Abstract
Intestinal Na+-nutrient cotransport depends on claudin-2 and claudin-15 mediated Na+ recycling. Expression of these proteins is coordinately regulated during postnatal development. While expression of claudin-2 and claudin-15 has been studied in inflammatory bowel disease (IBD) and celiac disease (CD), it has not been assessed in other malabsorptive diseases, and no reports have compared expression in children and adults. We used quantitative immunofluorescence microscopy to assess claudin-2 and claudin-15 expression in duodenal biopsies from children and adults with malabsorptive disease and healthy controls. Consistent with previous work in rodents, claudin-2 expression in healthy children was markedly greater, and claudin-15 expression was less, than that in adults. Claudin-2 expression was increased in adults with CD and downregulated in children with graft-versus-host disease (GVHD). In contrast, claudin-15 expression was reduced in adults with GVHD and common variable immunodeficiency (CVID). These data show that one of the two Na+/water pore-forming claudins is upregulated in CD and downregulated in GVHD and CVID. The specific claudin whose expression changes, however, reflects the age of the patient (child or adult). We conclude that contributions of claudin-2 and claudin-15 to pathophysiology of and responses to diarrhea in children and adults with GVHD and CVID differ from those in CD and IBD.
Keywords: celiac disease, graft versus host disease, common variable immunodeficiency, diarrhea, intestinal permeability, intestinal barrier
Introduction
The tight junction proteins claudin-2 and claudin-15 have been identified as essential regulators of paracellular Na+ flux and transcellular nutrient absorption.1, 2 Tsukita and colleagues have shown that knockout of claudin-2 alone does not have a marked effect on intestinal transport,1, 3 while knockout of claudin-15 results in marked hypertrophy of the intestine.4 One might therefore hypothesize that knockout of both claudin-2 and claudin-15 would result in a phenotype similar to claudin-15 knockout. However, double knockout mice died of within three weeks of birth as a result of inadequate Na+-dependent nutrient absorption, which drives the absorption of most nutrients.2, 5, 6 This was due to reduced Na+ within the gut lumen rather than loss of Na+-nutrient cotransporters. Malabsorptive death follows because normal diets do not contain sufficient Na+ to drive absorption. It is therefore essential that Na+ be recycled from the lamina propria back to the gut lumen in order to support additional rounds of transcellular transport. Paracellular efflux of Na+ from the lamina propria to the lumen is thus required for ongoing nutrient and water absorption. This Na+ recycling is accomplished by paracellular Na+ channels formed from claudin-2 and claudin-15.
Consistent with their essential roles, expression of claudin-2 and claudin-15 is tightly regulated during development in rodents.7 Claudin-2 is highly expressed at birth and is rapidly down regulated thereafter.7 Conversely, at birth claudin-15 is only expressed at low levels, but is markedly upregulated during growth. One may, therefore, imagine that claudin-15 supplants claudin-2 function during postnatal intestinal development. While the low levels of claudin-15 expressed are sufficient to allow claudin-2 knockout mice to survive the neonatal period, the limited claudin-2 expression seen in adults is insufficient to support nutrient absorption in claudin-15 knockout mice.1 The intestinal hypertrophy that occurs in claudin-15 knockout mice can therefore be understood as a physiologic adaptation whereby increases in mucosal surface area compensate for deficient nutrient and water absorption. Finally, the observation that knockout of both claudin-2 and claudin-15 leads to malabsorptive death indicates that these are the only claudin proteins that can adequately support Na+ recycling and nutrient absorption.
Previous studies have demonstrated that claudin-2 expression is upregulated in inflammatory bowel disease (IBD)8, 9 and celiac disease (CD).9–11 Similarly, one study has suggested that claudin-15 is modestly upregulated in CD.10 Claudin-2 and claudin-15 upregulation in these malabsorptive diseases may be a physiologic response representing efforts to increase absorption to compensate for reduced mucosal surface area. We therefore hypothesized that claudin-2 and claudin-15 expression might be increased in other malabsorptive diseases of the small intestine.
To better understand the pathophysiology of malabsorption and barrier dysfunction in disease, we assessed claudin-2 and claudin-15 expression in three diarrheal diseases. The first two, CD12 and graft-versus-host disease (GVHD),13–15 are characterized by increased intestinal permeability as well as malabsorptive diarrhea. The third, common variable immunodeficiency (CVID) is less well-defined and has not been studied in terms of intestinal permeability or tight junction protein expression.
