Abstract
Nanoradiosensitizers have been developed to enhance localization and precision of therapeutic radiation delivery. A specific volume of comprising surface atoms is known to be the radiosensitizing region. However, the shape-dependent local dose enhancement of nanoparticles is often underestimated and rarely reported. Here, a noble metal nanostructure, inspired by the photoperiodic day-flowers, was synthesized by metal reduction with bile acid molecules. The impact of high surface area of day-flower mimicking metallic nanoparticles (D-NP) on radiosensitizing effect was demonstrated with assays for ROS generation, cellular apoptosis, and clonogenic survival of human liver cancer cells (HepG2) cells. In comparison with lower-surface-area spherical night-flower mimicking metallic nanoparticles (N-NP), exposure of our D-NP to external beam radiation doses led to a significant increase in reactive oxygen species (ROS) production and radiosensitizing cell cycle synchronization, resulting in an enhanced cancer-cell-killing effect. In clonogenic survival studies, dose-enhancing factor (DEF) of D-NP was 16.5-fold higher than N-NP. Finally, we demonstrated in vivo feasibility of our D-NP as a potent nanoradiosensitizer and CT contrast agent for advanced image-guided radiation therapy.
Keywords: metallic nanoparticles, radiosensitizers, image-guided radiotherapy, radiation, cancer therapy
Graphical Abstract

Ionizing radiation therapy is one of the primary approaches to cancer treatment. It is considered to be a highly effective noninvasive therapy for the majority of cancer patients. Over half of cancer patients are given radiation as part and their disease management to reduce tumor burden.1 However, one of the greatest challenges in radiotherapy is to provide an effective radiation dose specially to tumor tissues and minimize exposure to surrounding normal tissues. For example, radiation therapies on gastrointestinal (GI) cancers including liver, colorectal, stomach, biliary system, pancreatic, and intestinal have shown limited success because of their close proximity to normal and healthy tissues. Furthermore, structures such as liver, biliary tracts, vasculatures, colon, stomach, and diaphragm, are prone to radiation injury. Iatrogenic radiation injury to normal tissues is a common sequence of radiation therapy in a majority of cancer patients. Subsequently, cancer survivors will suffer from a variety of acute and chronic adverse symptoms following radiotherapy. These symptoms significantly reduce quality of life as well as increase the cost burden of health care, especially for patients who require high radiation dosages. Therefore, the precise and accurate radiation delivery represents a remarkable technical challenge for clinicians. Recently, image-guided radiation therapy (IGRT) methods have been developed to localize tumors with increased sensitivity and specificity with the development of image-based treatment systems and high-resolution radiation delivery techniques.2 The IGRT methods utilizing state-of-the-art CT or MRI have been regarded as promising approaches for enhanced precision, accurate localization, and treatment; especially for tumor lesions embedded within soft tissues.3 However, the overall long-term morbidity and mortality in these patients treated with IGRT still remains dismal due to inadvertent irradiation of healthy surrounding tissue. Further improvements in IGRT approaches are necessary to reduce excessive radiation doses and achieve superior therapeutic efficacy and improve long-term treatment outcomes.
One of the promising new approach to enhance targeted local radiation and reduce the overall total radiation dose involves the use of high atomic number (Z) nanomaterials. These high-Z-element-based agents can be delivered to tumor, where they augment local radiation dose responses by strongly absorbing, scattering, and emitting radiation energy.4 High atomic number nanomaterials such as iodine (Z = 53), gadolinium (Z = 64), gold (Z = 79), and bismuth (Z = 83) have been utilized to enhance the photoelectric and Compton effect (subsequent emission of secondary electrons) to generate reactive oxygen species (ROS) and significantly increase radiation-induced DNA damage.5–8 High Z nanoparticles have been proven to be very effective radiosensitizers, because of their ability to generate more ROS per given dose of radiation than simple radiotherapy alone.9–11 Among all the materials used to date, various-sized spherical or thorny Au nanoparticles have been well-documented for enhancing radiosensitization.12,13 However, the shape-dependent local dose augmentation by nanoparticles is often underestimated and rarely reported. Most previous reports focus on tumor targeting and cellular uptake for enhancing radiosensitizing effects of nanoparticles rather than the aspects morphological dependency on radiosensitizing effects.14–16 Importance of structural contributions in radiosensitizing nanoparticles should be investigated thoroughly to develop more highly efficient radiosensitizers.
In nature, photoperiodic flowers such as mimosa or Ipomoea nil or Acacia Gerrardii control blooming time to enhance reproductive success.17,18 They open the petals for more sunlight exposure during the day and attracting symbiotic species for pollinators.19 Similar to the way these flowers are formed for effective light capturing, this natural morphology provides a clever scheme to engineer advanced radiosensitizing nanoparticles. Inspired by the structure and ecology of photoperiodic flowers, the fully bloomed day-time flower mimicking metallic nanoparticles (D-NP) were synthesized for high efficiency radiosensitization in IGRT applications. The number of nanopetals in D-NP provides vast amounts of specific surface atoms for absorbing radiation and producing higher levels of ROS. To elucidate the radiosensitizing effect of D-NP, we prepared a nanoparticle that resembles a spherical night-time flower mimicking metallic nanoparticles (N-NP) as a counterpart to D-NP as illustrated in Figure 1a. The noble structures of both D-NP and N-NP were readily synthesized with a one-pot reaction using amphiphilic cholic acid (CA, 1.8 mM), HAuCl4·3H2O (0.22 mM), AgNO3 (0.13 mM), and l-ascorbic acid (AA; 2.65 (D-NP) or 0.33 mM (N-NP)) following a modified protocol of previously reported.20 A higher concentration of l-ascorbic acid (AA; 2.65 mM) formed approximately ~150 nm D-NP by fast reduction of metal ions in a cholic acid (CA)-metal complex solution, but lower concentration of AA (0.33 mM) formed ~130 nm N-NP, as shown in Figure 1b, c. Small-angle X-ray scattering (SAXS) analysis revealed the details of formed D-NP and N-NP nanostructures. Guinier-Porod fitting of the SAXS curves21 indicated that the final morphology of the nanoparticles is a multilevel hierarchical nanostructure (Figure 1d and Figure S1). Higher q regions in the SAXS curves of both D-NP and N-NP show the primary particles as building blocks of hierarchical nanostructure. SAXS intensities of D-NP and N-NP at low q regions show a significant difference in their respective power-law slopes, indicating that the D-NP were formed from fused primary particles into an elongated petal-like nanostructure, whereas clusterlike secondary structures were observed in N-NP (Figure S1).22 High-resolution (HR) STEM images confirmed that D-NP is comprised of a fused-assembly of multiple nanocrystals, sized approximately 3–6 nm in diameter (Figure S2). XPS analysis confirmed the presence of Au/Ag with 1:1 molar ratios in both D-NP and N-NP (Figure 1e). The XPS binding energies of Au 4f7/2 and Au 4f5/2 appeared at 84.1 and 87.8 eV and those of Ag 3d5/2 and Ag 3d3/2 appeared at 368.3 and 375.3 eV, respectively.
