Abstract
Medications to treat major depressive disorder (MDD) are not equally effective across patients. Given that neural response to rewards is altered in MDD and given that reward-related circuitry is modulated by dopamine and serotonin, we examined, for the first time, whether reward-related neural activity moderated response to sertraline, an antidepressant medication that targets these neurotransmitters. 222 unmedicated adults with MDD randomized to receive sertraline (n=110) or placebo (n=112) in the EMBARC (Establishing Moderators and Biosignatures of Antidepressant Response in Clinical Care) study completed demographic and clinical assessments, and pre-treatment functional magnetic resonance imaging while performing a reward task. We tested whether an index of reward system function in the ventral striatum (VS), a key reward circuitry region, moderated differential response to sertraline versus placebo, assessed with the Hamilton Rating Scale for Depression over 8 weeks. We observed a significant moderation effect of the reward index, reflecting the temporal dynamics of VS activity, on Week-8 depression levels (Fs≥9.67,ps≤0.002). Specifically, VS responses that were abnormal with respect to predictions from reinforcement learning theory were associated with lower Week-8 depression symptoms in the sertraline versus placebo arms. Thus, a more abnormal pattern of pre-treatment VS dynamic response to reward expectancy (expected outcome value) and prediction error (difference between expected and actual outcome), likely reflecting serotonergic and dopaminergic deficits, was associated with better response to sertraline than placebo. Pre-treatment measures of reward-related VS activity may serve as objective neural markers to advance efforts to personalize interventions by guiding individual-level choice of antidepressant treatment.
Trial Registration:
Introduction
Despite over 50 years of treatment development and dissemination, depression has risen to rank as the number one leading cause of disability worldwide1. On average, antidepressant medications, as with evidenced based psychotherapies, outperform placebo in alleviating depressive symptoms, but only approximately 50–60% of patients respond to treatments, and only approximately 35% remit2. Currently, choice of antidepressant treatment is not informed by identified neural pathologies, but is often based on trial and error, which can exacerbate patients’ distress and raise costs3. Although clinical predictors of specific antidepressant treatments have been identified e.g.4, findings have not been consistently replicated, underscoring the need to identify objective neurobiological predictors to inform understanding of the neural mechanisms underlying response to specific antidepressants, and help lead to more targeted, efficient, and effective treatments for individuals with major depressive disorder (MDD).
Using neuroimaging to examine how functioning in neural circuits underlying reward processing relates to treatment response is an especially promising approach, as this neural circuitry is well-delineated5. Key neural regions involved in reward processing are the ventral striatum (VS), responding to reward anticipation and receipt6,7, medial prefrontal cortex, including ventromedial prefrontal cortex which encodes reward value8, orbitofrontal cortex which processes specific reward features (e.g., sensory attributes)9, ventrolateral prefrontal cortex which encodes the value of different decision-making options10 and links stimulus representations to specific reward outcomes11,12 and anterior cingulate cortical regions supporting reward-related effort-based decision making13,14. It is well established that reward circuitry is modulated not only by dopamine but also by serotonin15,16, neurotransmitters targeted by antidepressants. Recent animal studies have, for example, underscored the role of serotonin in reward processing via dorsal raphe projections to the ventral tegmental area and VS17. Dorsal raphe nuclei serotonergic neurons also contribute to non-social reward behavior18.
Many neuroimaging studies reported disrupted reward circuitry function in MDD19,20, including specific functional abnormalities in the VS and medial prefrontal cortex21,22, and recent studies employed reinforcement learning models to further elucidate these abnormalities23,24. According to these models, the prediction of future reward is updated based on the difference between expected reward and actual reward outcome during learning (i.e., the prediction error; PE)25. PE signals are tracked in the VS26 and, as learning proceeds, VS responding shifts from reward outcome to reward cues, consistent with conditioning. The rate of learning can differ between individuals, such that fast learners show a rapid transition from outcome-locked (i.e., PE) to cue-locked (i.e. reward expectancy; RE) responses, while slow learners show a slower transition. Across all individuals, this would manifest as an inverse relationship between RE and PE. In line with these reinforcement models and earlier work27, we previously reported an inverse relationship between RE- and PE-related VS activity in healthy individuals, consistent with a shift in VS responding from PE to RE28. This relationship was absent in depressed individuals, suggesting a disruption in normative conditioning. We replicated these findings in separate cohorts of 31 healthy individuals and 148 unmedicated individuals with MDD in the EMBARC (Establishing Moderators and Biosignatures of Antidepressant Response in Clinical Care) study, a multi-site, randomized, placebo-controlled trial of a selective serotonin reuptake inhibitor (SSRI), sertraline29. Further, we demonstrated in those healthy individuals the theoretically-predicted shift in VS response from PE to RE (i.e., an increase in RE and a decrease in PE-related activity) across two scans, separated by one week30. In both our prior study28 and EMBARC cohorts29, the temporal dynamics of RE and PE-related activity differentiated depressed and healthy individuals, but mean VS activity did not. Thus, an abnormal relationship between RE and PE-related VS activity may reflect an underlying pathophysiological process in MDD, which, in turn, may be associated with antidepressant treatment response.
Most treatment studies of neuroimaging markers in MDD have not examined reward paradigms, but rather included resting state or emotion-related paradigms and largely focused on frontolimbic regions involved in cognitive control and emotion processing31–33. Only two fMRI studies34–36 examined the extent to which reward-related neural measures predict treatment response in MDD. Both focused on psychotherapies or psychotherapies combined with medications; neither was a randomized trial with a control condition; and both reported that patterns of VS and medial prefrontal cortical activity were associated with depressive symptom change following treatment.
There are two other limitations of extant neuroimaging studies of antidepressant response in MDD. First, the majority of studies reported on general prediction of treatment response (i.e., associations between pre-treatment measures and subsequent response, irrespective of which treatment was received), rather than on moderators of differential response (i.e., the degree to which scores from a pre-treatment measure are associated with superior response in one treatment condition versus another, typically indicated by a significant pre-treatment-measure-by-treatment-condition interaction effect)37. One study38 did examine moderators of differential treatment response to escitalopram and CBT, using resting Positron Emission Tomography. Here, six regions moderated differential response between treatments, with the strongest pattern in the insula: insula hypermetabolism predicted better response to escitalopram and poorer response to CBT, whereas insula hypometabolism predicted the opposite pattern. No study has examined whether measures of reward circuitry activity moderate response to antidepressant medications and/or psychotherapies in MDD.
A second limitation concerns the use of placebo. Placebo response in antidepressant trials is substantial, and there is some evidence that it has increased over time39. Given the cost and side effects of most prescribed antidepressant medications versus placebo40, it is essential to identify markers (i.e., moderators) that predict differential response to active treatment versus placebo in order to identify individuals most likely to benefit from active medications. Yet, few previous neuroimaging-based treatment studies included a control condition of any sort, including placebo.
