Abstract
The glycocalyx is a dense and diverse coat of glycans and glycoconjugates responsible for maintaining cell surface integrity and regulating the interaction of cells with the external environment. Transmembrane mucins such as MUC1 and MUC16 comprise a major component of the epithelial glycocalyx and are currently used to monitor disease progression in cancer. At the ocular surface, multiple lines of evidence indicate that abnormal expression of the enzymes responsible for glycan biosynthesis during pathological conditions impairs the glycosylation of transmembrane mucins. It is now becoming clear that these changes contribute to modify the interaction of mucins with galectin-3, a multimeric lectin crucial for preserving the ocular surface epithelial barrier. This review highlights the potential of using the epithelial glycocalyx as a reliable source for the generation of biomarkers to diagnose and monitor ocular surface disease.
Keywords: biomarker, galectin, glycocalyx, transmembrane mucin, ocular surface
The surface of the eye is faced with the critical function of forming a protective barrier that prevents cellular damage and systemic infection while allowing the exchange of molecules with the external environment. It is formed by a highly specialized layer of corneal and conjunctival epithelial cells and it is covered by a tear film that contributes to moisturize and maintain a smooth refractive surface.1 A number of pathological conditions are known to impair the integrity of the ocular surface epithelia. Among them, dry eye is the most prevalent form of ocular surface disease affecting millions of adults worldwide.2 For many years the lack of biological measures of ocular surface disease has slowed down progress in clinical research and patient care, particularly in dry eye. However, recent technological advances have led to a resurgence of studies directed at finding minimally invasive biomarkers to assist in the diagnosis of ocular surface disease and to determine progression or responsiveness to therapy.3 This review provides an overview of the structure of the healthy ocular surface epithelial glycocalyx and its potential to serve as a source of biomarkers in pathological conditions.
THE GLYCOCALYX AS A BIOMARKER
The glycocalyx is a dense and diverse coat of glycans and glycoconjugates present in all animal cells. One of the more important characteristics of the glycocalyx is that it is dynamic, therefore, its composition changes to ensure an adequate response to physiological and pathological conditions. This feature has been extensively exploited in the field of cancer for the development of biomarkers with proven clinical value. Here, abnormal expression of transmembrane mucins has led to current diagnostic assays for the detection of a variety of tumors.4, 5 The cancer antigen 15–3 (CA15–3), the soluble product of the MUC1 gene, is the most commonly used marker in breast cancer and is used to monitor response to treatment and disease recurrence.5, 6 Similarly, the CA125 test is commonly used to detect elevated levels of MUC16 in blood, an early sign of ovarian cancer in patients at high risk of the disease.7 An additional FDA-approved test evaluates CA19–9, a sialylated Lewis carbohydrate antigen widely used for the diagnosis of pancreatic cancer.8, 9 Not surprisingly, the use of the glycocalyx as a source of biomarkers is not restricted exclusively to tumors. The vascular endothelial surface is covered with an abundant amount of glycoproteins, proteoglycans and associated plasma proteins. Increased serum levels of syndecan-1 and glycosaminoglycans resulting from a disrupted endothelial glycocalyx have been associated with deteriorating cardiac and renal function,10, 11 and organ failure in sepsis,12, 13 and have been consequently proposed as biomarkers to monitor disease progression.
