Abstract
Mammalian reproductive function includes puberty onset and completion, reproductive cyclicity, steroidogenesis, gametogenesis, fertilization, pregnancy and lactation; all are indispensable to perpetuate species. Reproductive cycles are critical for providing the hormonal milieu needed for follicular development and maturation of eggs but cycles, in and of themselves, do not guarantee ovulation will occur. Here we review the roles in female reproductive neuroendocrine function of two hypothalamic populations that produce the neuropeptide kisspeptin, demonstrating distinct roles in maintaining cycles and ovulation.
Keywords: ovulation, CRISPR, estradiol, reproduction, kisspeptin, AVPV, arcuate
The reproductive axis and estradiol feedback
Gonadotropin-releasing hormone (GnRH) neurons integrate central, peripheral and external cues to generate the central output that regulates fertility [1,2]. GnRH neurons reside primarily in the preoptic area (POA) and anterior hypothalamus. These neurons project to the median eminence and release GnRH near the primary capillaries of the hypophyseal portal vasculature, which carry this decapeptide to the pituitary where it activates the synthesis and secretion of the gonadotropins luteinizing hormone (LH) and follicle-stimulating hormone (FSH) [1,3]. GnRH is released in an episodic, or pulsatile, manner that is critical for pituitary function [4–6]. High GnRH pulse frequency favors LH synthesis and release, whereas low GnRH pulse frequency preferentially promotes FSH [7–9]. FSH and LH regulate gametogenesis and steroidogenesis [10]. The sex steroids, including estradiol, progesterone and testosterone, feed back to the brain to regulate GnRH release, and on the pituitary to regulate the responsiveness of gonadotropes to GnRH [11–16]. In males and during most of the female reproductive cycle, sex steroids suppress GnRH neuron activity and release via negative feedback [17–22]. During the preovulatory stage of the female cycle (late follicular phase or proestrus in rodents), a sustained elevation in estradiol causes a switch of estradiol action from negative to positive feedback, thus inducing elevated GnRH neuronal activity and causing a preovulatory surge of GnRH, and subsequent LH, release [6,23–25]. The LH surge triggers ovulation. There is some debate in higher primates about the necessity of the GnRH surge for ovulation, as an LH surge can occur without a change in episodic release in women [26], and an unchanging frequency of GnRH pulse administration can induce cycles in monkeys in which endogenous GnRH was ablated [4]. In this regard, it is worth noting that in monkeys GnRH surges have been observed along with the preovulatory or estradiol-induced LH surge [27,28]. In rodents and sheep, a surge release of GnRH is required for the LH surge [25,29,30]. The investigation of estradiol feedback regulation of the hypothalamus has focused mainly on ovarian estradiol as it is the predominant signal to generate the switch between negative and positive feedback. For a recent review on the possible role of local neurosteroids in these processes, the reader is directed to Terasawa, et al. [31].
With the help of the advanced genetic and other technical tools available, much of the work to understand central control of fertility has been done in rodents, specifically laboratory mice. To study systemic estradiol regulation, both stages of the estrous cycle characterized by negative (diestrus) and positive (proestrus) feedback, and hormone manipulation have been used [32,33]. With regard to the latter, one paradigm utilizes ovariectomy (OVX) with low estradiol replacement (OVX+E, negative feedback), followed by a subsequent estrogen injection several days later to induce positive feedback (OVX+E+E); this is referred as estradiol rise model [34]. Another paradigm utilizes OVX and with or without a constant high physiological level of estradiol [17]. This daily surge model exhibits a diurnal switch in estradiol feedback, with LH levels lower in estradiol-treated than OVX mice due to estradiol negative feedback in the morning (OVX+E AM), and higher in estradiol-treated mice in the afternoon due to positive feedback (OVX+E PM). As with LH in vivo, GFP-identified GnRH neurons in brain slices prepared from this model exhibit low firing rates and release frequency in OVX+E AM mice and high firing and release frequency in OVX+E PM mice [17,18]. Fast-synaptic transmission to and intrinsic membrane properties and ionic conductances of GnRH neurons are both altered by estradiol in this model [35–40]. Similar changes occur between positive feedback during the cycle [33,41]. Recent studies further demonstrated that GnRH neurons integrate fast-synaptic and intrinsic changes to increase firing rates during positive feedback [42].
Estrogen receptors involved in systemic estradiol feedback
The physiological responses to systemic estradiol in regulating reproductive functions are primarily mediated by two known subtypes of nuclear receptor, estrogen receptor α (ERα) and estrogen receptor β (ERβ) [43–45], as well as membrane-associated receptors (GPR30 and mER) [46–48]. In rodents and humans, the two nuclear receptors are encoded by Esr1 (human ESR1) and Esr2 (human ESR2), respectively. Both ERα and ERβ typically act as ligand-activated transcription factors [49], by either binding directly to estrogen response elements (EREs) or interacting with other proteins to alter gene expression [50,51]. ERα and ERβ can also modulate non-genomic membrane-associated signaling cascades [47,52–55]. Genomic and nongenomic actions of ERs are evident for many reproductive processes [50,56]. For example, signaling via the estrogen response element (ERE) is needed for estradiol-induced changes in GnRH neuron firing rate [57], whereas non-classical signaling also plays a role in this response at the pituitary [58]. Mice with global knockout of ERα or ERβ show distinct reproductive deficits. ERαKO mice are infertile and have disrupted reproductive tracts including hypoplastic uteri, and large hemorrhagic cysts and absence of corpora lutea in the ovaries [59]. In contrast, ERβKO mice are fertile but have fewer and smaller litters [59,60]. Further, ERαKO, but not βERKO, female mice exhibit atypically elevated serum LH in ovary-intact mice compared to their littermate controls. Likely related to this, estradiol replacement in OVX ERαKO females does not reduce LH [61]. A neuron-specific ERα KO mouse model shares similarly impaired negative feedback as the ERαKO mice, as well as disrupted positive feedback marked by an absence of estradiol-induced LH surge release [62]. Together these observations suggest that estradiol negative and positive feedback rely on estrogen signaling via ERα. Although tightly regulated by estradiol, GnRH neurons do not express detectable level of ERα; their response is thus at least in part attribute to estradiol action through upstream ERα-expressing neurons [63,64]. The upstream neurons that have by far been the subject of the most investigation for its role in estradiol feedback over the past fifteen years are kisspeptin neurons.
Kisspeptin signaling
The discovery of the link between KISS1 (produce kisspeptin) and KISS1R (produce kisspeptin receptors) genes and puberty and fertility comes from human studies. Patients carrying mutations in either of these genes exhibit idiopathic hypothalamic hypogonadism, impaired pubertal maturation, and low-amplitude LH pulses [65,66]. Transgenic mice that lack Kiss1 or Kiss1r exhibit a similar hypothalamic hypogonadism phenotype [66,67]. Kisspeptin is expressed in several organs including gonads, pancreas, colon, pituitary, and brain [68,69]. In mouse hypothalamus, kisspeptin expression is restricted to two regions: the arcuate nucleus and the anteroventral periventricular nucleus (AVPV) [70], Both populations express ERα, ~99% in the arcuate and ~70% in the AVPV [71]. Arcuate kisspeptin expression is similar in both sexes, whereas AVPV kisspeptin expression is more extensive in females [72]. When estradiol is elevated, kisspeptin mRNA expression is increased in the AVPV and decreased in the arcuate nucleus [73,74].
Projections from kisspeptin neurons to GnRH neurons vary with species [75–77]. In the mouse, kisspeptin fibers from the AVPV form direct appositions to GnRH cell bodies, whereas fibers from arcuate kisspeptin neurons are primarily apposed to GnRH processes that are running through the arcuate nucleus to the median eminence [78–80]. Both of these configurations support a direct kisspeptin-GnRH connection. In situ hybridization for Kiss1r and Kiss1r promoter-driven lacZ demonstrate GnRH neurons express kisspeptin receptors [81,82]. Bath application of kisspeptin to brain slices robustly increases GnRH firing activity and release [18,83,84]; kisspeptin injection in vivo increases GnRH release and subsequent LH release [85]. From a loss-of-function standpoint, blockade of kisspeptin action by injecting an antibody or a specific antagonist decreases GnRH activity, LH release and estrous cyclicity [72,86]. Deletion of Kiss1r from GnRH neurons recapitulates the Kiss1r KO phenotypes; re-introducing Kiss1r expression to GnRH neurons in Kiss1r KO mice rescue the deficits [87]. These observations indicate kisspeptin-GnRH circuitries are critical for normal reproduction. The differential regulation of kisspeptin by estradiol in arcuate and AVPV kisspeptin neurons set up the working hypothesis that these regions have distinct roles in mediating estradiol negative and positive feedback, respectively [88].
Arcuate kisspeptin neurons and estradiol negative feedback
Evidence that suggested a link between arcuate nucleus neurons and LH pulses came from early lesion studies, well before the discovery of kisspeptin: ablation of the arcuate nucleus abolished LH pulses in rats and monkeys [89,90]. Further, correlation of peaks in neuronal multi-unit activity (MUA) in the mediobasal hypothalamus (MBH), which contains the arcuate, with pulsatile LH release was demonstrated in several species including monkeys, goats, sheep and rodents [91–94]. In rats, sheep and goats, only a small percent of GnRH neurons are in the MBH, suggesting other neurons in MBH are involved in generating MUA peaks and perhaps pulse generation.