CD is an immune-mediated disorder16–18 in which dietary gluten-derived peptides trigger epithelial stress, immune activation, and T cell-mediated epithelial damage in susceptible individuals.19–21 The latter reduces epithelial survival and results in accelerated epithelial turnover that leads to villus atrophy, reduced and absorptive surface area, and loss of fully mature enterocytes, as their shortened survival does not permit complete differentiation.17, 18
GVHD occurs as a complication of hematopoietic stem cell (bone marrow) transplantation.22, 23 Similar to CD, GVHD patients suffer from malabsorptive diarrhea24 which, in many cases, is associated with villous atrophy.24 The hallmark feature of graft-versus-host disease is, however, crypt epithelial cell apoptosis due to direct attack by CD8+ T cells.24, 25 As with CD, diarrhea in GVHD is thought to reflect loss of both absorptive surface area and mature enterocytes. Despite these similarities, pathogenesis of CD and GVHD are quite different from one another.
CVID is one of the most common primary immunodeficiencies, with a prevalence of 1 case per 10,000 to 250,000 individuals, and represents a generalized defect in immunoglobulin synthesis.26 While the majority of cases are sporadic, a variety of genetic defects, primarily within the immune system, have been identified in familial CVID, which comprises ~10% of patients. CVID typically presents in the third and fourth decades of life, and ~25% of patients suffer from autoimmune disease. The histologic features of small bowel disease in CVID vary and can overlap significantly with both CD and GVHD.27 While many patients present with diarrhea and malabsorptive disease, the mechanisms are not well defined.
The aim of this study was to assess expression of the Na+/water pore-forming claudins, claudin-2 and claudin-15, in malabsorptive disease. We used duodenal biopsies from patients with these diagnoses and age-matched healthy controls to assess intestinal epithelial claudin-2 and claudin-15 expression in adults and children.
Materials and Methods
Ethics statement and tissue collection
Institutional approval was granted by the University of Chicago and Brigham and Women’s Hospital Institutional Review Boards. Data from this study are not available in a public repository but will be made available upon request. Paraffin embedded duodenal biopsies were obtained from pathology department archives. A computer search identified children and adults with diagnoses of normal (within normal limits), CD, GVHD, and CVID (adults only). After examination of clinical records, pathology reports, and haematoxylin and eosin- (H&E) stained slides, cases were arranged in high density tissue microarrays using an ATA-27 automated tissue arrayer (Beecher Instruments).
Immunohistochemical staining
Claudin-2 and claudin-15 immunostains were performed on 5 μm sections of formalin-fixed paraffin embedded duodenal biopsies. Slides were baked overnight at 60°C, washed thrice in xylene, transferred to 100% ethanol, and rehydrated through graded ethanol solutions. Antigen retrieval was performed for 15 minutes in a pressure cooker using Dako Target Retrieval Solutions S1699 and S2367 (Agilent Technologies, Santa Clara, CA). After cooling on ice followed by a wash in PBS, tissues were photobleached (under bright light) in 4.5% hydrogen peroxide, 0.005 N NaOH in PBS. The solution was replenished after 1 hour for a total photobleaching time of 2 hours. After a brief wash in PBS, slides were incubated in blocking buffer (DAKO X090930) for 10 minutes. Primary antibodies were prepared in antibody diluent (DAKO SB302283) and applied overnight at room temperature. Primary antibodies were. rabbit polyclonal anti-claudin-15, 0.25 μg/mL (Invitrogen 38–9200; lot# QJ228299); rabbit polyclonal anti-claudin-2, 0.25 μg/mL (Atlas Antibodies HPA05154; lot# R61400); mouse monoclonal anti-E-cadherin, 0.83 μg/mL (Abcam ab76055, clone M168, lot# GR317373–12). After three washes in PBS, secondary antibodies were diluted as above with 10 μg/ml Hoechst 33342 (Thermo Fisher Scientific) for 1 hour. Secondary antibodies were. AlexaFluor 594 AffiniPure donkey anti-rabbit IgG, 1.5 μg/ml (Jackson ImmunoResearch 711–586-152; lot# 136184) and AlexaFluor 488 AffiniPure donkey anti-mouse IgG, 1.5 μg/ml (Jackson ImmunoResearch 715–545-151; lot# 118237) and Hoescht (as above) were applied for 1 hour. The slides were then washed three times in PBS, dipped in water and mounted using ProLong Diamond antifade (Thermo Fisher Scientific).
Quantification of claudin 2 and 15 staining intensities
Claudin staining intensity was scored in crypt regions using Metamorph 7.8. From each tissue biopsy, a blinded observer selected three representative crypt regions on the basis of orientation, representative histology, image quality, and staining typical of the biopsy. Regions-of-interest were drawn to encompass approximately 2 μm of intracellular and extracellular space on either side of the apical membrane. After applying a unique threshold to exclude non-junctional staining, the mean intensity was calculated for each region. The average of the three measurements, less the threshold, was used as a single intensity score for each biopsy. These were normalized to the mean intensity of healthy adult biopsies.