THE OCULAR SURFACE EPITHELIAL GLYCOCALYX
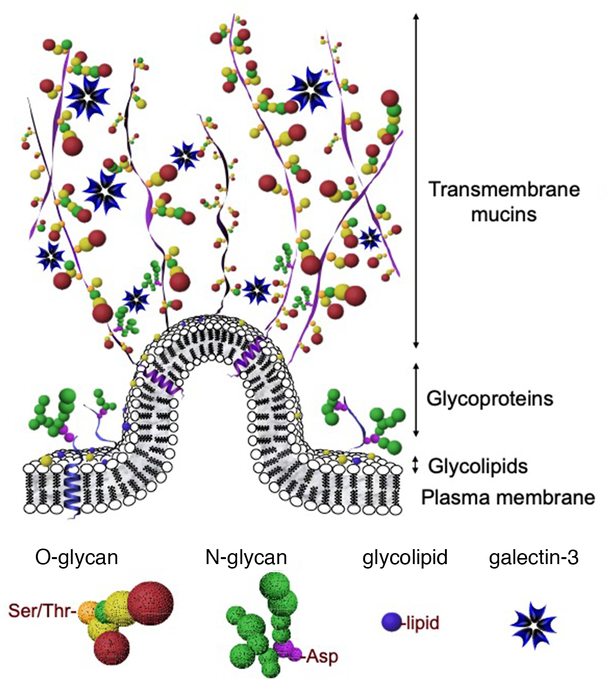
Research during the last decade has redefined and highlighted the contribution of apical plasma membranes on epithelial cells to the protection of the ocular surface. It is this area adjacent to the tear film interface where short membrane folds form to produce microplicae (Fig. 1). Transmembrane mucins emanate from the tips of these structures, extending up to 500 nm above the plasma membrane, far above other glycoconjugates, to form a distinct glycocalyx.14–16 These mucins have single membrane-spanning regions with large extracellular glycosylated domains containing tandem repeats of amino acids rich in serine and threonine residues. The most apical portion of the human ocular surface epithelia produces primarily three mucin genes named MUC1, MUC4 and MUC16.17 Approximately 55% of the mucin products are carbohydrate chains containing galactose, N-acetylgalactosamine, N-acetylglucosamine and terminal sialic acid derivatives.18, 19 Under physiological conditions, galactosyl residues on mucin glycans are cross-linked by the multimeric lectin galectin-3 on the apical cell glycocalyx. This interaction is mediated by two major classes of glycans, N-glycans and O-glycans, and is critical to maintain the epithelial barrier and to prevent cellular damage.20, 21 In addition to transmembrane mucins, the ocular surface glycocalyx is rich in glycoconjugates such as proteoglycans (i.e., heparan sulfate and chondroitin sulfate) and gangliosides.22, 23
FIG. 1. The ocular surface epithelial glycocalyx.
Transmembrane mucins emanating up to 500 nm above the plasma membrane interact with multimeric galectin-3 to promote epithelial integrity. Major classes of glycans at the ocular surface include O-glycans, commonly attached to serine (Ser) or threonine (Thr) amino acids, and N-glycans, attached to asparagine (Asn). Mucins, glycoproteins and glycolipids are embedded in the plasma membrane.
DISRUPTION OF THE GLYCOCALYX IN OCULAR SURFACE DISEASE
A number of studies have evaluated the content of transmembrane mucins in ocular surface disease, focusing primarily on their (i) cellular expression, (ii) protein levels in tear fluid, and (iii) glycosylation status. While changes in mucin glycosylation in dry eye appear to be well documented, there is no consensus on whether alteration of mucin transcription or protein biosynthesis is a good indicator of disease. An early study of the ocular surface of postmenopausal women with dry eye noted increased expression and biosynthesis in MUC1 and MUC16 that positively correlated with diagnostic tests.24 Subsequently, other investigators found no evidence that would support a correlation between MUC1 or MUC16 protein concentration, or MUC1 mRNA expression, with a range of symptomatic data in postmenopausal women.25 To complicate things further, an additional study reported decreased expression of MUC1 and MUC4 in patients with non-Sjögren’s dry eye.26 In Sjögren’s patients, our own studies failed to find differences in the levels of MUC1 or MUC4 expression compared to healthy individuals,27 although the amounts of both soluble MUC16 and MUC16 mRNA appear to increase.28 In addition to drying diseases, potential alteration of transmembrane mucins at the ocular surface has been reported in atopic keratoconjunctivitis,29 infection,30 pseudophakic bullous keratopathy,31 complete androgen insensitivity syndrome,32 and pterygium.33
It is now well established that changes in cell surface glycosylation occur during pathological processes at the ocular surface. These alterations arise from an abnormal expression of the enzymes responsible for glycan biosynthesis. During homeostatic conditions, the distribution of glycosyltransferases involved in the initiation of mucin-type O-glycosylation is exquisitely regulated in a cell-layer- and cell-type-specific manner.34 Likewise, the proper expression of N-glycosylation processing enzymes assures stability and barrier function of the MUC16 mucin.20 In ocular cicatricial pemphigoid, however, the expression of these enzymes is significantly altered as the ocular surface becomes keratinized.34, 35 This impairment in glycosyltransferase expression in ocular surface drying conditions has led to propose that mucin glycosylation could be used in the diagnosis, monitoring and management of epithelial dysfunction. Certainly, antibodies to mucin carbohydrates whose distribution is