Abstract
Background.
Small, basic peanut proteins are often poorly extracted in pH-neutral buffers that are optimal for the extraction of peanut storage proteins such as Ara h 1. As a result, such proteins are easily missed as potential allergens.
Objective:
To analyze the allergenic composition of the Basic Peanut Protein (BPP) fraction.
Methods.
A peanut extract prepared at pH 4 was fractionated by physicochemical procedures. Chemical analysis was performed by SDS-PAGE and mass spectrometry. Because immunoblotting was found to be inefficient for most of these small basic proteins, IgE binding activity was measured by coupling the fractions to CNBr-activated Sepharose, followed by incubation with sera from 55 Dutch peanut-allergic children and 125I-labeled anti-IgE.
Results.
Most IgE reactivity of the BPP fraction was due to the 5–7 kDa amino-terminal fragment of Ara h 1. This finding was confirmed by the use of the fragment in recombinant form, to which 25/55 of the sera was IgE-positive.
Conclusion.
The amino-terminal fragment of Ara h 1, a member of a family of small anti-microbial proteins, is an allergen independent of the carboxy-terminal fragment of Ara h 1.
Introduction
Peanut allergens largely belong to two physiological types: storage proteins and defense-related proteins. Some of the defense-related proteins, such as Lipid Transfer Proteins (LTPs), are small (<10 kDa) basic peanut proteins (BPPs). The aim of our study was to investigate which of these BPPs were most relevant in relation to peanut sensitization (i.e. induction of sIgE, irrespective of clinical symptomatology upon exposure).
Immunoblotting, which reliably detects the known LTPs, was previously found to be inefficient in identifying other IgE-binding proteins in the BPP fraction[1]. Therefore, we set out to identify IgE-binding BPPs using a traditional RAST-type assay based on covalent coupling of BPP fractions to CNBr-activated Sepharose beads and detection of bound IgE with 125I-labeled anti-IgE. In addition, we used RAST-inhibition to quantify IgE-binding allergenic activity. The IgE binding activity was compared with mass spectrometric analyses and we show that the amino-terminal fragment of Ara h 1 is a major IgE-binding component of peanut.
Some background information on the structure of Ara h 1, one of the major peanut allergens, may be desirable. It is a large (65 kDa) protein. The recombinant allergen used in our diagnostic test, is Ara h 1.0101. This is the full-length protein without the leader sequence and corresponds to the amino acid residues 26–626 encoded by the genomic sequence. We will refer to this full-length protein as rAra h 1. Ara h 1 purified from peanut extract largely lacks the amino-terminal amino acids 26–83 due to cleavage in the peanut by a vacuolar protease (for more information on vacuolar proteases producing multiple proteins from a precursor poly-protein, see Online Repository). We will refer to this processed carboxy-terminal fragment as natural Ara h 1 (nAra h 1, residues 84–626). The small protein corresponding to the amino-terminal amino acids 26–83 is the main topic of this paper. Since it is known in literature as a propeptide, we will refer to it as Arah1Pro. Its actual size in peanut extract proves to be smaller than predicted, because it is trimmed at both its termini.
Methods
Patients, serum samples and serological tests
The human sera are referred to by an ID code preceded by the ‘#’ sign. Most assays were performed with a BPP-positive reference serum. This BPP+ reference serum has been extensively used in the work presented in our preceding paper [1]. The BPP+ reference serum (total IgE 2743 kU/L) was obtained from a Dutch patient with a clinical peanut allergy. This serum was positive to rAra h 1, 2, 3, 6 and 8 and to rBet v 2 (84.7, 36.3, 5.8, 33.3, 10.0 and 1.26 kUA/L, respectively). IgE reactivity to rAra h 9, Pru p 3 and CCD were <0.35 kUA/L. The other reference serum used was an LTP-positive serum. The LTP+ reference serum (total IgE 131 kU/L) was obtained from a Dutch peach-allergic patient with a strong IgE reactivity to rPru p 3 (10.33 kUA/L) and a weaker reactivity to rAra h 9 (6.20 kUA/L), as measured using the ImmunoCAP (Thermo Fisher Scientific, Uppsala, Sweden). IgE reactivity to rAra h 1, 2, 3, 6 and 8 and to rBet v 2 and CCD were all <0.35 kUA/L. Regrettably, we have no information on the presence of symptoms upon peanut exposure. We used 55 sera from our panel of 64 Dutch pediatric patients to substantiate these results. These patients, with codes #01 to #64, were DBPCFC-tested, as described elsewhere [1]. For the ImmunoCAP results of these sera, see [1]. The study of these patients was approved by the local medical ethics review boards (METC, UMC Utrecht; project number 05/084) and informed consent was obtained for all subjects. IgE to the peanut extract and to the peanut fractions was measured using the Sanquin RAST [1] based on allergens coupled to CNBr-activated Sepharose and detected by 125I-anti-IgE.