Statistical Analysis
All statistical analyses used ANOVA (GraphPad Prism 5.0). Figures show one point for each subject and 95% confidence intervals for the group as a whole.
Results
Subjects and histopathology
We assessed duodenal biopsies from pediatric and adult subjects with normal biopsies, CD, acute GVHD, or CVID (Table I). Pediatric cases were analyzed separately, as studies in mice suggest that claudin-2 and claudin-15 expression would be increased and decreased, respectively, in young children. Those mouse studies focused on comparing mice before and after weaning. We therefore limited our pediatric population to children under 5 years of age.
Table I.
Patient demographics
| Normal | CD | GVHD | CVID | |
|---|---|---|---|---|
| Mean age (range) years n (F, M) | ||||
| Children | 1.2 (1–3) 15 F, 23 M | 17 (1–5) 5 F, 9 M | 2.3 (1–4) 1 F, 5 M | NA |
| Adults | 51 (30–80) 10 F, 5 M | 49 (27–76) 13 F, 3 M | 52 (26–70) 9 F, 8 M | 53 (22–72) 3 F, 7 M |
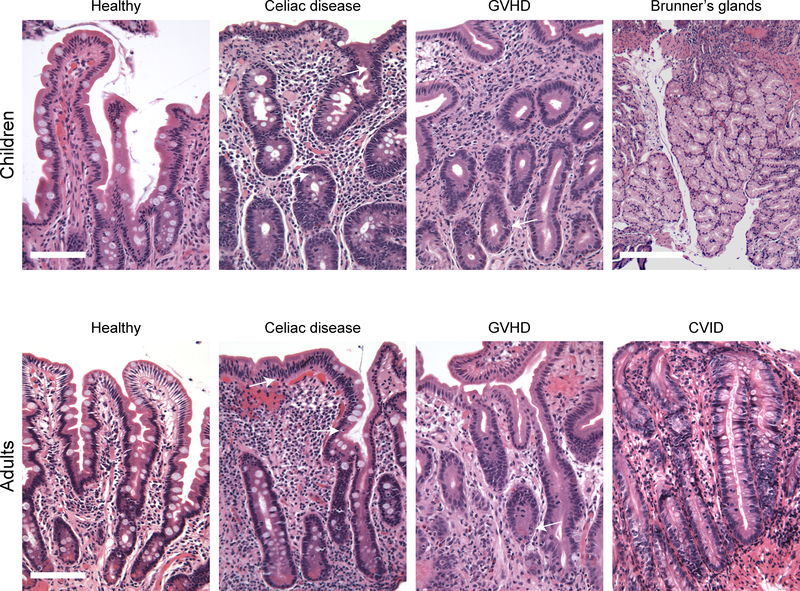
Histopathological analysis of CD patient biopsies showed typical features in both pediatric and adult cases. All patients with CD had either partial or total villus atrophy and were characterized by marked intraepithelial lymphocytosis. Crypt hyperplasia was modest. Dense lamina propria lymphoplasmacytic infiltrates were also present in all CD cases (Figure 1). Apoptotic crypt epithelial cells were not seen.
Figure 1: Duodenal biopsy histology.
Representative H&E images for each subject group are shown. White arrows indicate intraepithelial lymphocytes in CD biopsies and apoptotic crypt epithelial cells in GVHD. Note the absence of plasma cells in CVID. Scale: bar = 100 μm; Brunner’s gland scale bar = 250 μm.
GVHD cases were characterized by partial villus atrophy. The lamina propria was sparsely populated by immune cells and appeared somewhat fibrotic in most cases. Varying numbers of apoptotic crypt epithelial cells were present in all cases and tended to correlate with the degree of crypt hyperplasia, which ranged from mild to marked (Figure 1). Intraepithelial lymphocytes were not prominent in any cases.
The CVID biopsies studied here ranged from nearly normal appearing histology to histopathology similar to advanced CD. The latter included total villous atrophy, moderate crypt hyperplasia, and marked intraepithelial lymphocytosis. These cases tended to have a dense lamina propria lymphocytic infiltrate. Neither plasma cells nor apoptotic bodies were seen in any of the CVID biopsies.
Claudin-2
Claudin-2 expression in adult cases was limited to the crypt region. Although claudin-2 expression could be detected in villous enterocytes of control pediatric biopsies, villi were absent or substantially blunted in the CD and GVHD cases. Our scoring algorithm therefore focused on crypt epithelial cells. Only well-oriented crypts were studied. This allowed quantitative analysis of fluorescent intensity at intercellular junctions, i.e. tight junctions, which were easily recognized as bright punctae (Figure 2).
