of positive stimuli, decreased reward anticipation, and altered reinforcement learning, which are believed to culminate in decreased motivation to act94. Recently, computational approaches have been applied in an effort to further dissect these biases and unveil their component neurocomputational processes. Findings from this emerging literature suggest several impairments in reinforcement learning, or the process of maximizing reward and minimizing loss by modifying behavior in response to experience, which plays a central role in decision-making95. For instance, depressed patients exhibit both hyposensitivity to rewards and oversensitivity to punishments96,97, attributed to alterations in mesolimbic prediction error signaling97–99, and may exhibit a constrained option space (“tunnel vision”) during decision-making100. Broadly, positive valence system deficits are linked primarily to altered dopaminergic and/or opioid signaling within midbrain striatal circuits94,101, but may intersect with plasticity impairments and dysconnectivity of excitatory glutamatergic synapses in an overlapping prefrontal-mesolimbic circuit5 to produce the full spectrum of affective biases seen in depression.
Though the etiology of these affective information processing biases is not fully understood, the neuroplasticity model of depression suggests a two-fold process: 1) chronic stress, adversity, and negative life events (e.g., parental and/or social interactions) promote learning of negative associations and expectations via normative, experience-dependent plasticity mechanisms, which include evolutionarily preserved mechanisms that prioritize the learning and retention of negative and emotionally salient information102,103; 2) such learning is further entrenched through simultaneous, stress-induced decreases in overall plasticity within the prefrontal-limbic circuit, resulting in inflexible, intractable biases that are resistant to subsequent environmental inputs (e.g., positive/disconfirming information). Consistent with our integrative framework (Table 2), the neural substrates of affective biases have been posited to involve inadequate PFC regulation of stimulus-driven limbic responses8,47 and generalized deficiencies of cognitive inhibition7. Negative biases in attention42,43,104,105 and self-representations44 have been directly linked to reduced functional connectivity in prefrontal-limbic circuitry, suggesting these neural and behavioral features of depression could reflect a unitary plasticity impairment, expressed across levels of analysis. Biased patterns of information processing might then constitute a final pathway to negative affect and self-reported symptomatology (discussed further below), via their chronic and cumulative influence on the individual’s day-to-day perceptions and experiences82,106–109. Further integrative research is needed to evaluate this multi-domain hypothesis.
Table 2.
Summary of key findings supporting integrative neuroplasticity model
| Plasticity marker | Relevance: Key empirical findings linking marker to depression (see main text for details and citations) | Integration: Findings directly linking marker to other levels of analysis | Causality: Does manipulating marker lead to generalized depression relief? |
|---|---|---|---|
| Molecular/cellular | |||
| ↓Synapse number and function ↓Neurotrophic factors |
Chronic stress induces synaptic deficits in mPFC and hippocampus, which lead to depressive-like behaviors in animal models ↓prefrontal synapses in patients post-mortem Decreased BDNF in depressed patients |
-- |
Approach: Intravenous ketamine Evidence: Strong support12 |
| Neural network | |||
| ↓PFC-limbic circuit connectivity | Decreased PFC/limbic volumes, white matter integrity, intrinsic functional connectivity, and task-based connectivity in patients | ↓PFC-limbic intrinsic83 and task-based42,43,105 connectivity linked to ↑negative attentional bias ↓intrinsic connectivity within limbic regions linked to ↑lassitude153 |
Approach: Neurofeedback Evidence: Preliminary support154,155 Approach: Neuromodulation of PFC Evidence: Moderate-to-strong support156 |
| Cognitive Function | |||
| ↓cognitive/executive control memory impairments (prospective memory deficits; overgeneral autobiographical memory) |
Decreased executive functions Impaired and “over-general” memory recall |
↓Self-reported cognitive control linked to ↑negative attentional bias157 ↓Self-reported cognitive control linked to ↑depression severity via ↑rumination158 ↑Overgeneral memory linked to ↑rumination and ↓executive function159 |
Approach: Cognitive control training Evidence: Preliminary support75 Approach: Autobiographical episodic memory training Evidence: Preliminary support160 |
| Affective Information Processing | |||
| Attentional bias Interpretive bias Memory bias Biased self-associations Biased reward learning |
Behavioral task performance supports the existence of each form of bias in depressed patients | Attentional bias: see above ↑negative biases in inhibitory control linked to ↑rumination7 |
Approach: Cognitive bias modification Evidence: Mixed/limited support108 (possibly related to difficulties in robustly modifying biases with current approaches)161 Approach: Evaluative conditioning of self-associations Evidence: Collateral/preliminary support162 |
| Clinical/self-report | |||
| Inflexible thought patterns Inflexible behaviors |
Repetitive negative thinking patterns (e.g. rumination, depressive schemas); constrained behaviors (e.g., lassitude) reported routinely by depressed patients |
Lassitude and rumination: see above |
Approach: Cognitive therapy Evidence: Strong support114 Approach: Behavioral activation Evidence: Strong support119 |
Plasticity impairments in self-reported psychological symptoms
Though depression is a highly heterogeneous and complex syndrome, it is notable that the clinical phenomenology of depression includes multiple prominent markers of inflexible thought and behavior, which are broadly consistent with a decreased capacity for the brain to flexibly adjust and reorganize itself in response to a changing environment6. The two hallmark mood symptoms of depression—at least one of which is required to diagnose a major depressive episode110—are persistent dysphoric mood and persistent anhedonia (the inability to experience pleasure), mirroring the two major forms of information processing bias (positive and negative valence systems) discussed above. Rumination, a form of perseverative negative thought pattern, is a prominent risk factor for depression111. Similar perseverative thought patterns, such as worry, ruminative post-event processing, and obsessional thinking, are transdiagnostic features of other (often comorbid) negative affective conditions, leading to the hypothesis that repetitive negative thinking is a core, cross-cutting feature of disorders of negative affect112. Depressive “schemas,” the principle treatment target in gold-standard cognitive-behavioral interventions113, are likewise characterized by rigid, over-generalized negativity with regard to perceptions of self, future, and the world9. Efficacious cognitive-behavioral treatments therefore focus on the goal of expanding plasticity within the perceptions and conscious representations of the patient’s internal and external worlds, through repeated, deliberate practice in recognizing and correcting maladaptive, excessively negative thought patterns114. Mindfulness-based cognitive therapy, which can effectively forestall the return of depression among individuals with a depression history115,116, has similarly been characterized as an effort to increase mental flexibility in response to the environment, specifically through the practice of “de-centering,” or learning to perceive one’s thoughts, feelings and reactions from an objective, non-judgmental, and accepting stance117.