IgE to purified natural Ara h 1 was measured by a modified RAST protocol, in which two mouse monoclonal antibodies to Ara h 1 were coupled to CNBr-activated Sepharose and subsequently loaded with purified nAra h 1 (100 ng/test). The monoclonal antibodies were directed to non-overlapping epitopes. Both the monoclonal antibodies and the purified nAra h 1 were provided by Indoor Biotechnologies (Charlottesville, Va. USA). We loaded the anti-Ara h 1 coated beads separately with purified nAra h 1 and mixed these two bead suspensions. Per test we used the equivalent of 100 ng Ara h 1. We incubated these nAra h 1-coated beads with 50 μl patient serum overnight, washed, added 125I-labeled affinity-purified sheep anti-IgE, incubated overnight and washed. Sepharose-bound radioactivity was measured and converted to kUa/L using a human-mouse chimeric IgE antibody to Der p 2 as reference. IgE to recombinant Arah1Pro (see below) was measured by coupling the recombinant protein to CNBr-activated Sepharose. Per test we used the equivalent of 1 μg Arah1Pro.
Fractionation of the basic peanut protein (BPP) fraction
Details on the production of the BPP fractions are presented in the Online Repository.
Monitoring IgE-binding activity of peanut fractions by RAST and RAST inhibition
BPP fractions obtained by reversed phase (RP) chromatography (for details on these fractions, see Figure 2A) were tested initially by direct RAST. Of each fraction, 10 μg protein was coupled to 50 mg CNBr-activated Sepharose. The IgE-binding activity of each fraction was tested by incubating 0.5 mg allergen-coated Sepharose with the BPP+ reference serum or with the LTP+ reference serum, followed by washing and incubation with 125I anti-IgE.
Figure 2.
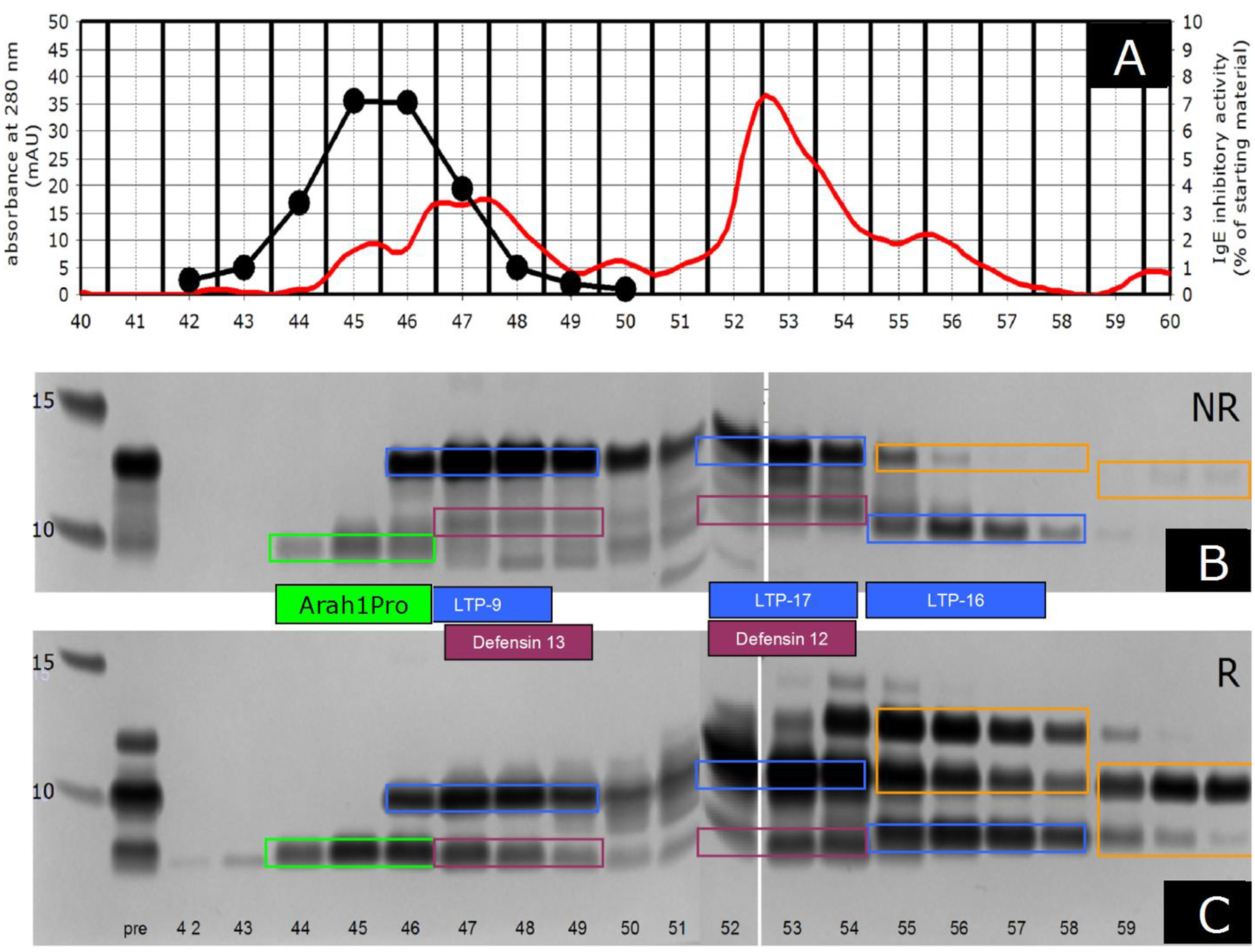
Frame A: Preparative reversed-phase (RP) chromatography of the peanut fraction purified by cation exchange and SEC. The black line indicates allergenic activity, as measured by RAST inhibition using the BPP+ reference serum. The allergenic activity is indicated on the y-axis on the right using the starting material (i.e. the peanut fraction purified by cation exchange and SEC) as 100% value. The red line indicates the optical