The current study examined whether the temporal dynamics of activity in reward circuitry moderated response to antidepressant medication versus placebo in MDD, using a well-validated monetary reward task used in studies of MDD28,41. Participants were individuals with MDD randomized to receive sertraline or placebo in EMBARC, who performed the reward task during functional magnetic resonance imaging (fMRI) prior to randomization.
For each participant, a specific reward index was computed to capture the hypothesized temporal dynamics of RE and PE-related VS activity during the task that are predicted from reinforcement learning models and our previous findings28,29. The reward index formula quantifies change in RE and PE-related activity from the 1st to 2nd half of the task with a higher score on this index reflecting a greater increase in neural response to RE and greater decrease in response to PE over the course of the task, associated with conditioning. Our main aim was to determine whether this index of baseline VS activity to reward moderated differential response to sertraline versus placebo, as measured by depressive symptoms at the end of the eight-week treatment course. VS activity is modulated by both serotonin and dopamine15,16. Furthermore, sertraline increases VS serotonin and dopamine levels42. We thus hypothesized that lower pre-treatment scores on the VS reward index, reflecting more abnormal response with respect to reinforcement learning theory in the serotonin- and dopamine-modulated VS, would be associated with better response to sertraline versus placebo after eight weeks of treatment. We also explored whether a reward index computed in other regions important for reward processing28,43, and mean levels of activity in the VS and in regions identified in wholebrain analyses to RE and PE across the entire task, predicted or moderated differential response to sertraline versus placebo.
Methods
Participants
296 unmedicated depressed individuals with MDD were randomized to receive sertraline or placebo in the EMBARC study. A complete list of inclusion/exclusion criteria and description of the EMBARC study design and rationale have been previously reported44.
The flow of participants is displayed in Figure 1 (see Supplementary Figure 1 for the study design). The final sample included 222 (148 females;mean age=36.5,SD=13.1) participants with baseline reward imaging data. The study was approved by the Institutional Review Boards at each of the four recruitment sites, and all participants gave written informed consent.
Figure 1. CONSORT Flow Diagram.
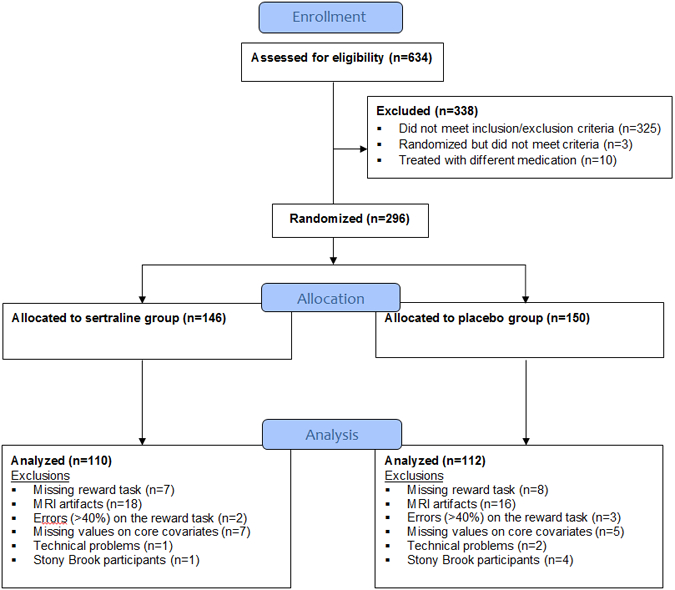
Of the 296 participants randomized, 15 did not complete the reward task, 34 were excluded for severe artifacts in neuroimaging data acquisition (including motion, inhomogeneity, and ghosting), 12 were excluded due to missing values on core baseline covariates, 5 were excluded for large number of omission errors (> 40%) on the reward task, and 3 were excluded for technical problems. Five (n=5) individuals recruited at a separate clinical site (Stony Brook University) were excluded because the small sample size did not allow adequate control for possible site effects. The final sample included n=222.
Reward task
The task41, 45 has been described in detail elsewhere29 and is summarized in Supplementary Information.
Image Acquisition
Neuroimaging data were collected using 3 Tesla magnetic resonance imaging (MRI) scanners at all sites. Imaging parameters and preprocessing procedures are reported in Supplementary Information.
Image Analysis: First-level model
The first-level model included 17 regressors: response (4-second presentations of question mark), anticipation in the 1st half of the task and 2nd half of the task (6-second presentations of arrow), outcome in the 1st half of the task and 2nd half of the task (1 second presentations of the number and feedback arrow), and baseline (3-second presentation of orienting cross). Four additional regressors represented reward expectancy (1st half), reward expectancy (2nd half), prediction error (1st half), and prediction error (2nd half). The reward expectancy regressors, coupled to the anticipation phase, reflected the expected value (EV) of the arrow, set to +0.5 for the up arrow condition (given the 50% chance of winning $1) and to −0.25 for the down arrow condition (given the 50% chance of losing 50 cents). The prediction error regressors, coupled to the outcome, were determined by the difference between the outcome and the EV (i.e., +0.5 for a win following an up arrow, −0.5 for no win following an up arrow, +0.25 for a no loss following a down arrow, −0.25 for a loss following a down arrow). The reward expectancy and prediction error regressors take into account all 24 trials of the task (12 for each half). To model omission errors, we included another regressor for trials in which a participant failed to respond, which lasted 17 seconds from the onset of the guessing phase of the trial (the question mark). This regressor replaced other trial events during this period. In addition, we included six motion regressors from the realignment phase.
Data Analysis
All analyses were intent-to-treat. The primary outcome measure was the 17-item version of the Hamilton Rating Scale for Depression 17-item (HRSD)46, which was assessed at weeks 1, 2, 3, 4, 6 and 8. As in previous analyses of the EMBARC data47, we analyzed HRSD data using multilevel linear models. With these models, growth curves and end-of-treatment (Week-8) depression scores are estimated from a combination of fixed and random effects. To optimally model the pattern of change over time, we examined linear, log-transformed, square-root transformed, and quadratic change trajectories. The best fitting model, determined by the Akaike Information Criteria, was the quadratic representation of time. As such, primary hypotheses focused on model-estimated depression scores at Week-8, and we report differences in the shape of the curvilinear trajectory in Supplementary Information. Intercepts, instantaneous slopes, and quadratic effects were included as random effects, and an unstructured covariance matrix was estimated to model the correlation among these effects. Full maximum likelihood estimation was used for all models, and degrees of freedom for hypothesis tests were estimated with the Kenward-Roger approximation48. Our analytic approach simultaneously examined variables as potential general predictors of symptoms at Week-8 (evidenced by a significant association with estimated Week-8 depression scores, irrespective of treatment assignment) and as potential moderators of differential symptom reduction between sertraline and placebo (evidenced by a significant interaction between the variable and treatment group assignment on estimated Week-8 depression scores)49. Analyses were conducted with SAS Version 9.4 PROC MIXED (SAS Institute, Cary, NC).