The behavioral repertoire reported by depressed patients likewise lacks flexibility and diversity, characterized by symptoms of social withdrawal, behavioral deactivation, lassitude, ‘vegetative symptoms’ (e.g., loss of appetite and libido), and amotivation110,118,119. Depressed patients report that a constrained set of possible actions appear viable, paralyzing action and decision-making120. Behavioral activation, a psychotherapy that aims to directly increase activity, can effectively treat depression through a concerted effort to expand on points of contact between the patient and their environment, thereby stimulating natural opportunities for positive reinforcement to be received119,121,122. Thus, plasticity and diversification within the realm of volitional actions may likewise be antithetical to the state of depression.
Testing predictions of the integrative model: Intravenous ketamine as a test case
Our integrative model predicts the falsifiable hypothesis that an agent that reverses molecular neuroplasticity deficits in animal models should exhibit not only clinical effects on depression, but also correlated, simultaneous reversals of impairments at each level of analysis (Table 1). While some of these markers may be quantifiable in animal models, allowing for greater experimental controls (e.g., neural networks; cognition), others may be unique to humans (e.g., information processing; self-report). The use of a rapid-acting agent may be particularly beneficial in human studies, because the influence of many environmental and situational confounds inherent to human research are temporally contained due to the rapidity of ketamine’s effects. However, ketamine research, like most pharmacological research, has focused largely on (a) molecular effects in animal models or (b) symptom-level effects in human patients. While molecular neuroplasticity effects are well-described in rodent models, attempts to extend molecular findings to humans (e.g., BDNF and synapse levels) have yielded inconsistent results3, in no small part because of the difficulty of assessing these endpoints in the living brain, the tissue of interest. The cognitive, information processing, and neural network domains offer relatively untapped opportunities to integratively understand how enhanced neuroplasticity might ultimately translate to a profound shift in human experience, i.e. rapid depression relief.
Preliminary findings on ketamine’s effects on neurocognition are consistent with the proposed model of neuroplasticity, suggesting clues as to how ketamine’s rapid clinical and neuronal/molecular effects might manifest in other domains.
Neural networks:
fMRI investigations in depressed patients have linked ketamine’s antidepressant effects to increased connectivity between mPFC and striatum123, between lateral PFC and subgenual ACC124, and to increased global connectivity between the PFC and the rest of the brain55. Convergent data show increased PFC glucose metabolism 24-hours post-ketamine125,126, increased fMRI PFC activations acutely (during infusion itself)127, and decreased limbic responses to angry (relative to happy) faces among ketamine responders128. Increased connectivity between the default mode network (DMN), which encompasses the medial PFC, and the insula have also been reported in depressed patients 2 days following ketamine, suggesting sustained normalization of the DMN’s relationship to other networks129.
Cognitive function:
Two studies in rats130,131 extend molecular findings in animal models to behavioral indices with possible relevance to cognitive flexibility in human patients. Following stress, rats randomized to ketamine (compared to saline) exhibited enhanced cognitive flexibility, as indexed by improved set-shifting task performance. In small, uncontrolled samples, convergent findings in depressed patients further suggest cognitive flexibility on objective cognitive tests improves acutely following ketamine132,133.
Affective information processing:
Ketamine rapidly induces flexibility in implicit representations of self on the Implicit Association Test134,135, and these shifts correlated with self-reported symptom improvements110, suggesting that ketamine can immediately impact the structure of implicit mental representations relevant to one’s concept of self, a core form of cognitive bias in depression. Although in a separate study, ketamine failed to illustrate a similar impact on attentional bias towards negative cues128, the use of a reaction time measure (the dot-probe task) with notoriously poor psychometric properties136–138 may have impeded the ability to sensitively detect changes over time.
Further integration across multiple markers of plasticity is needed to build support for an integrative model, as highlighted by the small number of direct “integration” links in Table 2. Of note, to accurately test for relationships in individual differences expressed across patients with correlational and mediational investigations, larger sample sizes are likely required relative to those necessary simply to establish the antidepressant efficacy of ketamine. Ongoing work in depression (e.g., R01MH113857;) aims to comprehensively characterize and link ketamine’s depression-relevant effects across molecular, neural network, cognitive, implicit processing, and symptomatic levels of analysis in human patients, in the hopes of establishing a multi-domain neuroplasticity signature.
Testing predictions of the integrative model: Experimental manipulation at each level
In addition to manipulating molecular neuroplasticity targets (e.g., pharmacologically), a complimentary approach is to evaluate the causal influence of each posited form of plasticity by manipulating it directly (e.g., through mechanistic intervention; see Table 2:’Causality’ for specific examples and evidence base) and observing its influence on clinical depression and all other levels of analysis. Leveraging RCT designs and explicit tests of ‘target engagement’ (to validate that the targeted neural, cognitive, or information processing manipulation was successful), this approach has the potential to distinguish causal influences on depression from depression correlates and to build support for a causal chain of neuroplasticity decrements leading to depression symptoms.
Generalizability and Transdiagnostic Relevance
Though the current review focuses on depression, it is notable that chronic stress is a risk factor for virtually all psychiatric conditions139. Likewise, the neural, cognitive, information processing, and psychological patterns discussed above have broad transdiagnostic relevance, particularly with respect to other disorders of negative affect (e.g., anxiety, trauma-related conditions)140—that likewise respond to pharmacologic interventions with plasticity-enhancing properties141,142. Equally noteworthy, however, is the marked heterogeneity of depressed patients, which is evident at the neural network143,144, cognitive145, information processing145,146, and clinical147 levels, and may help to explain the fact that no known intervention strategy is effective for all depressed patients. Rather than assuming a unitary etiology, and a correspondingly uniform pathway to recovery from depression, a focus on establishing correlations across levels of analysis—both before and after administering plasticity-enhancing interventions—will help to clarify the degree to which an integrative neuroplasticity model may be relevant to some individual forms of depression and negative affect, but not others140.