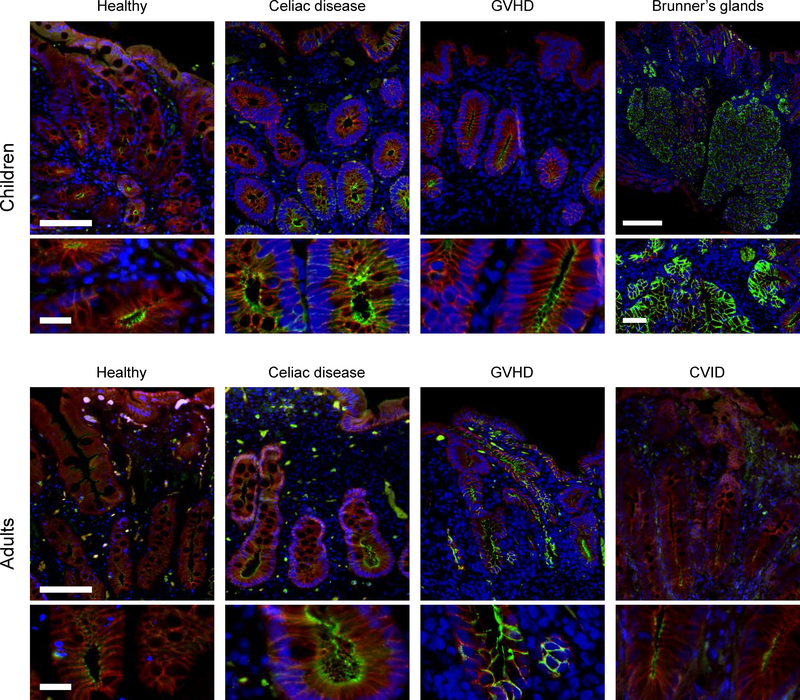
Figure 2: Claudin-2 expression.
Representative claudin-2 immunofluorescence images for each subject group are shown. Immunofluorescence shows claudin-2 (green), E-cadherin (red), and DNA (blue). Note the intense claudin-2 expression within Brunner’s gland epithelia. Scale bar = 100 μm; inset bar = 20 μm. Brunner’s gland scale: bar = 250 μm; inset bar = 50 μm.
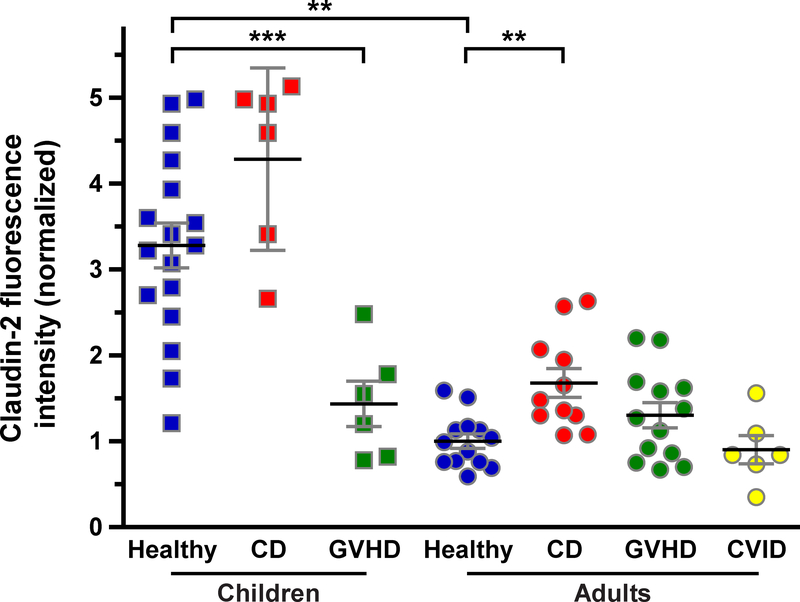
Claudin-2 expression in healthy children was 3.3-fold of that in healthy adults (P<0.005; Figure 3). Moreover, claudin-2 was highly expressed in Brunner’s glands of children and adults. Consistent with a previous report,10 we detected a 68% increase in tight junction-associated claudin-2 within duodenal biopsies of adult CD patients (P<0.005; Figure 3). We did not, however, detect any change in tight junction-associated claudin-2 expression when pediatric CD cases and healthy controls were compared. Nevertheless, claudin-2 expression in healthy and CD pediatric subjects remained far greater than in adult CD patients (2.6-fold; P<0.001; Figure 3).
Figure 3: Quantitative analysis of claudin-2 expression.
Claudin-2 fluorescence intensity in children and adults. n = children: 17, 6, 6 (healthy, CD, GVHD); adults: 13, 11, 13, 6 (healthy, CD, GVHD, CVID). **, P<0.005; ***, P <0.001.
In contrast to CD, changes in claudin-2 expression differed between pediatric and adult GVHD patients. Claudin-2 expression was reduced by 57% in children with GVHD (P<0.001; Figure 3) but was not significantly changed in adults GVHD patients (Figure 3).