density at 280 nm. Frames B and C: Silver-stained SDS-PAGE (B: NR=non-reduced; C: R=Reduced) of the starting material (“pre”) and the RP fractions. The green, blue and purple colored rectangles indicate our provisional assignments of the allergens mentioned in the text. Arah1Pro is the propeptide of Ara h 1. LTP-9, 16 and 17 are Ara h 9, Ara h 16 and Ara h 17; Defensin 12 and 13 are Ara h 12 and Ara h 13. The proteins indicated by the orange-colored rectangle of fractions 59–61 have not been investigated.
For the initial screening also two LTP-enriched fractions (fraction 53 and 56 in Figure 2A) were used in the direct RAST protocol to test for LTP-related IgE binding in sera from the panel of Dutch pediatric patients[1].
For RAST inhibition, the allergen sample under investigation was titrated and pre-incubated with the BPP+ reference serum. Residual IgE binding was measured by incubation with Sepharose-coupled fraction 45 (see in Figure 2A), followed by washing and incubation with 125I anti-IgE.
Sample characterization by Mass Spectrometry
The methods used for Mass Spectrometry are specified in the Online Repository. Representative spectra are shown in figures E3, E5 and E6 in the Online Repository.
Production and purification of recombinant Arah1Pro
Recombinant Arah1Pro was prepared as described in the Online Repository. The connectivity of the disulphide bridges was investigated by nuclear magnetic resonance and mass spectrometry.
Results
By mass-spectroscopic analyses (Wildner et al., manuscript in preparation) we identified two novel peanut LTPs, which have been provisionally accepted by the WHO/IUIS Allergen Nomenclature Committee in their database (www.allergen.org) as Ara h 16 (7011 Da, 35% identity to Ara h 9) and Ara h 17 (9349 Da, 62% identity to Ara h 9). Based on the high allergenicity of other LTPs, particularly peach LTP (Pru p 3) [2], we initially focused on the IgE-binding activity of the three identified peanut LTPs in the BPP fraction. However, as shown in figure E1 in the Online repository, in the Dutch pediatric panel, these LTPs were found to be only minor contributors to the IgE-binding activity among the group of small basic peanut proteins.