Predictors and moderators of interest
The main variable of interest was a reward index that measured temporal change in RE and PE-related VS activity during the task. Higher scores on this index reflected a pattern of greater increase in neural response to RE and greater decrease in response to PE over the course of the task - the pattern predicted by reinforcement learning theory. We calculated a separate reward index for the right and left VS using the formula: [RE-related VS activity (2nd half of task) - RE-related VS activity (1st half of task)] + [PE-related VS activity (1st half) - PE-related VS activity (2nd half)]. We examined prediction effects of right and left VS activity related to this index on estimated Week-8 depression scores across sertraline and placebo groups, as well as reward-index-by-group interactions (i.e., moderator effects). We also examined prediction and moderation effects of right and left VS activity related to each separate sub-index (i.e., the RE and PE change components) of the reward index (see Supplementary Figure 2 for distribution histograms for the VS reward index and sub-indices). We used False Discovery Rate corrections50–52 to control for multiple comparisons for all primary statistical analyses, correcting for a total of 20 tests (10 prediction effects and 10 moderation effects). Variability across sites was evaluated by examining whether the primary statistic of interest, the treatment-by-reward-index interaction, was itself significantly moderated by site and if it (the treatment-by-reward-index interaction) remained significant averaging across any observed site differences.
Secondary analyses examined prediction and moderation effects of the reward index (and RE and PE sub-indices) in additional regions important for reward processing43,28 and of mean activity (i.e., across the 1st and 2nd half of the task) to RE and PE in the right and left VS, and in regions that emerged in exploratory whole brain (family-wise error correction p<0.05) analyses of mean activity to RE and PE (Supplementary Information).
In addition, we conducted exploratory logistic regression analyses of categorical response at Week-8 with reward indices × group interactions, and covariates as above (Supplementary Information).
Baseline demographic/clinical measure covariates
The following baseline measures were used as covariates, as in previous EMBARC studies47: 1) Randomization group (sertraline, placebo), 2) Site, 3) Race (Caucasian, non-Caucasian), 4) Sex, 5) Age, 6) Employment status, 7) Education (years), 8) Marital status, 9) Chronicity, 10) Anhedonia (The Snaith–Hamilton Pleasure Scale)53, 11) Anxiety severity (The Mood and Anxiety Symptom Questionnaire Anxious Arousal Scale)54, and 12) Baseline HRSD.
Results
Demographic/Clinical Measures
Table 1 displays demographic and clinical measures for the sertraline (n=110) and placebo (n=112) groups. On average, participants were in their mid-thirties with a college education and moderate levels of depressive symptoms. Approximately two-thirds were Caucasian, two-thirds were female, the majority were single, and nearly 60% were employed. There were no significant group differences for any measures (all ps>0.05); however, we observed a statistically non-significant trend for a greater proportion of males in the placebo group (p=0.06). We observed no significant differences on any measures between the study sample (n=222) and individuals excluded from analysis (n=74;Supplementary Table 2). Sex was included as a covariate, as noted above.
Table 1.
Demographic and clinical measures for the sertraline and placebo groups.
| SERT (n=110) | PBO (n= 112) | Group comparison | ||||
|---|---|---|---|---|---|---|
| Frequency | Percent | Frequency | Percent | Statistic | P-value | |
| Sex (female/male) | 80/30 | 73%/ 27% | 68/44 | 61%/ 39% | X2(1)=3.6 | p=0.06 |
| Employment (yes/no) | 61/49 | 55%/ 45% | 68/44 | 61%/ 39% | X2(1)=.63 | p=0.43 |
| Marital status (yes/no) | 19/91 | 17%/ 83% | 28/84 | 25%/ 75% | X2(1)=1.99 | p=0.16 |
| Race (Caucasian, non-Caucasian) | 71/39 | 65%/ 35% | 78/34 | 70%/ 30% | X2(1)=.65 | p=0.42 |
| Chronicity (chronic/non-chronic) | 55/55 | 50%/ 50% | 57/55 | 51%/ 49% | X2(1)=.02 | p=0.89 |
| Mean | SD | Mean | SD | |||
| Age | 36.84 | 13.17 | 36.94 | 12.35 | t(220)= −.06 | p=0.95 |
| Education (years) | 14.96 | 2.68 | 15.29 | 2.74 | t(220)= −.93 | p=0.35 |
| HRSD baseline | 18.59 | 4.36 | 18.91 | 4.17 | t(220)= −.56 | p=0.58 |
| SHAPS | 33.65 | 5.17 | 32.75 | 5.64 | t(220)= 1.25 | p=0.21 |
| MASQ-AA | 17.82 | 5.89 | 17.01 | 5.22 | t(220)= 1.08 | p=0.28 |
HRSD = Hamilton Rating Scale for Depression; SHAPS= Snaith–Hamilton Pleasure Scale (the four response categories were coded as separate scores (ranging from 0 to 3); MASQ-AA= Mood and Anxiety Symptom Questionnaire Anxious Arousal Scale
Effects of right and left VS reward indices on Week-8 depression scores
We observed no evidence of a general predictor effect of VS reward index on Week-8 depression scores (F(1,198)=0.31,p=0.58,right VS;F(1,193)=0.53,p=0.47,left VS;Supplementary Table 3, Supplementary Table 4). We did observe that both the right (F(1,198)=9.67,p=0.002) and the left (F(1,193)=12.93,p=0.0004) VS reward index moderated treatment effects: lower reward index values were associated with lower estimated Week-8 scores in the sertraline versus placebo groups (Figure 2). The moderation effect of the reward index on the left did not differ significantly across the four sites, and it remained significant on average across any observed site differences (Supplementary Information). The same was not true of the reward index on the right (Supplementary Information) and will not be discussed further. On the left, the difference between sertraline and placebo was estimated to cross the National Institute for Clinical Excellence55 threshold for a clinically significant difference (raw HRSD difference≥3 points) at an index level of z=−0.21 (t(193)=2.38,p=0.02,d=0.32,95% CI:0.06–0.58) on the left (Figure 3a). Thus, patients below this threshold are expected to have a superior response to sertraline than to placebo, or the nonspecific effects of treatment. The lower a particular patient’s index score is, the larger the expected advantage of the active medication, sertraline. By contrast, patients above the threshold are not expected to have a clinically meaningfully better response to the active ingredients of sertraline compared to the non-specific effects provided by placebo (Figure 3b).