Only adult cases of CVID were identified, consistent with the natural history of this disease. Despite the dense lymphocytic infiltrates within the lamina propria, claudin-2 expression in CVID was similar to that in healthy adults (Figure 3).
Claudin-15
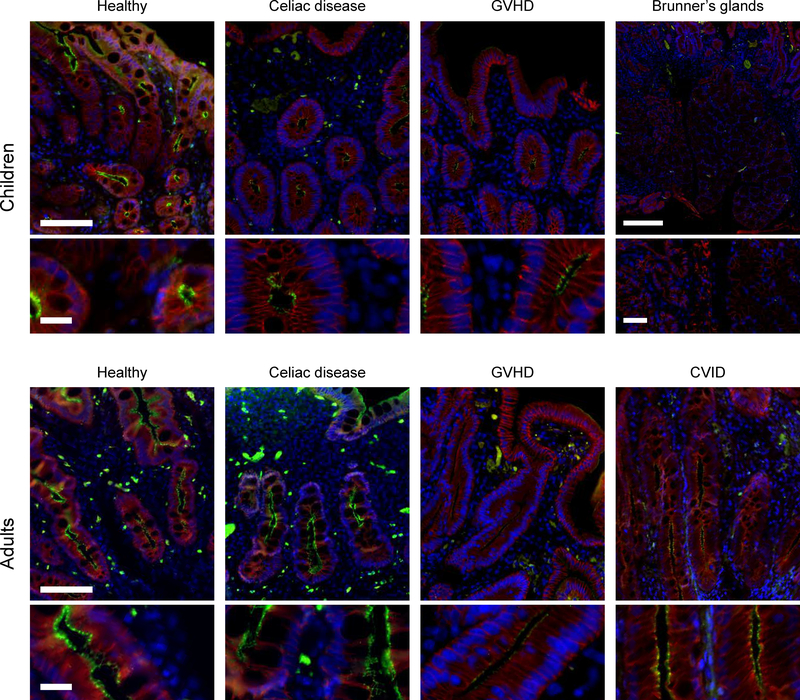
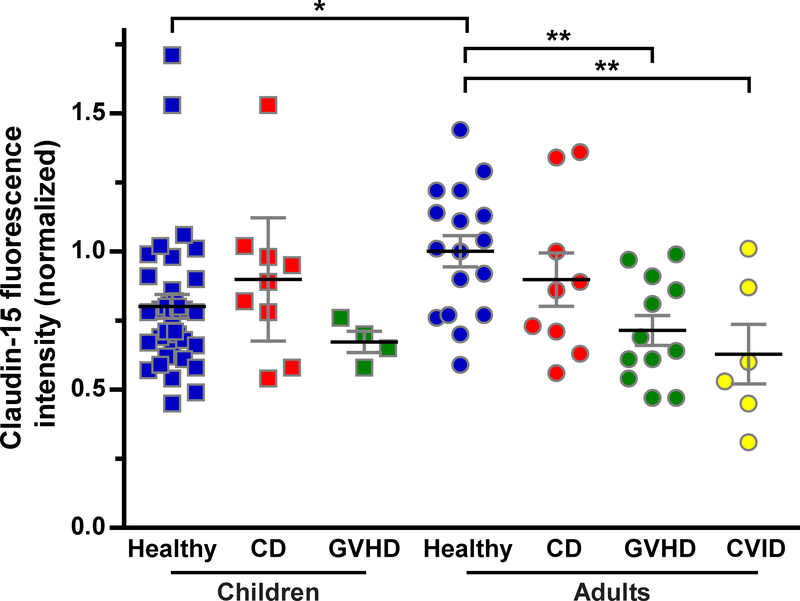
The overall distribution of claudin-15 within villus and crypt epithelial cells of healthy pediatric and adult biopsies was similar to that of claudin-2 (Figure 4), but claudin-15 expression was greater in adults (P<0.05; Figure 5). Claudin-15 expression was not detected in Brunner’s glands (Figure 4).
Figure 4: Claudin-15 expression.
Representative claudin-15 immunofluorescence images for each subject group are shown. Immunofluorescence shows claudin-15 (green), E-cadherin (red), and DNA (blue). Note the lack of claudin-15 expression within Brunner’s gland epithelia. Scale bar = 100 μm; inset bar = 20 μm. Brunner’s gland scale: bar = 250 μm; inset bar = 50 μm.
Figure 5: Quantitative analysis of claudin-15 expression.
Claudin-15 fluorescence intensity in children and adults. n = children: 35, 9, 4 (healthy, CD, GVHD); adults: 17, 9, 12, 6 (healthy, CD, GVHD, CVID) *, P <0.05; **, P<0.005.