Identification of a role of kisspeptin in fertility refocused attention on the arcuate region, as arcuate kisspeptin neurons exhibit two characteristics needed for steroid regulated GnRH/LH pulse frequency. First, their activity is associated with pulsatile LH release [95]. Second, they can directly sense steroid feedback [77]. This brings up the intriguing possibility that generation and steroid regulation of GnRH pulses may be combined into one system. Arcuate kisspeptin neurons coexpress two additional peptides involved in regulating reproduction: neurokinin B (NKB) and dynorphin A, and are often referred to as KNDy neurons [77,96,97]. Intracerebroventricular (ICV) injection of NKB and dynorphin alters LH pulse frequency and associated MUA activity peaks in goats, and LH pulse frequency in ewes: NKB increased the frequency of MUA peaks in the arcuate and LH pulse frequency. In contrast, dynorphin inhibited MUA peaks and LH secretion, whereas blocking its action increased frequency of both central and pituitary output [98,99]. Bath application of these two peptides to mouse brain slides has a corresponding effect on KNDy neurons; NKB is excitatory, dynorphin inhibitory [100–102]. These changes may be attributable to direct action of the peptides on KNDy neurons, as these neurons form interconnect circuits [102] and most KNDy neurons express the NKB receptor, NK3R, with a smaller percentage of these cells expressing the dynorphin-specific kappa-opioid receptor (KOR) [97,103]. Long-term monitoring of KNDy neuron activity revealed that they exhibit spontaneous peaks and nadirs in firing rate [104]. This pattern can be altered when NK3R is activated; blocking KOR, however, did not affect the patterns [104]. This difference may be explained by the observation that fewer KNDy neurons express KOR than NK3R [105], although the higher efficacy of in vivo KOR blockade vs in brain slices may indicate dynorphin acts via cell populations not present in the brain slice to inhibit LH pulses in vivo. Together these observations point to a hypothesis that KNDy neurons form an interconnected network that is modulated by its peptide products to determine their rhythmic output to GnRH neurons, thus affecting LH pulses.
To test the sufficiency of KNDy neurons to trigger a pulse of LH release, studies were conducted using Cre-dependent mice and adeno-associated viral (AAV) vectors. Activation of KNDy neurons using optogenetic channelrhodopsin2 (ChR2) in vivo triggers a pulse of LH release, confirming their capability to elicit LH release either directly or indirectly [106]. The calcium indicator GCaMP6 and fiber photometry were utilized to estimate bulk arcuate kisspeptin neuron activity based on fluctuations in calcium-sensitive fluorescence. Increases in fluorescence of KNDy neurons correlates with LH pulse release [95,107]. From a loss-of-function standpoint, KNDy neuron ablation was achieved by delivering diphtheria toxin A to mice expressing the toxin receptor in kisspeptin cells. When done on postnatal day 20, when the diphtheria toxin receptor is primarily expressed in arcuate but not AVPV kisspeptin neurons [108], these mice exhibit persistent diestrus as adults, suggesting KNDy neurons are required for establishing/maintaining cyclicity [109]; of note, the integrity of the AVPV kisspeptin population was not assessed in adult mice in these studies. Similarly, blocking the release of neuropeptides and neurotransmitters from KNDy neurons using a Cre-dependent AAV expressing the light chain of tetanus toxin halted the reproductive cycle, with mice again remaining in diestrus; these mice also had decreased LH levels [110]. These in vivo studies further suggest KNDy neurons are at least a component of the pulse-generating or conveying system.
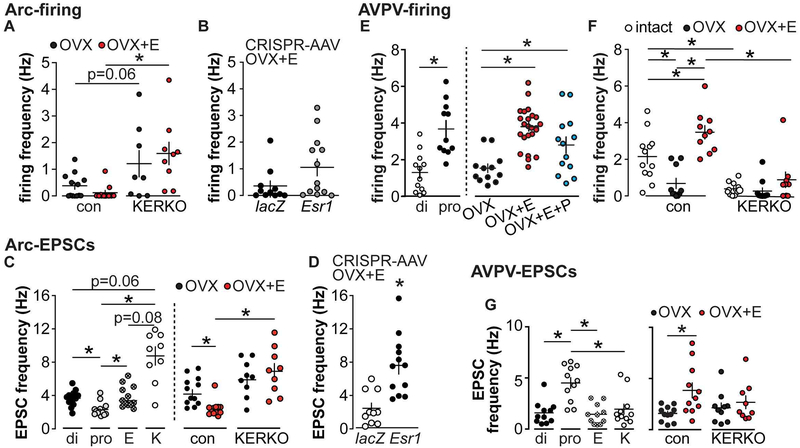
Besides displaying rhythmic activity, KNDy neurons are also capable of receiving and being regulated by steroids, including estradiol. This modulation happens at multiple levels. First, estradiol decreases kisspeptin, NKB and NK3R expression in these cells [73,74,97]. Second, estradiol reduces the excitatory effect of NKB and enhances the inhibitory effect of dynorphin on KNDy neuron firing rate [100,111]. The effects of steroids on KNDy neuron firing rate is complicated. In early short-term extracellular recordings of these cells, results were inconsistent but typically revealed no effect of castration in females or males [100,101]. More recent work examining OVX vs OVX+E females demonstrated a trend for estradiol to reduce firing rate; no statistical difference was revealed in two-way ANOVA analysis (Figure 1A) [112], but a direct comparison of these groups with greater power in a recent preliminary report revealed a suppression in OVX+E females [113]. There is only one study of long-term firing pattern, which was done in male mice. This work suggest sex steroids, including estradiol, modulate firing patterns of KNDy neurons but not the overall mean firing rate over a couple hours [104]. Fast synaptic inputs to KNDy neurons are also regulated by estradiol, as estradiol decreases glutamatergic input frequency (Figure 1C) and GABAergic input amplitude to KNDy neurons [112,114]. These suggest that estradiol may alter KNDy neuron activity both directly and via modulating afferent systems. With regard to the latter, the interconnected nature of KNDy neurons and their use of glutamate as a cotransmitter [71] may indeed be a direct KNDy neuron network effect, whereas GABA changes are more likely via distinct cells.
Figure 1.
Estradiol regulation of firing rate and EPSC frequency in arcuate and AVPV kisspeptin neurons of the hypothalamus. A, Firing rate of arcuate kisspeptin neurons is elevated in cells from KERKO compared to control mice. B, Firing rate is not different between cells from Arc-AAV-Esr1 and Arc-AAV-lacZ OVX+E mice. C, Spontaneous glutamatergic EPSC frequency is regulated by cycle stage (left) and estradiol (right) in arcuate kisspeptin neurons. E, estrus, K, KERKO. D, Knockdown of ERα targeted to the arcuate increases glutamatergic inputs to arcuate KNDy neurons. E, Firing rate of AVPV kisspeptin neurons is elevated during proestrus (left) and by estradiol (right). F, The firing rate decreases in cells from KERKO compared to control and is no longer estradiol-sensitive. G, Spontaneous glutamatergic EPSC frequency is regulated by cycle stage (left) and estradiol (right) in AVPV kisspeptin neurons. *, p<0.05; adapted from [112,119,137] with permission.
The importance of estradiol-sensing in KNDy neurons is demonstrated by two Cre-lox genetic mouse models: a kisspeptin-specific ERα knockout (KERKO) and an NKB (Tac2 gene produce)-specific ERα knockout (TERKO) [115,116]. In KERKO mice, deletion of ERα from kisspeptin cells leads to advanced vaginal opening during development but disrupted cyclicity (persistent estrus) in adults [116]. In this model, however, ERα is removed from both AVPV and arcuate kisspeptin neurons as well as other kisspeptin cells located centrally and peripherally, making the interpretation of a specific role of arcuate kisspeptin neurons difficult. In the TERKO model, ERα is removed largely from the arcuate kisspeptin neurons in the brain [115]. TERKO mice also exhibited advanced vaginal opening and prolonged estrus, similar to the KERKO mice, suggesting the phenotypes may largely attribute to KNDy neurons, and that ERα in kisspeptin cells, particularly KNDy neurons, is critical for reproductive function including puberty and cyclicity.
KERKO mice have also been used to study the mechanisms of how estradiol modulates KNDy neuron activity and LH pulse generation. To test if KERKO mice are able to respond to negative feedback regulation, plasma LH levels were measured in OVX and OVX+E control and KERKO mice. The post-OVX rise in KERKO mice was reduced compared to controls, but estradiol was able to reduce LH levels in both groups [117]. These findings led to the postulate that arcuate kisspeptin neurons may not be necessary to mediate negative feedback. Examination of LH pluses with frequent sampling revealed elevated pulse frequency in ovary-intact KERKO mice compared to estrous controls, suggesting frequency modulating effects of estradiol are likely at least in part mediated by ERα in kisspeptin cells [112]. Interestingly, KERKO mice are also less responsive to kisspeptin and GnRH challenge in terms of LH release [112]. Biophysical studies of KNDy neurons in KERKO mice further reveal several critical roles ERα plays. KNDy neurons in KERKO mice exhibit elevated firing rate (Figure 1 A), and received elevated spontaneous and action potential-independent glutamatergic transmission compared to controls [112] (Figure 1 C). Further, when OVX vs OVX+E mice were compared, estradiol suppressed glutamatergic transmission and firing rate in controls but not KERKO mice (Figure 1 A and C). This suggests the lack of ERα in kisspeptin cells leads to a lack of response to estradiol in these cells [112]. The loss of ERα signaling and subsequent elevated LH pulse frequency may contribute to the disrupted cyclicity [112], as modulation of GnRH/LH frequency is critical for maintaining normal cyclicity.