Conclusion
Neuroplasticity, or the brain’s capacity to flexibly adjust and reorganize itself in response to a changing environment, is a fundamental contributor to adaptive functioning. Impairments of neuroplasticity often characterize disorders of negative affect including depression3,4 and multiple efficacious therapies often reverse such deficits10,148–151. Our review highlights conceptual convergence of findings across relatively disparate literatures in basic neuroscience, cognitive science, and clinical psychology. Direct empirical links remain quite preliminary (Table 2). We hope to stimulate future studies with a broader integrative focus to empirically elucidate the intermediary mechanisms that allow neuronal and molecular changes at the cellular level to propagate up through a complex, circuit-based neurocognitive system. Ideally this work will suggest new treatment avenues to provide relief from pervasive, chronic, inflexible, and debilitating patterns of mood and behavior. Such novel treatments might aim, for example, to synergistically target more than one form of plasticity deficit simultaneously through theory-driven, somatic-behavioral treatment pairings152.
Acknowledgements:
This project was supported in part by National Institute of Mental Health grant number R01MH113857 (RBP).
Footnotes
Conflict of Interest: Dr. Price and Dr. Duman report no conflicts of interest or competing financial interests.
References
- 1.Chisholm D, Sweeny K, Sheehan P, Rasmussen B, Smit F, Cuijpers P et al. Scaling-up treatment of depression and anxiety: a global return on investment analysis. Lancet Psychiatry 2016; 3(5): 415–424. [DOI] [PubMed] [Google Scholar]
- 2.Wang PS, Lane M, Olfson M, Pincus HA, Wells KB, Kessler RC. Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62(6): 629–640. [DOI] [PubMed] [Google Scholar]
- 3.Abdallah CG, Sanacora G, Duman RS, Krystal JH. Ketamine and rapid-acting antidepressants: a window into a new neurobiology for mood disorder therapeutics. Annu Rev Med 2015; 66: 509–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duman RS, Aghajanian GK, Sanacora G, Krystal JH. Synaptic plasticity and depression: new insights from stress and rapid-acting antidepressants. Nat Med 2016; 22(3): 238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science 2012; 338(6103): 68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kashdan TB, Rottenberg J. Psychological flexibility as a fundamental aspect of health. Clin Psychol Rev 2010; 30(7): 865–878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joormann J Cognitive inhibition and emotion regulation in depression. Curr Dir Psychol Sci 2010; 19(3): 161–166. [Google Scholar]
- 8.Disner S, Beevers C, Haigh EAP, Beck AT. Neural mechanisms of the cognitive model of depression. 2011; 12: 467–477. [DOI] [PubMed] [Google Scholar]
- 9.Beck AT, Bredemeier K. A Unified Model of Depression: Integrating Clinical, Cognitive, Biological, and Evolutionary Perspectives. Clin Psychol Science 2016; 4(4): 596–619. [Google Scholar]
- 10.Moda-Sava RN, Murdock MH, Parekh PK, Fetcho RN, Huang BS, Huynh TN et al. Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science 2019; 364(6436). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CM, Perez AM et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: A Two-site randomized controlled trial. Am J Psychiatry 2013; 170(10): 1134–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu Y, Hackett M, Carter G, Loo C, Galvez V, Glozier N et al. Effects of low-dose and very low-dose dose ketamine among patients with major depression: a systematic review and meta-analysis. Int J Neuropsychopharmacol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beurel E, Nemeroff CB. Interaction of stress, corticotropin-releasing factor, arginine vasopressin and behaviour. Curr Top Behav Neurosci 2014; 18: 67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luscher B, Fuchs T. GABAergic control of depression-related brain states. Adv Pharmacol 2015; 73: 97–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Waters RP, Rivalan M, Bangasser DA, Deussing JM, Ising M, Wood SK et al. Evidence for the role of corticotropin-releasing factor in major depressive disorder. Neurosci Biobehav Rev 2015; 58: 63–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fee C, Banasr M, Sibille E. Somatostatin-Positive Gamma-Aminobutyric Acid Interneuron Deficits in Depression: Cortical Microcircuit and Therapeutic Perspectives. Biol Psychiatry 2017; 82(8): 549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wohleb ES, Franklin T, Iwata M, Duman RS. Integrating neuroimmune systems in the neurobiology of depression. Nat Rev Neurosci 2016; 17(8): 497–511. [DOI] [PubMed] [Google Scholar]
- 18.McEwen BS, Nasca C, Gray JD. Stress Effects on Neuronal Structure: Hippocampus, Amygdala, and Prefrontal Cortex. Neuropsychopharmacology 2016; 41(1): 3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anacker C, Hen R. Adult hippocampal neurogenesis and cognitive flexibility - linking memory and mood. Nat Rev Neurosci 2017; 18(6): 335–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koo JW, Chaudhury D, Han MH, Nestler EJ. Role of Mesolimbic Brain-Derived Neurotrophic Factor in Depression. Biol Psychiatry 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cathomas F, Murrough JW, Nestler EJ, Han MH, Russo SJ. Neurobiology of Resilience: Interface Between Mind and Body. Biol Psychiatry In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Duman RS, Sanacora G, Krystal JH. Altered Connectivity in Depression: GABA and Glutamate Neurotransmitter Deficits and Reversal by Novel Treatments. Neuron 2019; 102(1): 75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnone D Functional MRI findings, pharmacological treatment in major depression and clinical response. Prog Neuropsychopharmacol Biol Psychiatry 2019; 91: 28–37. [DOI] [PubMed] [Google Scholar]