Claudin-15 expression was not affected by CD in either children or adults (Figure 5). Claudin-15 expression was, however, significantly reduced in biopsies from adults with GVHD or CVID (P<0.005; Figure 5). As a result, claudin-15 expression in adults with GVHD or CVID was similar to that in healthy, CD, and GVHD children (Figure 5). Claudin-15 expression was unchanged in children with GVHD (Figure 5).
Discussion
This study addresses some issues that have been studied previously,10, 11 but assesses them from significantly different perspectives. First, although claudin-2 and claudin-15 expression within the intestine have been assessed during development, prior analyses were done in mice.1, 7 No previous reports have used quantitative morphometry to assess claudin-2 and claudin-15 expression in intestinal biopsies from healthy human subjects.
One previous study assessed claudin-2 and claudin-15 expression in adults with CD using western blots of whole biopsies.10 A separate study assessed the proportion of cells expressing claudin-2 expression in pediatric CD; claudin-2 was primarily intracellular in that study.11 Although total claudin within the biopsy and the proportion of epithelial cells expressing a claudin can be informative, it is ultimately the number of claudin molecules at the epithelial tight junction that define paracellular permeability. Our analyses of tight junction-associated claudin-2 and claudin-15 therefore provide substantive new insight into the effects of disease on paracellular permeability.
Given the importance of claudin-2 and claudin-15 to Na+, water, and nutrient transport, further definition of their expression patterns in health and disease is required to understand the pathophysiology of malabsorptive diarrhea. Rather than focusing exclusively on CD, we chose to compare CD with GVHD, another malabsorptive disease resulting from epithelial damage inflicted by cytolytic T cells.24, 25 In adult cases, we also assessed claudin-2 and claudin-15 expression in patients with CVID (which typically presents well after puberty), an uncommon cause of malabsorptive diarrhea.
Our data are consistent with quantitative PCR data from mice.7 Our finding that claudin-2 expression is reduced and claudin-15 expression is increased during human development is also similar to immunostain data from mouse jejunum reported by two groups who analyzed expression at either 1 and 28 days or 2 and 8 weeks of life.1, 7 The primary difference between our results in normal human children and adults and previous analyses of mice was that we detected expression of both claudins within villous enterocytes at all ages. This may be because the children studied here were post-weaning, while the mouse studies focused on times before weaning. Our data therefore demonstrate that claudin-2 downregulation and claudin-15 upregulation occur during human postnatal development and are independent of weaning.
One potential explanation for increased claudin-2 expression in children could be that it provides an advantage over claudin-15 during rapid growth, where demands on Na+-nutrient cotransport systems are maximized. Recent work showing that claudin-2 and claudin-15 differ biophysically, with the claudin-2 channel accommodating only dehydrated or partially dehydrated Na+ and the larger claudin-15 channel accommodating both water and hydrated Na+,28 may shed further light on this. That work found that water and Na+ fluxes across the claudin-15 channel inhibit one another; this was not true for claudin-2. We speculate that this difference allows claudin-2 to be a more efficient conductor of paracellular Na+ efflux into the lumen, which is necessary for ongoing Na+-nutrient cotransport. This idea is supported by our report that claudin-2 overexpression in mice enhances stool water and Na+ in healthy mice.29 Our observation that claudin-2, but not claudin-15, is highly expressed in Brunner’s glands, which secrete bicarbonate-rich fluid to hydrate and neutralize the pH of gastric contents is also consistent with such a functional difference between these Na+/water pore-forming claudins.
Because our study focused on altered expression patterns in disease, where villi are often absent, the analyses concentrated on claudin expression within the crypt epithelium. We also focused on claudin proteins at the tight junction. This is an advantage of our study that likely explains the more modest increases in claudin-2 and claudin-15 expression we detected in human CD relative to previous work that depended on western blots and did not provide spatial resolution.10, 30 Thus, for claudin-2, which is primarily expressed in adult crypt epithelium, the presence of villous epithelium in the western blot specimens from healthy adults may have led to an underestimate of expression within crypt cells. The nearly 200% increase in claudin-2 expression in adult CD reported by that study may therefore be overstated.10 Our analyses, which focused on individual intercellular junctions within duodenal crypts, were unaffected by such biases, and found a somewhat smaller 68% increase in claudin-2 expression in adult CD. Similar to previous studies,10, 11 we also detected cytoplasmic claudin-2 pools but did not include these in our analyses of tight junction-associated claudin-2.