Figure 2. Estimated Depression Scores over Time as a Function of Treatment and the Left Ventral Striatal (VS) Reward Index.
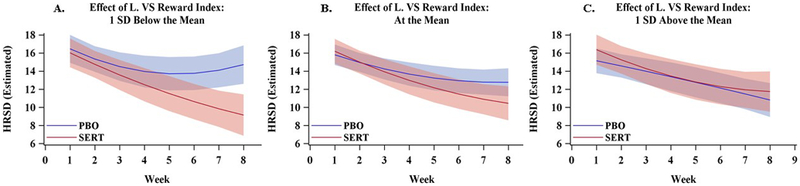
Dark lines represent estimated Hamilton Rating Scale for Depression (HRSD) scores and cones represent 95% confidence intervals. Values were estimated from the primary multilevel statistical model at three levels of the left VS reward index, 1 SD below the mean (Panel A), the mean (Panel B), and 1 SD above the mean (Panel C).
Figure 3. Difference in Estimated Week-8 Depression Scores between the Sertraline and Placebo Groups as a Function of Left Ventral Striatal (VS) Reward Index.
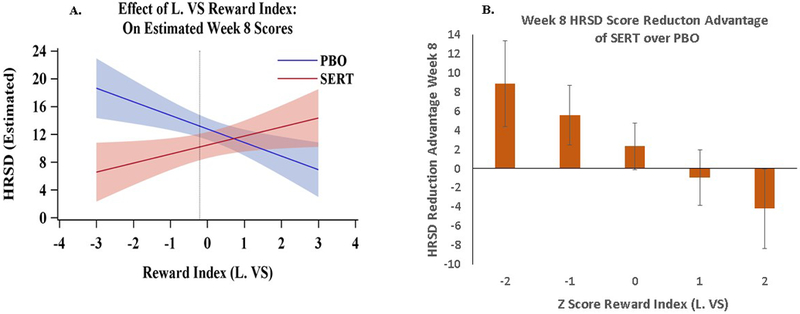
Panel A depicts the estimated Hamilton Rating Scale for Depression (HRSD) scores at Week-8 (y-axis) from across the full range of left VS index scores in the sample (x-axis). The dotted vertical line represents the point below which the sertraline (SERT) and placebo (PBO) difference crosses the NICE threshold for a clinically significant difference (HRSD > 3 points). Individuals below that cutoff are expected to respond better to sertraline than to placebo. Cones represent 95% confidence intervals. Panel B. Represents point estimates for the differences in Week 8 HRSD scores between sertraline and placebo at particular values of the reward index. Error bars represent 95% confidence intervals. This graph indicates, for example, that for a depressed individual with a pre-treatment ventral striatal reward index z score at −2 or below, there will be a likelihood of having an additional 8.8 point reduction in their HRSD score after 8 weeks of taking sertraline relative to those with the same z-score who received placebo.
Effects of the right and left VS reward expectancy (RE) sub-indices on Week-8 depression scores
We observed no evidence of a general predictor effect of VS RE sub-index on Week-8 depression scores (F(1,192)≤0.01,p=0.96,right VS;F(1,192)=0.44,p=0.51,left VS;Supplementary Table 5, Supplementary Table 6), but we observed a significant moderation effect for left VS RE sub-index (F(1,192)=7.68,p=0.006;Figure 4) on estimated Week-8 depression scores and a moderation effect for the right that did not meet false discovery rate-corrected significance (F(1,192)=6.16,p=0.014,FDR threshold:p=0.0139). Focusing on the effect in the left VS, lower values on this sub-index were associated with lower Week-8 depression scores in the sertraline, relative to the placebo, group (Figure 4). As above, the moderation effect of the RE sub-index on the left did not differ significantly across the four sites, and it remained significant on average across any observed site differences (Supplementary Information). The difference between sertraline and placebo was estimated to cross the National Institute for Clinical Excellence55 threshold for a clinically significant difference (raw HRSD difference≥3 points) at an RE sub-index level of z=−0.38 (t(194)=2.31,p=0.02,d=0.31,95% CI:0.05–0.57) on the left (Figure 4).
Figure 4. Estimated Depression Scores as a Function of Treatment and the Left Ventral Striatal (VS) Reward Expectancy (RE) Sub-index.
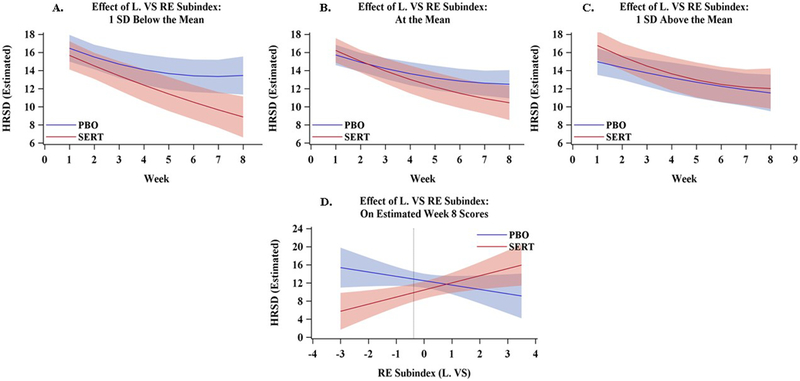
Dark lines represent estimated Hamilton Rating Scale for Depression (HRSD) scores and cones represent 95% confidence intervals. Values were estimated from the primary multilevel statistical model at three levels of the left RE sub-index, 1 SD below the mean (Panel A), the mean (Panel B), and 1 SD above the mean (Panel C). Panel D depicts the estimated HRSD scores at Week-8 (y-axis) from across the full range of left RE sub-index scores in the sample (x-axis). The dotted vertical line represents the point below which the sertraline (SERT) and placebo (PBO) difference crosses the NICE threshold for a clinically significant difference (HRSD ≥ 3 points).
Effects of the right and left VS prediction error (PE) sub-index on Week-8 depression scores
We observed no evidence of either a general predictor or moderation effect of right or left VS PE sub-index on estimated Week-8 depression scores (all Fs≤2.76,all ps>0.098).
Effect of covariates
The moderation effects of the left VS reward index and left VS RE sub-index remained significant when excluding all covariates not significantly associated with our primary outcome of interest, estimated week-8 depression scores, for each index (Supplementary Tables 7-8).
Other regions
We observed no evidence of general predictor or moderation effects of reward index and RE and PE sub-indices related activity on Week-8 depression scores in any other regions of interest except for one predictor effect for the reward index in the right orbitofrontal cortex, which only just crossed the FDR-corrected significance threshold (F(1,199)=6.28,p=0.013,FDR threshold:139; Supplementary Information).