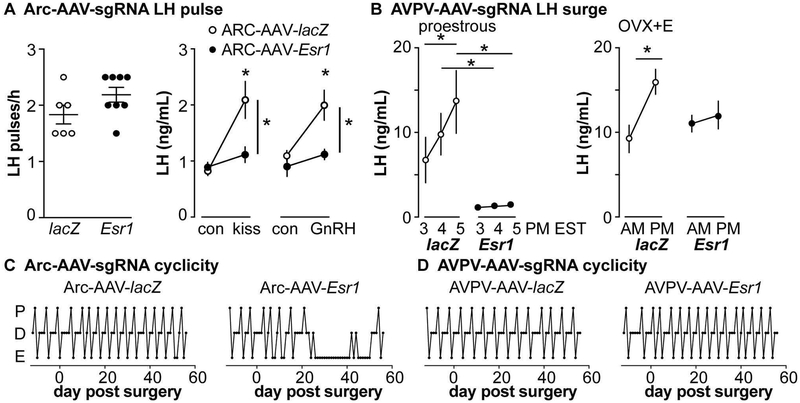
Although informative to understand estradiol negative feedback on GnRH-KNDy network, this Cre-lox based KERKO model has its own caveats. Specifically, it is impossible to distinguish activational and organizational roles of ERα, as ERα is deleted as soon as the kisspeptin gene turns on, before birth in KNDy neurons and before puberty in AVPV kisspeptin neurons [108,118]. Further, ERα is deleted from all kisspeptin cells. Spatial and temporal precision is needed to dissect the role of KNDy neurons in reproduction. To overcome these caveats, a CRISPR-Cas9 approach was employed to reduce ERα in the arcuate kisspeptin neurons in adult mice [119]. This model utilized the Cre-lox system to express the Cas9 protein in kisspeptin cells. Then, in adult mice, an AAV vector that expresses an sgRNA that targets Esr1 (ERα gene) was injected into the arcuate region (Arc-AAV-Esr1). As a control, an AAV vector targeting the lacZ gene was introduced in the same manner (Arc-AAV-lacZ); these mice were not different from control females for the parameters measured [119]. To reduce the caveats of CRISPR off-target effects, two independent sgRNAs were used and independently tested; no detectable differences were found between these. This approach achieved partial (~65%) knockdown of ERα in kisspeptin cells specifically in the arcuate region. Despite the partial nature of this knockdown, these Arc-AAV-Esr1 mice exhibited disrupted reproductive cyclicity, spending prolonged time in estrus, and reduced response to kisspeptin and GnRH administration compared to Arc-AAV-lacZ; both of these responses are similar to the KERKO mode (Figure 2 A and C) [112,115,116,119]. In contrast to KERKO mice, which exhibit increased LH-pulse frequency, no changes of pulse frequency were observed in Arc-AAV-Esr1 mice (Figure 2A). This may be attributable to these mice being singly housed, whereas the KERKO mice examined for LH pulses were group housed; single housing can increase stress, which makes pulses harder to detect [120].
Figure 2.
Distinct roles of arcuate and AVPV kisspeptin neurons in regulating estradiol feedback and reproductive function. A, Knockdown of ERα in arcuate kisspeptin neurons did not alter LH-pulse frequency on estrus (left) but desensitized the LH response to kisspeptin and GnRH challenge. B, Knockdown of ERα in AVPV kisspeptin neurons blunted the proestrous (left) and estradiol-induced (right) surges. C and D, Knockdown of ERα in arcuate (C) but not AVPV (D) kisspeptin neurons alter reproductive cyclicity. Adapted from [119].
In brain slices, KNDy neurons in Arc-AAV-Esr1 mice also shared several biophysical similarities with cells from KERKO mice. Specifically, these neurons tend to be more active (Figure 1 B, p<0.05 two-tailed t-test of log-normalized data, p<0.14 two-tailed Mann-Whitney U test of original data) and receive more glutamatergic inputs compared to Arc-AAV-lacZ (Figure 1 D) [112,119]. Taken together, ERα in KNDy neurons is required to maintain the typical function of the KNDy neuron network and female cycles, independent of developmental roles.
AVPV kisspeptin neurons and estradiol positive feedback
As with the arcuate region and pulses, the AVPV region was associated with surge generation long before the discovery of kisspeptin. AVPV neurons are sexually dimorphic, with more neurons in females, and many express ERα [121,122]. They exhibit increased cFos expression, an immediate early gene with expression often correlated with increased neuronal activity, during the LH surge when GnRH release is elevated [123,124]. Lesions of the AVPV block the preovulatory as well as the estradiol-induced LH surge [125–127]. AVPV kisspeptin neurons, as a subset of AVPV neurons, share these characteristics: they are sexually dimorphic, ERα positive (more than 70%), and express cFos during the LH surge [78,88,128–130]. At least 1/3 of AVPV kisspeptin neurons communicate with GnRH cell bodies; these neurons almost all express ERα [131]. Elevated activity often correlates with neurotransmitter and neuropeptide release, thus cFos expression may indicate increased activity and release from AVPV kisspeptin neurons to their efferent projections including GnRH neurons. Besides expressing the potent GnRH stimulator kisspeptin, AVPV kisspeptin neurons coexpress tyrosine hydroxylase (~70%), and utilize GABA (~75%) and glutamate (~20%) [71,132], both of which excite GnRH neurons, as cotransmitters [133,134]. The role of dopamine, a product of TH-expressing neurons, on GnRH neuron function is not very well defined. When TH is knocked out of kisspeptin cells, mice exhibit normal reproduction [135], suggesting kisspeptin and GABA might be the main resource for GnRH excitation [136]. Together these observations help to build a model that AVPV kisspeptin neurons are regulated by estradiol to increase activity during the GnRH/LH surge.
To investigate this model at a more mechanistic level, studies were conducted to test if AVPV kisspeptin neurons are more excitable during estradiol positive feedback (proestrus) compared to negative feedback (diestrus). Extracellular recordings of GFP-identified neurons in brain slices were made of GFP-identified AVPV kisspeptin cells. These neurons firing more action potentials and exhibit a greater degree of rapid action potential bursts on proestrus compared to diestrus and estrus (Figure 1 E, left) [137,138]. Hormonal manipulations (OVX+ E, OVX+ E+ progesterone) suggest that it is primarily estradiol that mediates these changes as OVX+E mice recapitulate the firing characteristics observed in cells from proestrous mice, whereas OVX+E+P mice are not different from those receiving only estradiol replacement (Figure 1 E, right). Several ionic conductances have been identified in AVPV kisspeptin neurons, including hyperpolarization-activated cation channels, T-type calcium channels, and persistent sodium channels [137,139,140]. All three of these channels have been demonstrated to promote burst firing and pace-making in other neurons [141–143]. Both electrophysiological recordings measuring ionic currents and mRNA expression of these ion channel genes in pooled AVPV kisspeptin cells suggest these conductances are upregulated by estradiol [137,139,140]. These increases in burst-related ionic conductances in AVPV kisspeptin neurons may contribute to the increased firing activity of these neurons during the time of the GnRH/LH surge provide a mechanism for how estradiol modulates AVPV kisspeptin neuronal firing to facilitate positive feedback. Besides exhibiting cycle/estradiol-dependent ionic conductances, AVPV kisspeptin neurons also received increased excitatory glutamatergic inputs (Figure 1 G) and decreased inhibitory GABAergic transmission during positive feedback [112,114], tilting the balance toward excitation during positive feedback. These observations suggest that estradiol-sensing positive feedback circuitry may extend beyond AVPV kisspeptin neurons, as their upstream inputs are also modulated by estradiol.
To test if stimulation of AVPV kisspeptin neurons is sufficient to generate LH secretion in vivo, ChR2 was targeted to these cells. Photostimulation of AVPV kisspeptin neurons for ~15 minutes induced LH release secretion of similar amplitude to the endogenous LH surge, but the time course was more similar to a pulse than a prolonged surge release [136]. These results demonstrate activation of AVPV kisspeptin cells likely increases GnRH and thereby LH release, but also suggest induction of surge release may need prolonged activation of AVPV kisspeptin neurons.
The KERKO model has also been used to study role of ERα in kisspeptin cells, including AVPV kisspeptin neurons, in positive feedback. KERKO mice remain in estrus and exhibit high circulating estradiol, thus an estradiol rise surge model was used to study estradiol positive feedback. KERKO mice failed to generate estradiol-induced LH surges, suggesting ERα in kisspeptin cells is required for estradiol positive feedback [117]. From a biophysical aspect, AVPV kisspeptin neurons from KERKO mice are less excitable (Figure 1 F) and receive fewer glutamatergic inputs compared to littermate controls (Figure 1 G). Further, these typically estradiol-sensitive parameters also no longer regulated by estradiol in OVX vs OVX+E mice (Figure 1 F and G) [112,119]. This indicates that ERα plays a necessary role in modulating the excitability of AVPV kisspeptin neurons to trigger LH surge [119]. Although informative, this model also has the caveats mentioned above regarding a lack of spatial and temporal precision. These caveats are potentially more serious in the AVPV population as kisspeptin cell number in the AVPV drops when estradiol is removed.