- 24.Castrén E Is mood chemistry? 2005; 6: 241–246. [DOI] [PubMed] [Google Scholar]
- 25.Castren E, Hen R. Neuronal plasticity and antidepressant actions. Trends Neurosci 2013; 36(5): 259–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Changeaux JP, Danchin A. Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature 1976; 264: 705–712. [DOI] [PubMed] [Google Scholar]
- 27.Wang Q, Timberlake MA 2nd Prall K, Dwivedi Y. The recent progress in animal models of depression. Prog Neuropsychopharmacol Biol Psychiatry 2017; 77: 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ota K, Liu R, Voleti B, Maldonado-Aviles J, Duric V, Iwata M et al. REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat Med 2014; 20: 531–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wohleb ES, Terwilliger R, Duman CH, Duman RS. Stress-Induced Neuronal Colony Stimulating Factor 1 Provokes Microglia-Mediated Neuronal Remodeling and Depressive-like Behavior. Biol Psychiatry 2018; 83(1): 38–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roozendaal B, McEwen BS, Chattarji S. Stress, memory and the amygdala. 2009; 10: 423–433. [DOI] [PubMed] [Google Scholar]
- 31.Patel D, Anilkumar S, Chattarji S, Buwalda B. Repeated social stress leads to contrasting patterns of structural plasticity in the amygdala and hippocampus. Behav Brain Res 2018; 347: 314–324. [DOI] [PubMed] [Google Scholar]
- 32.Caddy C, Amit BH, McCloud TL, Rendell JM, Furukawa TA, McShane R et al. Ketamine and other glutamate receptor modulators for depression in adults. Cochrane Database Syst Rev 2015; (9): CD011612. [DOI] [PubMed] [Google Scholar]
- 33.McCloud TL, Caddy C, Jochim J, Rendell JM, Diamond PR, Shuttleworth C et al. Ketamine and other glutamate receptor modulators for depression in bipolar disorder in adults. Cochrane Database Syst Rev 2015; (9): CD011611. [DOI] [PubMed] [Google Scholar]
- 34.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 2016; 533(7604): 481–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010; 329(5994): 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu RJ, Duman C, Kato T, Hare B, Lopresto D, Bang E et al. GLYX-13 Produces Rapid Antidepressant Responses with Key Synaptic and Behavioral Effects Distinct from Ketamine. Neuropsychopharmacology 2017; 42(6): 1231–1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Burgdorf J, Zhang XL, Nicholson KL, Balster RL, Leander JD, Stanton PK et al. GLYX-13, a NMDA receptor glycine-site functional partial agonist, induces antidepressant-like effects without ketamine-like side effects. Neuropsychopharmacology 2013; 38(5): 729–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB et al. Ketamine and Other NMDA Antagonists: Early Clinical Trials and Possible Mechanisms in Depression. Am J Psychiatry 2015; 172(10): 950–966. [DOI] [PubMed] [Google Scholar]
- 39.Abdallah C, Averill L, Gueorguieva R, Goktas S, Purohit P, Ranganathan M et al. Rapamycin, an immunosuppressant and mTORC1 inhibitor, triples the antidepressant response rate to ketamine at two weeks following treatment. BioRXiv 2018. [Google Scholar]
- 40.Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry 2008; 64(6): 527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kang HJ, Voleti B, Hajszan T, Rajkowska G, Stockmeier CA, Licznerski P et al. Decreased expression of synapse-related genes and loss of synapses in major depressive disorder. Nat Med 2012; 18(9): 1413–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Price RB, Eldreth DA, Mohlman J. Deficient prefrontal attentional control in late-life generalized anxiety disorder: an fMRI investigation. Translational Psych 2011; 1: e46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Price RB, Allen KB, Silk JS, Ladouceur CD, Ryan ND, Dahl RE et al. Vigilance in the laboratory predicts avoidance in the real world: A dimensional analysis of neural, behavioral, and ecological momentary data in anxious youth. Dev Cogn Neurosci 2016; 19: 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: Related and independent features. Biol Psychiatry 2007; 61(2): 198–209. [DOI] [PubMed] [Google Scholar]
- 45.Gotlib IH, Joormann J. Cognition and depression: current status and future directions. Annu Rev Clin Psychol 2010; 6: 285–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dozois D, Beck A. Cognitive schemas, beliefs and assumptions In: Dobson K, Dozois D (eds). Risk factors in depression. Elsevier/Academic Press: Oxford, England, 2008, pp 121–143. [Google Scholar]
- 47.de Raedt R, Koster EHW. Understanding vulnerability for depression from a cognitive neuroscience perspective: A reappraisal of attentional factors and a new conceptual framework. Cogn Affect Behav Neurosci 2010; 10: 50–70. [DOI] [PubMed] [Google Scholar]
- 48.Stange JP, Connolly SL, Burke TA, Hamilton JL, Hamlat EJ, Abramson LY et al. Inflexible Cognition Predicts First Onset of Major Depressive Episodes in Adolescence. Depress Anxiety 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmaal L, Veltman DJ, van Erp TG, Samann PG, Frodl T, Jahanshad N et al. Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry 2016; 21(6): 806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schmaal L, Hibar DP, Samann PG, Hall GB, Baune BT, Jahanshad N et al. Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry 2017; 22(6): 900–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen G, Guo Y, Zhu H, Kuang W, Bi F, Ai H et al. Intrinsic disruption of white matter microarchitecture in first-episode, drug-naive major depressive disorder: A voxel-based meta-analysis of diffusion tensor imaging. Prog Neuropsychopharmacol Biol Psychiatry 2017; 76: 179–187. [DOI] [PubMed] [Google Scholar]
- 52.Park HJ, Friston K. Structural and functional brain networks: from connections to cognition. 2013; 342(6158): 1238411. [DOI] [PubMed] [Google Scholar]
- 53.Sporns O, Chialvo DR, Kaiser M, Hilgetag CC. Organization, development and function of complex brain networks. Trends Cogn Sci 2004; 8(9): 418–425. [DOI] [PubMed] [Google Scholar]