Upon reversed phase (RP) fractionation, the strongest IgE-binding activity of the BPP fraction was found in fractions eluting before the LTPs (Figure 1 and 2). Upon fractionation by size-exclusion chromatography (SEC), most IgE-binding activity was found in fractions eluting slightly after the LTPs (results not shown), This chromatographic behavior was similar to that of the two peanut defensins Ara h 12 and Ara h 13 [3]. However, analysis by mass spectrometry indicated that the strongest IgE-reactive RP fraction, which eluted just before the defensins, contained peptides derived from Arah1Pro, but were smaller.
Figure 1.
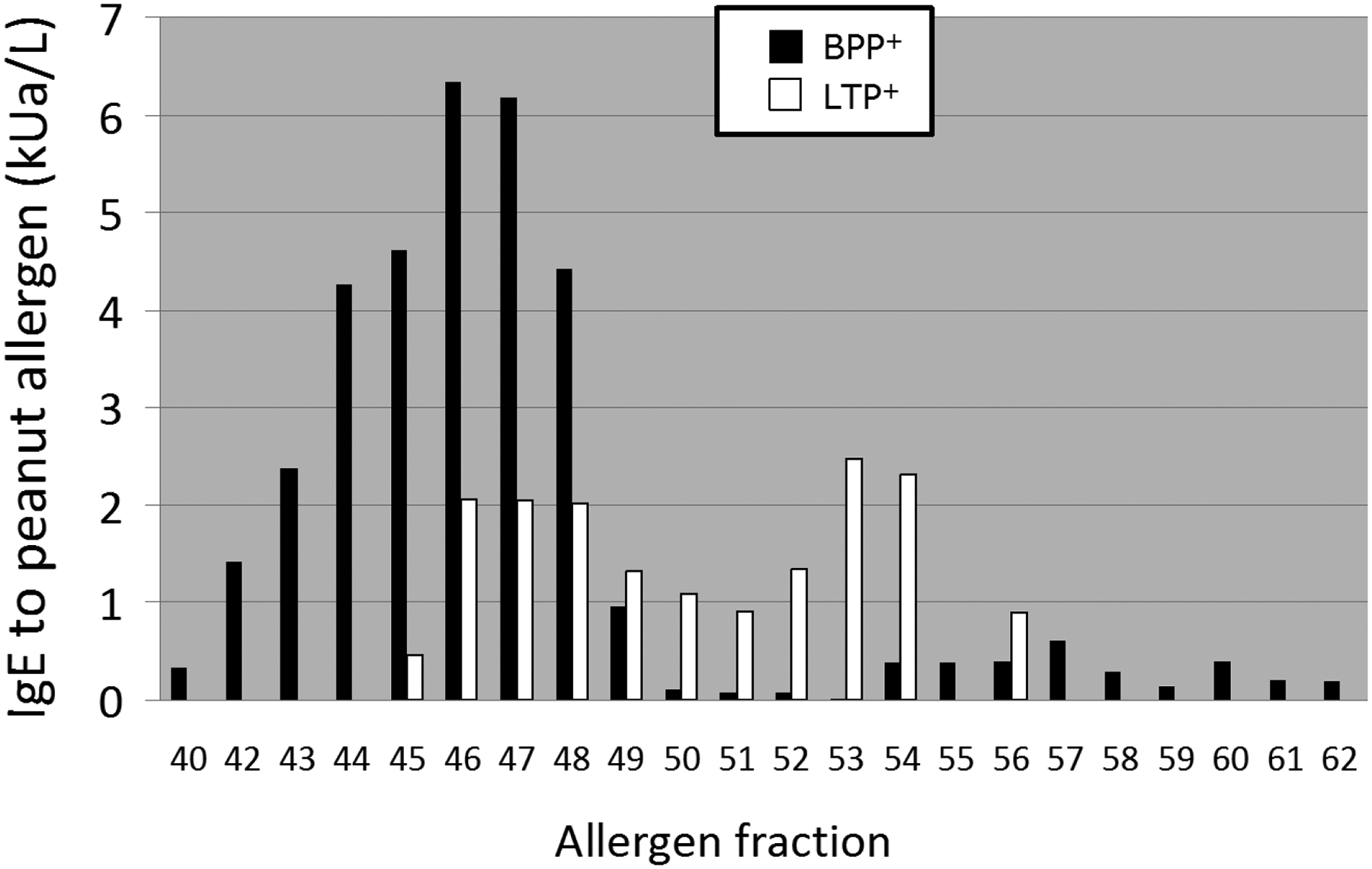
IgE-binding activity of Sepharose-coupled reversed-phase chromatography column fractions for the two reference sera BPP+ (black bars) and LTP+ (white bars).