Figure 1.
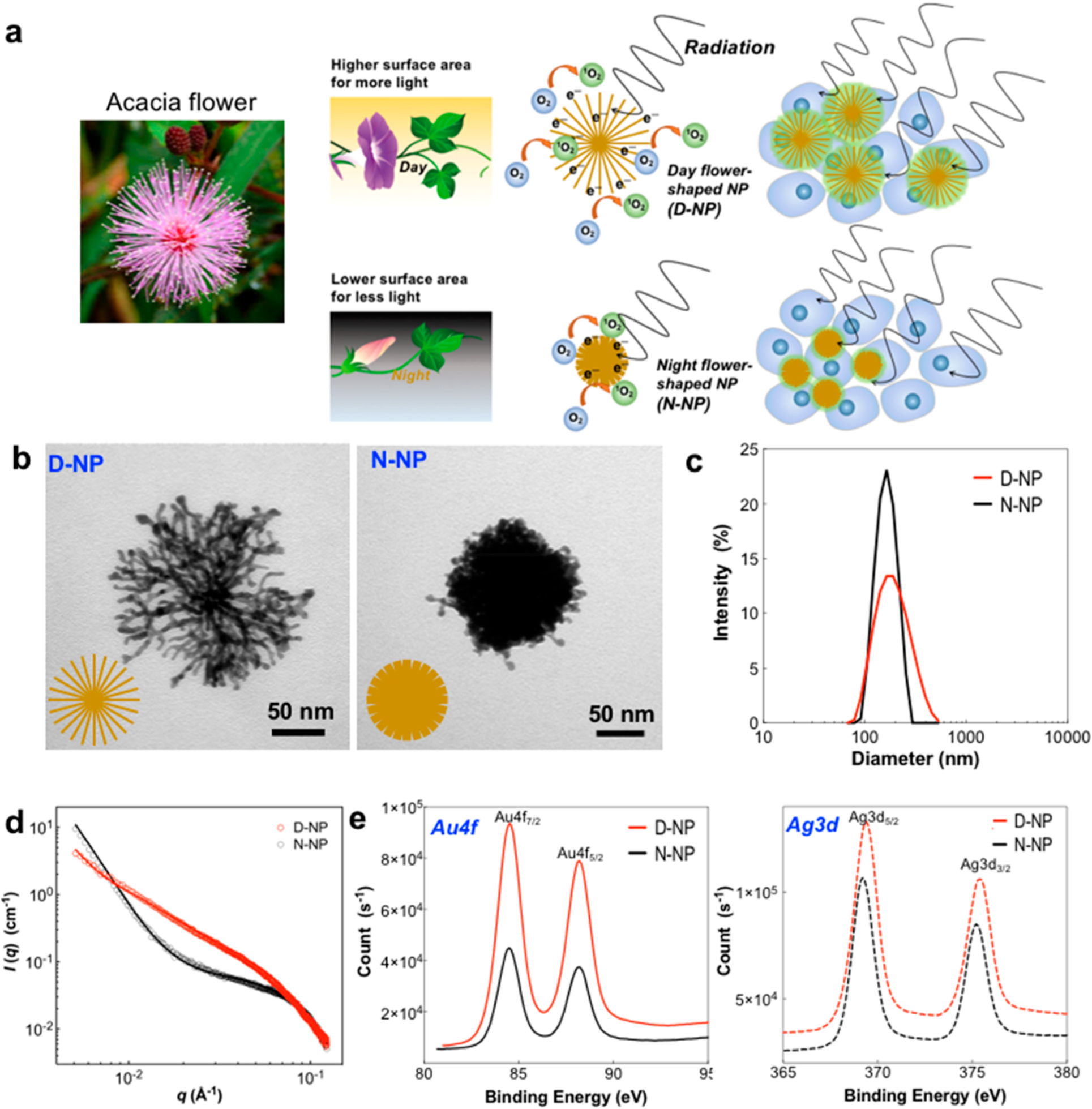
(a) Schematics of photoperiodic flower-mimicking metallic nanoparticles including day-flower-mimicking metallic nanoparticles (D-NP) and night-flower-mimicking metallic nanoparticles (N-NP). The number of nanopetals in D-NP provides higher specific surface atoms for radiation absorption, which in turn produces higher levels of radiosensitizing ROS. (b) TEM images and (c) hydrodynamic size of D-NP and N-NP synthesized by a one-pot method using cholic acid, HAuCl4, 3H2O,AgNO3, and l-ascorbic acid. (d) SAXS data obtained in samples of D-NP and N-NP and their Guinier–Porod model fitting. (e) X-ray photoelectron spectroscopy (XPS) data of D-NP and N-NP.