altered in dry eye disease are available. An example is the H185 monoclonal antibody. This antibody, developed by Ilene Gipson in the nineties, binds to apical membranes of apical cells of human corneal, conjunctival, laryngeal, and vaginal epithelium, particularly at the tips of microplicae.36 Subsequent structural characterization has demonstrated that the H185 epitope is an O-acetyl sialic acid derivative on MUC16.18, 37 Altered binding of the antibody to conjunctival epithelium correlates with severity in patients with dry eye symptoms,38 and the antigen can be detected at elevated concentrations in pathological tears.24 In addition, the H185 antibody has been used to monitor the progression of keratinization following the treatment of superior limbic keratoconjunctivitis.39 Other antibodies against carbohydrate epitopes that bind to the ocular surface epithelia include KL-6 and CA19–9. KL-6 recognizes a sialylated carbohydrate epitope on MUC1 and produces a mosaic pattern in impression cytology samples of patients with dry eye disease40 whereas CA19–9, used in the diagnosis of pancreatic cancer, diminishes in their tear fluid.41
GALECTIN-3 AS A DISEASE BIOMARKER
Galectin-3 is a β-galactoside-binding lectin highly expressed by the ocular surface epithelia.42 Despite being a soluble protein without a transmembrane domain, it localizes to cell membranes, predominantly on the apical portion of the stratified corneal and conjunctival epithelia.21 Altered expression levels of galectin-3 are observed in heart disease, kidney disease and cancer, and because the protein can be found on biological fluids including serum and urine, it has been proposed as a diagnostic biomarker. Of interest is that galectin-3 does not exhibit circadian variation and its expression is not associated with age, body mass index or sex.43 In the eye, early studies suggested that galectin-3 was present only in tears from patients with ocular surface disorders.44 Further investigation demonstrated that the concentration of galectin-3 in the normal tear fluid is 0.12 ng/μg total protein but significantly increases to 0.38 ng/μg total protein in patients with dry eye.45 Importantly, this study also showed an association between elevated expression of MMP9 in dry eye subjects and the presence of cleaved galectin-3 in the tear fluid, as no degradation of galectin-3 was observed in tears of healthy subjects. Several cell types could contribute to the elevated concentration of galectin-3 in tears of patients with ocular surface disease, including immune and epithelial cells. We have hypothesized that disruption of mucin glycosylation on the epithelial glycocalyx could result in decreased affinity towards galectin-3 and its subsequent release into the tear fluid.46 Certainly, the individual contribution of these and other cell types to galectin-3 content in biological fluids merits further investigation.
CONCLUSION
Key events during disease development and progression are changes in tissue glycosylation and the release, in many instances, of glycans and glycoconjugates into extracellular fluids. These compounds have constituted a valuable resource for the development of biomarkers.47 In this context, attempts have been made to use tear fluid glycans as a diagnostic indicator of skin conditions and diabetes.48, 49 The epithelial glycocalyx is a reliable source for the generation of biomarkers. Research during the past decade has established that the ocular surface epithelial glycocalyx is sensitive to disruption during pathological states. The data generated indicate that the glycosylation of transmembrane mucins is impaired in ocular surface disease and supports the use of mucin glycan antibodies as novel tools for biomarker discovery in the ophthalmic field. Further, new advances indicate that disruption of the epithelial glycocalyx negatively affects the mucin-galectin interaction. Monitoring the integrity of galectin-3, and its release into the tear film, could be used to determine the severity and progression of dry eye, and in the prediction of treatment response. Many other components of the ocular surface epithelial glycocalyx remain understudied, and include proteoglycans, glycosaminoglycans, and glycosphingolipids.50 Further exploration efforts should have important clinical implications for the diagnosis and treatment of ocular surface disease.
Acknowledgments
Supported by the National Institutes of Health, NEI Grant R01EY026147.