The CRISPR approach again provides space and time-specific regulation of ERα in AVPV kisspeptin neurons. Reduction of Esr1 in the AVPV region was achieved as above using kisspeptin-Cre targeting of Cas9 and delivery of sgRNAs targeting the Esr1 gene (AVPV-AAV-Esr1) in adulthood [119]. In AAV-AVPV- Esr1 mice, ~35% of kisspeptin neurons express ERα vs 70% in control mice that received AAV-AVPV-lacZ. To test if ERα deletion in AVPV kisspeptin neurons alters neuronal firing properties in OVX+E mice, whole-cell recordings were paired with post hoc testing (immunofluorescence of biocytin labeled recorded cells or single-cell qPCR) to determine expression of ERα in each recorded cell. In OVX+E mice with AAV-AVPV-Esr1, only ERα negative AVPV kisspeptin neurons exhibited decreased firing rate and bursts compared to AVPV-AAV-lacZ infected cells and AAV-AVPV-Esr1 uninfected cells [119]. These responses are similar to changes that occur in these cells in KERKO mice, suggesting the primary effect of estradiol on the intrinsic electrophysiological properties of these cells is activational.
From a systemic aspect, these mice maintained normal cyclicity for at least two months post surgery; in contrast, disruptions of cyclicity when the arcuate kisspeptin population was targeted began to emerge within three weeks (Figure 2 D) [119]. It is possible that the remaining ERα expressing AVPV kisspeptin neurons are sufficient to maintain cyclicity. Alternatively, cyclicity may be maintained by other neurons, such as the arcuate KNDy neurons. In a recent study, genetic deletion of ERα broadly in the preoptic area and AVPV region, but not arcuate of adult female mice produced persistent estrus and decreased the amplitude of the estradiol-induced LH surge using the estradiol rise surge-induction model [144]. In the area covered by this knockdown many cells besides AVPV kisspeptin neurons express ERα [145] making it difficult to ascribe these results to a specific cell type.
Despite having normal cycles, AVPV-AAV-Esr1 mice had, at best, blunted LH surges, both proestrus and estradiol induced (Figure 2 B). Although a reduced proestrous surge could be attributable to reduced estradiol levels, this caveat is minimized by the demonstration that estradiol-induced surges are also reduced. Further, the estradiol levels produced in the AVPV-AAV-Esr1 mice are sufficient to induce vaginal cornification. Consistent with blunted LH surges, ovarian histology showed reduced or absent corpra lutea in two-thirds of knockdown mice compared to AVPV-AAV-lacZ control mice [119]. These results support and extend much research in the field by demonstrating that ERα in AVPV kisspeptin neurons is important for positive feedback and LH surge generation.
Conclusion and future directions
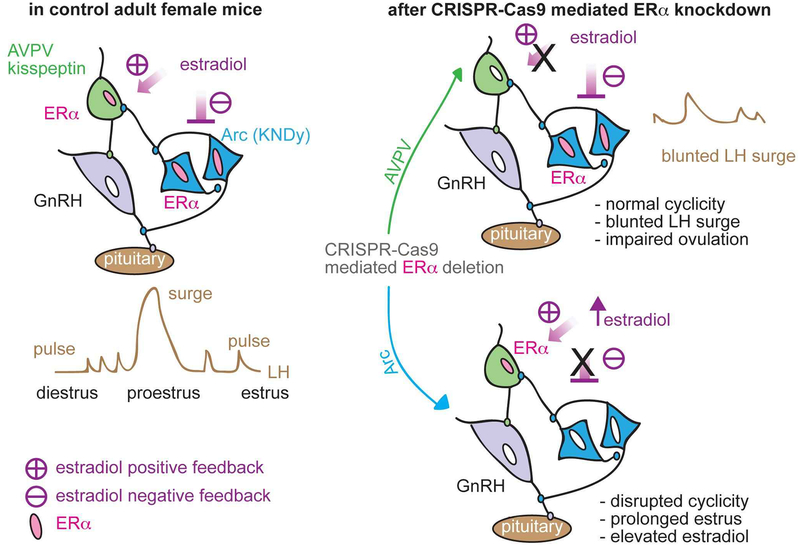
Application of modern genetic approaches to the long-existing questions of where estradiol acts to bring about negative and positive feedback has brought more insights into the regulation of GnRH/LH pulses and surges. There is now strong evidence that arcuate and AVPV kisspeptin neurons play distinct roles in mediating the response to systemic changes in estradiol, regulating cyclicity and the LH surge, respectively. Because CRISPR-mediated changes were induced in adults, we can conclude that the observations are not attributable to a loss of ERα action during development (Figure 3).
Figure 3.
Schematic diagram of estradiol feedback regulation on ERα in AVPV and arcuate kisspeptin neurons in adulthood. Knockdown of ERα in AVPV kisspeptin neurons blunted LH surge but did not alter reproductive cyclicity whereas knockdown of ERα in arcuate kisspeptin neurons disrupted the cyclicity. From [119].
Several major areas of investigation remain regarding the kisspeptin-GnRH circuitry. First, AVPV and arcuate kisspeptin neurons receive and process estradiol signals, but we do not know if estradiol action in their estrogen-sensitive afferents is critical. It is possible that reducing ERα in our target kisspeptin populations merely blocked processing of incoming signals from the true first-order responding cells. Are these signals from upstream cells required and, if so, what and where are these neurons. Further, do AVPV kisspeptin neurons require the input from arcuate kisspeptin neurons to mediate the switch from negative to positive feedback? Multi-region spectrum-specific fiber photometry approaches may be applied to monitor simultaneously the activity of AVPV and arcuate neuronal activity in each distinct reproductive stage [146,147]. This approach could also be utilized to test if there is synchrony between arcuate kisspeptin neurons and GnRH neurons.
Second, how AVPV and arcuate kisspeptin neurons convey their feedback modulation to GnRH neurons is not completely understood. Does increased AVPV and/or arcuate kisspeptin neuronal activity lead to increased neurosecretion? Assuming from work on other systems that the answer is yes, are the cotransmitters and other peptides in these cells important or is kisspeptin the primary player? Further, is all of the kisspeptin communication received directly by GnRH neurons? Studies in global Kiss1r knockout mice have suggested that replacing these receptors only in GnRH neurons restore fertility [87]. Further work on these mice, however, has demonstrated that aspects of steroid feedback, gonadal structure, and gonadotropin release are not fully restored [148]. Consistent with this latter finding, kisspeptin treatment increases fast synaptic transmission to GnRH neurons, indicating there are indirect pathways involving at least GABA and glutamate through which this neuromodulator may influence GnRH output [149]. One population of interest in this regard are neurons that utilize nitric oxide (NO) as a neurotransmitter; NO has been proposed as a synchrony signal to GnRH neurons [150,151]. Further, nitric oxide synthase (nNOs) expressing neurons in the median preoptic nucleus (MnPO) region also express Kiss1r [152], making them another possible intermediate between kisspeptin and GnRH neurons. Comprehensive projection mapping and direct stimulation and/or inhibition of nNOs neurons in vivo are needed to test this postulate.
Third, it is still not clear how estradiol regulates gene expression profiles to change intrinsic neuronal activity of AVPV and arcuate kisspeptin neurons. Of particular interest in this regard are the mechanisms underlying the many-hour delay from achieving a surge-inducing level of estradiol and the onset of the GnRH/LH surge. The length of this delay and the ability to remove estradiol before the surge is initiated without affecting it imply genomic mechanisms [12], but detailed temporal profiling of gene expressing spanning this gap is lacking. Advanced single-cell and/or single-nucleus sequencing approaches may shed light on the steps involved in estradiol feedback regulation on these cells. Recently, two droplet-based single-cell RNA-sequencing studies of the arcuate and POA region provide intriguing data for identify different populations of neurons and their transcriptomes, including the two kisspeptin populations; this detailed information allows potential reimagining of how different neuronal populations are related to one another [153,154]. Future studies should include studying these neurons at precise times under different hormone treatments and/or distinct cycle stages to reveal the time course of estradiol-dependent gene expression profiles, and generate hypotheses for future physiological investigations.
Support
Supported by National Institute of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development R01 HD41469.
Footnotes
Reprints requests should be addressed to corresponding author
Disclosure statement: The authors have nothing to disclose.