- 54.Kaiser RH, Andrews-Hanna JR, Wager TD, Pizzagalli DA. Large-Scale Network Dysfunction in Major Depressive Disorder: A Meta-analysis of Resting-State Functional Connectivity. JAMA Psychiatry 2015; 72(6): 603–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Abdallah CG, Averill LA, Collins KA, Geha P, Schwartz J, Averill C et al. Ketamine treatment and global brain connectivity in Major Depression. Neuropsychopharmacology In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hirshfeld-Becker DR, Gabrieli JDE, Shapero BG, Biederman J, Whitfield-Gabrieli S, Chai XJ. Intrinsic Functional Brain Connectivity Predicts Onset of Major Depression Disorder in Adolescence: A Pilot Study. Brain Connect 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jiang X, Shen Y, Yao J, Zhang L, Xu L, Feng R et al. Connectome analysis of functional and structural hemispheric brain networks in major depressive disorder. Transl Psychiatry 2019; 9(1): 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greicius MD, Flores BH, Menon V, Glover GH, Solvason HB, Kenna H et al. Resting-state functional connectivity in major depression: abnormally increased contributions from subgenual cingulate cortex and thalamus. Biol Psychiatry 2007; 62(5): 429–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rive MM, van Rooijen G, Veltman DJ, Phillips ML, Schene AH, Ruhe HG. Neural correlates of dysfunctional emotion regulation in major depressive disorder. A systematic review of neuroimaging studies. Neurosci Biobehav Rev 2013; 37(10 Pt 2): 2529–2553. [DOI] [PubMed] [Google Scholar]
- 60.Carballedo A, Scheuerecker J, Meisenzahl E, Schoepf V, Bokde A, Moller HJ et al. Functional connectivity of emotional processing in depression. J Affect Disord 2011; 134(1–3): 272–279. [DOI] [PubMed] [Google Scholar]
- 61.Anand A, Li Y, Wang Y, Wu J, Gao S, Bukhari L et al. Activity and connectivity of brain mood regulating circuit in depression: a functional magnetic resonance study. Biol Psychiatry 2005; 57(10): 1079–1088. [DOI] [PubMed] [Google Scholar]
- 62.Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JD et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage 2004; 23(2): 483–499. [DOI] [PubMed] [Google Scholar]
- 63.Zilverstand A, Parvaz MA, Goldstein RZ. Neuroimaging cognitive reappraisal in clinical populations to define neural targets for enhancing emotion regulation. A systematic review. Neuroimage 2017; 151: 105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pico-Perez M, Radua J, Steward T, Menchon JM, Soriano-Mas C. Emotion regulation in mood and anxiety disorders: A meta-analysis of fMRI cognitive reappraisal studies. Prog Neuropsychopharmacol Biol Psychiatry 2017; 79(Pt B): 96–104. [DOI] [PubMed] [Google Scholar]
- 65.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci 2007; 27(33): 8877–8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thompson SM, Kallarackal AJ, Kvarta MD, Van Dyke AM, LeGates TA, Cai X. An excitatory synapse hypothesis of depression. Trends Neurosci 2015; 38(5): 279–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koechlin E Prefrontal executive function and adaptive behavior in complex environments. Curr Opin Neurobiol 2016; 37: 1–6. [DOI] [PubMed] [Google Scholar]
- 68.Wagner S, Muller C, Helmreich I, Huss M, Tadic A. A meta-analysis of cognitive functions in children and adolescents with major depressive disorder. Eur Child Adolesc Psychiatry 2015; 24(1): 5–19. [DOI] [PubMed] [Google Scholar]
- 69.Wagner S, Doering B, Helmreich I, Lieb K, Tadic A. A meta-analysis of executive dysfunctions in unipolar major depressive disorder without psychotic symptoms and their changes during antidepressant treatment. Acta psychiatrica Scandinavica 2012; 125(4): 281–292. [DOI] [PubMed] [Google Scholar]
- 70.McClintock SM, Husain MM, Greer TL, Cullum CM. Association between depression severity and neurocognitive function in major depressive disorder: a review and synthesis. Neuropsychology 2010; 24(1): 9–34. [DOI] [PubMed] [Google Scholar]
- 71.Roca M, Vives M, Lopez-Navarro E, Garcia-Campayo J, Gili M. Cognitive impairments and depression: a critical review. Actas Esp Psiquiatr 2015; 43(5): 187–193. [PubMed] [Google Scholar]
- 72.Smith EE, Jonides J. Storage and executive processes in the frontal lobes. Science 1999; 283(5408): 1657–1661. [DOI] [PubMed] [Google Scholar]
- 73.Fuster JM. The prefrontal cortex 3rd edn Lippincott-Raven: Philadelphia, PA, 1997. [Google Scholar]
- 74.Murrough JW, Iacoviello B, Neumeister A, Charney DS, Iosifescu DV. Cognitive dysfunction in depression: neurocircuitry and new therapeutic strategies. Neurobiol Learn Mem 2011; 96(4): 553–563. [DOI] [PubMed] [Google Scholar]
- 75.Motter JN, Pimontel MA, Rindskopf D, Devanand DP, Doraiswamy PM, Sneed JR. Computerized cognitive training and functional recovery in major depressive disorder: A meta-analysis. J Affect Disord 2016; 189: 184–191. [DOI] [PubMed] [Google Scholar]
- 76.Siegle GJ, Price RB, Jones N, Ghinassi F, Painter T, Thase ME. You gotta work at it: Pupillary indices of task focus are prognostic for response to a neurocognitive intervention for depression. Clin Psychol Sci 2014; 2(4): 455–471. [Google Scholar]
- 77.Zhou FC, Wang YY, Zheng W, Zhang Q, Ungvari GS, Ng CH et al. Prospective memory deficits in patients with depression: A meta-analysis. 2017; 220: 79–85. [DOI] [PubMed] [Google Scholar]
- 78.Van Vreeswijk MF, De Wilde EJ. Autobiographical memory specificity, psychopathology, depressed mood and the use of the Autobiographical Memory Test: a meta-analysis. 2004; 42(6): 731–743. [DOI] [PubMed] [Google Scholar]
- 79.Pollock LR, Williams JM. Effective problem solving in suicide attempters depends on specific autobiographical recall. Suicide Life Threat Behav 2001; 31(4): 386–396. [DOI] [PubMed] [Google Scholar]