The measured Brunauer–Emmett–Teller (BET) surface area of the high nanopetaled D-NP was 113.0 m2/g, significantly higher than that of N-NP (15.6 m2/g) (Table 1). To the best of our knowledge, D-NP is the highest-surface-area Au-based nanoparticle in existence prepared by a simple one-pot synthesis. D-NP is structurally formed with a multitude of nanopetals fused by primary metal nanoparticles (around 2–3 nm) during crystal growth. Enhanced roughness in D-NP’s extended portions of nanopetals contribute to its high BET surface area. Radiosensitizing dose enhancing effects and ROS generation are attributed to the expanded volumes of high Z surface atoms.15 Because of the extensive surface area of D-NP, it provides 30 times more radiosensitizing surface atoms compared to spherical N-NP (Table 1 and the Supporting Information).
Table 1.
Measured Surface Area and Calculated Radiosensitizing Surface Atoms of D-NP and N-NP
| sample | measured BET surface area (m2/g) | no. of atoms per particlea | no, of radiosensitizing surface atomsa | percentage of radiosensitizing surface atoms (%)a |
|---|---|---|---|---|
| N-NP | 15.6 | 9.25 × 106 | 1.0 × 105 | 1.2 |
| D-NP | 113.0 | 9.25 × 106 | 2.7 × 105 | 29.7 |
Estimated values with generalized radial and spherical NP model (see the Supporting Information).
The widely exposed surface atoms in D-NP directly contribute to the generation of high concentrations of active ROS upon irradiation. Radiation induced ROS generation by D-NP and N-NP sample solutions were quantified with 1,3-diphenylisobenzofuran (DPBF) photobleaching dye at an absorbance of 412 nm (Figure 2a). D-NP demonstrated remarkable ROS generation, of about 15–20-fold higher than the ROS generated from N-NP within a radiation dose range of 3–6 Gy (Figure 2a).23–25 Strong radiosensitizing ROS generation of D-NP saturated DPBF dye from 3 Gy radiation while other samples still increased with radiation doses. Subsequently, the radiosensitizing ROS generation of D-NP resulted in an enhanced cell-killing effect of radiation therapy. Prior to evaluating radiosensitizing cell-killing effects of D-NP, the concentration-dependent cytotoxicity of nanoparticles was tested in MTT assay. When we treated HepG2 cells with various amounts of nanoparticles, the lethal doses, 50% of both D-NP and N-NP were measured to be LD50 ≈ 18 μg/mL. (Figure S3). In our flow cytometry studies for measuring apoptosis of cells, an apoptosis rate less than 20% after HepG2 cell incubation with D-NP and N-NP (5.2 mg/mL) for 48 h was maintained without radiation treatment. When HepG2 human hepatocellular carcinoma cells treated with D-NP were irradiated with a single fraction of radiation (6 Gy), a significantly increased level of cancer cell apoptosis was appreciated (Figure 2b). A group treated with D-NP and 6 Gy radiation showed an apoptosis rate of 78%, which was 7 times higher value than the radiation only group (6 Gy) (Figure 2b). Though the enhanced cell killing effects are mainly attributed to elevated ROS generation; cellular uptake and cell cycle synchronization effects which are critical for the radiosensitizing effect of nanoparticles were also observed. Here, we confirmed that the difference in nanoparticle structure have a negligible effect with a cellular uptake test of D-NP and N-NP. Though intracellular pathways were not studied here, similar amounts (~2.8 pg/cell) of Au and Ag from nanoparticles were measured in both groups treated with D-NP and N-NP after 24 h coincubation period (Figure 2c). The high surface area of D-NP induced a shorter G0/G1 phase and extended the radiosensitive G2/M phase in cancer cells compared with nontreated cells or N-NP treated cells before the radiation treatment (Figure 2d). This D-NP induced cell cycle synchronization effect contributes to its cell killing efficiency upon irradiation therapies. For the quantification of D-NP’s radiosensitizing effect, the dose enhancement factor (DEF) was measured using the clonogenic assay (also called CFU assay for colony forming unit), the gold standard evaluating radiosensitizers. HepG2 human hepatoma cells given specific radiation doses via a 160 kV X-ray beam, demonstrated a superior DEF of 29.7 in D-NP compared to a 1.8 DEF for N-NP at the same nanoparticle concentration (Figure 2e). We believe that the enhanced cell-killing efficiency in D-NP resulted from superior ROS generation secondary to its high surface area and a cell cycle synchronization effect.