Discussion
This is the first study to show that reward-related neural measures moderate response to an antidepressant relative to placebo in MDD. Specifically, an index of baseline activity in left VS, reflecting the extent to which changes in response to RE and PE during a monetary reward task followed the pattern predicted by reinforcement learning models, moderated response to sertraline versus placebo. Lower reward index values, reflecting more abnormal VS functioning, were associated with better response, i.e., lower depressive symptoms at Week-8, in the sertraline versus placebo group. Moreover, this effect was robust to different combinations of demographic and clinical covariates in statistical models.
Reward neural circuitry is well-delineated, modulated by dopamine and serotonin15,16,17,18, as highlighted in the introduction, and associated with functional abnormalities in MDD19. Additionally, sertraline increases extracellular dopamine concentrations in the striatum42. While SSRIs may diminish dopaminergic activity, at least in the ventral tegmental area, through the inhibitory actions resulting from activation of serotonin receptors56, sertraline may overcome these inhibitory effects on dopamine signaling, due to its greater affinity for dopamine transporters than other SSRIs57. Thus, abnormal patterns of bilateral RE- and PE-related VS activity in MDD may reflect abnormally low pre-treatment serotonin and dopamine modulation of reward circuitry. Individuals showing greater pre-treatment magnitude of abnormalities in these regions during reward processing, specifically an absence of increases in RE-related and decreases in PE-related activity over time, may show better response to sertraline than placebo because of ameliorating effects of such medication on these neural abnormalities via changes in serotonin and dopamine levels in the VS. Moderator effects of reward index-related activity were specific to the VS and not observed in other regions implicated in reward processing. These findings suggest that abnormalities in pre-treatment serotonin and dopamine modulation may impact the VS in particular, and highlight the VS as a key region in which abnormal reward index-related activity may be a useful clinical moderator of differential treatment response.
Further analyses revealed that the left VS RE sub-index, with the right VS RE sub-index just missing the corrected significance threshold, but not the VS PE sub-indices, was a significant moderator of differential response to sertraline versus placebo. The VS RE sub-index in particular may reflect the extent of reward learning (i.e. conditioning) over the course of the task, as VS activity shifts to the expectancy rather than the outcome phase of the trial. For this reason, the VS RE sub-index may be a better moderator of response than the VS PE sub-index. Thus, abnormal serotonergic and dopaminergic modulation of VS activity may manifest as a failure to show an increase in RE-related VS activity over time, which sertraline may help ameliorate.
The inclusion of a placebo comparison was a critical feature of the study design because it allowed identification of neural marker moderators of differential response to a specific active medication, sertraline, versus placebo. Such markers are important because they point to potential neural mechanisms targeted by the medication, rather than mechanisms associated with nonspecific response to treatment in general. We hypothesized that a critical mechanism of action of sertraline may be to normalize reward circuitry activity for those depressed individuals who show serotonergically and dopaminergically-modulated abnormalities in the functioning of this circuitry at baseline. Future work can test this hypothesis directly, but, if confirmed, such findings would identify targets for guiding and potentially monitoring personalized treatments. Given that active treatments for depression are on average only modestly more efficacious than placebo58, there is a critical need to identify such targets so that antidepressant treatments can be more effectively and efficiently prescribed to maximize response in individuals with MDD.
We excluded 74 of the 296 participants randomized. Data loss in this range is common in neuroimaging studies of acutely depressed individuals38. Importantly, we did not observe any differences between included and excluded groups on demographic or clinical measures. Only pre-treatment neural measures were examined. Although the within-session reward index used in the current study was formed on the basis of theoretical predictions and on observations in healthy individuals across separate scans, more work should validate and refine this measure to capture the precise dynamics of normative reward-related responding over time. Although the direction of the effect of interest was similar in both hemispheres, it was more robust in the left hemisphere. This may reflect the left hemisphere’s role in encoding approach-related emotions59, such as reward, but can be a focus of future studies. Finally, whereas inclusion of a placebo condition is a strength, there was no active treatment comparator. A different treatment may be even more effective than sertraline for individuals showing abnormal reward-related neural function.
In summary, we observed a moderation effect of reward-related left VS activity on antidepressant response. Specifically, a more abnormal pre-treatment pattern of dynamic VS response to RE and PE, likely reflecting underlying deficits in serotonergic and dopaminergic neurotransmission, was associated with better response to sertraline versus placebo. These findings suggest that pre-treatment measures of individual-level reward-related neural activity, especially within the VS, have potential to serve as objective, neural markers to advance efforts to personalize interventions by guiding individual-level choice of antidepressant treatment.
Supplementary Material
Acknowledgements:
Research reported in this publication was supported by the National Institute of Mental Health of the National Institutes of Health under award numbers U01MH092221 (Trivedi, M.H.) and U01MH092250 (McGrath, P.J., Parsey, R.V., Weissman, M.M.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was supported by the EMBARC National Coordinating Center at UT Southwestern Medical Center and the Data Center at Columbia University and Stony Brook University. Valeant Pharmaceuticals donated the Wellbutrin XL used in the study.