- 80.Williams JM, Ellis NC, Tyers C, Healy H, Rose G, MacLeod AK. The specificity of autobiographical memory and imageability of the future. Mem Cognit 1996; 24(1): 116–125. [DOI] [PubMed] [Google Scholar]
- 81.McEwen BS, Sapolsky RM. Stress and cognitive function. 1995; 5: 205–216. [DOI] [PubMed] [Google Scholar]
- 82.Mathews A, MacLeod C. Cognitive vulnerability to emotional disorders. Annu Rev Clin Psychol 2005; 1: 167–195. [DOI] [PubMed] [Google Scholar]
- 83.Peckham AD, McHugh RK, Otto MW. A meta-analysis of the magnitude of biased attention in depression. Depress Anxiety 2010; 27(12): 1135–1142. [DOI] [PubMed] [Google Scholar]
- 84.Gilboa-Schechtman E, Erhard-Weiss D, Jeczemien P. Interpersonal deficits meet cognitive biases: memory for facial expressions in depressed and anxious men and women. Psychiatry Res 2002; 113: 279–293. [DOI] [PubMed] [Google Scholar]
- 85.Ridout N, Astell AJ, Reid IC, Glen T, O’Carroll RE. Memory bias for emotional facial expressions in major depression. Cogn Emot 2003; 17: 101–122. [DOI] [PubMed] [Google Scholar]
- 86.Hirsch CR, Meeten F, Krahe C, Reeder C. Resolving ambiguity in emotional disorders: The nature and role of interpretation biases. Annu Rev Clin Psychol 2016; 12: 281–305. [DOI] [PubMed] [Google Scholar]
- 87.Milders M, Bell S, Platt J, Serrano R, Runcie O. Stable expression recognition abnormalities in unipolar depression. Psychiatry Res 2010; 179. [DOI] [PubMed] [Google Scholar]
- 88.Persad SM JP Differences between depressed and nondepressed individuals in the recognition of and response to facial emotional cues. J Abnorm Psychol 1993; 102: 358–368. [DOI] [PubMed] [Google Scholar]
- 89.van Randenborgh A, Pawelzik M, Quirin M, Kuhl J. Bad Roots to Grow: Deficient Implicit Self-Evaluations in Chronic Depression With an Early Onset. J Clin Psychol 2016; 72(6): 580–590. [DOI] [PubMed] [Google Scholar]
- 90.Franck E, De Raedt R, De Houwer J. Implicit but not explicit self-esteem predicts future depressive symptomatology. Behav Res Ther 2007; 45(10): 2448–2455. [DOI] [PubMed] [Google Scholar]
- 91.Phillips WJ, Hine DW, Thorsteinsson EB. Implicit cognition and depression: a meta-analysis. Clin Psychol Rev 2010; 30(6): 691–709. [DOI] [PubMed] [Google Scholar]
- 92.Nock MK, Park JM, Finn CT, Deliberto TL, Dour HJ, Banaji MR. Measuring the suicidal mind: implicit cognition predicts suicidal behavior. Psychol Sci 2010; 21: 511–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cha CB, Najmi S, Park JM, Finn CT, Nock MK. Attentional bias toward suicide-related stimuli predicts suicidal behavior. J Abnorm Psychol 2010; 119(3): 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barch DM, Pagliaccio D, Luking K. Mechanisms Underlying Motivational Deficits in Psychopathology: Similarities and Differences in Depression and Schizophrenia. Curr Top Behav Neurosci 2016; 27: 411–449. [DOI] [PubMed] [Google Scholar]
- 95.Chen C, Takahashi T, Nakagawa S, Inoue T, Kusumi I. Reinforcement learning in depression: A review of computational research. Neurosci Biobehav Rev 2015; 55: 247–267. [DOI] [PubMed] [Google Scholar]
- 96.Dombrovski AY, Szanto K, Clark L, Aizenstein HJ, Chase HW, Reynolds CF 3rd, et al. Corticostriatothalamic reward prediction error signals and executive control in late-life depression. Psychol Med 2015; 45(7): 1413–1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dombrovski AY, Szanto K, Clark L, Reynolds CF, Siegle GJ. Reward signals, attempted suicide, and impulsivity in late-life depression. JAMA Psychiatry 2013; 15213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gradin VB, Kumar P, Waiter G, Ahearn T, Stickle C, Milders M et al. Expected value and prediction error abnormalities in depression and schizophrenia. Brain 2011; 134(Pt 6): 1751–1764. [DOI] [PubMed] [Google Scholar]
- 99.Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD. Abnormal temporal difference reward-learning signals in major depression. Brain 2008; 131(Pt 8): 2084–2093. [DOI] [PubMed] [Google Scholar]
- 100.Dombrovski AY, Hallquist MN. The decision neuroscience perspective on suicidal behavior: evidence and hypotheses. Curr Opin Psychiatry 2017; 30(1): 7–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Berridge KC. Motivation concepts in behavioral neuroscience. Physiol Behav 2004; 81(2): 179–209. [DOI] [PubMed] [Google Scholar]
- 102.McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. 2004; 27: 1–28. [DOI] [PubMed] [Google Scholar]
- 103.Cahill L, Alkire MT. Epinephrine enhancement of human memory consolidation: interaction with arousal at encoding. 2003; 79: 194–198. [DOI] [PubMed] [Google Scholar]
- 104.Beevers CG, Clasen PC, Enock PM, Schnyer DM. Attention bias modification for major depressive disorder: Effects on attention bias, resting state connectivity, and symptom change. J Abnorm Psychol 2015; 124(3): 463–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.White LK, Sequeira S, Britton JC, Brotman MA, Gold AL, Berman E et al. Complementary Features of Attention Bias Modification Therapy and Cognitive-Behavioral Therapy in Pediatric Anxiety Disorders. Am J Psychiatry 2017; 174(8): 775–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mathews A, MacLeod C. Cognitive approaches to emotion and emotional disorders. Annu Rev Psychol 1994; 45: 25–50. [DOI] [PubMed] [Google Scholar]
- 107.MacLeod C Cognitive bias modification procedures in the management of mental disorders. Curr Opin Psychiatry 2012; 25(2): 114–120. [DOI] [PubMed] [Google Scholar]
- 108.Jones EB, Sharpe L. Cognitive bias modification: A review of meta-analyses. J Affect Disord 2017; 223: 175–183. [DOI] [PubMed] [Google Scholar]
- 109.Harmer CJ, Goodwin GM, Cowen PJ. Why do antidepressants take so long to work? A cognitive neuropsychological model of antidepressant drug action. 2009; 195: 102–108. [DOI] [PubMed] [Google Scholar]
- 110.Association AP. Diagnostic and statistical manual of mental disorders. American Psychiatric Publishing: Arlington, VA, 2013. [Google Scholar]