To exclude the possibility of in vitro breakdown, we prepared peanut extract in the presence of a protease inhibitor cocktail and immediately isolated the 3.5–7 kDa fraction. The fractions with proteins smaller than LTPs (as judged by SDS-PAGE) were pooled, concentrated and separated by SP chromatography. The fractions were tested by RAST-inhibition, using Sepharose-coupled fraction 45 (see Figure 2A) on the solid phase and the BPP+ reference serum as IgE source. Mass spectrometry analysis of peptides related to Ara h 1 indicated that these fractions contained trimmed Arah1Pro-derived peptides with calculated isoelectric points between 7.7 and 8.3 (figures E4 and E7). The observations support the notion that not only the propeptide, but also the amino- and carboxy-terminally trimmed peptides, are formed in planta.
To exclude that the IgE reactivity to the fraction containing Ara h 1Pro was due to contaminating proteins, we produced the Arah1Pro as recombinant protein and tested it for IgE binding. Using a cut-off of 0.35 kUa/L, 25/55 sera were positive for rArah1Pro, compared to 17 for nAra h 1 (Figure 3). This is compatible with the conclusion that rArah1Pro and/or trimmed variants are a major source of the IgE-binding activity in the BPP fraction. Six of the 25 Arah1Pro-positive children (24%) passed the DBPCFC (i.e. were peanut-tolerant), compared to 2/17=11.8% for nAra h 1.
Figure 3.
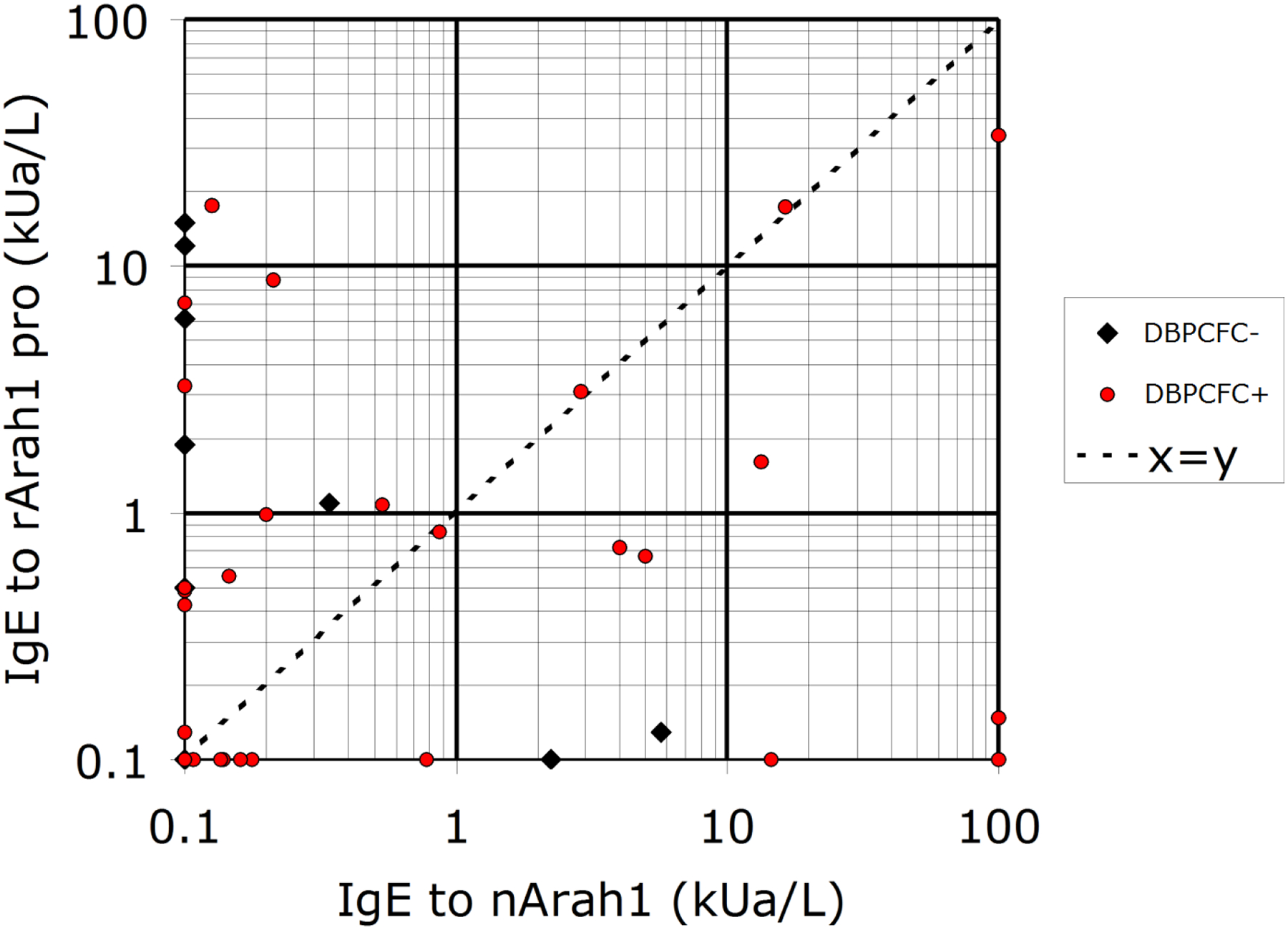
Comparison between IgE binding to the rArah1pro and natural Ara h 1 purified from peanut extract. Of all sera tested, 17 were double-negative. The data are based on 55 sera from the Dutch pediatric panel [1], of which 36 were positive in the DBPCFC.
Discussion
Our results with Arah1Pro indicate that IgE-binding activity of small basic anti-microbial proteins is easily overlooked. Relevant factors for their optimal identification are extraction at low pH, IgE detection techniques other than immunoblotting and ways to deal with size heterogeneity due to proteolytic trimming. The latter feature makes it very hard to prepare a sufficiently pure natural protein, so the production of a recombinant protein is essential. With these cysteine-rich proteins the connectivity of the disulphides needs to be established. The potential initial production as part of a poly-protein is also a complicating factor.