Footnotes
Conflict of Interest Disclosures:
The authors declare no conflict of interest. Financial disclosures are included below: Dr. Deckersbach has received research funding from the Depressive and Bipolar Disorder Alternative Treatment study, the International OCD Foundation, NARSAD, NIMH, TSA, and Tufts University; he has received honoraria, consultation fees, and/or royalties from Boston University, BrainCells, Catalan Agency for Health Technology Assessment and Research, Clintara, LLC, the Massachusetts General Hospital Psychiatry Academy, the Massachusetts Medical Society, the National Association of Social Workers-Massachusetts, the National Institute on Drug Abuse, NIMH, Oxford University Press, Systems Research and Applications Corporation, and Tufts University; and he has participated in research funded by the Agency for Healthcare Research and Quality, Cyberonics, Forest Research Institute, Janssen Pharmaceuticals, Medtronic, NIA, NIH, Northstar, Shire Development, and Takeda. Dr. Carmody received an honorarium from the University of Texas at San Antonio. Dr. Kurian has received research funding/grants from Evotec, Forest Pharmaceuticals, Johnson and Johnson, Naurex, NIMH, Pfizer, Rexahn, and Targacept. Dr. McInnis received funding from the NIMH and consulting fees from Janssen and Otsuka Pharmaceuticals. Dr. Oquendo receives royalties from Research Foundation of Mental Hygiene for the commercial use of the Columbia-Suicide Severity Rating Scale. Her family owns stock in Bristol Myers Squibb. Dr. Fava reports lifetime disclosures of research support from Abbott Laboratories, Acadia Pharmaceuticals, Research Original Investigation Rostral Anterior Cingulate Cortex Activity in Relation to Symptom Improvement in Depression E6 JAMA Psychiatry Published online April 11, 2018 (Reprinted) jamapsychiatry.com © 2018 American Medical Association. All rights reserved. Downloaded From: on 04/17/2018 Alkermes Inc, American Cyanamid, Aspect Medical Systems, AstraZeneca, Avanir Pharmaceuticals, AXSOME Therapeutics, Biohaven, BioResearch, BrainCells Inc, Bristol-Myers Squibb, CeNeRx BioPharma, Cephalon, Cerecor, Clintara LLC, Covance, Covidien, Eli Lilly and Company, EnVivo Pharmaceuticals Inc, Euthymics Bioscience Inc, Forest Pharmaceuticals Inc, FORUM Pharmaceuticals, Ganeden Biotech Inc, GlaxoSmithKline, Harvard Clinical Research Institute, Hoffman-LaRoche, Icon Clinical Research, i3 Innovus/Ingenix, Janssen R&D LLC, Jed Foundation, Johnson & Johnson Pharmaceutical Research & Development, Lichtwer Pharma GmbH, Lorex Pharmaceuticals, Lundbeck Inc, Marinus Pharmaceuticals, MedAvante,Methylation Sciences Inc, National Alliance for Research on Schizophrenia and Depression (NARSAD), National Center for Complementary and Alternative Medicine, National Coordinating Center for Integrated Medicine, National Institute of Drug Abuse, NIMH, Neuralstem Inc, NeuroRx, Novartis AG, Organon Pharmaceuticals, Otsuka Pharmaceutical Development Inc, PamLab LLC, Pfizer Inc, Pharmacia-Upjohn, Pharmaceutical Research Associates Inc, Pharmavite LLC, PharmoRx Therapeutics, Photothera, Reckitt Benckiser, Roche Pharmaceuticals, RCT Logic LLC (formerly Clinical Trials Solutions LLC), Sanofi-Aventis US LLC, Shire, Solvay Pharmaceuticals Inc, Stanley Medical Research Institute, Synthelabo, Taisho Pharmaceuticals, Takeda Pharmaceuticals, Tal Medical, VistaGen, andWyeth-Ayerst Laboratories; served on an advisory board or as a consultant for Abbott Laboratories, Acadia, Affectis Pharmaceuticals AG, Alkermes Inc, Amarin Pharma Inc, Aspect Medical Systems, AstraZeneca, Auspex Pharmaceuticals, Avanir Pharmaceuticals, AXSOME Therapeutics, Bayer AG, Best Practice Project Management Inc, Biogen, BioMarin Pharmaceuticals Inc, Biovail Corporation, BrainCells Inc, Bristol-Myers Squibb, CeNeRx BioPharma, Cephalon Inc, Cerecor, CNS Response Inc, Compellis Pharmaceuticals, Cypress Pharmaceutical Inc, DiagnoSearch Life Sciences (P) Ltd, Dinippon Sumitomo Pharma Co Inc, Dov Pharmaceuticals Inc, Edgemont Pharmaceuticals Inc, Eisai Inc, Eli Lilly and Company, EnVivo Pharmaceuticals Inc, ePharmaSolutions, EPIX Pharmaceuticals Inc, Euthymics Bioscience Inc, Fabre-Kramer Pharmaceuticals Inc, Forest Pharmaceuticals Inc, Forum Pharmaceuticals, GenOmind LLC, GlaxoSmithKline, Grunenthal GmbH, Indivior, i3 Innovus/Ingenis, Intracellular, Janssen Pharmaceutica, Jazz Pharmaceuticals Inc, Johnson & Johnson Pharmaceutical Research & Development LLC, Knoll Pharmaceuticals Corp, Labopharm Inc, Lorex Pharmaceuticals, Lundbeck Inc, Marinus Pharmaceuticals, MedAvante Inc, Merck & Co Inc, MSI Methylation Sciences Inc, Naurex Inc, Navitor Pharmaceuticals Inc, Nestle Health Sciences, Neuralstem Inc, Neuronetics Inc, NextWave Pharmaceuticals, Novartis AG, Nutrition 21, Orexigen Therapeutics Inc, Organon Pharmaceuticals, Osmotica, Otsuka Pharmaceuticals, Pamlab LLC, Pfizer Inc, PharmaStar, Pharmavite LLC, PharmoRx Therapeutics, Precision Human Biolaboratory, Prexa Pharmaceuticals Inc, Pharmaceutical Product Development, Purdue Pharma, Puretech Ventures, PsychoGenics, Psylin Neurosciences Inc, RCT Logic LLC (formerly Clinical Trials Solutions LLC), Relmada Therapeutics Inc, Rexahn Pharmaceuticals Inc, Ridge Diagnostics Inc, Roche, Sanofi-Aventis US LLC, Sepracor Inc, Servier Laboratories, Schering-Plough Corporation, Shenox Pharmaceuticals, Solvay Pharmaceuticals Inc, Somaxon Pharmaceuticals Inc, Somerset Pharmaceuticals Inc, Sunovion Pharmaceuticals, Supernus Pharmaceuticals Inc, Synthelabo, Taisho Pharmaceuticals, Takeda Pharmaceutical Company Limited, Tal Medical Inc, Tetragenex, Teva Pharmaceuticals, TransForm Pharmaceuticals Inc, Transcept Pharmaceuticals Inc, Usona Institute Inc, Vanda Pharmaceuticals Inc, Versant Venture Management LLC, and VistaGen; has received compensation for speaking or publishing from Adamed Co, Advanced Meeting Partners, American Psychiatric Association, American Society of Clinical Psychopharmacology, AstraZeneca, Belvoir Media Group, Boehringer Ingelheim GmbH, Bristol-Myers Squibb, Cephalon Inc, CME Institute/Physicians Postgraduate Press Inc, Eli Lilly and Company, Forest Pharmaceuticals Inc, GlaxoSmithKline, Imedex LLC, MGH Psychiatry Academy/Primedia, MGH Psychiatry Academy/Reed Elsevier, Novartis AG, Organon Pharmaceuticals, Pfizer Inc, PharmaStar, United BioSource Corp, andWyeth-Ayerst Laboratories; has equity holdings in Compellis and PsyBrain Inc and hold patents for Sequential Parallel Comparison Design, licensed by MGH to Pharmaceutical Product Development LLC (US_7840419, US_7647235, US_7983936, US_8145504, and US_8145505). Dr. Weissman received funding from the NIMH, NARSAD, the Sackler Foundation, and the Templeton Foundation and royalties from the Oxford University Press, Perseus Press, the American Psychiatric Association Press, and MultiHealth Systems. Dr. Trivedi has consulted for or served on the advisory board of AcademyHealth, Alkeremes Inc., Akili Interactive, Allergan Pharmaceuticals, Arcadia Pharmaceuticals, Avanir Pharmaceuticals, Johnson & Johnson Pharmaceutical Research & Development, Lundbeck Research USA, Medscape, Merck & Co. Inc, Otsuka America Pharmaceutical Inc., One Carbon Therapeutics, Takeda Global Research; and he has received grants from Agency for Healthcare Research and Quality (AHRQ), Cancer Prevention and Research Institute of Texas (CPRIT), National Institute of Mental Health (NIMH), National Institute of Drug Abuse (NIDA), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Center for Advancing Translational Sciences (NCATS), Johnson & Johnson, Patient-Centered Outcomes Research Institute (PCORI). All other authors report no financial relationships with commercial interests.