- 111.Nolen-Hoeksema S, Morrow J, Fredrickson BL. Response styles and the duration of episodes of depressed mood. J Abnorm Psychol 1993; 102(1): 20–28. [DOI] [PubMed] [Google Scholar]
- 112.McEvoy PM, Mahoney AEJ, Moulds ML. Are worry, rumination, and post-event processing one and the same? Development of the repetitive thinking questionnaire. J Anxiety Disord 2010; 24: 509–519. [DOI] [PubMed] [Google Scholar]
- 113.Beck JG. Cognitive Therapy: Basics and Beyond. The Guilford Press: New York, NY, 1995. [Google Scholar]
- 114.Beck AT, Dozois DJ. Cognitive therapy: current status and future directions. Annu Rev Med 2011; 62: 397–409. [DOI] [PubMed] [Google Scholar]
- 115.Teasdale JD, Segal ZV, Williams JMG, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol 2000; 68(4): 615–623. [DOI] [PubMed] [Google Scholar]
- 116.Segal ZV, Walsh KM. Mindfulness-based cognitive therapy for residual depressive symptoms and relapse prophylaxis. Curr Opin Psychiatry 2016; 29(1): 7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Segal ZV, Teasdale JD, Williams JMG. Mindfulness-Based Cognitive Therapy: Theoretical Rationale and Empirical Status. 2004. [Google Scholar]
- 118.Davidson J, Turnbull CD. Diagnostic significance of vegetative symptoms in depression. Br J Psychiatry 1986; 148: 442–446. [DOI] [PubMed] [Google Scholar]
- 119.Dimidjian S, Barrera M Jr., Martell C, Munoz RF, Lewinsohn PM. The origins and current status of behavioral activation treatments for depression. Annu Rev Clin Psychol 2011; 7: 1–38. [DOI] [PubMed] [Google Scholar]
- 120.Ratcliffe M Experiences of Depression: A Study in Phenomenology. Oxford University Press: Oxford, UK, 2015. [Google Scholar]
- 121.Martell CR, Dimidjian S, Herman-Dunn R. Behavioral Activation for Depression: A Clinician’s Guide. The Guilford Press: New York, 2010. [Google Scholar]
- 122.Dimidjian S, Hollon SD, Dobson KS, Schmaling KB, Kohlenberg RJ, Addis ME et al. Randomized trial of behavioral activation, cognitive therapy, and antidepressant medication in the acute treatment of adults with major depression. J Consult Clin Psychol 2006; 74: 658–670. [DOI] [PubMed] [Google Scholar]
- 123.Murrough JW, Collins KA, Fields J, DeWilde KE, Phillips ML, Mathew SJ et al. Regulation of neural responses to emotion perception by ketamine in individuals with treatment-resistant major depressive disorder. Transl Psychiatry 2015; 5: e509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Gartner M, Aust S, Bajbouj M, Fan Y, Wingenfeld K, Otte C et al. Functional connectivity between prefrontal cortex and subgenual cingulate predicts antidepressant effects of ketamine. Eur Neuropsychopharmacol 2019; 29(4): 501–508. [DOI] [PubMed] [Google Scholar]
- 125.Li CT, Chen MH, Lin WC, Hong CJ, Yang BH, Liu RS et al. The effects of low-dose ketamine on the prefrontal cortex and amygdala in treatment-resistant depression: A randomized controlled study. Hum Brain Mapp 2016; 37(3): 1080–1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Ionescu DF, Felicione JM, Gosai A, Cusin C, Shin P, Shapero BG et al. Ketamine-Associated Brain Changes: A Review of the Neuroimaging Literature. Harv Rev Psychiatry 2018; 26(6): 320–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Downey D, Dutta A, McKie S, Dawson GR, Dourish CT, Craig K et al. Comparing the actions of lanicemine and ketamine in depression: key role of the anterior cingulate. Eur Neuropsychopharmacol 2016; 26(6): 994–1003. [DOI] [PubMed] [Google Scholar]
- 128.Reed JL, Nugent AC, Furey ML, Szczepanik JE, Evans JW, Zarate CA Jr., Ketamine normalizes brain activity during emotionally valenced attentional processing in depression. 2018; 20: 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Evans JW, Szczepanik J, Brutsche N, Park LT, Nugent AC, Zarate CA Jr. Default Mode Connectivity in Major Depressive Disorder Measured Up to 10 Days After Ketamine Administration. Biol Psychiatry 2018; 84(8): 582–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jett JD, Boley AM, Girotti M, Shah A, Lodge DJ, Morilak DA. Antidepressant-like cognitive and behavioral effects of acute ketamine administration associated with plasticity in the ventral hippocampus to medial prefrontal cortex pathway. Psychopharmacol 2015; 232(17): 3123–3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Nikiforuk A, Popik P. Ketamine prevents stress-induced cognitive inflexibility in rats. Psychoneuroendocrinology 2014; 40: 119–122. [DOI] [PubMed] [Google Scholar]
- 132.Shiroma PR, Albott CS, Johns B, Thuras P, Wels J, Lim KO. Neurocognitive performance and serial intravenous subanesthetic ketamine in treatment-resistant depression. Int J Neuropsychopharmacol 2014; 17(11): 1805–1813. [DOI] [PubMed] [Google Scholar]
- 133.Permoda-Osip A, Kisielewski J, Bartkowska-Sniatkowska A, Rybakowski JK. Single ketamine infusion and neurocognitive performance in bipolar depression. Pharmacopsychiatry 2015; 48(2): 78–79. [DOI] [PubMed] [Google Scholar]
- 134.Price RB, Iosifescu DV, Murrough JW, Chang LC, Al Jurdi RK, Iqbal SZ et al. Effects of ketamine on explicit and implicit suicidal cognition: a randomized controlled trial in treatment-resistant depression. Depress Anxiety 2014; 31(4): 335–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Price RB, Nock MK, Charney DS, Mathew SJ. Effects of intravenous ketamine on explicit and implicit measures of suicidality in treatment-resistant depression. Biol Psychiatry 2009; 66(5): 522–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Price RB, Kuckertz JM, Siegle GJ, Ladouceur CD, Silk JS, Ryan ND et al. Empirical recommendations for improving the stability of the dot-probe task in clinical research. Psychol Assess 2015; 27(2): 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Price RB, Brown V, Siegle GJ. Computational Modeling Applied to the Dot-Probe Task Yields Improved Reliability and Mechanistic Insights. Biol Psychiatry 2019; 85(7): 606–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Rodebaugh TL, Scullin RB, Langer JK, Dixon DJ, Huppert JD, Bernstein A et al. Unreliability as a threat to understanding psychopathology: The cautionary tale of attentional bias. J Abnorm Psychol 2016; 125(6): 840–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Auxéméry Y Post-traumatic psychiatric disorders: PTSD is not the only diagnosis. Presse Med 2018; 47: 423–430. [DOI] [PubMed] [Google Scholar]