In the case of Arah1Pro we find it likely that it acts as a predominantly independent molecular sensitizer and suggest that it in that sense may be relevant to regard it as a distinct peanut allergen. A similar conclusion was reached by Downs et al for the propeptide from walnut vicilin[4]. It is, however, important to stress that the immunoblots shown in their paper do not provide proof of IgE-binding to a propeptide specifically. The walnut fraction that was tested for IgE binding was obtained by SEC fractionation, without further subfractionation. This fraction evidently contains the walnut vicilin propeptide, but presumably also other proteins such as defensin-related allergens which have a molecular weight of 5–6 kDa[3].
A potential weakness of the current data set is that the results for IgE to nAra h 1 could be influenced by trace amounts of potent contaminating allergens such as Ara h 2. We minimized such a contamination effect by using a modified RAST protocol based on capture of the purified allergen by monoclonal anti-Ara h 1 coupled to the solid phase and using a low dose of allergen (100 ng/test). As a consequence of this low dose, IgE levels may be underestimated in sera with a high level of IgG to nAra h 1.
Several small cysteine-rich BPPs that have been described as allergens are defense-related proteins belonging to the defensin-, LTP- or alpha-hairpinin family [5]. Arah1Pro with its repeated CXXXC motifs presumably belongs to the latter[6]. Figure 4 shows a cartoon of the structure of Arah1Pro (based on mass spectrometry and a preliminary nuclear magnetic resonance analysis) and the three IgE epitopes-predicted from synthetic peptides AKSSPYQKKT, QEPDDLKQKA and LEYDPRLVYD ([7,8]; the first two peptides contained no cysteines; the single cysteine in the 3rd sequence was replaced for technical reasons by leucine). It will be interesting to see how efficient these peptides inhibit IgE binding to fully folded rArah1Pro. We expect that some IgE epitopes depend on the intact double-helical hairpin configuration.
Figure 4.