References
- 1.Fink G: Neuroendocrine Regulation of Pituitary Function; in : Neuroendocrinology in Physiology and Medicine. Totowa, NJ, Humana Press, 2000, pp 107–133. [Google Scholar]
- 2.Plant TM, Zeleznik AJ, Herbison AE: Physiology of the Adult Gonadotropin-Releasing Hormone Neuronal Network. Knobil and Neill’s Physiology of Reproduction 2015;399–467. [Google Scholar]
- 3.Silverman A, Livne I, Witkin JW: The gonadotrophin-releasing hormone (GnRH), neuronal systems: immunocytochemistry and in situ hybridization; in Neill J (ed): Physiology of reproduction, 2nd edition, ed 2nd New York, Raven Press, 1994, pp 1683–1706. [Google Scholar]
- 4.Knobil E, Plant TM, Wildt L, Belchetz PE, Marshall G: Control of the rhesus monkey menstrual cycle: permissive role of hypothalamic gonadotropin-releasing hormone. Science 1980;207:1371–3. [DOI] [PubMed] [Google Scholar]
- 5.Clarke IJ, Cummins JT: The temporal relationship between gonadotropin releasing hormone (GnRH) and luteinizing hormone (LH) secretion in ovariectomized ewes. Endocrinology 1982;111:1737–1739. [DOI] [PubMed] [Google Scholar]
- 6.Moenter SM, Brand RC, Karsch FJ: Dynamics of gonadotropin-releasing hormone (GnRH) secretion during the GnRH surge: insights into the mechanism of GnRH surge induction. Endocrinology 1992;130:2978–84. [DOI] [PubMed] [Google Scholar]
- 7.Marshall JC, Griffin ML: The role of changing pulse frequency in the regulation of ovulation. Human Reproduction 1993;8:57–61. [DOI] [PubMed] [Google Scholar]
- 8.Haisenleder DJ, Khoury S, Zmeili SM, Papavasiliou S, Ortolano GA, Dee C, et al. : The Frequency of Gonadotropin-Releasing Hormone Secretion Regulates Expression of a and Luteinizing Hormone β-Subunit Messenger Ribonucleic Acids in Male Rats. Molecular Endocrinology 1987;1:834–838. [DOI] [PubMed] [Google Scholar]
- 9.Wildt L, Hausler A, Marshall G, Hutchison JS, Plant TM, Belchetz PE, et al. : Frequency and amplitude of gonadotropin-releasing hormone stimulation and gonadotropin secretion in the rhesus monkey. Endocrinology 1981;109:376–385. [DOI] [PubMed] [Google Scholar]
- 10.Plant TM, Zeleznik AJ: Knobil and Neill’s physiology of reproduction 4th edition. Ed 4thEdition Elsevier, 2015. [Google Scholar]
- 11.Goodman RL, Daniel K: Modulation of pulsatile luteinizing hormone secretion by ovarian steroids in the rat. Biology of reproduction 1985;32:217–25. [DOI] [PubMed] [Google Scholar]
- 12.Evans NP, Dahl GE, Padmanabhan V, Thrun LA, Karsch FJ: Estradiol Requirements for Induction and Maintenance of the Gonadotropin-Releasing Hormone Surge: Implications for Neuroendocrine Processing of the Estradiol Signal. Endocrinology 1997;138:5408–5414. [DOI] [PubMed] [Google Scholar]
- 13.Leipheimer RE, Bona-Gallo A, Gallo RV.: Ovarian Steroid Regulation of Pulsatile Luteinizing Hormone Release during the Interval between the Mornings of Diestrus 2 and Proestrus in the Rat. Neuroendocrinology 2008;41:252–257. [DOI] [PubMed] [Google Scholar]
- 14.Adams TE, Norman RL, Spies HG: Gonadotropin-Releasing Hormone Receptor Binding and Pituitary Responsiveness in Estradiol-Primed Monkeys. Science 213:1388–1390. [DOI] [PubMed] [Google Scholar]
- 15.Barrell GK, Moenter SM, Cahaty A, Karsch FJ: Seasonal Changes of Gonadotropin-Releasing Hormone Secretion in the Ewe1. Biology of Reproduction 1992;46:1130–1135. [DOI] [PubMed] [Google Scholar]
- 16.Hileman SM, Jackson GL: Regulation of gonadotrophin-releasing hormone secretion by testosterone in male sheep. Journal of reproduction and fertility Supplement 1999;54:231–42. [PubMed] [Google Scholar]
- 17.Christian CA, Mobley JL, Moenter SM: Diurnal and estradiol-dependent changes in gonadotropin-releasing hormone neuron firing activity. Proceedings of the National Academy of Sciences of the United States of America 2005;102:15682–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glanowska KM, Venton BJ, Moenter SM: Fast scan cyclic voltammetry as a novel method for detection of real-time gonadotropin-releasing hormone release in mouse brain slices. The Journal of neuroscience: the official journal of the Society for Neuroscience 2012;32:14664–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caraty A, Locatelli A, Martin GB: Biphasic response in the secretion of gonadotrophin-releasing hormone in ovariectomized ewes injected with oestradiol. The Journal of endocrinology 1989;123:375–82. [DOI] [PubMed] [Google Scholar]
- 20.Pielecka J, Quaynor SD, Moenter SM: Androgens Increase Gonadotropin-Releasing Hormone Neuron Firing Activity in Females and Interfere with Progesterone Negative Feedback. Endocrinology 2006;147:1474–1479. [DOI] [PubMed] [Google Scholar]
- 21.Pielecka J, Moenter SM: Effect of Steroid Milieu on Gonadotropin-Releasing Hormone-1 Neuron Firing Pattern and Luteinizing Hormone Levels in Male Mice. Biology of Reproduction 2006;74:931–937. [DOI] [PubMed] [Google Scholar]
- 22.Karsch FJ, Cummins JT, Thomas GB, Clarke IJ: Steroid feedback inhibition of pulsatile secretion of gonadotropin-releasing hormone in the ewe. Biology of reproduction 1987;36:1207–18. [DOI] [PubMed] [Google Scholar]
- 23.Moenter SM, Caraty A, Karsch FJ: The estradiol-induced surge of gonadotropin-releasing hormone in the ewe. Endocrinology 1990;127:1375–84. [DOI] [PubMed] [Google Scholar]
- 24.Crowder ME, Nett TM: Pituitary Content of Gonadotropins and Receptors for Gonadotropin-Releasing Hormone (GnRH) and Hypothalamic Content of GnRH during the Periovulatory Period of the Ewe. Endocrinology 1984;114:234–239. [DOI] [PubMed] [Google Scholar]
- 25.Sarkar DK, Chiappa SA, Fink G, Sherwood NM: Gonadotropin-releasing hormone surge in pro-oestrous rats. Nature 1976;264:461–3. [DOI] [PubMed] [Google Scholar]
- 26.Adams JM, Taylor AE, Schoenfeld DA, Crowley WF, Hall JE: The midcycle gonadotropin surge in normal women occurs in the face of an unchanging gonadotropin-releasing hormone pulse frequency. The Journal of Clinical Endocrinology & Metabolism 1994;79:858–864. [DOI] [PubMed] [Google Scholar]
- 27.Pau KY, Berria M, Hess DL, Spies HG: Preovulatory gonadotropin-releasing hormone surge in ovarian-intact rhesus macaques. Endocrinology 1993;133:1650–1656. [DOI] [PubMed] [Google Scholar]
- 28.Xia L, Van Vugt D, Alston EJ, Luckhaus J, Ferin M: A surge of gonadotropin-releasing hormone accompanies the estradiol-induced gonadotropin surge in the rhesus monkey. Endocrinology 1992; 131:2812–2820. [DOI] [PubMed] [Google Scholar]
- 29.Karsch FJ, Bowen JM, Caraty A, Evans NP, Moenter SM: Gonadotropin-releasing hormone requirements for ovulation. Biology of reproduction 1997;56:303–9. [DOI] [PubMed] [Google Scholar]
- 30.Kaynard AH, Malpaux B, Robinson JE, Wayne NL, Karsch FJ: Importance of Pituitary and Neural Actions of Estradiol in Induction of the Luteinizing Hormone Surge in the Ewe. Neuroendocrinology 1988;48:296–303. [DOI] [PubMed] [Google Scholar]
- 31.Terasawa E: Neuroestradiol in regulation of GnRH release. Hormones and Behavior 2018;104:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dror T, Franks J, Kauffman AS: Analysis of Multiple Positive Feedback Paradigms Demonstrates a Complete Absence of LH Surges and GnRH Activation in Mice Lacking Kisspeptin Signaling. Biology of Reproduction 2013;88:146–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Silveira MA, Burger LL, DeFazio RA, Wagenmaker ER, Moenter SM: GnRH Neuron Activity and Pituitary Response in Estradiol-Induced vs Proestrous Luteinizing Hormone Surges in Female Mice. Endocrinology 2017;158:356–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bronson FH: The regulation of luteinizing hormone secretion by estrogen: relationships among negative feedback, surge potential, and male stimulation in juvenile, peripubertal, and adult female mice. Endocrinology 1981;108:506–16. [DOI] [PubMed] [Google Scholar]
- 35.Pielecka-Fortuna J, DeFazio RA, Moenter SM: Voltage-gated potassium currents are targets of diurnal changes in estradiol feedback regulation and kisspeptin action on gonadotropin-releasing hormone neurons in mice. Biology of reproduction 2011;85:987–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun J, Chu Z, Moenter SM: Diurnal in vivo and rapid in vitro effects of estradiol on voltage-gated calcium channels in gonadotropin-releasing hormone neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience 2010;30:3912–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang C, Bosch MA, Rick EA, Kelly MJ, Rønnekleiv OK: 17Beta-estradiol regulation of T-type calcium channels in gonadotropin-releasing hormone neurons. The Journal of neuroscience: the official journal of the Society for Neuroscience 2009;29:10552–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chu Z, Takagi H, Moenter SM: Hyperpolarization-activated currents in gonadotropin-releasing hormone (GnRH) neurons contribute to intrinsic excitability and are regulated by gonadal steroid feedback. The Journal of neuroscience: the official journal of the Society for Neuroscience 2010;30:13373–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Christian CA, Pielecka-Fortuna J, Moenter SM: Estradiol suppresses glutamatergic transmission to gonadotropin-releasing hormone neurons in a model of negative feedback in mice. Biology of reproduction 2009;80:1128–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christian CA, Moenter SM: Estradiol induces diurnal shifts in GABA transmission to gonadotropin-releasing hormone neurons to provide a neural signal for ovulation. The Journal of neuroscience: the official journal of the Society for Neuroscience 2007;27:1913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Adams C, Stroberg W, DeFazio RA, Schnell S, Moenter SM: Gonadotropin-Releasing Hormone (GnRH) Neuron Excitability Is Regulated by Estradiol Feedback and Kisspeptin. The Journal of Neuroscience 2018;38:1249–1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Adams C, DeFazio RA, Christian CA, Milescu LS, Schnell S, Moenter SM: Changes in Both Neuron Intrinsic Properties and Neurotransmission Are Needed to Drive the Increase in GnRH Neuron Firing Rate during Estradiol-Positive Feedback. The Journal of Neuroscience 2019;39:2091–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA: Cloning of a novel receptor expressed in rat prostate and ovary. Proceedings of the National Academy of Sciences of the United States of America 1996;93:5925–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tremblay GB, Tremblay A, Copeland NG, Gilbert DJ, Jenkins NA, Labrie F, et al. : Cloning, Chromosomal Localization, and Functional Analysis of the Murine Estrogen Receptor β. Molecular Endocrinology 1997;11:353–365. [DOI] [PubMed] [Google Scholar]