The caveats noted above with regard to comparisons between western blot and quantitative immunofluorescence data also apply to claudin-15. This may explain why we were unable to confirm a previous report that claudin-15 expression was upregulated in CD.10 We did, however, find that claudin-15 expression was downregulated in adult, but not pediatric, GVHD. Conversely, claudin-2 was downregulated in pediatric, but not adult, GVHD. This difference highlights the need to consider both the patient population and controls to which patient biopsies are compared when performing analyses such as these and, more importantly, suggests that the pathophysiology of diarrhea in these patient groups may differ. Moreover, the observation that Na+/water pore-forming claudins are downregulated in GVHD in both children and adults suggests that mechanisms of altered intestinal paracellular transport are significantly different between CD and GVHD. The ongoing crypt cell damage that characterizes GVHD may be one contributor to these differences.
Given the presumed immune-mediated diarrhea pathophysiology in CVID, we were surprised to find no difference in claudin-2 expression in these biopsies. We had anticipated that the dense lymphocytic infiltrates within the lamina propria in CVID would produce cytokines that increase claudin-2 expression.29, 31, 32 This was not the case, and claudin-15 expression was reduced. These data suggest that incomplete Na+-nutrient cotransport due to insufficient paracellular Na+ recycling may contribute to diarrheal disease in CVID. Although this clearly requires further study, it may be difficult, as CVID remains a relatively uncommon disease despite being frequent among immunodeficiencies.
In summary, these data confirm some, but not all, previous observations in adult CD patients, expand on and refine previous observations in pediatric CD patients and are the first to compare expression in children and adults. In addition, this is the first report to assess claudin expression in GVHD and CVID. While our sample size was small, the implications for advancing our understanding and ultimately influencing treatment of malabsorptive diarrhea are significant.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health (R01DK61631, R01DK68271, R24DK099803), the Harvard Digestive Disease Center (P30DK034854), and the University of Chicago Cancer Center (P30CA014599). AS and JRT were involved in case acquisition and tissue microarray development; MLDM, SPN, SY, and JRT were involved in data acquisition and analysis, figure preparation, and manuscript creation. All authors approved the final manuscript.
Footnotes
Competing Interests: No competing interests.
References
- 1.Tamura A, Hayashi H, Imasato M, et al. Loss of claudin-15, but not claudin-2, causes Na+ deficiency and glucose malabsorption in mouse small intestine. Gastroenterol 2011;140(3):913–923. [DOI] [PubMed] [Google Scholar]
- 2.Wada M, Tamura A, Takahashi N, et al. Loss of claudins 2 and 15 from mice causes defects in paracellular Na+ flow and nutrient transport in gut and leads to death from malnutrition. Gastroenterol 2013;144(2):369–380. [DOI] [PubMed] [Google Scholar]
- 3.Muto S, Hata M, Taniguchi J, et al. Claudin-2-deficient mice are defective in the leaky and cation-selective paracellular permeability properties of renal proximal tubules. Proc Natl Acad Sci USA 2010;107(17):8011–8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamura A, Kitano Y, Hata M, et al. Megaintestine in claudin-15-deficient mice. Gastroenterol 2008;134(2):523–534. [DOI] [PubMed] [Google Scholar]
- 5.Turner JR, Black ED. NHE3-dependent cytoplasmic alkalinization is triggered by Na(+)-glucose cotransport in intestinal epithelia. Am J Physiol - Cell Physiol 2001;281(5):C1533–1541. [DOI] [PubMed] [Google Scholar]
- 6.Lin R, Murtazina R, Cha B, et al. D-glucose acts via sodium/glucose cotransporter 1 to increase NHE3 in mouse jejunal brush border by a Na+/H+ exchange regulatory factor 2-dependent process. Gastroenterol 2011;140(2):560–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holmes JL, Van Itallie CM, Rasmussen JE, et al. Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr Patterns 2006;6(6):581–588. [DOI] [PubMed] [Google Scholar]
- 8.Weber CR, Nalle SC, Tretiakova M, et al. Claudin-1 and claudin-2 expression is elevated in inflammatory bowel disease and may contribute to early neoplastic transformation. Lab Invest 2008;88(10): 1110–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Luettig J, Rosenthal R, Barmeyer C, et al. Claudin-2 as a mediator of leaky gut barrier during intestinal inflammation. Tissue Barriers 2015;3(1–2):e977176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schumann M, Gunzel D, Buergel N, et al. Cell polarity-determining proteins Par-3 and PP-1 are involved in epithelial tight junction defects in coeliac disease. Gut 2012;61(2):220–228. [DOI] [PubMed] [Google Scholar]