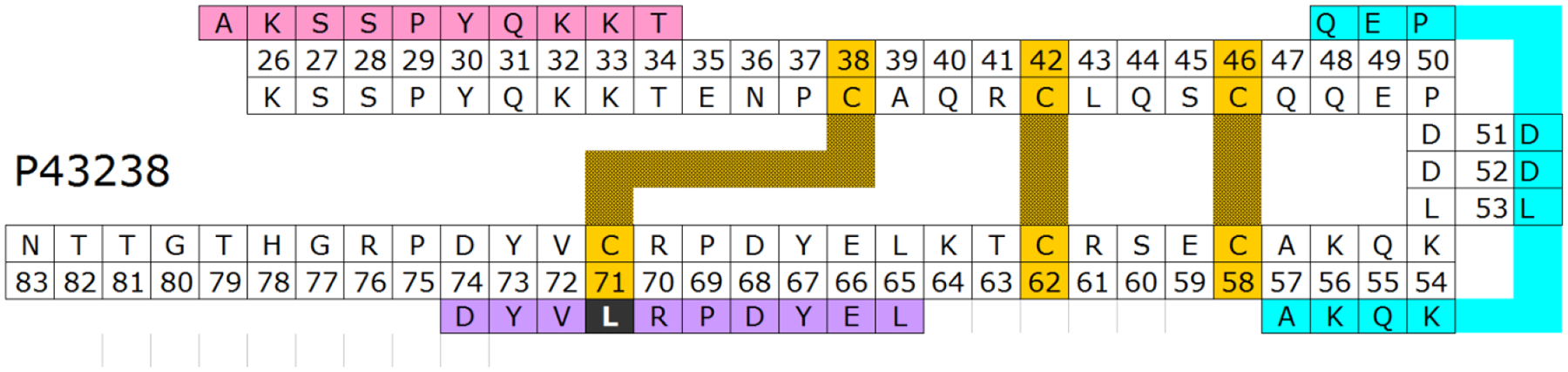
Arah1Pro cartoon showing the location of the three synthetic IgE-binding peptides (pink, cyan and purple) reported by Burks et al [7].
Ye at al [9] described an antifungal peptide from peanuts with sequence similarity to Arah1pro, which they named Hypogin 1. Using the Edman degradation procedure, they reported a partial amino-terminal sequence KSPYYQKKTENPQAQRQLQSDDQEPAKLKXKRGTSRDTXY. This sequence deviates from the Arah1Pro sequence, suggesting that even though the propeptide was present in their peanut fraction, it may not have been entirely pure. Further experiments are needed to substantiate the claim that Arah1pro has anti-microbial activity.
The more we know about these small cysteine-rich basic proteins, the more it becomes evident that it is essential to show IgE-binding to recombinantly expressed proteins to establish the IgE-binding activity of such proteins. Our results with the recombinant protein show that Arah1Pro has IgE-binding activity. In addition to IgE-binding it will be important to establish allergenic activity in basophil/ mast cell systems. The small size of these tightly-folded cysteine-rich proteins is likely to limit number of antibody molecules that fit on their surface, particularly if their N- and C-terminal termini are trimmed. Based on our results with rArah1Pro it is evident that it has a substantial IgE-binding activity. In view of its small size it will be interesting to investigate if its activity in biological assays is inferior to that of the larger allergens. If so, this might explain the results shown in Figure 3, which suggest that sIgE to Arah1Pro is more prevalent in peanut-tolerant children. Because of the high sensitivity of the bioassays, it is important to be aware of the effects of contamination by proteins of similar size in allergens preparations purified from natural source materials. Using recombinantly produced allergens is often crucial.
Supplementary Material
Acknowledgements
We thank Alexander C.Y. Foo for the preparation of the batch of rArah1pro used for these studies. We thank Serge Versteeg for performing the confirmatory rAra h 1 ImmunoCAP tests, Ronald van Ree, Laurian Jongejan, Jaap Akkerdaas and Serge Versteeg from the Academic Medical Center Amsterdam, Gabriele Gadermaier from the University of Salzburg and Stef Koppelman (University of Nebraska) for stimulating discussions.