Figure 2.
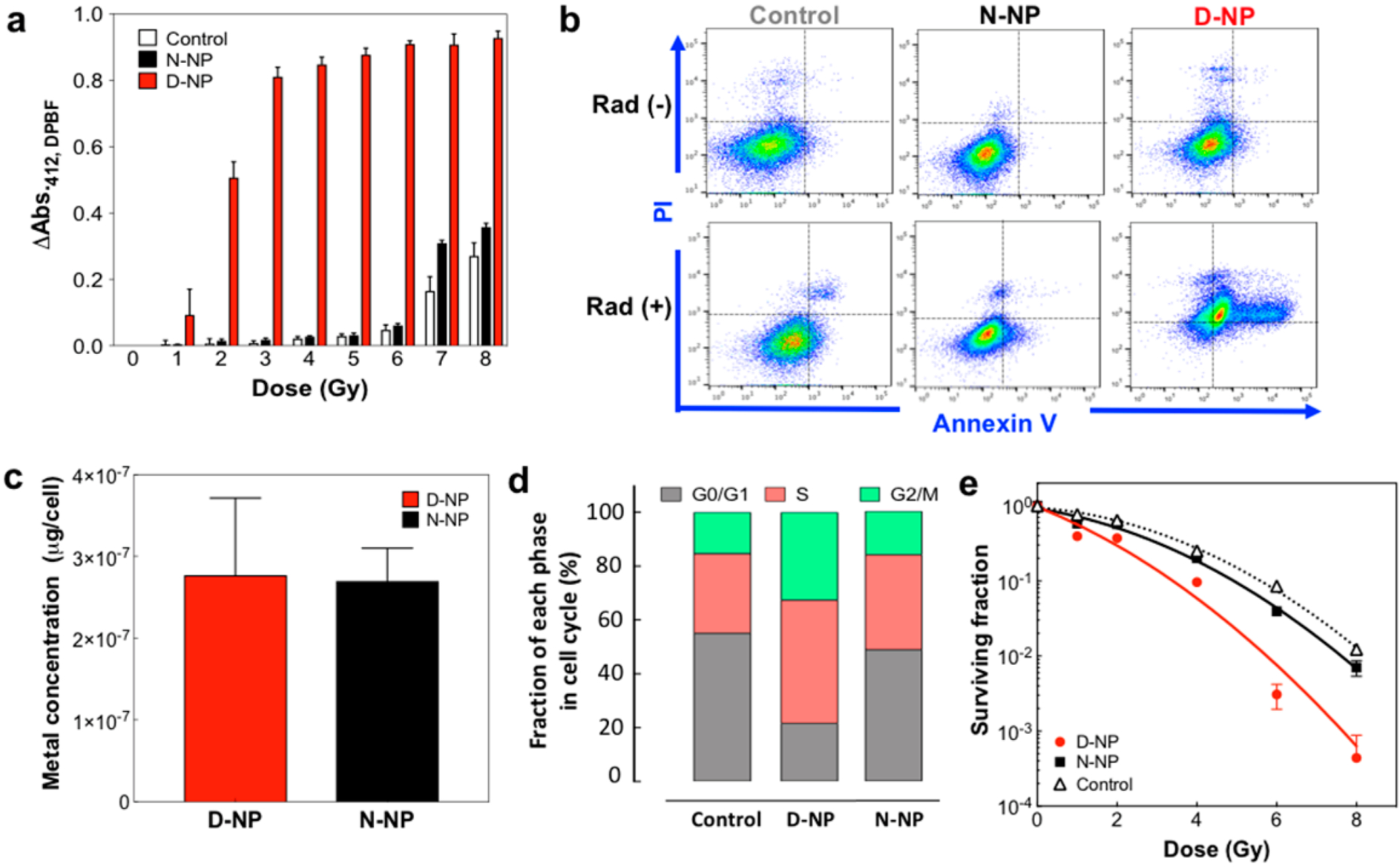
(a) Comparative reactive oxygen species (ROS) generation in samples of D-NP, N-NP, and control solution only with specific radiation doses from 1–8 Gy (each group n = 6). (b) Flow cytometry results showing apoptotic cell death. For control, N-NP and D-NP samples each had 6 Gy radiation or nonradiation. FACS analysis was performed using FITC Annexin-V and propidium iodide (PI) staining. (c) Amounts D-NP and N-NP in cells. Au and Ag elements in cells were measured by ICP-OES (each group n = 6). (d) Cell cycle changes after treatment with D-NP and N-NP. (e) Surviving fraction curves fitted within a linear–quadratic model. Radiation-dose-dependent surviving fraction of cells treated with D-NP and N-NP. HepG2 cells were treated with samples and different radiation doses of 160 kV X-ray source and were cultured for 10 days and stained with crystal violet. Stained cells were counted for the survival fraction (each group n = 6, P < 0.0001 (D-NP vs N-NP), 2-way ANOVA).
In the clinic/preclinics, IGRT procedures commonly involve imaging modalities like computed tomography (CT), magnetic resonance imaging (MRI), ultrasound, and optical imaging, while fiducial markers, electromagnetic transponders, and colored ink tattoos on the skin help align and target the radiation.26,27 The CT scan is one of the main cancer imaging methods worldwide. The quick scan time make it a vital tool for clinicians. CT imaging is used for screening and monitoring radio-therapeutic effect for many malignancies. They are used to measuring the size, shape and position of tumors as well as the surrounding tissues and bones. In a phantom study, D-NP showed excellent contrast effects on CT imaging at 50 kVp.28 The CT attenuation coefficient of D-NP was 41.1 HU/mg/mL, significantly higher than that of N-NP (27.4 HU/mg/mL) (Figure 3a). Although X-ray attenuation is not influenced by the size of nanoparticles,29 the significant higher surface area of D-NP and resulting structurally increased mass concentration in a unit area of agar phantom gels might enhanced the CT attenuation coefficient. The CT visibility shows promise as a high-resolution contrast agent to facilitate IGRT to minimize excessive radiation administration and avoid inadvertent tissue damage. Our image guided nanomedicine lab has been developing image-guided therapeutic delivery techniques for high accuracy tumor localization.20,28,30–32 We were able to demonstrate a potential for in vivo CT-IGRT applications of D-NP in a human hepatoma (HepG2) xenograft mouse model. D-NP’s radiopacity allowed for CT guided infusion and pinpoint irradiation via X-ray. Instead of systemic IV injection of D-NP, image guided intratumoral infusion of nanoparticles were performed for accurate localization and targeting of nanoparticles in the tumor.33,34 As shown in Figure 3b, c, MRI and CT fusion images helped identify and coordinate the tumor region (region-of-interest (ROI)) for targeted catheter infusion of D-NP and local radiation therapy (Figure 3b). Intraoperative CT imaging in short time scans (ca. 5 min/scan) and contrast enhanced (CE) CT signal from the infused D-NP delineated the target tumor volumes effectively and guided catheter directed intratumoral infusion (Figure 3c). Intraoperative CE-CT imaging also allows for prompt adjustments of the amount of D-NP or injection site (catheter placement) ifnecessary.33 After infusion, intratumoral uptake and distribution of infused D-NP was depicted by significant contrast enhancement of the delineated tumor region while N-NP showed less increase of CT number (Figure 3d and Figure S4). The locally distributed D-NP in tumor showed an effective radiosensitizing cancer cell killing effect at post 2-days of 10 Gy radiation dose. H&E and TUNEL stained tissue analysis (Figures 3e, f) showed significantly higher apoptosis rates in the D-NP treated cells compared to N-NP or only radiation therapy alone, in concurrence with our in vitro studies. Additional studies confirming dose-dependent long-term therapeutic effects should be evaluated in the future study using orthotopic liver cancer models.
Figure 3.