Supplementary information is available at MP’s website.
References
- 1.Organization WH. Global Health Estimates 2015: Disease burden by Cause, Age, Sex, by Country and by Region, 2000–2015. Geneva, Switzerland: 2016. [Google Scholar]
- 2.Trivedi MH, Rush AJ, Wisniewski SR, Nierenberg AA, Warden D, Ritz L et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. The American journal of psychiatry 2006; 163(1): 28–40. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg PE, Fournier AA, Sisitsky T, Pike CT, Kessler RC. The economic burden of adults with major depressive disorder in the United States (2005 and 2010). The Journal of clinical psychiatry 2015; 76(2): 155–162. [DOI] [PubMed] [Google Scholar]
- 4.Fournier JC, DeRubeis RJ, Shelton RC, Hollon SD, Amsterdam JD, Gallop R. Prediction of Response to Medication and Cognitive Therapy in the Treatment of Moderate to Severe Depression. Journal of consulting and clinical psychology 2009; 77(4): 775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2010; 35(1): 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron 2008; 58(2): 284–294. [DOI] [PubMed] [Google Scholar]
- 7.Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. The Journal of neuroscience : the official journal of the Society for Neuroscience 2001; 21(16): Rc159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grabenhorst F, Rolls ET. Value, pleasure and choice in the ventral prefrontal cortex. Trends in cognitive sciences 2011; 15(2): 56–67. [DOI] [PubMed] [Google Scholar]
- 9.McDannald MA, Takahashi YK, Lopatina N, Pietras BW, Jones JL, Schoenbaum G. Model-based learning and the contribution of the orbitofrontal cortex to the model-free world. The European journal of neuroscience 2012; 35(7): 991–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walton ME, Behrens TE, Noonan MP, Rushworth MF. Giving credit where credit is due: orbitofrontal cortex and valuation in an uncertain world. Annals of the New York Academy of Sciences 2011; 1239: 14–24. [DOI] [PubMed] [Google Scholar]
- 11.Boorman ED, Rajendran VG, O’Reilly JX, Behrens TE. Two Anatomically and Computationally Distinct Learning Signals Predict Changes to Stimulus-Outcome Associations in Hippocampus. Neuron 2016; 89(6): 1343–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee SW, O’Doherty JP, Shimojo S. Neural Computations Mediating One-Shot Learning in the Human Brain. PLOS Biology 2015; 13(4): e1002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bush G, Vogt BA, Holmes J, Dale AM, Greve D, Jenike MA et al. Dorsal anterior cingulate cortex: A role in reward-based decision making. Proceedings of the National Academy of Sciences of the United States of America 2002; 99(1): 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rushworth MF, Noonan MP, Boorman ED, Walton ME, Behrens TE. Frontal cortex and reward-guided learning and decision-making. Neuron 2011; 70(6): 1054–1069. [DOI] [PubMed] [Google Scholar]
- 15.Bressan RA, Crippa JA. The role of dopamine in reward and pleasure behaviour – review of data from preclinical research. Acta Psychiatrica Scandinavica 2005; 111(s427): 14–21. [DOI] [PubMed] [Google Scholar]
- 16.Kranz GS, Kasper S, Lanzenberger R. Reward and the serotonergic system. Neuroscience 2010; 166(4): 1023–1035. [DOI] [PubMed] [Google Scholar]
- 17.Dolen G, Darvishzadeh A, Huang KW, Malenka RC. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature 2013; 501(7466): 179–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Z, Zhou J, Li Y, Hu F, Lu Y, Ma M et al. Dorsal raphe neurons signal reward through 5-HT and glutamate. Neuron 2014; 81(6): 1360–1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russo SJ, Nestler EJ. The Brain Reward Circuitry in Mood Disorders. Nature reviews Neuroscience 2013; 14(9): 10.1038/nrn3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keren H, O’Callaghan G, Vidal-Ribas P, Buzzell GA, Brotman MA, Leibenluft E et al. Reward Processing in Depression: A Conceptual and Meta-Analytic Review Across fMRI and EEG Studies. The American journal of psychiatry 2018: appiajp201817101124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J et al. Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. The American journal of psychiatry 2006; 163(10): 1784–1790. [DOI] [PubMed] [Google Scholar]
- 22.Murrough JW, Abdallah CG, Anticevic A, Collins KA, Geha P, Averill LA et al. Reduced global functional connectivity of the medial prefrontal cortex in major depressive disorder. Human brain mapping 2016; 37(9): 3214–3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gradin VB, Kumar P, Waiter G, Ahearn T, Stickle C, Milders M et al. Expected value and prediction error abnormalities in depression and schizophrenia. Brain : a journal of neurology 2011; 134(Pt 6): 1751–1764. [DOI] [PubMed] [Google Scholar]
- 24.Kumar P, Waiter G, Ahearn T, Milders M, Reid I, Steele JD. Abnormal temporal difference reward-learning signals in major depression. Brain : a journal of neurology 2008; 131(Pt 8): 2084–2093. [DOI] [PubMed] [Google Scholar]
- 25.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science (New York, NY) 1997; 275(5306): 1593–1599. [DOI] [PubMed] [Google Scholar]
- 26.O’Doherty JP, Dayan P, Friston K, Critchley H, Dolan RJ. Temporal difference models and reward-related learning in the human brain. Neuron 2003; 38(2): 329–337. [DOI] [PubMed] [Google Scholar]
- 27.Montague PR, Dayan P, Sejnowski TJ. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. The Journal of neuroscience : the official journal of the Society for Neuroscience 1996; 16(5): 1936–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chase HW, Nusslock R, Almeida JR, Forbes EE, LaBarbara EJ, Phillips ML. Dissociable patterns of abnormal frontal cortical activation during anticipation of an uncertain reward or loss in bipolar versus major depression. Bipolar disorders 2013; 15(8): 839–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Greenberg T, Chase HW, Almeida JR, Stiffler R, Zevallos CR, Aslam HA et al. Moderation of the Relationship Between Reward Expectancy and Prediction Error-Related Ventral Striatal Reactivity by Anhedonia in Unmedicated Major Depressive Disorder: Findings From the EMBARC Study. The American journal of psychiatry 2015; 172(9): 881–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chase HW, Fournier JC, Greenberg T, Almeida JR, Stiffler R, Zevallos CR et al. Accounting for Dynamic Fluctuations across Time when Examining fMRI Test-Retest Reliability: Analysis of a Reward Paradigm in the EMBARC Study. PloS one 2015; 10(5): e0126326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lener MS, Iosifescu DV. In pursuit of neuroimaging biomarkers to guide treatment selection in major depressive disorder: a review of the literature. Annals of the New York Academy of Sciences 2015; 1344: 50–65. [DOI] [PubMed] [Google Scholar]