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
- # followed by a number
serum from the pediatric panel
- Arah1Pro
amino-terminal fragment of Ara h 1 (residues K26-N83 or K26-N77, depending on the Ara h 1 isoform)
- BPP
Basic peanut protein
- Da, kDa
(kilo)Dalton
- kUa/L
concentration of allergen-specific IgE
- LTP
Lipid transfer protein
- nAra h 1
the natural peanut allergen extracted from peanut (residues Q84 or R85 to N626)
- NR
Non-reduced = disulphide bridges intact
- R
Reduced = disulphide bridges broken
- rAra h 1
refers to peanut allergen, residues K26-N626, i.e. without the leader peptide M1-A25, but including the Arah1Pro segment
- rArah1Pro
Ara h 1.0101 (25–83, A25G): the recombinant version of the predicted amino-terminal split product of Ara h 1.0101
- RP
Reversed Phase (chromatography)
- SEC
Size exclusion chromatography, also known as gel filtration
- SP
SulphoProplyl (Cation exchange chromatography)
Footnotes
Conflict of interest
RA, GM, ND, JA, LE, TR and PB have no conflict of interest related to the presented work. JL is an employee of Thermo Fisher Scientific. AP is an employee of Indoor Biotechnologies Inc.
This study was supported in part by the Intramural Research Program of the National Institute of Environmental Health Sciences (Z01-ES102906–01, G Mueller).
Ethical approval and informed consent
The study was approved by the local medical ethics review boards (METC, UMC Utrecht; project number 05/084) and informed consent was obtained for all subjects.
References
- 1.Aalberse JA, Meijer Y, Derksen N, van der Palen-Merkus T, Knol E, Aalberse RC. Moving from peanut extract to peanut components: towards validation of component-resolved IgE tests. Allergy. 2013;68:748–56. [DOI] [PubMed] [Google Scholar]
- 2.Pastorello EA, Farioli L, Stafylaraki C, Mascheri A, Scibilia J, Pravettoni V, Primavesi L, et al. Anti-rPru p 3 IgE levels are inversely related to the age at onset of peach-induced severe symptoms reported by peach-allergic adults. Int Arch Allergy Immunol. 2013;162:45–9. [DOI] [PubMed] [Google Scholar]
- 3.Petersen A, Kull S, Rennert S, Becker WM, Krause S, Ernst M, Gutsmann T, Bauer J, Lindner B, Jappe U. Peanut defensins: Novel allergens isolated from lipophilic peanut extract. J Allergy Clin Immunol. 2015;136:1295–301. [DOI] [PubMed] [Google Scholar]
- 4.Downs ML, Semic-Jusufagic A, Simpson A, Bartra J, Fernandez-Rivas M, Rigby NM,Taylor SL, Baumert JL, Mills EN. Characterization of low molecular weight allergens from English walnut (Juglans regia). J Agric Food Chem. 2014;62:11767–75. [DOI] [PubMed] [Google Scholar]
- 5.Nolde SB, Vassilevski AA, Rogozhin EA, Barinov NA, Balashova TA, Samsonova OV, Baranov YV, Feofanov AV, Egorov TA, Arseniev AS, Grishin EV. Disulfide-stabilized helical hairpin structure and activity of a novel antifungal peptide EcAMP1 from seeds of barnyard grass (Echinochloa crus-galli). J Biol Chem. 2011;286:25145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J, Payne CD, Pouvreau B, Schaefer H, Fisher MF, Taylor NL, Berkowitz O (3), Whelan J (3), Rosengren KJ, Mylne JS. An Ancient Peptide Family Buried within Vicilin Precursors. ACS Chem Biol. 2019;14:979–993. [DOI] [PubMed] [Google Scholar]
- 7.Burks AW, Shin D, Cockrell G, Stanley JS, Helm RM, Bannon GA. Mapping and mutational analysis of the IgE-binding epitopes on Ara h 1, a legume vicilin protein and a major allergen in peanut hypersensitivity. Eur J Biochem. 1997;245:334–9. [DOI] [PubMed] [Google Scholar]
- 8.Flinterman AE, Knol EF, Lencer DA, Bardina L, den Hartog Jager CF, Lin J, Pasmans SG, Bruijnzeel-Koomen CA, Sampson HA, van Hoffen E, Shreffler WG. Peanut epitopes for IgE and IgG4 in peanut-sensitized children in relation to severity of peanut allergy. J Allergy Clin Immunol. 2008;121:737–743 [DOI] [PubMed] [Google Scholar]
- 9.Ye XY, Ng TB. Hypogin, a novel antifungal peptide from peanuts with sequence similarity to peanut allergen. J Pept Res. 2001;57:330–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