- 140.Williams LM. Precision psychiatry: a neural circuit taxonomy for depression and anxiety. Lancet Psychiat 2016; 3(5): 472–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Feder A, Parides MK, Murrough JW, Perez AM, Morgan JE, Saxena S et al. Efficacy of intravenous ketamine for treatment of chronic posttraumatic stress disorder: a randomized clinical trial. JAMA Psychiatry 2014; 71(6): 681–688. [DOI] [PubMed] [Google Scholar]
- 142.Glue P, Medlicott NJ, Harland S, Neehoff S, Anderson-Fahey B, Le Nedelec M et al. Ketamine’s dose-related effects on anxiety symptoms in patients with treatment refractory anxiety disorders. J Psychopharmacol 2017; 31(10): 1302–1305. [DOI] [PubMed] [Google Scholar]
- 143.Price RB, Gates K, Kraynak TE, Thase ME, Siegle GJ. Data-driven subgroups in depression derived from directed functional connectivity paths at rest. Neuropsychopharmacology 2017; 42(13): 2623–2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Price RB, Lane S, Gates K, Kraynak TE, Horner MS, Thase ME et al. Parsing heterogeneity in the brain connectivity of depressed and healthy adults during positive mood. Biol Psychiatry 2017; 81(4): 347–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Langenecker SA, Mickey BJ, Eichhammer P, Sen S, Elverman KH, Kennedy SE et al. Cognitive Control as a 5-HT1A-Based Domain That Is Disrupted in Major Depressive Disorder. Front Psychol 2019; 10: 691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Price RB, Rosen D, Siegle GJ, Ladouceur CD, Tang K, Allen KB et al. From anxious youth to depressed adolescents: Prospective prediction of 2-year depression symptoms via attentional bias measures. J Abnorm Psychol 2015; 125(2): 267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Fried EI, Nesse RM. Depression is not a consistent syndrome: An investigation of unique symptom patterns in the STAR*D study. J Affect Disord 2014; 172C: 96–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003; 301(5634): 805–809. [DOI] [PubMed] [Google Scholar]
- 149.Hoogendam JM, Ramakers GM, Di Lazzaro V. Physiology of repetitive transcranial magnetic stimulation of the human brain. 2010; 3(2): 95–118. [DOI] [PubMed] [Google Scholar]
- 150.Dukart J, Regen F, Kherif F, Colla M, Bajbouj M, Heuser I et al. Electroconvulsive therapy-induced brain plasticity determines therapeutic outcome in mood disorders. Proceedings of the National Academy of Sciences of the United States of America 2014; 111(3): 1156–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Cassilhas RC, Tufik S, de Mello MT. Physical exercise, neuroplasticity, spatial learning and memory. Cell Mol Life Sci 2016; 73(5): 975–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wilkinson S, Holtzheimer PE, Gao S, Kirwin D, Price R. Leveraging neuroplasticity to enhance adaptive learning: The potential for synergistic somatic-behavioral treatment combinations to improve clinical outcomes in depression. Biol Psychiatry 2019; 85: 454–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cullen KR, Westlund MK, Klimes-Dougan B, Mueller BA, Houri A, Eberly LE et al. Abnormal amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry 2014; 71(10): 1138–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Young KD, Zotev V, Phillips R, Misaki M, Drevets WC, Bodurka J. Amygdala real-time functional magnetic resonance imaging neurofeedback for major depressive disorder: A review. Psychiatry Clin Neurosci 2018; 72(7): 466–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Young KD, Siegle GJ, Misaki M, Zotev V, Phillips R, Drevets WC et al. Altered task-based and resting-state amygdala functional connectivity following real-time fMRI amygdala neurofeedback training in major depressive disorder. Neuroimage Clin 2018; 17: 691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Dell’osso B, Camuri G, Castellano F, Vecchi V, Benedetti M, Bortolussi S et al. Meta-Review of Metanalytic Studies with Repetitive Transcranial Magnetic Stimulation (rTMS) for the Treatment of Major Depression. Clin Pract Epidemiol Ment Health 2011; 7: 167–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. J Abnorm Psychol 2002; 111(2): 225–236. [DOI] [PubMed] [Google Scholar]
- 158.Hsu KJ, Beard C, Rifkin L, Dillon DG, Pizzagalli DA, Bjorgvinsson T. Transdiagnostic mechanisms in depression and anxiety: The role of rumination and attentional control. J Affect Disord 2015; 188: 22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sumner JA. The mechanisms underlying overgeneral autobiographical memory: an evaluative review of evidence for the CaR-FA-X model. Clin Psychol Rev 2012; 32(1): 34–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Hitchcock C, Werner-Seidler A, Blackwell SE, Dalgleish T. Autobiographical episodic memory-based training for the treatment of mood, anxiety and stress-related disorders: A systematic review and meta-analysis. Clin Psychol Rev 2017; 52: 92–107. [DOI] [PubMed] [Google Scholar]
- 161.Clarke PJ, Notebaert L, Macleod C. Absence of evidence or evidence of absence: reflecting on therapeutic implementations of attentional bias modification. BMC psychiatry 2014; 14(1): 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Franklin JC, Fox KR, Franklin CR, Kleiman EM, Ribeiro JD, Jaroszewski AC et al. A brief mobile app reduces nonsuicidal and suicidal self-injury: Evidence from three randomized controlled trials. J Consult Clin Psychol 2016; 84(6): 544–557. [DOI] [PubMed] [Google Scholar]