REFERENCES
- [1].Gipson IK: The ocular surface: the challenge to enable and protect vision: the Friedenwald lecture. Invest Ophthalmol Vis Sci 2007, 48:4390; 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gayton JL: Etiology, prevalence, and treatment of dry eye disease. Clin Ophthalmol 2009, 3:405–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Roy NS, Wei Y, Kuklinski E, Asbell PA: The Growing Need for Validated Biomarkers and Endpoints for Dry Eye Clinical Research. Invest Ophthalmol Vis Sci 2017, 58:BIO1–BIO19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Felder M, Kapur A, Gonzalez-Bosquet J, Horibata S, Heintz J, Albrecht R, Fass L, Kaur J, Hu K, Shojaei H, Whelan RJ, Patankar MS: MUC16 (CA125): tumor biomarker to cancer therapy, a work in progress. Mol Cancer 2014, 13:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Martin A, Corte MD, Alvarez AM, Rodriguez JC, Andicoechea A, Bongera M, Junquera S, Pidal D, Allende T, Muniz JL, Vizoso F: Prognostic value of pre-operative serum CA 15.3 levels in breast cancer. Anticancer Res 2006, 26:3965–71. [PubMed] [Google Scholar]
- [6].Duffy MJ, Evoy D, McDermott EW: CA 15–3: uses and limitation as a biomarker for breast cancer. Clin Chim Acta 2010, 411:1869–74. [DOI] [PubMed] [Google Scholar]
- [7].Bast RC Jr., Klug TL, St John E, Jenison E, Niloff JM, Lazarus H, Berkowitz RS, Leavitt T, Griffiths CT, Parker L, Zurawski VR Jr., Knapp RC: A radioimmunoassay using a monoclonal antibody to monitor the course of epithelial ovarian cancer. N Engl J Med 1983, 309:883–7. [DOI] [PubMed] [Google Scholar]
- [8].Steinberg W: The clinical utility of the CA 19–9 tumor-associated antigen. Am J Gastroenterol 1990, 85:350–5. [PubMed] [Google Scholar]
- [9].Partyka K, Maupin KA, Brand RE, Haab BB: Diverse monoclonal antibodies against the CA 19–9 antigen show variation in binding specificity with consequences for clinical interpretation. Proteomics 2012, 12:2212–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kim YH, Nijst P, Kiefer K, Tang WH: Endothelial Glycocalyx as Biomarker for Cardiovascular Diseases: Mechanistic and Clinical Implications. Curr Heart Fail Rep 2017, 14:117–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Vlahu CA, Lemkes BA, Struijk DG, Koopman MG, Krediet RT, Vink H: Damage of the endothelial glycocalyx in dialysis patients. J Am Soc Nephrol 2012, 23:1900–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Smart L, Bosio E, Macdonald SPJ, Dull R, Fatovich DM, Neil C, Arendts G: Glycocalyx biomarker syndecan-1 is a stronger predictor of respiratory failure in patients with sepsis due to pneumonia, compared to endocan. J Crit Care 2018, 47:93–8. [DOI] [PubMed] [Google Scholar]
- [13].Burke-Gaffney A, Evans TW: Lest we forget the endothelial glycocalyx in sepsis. Crit Care 2012, 16:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gipson IK, Argueso P: Role of mucins in the function of the corneal and conjunctival epithelia. Int Rev Cytol 2003, 231:1–49. [DOI] [PubMed] [Google Scholar]
- [15].Hilkens J, Ligtenberg MJ, Vos HL, Litvinov SV: Cell membrane-associated mucins and their adhesion-modulating property. Trends Biochem Sci 1992, 17:359–63. [DOI] [PubMed] [Google Scholar]
- [16].Hollingsworth MA, Swanson BJ: Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer 2004, 4:45–60. [DOI] [PubMed] [Google Scholar]
- [17].Mantelli F, Argueso P: Functions of ocular surface mucins in health and disease. Curr Opin Allergy Clin Immunol 2008, 8:477–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Argueso P, Sumiyoshi M: Characterization of a carbohydrate epitope defined by the monoclonal antibody H185: sialic acid O-acetylation on epithelial cell-surface mucins. Glycobiology 2006, 16:1219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chao CC, Butala SM, Herp A: Studies on the isolation and composition of human ocular mucin. Exp Eye Res 1988, 47:185–96. [DOI] [PubMed] [Google Scholar]