(a) Concentration-dependent CT-contrast images of D-NP and N-NP and a linear relationship between CT numbers and concentrations of D-NP and N-NP (each group n = 5). (b) MRI and CT image guidance at catheter-directed infusion of nanoradiosensitizers into the tumor. (c) Reconstructed CT body images allowed identification of the tumor region (region-of-interest (ROI)) and coordination for direct injection of our D-NP (each group n = 3, P < 0.0001 (pre- vs postinfusion), unpaired t test). (d) (right) CT images of pre and post infusion of D-NP. (Left) CT number changes of delineated tumor regions at pre- and postinfusion of D-NP. (e) Representative images of H&E and TUNEL stained tumor tissues in each group. (f) Comparative apoptotic area (%) of tumors from each animal groups (radiation+D-NP, radiation+N-NP and radiation only; human HepG2 hepatoma xenograft mouse model, at a radiation dose of 10 Gy) (each group n = 3, P < 0.0001 (radiation+D-NP vs radiation+N-NP), unpaired t test).
In conclusion, we synthesized and characterized a novel D-NP directed by CA biomolecules. The large surface area-to-volume ratio of D-NP exhibited superior radiosensitizing ROS generation and cell cycle synchronization.10,35 Significantly enhanced performance of D-NP as both a nanoradiosensitizer and CT imaging contrast agent will be strongly beneficial as an advanced IGRT technique. We expect high-surface-area D-NP radiosensitizer in conjunction with advanced IGRT represent a unique opportunity to augment tumoricidal effects on refractory tumors while sparing proximal normal tissues. Additionally, the D-NP could be potentiate the development of highly localized clinical radiation therapies such as selective internal 90Y radioembolization,36 brachy therapies,37 and combinational radio-immunotherapies.38
EXPERIMENTAL SECTION
Materials.
Hydrochloroauric acid (HAuCl4 3H2O), silver nitrate (AgNO3), and l-ascorbic acid (AA), were obtained from Sigma (St. Louis, USA). Sodium cholic acid was purchased from Pierce (Thermo Scientific, Rockford, USA). All the chemicals were analytical grade reagents and used without further purification. Milli-Q water was used during the study.
Preparation of D-NP and N-NP.
Ten milliliters of a queous sodium cholic acid solution (2 mM) was prepared with a magnetic stirrer. While stirring the solution for 350 rpm, 1 mL of HAuCl4·3H2O solution (10 mM) and 150 μL of AgNO3 solution (10 mM) were subsequently added. Then, 150 μL of 200 mM AA solution was added for D-NPs or 150 μL of 25 mM AA solution for N-NPs, respectively. The reaction mixture was stirred for 20 s and left undisturbed for 2 h. The nanoparticles were purified in ethanol dispersion by 3 times repetition of centrifugation at 12,000 rpm for 15 min and redispersion in fresh water with thiol PEG carboxylic acid. Finally, the PEGylated nanoparticles were dialyzed in a dialysis membrane (MWCO 3500) against fresh water for 1 day.
Characterization.
Samples were characterized with transmission electron microscopy (TEM, Tecnai Spirit G2, 120 kV, FEI and TEM, JEM-2100 FasTEM, JEOL) and scanning and transmission electron microscopy (STEM, HD-2300 Dual EDS Cryo STEM, Hitachi) for the analysis of morphologies and elemental mapping of Au, Ag, and the other elements. Hydrodynamic size of samples was measured with Zetasizer Nano ZSP (Malvern Inc.) Samples were analyzed with X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi, Thermo Scientific). The solutions of D-NP and N–NP were drop-casted on the slide glasses for the same concentrations (25 mg/mL) and left for 3 h to water got evaporated. Then, the samples were measured with XPS in 10 spots to get the photoelectron peaks of Au (scaning range = 80–90 eV) and Ag (scanning range = 560–580 eV) and averaged for the comparison. Sample surface area was measured with Brunauer-Emmett–Teller analysis (BET, 3Flex Surface Characterization Analyzer, Micromeritics).
Synchrotron X-ray Analysis.
X-ray scattering measurements were performed at APS 12-ID-B beamline. X-ray beam with energy of 14keV was used and scattering data were collected with Pilatus 2 M for SAXS. A solution sample was prepared in a flow cell made of a quartz capillary with diameter of 2 mm. X-ray beam was exposed for 0.2 s for 10 times to collect averaged scattering patterns.
Reactive Oxygen Species (ROS) Detection.
ROS generation was detected by measuring the bleach of 1,3-diphenylisobenzofuran (DPBF) widely used for this purpose. The mixture of 1 mL of DPBF (1 mM) in ethanol and 1 mL of 100 μg/mL of samples in water was prepared in microtubes. The samples in the rack were placed on the center of shelf in an X-ray irradiator (RS-2000, RadSource Technologies, Inc.) with the radiation output at 160 kV and 25 mM equipped 0.3 mm Cu filter. The dose of radiation was controlled by X-ray exposure time, which varied 0–10 Gy. An absorbance of DPBF depending on radiation dose was measured with an UV/vis spectrometer (Cytation3, BioTek). An absorbance of DPBF at 412 nm was calculated by subtracting the absorbance of nanoparticles at 412 nm from the measured sample absorbance. Radiation dose-dependent DPBF absorbance at 412 nm (ΔAbs.412 nm dpbf) was described by subtraction between absorbance at 0 Gy and absorbance at corresponding dose. The dose enhancement factor (DEF) was calculated by dividing the dose of the radiation alone by the dose of radiation after nanoparticle treatment needed for the same ΔAbs.412 nm of the radiation alone.
Characterization of CT Contrast Property.
CT-phantoms containing 0–40 mg/mL samples in water were prepared in the microtubes. CT-number in Hounsfield unit (HU) was measured in each sample with a CT scanner (NanoScan PET/CT, Mediso) and plotted for concentration dependent CT numbers of the samples.
Cell Culture.
The human HepG2 hepatoma cells (ATCC, Manassas, VA, USA) was cultured in Eagle’s minimum essential medium (EMEM) with 10% fetal bovine serum (FBS) and supplemented with 1% penicillin at 37 °C, under 5% CO2.
In Vitro Cytotoxicity Assessment.