- 11.Szakal DN, Gyorffy H, Arato A, et al. Mucosal expression of claudins 2, 3 and 4 in proximal and distal part of duodenum in children with coeliac disease. Virchows Arch 2010;456(3):245–250. [DOI] [PubMed] [Google Scholar]
- 12.Kohout P Small bowel permeability in diagnosis of celiac disease and monitoring of compliance of a gluten-free diet (gut permeability in celiac disease). Acta Medica (Hradec Kralove) 2001;44(3):101–104. [PubMed] [Google Scholar]
- 13.Nalle SC, Kwak HA, Edelblum KL, et al. Recipient NK cell inactivation and intestinal barrier loss are required for MHC-matched graft-versus-host disease. Sci TranslMed 2014;6(243):243ra287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nalle SC, Turner JR. Intestinal barrier loss as a critical pathogenic link between inflammatory bowel disease and graft-versus-host disease. Mucosal Immunol 2015;8(4):720–730. [DOI] [PubMed] [Google Scholar]
- 15.Koltun WA, Bloomer MM, Colony P, et al. Increased intestinal permeability in rats with graft versus host disease. Gut 1996;39(2):291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Green PH, Jabri B. Celiac disease. Annu Rev Med 2006;57:207–221. [DOI] [PubMed] [Google Scholar]
- 17.Guandalini S, Assiri A. Celiac disease: a review. JAMA Pediatr 2014;168(3):272–278. [DOI] [PubMed] [Google Scholar]
- 18.Green PH, Cellier C. Celiac disease. N Engl J Med 2007;357(17):1731–1743. [DOI] [PubMed] [Google Scholar]
- 19.Diosdado B, van Bakel H, Strengman E, et al. Neutrophil recruitment and barrier impairment in celiac disease: a genomic study. Clin Gastroenterol Hepatol 2007;5(5):574–581. [DOI] [PubMed] [Google Scholar]
- 20.Calleja S, Vivas S, Santiuste M, et al. Dynamics of Non-conventional Intraepithelial Lymphocytes-NK, NKT, and gammadelta T-in Celiac Disease: Relationship with Age, Diet, and Histopathology. Dig Dis Sci 2011. [DOI] [PubMed] [Google Scholar]
- 21.McAllister CS, Kagnoff MF. The immunopathogenesis of celiac disease reveals possible therapies beyond the gluten-free diet. Seminars in immunopathology 2012;34(4):581–600. [DOI] [PubMed] [Google Scholar]
- 22.Cogbill CH, Drobyski WR, Komorowski RA. Gastrointestinal pathology of autologous graft-versus-host disease following hematopoietic stem cell transplantation: a clinicopathological study of 17 cases. Mod Pathol 2011;24(1): 117–125. [DOI] [PubMed] [Google Scholar]
- 23.Sivula J, Cordova ZM, Tuimala J, et al. Toll-like receptor gene polymorphisms confer susceptibility to graft-versus-host disease in allogenic hematopoietic stem cell transplantation. Scand J Immunol 2012;76(3):336–341. [DOI] [PubMed] [Google Scholar]
- 24.Washington K, Jagasia M. Pathology of graft-versus-host disease in the gastrointestinal tract. Hum Pathol 2009;40(7):909–917. [DOI] [PubMed] [Google Scholar]
- 25.Sung D, Iuga AC, Kato T, et al. Crypt apoptotic body counts in normal ileal biopsies overlap with graft-versus-host disease and acute cellular rejection of small bowel allografts. Hum Pathol 2016;56:89–92. [DOI] [PubMed] [Google Scholar]
- 26.Daniels JA, Lederman HM, Maitra A, et al. Gastrointestinal tract pathology in patients with common variable immunodeficiency (CVID): a clinicopathologic study and review. Am JSurgPathol 2007;31(12):1800–1812. [DOI] [PubMed] [Google Scholar]
- 27.Washington K, Stenzel TT, Buckley RH, et al. Gastrointestinal pathology in patients with common variable immunodeficiency and X-linked agammaglobulinemia. Am J Surg Pathol 1996;20(10): 1240–1252. [DOI] [PubMed] [Google Scholar]
- 28.Rosenthal R, Gunzel D, Piontek J, et al. Claudin-15 forms a water channel through the tight junction with distinct function compared to claudin-2. Acta Physiol (Oxf) 2019:e13334. [DOI] [PubMed] [Google Scholar]
- 29.Tsai PY, Zhang B, He WQ, et al. IL-22 Upregulates Epithelial Claudin-2 to Drive Diarrhea and Enteric Pathogen Clearance. Cell Host Microbe 2017;21(6):671–681 e674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schumann M, Siegmund B, Schulzke JD, et al. Celiac Disease: Role of the Epithelial Barrier. Cell Mol Gastroenterol Hepatol 2017;3(2):150–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Heller F, Florian P, Bojarski C, et al. Interleukin-13 is the key effector Th2 cytokine in ulcerative colitis that affects epithelial tight junctions, apoptosis, and cell restitution. Gastroenterol 2005;129(2):550–564. [DOI] [PubMed] [Google Scholar]
- 32.Weber CR, Raleigh DR, Su L, et al. Epithelial myosin light chain kinase activation induces mucosal interleukin-13 expression to alter tight junction ion selectivity. J Biol Chem 2010;285(16): 12037–12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.