- [20].Taniguchi T, Woodward AM, Magnelli P, McColgan NM, Lehoux S, Jacobo SMP, Mauris J, Argueso P: N-Glycosylation affects the stability and barrier function of the MUC16 mucin. J Biol Chem 2017, 292:11079–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Argueso P, Guzman-Aranguez A, Mantelli F, Cao Z, Ricciuto J, Panjwani N: Association of cell surface mucins with galectin-3 contributes to the ocular surface epithelial barrier. J Biol Chem 2009, 284:23037–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bairaktaris G, Lewis D, Fullwood NJ, Nieduszynski IA, Marcyniuk B, Quantock AJ, Ridgway AE: An ultrastructural investigation into proteoglycan distribution in human corneas. Cornea 1998, 17:396–402. [DOI] [PubMed] [Google Scholar]
- [23].Nilsson EC, Storm RJ, Bauer J, Johansson SM, Lookene A, Angstrom J, Hedenstrom M, Eriksson TL, Frangsmyr L, Rinaldi S, Willison HJ, Pedrosa Domellof F, Stehle T, Arnberg N: The GD1a glycan is a cellular receptor for adenoviruses causing epidemic keratoconjunctivitis. Nat Med 2011, 17:105–9. [DOI] [PubMed] [Google Scholar]
- [24].Gipson IK, Spurr-Michaud SJ, Senchyna M, Ritter R 3rd, Schaumberg D: Comparison of mucin levels at the ocular surface of postmenopausal women with and without a history of dry eye. Cornea 2011, 30:1346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Srinivasan S, Heynen ML, Martell E, Ritter R 3rd, Jones L, Senchyna M: Quantification of MUCIN 1, cell surface associated and MUCIN16, cell surface associated proteins in tears and conjunctival epithelial cells collected from postmenopausal women. Mol Vis 2013, 19:970–9. [PMC free article] [PubMed] [Google Scholar]
- [26].Corrales RM, Narayanan S, Fernandez I, Mayo A, Galarreta DJ, Fuentes-Paez G, Chaves FJ, Herreras JM, Calonge M: Ocular mucin gene expression levels as biomarkers for the diagnosis of dry eye syndrome. Invest Ophthalmol Vis Sci 2011, 52:8363–9. [DOI] [PubMed] [Google Scholar]
- [27].Argueso P, Balaram M, Spurr-Michaud S, Keutmann HT, Dana MR, Gipson IK: Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjogren syndrome. Invest Ophthalmol Vis Sci 2002, 43:1004–11. [PubMed] [Google Scholar]
- [28].Caffery B, Joyce E, Heynen ML, Jones L, Ritter R 3rd, Gamache DA, Senchyna M: MUC16 expression in Sjogren’s syndrome, KCS, and control subjects. Mol Vis 2008, 14:2547–55. [PMC free article] [PubMed] [Google Scholar]
- [29].Dogru M, Matsumoto Y, Okada N, Igarashi A, Fukagawa K, Shimazaki J, Tsubota K, Fujishima H: Alterations of the ocular surface epithelial MUC16 and goblet cell MUC5AC in patients with atopic keratoconjunctivitis. Allergy 2008, 63:1324–34. [DOI] [PubMed] [Google Scholar]
- [30].Govindarajan B, Menon BB, Spurr-Michaud S, Rastogi K, Gilmore MS, Argueso P, Gipson IK: A metalloproteinase secreted by Streptococcus pneumoniae removes membrane mucin MUC16 from the epithelial glycocalyx barrier. PLoS One 2012, 7:e32418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Glasgow BJ, Gasymov OK, Casey RC: Exfoliative epitheliopathy of bullous keratopathy with breaches in the MUC16 Glycocalyx. Invest Ophthalmol Vis Sci 2009, 50:4060–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Mantelli F, Moretti C, Micera A, Bonini S: Conjunctival mucin deficiency in complete androgen insensitivity syndrome (CAIS). Graefes Arch Clin Exp Ophthalmol 2007, 245:899–902. [DOI] [PubMed] [Google Scholar]
- [33].Kase S, Kitaichi N, Furudate N, Yoshida K, Ohno S: Increased expression of mucinous glycoprotein KL-6 in human pterygium. Br J Ophthalmol 2006, 90:1208–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Argueso P, Tisdale A, Mandel U, Letko E, Foster CS, Gipson IK: The cell-layer- and cell-type-specific distribution of GalNAc-transferases in the ocular surface epithelia is altered during keratinization. Invest Ophthalmol Vis Sci 2003, 44:86–92. [DOI] [PubMed] [Google Scholar]