- 45.Koike S, Sakai M, Muramatsu M: Molecular cloning and characterization of rat estrogen receptor cDNA. Nucleic acids research 1987;15:2499–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Micevych PE, Mermelstein PG, Sinchak K: Estradiol membrane-initiated signaling in the brain mediates reproduction. Trends in neurosciences 2017;40:654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Micevych PE, Kelly MJ: Membrane Estrogen Receptor Regulation of Hypothalamic Function. Neuroendocrinology 2012;96:103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Olde B, Leeb-Lundberg LMF: GPR30/GPER1: searching for a role in estrogen physiology. Trends in Endocrinology & Metabolism 2009;20:409–416. [DOI] [PubMed] [Google Scholar]
- 49.Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schütz G, Umesono K, et al. : The nuclear receptor superfamily: the second decade. Cell 1995;83:835–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Björnström L, Sjöberg M: Mechanisms of Estrogen Receptor Signaling: Convergence of Genomic and Nongenomic Actions on Target Genes. Molecular Endocrinology 2005;19:833–842. [DOI] [PubMed] [Google Scholar]
- 51.Ayaz G, Yasar P, Olgun CE, Karakaya B, Kars G, Razizadeh N, et al. : Dynamic transcriptional events mediated by estrogen receptor alpha. Frontiers in bioscience (Landmark edition) 2019;24:245–276. [DOI] [PubMed] [Google Scholar]
- 52.Qiu J, Ronnekleiv O, Kelly M: Modulation of hypothalamic neuronal activity through a novel G-protein-coupled estrogen membrane receptor. Steroids 2008;73:985–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chu Z, Andrade J, Shupnik MA, Moenter SM: Differential regulation of gonadotropin-releasing hormone neuron activity and membrane properties by acutely applied estradiol: dependence on dose and estrogen receptor subtype. The Journal of neuroscience: the official journal of the Society for Neuroscience 2009;29:5616–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mhyre AJ, Dorsa DM: Estrogen activates rapid signaling in the brain: Role of estrogen receptor α and estrogen receptor β in neurons and glia. Neuroscience 2006;138:851–858. [DOI] [PubMed] [Google Scholar]
- 55.Moss RL, Gu Q, Wong M: Estrogen: nontranscriptional signaling pathway. Recent progress in hormone research 1997;52:33–68; discussion 68–9. [PubMed] [Google Scholar]
- 56.Kelly MJ, Qiu J: Estrogen signaling in hypothalamic circuits controling reproduction. Brain Research 2010;1364:44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Christian CA, Glidewell-Kenney C, Jameson JL, Moenter SM: Classical estrogen receptor α signaling mediates negative and positive feedback on gonadotropin-releasing hormone neuron firing. Endocrinology 2008;149:5328–5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Glidewell-Kenney C, Hurley LA, Pfaff L, Weiss J, Levine JE, Jameson JL: Nonclassical estrogen receptor signaling mediates negative feedback in the female mouse reproductive axis. Proceedings of the National Academy of Sciences 2007;104:8173–8177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lubahn DB, Moyer JS, Golding TS, Couse JF, Korach KS, Smithies O: Alteration of reproductive function but not prenatal sexual development after insertional disruption of the mouse estrogen receptor gene. Proceedings of the National Academy of Sciences of the United States of America 1993;90:11162–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Krege JH, Hodgin JB, Couse JF, Enmark E, Warner M, Mahler JF, et al. : Generation and reproductive phenotypes of mice lacking estrogen receptor beta. Proceedings of the National Academy of Sciences of the United States of America 1998;95:15677–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wersinger SR, Haisenleder DJ, Lubahn DB, Rissman EF: Steroid Feedback on Gonadotropin Release and Pituitary Gonadotropin Subunit mRNA in Mice Lacking a Functional Estrogen Receptor a. Endocrine 1999;11:137–144. [DOI] [PubMed] [Google Scholar]
- 62.Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone H-J, Todman MG, et al. : Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron 2006;52:271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Noel SD, Keen KL, Baumann DI, Filardo EJ, Terasawa E: Involvement of G Protein-Coupled Receptor 30 (GPR30) in Rapid Action of Estrogen in Primate LHRH Neurons. Molecular Endocrinology 2009;23:349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hrabovszky E, Steinhauser A, Barabas K, Shughrue PJ, Petersen SL, Merchenthaler I, et al. : Estrogen receptor-beta immunoreactivity in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology 2001;142:3261–4. [DOI] [PubMed] [Google Scholar]
- 65.de Roux N, Genin E, Carel J-C, Matsuda F, Chaussain J-L, Milgrom E: Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proceedings of the National Academy of Sciences 2003;100:10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Shagoury JK, et al. : The GPR54 Gene as a Regulator of Puberty. New England Journal of Medicine 2003;349:1614–1627. [DOI] [PubMed] [Google Scholar]
- 67.d’Anglemont de Tassigny X, Fagg LA, Dixon JPC, Day K, Leitch HG, Hendrick AG, et al. : Hypogonadotropic hypogonadism in mice lacking a functional Kiss1 gene. Proceedings of the National Academy of Sciences 2007;104:10714–10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ohtaki T, Shintani Y, Honda S, Matsumoto H, Hori A, Kanehashi K, et al. : Metastasis suppressor gene KISS-1 encodes peptide ligand of a G-protein-coupled receptor. Nature 2001;411:613–617. [DOI] [PubMed] [Google Scholar]
- 69.Bhattacharya M, Babwah AV.: Kisspeptin: Beyond the Brain. Endocrinology 2015;156:1218–1227. [DOI] [PubMed] [Google Scholar]
- 70.Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA: Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. The Journal of neuroscience: the official journal of the Society for Neuroscience 2006;26:6687–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cravo RM, Margatho LO, Osborne-Lawrence S, Donato J, Atkin S, Bookout AL, et al. : Characterization of Kiss1 neurons using transgenic mouse models. Neuroscience 2011;173:37–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roseweir AK, Kauffman AS, Smith JT, Guerriero KA, Morgan K, Pielecka-Fortuna J, et al. : Discovery of Potent Kisspeptin Antagonists Delineate Physiological Mechanisms of Gonadotropin Regulation. Journal of Neuroscience 2009;29:3920–3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA: Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 2005;146:3686–3692. [DOI] [PubMed] [Google Scholar]
- 74.Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, et al. : Differential Regulation of KiSS-1 mRNA Expression by Sex Steroids in the Brain of the Male Mouse. Endocrinology 2005; 146:2976–2984. [DOI] [PubMed] [Google Scholar]
- 75.Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, et al. : Variation in Kisspeptin and RFamide-Related Peptide (RFRP) Expression and Terminal Connections to Gonadotropin-Releasing Hormone Neurons in the Brain: A Novel Medium for Seasonal Breeding in the Sheep. Endocrinology 2008;149:5770–5782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ramaswamy S, Guerriero KA, Gibbs RB, Plant TM: Structural interactions between kisspeptin and GnRH neurons in the mediobasal hypothalamus of the male rhesus monkey (Macaca mulatta) as revealed by double immunofluorescence and confocal microscopy. Endocrinology 2008;149:4387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lehman MN, Coolen LM, Goodman RL: Minireview: Kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology 2010;151:3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clarkson J, Herbison AE: Postnatal Development of Kisspeptin Neurons in Mouse Hypothalamus; Sexual Dimorphism and Projections to Gonadotropin-Releasing Hormone Neurons. Endocrinology 2006;147:5817–5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yeo SH, Herbison AE: Projections of arcuate nucleus and rostral periventricular kisspeptin neurons in the adult female mouse brain. Endocrinology 2011;152:2387–2399. [DOI] [PubMed] [Google Scholar]
- 80.Ciofi P, Leroy D, Tramu G: Sexual dimorphism in the organization of the rat hypothalamic infundibular area. Neuroscience 2006;141:1731–1745. [DOI] [PubMed] [Google Scholar]