- 32.Fonseka TM, MacQueen GM, Kennedy SH. Neuroimaging biomarkers as predictors of treatment outcome in Major Depressive Disorder. J Affect Disord 2017. [DOI] [PubMed] [Google Scholar]
- 33.Pizzagalli DA. Frontocingulate dysfunction in depression: toward biomarkers of treatment response. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2011; 36(1): 183–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forbes EE, Olino TM, Ryan ND, Birmaher B, Axelson D, Moyles DL et al. Reward-Related Brain Function as a Predictor of Treatment Response in Adolescents with Major Depressive Disorder. Cognitive, affective & behavioral neuroscience 2010; 10(1): 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walsh E, Carl H, Eisenlohr-Moul T, Minkel J, Crowther A, Moore T et al. Attenuation of Frontostriatal Connectivity During Reward Processing Predicts Response to Psychotherapy in Major Depressive Disorder. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 2017; 42(4): 831–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carl H, Walsh E, Eisenlohr-Moul T, Minkel J, Crowther A, Moore T et al. Sustained anterior cingulate cortex activation during reward processing predicts response to psychotherapy in major depressive disorder. J Affect Disord 2016; 203: 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Phillips ML, Chase HW, Sheline YI, Etkin A, Almeida JRC, Deckersbach T et al. Identifying Predictors, Moderators, and Mediators of Antidepressant Response in Major Depressive Disorder: Neuroimaging Approaches. The American journal of psychiatry 2015; 172(2): 124–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGrath CL, Kelley ME, Holtzheimer PE, Dunlop BW, Craighead WE, Franco AR et al. Toward a neuroimaging treatment selection biomarker for major depressive disorder. JAMA psychiatry 2013; 70(8): 821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walsh BT, Seidman SN, Sysko R, Gould M. Placebo response in studies of major depression: variable, substantial, and growing. Jama 2002; 287(14): 1840–1847. [DOI] [PubMed] [Google Scholar]
- 40.Anderson HD, Pace WD, Libby AM, West DR, Valuck RJ. Rates of 5 common antidepressant side effects among new adult and adolescent cases of depression: a retrospective US claims study. Clinical therapeutics 2012; 34(1): 113–123. [DOI] [PubMed] [Google Scholar]
- 41.Forbes EE, Hariri AR, Martin SL, Silk JS, Moyles DL, Fisher PM et al. Altered striatal activation predicting real-world positive affect in adolescent major depressive disorder. The American journal of psychiatry 2009; 166(1): 64–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kitaichi Y, Inoue T, Nakagawa S, Boku S, Kakuta A, Izumi T et al. Sertraline increases extracellular levels not only of serotonin, but also of dopamine in the nucleus accumbens and striatum of rats. European Journal of Pharmacology 2010; 647(1): 90–96. [DOI] [PubMed] [Google Scholar]
- 43.Garrison J, Erdeniz B, Done J. Prediction error in reinforcement learning: a meta-analysis of neuroimaging studies. Neuroscience and biobehavioral reviews 2013; 37(7): 1297–1310. [DOI] [PubMed] [Google Scholar]
- 44.Trivedi MH, McGrath PJ, Fava M, Parsey RV, Kurian BT, Phillips ML et al. Establishing moderators and biosignatures of antidepressant response in clinical care (EMBARC): Rationale and design. Journal of psychiatric research 2016; 78: 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of neurophysiology 2000; 84(6): 3072–3077. [DOI] [PubMed] [Google Scholar]
- 46.Hamilton M A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry 1960; 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pizzagalli DA, Webb CA, Dillon DG, et al. Pretreatment rostral anterior cingulate cortex theta activity in relation to symptom improvement in depression: A randomized clinical trial. JAMA psychiatry 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kenward MG, Roger JH. Small Sample Inference for Fixed Effects from Restricted Maximum Likelihood. Biometrics 1997; 53(3): 983–997. [PubMed] [Google Scholar]
- 49.Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Archives of general psychiatry 2002; 59(10): 877–883. [DOI] [PubMed] [Google Scholar]
- 50.Benjamini Y, Yekutieli D. The control of the false discovery rate in multiple testing under dependency. Ann Statist 2001; 29(4): 1165–1188. [Google Scholar]
- 51.Narum SR. Beyond Bonferroni: less conservative analyses for conservation genetics. Conservation Genetics 2006; 7(5): 811–811. [Google Scholar]
- 52.Shaffer J Multiple Hypothesis Testing. Annual Review of Psychology 1995; 46(1): 561–584. [Google Scholar]
- 53.Snaith RP, Hamilton M, Morley S, Humayan A, Hargreaves D, Trigwell P. A scale for the assessment of hedonic tone the Snaith-Hamilton Pleasure Scale. The British journal of psychiatry : the journal of mental science 1995; 167(1): 99–103. [DOI] [PubMed] [Google Scholar]
- 54.Watson D, Clark LA. The Mood and Anxiety Symptom Questionnaire University of Iowa: Iowa City, Iowa, 1991. [Google Scholar]
- 55.Excellence NIfC. Depression: Management of Depression in Primary and Secondary Care. National Institute for Clinical Excellence: London, England, 2004. [Google Scholar]
- 56.Dremencov E, El Mansari M, Blier P. Effects of sustained serotonin reuptake inhibition on the firing of dopamine neurons in the rat ventral tegmental area. Journal of psychiatry & neuroscience : JPN 2009; 34(3): 223–229. [PMC free article] [PubMed] [Google Scholar]
- 57.Goodnick PJ, Goldstein BJ. Selective serotonin reuptake inhibitors in affective disorders--I. Basic pharmacology. Journal of psychopharmacology (Oxford, England) 1998; 12(3 Suppl B): S5–20. [DOI] [PubMed] [Google Scholar]
- 58.Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. The Lancet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pizzagalli DA, Sherwood RJ, Henriques JB, Davidson RJ. Frontal brain asymmetry and reward responsiveness: a source-localization study. Psychological science 2005; 16(10): 805–813. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