A 100 μL solution of human HepG2 hepatoma cells with the concentration of 1 × 105 /mL was plated in each well of a 96-well plate and cultured for 12 h. The 20 μL solutions of nanoparticles with the concentration range of 0–500 μg/mL were treated onto the cells and incubated for 24 h. The cell media was discarded and cells were washed with fresh PBS solutions to remove supernatant and debris. Cells were then treated with 50 μL of MTT solution (5 mg/mL solution in PBS) and incubated for 2 h. After incubation, 100 μL of DMSO was added to the wells. The plate was sealed with aluminum foil to prevent light exposure and left for 30 min to dissolve the MTT formazan. Finally, the plate was read at 590 nm (O.D.) with the plate reader (BioTek Inc., Crawfordsville, IN, USA).
In Vitro ROS Measurement in Cells.
Five milliliters of a solution of human HepG2 hepatoma cells with the concentration of 5 × 105 /mL was added in the tissue culture flask and incubated for 12 h. When cells were attached on the bottom of flask, 5 mL of 100 μg/mL solution of the samples (D-NP or N-NP) or control (no nanoparticles) was added after removal of the media and incubated for 5 h. The cells in each flask were then trypsinized and washed with PBS buffer by centrifugation and redispersion in 1 mL of fresh PBS buffer in the microtubes. Ten micromolar DCF-DA solution was added into each microtube and incubated at 37 °C for 30 min. Then, the cells were irradiated with X-ray for 4 Gy by placing the microtubes in the center of the X-ray irradiator for the radiation. The cells were incubated at 37 °C for 15 min and analyzed with flow cytometry (LSRFortessa 6-Laser, BD Biosciences) at excitation 485 nm.
In Vitro Flow Cytometry Assessment.
Five milliliters of a solution of human HepG2 hepatoma cells with the concentration of5 × 105 /mL was plated on tissue culture flask and incubated for 12 h. When cells were attached on the bottom of flask, 5 mL of 100 μg/mL samples (D-NP or N-NP) was added after removal of the media and incubated for 5 h. For the treatment of X-ray radiation, the flask was placed onto the center of the shelf in an X-ray irradiator for the radiation treatment. The cells were collected after culturing further for 3 days. The cells were trypsinization and washed for twice by centrifugation at 1500 rpm and redispersion in fresh PBS to remove trypsin. After recentrifugation to wash cells and make cell pellets, cells were resuspended in the 100 μL of 1× Annexin-binding buffer. Five microliters of FITC Annexin V and 1 μL of 100 μg/mL propidium iodide solution were added to the cells and cell incubation was performed at room temperature for 15 min. Lastly, 400 μL of 1× Annexin-binding buffer was added to the cells and the stained cells were kept on ice before measurement with flow cytometry (LSRFortessa 6-Laser, BD Biosciences). The fluorescence emission was set at 530 nm (FL1) and >575 nm (FL3) and 50 000 events were counted to observe the population of cells in three groups: live cells, apoptotic cells, and dead cells.
In Vitro Assessment of Cell Cycle Study.
Five milliliters of a solution of human HepG2 hepatoma cells with the concentration of5 × 105 /mL was plated on tissue culture flask and incubated for 12 h. When cells were attached on the bottom of flask, 5 mL of 100 μg/mL of samples (D-NP or N-NP) were added after removal of the media and incubated for 24 h. The cells were then trypsinized and washed for twice by centrifugation at 1500 rpm and redispersed in the fresh PBS to remove trypsin. After recentrifugation for cell sedimentation, cells were fixed with ice-cold 80% ethanol aqueous solution and stored in −20 °C freezer for 12 h. After fixation, the fresh PBS was added to the cells and the cells were washed by centrifugation and redispersion in the PBS. In the resuspended cell pellet, PI staining solution was added and the cells were incubated for 20 min at 37 °C and covered with foil and stored at 4 °C before analysis with flow cytometer flow cytometry (LSRFortessa 6-Laser, BD Biosciences). The fluorescence emission was set at 488 nm (FL2) and 40,000 events were counted to observe the population of cells. The cells cycle of the PI stained cells was analyzed with FlowJo10.4.1: the phase fractions of G0/G1, S, and G2/M were determined by the curve fittings of the FlowJo software.
In Vitro Assessment of Clonogenic Study.
Two milliliters of human HepG2 hepatoma cells with a concentration of 500/mL was plated in each well of a 6-well plate and incubated for 12 h; next 200 μL of 100 μg/mL samples (D-NP or N-NP) was added into the well and incubated for 5 h. The medium containing free nanoparticles was removed and replaced with fresh cell-culturing medium. For the treatment of X-ray radiation, the well-plate was placed onto the center of the shelf in an X-ray irradiator and was exposed upon a radiation dose of 0–8 Gy, which was controlled by X-ray exposure time. Cell culture media in the wells were replaced in 2 day-interval during 10 days of incubation. The colonies on the wells were washed with PBS buffer and treated with 1 mL of 25%v/v fixative solution containing acetic acid in methanol in each well. After 5 min of fixation, the fixative was removed from the wells and 1 mL of 0.5% solution containing crystal violet in methanol was treated in each well for 15 min. After staining the colonies, the wells were cleansed with tap water and left on the shelves to be dried. Each well was analyzed its number of colonies (>10 cells) and plotted by considering the number of original plating on the well. The surviving fraction was calculated by dividing the number of counted colonies by the number of seeded cells and plating efficiency.
In Vivo Assessment of CT-Image Guidance and Radiation Therapy.
D-NP and N-NP were applied to the animal; human HepG2 hepatoma xenograft mice model and procedures for CT-image guided radiation therapy were approved by IACUC at Northwestern University before in vivo assessment. One hundred microliters of human HepG2 hepatoma cells (2 × 105) suspended in EMEM media was subcutaneously injected on the right flanks of 2–3 weeks old immunocompromised CB17-Prkd c SCID/J mice and 2 weeks were allowed for tumor growth (0.5–1 cm diameter). There were four groups of experiments and each group consists of three animals: nontreated (control), radiation only, radiation after D-NP treated, and radiation after N-NP treated. Ten microliters of nanoparticles (5 mg/mL) in PBS dispersion was injected into the tumor via catheter that has guided the depth and angle of infusion using CT. In vivo contrast to noise ratio (CNR) was calculated by obtaining image contrast between nanoparticles accumulated region and tumor region, which were estimated by signal-to-noise ratio (SNR) with the equation, CNR = SNRNP − SNRtumor. After injection of the nanoparticles, mice were sedated with ketamine and xylazine and irradiated with a single dose of radiation (10 Gy) on the tumor region while shielding the rest of body using a lead shield (Braintree scientific, MA, USA). The mice were then euthanized within 48 h after treatment and organs were collected for histological analysis.