- 81.Herbison AE, d’Anglemont de Tassigny X, Doran J, Colledge WH: Distribution and Postnatal Development of Gpr54 Gene Expression in Mouse Brain and Gonadotropin-Releasing Hormone Neurons. Endocrinology 2010;151:312–321. [DOI] [PubMed] [Google Scholar]
- 82.Lee DK, Nguyen T, O’Neill GP, Cheng R, Liu Y, Howard AD, et al. : Discovery of a receptor related to the galanin receptors. FEBS letters 1999;446:103–7. [DOI] [PubMed] [Google Scholar]
- 83.Pielecka-Fortuna J, Chu Z, Moenter SM: Kisspeptin acts directly and indirectly to increase gonadotropin-releasing hormone neuron activity and its effects are modulated by estradiol. Endocrinology 2008;149:1979–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Han S, Gottsch ML, Lee KJ, Popa SM, Smith JT, Jakawich SK, et al. : Activation of gonadotropin-releasing hormone neurons by kisspeptin as a neuroendocrine switch for the onset of puberty. The Journal of neuroscience: the official journal of the Society for Neuroscience 2005;25:11349–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Messager S, Chatzidaki EE, Ma D, Hendrick AG, Zahn D, Dixon J, et al. : Kisspeptin directly stimulates gonadotropin-releasing hormone release via G protein-coupled receptor 54. Proceedings of the National Academy of Sciences of the United States of America 2005;102:1761–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kinoshita M, Tsukamura H, Adachi S, Matsui H, Uenoyama Y, Iwata K, et al. : Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology 2005;146:4431–6. [DOI] [PubMed] [Google Scholar]
- 87.Kirilov M, Clarkson J, Liu X, Roa J, Campos P, Porteous R, et al. : Dependence of fertility on kisspeptin-Gpr54 signaling at the GnRH neuron. Nature Communications 2013;4:2492. [DOI] [PubMed] [Google Scholar]
- 88.Oakley AE, Clifton DK, Steiner RA: Kisspeptin signaling in the brain. Endocrine Reviews 2009;30:713–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Plant TM, Krey LC, Moossy J, McCormack JT, Hess DL, Knobil E: The Arcuate Nucleus and the Control of Gonadotropin and Prolactin Secretion in the Female Rhesus Monkey (Macaca mulatta). Endocrinology 1978;102:52–62. [DOI] [PubMed] [Google Scholar]
- 90.Soper BD, Weick RF: Hypothalamic and Extrahypothalamic Mediation of Pulsatile Discharges of Luteinizing Hormone in the Ovariectomized Rat. Endocrinology 1980;106:348–355. [DOI] [PubMed] [Google Scholar]
- 91.Mori Y, Nishihara M, Tanaka T, Shimizu T, Yamaguchi M, Takeuchi Y, et al. : Chronic Recording of Electrophysiological Manifestation of the Hypothalamic Gonadotropin-Releasing Hormone Pulse Generator Activity in the Goat. Neuroendocrinology 1991;53:392–395. [DOI] [PubMed] [Google Scholar]
- 92.Kawakami M, Uemura T, Hayashi R: Electrophysiological Correlates of Pulsatile Gonadotropin Release in Rats. Neuroendocrinology 1982;35:63–67. [DOI] [PubMed] [Google Scholar]
- 93.Wilson RC, Kesner JS, Kaufman J-M, Uemura T, Akema T, Knobil E: Central Electrophysiologic Correlates of Pulsatile Luteinizing Hormone Secretion in the Rhesus Monkey. Neuroendocrinology 1984;39:256–260. [DOI] [PubMed] [Google Scholar]
- 94.Martin GB, Thiery JC: Hypothalamic multiunit activity and LH secretion in conscious sheep. Experimental brain research 1987;67:469–78. [DOI] [PubMed] [Google Scholar]
- 95.Clarkson J, Han SY, Piet R, McLennan T, Kane GM, Ng J, et al. : Definition of the hypothalamic GnRH pulse generator in mice. Proceedings of the National Academy of Sciences 2017;114:E10216–E10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Navarro VM, Castellano JM, McConkey SM, Pineda R, Ruiz-Pino F, Pinilla L, et al. : Interactions between kisspeptin and neurokinin B in the control of GnRH secretion in the female rat. American Journal of Physiology-Endocrinology and Metabolism 2011; 300:E202–E210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Navarro VM, Gottsch ML, Chavkin C, Okamura H, Clifton DK, Steiner RA: Regulation of Gonadotropin-releasing hormone secretion by kisspeptin/Dynorphin/Neurokinin B neurons in the arcuate uucleus of the mouse. Journal of Neuroscience 2009;29:11859–11866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wakabayashi Y, Nakada T, Murata K, Ohkura S, Mogi K, Navarro VM, et al. : Neurokinin B and dynorphin A in kisspeptin neurons of the arcuate nucleus participate in generation of periodic oscillation of neural activity driving pulsatile gonadotropin-releasing hormone secretion in the goat. Journal of Neuroscience 2010;30:3124–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Goodman RL, Hileman SM, Nestor CC, Porter KL, Connors JM, Hardy SL, et al. : Kisspeptin, neurokinin B, and dynorphin act in the arcuate nucleus to control activity of the GnRH pulse generator in ewes. Endocrinology 2013;154:4259–4269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ruka KA, Burger LL, Moenter SM: Regulation of arcuate neurons coexpressing kisspeptin, neurokinin B, and dynorphin by modulators of neurokinin 3 and κ-opioid receptors in adult male mice. Endocrinology 2013;154:2761–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.De Croft S, Piet R, Mayer C, Mai O, Boehm U, Herbison AE: Spontaneous kisspeptin neuron firing in the adult mouse reveals marked sex and brain region differences but no support for a direct role in negative feedback. Endocrinology 2012;153:5384–5393. [DOI] [PubMed] [Google Scholar]
- 102.Qiu J, Nestor CC, Zhang C, Padilla SL, Palmiter RD, Kelly MJ, et al. : High-frequency stimulation-induced peptide release synchronizes arcuate kisspeptin neurons and excites GnRH neurons. eLife 2016;5:e16246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Krajewski SJ, Anderson MJ, Iles-Shih L, Chen KJ, Urbanski HF, Rance NE: Morphologic evidence that neurokinin B modulates gonadotropin-releasing hormone secretion via neurokinin 3 receptors in the rat median eminence. The Journal of Comparative Neurology 2005;489:372–386. [DOI] [PubMed] [Google Scholar]
- 104.Vanacker C, Moya MR, DeFazio RA, Johnson ML, Moenter SM: Long-term recordings of arcuate nucleus kisspeptin neurons reveal patterned activity that is modulated by gonadal steroids in male mice. Endocrinology 2017; DOI: 10.1210/en.2017-00382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Navarro VM, Gottsch ML, Wu M, Garcla-Galiano D, Hobbs SJ, Bosch MA, et al. : Regulation of NKB pathways and their roles in the control of Kiss1 neurons in the arcuate nucleus of the male mouse. Endocrinology 2011;152:4265–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Han SY, McLennan T, Czieselsky K, Herbison AE: Selective optogenetic activation of arcuate kisspeptin neurons generates pulsatile luteinizing hormone secretion. Proceedings of the National Academy of Sciences 2015;112:13109–13114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Han SY, Kane G, Cheong I, Herbison AE: Characterization of GnRH Pulse Generator Activity in Male Mice Using GCaMP Fiber Photometry. Endocrinology 2019;160:557–567. [DOI] [PubMed] [Google Scholar]
- 108.Kumar D, Freese M, Drexler D, Hermans-Borgmeyer I, Marquardt A, Boehm U: Murine arcuate nucleus kisspeptin neurons communicate with GnRH neurons in utero. Journal of Neuroscience 2014;34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mayer C, Boehm U: Female reproductive maturation in the absence of kisspeptin/GPR54 signaling. Nature Neuroscience 2011;14:704–710. [DOI] [PubMed] [Google Scholar]
- 110.Padilla SL, Perez JG, Ben-Hamo M, Johnson CW, Sanchez REA, Bussi IL, et al. : Kisspeptin neurons in the arcuate nucleus of the hypothalamus orchestrate circadian rhythms and metabolism. Current biology: CB 2019;29:592–604.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ruka KA, Burger LL, Moenter SM: Both estrogen and androgen modify the response to activation of neurokinin-3 and K-opioid receptors in arcuate kisspeptin neurons from male mice. Endocrinology 2016;157:752–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang L, Burger LL, Greenwald-Yarnell ML, Myers MG, Moenter SM: Glutamatergic transmission to hypothalamic kisspeptin neurons is differentially regulated by estradiol through estrogen receptor a in adult female mice. The Journal of Neuroscience 2018;38:1061 LP - 1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Phumsatitpong C M S: Corticotropin-releasing hormone (CRH) has no effect on arcuate kisspeptin neuron firing activity in female mice. Society for Neuroscience 2019; [Google Scholar]
- 114.DeFazio RA, Elias CF, Moenter SM: GABAergic transmission to kisspeptin neurons is differentially regulated by time of day and estradiol in female mice. The Journal of neuroscience: the official journal of the Society for Neuroscience 2014;34:16296–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Greenwald-Yarnell ML, Marsh C, Allison MB, Patterson CM, Kasper C, MacKenzie A, et al. : ERα in Tac2 neurons regulates puberty onset in female mice. Endocrinology 2016;157:1555–1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mayer C, Acosta-Martinez M, Dubois SL, Wolfe A, Radovick S, Boehm U, et al. : Timing and completion of puberty in female mice depend on estrogen receptor alpha-signaling in kisspeptin neurons. Proceedings of the National Academy of Sciences of the United States of America 2010;107:22693–22698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dubois SL, Acosta-Martlnez M, DeJoseph MR, Wolfe A, Radovick S, Boehm U, et al. : Positive, but not negative feedback actions of estradiol in adult female mice require estrogen receptor a in kisspeptin neurons. Endocrinology 2015;156(3):1111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Semaan SJ, Murray EK, Poling MC, Dhamija S, Forger NG, Kauffman AS: BAX-dependent and BAX-independent regulation of Kiss1 neuron development in mice. Endocrinology 2010; 151:5807–5817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang L, Vanacker C, Burger LL, Barnes T, Shah YM, Myers MG, et al. : Genetic dissection of the different roles of hypothalamic kisspeptin neurons in regulating female reproduction. eLife 2019;8 DOI: 10.7554/eLife.43999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Czieselsky K, Prescott M, Porteous R, Campos P, Clarkson J, Steyn FJ, et al. : Pulse and surge profiles of luteinizing hormone secretion in the mouse. Endocrinology 2016;157:4794–4802. [DOI] [PubMed] [Google Scholar]