- [35].Woodward AM, Lehoux S, Mantelli F, Di Zazzo A, Brockhausen I, Bonini S, Argueso P: Inflammatory Stress Causes N-Glycan Processing Deficiency in Ocular Autoimmune Disease. Am J Pathol 2019, 189:283–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Watanabe H, Fabricant M, Tisdale AS, Spurr-Michaud SJ, Lindberg K, Gipson IK: Human corneal and conjunctival epithelia produce a mucin-like glycoprotein for the apical surface. Invest Ophthalmol Vis Sci 1995, 36:337–44. [PubMed] [Google Scholar]
- [37].Argueso P, Spurr-Michaud S, Russo CL, Tisdale A, Gipson IK: MUC16 mucin is expressed by the human ocular surface epithelia and carries the H185 carbohydrate epitope. Invest Ophthalmol Vis Sci 2003, 44:2487–95. [DOI] [PubMed] [Google Scholar]
- [38].Danjo Y, Watanabe H, Tisdale AS, George M, Tsumura T, Abelson MB, Gipson IK: Alteration of mucin in human conjunctival epithelia in dry eye. Invest Ophthalmol Vis Sci 1998, 39:2602–9. [PubMed] [Google Scholar]
- [39].Watanabe H, Maeda N, Kiritoshi A, Hamano T, Shimomura Y, Tano Y: Expression of a mucin-like glycoprotein produced by ocular surface epithelium in normal and keratinized cells. Am J Ophthalmol 1997, 124:751–7. [DOI] [PubMed] [Google Scholar]
- [40].Hayashi Y, Kao WW, Kohno N, Nishihara-Hayashi M, Shiraishi A, Uno T, Yamaguchi M, Okamoto S, Maeda M, Ikeda T, Hamada H, Kondo K, Ohashi Y: Expression patterns of sialylated epitope recognized by KL-6 monoclonal antibody in ocular surface epithelium of normals and dry eye patients. Invest Ophthalmol Vis Sci 2004, 45:2212–7. [DOI] [PubMed] [Google Scholar]
- [41].Garcher C, Bron A, Baudouin C, Bildstein L, Bara J: CA 19–9 ELISA test: a new method for studying mucus changes in tears. Br J Ophthalmol 1998, 82:88–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mantelli F, Schaffer L, Dana R, Head SR, Argueso P: Glycogene expression in conjunctiva of patients with dry eye: downregulation of Notch signaling. Invest Ophthalmol Vis Sci 2009, 50:2666–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dong R, Zhang M, Hu Q, Zheng S, Soh A, Zheng Y, Yuan H: Galectin-3 as a novel biomarker for disease diagnosis and a target for therapy (Review). Int J Mol Med 2018, 41:599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Hrdlickova-Cela E, Plzak J, Smetana K Jr., Melkova Z, Kaltner H, Filipec M, Liu FT, Gabius HJ: Detection of galectin-3 in tear fluid at disease states and immunohistochemical and lectin histochemical analysis in human corneal and conjunctival epithelium. Br J Ophthalmol 2001, 85:1336–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Uchino Y, Mauris J, Woodward AM, Dieckow J, Amparo F, Dana R, Mantelli F, Argueso P: Alteration of galectin-3 in tears of patients with dry eye disease. Am J Ophthalmol 2015, 159:1027–35 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Argueso P: Glycobiology of the ocular surface: mucins and lectins. Jpn J Ophthalmol 2013, 57:150–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Hua S, An HJ: Glycoscience aids in biomarker discovery. BMB Rep 2012, 45:323–30. [DOI] [PubMed] [Google Scholar]
- [48].Nguyen-Khuong T, Everest-Dass AV, Kautto L, Zhao Z, Willcox MD, Packer NH: Glycomic characterization of basal tears and changes with diabetes and diabetic retinopathy. Glycobiology 2015, 25:269–83. [DOI] [PubMed] [Google Scholar]
- [49].An HJ, Ninonuevo M, Aguilan J, Liu H, Lebrilla CB, Alvarenga LS, Mannis MJ: Glycomics analyses of tear fluid for the diagnostic detection of ocular rosacea. J Proteome Res 2005, 4:1981–7. [DOI] [PubMed] [Google Scholar]
- [50].Rodriguez Benavente MC, Argueso P: Glycosylation pathways at the ocular surface. Biochem Soc Trans 2018, 46:343–50. [DOI] [PMC free article] [PubMed] [Google Scholar]