Histology and Immunohistochemistry.
To investigate the efficacy of radiation therapy with D-NP or N-NP, tumors harvested from mice after treatments were sliced with microtome sectioning. Five-micrometer slices through the center of each tumor were used for staining with hematoxylin and eosin (H&E) and terminal deoxy-nucleotidyl transferase-mediated dUTP nick end labeling (TUNEL). All slides were analyzed with a TissueFAXS microscope (TissueG-nostics GmbH, Vienna, Austria).
Supplementary Material
ACKNOWLEDGMENTS
The financial support from NIH/NCI-R21CA173491 (D.-H.K.), NIH/NCI-R21CA185274 (D.-H.K.), and NIH/NIBIB-R21EB017986 (D.-H.K.) are greatly acknowledged. This work used resources of the Advanced Photon Source, a U.S. Department of Energy (DOE) Office of Science User Facility operated for the DOE Office of Science by Argonne National Laboratory under Contract DE-AC02-06CH11357. This work was also supported by the Center for Translational Imaging and Mouse Histology and Phenotyping Laboratory at Northwestern University.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsami.8b09596.
Supplementary notes about SAXS curve fitting and calculation of the numbers of radiosensitizing surface atoms, and supplementary figures of CT images of pre-and postinjection of N-NPs and viability of cells after treatment of D-NP or N-NP (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Delaney GP; Barton MB Evidence-Based Estimates of the Demand for Radiotherapy. Clin. Oncol. (R. Coll. Radiol.) 2015, 27, 7076. [DOI] [PubMed] [Google Scholar]
- (2).Jaffray DA Image-Guided Radiotherapy: from Current Concept to Future Perspectives. Nat. Rev. Clin. Oncol 2012, 9, 688–699. [DOI] [PubMed] [Google Scholar]
- (3).Detappe A; Thomas E; Tibbitt MW; Kunjachan S; Zavidij O; Parnandi N; Reznichenko E; Lux F; Tillement O; Berbeco R Ultrasmall Silica-Based Bismuth Gadolinium Nanoparticles for Dual Magnetic Resonance-Computed Tomography Image Guided Radiation Therapy. Nano Lett. 2017, 17, 1733–1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Hainfeld JF; Dilmanian FA; Slatkin DN; Smilowitz HM Radiotherapy Enhancement with Gold Nanoparticles. J. Pharm. Pharmacol 2008, 60, 977–985. [DOI] [PubMed] [Google Scholar]
- (5).Xiao FX; Zheng Y; Cloutier P; He YH; Hunting D; Sanche L On the Role of Low-Energy Electrons in the Radiosensitization of DNAby Gold Nanoparticles. Nanotechnology 2011, 22, 465101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Jain S; Coulter JA; Hounsell AR; Butterworth KT; McMahon SJ; Hyland WB; Muir MF; Dickson GR; Prise KM; Currell FJ; O’Sullivan JM; Hirst DG Cell-Specific Radiosensitization by Gold Nanoparticles at Megavoltage Radiation Energies. Int. J. Radiat. Oncol. Biol. Phys 2011, 79, 531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Pronschinske A; Pedevilla P; Murphy CJ; Lewis EA; Lucci FR; Brown G; Pappas G; Michaelides A; Sykes ECH Enhancement of Low-Energy Electron Emission in 2D Radioactive Films. Nat. Mater 2015, 14, 904–907. [DOI] [PubMed] [Google Scholar]
- (8).Jain S; Hirst DG; O’Sullivan JM Gold Nanoparticles as Novel Agents for Cancer Therapy. Br. J. Radiol 2012, 85, 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Rose JH; Norman A; Ingram M; Aoki C; Solberg T; Mesa A First Radiotherapy of Human Metastatic Brain Tumors Delivered by a Computerized Tomography Scanner (CTRx). Int. J. Radiat. Oncol., BiolPhys 1999, 45, 1127–32. [DOI] [PubMed] [Google Scholar]
- (10).Jain S; Hirst DG; O’Sullivan JM Gold Nanoparticles as Novel Agents for Cancer Therapy. Br. J. Radiol 2012, 85, 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Cho SH; Jones BL; Krishnan S The Dosimetric Feasibility of Gold Nanoparticle-Aided Radiation Therapy (GNRT) via Brachytherapy Using Low-Energy Gamma-/X-ray Sources. Phys. Med. Biol 2009, 54, 4889–4905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Popovtzer A; Mizrachi A; Motiei M; Bragilovski D; Lubimov L; Levi M; Hilly O; Ben-Aharon I; Popovtzer R Actively Targeted Gold Nanoparticles as Novel Radiosensitizer Agents: an in vivo Head and Neck Cancer Model. Nanoscale 2016, 8, 2678–2685. [DOI] [PubMed] [Google Scholar]
- (13).Butterworth KT; McMahon SJ; Currell FJ; Prise KM Physical Basis and Biological Mechanisms of Gold Nanoparticle Radiosensitization. Nanoscale 2012, 4, 4830–4838. [DOI] [PubMed] [Google Scholar]
- (14).Ma N; Wu FG; Zhang X; Jiang YW; Jia HR; Wang HY; Li YH; Liu P; Gu N; Chen Z Shape-Dependent Radiosensitization Effect of Gold Nanostructures in Cancer Radiotherapy: Comparison of Gold Nanoparticles, Nanospikes, and Nanorods. ACS Appl. Mater. Interfaces 2017, 9, 13037–13048. [DOI] [PubMed] [Google Scholar]
- (15).Jackson P; Periasamy S; Bansal V; Geso M Evaluation of the Effects of Gold Nanoparticle Shape and Size on Contrast Enhancement in Radiological imaging. Australas. Phys. Eng. Sci. Med 2011, 34, 243–249. [DOI] [PubMed] [Google Scholar]