- 121.Watson RE, Langub MC, Engle MG, Maley BE: Estrogen-receptive neurons in the anteroventral periventricular nucleus are synaptic targets of the suprachiasmatic nucleus and peri-suprachiasmatic region. Brain Research 1995;689:254–264. [DOI] [PubMed] [Google Scholar]
- 122.Brock O, De Mees C, Bakker J: Hypothalamic expression of oestrogen receptor a and androgen receptor is sex-, age- and region-dependent in mice. Journal of neuroendocrinology 2015;27:264–76. [DOI] [PubMed] [Google Scholar]
- 123.Gu GB, Simerly RB: Projections of the sexually dimorphic anteroventral periventricular nucleus in the female rat. The Journal of comparative neurology 1997;384:142–64. [PubMed] [Google Scholar]
- 124.Le WW, Berghorn KA, Rassnick S, Hoffman GE: Periventricular Preoptic Area Neurons Coactivated with Luteinizing Hormone (LH)-Releasing Hormone (LHRH) Neurons at the Time of the LH Surge Are LHRH Afferents 1. Endocrinology 1999;140:510–519. [DOI] [PubMed] [Google Scholar]
- 125.Wiegand SJ, Terasawa E: Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology 1982;34:395–404. [DOI] [PubMed] [Google Scholar]
- 126.Wiegand SJ, Terasawa E, Bridson WE, Goy RW: Effects of Discrete Lesions of Preoptic and Suprachiasmatic Structures in the Female Rat. Neuroendocrinology 1980;31:147–157. [DOI] [PubMed] [Google Scholar]
- 127.Wiegand SJ, Terasawa E, Bridson WE: Persistent estrus and blockade of progesterone-induced lh release follows lesions which do not damage the suprachiasmatic nucleus. Endocrinology 1978; 102:1645–1648. [DOI] [PubMed] [Google Scholar]
- 128.Kauffman AS, Gottsch ML, Roa J, Byquist AC, Crown A, Clifton DK, et al. : Sexual Differentiation of Kiss1 Gene Expression in the Brain of the Rat. Endocrinology 2007;148:1774–1783. [DOI] [PubMed] [Google Scholar]
- 129.Clarkson J, d’Anglemont de Tassigny X, Moreno AS, Colledge WH, Herbison AE: Kisspeptin-GPR54 signaling is essential for preovulatory gonadotropin-releasing hormone neuron activation and the luteinizing hormone surge. The Journal of neuroscience: the official journal of the Society for Neuroscience 2008;28:8691–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Robertson JL, Clifton DK, De La Iglesia HO, Steiner R a., Kauffman AS: Circadian regulation of Kiss1 neurons: Implications for timing the preovulatory gonadotropin-releasing hormone/luteinizing hormone surge. Endocrinology 2009;150:3664–3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kumar D, Candlish M, Periasamy V, Avcu N, Mayer C &, Boehm U: Specialized subpopulations of kisspeptin neurons communicate with GnRH neurons in female mice. Endocrinology 2015; 156:32–38. [DOI] [PubMed] [Google Scholar]
- 132.Clarkson J, Herbison AE: Dual Phenotype Kisspeptin-Dopamine Neurones of the Rostral Periventricular Area of the Third Ventricle Project to Gonadotrophin-Releasing Hormone Neurones. Journal of Neuroendocrinology 2011;23:293–301. [DOI] [PubMed] [Google Scholar]
- 133.DeFazio RA, Heger S, Ojeda SR, Moenter SM: Activation of A-type gamma-aminobutyric acid receptors excites gonadotropin-releasing hormone neurons. Molecular endocrinology (Baltimore, Md) 2002;16:2872–91. [DOI] [PubMed] [Google Scholar]
- 134.Kuehl-Kovarik MC, Pouliot WA, Halterman GL, Handa RJ, Dudek FE, Partin KM: Episodic Bursting Activity and Response to Excitatory Amino Acids in Acutely Dissociated Gonadotropin-Releasing Hormone Neurons Genetically Targeted with Green Fluorescent Protein. J Neurosci 2002;22:2313–2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stephens SBZ, Rouse ML, Tolson KP, Liaw RB, Parra RA, Chahal N, et al. : Effects of Selective Deletion of Tyrosine Hydroxylase from Kisspeptin Cells on Puberty and Reproduction in Male and Female Mice. eNeuro 2017;4:ENEURO.0150–17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Piet R, Kalil B, McLennan T, Porteous R, Czieselsky K, Herbison AE: Dominant Neuropeptide Cotransmission in Kisspeptin-GABA Regulation of GnRH Neuron Firing Driving Ovulation. The Journal of neuroscience: the official journal of the Society for Neuroscience 2018;38:6310–6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang L, DeFazio RA, Moenter SM: Excitability and burst generation of AVPV kisspeptin neurons are regulated by the estrous cycle via multiple conductances modulated by estradiol action. eNeuro 2016;3:e0094–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Piet R, Boehm U, Herbison AE, Zhang C, Tonsfeldt KJ, Qiu J, et al. : Estrous cycle plasticity in the hyperpolarization-activated current Ih Is mediated by circulating 17 -estradiol in preoptic area kisspeptin neurons. Journal of Neuroscience 2013;33:10828–10839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang C, Bosch MA, Qiu J, Rønnekleiv OK, Kelly MJ: 17β-Estradiol Increases Persistent Na + Current and Excitability of AVPV/PeN Kiss1 Neurons in Female Mice. Molecular Endocrinology 2015;29:518–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang C, Tonsfeldt KJ, Qiu J, Bosch MA, Kobayashi K, Steiner RA, et al. : Molecular mechanisms that drive estradiol-dependent burst firing of Kiss1 neurons in the rostral periventricular preoptic area. American journal of physiology Endocrinology and metabolism 2013;305:E1384–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Suzuki S, Rogawski M a: T-type calcium channels mediate the transition between tonic and phasic firing in thalamic neurons. Proceedings of the National Academy of Sciences of the United States of America 1989;86:7228–7232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Khaliq ZM, Bean BP: Pacemaking in dopaminergic ventral tegmental area neurons: depolarizing drive from background and voltage-dependent sodium conductances. The Journal of neuroscience: the official journal of the Society for Neuroscience 2010;30:7401–7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Krahe R, Gabbiani F: Burst firing in sensory systems. Nature reviews Neuroscience 2004;5:13–23. [DOI] [PubMed] [Google Scholar]
- 144.Porteous R, Herbison AE: Genetic deletion of Esr1 in the mouse preoptic area disrupts the LH surge and estrous cyclicity. Endocrinology 2019; DOI: 10.1210/en.2019-00284 [DOI] [PubMed] [Google Scholar]
- 145.Cheong RY, Czieselsky K, Porteous R, Herbison AE: Expression of ESR1 in glutamatergic and GABAergic neurons is essential for normal puberty onset, estrogen feedback, and fertility in female mice. Journal of Neuroscience 2015;35:14533–14543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Meng C, Zhou J, Papaneri A, Peddada T, Xu K, Cui G: Spectrally Resolved Fiber Photometry for Multi-component Analysis of Brain Circuits. Neuron 2018;98:707–717.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Sych Y, Chernysheva M, Sumanovski LT, Helmchen F: High-density multi-fiber photometry for studying large-scale brain circuit dynamics. Nature methods 2019;16:553–560. [DOI] [PubMed] [Google Scholar]
- 148.León S, Barroso A, Vázquez MJ, García-Galiano D, Manfredi-Lozano M, Ruiz-Pino F, et al. : Direct Actions of Kisspeptins on GnRH Neurons Permit Attainment of Fertility but are Insufficient to Fully Preserve Gonadotropic Axis Activity. Scientific Reports 2016;6:19206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pielecka-Fortuna J, Moenter SM: Kisspeptin Increases Y-Aminobutyric Acidergic and Glutamatergic Transmission Directly to Gonadotropin-Releasing Hormone Neurons in an Estradiol-Dependent Manner. Endocrinology 2010;151:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chachlaki K, Garthwaite J, Prevot V: The gentle art of saying NO: how nitric oxide gets things done in the hypothalamus. Nature Reviews Endocrinology 2017;13:521–535. [DOI] [PubMed] [Google Scholar]
- 151.Clasadonte J, Poulain P, Beauvillain J-C, Prevot V: Activation of Neuronal Nitric Oxide Release Inhibits Spontaneous Firing in Adult Gonadotropin-Releasing Hormone Neurons: A Possible Local Synchronizing Signal. Endocrinology 2008;149:587–596. [DOI] [PubMed] [Google Scholar]
- 152.Hanchate NK, Parkash J, Bellefontaine N, Mazur D, Colledge WH, d’Anglemont de Tassigny X, et al. : Kisspeptin-GPR54 signaling in mouse NO-synthesizing neurons participates in the hypothalamic control of ovulation. Journal of Neuroscience 2012;32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Moffitt JR, Bambah-Mukku D, Eichhorn SW, Vaughn E, Shekhar K, Perez JD, et al. : Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science 2018;362:eaau5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Campbell JN, Macosko EZ, Fenselau H, Pers TH, Lyubetskaya A, Tenen D, et al. : A molecular census of arcuate hypothalamus and median eminence cell types. Nature neuroscience 2017;20:484–496. [DOI] [PMC free article] [PubMed] [Google Scholar]