- (16).Zhang XD; Wu D; Shen X; Chen J; Sun YM; Liu PX; Liang XJ Size-Dependent Radiosensitization of PEG-Coated Gold Nanoparticles for Cancer Radiation Therapy. Biomaterials 2012, 33, 6408–6419. [DOI] [PubMed] [Google Scholar]
- (17).Glazinska P; Zienkiewicz A; Wojciechowski W; Kopcewicz J The Putative miR172 Target Gene InAPETALA2-Like is Involved in the Photoperiodic Flower Induction of Ipomoea nil. J. Plant Physiol 2009, 166, 1801–1813. [DOI] [PubMed] [Google Scholar]
- (18).Alqarni AS; Awad AM; Owayss AA Evaluation of Acacia Gerrardii Benth. (Fabaceae: Mimosoideae) as a Honey Plant under Extremely Hot-Dry Conditions: Flowering Phenology, Nectar Yield and Honey Potentiality. J. Anim. Plant Sci 2015, 25, 1667–1674. [Google Scholar]
- (19).Imaizumi T; Kay SA Photoperiodic Control of Flowering: Not Only by Coincidence. Trends Plant Sci. 2006, 11, 550–558. [DOI] [PubMed] [Google Scholar]
- (20).Kim DH; Larson AC Deoxycholate Bile Acid Directed Synthesis of Branched Au Nanostructures for Near Infrared Photo-thermal Ablation. Biomaterials 2015, 56, 154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Hammouda B A new Guinier-Porod Model. J. Appl. Crystallogr 2010, 43, 716–719. [Google Scholar]
- (22).Li T; Senesi AJ; Lee B Small Angle X-ray Scattering for Nanoparticle Research. Chem. Rev 2016, 116, 11128–11180. [DOI] [PubMed] [Google Scholar]
- (23).Al Zaki A; Joh D; Cheng Z; De Barros AL; Kao G; Dorsey J; Tsourkas A Gold-Loaded Polymeric Micelles for Computed Tomography-Guided Radiation Therapy Treatment and Radiosensitization. ACS Nano 2014, 8, 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Misawa M; Takahashi J Generation of Reactive Oxygen Species Induced by Gold Nanoparticles Under X-ray and UV Irradiations. Nanomedicine 2011, 7, 604–614. [DOI] [PubMed] [Google Scholar]
- (25).Pronschinske A; Pedevilla P; Murphy CJ; Lewis EA; Lucci FR; Brown G; Pappas G; Michaelides A; Sykes EC Enhancement of Low-Energy Electron Emission in 2D Radioactive Films. Nat. Mater 2015, 14, 904–907. [DOI] [PubMed] [Google Scholar]
- (26).Gupta T; Narayan CA Image-Guided Radiation Therapy: Physician’s Perspectives. J. Med. Phys 2012, 37, 174–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Baumann M; Krause M; Overgaard J; Debus J; Bentzen SM; Daartz J; Richter C; Zips D; Bortfeld T Radiation Oncology in the Era of Precision Medicine. Nat. Rev. Cancer 2016, 16, 234–249. [DOI] [PubMed] [Google Scholar]
- (28).Park W; Cho S; Huang X; Larson AC; Kim DH Branched Gold Nanoparticle Coating of Clostridium novyi-NT Spores for CT-Guided Intratumoral Injection. Small 2017, 13, 1602722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Ross RD; Cole LE; Tilley JMR; Roeder RK Effects of Functionalized Gold Nanoparticle Size on X-ray Attenuation and Substrate Binding Affinity. Chem. Mater 2014, 26, 1187–1194. [Google Scholar]
- (30).Lee J; Gordon AC; Kim H; Park W; Cho S; Lee B; Larson AC; Rozhkova EA; Kim DH Targeted Multimodal Nano-reporters for Pre-procedural MRI and Intra-operative Image-guidance. Biomaterials 2016, 109, 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Cho S; Park W; Kim DH Silica-Coated Metal Chelating-Melanin Nanoparticles as a Dual-Modal Contrast Enhancement Imaging and Therapeutic Agent. ACS Appl. Mater. Interfaces 2017, 9, 101–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Kim DH; Guo Y; Zhang Z; Procissi D; Nicolai J; Omary RA; Larson AC Temperature-Sensitive Magnetic Drug Carriers for Concurrent Gemcitabine Chemohyperthermia. Adv. Healthcare Mater 2014, 3, 714–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Kim D-H Image-Guided Cancer Nanomedicine. J. Imaging 2018, 4, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Kanesa-thasan A; Tyler PD; Nicolai J; Procissi D; Chen J; Lewandowski RJ; Salem R; Larson AC; Omary RA Comparison of image-guided intratumoral versus intravenous delivery of therapeutic nanoparticles for the treatment of hepatocellular carcinoma. Journal of Vascular and Interventional Radiology 2013, 24 (4), S142. [Google Scholar]
- (35).Retif P; Pinel S; Toussaint M; Frochot C; Chouikrat R; Bastogne T; Barberi-Heyob M Nanoparticles for Radiation Therapy Enhancement: the Key Parameters. Theranostics 2015, 5, 1030–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Salem R; Mazzaferro V; Sangro B Yttrium 90 Radioembolization for the Treatment of Hepatocellular Carcinoma: Biological Lessons, Current Challenges, and Clinical Perspectives. Hepatology 2013, 58, 2188–2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Su XY; Liu PD; Wu H; Gu N Enhancement of Radiosensitization by Metal-Based Nanoparticles in Cancer Radiation Therapy. Cancer Biol. Med 2014, 11, 86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Kim KJ; Kim JH; Lee SJ; Lee EJ; Shin EC; Seong J Radiation Improves Antitumor Effect of Immune Checkpoint Inhibitor in Murine Hepatocellular Carcinoma Model. Oncotarget 2017, 8, 41242–41255. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


